Review Article - Interventional Cardiology (2023)
Dual effects of leptin in myocardial ischemia
- Corresponding Author:
- Ekaterina A. Polyakova
Pavlov First Saint-Petersburg State Medical University, Saint-Petersburg, Russia,
E-mail: polyakova_ea@yahoo.com
Received date: 07-Aug-2023, Manuscript No. FMIC-23-109410; Editor assigned: 09-Aug-2023, PreQC No. FMIC-23-109410 (PQ); Reviewed date: 23-Aug-2023, QC No. FMIC-23-109410; Revised date: 30-Aug-2023, Manuscript No. FMIC-23-109410 (R); Published date: 07-Sep-2023, DOI: 10.37532/1755- 5310.2023.15(S1 ).454
Abstract
Leptin, a peptide secreted by adipocytes, with an important role in body weight regulation through its effects on food intake and energy balance. Leptin is mostly synthesised in white adipose tissue. High levels of leptin potentiates the effects of many atherogenic factors, such as platelet aggregation, immune cells migration, inflammation, proliferation, hypertrophy of vascular smooth muscle cells, and endothelial cell dysfunction in obese patients. Despite, leptin is an obesity-associated adipokine that has been implicated in cardiac protection against ischemia-reperfusion injury. This review analyzes the positive and negative effects of leptin on myocardial ischemia, the main mechanism of coronary artery disease.
Keywords
Leptin • Obesity • Myocardial ischemia • Coronary artery disease
Introduction
Leptin, a protein secreted by adipocytes, plays an important role in body weight regulation through its effects on energy balance and food intake. Leptin is mainly synthesized in white adipose tissue, although the expression levels and secretion rates differ between body fat depots [1].
There is a positive association between body mass index and level of leptin [1-3]. The association between adipose tissue mass and atherogenesis was proved in obese leptin-deficient ob/ob mice in an early study [4]. Obesity associated Hyperleptinemia is a significant marker that predicts cardiovascular diseases outcomes [5]. Leptin may be one of important factors in the regulation of myocardial function, systemic haemodynamics and myocardial metabolism and in the case of hyperexpress has been associated with pathological cardiovascular conditions, including heart failure and coronary artery disease [3,5]. Leptin also has important effects on systemic haemodynamics and myocardial metabolism, which may also have profound effects on cardiac function [6]. However, the obesity paradox presents that in patients with heart and vascular events and a high risk of mortality, obesity confers a protective effect despite its deleterious role in development of disease, and the obesity paradox is a widely disputed phenomenon [6]. Evidence for the obesity paradox has been presented for a variety of diseases, with some researchers arguing that this could be explained by subject selection bias [7].
Literature Review
Anti-ischemic effects of leptin on the myocardium
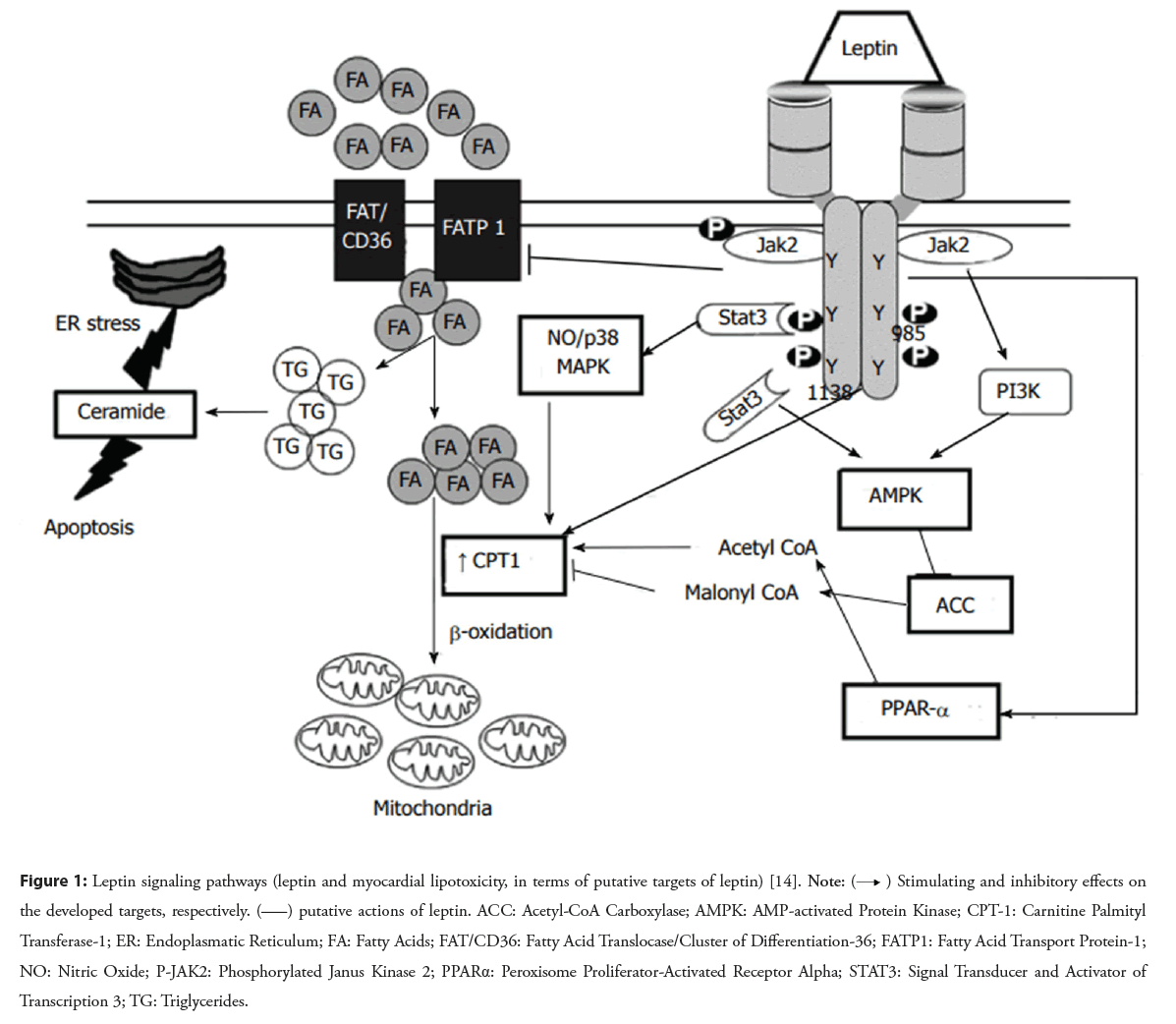
The metabolic effects of leptin are known to depend on nutritional status; therefore, most of the leptin-induced changes observed during leptin treatment in fasted animals are absent in fed animals [8,9]. Leptin administration is able to reduce body weight in ob/ob mice, which may be due to leptin-induced improvement in glycemic control. Furthermore, leptin in low dose improves glycemic control without weight loss in ob/ob mice, independently from the leptin-induced reduction in food intake [10]. However, the chronic administration of leptin in high-dose increases mean heart rate and arterial pressure. Some work has shown that it possibly involving cardiac leptin receptors [11,12]. The leptin receptors are expressed in the myocardium and leptin is synthesized in epicardial fat, leptin enters the coronary circulation and provides vasocrine action and physiological effects on the myocardium [13]. Cardiac protection induced by the low dose leptin injected during reperfusion in the rat isolated heart experiment is realized through a JAK/STAT signalling pathway activation and with mitochondrial permeability transition pore inhibition shown in the Figure 1 [14,15].
Figure 1: Leptin signaling pathways (leptin and myocardial lipotoxicity, in terms of putative targets of leptin) [14]. Note:  Stimulating and inhibitory effects on the developed targets, respectively.
Stimulating and inhibitory effects on the developed targets, respectively.  putative actions of leptin. ACC: Acetyl-CoA Carboxylase; AMPK: AMP-activated Protein Kinase; CPT-1: Carnitine Palmityl Transferase-1; ER: Endoplasmatic Reticulum; FA: Fatty Acids; FAT/CD36: Fatty Acid Translocase/Cluster of Differentiation-36; FATP1: Fatty Acid Transport Protein-1;
NO: Nitric Oxide; P-JAK2: Phosphorylated Janus Kinase 2; PPARα: Peroxisome Proliferator-Activated Receptor Alpha; STAT3: Signal Transducer and Activator of
Transcription 3; TG: Triglycerides.
putative actions of leptin. ACC: Acetyl-CoA Carboxylase; AMPK: AMP-activated Protein Kinase; CPT-1: Carnitine Palmityl Transferase-1; ER: Endoplasmatic Reticulum; FA: Fatty Acids; FAT/CD36: Fatty Acid Translocase/Cluster of Differentiation-36; FATP1: Fatty Acid Transport Protein-1;
NO: Nitric Oxide; P-JAK2: Phosphorylated Janus Kinase 2; PPARα: Peroxisome Proliferator-Activated Receptor Alpha; STAT3: Signal Transducer and Activator of
Transcription 3; TG: Triglycerides.
One of the works shows that the addition of 1.0 nM leptin to the standard perfusion solution exerted a significant infarct-limiting, preserved post-ischemic ventricular function, and prevented reperfusion arrhythmia compared to the addition of 3.1 nM leptin [16].
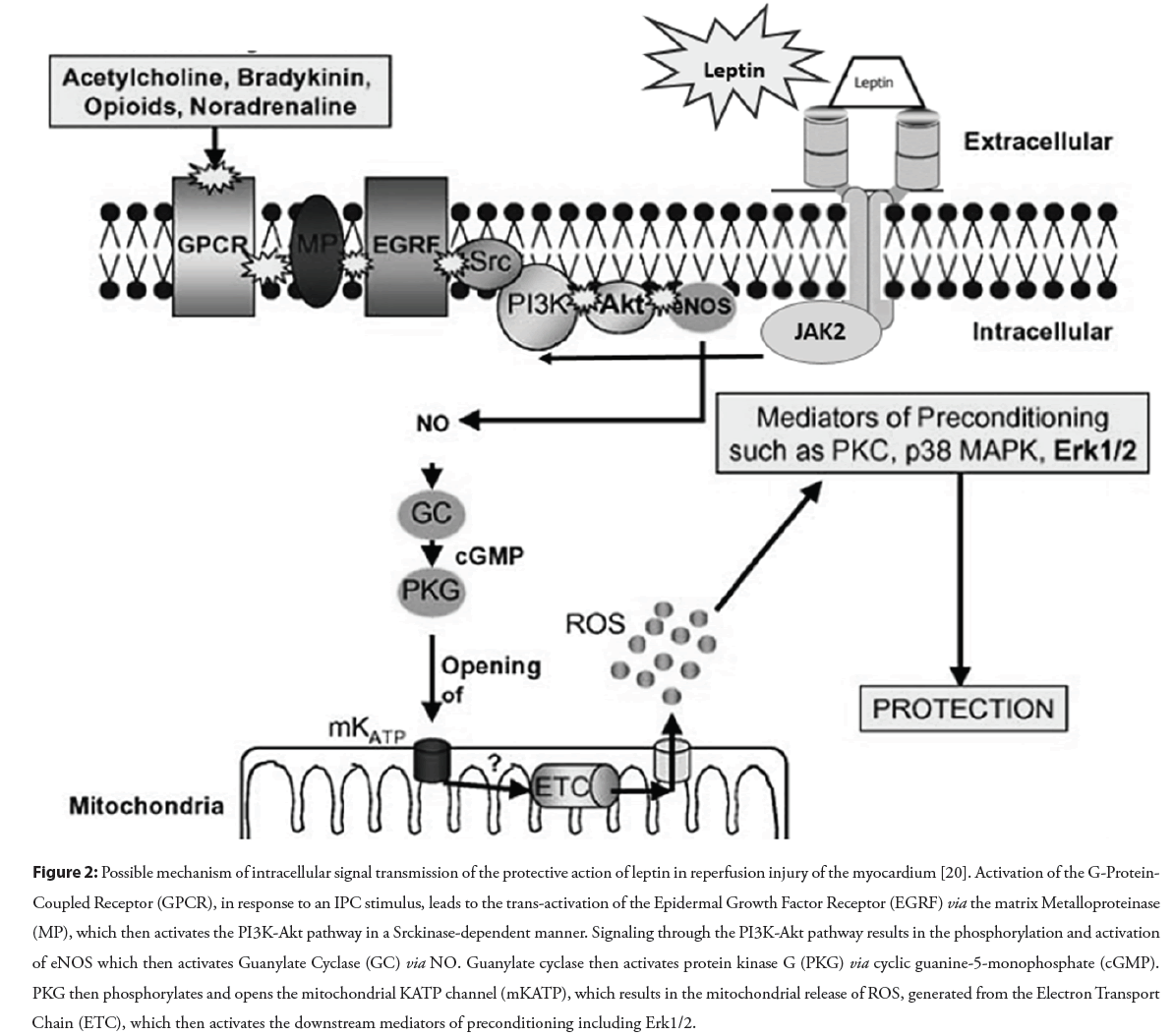
The acute intraperitoneal administration of leptin was associated with a protective action against ischemia/reperfusion injury in various organs, including the heart, kidneys, brain, gut in rodent models [17-21]. Leptin-induced cardioprotection involves activation of the reperfusion injury salvage kinase pathway through p44/42 mitogen-activated protein kinase and phosphatidylinositol 3-kinase cellular Akt/protein kinase B (Akt), suppression of central leptin– proopiomelanocortin pathway, and the mitochondrial permeability transition pore opening, which can regulate transcription, translation, metabolism, and other cell mechanisms considered prerequisites for heart protection shown in the Figure 2 [20-22].
Figure 2: Possible mechanism of intracellular signal transmission of the protective action of leptin in reperfusion injury of the myocardium [20]. Activation of the G-Protein- Coupled Receptor (GPCR), in response to an IPC stimulus, leads to the trans-activation of the Epidermal Growth Factor Receptor (EGRF) via the matrix Metalloproteinase (MP), which then activates the PI3K-Akt pathway in a Srckinase-dependent manner. Signaling through the PI3K-Akt pathway results in the phosphorylation and activation of eNOS which then activates Guanylate Cyclase (GC) via NO. Guanylate cyclase then activates protein kinase G (PKG) via cyclic guanine-5-monophosphate (cGMP). PKG then phosphorylates and opens the mitochondrial KATP channel (mKATP), which results in the mitochondrial release of ROS, generated from the Electron Transport Chain (ETC), which then activates the downstream mediators of preconditioning including Erk1/2.
It is important to note that the leptin signaling in obesity-induced leptin resistance can leads to an atherogenic phenotype, while in normal weight subjects leptin is considered protective against endothelium neointima formation in the healthy state [23].
Leptin treatment significantly reduces apoptosis in ob/ob mice in isolated cardiomyocytes; although ob/ob and db/db mice generally have normal left ventricle systolic function, they appear to have left ventricle diastolic functional abnormalities [24]. Leptin levels are elevated after myocardial infarction. The addition of leptin to cardiomyocytes prior to hypoxia in long-lived transgenic αMUPA mice reduces the expression of genes encoding proinflammatory cytokines, tumour necrosis factor-alpha, interleukin-1-beta implicated in non-apoptotic and apoptotic cell death [25,26].
Leptin administration in physiological studies may lead to unfavorable neurohormonal changes via sympathetic nervous system activation in rats. These effects include increased resting blood pressure and heart rate [27]. The resultant elevation in blood pressure may cause left ventricle hypertrophy. However, in a mouse model of obesity, leptin had protective effects against left ventricle hypertrophy [24]. In addition, the physiological effects of leptin include increased energy expenditure and decreased appetite, both of which can result in weight loss and improvement in metabolic derangements such as hyperinsulinemia and lipotoxicity, sleep apnea, hypertension and can reduce post-infarction heart failure severity [28,29].
There is evidence that 1.0 nM of leptin added to the standard perfusion solution was accompanied by a significant reduction in infarct size in Langendorff-perfused rat hearts, which is consistent with the findings in mouse model [15,16]. Thus, low-dose and short time leptin administration associated with reduce cardiac ischemic injury severity makes leptin as a probably therapeutic agent in the treatment of myocardial infarction [15]. But, high leptin concentrations and long-term leptin treatment have been associated with decreased oxidation and enlarged cellular fatty acid uptake, which over time results in lipotoxic cardiomyocyte damage, intracellular lipid accumulation and culminates in contractile failure [30]. Though in the unstressed heart myocardial metabolism was normal, cardiac specific leptin receptors loss completely inhibited the switch to increased glucose oxidation and glycolysis after myocardial infarction [31]. Study on experimentally induced acute anterior myocardial infarction in control mice and those with cardiac-specific deletion of the leptin receptor have provided evidence more left ventricle dysfunction and a higher mortality rate in mice lacking the leptin receptor in the heart. Data provide evidence of the beneficial effects of acute leptin administration in those mice [32]. Rodents with acute myocardial ischemia demonstrate preservation of heart function when cardiac leptin activity is counteracted [33,34].
The amount of epicardial and intramuscular myocardial fat is associated with body mass index and severity of obesity [35]. Intramuscular fat accumulation in the myocardium in obese patients may be associated with leptin affects heart rate if leptin receptors are present in the sinus node and on repolarization in the ventricle. Also the indirect pathways through sympathetic nervous system tone, leptin can decrease heart rate and increase the QT interval through leptin receptors independent of β-adrenoreceptor activation. During the inhibition of β-adrenoreceptor, high leptin concentrations in the myocardium can lead to a prolonged QT interval, ventricular arrhythmia and deep bradycardia, [36]. The dose of leptin to trigger norepinephrine release is approximately 1 μg/kg when it intracerebroventricular administration [37]. Experimental data found that two doses (1.0 nM and 3.1 nM) of leptin did not react the heart rate [16]. The dose of leptin to trigger norepinephrine release is approximately 1 μg/kg when it intracerebroventricular administration [37]. Leptin can decrease heart rate if sympathetic signalling is impaired due to drug effect or genetic [36].
High concentrations of leptin are accompanied by an increase in sympathetic activity, leading to an increased sinus node activity when the indirect and direct actions of leptin are superimposed and little heart rate change. The combination of indirect (β-adrenoreceptor) and direct (leptin receptor) effects of leptin not only provides an explanation for the long QT and slow heart rate in Zucker obese rats, it also explains sinus node dysfunction in obese patients [38]. Also one experiment showed that the total duration of reperfusion ventricular tachycardia was lower in the leptin group compare with control and the mean heart rate in reperfusion-induced ventricular tachycardia was significantly lower in the isolated rat hearts, which perfused with 1.0 nM leptin dose than in the control [16]. The short-term administration of leptin reduces myocardial infarct size in experimental rat model on isolated perfused hearts [11,34 ] and attenuates cardiomyocyte apoptosis after acute ischemia by reducing caspase-3 activity and by increasing survivin and bcl-2 gene expression [12,34]. Leptin at a concentration close to physiological has also been shown to have cardioprotective properties in several in vitro and ex vivo studies in mice [34,39].
Evaluation of the molecular pathways of cardiovascular disease associated with obesity is important for both improving management and prevention. So, some specific myocardial miRNAs such as miRNA-208a, miRNA-144, miRNA-499, miRNA-378 are known, however, the effect of obesity and changes in the concentration of adipose tissue hormones, in particular leptin, on cardiac miRNAs in ischemia remain obscure [40]. It is described that the expression of miRNA-208a in the myocardium depending on the concentration of leptin in rats after modelling global myocardial ischemia. MiRNA-208a is that regulates β-myosin heavy chain content via Mediator complex subunit 13 (MED13) and systemic energy homeostasis [41]. MiRNA-208a repress MED13 inducing thyroid hormone and stress-responsive signalling pathways in the myocardium. Myocardial MED13 acts to regulate cardiac remodeling as well as to increase weight loss by enhancing adipose tissue metabolism [41]. A low leptin concentration leads to a miRNA-208a expression decrease, and a high leptin concentration accompanied by an increase of miRNA- 208a myocardial expression [16]. This may be one of more obesity paradox explanations, when moderate obesity is observed to reduce the cardiovascular risk.
Thus, leptin is a potential therapeutic target in pathological conditions associated with myocardial ischemia and metabolic disorders. Therefore, the translation potential of experimental data aimed at the myocardial infarct size modification can be improved by using cell and animal models with Hyperleptinemia.
Pro-ischemic effects of leptin on the myocardium
As discussed above, many patients with myocardial infarction and obesity have an increased plasma leptin level and negative leptin effect demonstrated in an experimental result [42]. A high level of leptin in the blood leads to an unfavourable course of a myocardial ischemia is observed, since increased deterioration of cardiac structure and function, hypertrophy, apoptosis, impairment of glucose, fatty acid and energy metabolism, which further accelerated damage due to heart attack [42].
Serum leptin concentrations have been described as reaching ≥ 200 ng/mL in morbidly obese individuals compared to 10 ng/ mL in non-obese individuals [43]. A very high concentration of leptin in the blood potentiates inflammation, migration, hypertrophy, proliferation of vascular smooth muscle cells, endothelial cell dysfunction, platelet aggregation, reactive oxygen species formation, and decreased paraoxonase activity associated with atherogenesis. Hyperleptinemia modulates the expression of vascular genes associated with abnormal angiogenesis and atherosclerosis, including growth factors, extracellular matrix proteins and cytokines [44,45].
In addition, several signalling pathways have been shown to be involved in cardiac regulation by leptin, including mitogen- activated protein kinase, nitric oxide, Janus activated kinase, signal transducer and activator of transcription 3, which are associated with a myocardial remodeling [46]. The chronic blockade of leptin limits the development of postischemic cardiac misregulation in rats [47]. Of note, fibroblast growth factor-21, a stress-responsive transcription factor, is also known to mediate the negative effects of leptin [48].
Myocardial ischemic injury activates the Renin-Angiotensin- Aldosterone System (RAAS), resulting in the release of endothelin-1 and angiotensin II, which drive myocardial remodeling via leptin mediation and induction [49]. Hyperleptinemia induced in rodents experiment has been reported to result in post-ischemic myocardial remodeling, myocardial dysfunction and hypertension [13]. What was not described with short-term and low dose leptin administration [14,32]. Clinical evidence suggests that high blood levels of leptin correlate with obesity, myocardial infarction, heart failure and cardiovascular morbidity [50,51]. Moreover, elevated blood levels of leptin are often considered a surrogate cardiovascular risk factor [52].
Prolonged Hyperleptinemia in rats leads to an increase in heart rate and blood pressure, disturbed left ventricle function, myocardial hypertrophy, ischemic arrhythmias, increased size of myocardial infarction zone, associated with dyslipidemia and systemic inflammation. But, the pharmacological inhibition of the JAK/STAT pathway reverses leptin-induced changes in certain metabolic and hemodynamic parameters [34]. Earlier studies have shown insights regarding the mechanisms by which high leptin and STAT signalling is implicated in the development of myocardial ischemic injury [13,42,50].
High blood leptin concentration plays a variable role in the regulation of metabolic and inflammatory status and hemodynamic during ischemic myocardial damage, and can produce opposite effects under different contexts or conditions [47,53]. On the one hand, leptin may exert cardioprotective effects when administered acutely as discussed in the previous section of the mini-review [54]. On the other hand, evidence suggests that long-time elevated leptin level may be associated with negative effects through hemodynamic factors, such as increased blood pressure and heart rate [55,56], metabolic changes including glucose utilisation impairment and augmented fatty acid [57,58], structural cardiac changes, such as cardiac lipid accumulation [58], induced cardiac apoptosis [59], increased myocardial hypertrophy [60]. Obesity- related Hyperleptinemia is accompanied by inflammatory profile with increased circulating levels of TNF-alpha, IL-6, IL-12, and reduced concentrations of IL-10 [57]. In addition, one of the results in a rat model mimicked the clinical scenarios of patients who experience acute myocardial ischemia coinciding with leptin overexpression associated with a pre-existing inflammatory state [34]. Adverse effects associated with this unfavorable association have been observed in patients suffering from rheumatoid arthritis [61], or inflammatory bowel disease [62], who exhibit a disproportionally higher degree of post infarction heart failure. The worse outcome of acute myocardial ischemia may be associated with an excess leptin concentration in the blood [34], and mimics with the classical insulin resistance and leptin resistance features of human obesity [63].
More intense ischemic myocardial injury in chronic Hyperleptinemia in both murine and humans may be associated with hypertrophy of cardiomyocytes, which appears to be mediated by the activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol-3 kinase [64].
Autocrine and paracrine leptin signalling plays a significant role in cardiomyocyte metabolism [42]. This may justify the more pronounced myocardial ischemic damage in animals receiving long-time leptin administration. Hyperleptinemic rats had a larger size of myocardial infarction and higher concentration of troponin-I compared to the controls [34]. Moreover, the obese Zucker rats exhibited larger myocardial infarct size following ischemia and reperfusion than the control ones [65], as well as the high leptin level, which were observed in fat-fed hypertensive rats compared to hypertensive-glucose intolerant animals with lower leptin levels, Hyperleptinemia resulted in larger myocardial infarct zone [66]. More importantly, the long-term inhibition of the JAK/ STAT pathway (at a JSI-124 dose of 1 mg/kg/d) in rats with high leptin blood level did not reduce ischemic myocardial injury and inflammation in myocardial infarction model [34]. It is likely that inhibition of the JAK/STAT pathway does not reduce myocardial injury in leptin-treated rats, possibly due to the low dose of JSI-124 or regulation of these processes through other signalling pathways.
The upregulation of leptin and the associated elevation of atherogenic lipoproteins, IL-6, TNF-alpha are known to be involved in the pathological mechanisms of myocardial ischemic damage [57]. Recently, the involvement of leptin in the mechanisms of coronary artery disease has also been demonstrated [67,68]. A multicentre retrospective study suggested that blood leptin elevation is a representative risk factor for coronary artery disease in obese patients [69].
Discussion and Conclusion
Leptin is an important adipokine in the regulation of cardiac function and at physiological concentrations may protect against post-ischemic cardiac injury. Leptin administered simultaneously and at a low concentration had an anti-ischemic effect: It limited the size of the infarct area and preserved the post-ischemic function of the ventricles.
High leptin level with observed in obese patients mediating leptin-associated mechanisms of atherogenesis, metabolic damage, arrhythmogenesis and myocardial ischemic damage both in experimental animal models and humans. Hyperleptinemia preceding myocardial ischemia and reperfusion potentiates myocardial remodeling. This occurs due to cardiomyocyte hypertrophy and cardiac remodeling and may lead to augmented of post-infarction heart failure. These data can become the basis for the development of a new technology for the prevention and reduction of ischemic damage to the myocardium during ischemia and reperfusion.
Further research will be required to determine the involvement of various signalling pathways in leptin induced mechanisms and the interactions between them. In addition, these results also imply that drugs targeting the leptin pathways could provide new treatment strategy for coronary artery disease, especially in obese people.
Conflicts of Interest
No authors have any conflicts of interest during the publication of the manuscript.
References
- Dornbush S, Aeddula NR. Physiology, leptin. (2021).
- Benbaibeche H, Bounihi A, Koceir EA, et al. Leptin level as a biomarker of uncontrolled eating in obesity and overweight. Ir J Med Sci. 190(1):155-161 (2021)
- Chai S, Chen Y, Xin S, et al. Positive association of leptin and artery calcification of lower extremity in patients with type 2 diabetes mellitus: A pilot study. Front Endocrinol (Lausanne). 12:583575 (2021)
- Wang B, Chandrasekera PC, Pippin JJ, et al. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr Diabetes Rev. 10(2):131-145 (2014)
- Polyakova EA, Mikhaylov EN, Sonin DL, et al. Neurohumoral, cardiac and inflammatory markers in the evaluation of heart failure severity and progression. J Geriatr Cardiol 2021; 18(1): E1-E20 (2021)
- Pereira S, Cline DL, Glavas MM, et al. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr Rev. 42(1):1-28 (2021)
- Cortigiani L, Haberka M, Ciampi Q, et al. The obesity paradox in the stress echo lab: fat is better for hearts with ischemia or coronary microvascular dysfunction. Int J Obes (Lond). 45(2):308-315 (2021)
- Ramos-Lobo AM, Donato J Jr. The role of leptin in health and disease. Temperature (Austin). 4(3):258-291 (2017)
- Overton JM, Williams TD, Chambers JB, et al. Central leptin infusion attenuates the cardiovascular and metabolic effects of fasting in rats. Hypertension. 37(2 Pt 2):663-669 (2001)
- Neumann UH, Kwon MM, Baker RK, et al. Leptin contributes to the beneficial effects of insulin treatment in streptozotocin-diabetic male mice. Am J Physiol Endocrinol Metab. 15(6):E1264-E1273 (2018)
- Shirasaka T, Takasaki M, Kannan H, et al. Cardiovascular effects of leptin and orexins. Am J Physiol Regul Integr Comp Physiol. 284(3):R639-R651(2003)
- Ren J. Leptin and hyperleptinemia - from friend to foe for cardiovascular function. J Endocrinol. 181(1):1-10 (2004)
- Chen H, Liu L, Li M, et al. Epicardial adipose tissue-derived leptin promotes myocardial injury in metabolic syndrome rats through PKC/NADPH oxidase/ROS pathway. J Am Heart Assoc. 12(15):e029415 (2023).
- Hall ME, Harmancey R, Stec DE, et al. Lean heart: Role of leptin in cardiac hypertrophy and metabolism. World J Cardiol.7(9): 511-524 (2015)
- Smith CC, Dixon RA, Wynne AM, et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 299(4):H1265- H1270 (2010)
- Polyakova EA, Mikhaylov EN, Minasian SM, et al. Anti-Ischemic effect of leptin in the isolated rat heart subjected to global ischemia-reperfusion: Role of cardiac-specific miRNAS. Cardiogenetics. 13:1-13 (2023).
- Brzozowski T, Konturek PC, Pajdo R, et al. Brain-gut axis in gastroprotection by leptin and cholecystokinin against ischemia-reperfusion induced gastric lesions. J Physiol Pharmacol. 52(4):583-602 (2001).
- Erkasap S, Erkasap N, Koken T, et al. Effect of leptin on renal ischemia reperfusion damage in rats. J Physiol Biochem. 60(2):79-84 (2004).
- Zhang F, Wang S, Signore AP, et al. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 38(8):2329-2336 (2007).
- Gava FN, da Silva AA, Dai X, et al. Restoration of cardiac function after myocardial infarction by long-term activation of the CNS leptin-melanocortin system. JACC Basic Transl Sci. 6(1):55-70 (2021).
- Lopaschuk GD. Targeting the brain to protect the heart. JACC Basic Transl Sci. 6(1):71-73 (2021).
- Hausenloy DJ, Tsang A, Yellon DM, et al. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 15(2):69-75 (2005).
- Landecho MF, Tuero C, Valentí V, et al. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients. 11(11):2664 (2019).
- Hall ME, Maready MW, Hall JE, et al. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab. 307(3):E316-E325 (2014).
- Tang D, Kang R, Berghe TV, et al. The molecular machinery of regulated cell death. Cell Res. 29: 347-364 (2019).
- Abd Alkhaleq H, Kornowski R, Waldman M, et al. Leptin modulates gene expression in the heart and cardiomyocytes towards mitigating ischemia-induced damage. Exp Cell Res. 397(2):112373 (2020).
- Shek EW, Brands MW, Hall JE, et al. Chronic leptin infusion increases arterial pressure. Hypertension. 31(1 Pt 2):409-414 (1998).
- Shimada YJ. Is leptin protective against heart failure with preserved ejection fraction? A complex interrelationship among leptin, obesity, and left ventricular hypertrophy. Hypertens Res. 42(2):141-142 (2019).
- Tabucanon T, Wilcox J, Tang WHW, et al. Does weight loss improve clinical outcomes in overweight and obese patients with heart failure? Curr Diab Rep. 20(12):75 (2020).
- Palanivel R, Eguchi M, Shuralyova I, et al. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism. 55(8):1067-1075 (2006).
- Witham W, Yester K, O'Donnell CP, et al. Restoration of glucose metabolism in leptin-resistant mouse hearts after acute myocardial infarction through the activation of survival kinase pathways. J Mol Cell Cardiol. 53(1):91-100 (2012).
- McGaffin KR, Witham WG, Yester KA, et al. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 89(1):60-71 (2011).
- Minasian SM, Galagudza MM, Dmitriev YV, et al. Myocardial protection against global ischemia with Krebs-Henseleit buffer-based cardioplegic solution. J Cardiothorac Surg. (2013).
- Polyakova EA, Mikhaylov EN, Galagudza MM, et al. Hyperleptinemia results in systemic inflammation and the exacerbation of ischemia-reperfusion myocardial injury. Heliyon. 7(11):e08491 (2021).
- Szczepaniak LS, Victor RG, Orci L, et al. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res 101:759-767 (2007).
- Lin YC, Huang J, Hileman S, et al. Leptin decreases heart rate associated with increased ventricular repolarization via its receptor. Am J Physiol Heart Circ Physiol. 309(10):H1731- H1739 (2015).
- Satoh N, Ogawa Y, Katsuura G, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: Leptin-induced increase in catecholamine secretion. Diabetes. 48:1787-1793 (1999).
- Lin YC, Huang J, Kan H, et al. Defective calcium inactivation causes long QT in obese insulin-resistant rat. Am J Physiol Heart Circ Physiol.302:H1013-H1022 (2012).
- Avraham Y, Davidi N, Porat M, et al. Leptin reduces infarct size in association with enhanced expression of CB2, TRPV1, SIRT-1 and leptin receptor. Curr Neurovasc Res. 7(2):136-43 (2010).
- Gan M, Zhang S, Fan Y, et al. The Expression of microRNA in adult rat heart with isoproterenol-induced cardiac hypertrophy. Cells. 9(5):1173 (2020).
- Soci UPR, Cavalcante BRR, Improta-Caria AC, et al. The epigenetic role of mirnas in endocrine crosstalk between the cardiovascular system and adipose tissue: A bidirectional view. Front Cell Dev Biol.10:910884 (2022).
- Kang KW, Ok M, Lee SK, et al. Leptin as a key between obesity and cardiovascular disease. J Obes Metab Syndr. 29(4):248-259 (2020).
- Berzabá-Evoli E, Zazueta C, Cruz Hernández JH, et al. Leptin modifies the rat heart performance associated with mitochondrial dysfunction independently of its prohypertrophic effects. Int J Endocrinol. (2018).
- Montserrat-de la Paz S, Pérez-Pérez A, Vilariño-García T, et al. Nutritional modulation of leptin expression and leptin action in obesity and obesity-associated complications. J Nutr Biochem 89: 108561 (2021).
- Ganguly R, Khanal S, Mathias A, et al. TSP-1 (Thrombospondin-1) Deficiency Protects ApoE-/- Mice Against Leptin-Induced Atherosclerosis. Arterioscler Thromb Vasc Biol. (2020).
- Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and obesity: Role and clinical implication. Front Endocrinol (Lausanne) 12:585887 (2021).
- Gan XT, Zhao G, Huang CX, et al. Identification of fat mass and obesity associated FTO protein expression in cardiomyocytes: Regulation by leptin and its contribution to leptin-induced hypertrophy. PLoS One. 8(9):e74235 (2013).
- Asrih M, Veyrat-Durebex C, Poher AL, et al. Leptin as a potential regulator of FGF21. Cell Physiol Biochem. 38(3):1218-1225 (2016).
- Sapouckey SA, Morselli LL, Deng G, et al. Exploration of cardiometabolic and developmental significance of angiotensinogen expression by cells expressing the leptin receptor or agouti-related peptide. Am J Physiol Regul Integr Comp Physiol. 318(5):R855-R869 (2020).
- Segers VFM, De Keulenaer GW. Autocrine signaling in cardiac remodeling: A rich source of therapeutic targets. J Am Heart Assoc. 10(3):e019169 (2021).
- Syed AH, Lohana S, Aung NH, et al. Correlation of leptin with acute myocardial infarction: A case control study. Cureus. 12(12):e12190 (2020).
- Gutiérrez-Cuevas J, Sandoval-Rodriguez A, Meza-Rios A, et al. Molecular mechanisms of obesity-linked cardiac dysfunction: An up-date on current knowledge. Cells. 10(3):629 (2021).
- Poetsch MS, Strano A, Guan K, et al. Role of leptin in cardiovascular diseases. Front Endocrinol (Lausanne).11:354 (2020).
- Smith CC, Mocanu MM, Davidson SM, et al. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 149(1): 5-13 (2006).
- Gruber T, Pan C, Contreras RE, et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 33(6):1155-1170 (2021).
- Carlyle M, Jones OB, Kuo JJ, et al. Chronic cardiovascular and renal actions of leptin: Role of adrenergic activity. Hypertension. 39(2 Pt 2):496-501 (2002).
- Leon-Cabrera S, Solís-Lozano L, Suárez-Álvarez K, et al. Hyperleptinemia is associated with parameters of low-grade systemic inflammation and metabolic dysfunction in obese human beings. Front Integr Neurosci. 7:62 (2013).
- Rodríguez-Calvo R, Samino S, Guaita-Esteruelas S, et al. Plasma glucose, triglycerides, VLDL, leptin and resistin levels as potential biomarkers for myocardial fat in mice. Clin Investig Arterioscler. 32(1): 8-14 (2020).
- Barouch LA, Gao D, Chen L, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 98(1):119-24 (2006).
- Rubio B, Mora C, Pintado C, et al. The nutrient sensing pathways FoxO1/3 and mTOR in the heart are coordinately regulated by central leptin through PPARβ/δ. Implications in cardiac remodeling. Metabolism 115:154453 (2021).
- Rawla P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia. 57(1):27-36 (2019).
- Biondi RB, Salmazo PS, Bazan SGZ, et al. Cardiovascular risk in individuals with inflammatory bowel disease. Clin Exp Gastroenterol.13:107-113 (2020).
- Pretz D, Le Foll C, Rizwan MZ, et al. Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity. FASEB J. 35(2):e21216 (2021).
- Tajmir P, Ceddia RB, Li RK, et al. Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology 145(4):1550-1555 (2004).
- Katakam PV, Jordan JE, Snipes JA, et al. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol. 292:R920- R926 (2007).
- Mozaffari MS, Schaffer SW. Myocardial ischemic-reperfusion injury in a rat model of metabolic syndrome. Obesity. 16(10):2253-2258 (2008).
- Mohamadshahi M, Haybar H, Mousavi-Borazjani A, et al. The association between dietary patterns with severity of coronary artery stenosis, serum leptin-to-adiponectin ratio, and some related risk factors in patients with coronary artery disease. J Diabetes Metab Disord. 220(1):697-708 (2021).
- Xiao P, Shi J, Liu X, et al. Associations of leptin and leptin receptor genetic variants with coronary artery disease: A meta-analysis. Biosci Rep. 39(6) (2019).
- Chai SB, Sun F, Nie XL, et al. Leptin and coronary heart disease: A systematic review and meta-analysis. Atherosclerosis. 233(1): 3-10 (2014).