Review Article - Interventional Cardiology (2023) Volume 15, Issue 2
Existing relationship between viral infection and atherosclerosis
- Corresponding Author:
- Alexander V. Blagov
Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia
E-mail: al.blagov2014@gmail.com; - Alexander N. Orekhov
Petrovsky Scientific Center of Surgery, 2 Abrikosovsky Lane, 119991 Moscow, Russia
E-mail: a.h.opexob@ gmail.com
Received date: 09-Mar-2023, Manuscript No. FMIC-23-91193; Editor assigned: 10-Mar-2023, PreQC No. FMIC-23-91193 (PQ); Reviewed date: 24-Mar-2023, QC No. FMIC-23-91193; Revised date: 31-Mar-2023, Manuscript No. FMIC-23-91193 (R); Published date: 10-Apr-2023, DOI: 10.37532/1755- 5310.2023.15(2).685
Abstract
Atherosclerosis is manifested by thickening of the walls of the arteries and narrowing of their channels due to the accumulation of plaques. This is one of the most important indicators of cardiovascular disease. It can be caused by various factors such as smoking, a high cholesterol diet, hypertension, hyperglycemia, and genetic factors. However, atherosclerosis can also develop due to infection. Some bacteria and viruses have been reported to cause the development of atherosclerosis. Examples of these viruses are influenza viruses, herpes viruses, hepatitis viruses or papillomaviruses, which are widely distributed and known throughout the world. Moreover, many patients with Coronavirus Disease (COVID-19) showed symptoms of cardiovascular disease. The significance of various viral infections in the etiology and pathogenesis of atherosclerosis will be considered and analyzed in detail in this review.
Keywords
Atherosclerosis; Viral infection; LDL; COVID-19
Abbreviations
ASCVD: Atherosclerotic Cardiovascular Disease; LDL: Low-Density Lipoproteins; HIV: Human Immunodeficiency Virus; HSV: Herpes Simplex Virus; TLR: Toll-Like Receptor; NK cells: Natural Killer cells; TCR: T-Cell Receptor; BCR: B-Cell Receptor; DC: Dendric Cells; CMV: Cytomegalovirus; SMC: Smooth Muscle Cells; EC: Endothelial Cells; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; ROS: Reactive Oxygen Species; HPV: Human Papilloma Virus
Introduction
Atherosclerosis is a chronic disease characterized by thickening of the walls of arteries and their narrowing of channels due to the accumulation of plaques, which mainly consist of fats, cholesterol, mineral crystals, and cellular decay products [1]. It is primarily a lipid-dependent process initiated by the accumulation of low-density lipoproteins and residual lipoprotein particles, as well as an active inflammatory process in the focal areas of the arteries, especially in areas of impaired non-laminar blood flow at the arterial bi-furcation points, and is considered the main cause of Atherosclerotic Cardiovascular Disease (ASCVD), leading to heart attacks, stroke, and peripheral arterial disease [2,3].
Atherosclerosis mainly develops as a result of a continuous process of damage to the arterial wall due to the retention of lipids by entrapment of the intima by a matrix such as proteoglycans, which leads to modification, which in turn exacerbates chronic inflammation in vulnerable areas of the arteries and plays an important role in all phases, progression of atherogenesis [4]. This process begins with nascent fatty streaks in the intima of the arteries, which develop into fibrous plaques and develop into complex atherosclerotic lesions prone to rupture. In addition, stenosis due to inward expansion of atheroma can lead to occlusion of vessels, such as coronary [5].
And atherosclerosis has a multifactorial etiology. The most common risk factors include hypercholesterolemia (LDL cholesterol), arterial hypertension, diabetes mellitus, cigarette smoking, age (men over 45 and women over 55), male gender, and a strong family history [6]. In addition, sedentary lifestyles, obesity, diets high in saturated and trans fatty acids, and some genetic mutations contribute to the risk [7]. It has also recently been suggested that inflammation and infection may themselves trigger the development of atherosclerosis in the absence of other risk factors. Some viral agents are reported to be able to influence the progression of atherosclerosis [8]. In this review, the characteristics of viruses and molecular mechanisms that are considered to be related to the pathogenesis of atherosclerosis through viral infection will be considered.
Literature Review
General model of viral infection and antiviral immune response
Most human viruses replicate only in certain target tissues, which is mainly a con-sequence of the distribution of viral receptors. Many viruses use two receptors, such as the use of the HIV CD4 and CCR5 co-receptor [9]. Once attached to a cell receptor, viruses can fuse with the cell membrane or undergo endocytosis and then enter the cytoplasm or nucleus by fusion with the vesicular membrane (enveloped viruses such as HSV and HIV), or move across the cell membrane, or induce lysis of the endocytic vesicle immediately in the cytoplasm (non-enveloped viruses such as Norwalk virus and poliovirus). Viruses then use host cell machinery and specialized proteins encoded by the virus to rapidly replicate within the cell. Once replicated inside a cell, many viruses cause cytolysis to promote the release of new infectious virions (e.g. poxviruses, polio and herpes viruses). Other viruses are released from infected cells by budding through the cell membrane in the absence of cell death (e.g. HIV and influenza virus). However, once in the body, viruses encounter numerous innate defense mechanisms and activate components of adaptive immunity. The latter usually ensures that clinical disease, if not infection, does not occur [9].
Viral infection induces an extensive set of defense mechanisms in the host. Innate defenses come into play to block or inhibit initial infection, protect cells from infection, or kill virus-infected cells, and occur long before adaptive immunity emerges. Innate immune defenses are initiated by pathogen recognition receptors of the Toll-Like Receptor (TLR) family. Various TLR molecules recognize certain viral products, such as single and double stranded RNAs (TLR 3 and TLR7/8, respectively) or double stranded DNAs (TLR9) [10].
The innate defense system consists of many cellular components and many specialized proteins. The oldest known and best studied antiviral proteins are the α/β IFNs, which act by binding to the type I IFN receptor and result in the transcription of over 100 IFNstimulated genes. Type I IFNs also activate Natural Killer (NK) cells and induce other cytokines such as Interleukin (IL)-12 that promote the NK response. NK cells produce pro- inflammatory cytokines, they can kill infected cells and interact with Dendritic Cells (DC) and are also an important component of innate defense against viruses [11].
In addition to IFN-α/β, several other host proteins are involved in antiviral defense. These include natural antibodies, which may play a role in protecting against certain viral infections, as well as complement proteins. Some viruses can be directly inactivated by complement activation or killed by phagocytic cells that bind and engulf complement bound virions. Several cytokines and chemokines induced by viral infection also play a role in defense. These include the cytokines TNF-α, IFN-γ, IL-12, IL-6, and chemokines such as MIP-1α. In particular, IL-12 is a potent inducer of IFN-γ from NK cells. Inflammatory chemokines may also play an important role in innate antiviral defense by orchestrating macrophage, neutrophil, DC, and NK responses at the site of infection [9].
Innate immunity generally serves to slow rather than stop viral infection, allowing time for an adaptive immune response to begin. Lymphocytes are the central component of the adaptive immune system. Thanks to specialized receptors on the surface of lymphocytes (T-Cell Receptor (TCR), B-Cell Receptor (BCR)/ antibody molecule), these cells are able to recognize antigenic peptides of microbial origin, while maintaining tolerance to their own proteins. These cells can directly lyse or destroy virus-infected cells or produce antiviral agents such as cytokines (IFN, TNF) that enhance the defense mechanisms of the host’s innate immune system [12].
Chronic viral infections and atherosclerosis
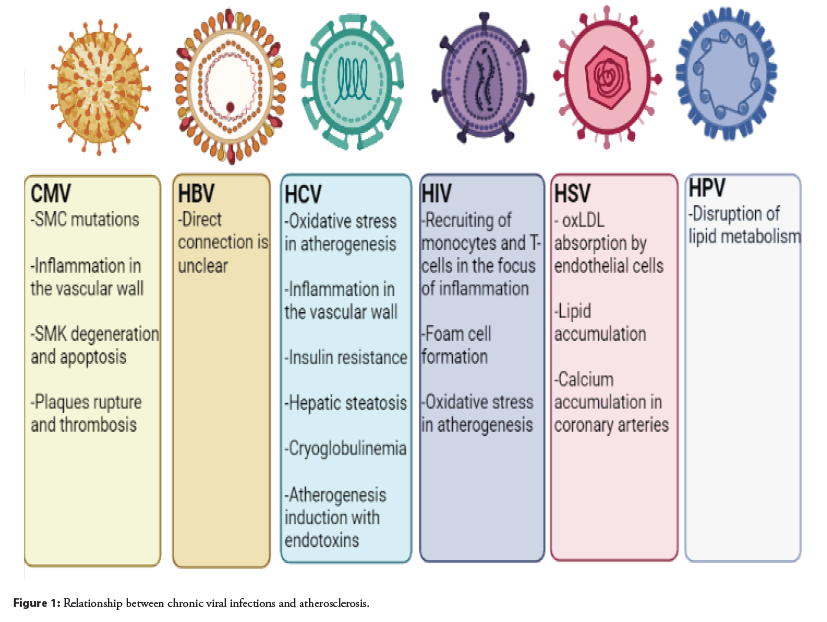
Cytomegalovirus: Cytomegalovirus (CMV) is a widespread virus whose manifestations range from asymptomatic to severe target organ dysfunction in immunocompromised patients with congenital CMV infection. CMV is a double-stranded DNA virus and belongs to the herpesviruses. Like other herpesviruses, after recovery from the initial infection, CMV remains dormant in the host. Viral reactivation occurs during the compromise of the immune system with immunosuppression [13].
CMV infection can induce a chronic immune inflammatory response [14]. Latent CMV infection periodically reactivates, resulting in a chronic immune or inflammatory response that damages the endothelium and vascular inner membrane, leading to Smooth Muscle Cell (SMC) proliferation and mutation. The formation of immune complexes of antibodies with the CMV antigen deposited in the vascular wall in atherosclerosis can induce vascular Endothelial Cells (EC), macrophages, foam cells, SMC and T-lymphocytes to express monocytic proteins CCL-2,-3,- 4 and-5, as well as macrophage colony- stimulating factor [15]. Moreover, they also stimulate macrophages to produce and release interleukins IL-1,-6,-8,-10 and-12, Tumor Necrosis Factor alpha (TNF-α), and other inflammatory cellular factors that induce cellular and humoral immune responses, and accelerate the release of C-Reactive Protein (CRP), causing an inflammatory chain reaction. Activation of blood mononuclear cells causes their migration to the intima. Among them, CCL-2 is the most important and most potent inducer of monocyte migration [16]. These cells stimulate CCL-2 expression in vitro via oxidized Low- Density Lipoprotein (ox-LDL). Another study showed that due to intermittent CMV activation in arterial SMCs induced by local immune responses and inflammation, SMCs exhibit degeneration and apoptosis induced by inflammatory substances, leading to plaque instability prone to rupture and bleeding, as well as acute coronary syndrome [17]. It has also been shown that CMV products activate the Cyclooxygenase-2 (COX-2) promoter, increasing the release of arachidonic acid. ROS are produced by COX-2-dependent pathway and cause damage to the vascular endothelium, activation, adhesion and aggregation of platelets, increased release and increased activity of tissue type plasminogen activator, which leads to plaque thrombosis [14].
Hepatitis B Virus: Hepatitis B Virus (HBV) is a partially double stranded DNA virus that transforms from pregenomic Ribonucleic Acid (RNA) to DNA by reverse transcription during its life cycle. The genome consists of an outer lipid envelope and an inner nucleocapsid core encoded by four overlapping open reading frames named C, X, P, and S [18]. The hepatitis B virus is transmitted by percutaneous inoculation or through mucous membranes with infected body fluids. After infection with the hepatitis B virus, most adults can recover from the infection. Patients may have acute symptomatic disease or have asymptomatic disease that is detected during HBV screening [19].
Early research on the association between HBV and atherosclerosis has yielded conflicting results. Evidence was obtained that showed no association between the risk of atherosclerosis and various serological markers of HBV infection, including HBsAg, as well as anti-HBs [20]. However, this study had some important limitations, especially regarding the adequacy of serological markers of HBV infection and time period. In a more recent study, HBsAg seropositivity was associated with a reduced risk of ischemic stroke and MI and an increased risk of hemorrhagic stroke. However, this association appeared to be secondary to HBV-associated liver dysfunction and did not support the hypothesis that HBV infection per se is associated with an increased risk of MI or ischemic stroke [21].
Dividing HBsAg positive participants into 2 groups based on the presence of liver dysfunction allowed us to differentiate any proinflammatory effect of HBV infection itself on CVD risk from a possible anticoagulant effect of liver dysfunction caused by secondary chronic HBV infection. This latter effect has been demonstrated by a higher risk of hemorrhagic stroke and a lower risk of myocardial infarction and ischemic stroke, especially in those with severe liver dysfunction among HBsAg positive men [21].
However, direct evidence of a link between HBV and atherosclerosis has not yet been obtained, which requires additional, more extensive studies.
Hepatitis C Virus: Hepatitis C Virus (HCV) is a spherical, enveloped positive strand Ribonucleic Acid (RNA) virus approximately 55 nm in diameter. It is a member of the Flaviviridae family, but should be classified as a separate Hepacivirus genus. The genome is approximately 9.6 kb long. It codes for a polyprotein which is then converted into at least ten proteins. Most patients (80% to 85%) who become acutely infected are unable to clear the virus and as a result the disease progresses to a chronic form. Consequences of chronic infection include cirrhosis, portal hypertension, hepatic decompensation with encephalopathy, and hepatocellular carcinoma [22].
Atherosclerosis is a chronic inflammatory disease and the mechanisms by which HCV can induce or promote atherosclerosis are not fully understood. Chronic HCV infection causes hepatic and systemic inflammation, and it has been suggested that direct and indirect mechanisms may be involved in the development of atherosclerosis through increased levels of pro-atherogenic chemokines and cytokines and induction of pro atherogenic metabolic factors [23]. HCV has been shown to live and multiply in carotid plaques, supporting the hypothesis that HCV plays a direct pro-atherogenic role by causing arterial inflammation, probably through the pro inflammatory cytokine inter leukin 1β [24]. In addition, HCV structural and nonstructural proteins play an important role in the initiation and maintenance of chronic inflammation and in the occurrence of oxidative stress triggering atherogenesis [24]. HCV also affects glucose and lipid metabolism, leading to Insulin Resistance (IR), diabetes, and hepatic steatosis, which are known factors that cause atherosclerosis [25]. A high ratio of TNF-α/adiponectin was found in patients with HCV, which is associated with the development of IR and atherosclerosis. It has been reported that atherosclerosis in HCV patients has also been associated with severe hepatic fibrosis [26].
Cryoglobulinemia occurs with high frequency in patients with chronic HCV infection, and the virus plays an etiological and pathogenetic role in its development [27]. The presence of cryoglobulinemia is associated with vacuities and cardiovascular events. It has recently been shown that endotoxinemia causes a proatherogenic inflammatory state and increased levels of oxidative stress, which contributes to the development of atherosclerosis in patients with chronic HCV [28].
Human Immunodeficiency Virus: The Human Immunodeficiency Virus (HIV) is an enveloped retrovirus containing 2 copies of the single-stranded RNA genome. It causes Acquired Immunodeficiency Syndrome (AIDS), which is the last stage of HIV infection. Two to four weeks after HIV enters the body, the patient may complain of symptoms of the primary infection [29]. After this, a long-term chronic HIV infection occurs, which can last for decades. AIDS is mainly characterized by opportunistic infections and tumors, which are usually fatal if left untreated [30].
Much evidence has been obtained indicating that HIV accelerates the development of atherosclerosis. The main link in HIVassociated atherosclerosis is likely to focus on macrophages and their critical role in inflammation and plaque formation. Migration and adhesion of monocytes to the sub endothelial space and removal of oxcLDL leading to transformation into foam cells are critical steps in atherogenesis [31]. Chemokines and their receptors play an important role in the migration of macrophages into the vasculature. Inhibition of their activity (e.g. CCL2, CX3CR1 and CCR5) resulted in a marked decrease in atherogenesis, and the size of the atherosclerotic lesion was strongly correlated with the number of circulating monocytes. This indicates that these chemokines and adhesion molecules account for nearly all of the migration and accumulation of monocytes in atherosclerotic arteries and highlights the importance of macrophage migration in atherogenesis [32]. During HIV infection, chemokines are elevated and may play a role in recruiting monocytes into the vasculature, thereby promoting HIV-associated atherogenesis [33]. The HIV protein Nef interferes with the normal function of the ATPbinding cassette transporter A1 (ABCA1) by interfering with the efflux of cholesterol from infected macrophages. This disruption leads to lipid accumulation and transformation of macrophages into foam cells in vitro and in vivo [34].
In HIV-infected patients with viral suppression, the percentage of activated T cells (CD38+HLA-DR+) was still 2-fold higher compared to the HIV control group [35]. And normally high levels of T cell activation contribute to the progressive loss of CD4+T-cells, even without measurable viremia [36]. Chronic immune activation may contribute to the initiation of endothelial activation and subsequent atherogenesis, and these markers of T cell activation have been correlated with atherosclerosis in several studies. In atherosclerosis, T cells are recruited along with macrophages into the endothelium. In plaques, T cells produce pro atherogenic mediators that promote lesion growth and exacerbate atherogenesis [37].
ROS play a significant role in the development of atherosclerosis.
Both in vitro and in vivo models of HIV infection, as well as primary patient samples, demonstrate increased levels of ROS and induced Oxidative Stress (OS). ROS levels and their effects (e.g. oxcLDL and oxidized nucleic acids) are higher in previously untreated patients compared to those treated with antiretroviral therapy [38]. The increase in ROS is the result of both an increase in production and a decrease in antioxidant capacity. HIV infected patients with elevated OS due to mutations affecting antioxidant capacity have an increased plasma HIV viral load, reduced CD4+ T-cell counts, and increased cytotoxicity. Many HIV proteins, including Nef, increase ROS production, causing endothelial dysfunction [39].
Herpes Simplex Virus: Herpes Simplex Virus (HSV) infections are widespread among people throughout the world [40]. The infection persists throughout life and is characterized by periodic reactivations at the site of infection. HSV type 1 is mainly transmitted by oral-to-oral contact and usually causes otolabial herpes. The type 1 virus also causes rarer conditions such as keratitis and other eye complications, as well as encephalitis. Infection of the genitals with HSV type 1 through oral-genital contact is becoming more common, although reactivation occurs less frequently than with HSV type 2. HSV type 2 is almost entirely sexually transmitted, causing genital herpes [41].
Autopsy, biopsy, metadata analysis, and laboratory data confirmed that HSV infection can initiate and progress the development of atherosclerosis. The higher detection of HSV-1 DNA at autopsy and atherosclerosis biopsy means that the virus is closely associated with the disease [42]. The Meta analysis also suggests that HSV may increase the incidence of atherosclerosis. From human samples, it was confirmed that HSV-1 was more significantly detected in groups with atherosclerosis compared to groups without atherosclerosis [42]. HSV activates the Lectin-Like Receptor for Oxidized LDL-1 (LOX-1), which is the main protein receptor for oxidized LDL, stimulates the absorption of oxLDL by endothelial cells, provokes the accumulation of lipids and their metabolism due to increased acquisition of saturated cholesterol esters and triacylglycerols, induces accumulation of calcium in the coronary arteries and causes the development of thrombosis, which are associated with the development of atherosclerosis [43]. There is no doubt that HSV is involved in the pathogenesis of atherosclerosis. Vaccines and specific treatments against these types of viruses are not yet available, but antiviral drugs such as acyclovir, famciclovir, and valaciclovir are the most popular drugs to relieve symptoms in people infected with HSV, although these drugs cannot completely cure the infection [44].
Human Papillomavirus: Human Papillomavirus (HPV) is a non-enveloped; double stranded circular DNA virus that causes multiple epithelial lesions and cancer. It can present as cutaneous and anogenital warts, which, depending on the subtype, may progress to carcinoma [45]. There is some evidence that vaginal HPV infections increase the risk of cardiovascular disease. One study identified HPV in 55% of atheromatous coronary arteries in a small sample of postmortem donors, and HPV E7 protein was found in smooth muscle cells, plasma cells, and foamy macrophages located in these plaques. These observations raise the question of possible mechanisms by which HPV may contribute to the formation of atheromatous plaques [46].
The prevailing opinion is that HPV remains limited to its epithelial lesions, but some authors report the detection of HPV DNA and proteins on endothelial cells and circulating blood leukocytes [47]. If these observations reflect the true spread of the virus, they may help explain the presence of HPV in atheromatous lesions. On the other hand, if HPV remains limited to mucosal lesions, it may be involved in CAD by promoting systemic inflammatory status and dysregulating host metabolism, in particular lipid metabolism [47].
Recently, a study has been conducted that supports the hypothesis that HPV infection is associated with CHD and that there is a specific association with high risk HPV types. If HPV does reach the coronary arteries and is present in atheromatous plaques, it may contribute to their development through its classical cellular targets, p53, and the Retinoblastoma protein (pRb). Loss of p53 function in macrophages has been found to be strongly associated with an increase in atherosclerotic lesions. In addition, pRb knockout animals showed an increased development of atherosclerosis [46].
The results also show lower HDL and higher systemic blood pressure in HPV-positive compared to HPV-negative groups. Statistical correlations with HPV infection were very significant and require explanation, which may include HPV interference with lipid metabolism. However, the results obtained are very limited, and the lack of additional data on cardiovascular diseases does not allow drawing accurate conclusions in this regard [47]. The relationship between chronic viral infections and atherosclerosis is shown in Figure 1.
Acute viral infections and atherosclerosis
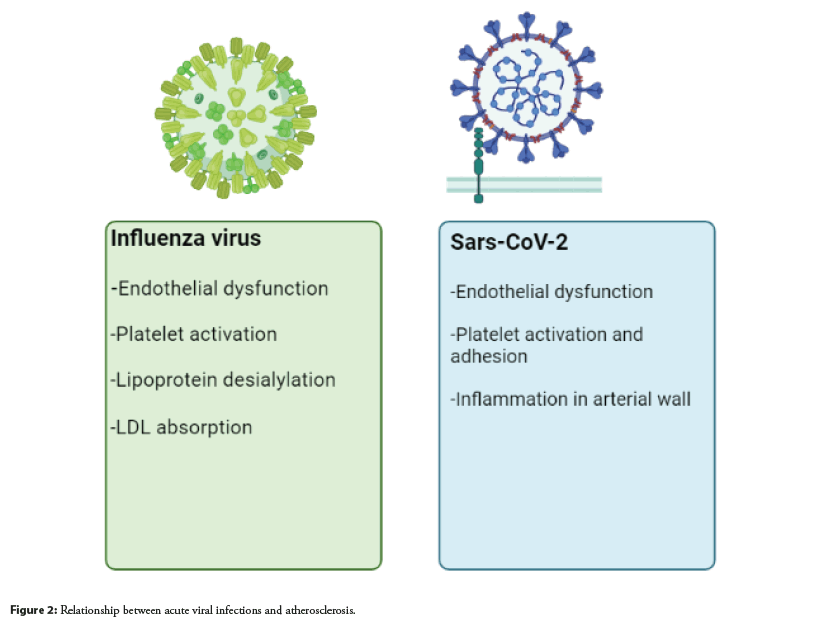
Influenza Virus: Influenza is an infectious viral disease that affects the upper and lower respiratory tract. It is caused by a wide range of influenza viruses. Some of these viruses can infect humans and some are species specific. These viruses are transmitted through respiratory droplets released from the mouth and respiratory system during coughing, talking and sneezing. Influenza viruses are about 80-120 nm in size and are roughly spherical/ovoid in shape. All of them contain a single stranded negative sense segmented RNA genome. They encode 11 proteins, including nine structural polymerase proteins polymerase basic protein 1 (PB1), PB1-F2, PB2 and Polymerase Acidic protein (PA); surface glycoproteins Hem Agglutinin (HA) and Neuraminidase (NA); Nucleocapsid Protein and Nucleo Proteins (NP); matrix proteins, M1; ion pore protein, M2) and two nonstructural proteins (NS1 and NS2), respectively [48].
Influenza viruses that attack the respiratory system usually cause fever, sore throat, muscle aches, cough, fatigue, and runny nose; they can also cause severe symptoms such as acute lung injury, pulmonary edema, hypoxemia, acute respiratory failure, and even heart failure. Vascular collapse with thrombosis and acute myocardial infection [49]. They induce life threatening systemic inflammatory syndromes by elevating adhesion molecules, chemokines, inflammatory mediators, and cytokines such as Tumor Necrosis Factor (TNF), Interleukin-1 beta (IL-1β), and Interleukin-6 (IL-6) [50]. Neutrophils secrete Neutrophil Extracellular Traps (NETs), which have a cytotoxic effect on lung endothelial cells and ultimately damage organs [51]. Moreover, viruses can induce apoptosis of epithelial cells and rearrange the structure of the endothelium, causing endothelial permeability and vascular leakage through hyper activation of cytokines and chemokines. At a diagnostic and practical level, it has been shown that influenza infection and acute myocardial infarction are strongly interrelated, and it has been reported that atherosclerosis can be caused by a thrombogenic environment through platelet activation and endothelial dysfunction caused by influenza infections [52]. In addition, neuraminidase, a group of enzymes that degrade sialic acid during virus exit from the host cell, can induce lipoprotein desialylation, increase LDL uptake, and thus enhance the development of atherosclerosis by increasing blood clots [53].
SARS-CoV-2: SARS-CoV-2 is a single stranded RNA virus belonging to the Coronaviridae family. Genome sequencing revealed the genetic similarity of the new virus with the previously known SARS-CoV (~79%) and MERS-CoV (~50%) coronaviruses. The S-protein of the SARS-CoV-2 virus has a binding affinity for the Angiotensin Converting Enzyme 2 (ACE2) receptor, and its affinity for this receptor is 10-20 times higher than that of SARS-CoV, which provides a high contaminating ability. Transmembrane type 2 serine protease (TMPRSS2) of host cells promotes viral uptake by cleaving ACE2 and activating the SARS-CoV-2 S protein, which mediates viral entry into cells [54].
The pathogenicity of SARS-CoV-2 is inextricably linked with the amount of systemic release of pro inflammatory cytokines [55]. “Cytokine storm” is caused by an inadequate host immune response to SARS-CoV-2 with excessive production of CD14++ and CD16+ inflammatory monocytes exacerbated by hyper activation of the T-helper (Th) 1-lymphocyte pathway [56]. Aberrant activation of innate immune pathways causes a complex multiorgan disease with thromboinflammatory manifestations; histologically, infected tissues are infiltrated with a large number of monocytes and neutrophils with Thrombotic Microangiopathy (TMA) as an expression of microvascular endothelial damage [57].
A disrupted inflammatory response with growing and consistent evidence supporting the central role played by complement dysregulation found in the natural course of COVID-19 causes hyperinflammation, immunothrombosis, and microvascular endothelial damage [58,59].
In pathological studies, complement deposition was observed in various tissues of deceased patients with COVID-19, with particular involvement of lung cells [60]. In particular, the damage mediated by the membrane attack complex (MAC or C5b-C9) seen in complement mediated disease is shown, with the identification of endothelial cell abnormalities typical of neutrophils with Thrombotic Microangiopathy (TMA) [61].
An elevated serum level of C5a, a serine protease important for the complement mediated pro inflammatory response, is a potential biomarker for predicting disease severity [62]. Activation of this last protease triggers thrombus inflammation with recruitment and activation of neutrophils in a process called Neutrophil Extracellular Trap (NET) release or NETosis; a potent activator of cultured endothelial cells in COVID-19, potentially leading to microvasculature occlusion. Like circulating complement components, serum NET levels positively correlate with disease severity due to cytotoxic effects on epithelial and endothelial cells [63].
Dysregulation of the interaction between NET and complement, which leads to an aberrant immune response, has recently been investigated and found that selective complement inhibitors can reduce and attenuate neutrophil activation and NETosis [63]. In addition to being involved in severe COVID-19, NETs may play a critical role in atherogenesis with multiple implications. The useful cholesterol efflux capacity imparted by HDL particles may be reduced due to the induction of oxidative stress. Platelet adhesion, activation, and aggregation can be stimulated by NET, which further promotes the accumulation of prothrombotic molecules such as von Willebrand factors and fibrinogen [64]. The relationship between acute viral infections and atherosclerosis is shown in Figure 2.
Discussion
Atherosclerosis is a chronic inflammatory disease manifested by thickening of arterial walls and narrowing of their channels due to plaque accumulation [65]. It is widely known that atherosclerosis can develop with smoking, a high cholesterol diet, hypertension, or hyperglycemia; but viral infection can also provoke and exacerbate the development of atherosclerosis [66]. However, information regarding the mechanisms underlying the development of atherosclerosis in viral infection is still limited, and current understanding is mainly based on statistical reports from various clinical studies. Many aspects of their molecular effects are thought to be related to general inflammatory and immunological events [67]. Moreover, according to the Centers for Disease Control, in patients with Coronavirus Infection (COVID-19) caused by the novel Severe Acute Respiratory Syndrome (SARS) Coronavirus-2 (CoV-2), cardiovascular disease is one of the most common comorbidities, which strongly indicates cardiovascular and atherosclerotic manifestations of SARS CoV-2 [68].
For all viral infections identified in this review (with the exception of HBV), pathological ways of enhancing the progression of atherosclerosis have been identified. Some of these ways are manifested in an increase in the inflammatory response; others in a disruption of lipid metabolism, thirds are associated with an increased absorption of oxLDL and the development of thrombosis and etc. Understanding these relationships may lead to better medication prescription for patients with comorbid conditions and the development of new drugs for them. The review [69], considers 2 models of the effect of viruses on the pathogenesis of atherosclerosis: In the first case, viruses infect vascular cells, causing inflammation in the endothelium; in the second case, they spread to various tissues, causing systemic inflammation. In addition, it is important to study the correlation between the development of a viral infection in atherosclerosis and the modulation of the activity of a number of genes directly related to the progression of atherosclerosis, for example, Sirtuin 1. The product of this gene is involved in the regulation of many processes important for the maintaining of life, including glucose and lipid metabolism, apoptosis and aging [70,71]. The regulation of lipid and carbohydrate metabolism may be the key to understanding the relationship between the modulation of Sirtuin 1 activity during viral infection and its role in the development of atherosclerosis [72]. From our point of view, for a better further understanding of the relationship between viral infections and atherosclerosis, it is necessary to do a lot of work to collect comorbidity statistics in patients who have had or have a viral disease, and at the same time was treated of an atherosclerosis syndrome.
Conclusion
This review looked at specific viruses that are involved in the progression of atherosclerosis which can give a general picture of this relationship. Common pathways for the impact of viruses that cause acute viral respiratory infection on the pathogenesis of atherosclerosis are the initiation of endothelial dysfunction and thrombosis. Viruses that cause chronic infection can increase inflammation in the vascular endothelium, induce oxLDL uptake and vascular calcification, and promote endothelial cell apoptosis and atherosclerotic plaque rupture. Although there have been studies proving a link between viral infection and the progression of atherosclerosis, the exact mechanisms are still not completely clear. Therefore, more research is needed for getting precise knowledge of the relationship between atherosclerosis and viral infection.
Author Contributions
Writing-original draft preparation, A.V.B.,M.A.P.; writingreview and editing, V.N.S.,E.B.Z., A.V.G.; supervision, A.N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant#22-25-00480).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul S, Lancaster GI, Meikle PJ, et al. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog Lipid Res.74:186-195 (2019).
- Ala-Korpela M. The culprit is the carrier, not the loads: cholesterol, triglycerides and apolipoprotein B in atherosclerosis and coronary heart disease. Int J Epidemiol. 48(5):1389-1392 (2019).
- Branchetti E, Bavaria JE, Grau JB, et al. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc biol. 34(10):2349-2357 (2014).
- Poggio P, Branchetti E, Grau JB, et al. Osteopontin-CD44v6 interaction mediates calcium deposition via phospho-Akt in valve interstitial cells from patients with noncalcified aortic valve sclerosis. Arterioscler Thromb Vasc biol. 34(9):2086-2094 (2014).
- Doodnauth SA, Grinstein S, Maxson ME, et al. Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Philos Trans R Soc Lond B Biol Sci. 374(1765):20180147 (2019).
- Reiss AB, Grossfeld D, Kasselman LJ, et al. Adenosine and the cardiovascular system. Am J Cardiovasc Drugs. 19(5):449-464 (2019).
- Zanobini M, Saccocci M, Tamborini G, et al. Postoperative echocardiographic reduction of right ventricular function: Is pericardial opening modality the main culprit? Biomed Res Int. 2017:4808757 (2017).
- Campbell LA, Rosenfeld ME. Infection and atherosclerosis development. Arch Med Res. 46(5):339-350 (2015).
- Williams MA, Fujinami RS. Immunopathology.(2014)
- Notarangelo LD. Human genetic defects resulting in increased susceptibility to viral infections. 375-388 (2016).
- Kee BL, Morman RE, Sun M, et al. (2020). Transcriptional regulation of natural killer cell development and maturation. Adv Immunol.146:1-28 (2020).
- Sharma S, Thomas PG. The two faces of heterologous immunity: protection or immunopathology. J Leukoc Biol. 95(3):405-441 (2013).
- Ngai JJ, Chong KL, Oli Mohamed S, et al. Cytomegalovirus retinitis in primary immune deficiency disease. Case Rep Ophthalmol Med. 2018:8125806 (2018).
- Du Y, Zhang G, Liu Z, et al. Human cytomegalovirus infection and coronary heart disease: A systematic review. Virology Journal. 15(1):31 (2018).
- Popović M, Smiljanić K, Dobutović B, et al. Human cytomegalovirus infection and atherothrombosis. J Thromb Thrombolysis. 33(2):160-172 (2011).
- Bakst RL, Xiong H, Chen CH, et al. Inflammatory monocytes promote perineural invasion via CCL2-mediated recruitment and cathepsin b expression. Cancer Res. 77(22):6400-6414 (2017).
- Izadi M, Fazel M, Saadat SH, et al. (2012). Cytomegalovirus localization in atherosclerotic plaques is associated with acute coronary syndromes: Report of 105 patients. Methodist Debakey Cardiovasc J. 8(2):42-46 (2012).
- McNaughton AL, D'Arienzo V, Ansari MA, et al. Insights from deep sequencing of the HBV genome-unique, tiny, and misunderstood. Gastroenterology. 156(2):384-399 (2018).
- Wang J, Zhang P, Zeng J, et al. (2019). Occurrence of occult hepatitis B virus infection associating with envelope protein mutations according to anti-HBs carriage in blood donors. Int J Infect Dis. 92:38-45 (2019).
- Völzke H, Schwahn C, Wolff B, et al. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 174(1):99-103 (2004).
- Jung SH, Lee KT. Atherosclerosis by virus infection-A short review. Biomedicines. 10(10):263 (2022).
- Galati G, Muley M, Vigano M, et al. (2019). Occurrence of hepatocellular carcinoma after direct-acting antiviral therapy for hepatitis C virus infection: Literature review and risk analysis. Expert Opin Drug Saf. 18(7):603-610 (2019).
- Zampino R, Marrone A, Restivo L, et al. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 5(10):528 (2013).
- Adinolfi LE. Chronic hepatitis C virus infection and atherosclerosis: Clinical impact and mechanisms. World J Gastroenterol. 20(13):3410 (2014).
- Adinolfi LE, Restivo L, Zampino R, et al. (2012). Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 221(2):496-502 (2012).
- Petta S, Torres D, Fazio G, et al. Carotid atherosclerosis and chronic hepatitis C: A prospective study of risk associations. Hepatology. 55(5):1317-1323 (2012).
- Zampino R, Marrone A, Rinaldi L, et al. Endotoxinemia contributes to steatosis, insulin resistance and atherosclerosis in chronic hepatitis C: The role of pro-inflammatory cytokines and oxidative stress. Infection. 46(6):793-799 (2018).
- Ragab G, Hussein MA. Vasculitic syndromes in hepatitis C virus: A review. J Adv Res. 8(2):99-11 (2017).
- Brew BJ, Garber JY. Neurologic sequelae of primary HIV infection. Handb Clin Neurol. 65-74 (2018).
- Capriotti T. HIV/AIDS. Home Healthcare Now. 36(6):348-355 (2018).
- Westhorpe CLV, Maisa A, Spelman T, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 92(2):133-138 (2013).
- Hamon P, Loyher PL, Baudesson de Chanville C, et al. CX3CR1-dependent endothelial margination modulates Ly6C high monocyte systemic deployment upon inflammation in mice. Blood. 129(10):1296-1307 (2017).
- Chow DC, Kagihara JM, Zhang G, et al. Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clinical Trials. 17(3):114-122 (2016).
- Pushkarsky T, Shilov E, Kruglova N, et al. Short communication: Accumulation of neutral lipids in liver and aorta of nef -transgenic mice. AIDS Res Hum Retroviruses. 33(1): 57-60 (2017).
- Karim R, Mack WJ, Kono N, et al. (2014). T-cell activation, both pre and post-haart levels, correlates with carotid artery stiffness over 6.5 years among HIV-infected women in the WIHS. J Acquir Immune Defic Syndr. 67(3):349-356 (2014).
- Grome HN, Barnett L, Hagar CC, et al. Association of T cell and macrophage activation with arterial vascular health in HIV. AIDS Res Hum Retroviruses. 33(2):181-186 (2017).
- Guaraldi G, Luzi K, Bellistrì GM, et al. CD8 T-cell activation is associated with lipodystrophy and visceral fat accumulation in antiretroviral therapy-treated virologically suppressed HIV-infected patients. J Acquir Immune Defic Syndr. 64(4):360-366 (2013).
- Watanabe LM, Barbosa Júnior F, Jordão AA, et al. Influence of HIV infection and the use of antiretroviral therapy on selenium and selenomethionine concentrations and antioxidant protection. Nutrition. 32(11-12):1238-1242 (2016).
- Kearns A, Gordon J, Burdo TH, et al. (2017). HIV-1-Associated atherosclerosis: Unraveling the missing link. J Am Coll Cardiol .69(25):3084-3098 (2017).
- Looker KJ, Magaret AS, Turner KME, et al. (2015). Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 10(1): e114989 (2015).
- James C, Harfouche M, Welton NJ, et al.(2020). Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bulletin of the World Health Organization. 98(5):315-329 (2020).
- Wu Y. Peng Sun, Wang Y, et al. (2016). Herpes simplex virus type 1 and type 2 infection increases atherosclerosis risk: Evidence based on a meta-analysis. BioMed Research International, 2016:1-9 (2016).
- Hechter RC, Budoff M, Hodis HN, et al. (2012). Herpes simplex virus type 2 (HSV-2) as a coronary atherosclerosis risk factor in HIV-infected men: Multicenter AIDS cohort study. Atherosclerosis. 223(2):433-436 (2012).
- Sadowski LA, Upadhyay R, Greeley ZW, et al. Current drugs to treat infections with herpes simplex viruses-1 and -2. Viruses. 13(7):1228 (2021).
- Bradbury M, Xercavins N, García-Jiménez A, et al. Vaginal intraepithelial neoplasia. J Low Genit Tract Dis. 23(1):7-12 (2018).
- Lawson JS, Glenn WK, Tran DD, et al. Identification of human papilloma viruses in atheromatous coronary artery disease. Front Cardiovasc Med. 2:17 (2015).
- Brito LMO, Brito HO, Corrêa RDGCF, et al. Human papillomavirus and coronary artery disease in climacteric women: Is there an association? TheScientificWorldJournal. 2019:1872536 (2019).
- Alguacil-Ramos AM, Portero -Alonso A, Pastor- Villalba E, et al. Rapid assessment of enhanced safety surveillance for influenza vaccine. Public Health. 168:137-141 (2019).
- Peretz A, Azrad M, Blum A. Influenza virus and atherosclerosis. QJM. 112(10):749-755 (2019).
- Indalao IL, Sawabuchi T, Takahashi E, et al. IL-1β is a key cytokine that induces trypsin upregulation in the influenza virus–cytokine–trypsin cycle. Arch Virol. 162(1):201-211 (2016).
- Warren-Gash C, Hayward AC, Hemingway H, et al. Influenza infection and risk of acute myocardial infarction in england and wales: a caliber self-controlled case series study. J Infect Dis. 206(11):1652-1659 (2012).
- Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 378(4):345-353 (2018).
- Demina EP, Smutova V, Pan X, et al. Neuraminidases 1 and 3 trigger atherosclerosis by desialylating low-density lipoproteins and increasing their uptake by macrophages. J Am Heart Assoc. 10 (4):e018756 (2021).
- Vinciguerra M, Romiti S, Sangiorgi GM, et al. SARS-CoV-2 and Atherosclerosis: Should COVID-19 Be Recognized as a New Predisposing Cardiovascular Risk Factor? J Cardiovasc Dev Dis. 8(10):130 (2021).
- Pum A, Ennemoser M, Adage T, et al. Cytokines and chemokines in sars-cov-2 infections-therapeutic strategies targeting cytokine storm. Biomolecules. 11(1):91.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet. 395(10229):1033-1034 (2020).
- Gavriilaki E, Brodsky RA. Severe COVID-19 infection and thrombotic microangiopathy: Success does not come easily. Br J Haematol. 189(6) (2020).
- Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID-19: Friend and foe? JCI insight.5 (15):e140711 (2020).
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 220:1-13 (2020).
- Yan B, Freiwald T, Chauss D, et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. 6(58):eabg0833 (2021).
- Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 12(1) (2021).
- Carvelli J, Demaria O, Vély F, et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 588(7836):146-150 (2020).
- Zuo Y, Kanthi Y, Knight JS, et al. The interplay between neutrophils, complement, and microthrombi in COVID-19. Best Pract Res Clin Rheumatol. 35(1):101661 (2021).
- Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis. (2019).
- Libby P. The changing landscape of atherosclerosis. Nature. 592(7855):524-533 (2021).
- Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis. J Am Coll Cardiol. 72(17):2071-2081 (2018).
- Pothineni NVK, Subramany S, Kuriakose K, et al. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 38(43):3195-3201 (2017).
- Vinciguerra M, Romiti S, Fattouch K, et al. Atherosclerosis as pathogenetic cubstrate for Sars-Cov2 cytokine storm. Journal of Clinical Medicine. 9(7):2095 (2020).
- Hemmat N, Ebadi A, Badalzadeh R, et al. Viral infection and atherosclerosis. Eur J Clin Microbiol Infect Dis. 37(12):2225-2233 (2018).
- Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations.
- Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. (2017).
- Budayeva HG, Rowland EA, Cristea IM, et al. Intricate roles of mammalian sirtuins in defense against viral pathogens. J Virol. 90(1):5-8 (2015).