Review Article - Imaging in Medicine (2012) Volume 4, Issue 1
MRI of atherosclerosis: from mouse to man
Alkystis Phinikaridou* and René M Botnar
Division of Imaging Science& Biomedical Engineering, King’s College London, The Rayne Institute, 4th Floor, Lambeth Wing, St Thomas’ Hospital, London SE1 7EH, UK
- *Corresponding Author:
- Alkystis Phinikaridou
Division of Imaging Science& Biomedical Engineering King’s College London
The Rayne Institute, 4th Floor Lambeth Wing, St Thomas’ Hospital London SE1 7EH, UK
Tel.: +44 207 188 8386
Fax: +44 207 188 5442
Email: alkystis.1.phinikaridou@kcl.ac.uk
Abstract
Atherosclerosis and its thrombotic complications still remain the major cause of morbidity and mortality in western societies. Atherogenesis in humans generally occurs over decades, and lesion evolution and growth may vary according to heredity, gender, lifestyle and environmental conditions. However, the development of animal models of experimental atherosclerosis and the emergence of several imaging modalities have provided indispensable knowledge to our understanding of the fundamental mechanisms of disease progression and allowed the in vivo detection of atherosclerosis in animals and humans. MRI has evolved as one of the leading noninvasive imaging modalities to visualize the vessel wall with high spatial resolution and without ionizing radiation. This article summarizes the currently available animal models of experimentally induced atherosclerosis and the application of MRI in preclinical and clinical imaging studies.
Keywords
animal model; atherosclerosis; contrast agent; molecular imaging; MRI; thrombosis
Atherosclerosis is a progressive arterial disease characterized by intimal thickening from the accumulation of lipids [1], smooth muscle cells, lipid-filled macrophages, monocytes, lymphocytes, erythrocytes, platelets [2–4] and extracellular matrix proteins (collagen, elastin, proteoglycans) [5,6]. It is considered the major contributor to the development of cardiovascular disease, the leading cause of death in the USA [7] and worldwide [8].
Histological studies using excised human vessels and atherosclerotic animal models have provided valuable information regarding the pathophysiology of atherosclerosis and thrombosis. Human vessels collected at autopsy were used by the American Heart Association Committee on Vascular Lesions to stratify the severity of atherosclerotic plaques based on compositional and morphological criteria [9–11]. This classification system was later modified by Virmani et al. [12]. It has also been shown that acute cardiovascular events and sudden death related to atherosclerosis are due to disruption of vulnerable or high-risk plaques and subsequent thrombosis, which may quickly cause luminal occlusion. Conversely, stable plaques can remain clinically asymptomatic. Currently, three distinct histological features: plaque rupture, plaque erosion and calcified nodules, have been associated with luminal thrombosis. Ruptured human plaques, also termed thin-cap atheromas, usually have:
A thin (<65 μm in the coronary arteries) [13–15], inflamed [16,17] fibrous cap infiltrated by macrophages;
A large lipid core (>40% of the total lesion area);
Increased neovessels [18];
Medial and adventitial disorganization [19];
Intraplaque hemorrhage [20];
Positive/outward vessel wall remodeling [21].
Unlike plaque rupture, in eroded plaques the thrombus forms over an intima lacking endothelial cells and a fibrous cap rich in smooth muscle cells, proteoglycans and type 3 collagen fibers [22]. Finally, atherothrombi may also occur as a result of calcified nodules that bulge into the lumen through a disrupted fibrous cap [12].
Despite the incremental understanding of the pathophysiology of atherosclerosis, histological studies are limited by their retrospective nature. Several studies have shown the feasibility of both invasive (angiography, angioscopy, intravascular ultrasound [IVUS], optimal coherence tomography, thermography, Raman spectroscopy, near-infrared spectroscopy) and noninvasive (B‑mode ultrasound tomography, CT, PET, MRI) imaging modalities for in vivo vessel wall imaging and characterization of atherosclerosis. Of these techniques, angiography and IVUS have been widely used in clinical practice primarily to estimate the degree of luminal stenosis and stratify patients in different intervention groups. However, angiographic studies of coronary arteries, performed before and after nonfatal myocardial infarction, showed that at the site of thrombosis, the pre-existing lesion frequently resulted in less than 50% stenosis [23,24] and frequently did not cause angina or a positive treadmill test. Only 20% of acute complete occlusions occur in lesions with a stenosis greater than 75% [25].
Therefore, there is a need for the development of a noninvasive imaging modality that would allow not only the estimation of luminal stenosis but also a compositional characterization of atherosclerotic plaque. This review article will focus on the different animal models currently available for studying atherosclerosis and the applications of noncontrast-enhanced, contrastenhanced and molecular MRI for preclinical and clinical use.
Animal models of atherosclerosis: advantages & disadvantages
The complexity and slow progression of atherosclerosis in humans and the unpredictable nature of plaque disruption have necessitated the development of animal models for understanding the molecular and cellular pathways involved in disease progression and the clinical manifestations, as well as the development of diagnostic procedures and therapeutic interventions. Unlike in humans, animal models allow the development of the disease in a reasonable time span and under precise settings where environmental, genetic and dietary variables can be controlled. Furthermore, animals allow the evaluation of risk factors independently or in combinations, in the presence or absence of other intercurrent diseases. Many requirements need to be satisfied in order to make an animal model suitable for the study of atherosclerosis. Some of the factors include: strain availability, susceptibility to disease, ease in handling, breeding and maintenance cost, reproducibility of results, anatomical, morphological and biochemical similarities to the human disease.
Anitschkow and Chalatow were among the first researchers to induce experimental atherosclerosis in animals by feeding rabbits an enriched cholesterol diet [1,26]. Since then, several other experimental conditions have been used to induce lesions in different animal species including dietary, physical, chemical, immunological and transgenic approaches applied individually or in combinations, simultaneously or sequentially. A summary of the different animal models available for studying atherosclerosis together with their basic characteristics and uses is illustrated in Table 1.
MRI of atherosclerosis in animal models & humans
Over the last decades extensive research has been dedicated to developing MR methods for in vivo imaging of atherosclerosis in animal models and humans. The major applications of MRI in characterizing animal and human atherosclerosis are described below and are summarized in Tables 2 & 3.
Assessment of plaque burden & composition
MRI has been applied to characterize plaque composition on the basis of biophysical and biochemical factors (T1 and T2 relaxation times), proton density, physical state, molecular motion, fibrous protein content (magnetization transfer) and diffusion coefficients (diffusion-weighted imaging) both in vivo and ex vivo [27–34]. In addition, in vivo techniques such as the black-blood pulse sequence and the use of phased-array receiver coils have improved the delineation of the arterial lumen from the vascular wall, which is critical for lesion visualization [35]. Validation studies were first performed in experimental models including mice [36–41], cholesterol-fed rabbits [42–52] and pigs [53]. In humans, validation of the in vivo MRI findings was performed mainly by using ex vivo carotid endarterectomy specimens. Several studies showed that the combination of multiple MR contrast weightings (proton density, T1‑weighted, T2‑weighted and time of flight) can be used to identify plaque components [54–59] based on their relative signal intensities and relaxation times. Multicontrast in vivo MRI has been used to evaluate plaque size [60] and components including the lipid core, fibrous cap, calcification [54,55,61–64], intraplaque hemorrhage [65,66] as well as features associated with symptomatic human carotid plaques [67]. Furthermore, diffusionweighted imaging is another technique used to generate contrast between plaque components based on the characteristic diffusion coefficients of water in each tissue [68,69]. Lastly, magnetization transfer between restricted and free-water protons was also used to discriminate the collagenous fibrous cap and the media from the lipid core and adventitia [70].
Several contrast agents have been used to improve the conspicuity of atherosclerotic plaques. Contrast-enhanced MRI using gadolinium diethylenetriamine penta-acetic acid (Gd‑DTPA) has been used to increase the sensitivity of MRI and further improve the identification of plaque components. Gd‑DTPA has been used for the discrimination between the fibrous cap and the lipid core [71–73] and the visualization of coronary atherosclerosis [74–76].
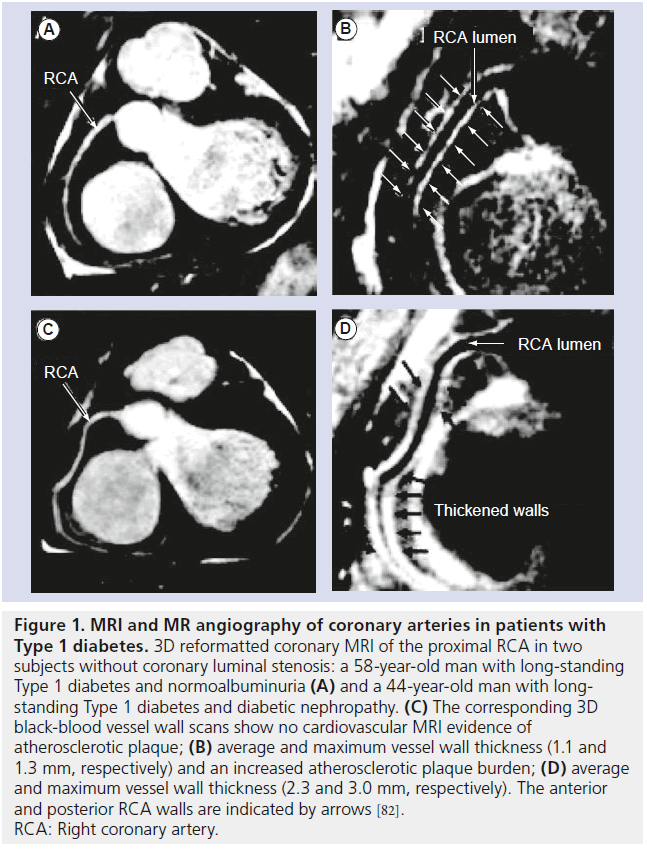
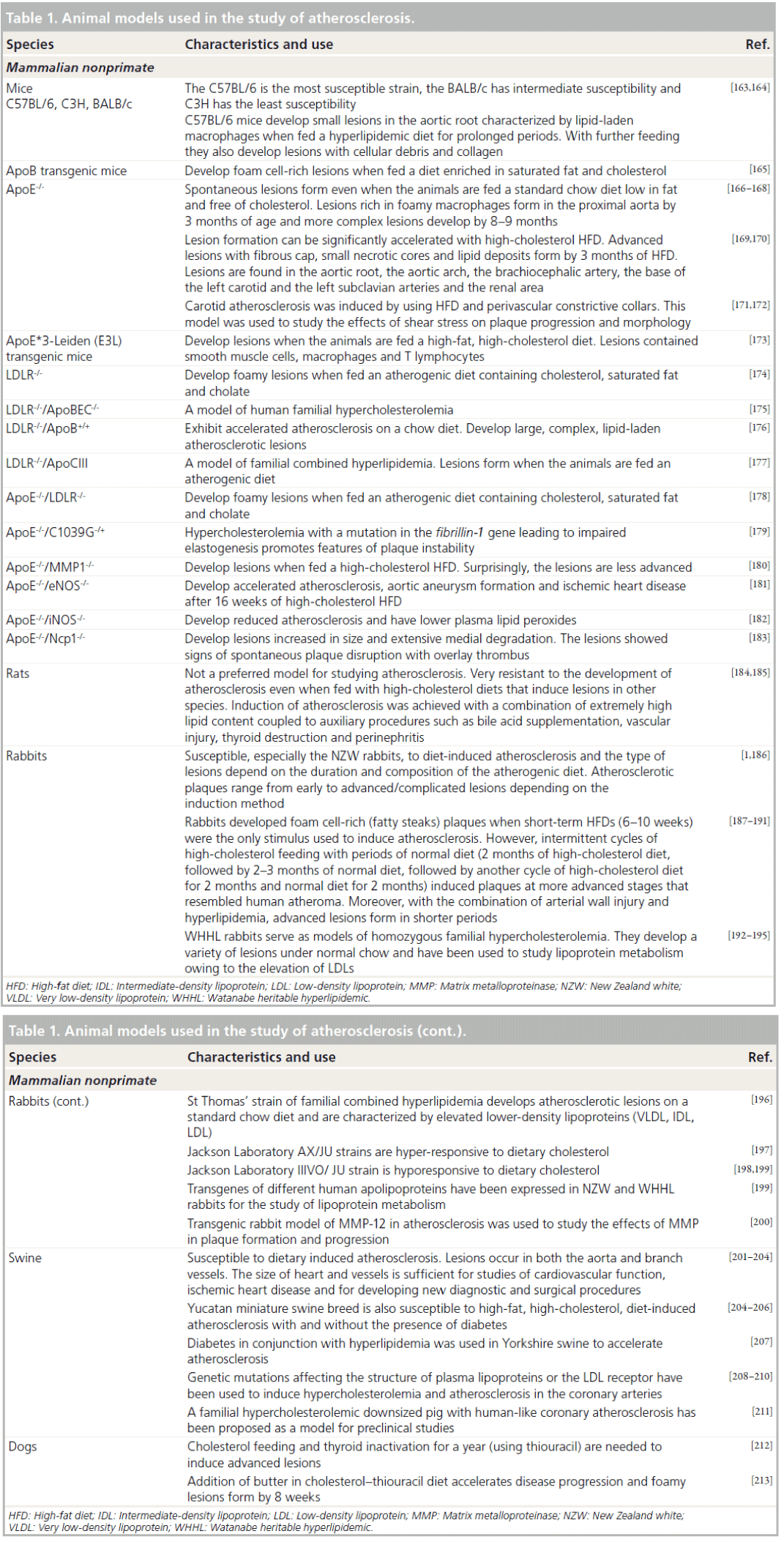
MRI and MR angiography of coronary arteries still remains challenging owing to cardiac motion, the small caliber and the tortuous structure of the vessels. However, advanced pulse sequence design with navigator gating, with and without breath-holds, has allowed the visualization of the coronary lumen and vessel wall [77–86]. For example, coronary MRI of asymptomatic Type 1 diabetics revealed greater coronary plaque burden in subjects with nephropathy compared with those with normoalbuminuria (Figure 1) [82].
Figure 1. MRI and MR angiography of coronary arteries in patients with Type 1 diabetes.3D reformatted coronary MRI of the proximal RCA in two subjects without coronary luminal stenosis: a 58-year-old man with long-standing Type 1 diabetes and normoalbuminuria (A) and a 44-year-old man with longstanding Type 1 diabetes and diabetic nephropathy. (C) The corresponding 3D black-blood vessel wall scans show no cardiovascular MRI evidence of atherosclerotic plaque; (B) average and maximum vessel wall thickness (1.1 and 1.3 mm, respectively) and an increased atherosclerotic plaque burden; (D) average and maximum vessel wall thickness (2.3 and 3.0 mm, respectively). The anterior and posterior RCA walls are indicated by arrows [82]. RCA: Right coronary artery.
Assessment of endothelial activation & permeability
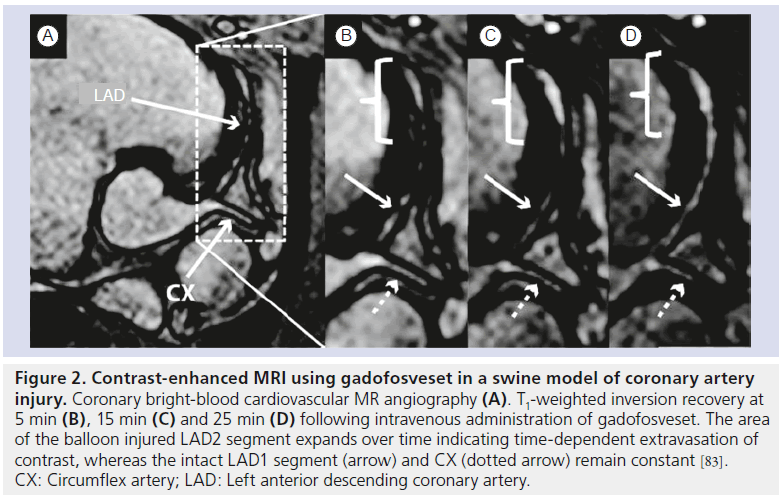
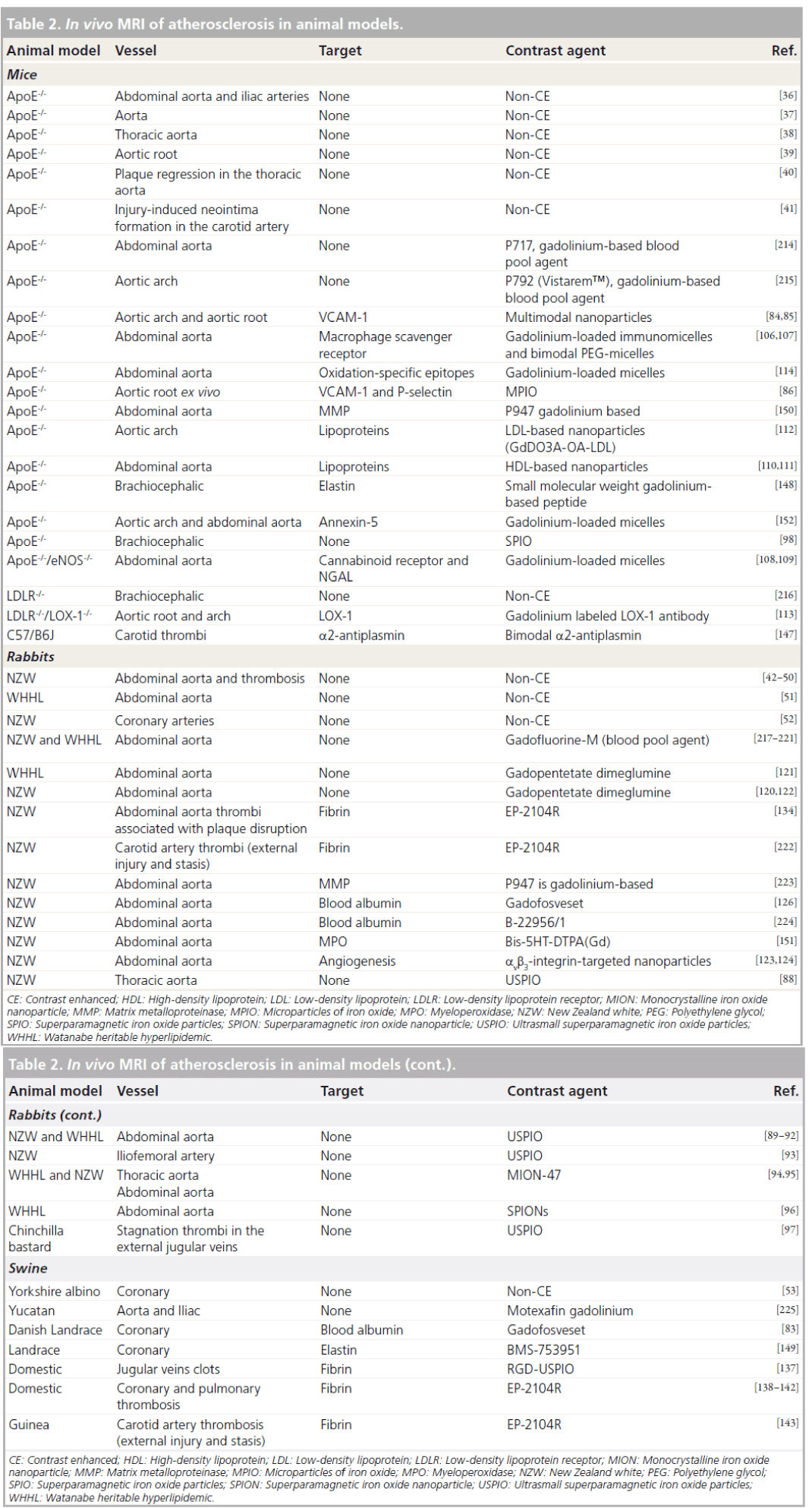
Increase in endothelial permeability and upregulation of adhesion molecules (VCAM‑1, ICAM‑1, P‑selectin) on the endothelial surface occurs in the early stages of atherosclerosis. Increased endothelial leakage allows blood molecules such as low-density lipoprotein (LDL) particles to passively diffuse into the vessel wall whereas expression of adhesion molecules is responsible for the receptor-mediated recruitment of leukocytes. Recently, gadofosveset, a gadolinium-based agent that reversibly binds to blood albumin has been shown to be associated with damaged endothelial cells in a swine model of coronary injury (Figure 2) [83]. Furthermore, multimodal nanoparticles (VIPN‑28) [84,85] and microparticles of iron oxide [86] targeting the VCAM‑1 receptor and/or P‑selectin have been used to image activated endothelium in mouse atherosclerotic plaques. Interestingly, a recent study showed that plaque permeation by contrast agents was strongly dependent on particle size [87].
Assessment of plaque macrophages & lipoproteins
Figure 2. Contrast-enhanced MRI using gadofosveset in a swine model of coronary artery injury. Coronary bright-blood cardiovascular MR angiography (A). T1-weighted inversion recovery at 5 min (B), 15 min (C) and 25 min (D) following intravenous administration of gadofosveset. The area of the balloon injured LAD2 segment expands over time indicating time-dependent extravasation of contrast, whereas the intact LAD1 segment (arrow) and CX (dotted arrow) remain constant [83]. CX: Circumflex artery; LAD: Left anterior descending coronary artery.
Macrophages are key players in the initiation, progression and complication of atherosclerosis. Superparamagnetic iron oxide particles of different sizes stabilized with different surface-coating materials (e.g., dextran or citrate) have been used to estimate the macrophage content of atherosclerotic plaques by becoming nonspecifically endocytosed by macrophages in hyperlipidemic rabbits [88–97], mice [98] and human carotid plaques [99–105]. Macrophages have also been imaged by using gadolinium-loaded micelles targeting the macrophage scavenger receptor in mouse plaques [106,107]. Atherosclerotic plaque macrophages also express the peripheral cannabinoid receptor (CB2‑R) and promote fibrous cap degradation by secretion of NGAL. CB2‑R- and NGALtargeted gadolinium-loaded micelles were shown to enhance murine atherosclerotic plaques with a vulnerable phenotype [108,109]. Gadoliniumloaded recombinant high-density lipoproteinlike nanoparticles [110,111] and LDL-based nanoparticles (GdDO3A‑OA‑LDL) [112] have also been developed to image atherosclerosis in mice. Furthermore, LOX‑1 and oxidized plaque LDL particles have been imaged using antibodies that bind to LOX‑1 receptor [113] and oxidation specific epitopes [114], respectively.
Assessment of plaque neovascularization
Aoki et al. were the first to observe a band of enhancement corresponding to the outer vessel wall, after injection of Gd‑DTPA, which was attributed to angiogenesis of the wall itself [115]. Enhancement of the outer rim was minimal in early phases of the disease and gradually increased. Subsequently, several other studies have demonstrated a correlation between Gd‑DTPA uptake and plaque neovascularization, inf lammation, endothelial permeability and fibrosis both in human [74,76,116–119] and animal models [117,120–122]. Gadolinium-based nanoparticles that target αvb3 integrins have also been engineered to selectively image plaque angiogenesis and as vehicles for antiangiogenic drug delivery in rabbit aortas [123,124]. Recently, the uptake of gadofosveset was shown to correlate with neovessel density in human carotid [125] and rabbit aortic plaques [126].
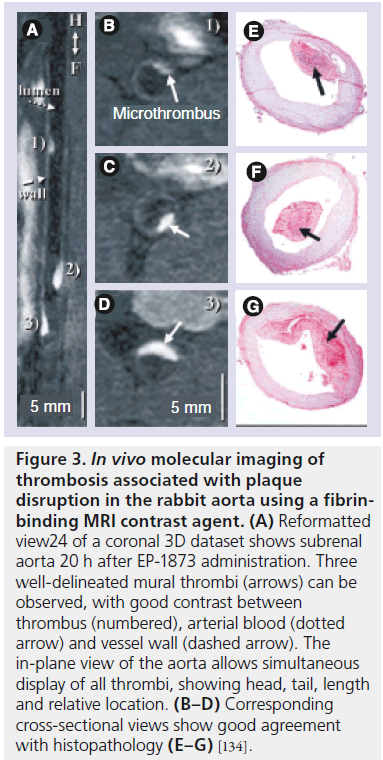
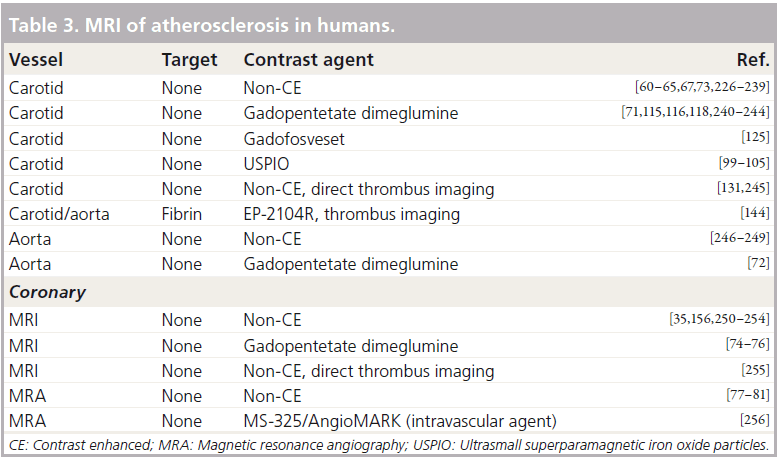
Figure 3. In vivo molecular imaging of thrombosis associated with plaque disruption in the rabbit aorta using a fibrinbinding MRI contrast agent. (A) Reformatted view24 of a coronal 3D dataset shows subrenal aorta 20 h after EP-1873 administration. Three well-delineated mural thrombi (arrows) can be observed, with good contrast between thrombus (numbered), arterial blood (dotted arrow) and vessel wall (dashed arrow). The in-plane view of the aorta allows simultaneous display of all thrombi, showing head, tail, length and relative location. (B–D) Corresponding cross-sectional views show good agreement with histopathology (E–G) [134].
Assessment of plaque intraplaque hemorrhage & thrombus
Intraplaque hemorrhage and thrombosis are major components of plaque vulnerability. Most MRI studies have focused on the detection of hematoma [127,128], venous thrombosis [129,130], intraplaque hemorrhage [131] and arterial thrombi [132,133] based on the temporal changes of T1 and T2 relaxation of different oxygenation states of hemoglobin in erythrocytes. Subsequently, the conspicuity of thrombi has been significantly increased by using fibrin- (Figure 3; rabbit model) [134–144], platelet- [97,145,146] and a2-antiplasmin-targeting contrast agents [147].
Assessment of plaque extracellular matrix
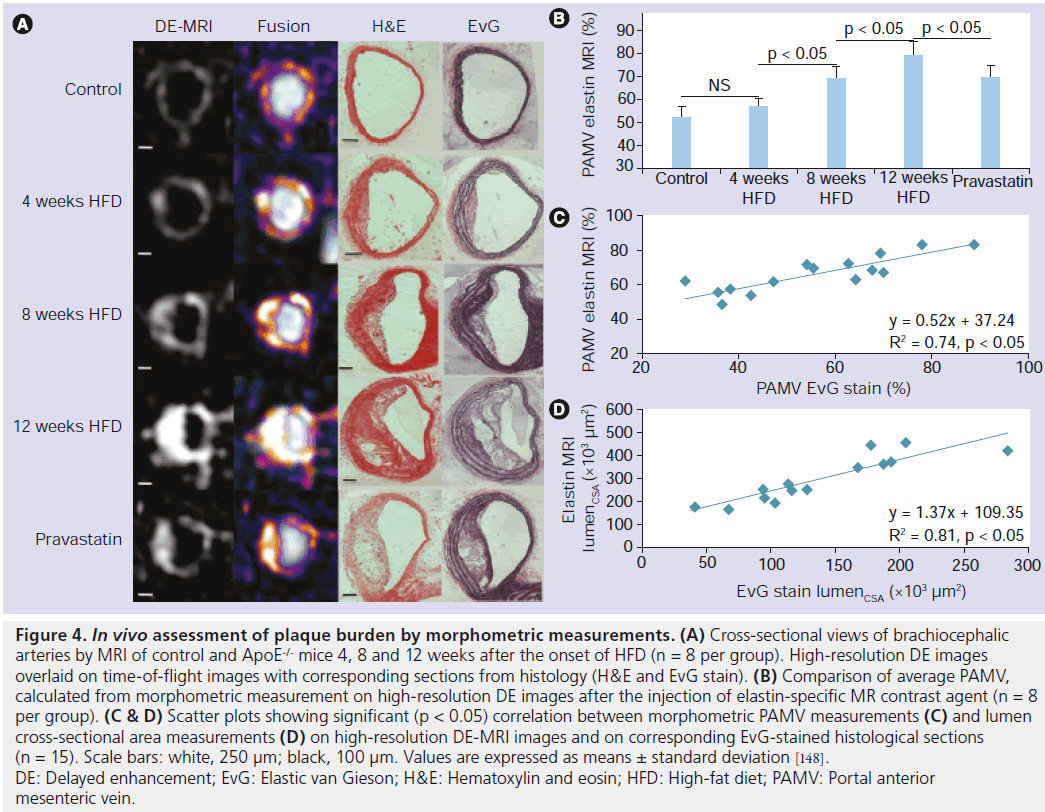
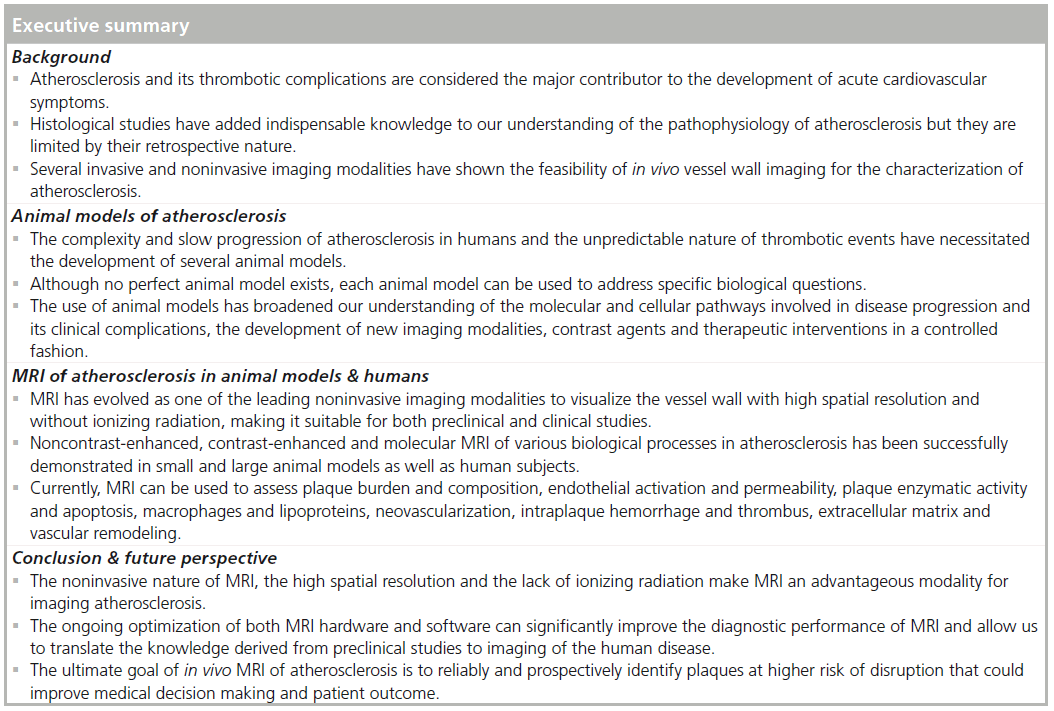
The fine-tuned balance in the production and degradation of extracellular matrix proteins (collagen, elastin, proteoglycans) is essential for plaque development and stability. Recently, with the development of a small molecular weight, gadolinium- based, elastin-targeting contrast agent, MRI of the vessel wall at all stages of atherosclerosis has become feasible in mouse atherosclerotic plaques (Figure 4) [148] and in a swine model of coronary injury [149].
Assessment of plaque enzymatic activity & apoptosis
Activated matrix metalloproteinases degrade the extracellular matrix and weaken the fibrous cap leading to plaque vulnerability. In vivo and ex vivo MRI for the characterization for matrix metalloproteinase-rich plaques was achieved with the use of a gadolinium-based matrix metalloproteinase- sensitive MRI contrast (P947) [150].
Figure 4.In vivo assessment of plaque burden by morphometric measurements. (A) Cross-sectional views of brachiocephalic arteries by MRI of control and ApoE-/- mice 4, 8 and 12 weeks after the onset of HFD (n = 8 per group). High-resolution DE images overlaid on time-of-flight images with corresponding sections from histology (H&E and EvG stain). (B) Comparison of average PAMV, calculated from morphometric measurement on high-resolution DE images after the injection of elastin-specific MR contrast agent (n = 8 per group). (C & D) Scatter plots showing significant (p < 0.05) correlation between morphometric PAMV measurements (C) and lumen cross-sectional area measurements (D) on high-resolution DE-MRI images and on corresponding EvG-stained histological sections (n = 15). Scale bars: white, 250 µm; black, 100 µm. Values are expressed as means ± standard deviation [148]. DE: Delayed enhancement; EvG: Elastic van Gieson; H&E: Hematoxylin and eosin; HFD: High-fat diet; PAMV: Portal anterior mesenteric vein.
Myeloperoxidase is another important enzyme secreted by activated macrophages at multiple stages of plaque development. Recently, in vivo MRI of myeloperoxidase has been achieved with the development of the gadolinium-based myeloperoxidase sensor bis‑5HT‑DTPA(Gd) in rabbit atherosclerotic plaques [151]. Lastly, cellular apoptosis is also a key feature of plaque progression and stability. Imaging of apoptosis has been shown in atherosclerotic mice using gadolinium-loaded micelles targeting annexin‑5 [152].
Assessment of vascular remodeling
Positive remodeling has been recognized as a possible mechanism to alleviate luminal narrowing based on autopsy studies [153–155]. In previous in vivo MRI studies of patients with subclinical coronary atherosclerosis [156,157] and of Watanabe hypercholesterolemic rabbits [121], positive remodeling was observed as an increase in the vessel wall area, determined by the outer vessel wall contour, with concurrent preservation of the lumen area. More recently, MRI characterization of vessel wall remodeling and its association with plaque vulnerability, using standardized cut off values, has been shown in atherosclerotic rabbits (Figure 5) [122].
Conclusion & future perspective
Figure 5.In vivo assessment of plaque burden by morphometric measurements. (A) Cross-sectional views of brachiocephalic arteries by MRI of control and ApoE-/- mice 4, 8 and 12 weeks after the onset of HFD (n = 8 per group). High-resolution DE images overlaid on time-of-flight images with corresponding sections from histology (H&E and EvG stain). (B) Comparison of average PAMV, calculated from morphometric measurement on high-resolution DE images after the injection of elastin-specific MR contrast agent (n = 8 per group). (C & D) Scatter plots showing significant (p < 0.05) correlation between morphometric PAMV measurements (C) and lumen cross-sectional area measurements (D) on high-resolution DE-MRI images and on corresponding EvG-stained histological sections (n = 15). Scale bars: white, 250 µm; black, 100 µm. Values are expressed as means ± standard deviation [148]. DE: Delayed enhancement; EvG: Elastic van Gieson; H&E: Hematoxylin and eosin; HFD: High-fat diet; PAMV: Portal anterior mesenteric vein.
Noncontrast-enhanced, contrast-enhanced and molecular MRI of various biological processes in atherosclerosis have been successfully demonstrated in small and large animal models as well as human subjects. The use of animal models allows the development of new imaging protocols, contrast agents and therapeutic interventions in a controlled fashion. Furthermore, it provides specimens for ex vivo validation studies. The noninvasive nature of MRI, the high spatial resolution and the lack of ionizing radiation make MRI an advantageous imaging modality for both preclinical and clinical studies. The development of higher field scanners and dedicated coils that allow for higher signal:noise ratio, the incorporation of multiple elements in the coils that allow higher acceleration factors, and the ongoing development of pulse sequences can significantly improve the diagnostic performance of MRI and allow translation of the knowledge derived from preclinical studies to imaging of the human disease. The ultimate goal of in vivo MRI of atherosclerosis is to reliably and prospectively identify plaques at higher risk for disruption that could improve medical decision making and patient outcome.
Currently, the use of most new contrast agents has been limited to preclinical models for investigating imaging protocols and elucidating the underlying biological processes involved in disease progression in a longitudinal noninvasive manner. Despite the exciting and promising results derived from the preclinical studies very few of these agents progressed to the clinical setting [158,159]. Important limitations that impede the translation to the clinical arena include scalability, cost, safety, favorable pharmacokinetics and regulatory guidelines [160]. Recently, two major prospective clinical studies that examined coronary atherosclerotic vessels in humans revealed that independent predictors including a large plaque burden, a small lumen area and a thin cap fibroatheroma (PROSPECT study) [161] and remodeling index (VIVA study) [162] were associated with future major adverse cardiac events as classified by radiofrequency IVUS. As shown in this review, similar measurements have been derived with native noncontrast and molecular MRI both in a preclinical and clinical setting. Although IVUS has superior spatial resolution compared with MRI it is invasive and therefore not suitable as a screening method. To this end, we envision the future use of noncontrast and molecular MRI as a noninvasive test for risk assessment and monitoring of interventions in subjects with suspected atherosclerosis by morphologic and biological plaque characterization.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: * of interest * of considerable interest
- Anitschkow N. [Über die veränderungen der kaninchenaorta bei experimenteller cholesterinsteatose]. Beiträge zur Pathologischen Anatomie und zur Allgemeinen Pathologie 56, 379–404 (1913). nn One of the first publications demonstrating that cholesterol-rich diets induce experimental atherosclerosis in rabbits.
- Ross R. Atherosclerosis – an inflammatory disease. N. Engl. J. Med. 340(2), 115–126 (1999).
- Lusis AJ. Atherosclerosis. Nature 407(6801), 233–241 (2000).
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 105(9), 1135–1143 (2002).
- Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J. Atheroscleros. Thrombos. 10(5), 267–274 (2003).
- Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler. Thromb. Vasc. Biol. 23(4), 582–587 (2003).
- Lloyd-Jones D, Adams R, Carnethon M et al. Heart disease and stroke statistics – 2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation 119(3), 480–486 (2009).
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet 349(9063), 1436–1442 (1997).
- Stary HC, Chandler AB, Dinsmore RE et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 15(9), 1512–1531 (1995).
- Stary HC, Chandler AB, Dinsmore RE et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92(5), 1355–1374 (1995).
- Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 20(5), 1177–1178 (2000).
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20(5), 1262–1275 (2000).
- Constantinides P. Plaque fissures in human coronary thrombosis. J. Atheroscler. Res. 6, 1–17 (1966). & Provided histological evidence that plaque rupture was the underlying cause of most acute cardiovascular events in subjects that have died suddenly.
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 336(18), 1276–1282 (1997).
- Kolodgie FD, Burke AP, Farb A et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 16(5), 285–292 (2001).
- Boyle JJ. Association of coronary plaque rupture and atherosclerotic inflammation. J. Pathol. 181(1), 93–99 (1997).
- van der Wal AC, Becker AE, van der Loos C, Das P. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89, 36–44 (1994).
- Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation 113(18), 2245–2252 (2006).
- Moreno PR, Purushothaman KR, Fuster V, O’Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation 105(21), 2504–2511 (2002).
- Kolodgie FD, Gold HK, Burke AP et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 349(24), 2316–2325 (2003).
- Pasterkamp G, Schoneveld AH, Hijnen DJ et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis 150(2), 245–253 (2000).
- Farb A, Burke AP, Tang AL et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 93(7), 1354–1363 (1996). & Provided histological evidence that plaque erosion without rupture can cause thrombosis leading to sudden death in humans.
- Ambrose JA, Tannenbaum MA, Alexopoulos D et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J. Am. Coll. Cardiol. 12(1), 56–62 (1988).
- Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am. J. Cardiol. 69(8), 729–732 (1992).
- Moise A, Lesperance J, Theroux P, Taeymans Y, Goulet C, Bourassa MG. Clinical and angiographic predictors of new total coronary occlusion in coronary artery disease: analysis of 313 nonoperated patients. Am. J. Cardiol. 54(10), 1176–1181 (1984).
- Anitschkow N, Chalatow S. [Ueber experimentelle cholesterinsteatose und ihre bedeutung für die entstehung einiger pathologischer prozesse.] Centrbl. Allg. Pathol. Pathol. Anat. 24, 1–9 (1913).
- Fayad ZA, Fuster V, Nikolaou K, Becker C. Computed tomography and magnetic resonance imaging for noninvasive coronary angiography and plaque imaging: current and potential future concepts. Circulation 106(15), 2026–2034 (2002).
- Choudhury RP, Fayad ZA. Imaging of atherosclerosis. Coronary wall imaging with MRI. J. Cardiovasc. Risk 9(5), 263–270 (2002).
- Choudhury RP, Fuster V, Badimon JJ, Fisher EA, Fayad ZA. MRI and characterization of atherosclerotic plaque: emerging applications and molecular imaging. Arterioscler. Thromb. Vasc. Biol. 22(7), 1065–1074 (2002).
- Fayad ZA. The assessment of the vulnerable atherosclerotic plaque using MR imaging: a brief review. Int. J. Cardiovasc. Imag. 17(3), 165–177 (2001).
- Fayad ZA. MR imaging for the noninvasive assessment of atherothrombotic plaques. Magn. Reson. Imaging Clin. N. Am. 11(1), 101–113 (2003).
- Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ. Res. 89(4), 305–316 (2001).
- Yuan C, Kerwin WS, Yarnykh VL et al. MRI of atherosclerosis in clinical trials. NMR Biomed. 19(6), 636–654 (2006).
- Yuan C, Hatsukami TS, Obrien KD. High-resolution magnetic resonance imaging of normal and atherosclerotic human coronary arteries ex vivo: discrimination of plaque tissue components. J. Investig. Med. 49(6), 491–499 (2001).
- Fayad ZA, Fuster V, Fallon JT et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 102(5), 506–510 (2000). & Demonstrated the usefulness of the black-blood pulse sequence for delineation of the vessel wall from the blood and the visualization of atherosclerosis.
- Fayad ZA, Fallon JT, Shinnar M et al. Noninvasive in vivo high-resolution magnetic resonance imaging of atherosclerotic lesions in genetically engineered mice. Circulation 98(15), 1541–1547 (1998).
- Choudhury RP, Aguinaldo JG, Rong JX et al. Atherosclerotic lesions in genetically modified mice quantified in vivo by non-invasive high-resolution magnetic resonance microscopy. Atherosclerosis 162(2), 315–321 (2002).
- Wiesmann F, Szimtenings M, Frydrychowicz A et al. High-resolution MRI with cardiac and respiratory gating allows for accurate in vivo atherosclerotic plaque visualization in the murine aortic arch. Magn. Reson. Med. 50(1), 69–74 (2003).
- Itskovich VV, Choudhury RP, Aguinaldo JG et al. Characterization of aortic root atherosclerosis in ApoE knockout mice: high-resolution in vivo and ex vivo MRM with histological correlation. Magn. Reson. Med. 49(2), 381–385 (2003).
- Trogan E, Fayad ZA, Itskovich VV et al. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 24(9), 1714–1719 (2004).
- Manka DR, Gilson W, Sarembock I, Ley K, Berr SS. Noninvasive in vivo magnetic resonance imaging of injury-induced neointima formation in the carotid artery of the apolipoprotein-E null mouse. J. Magn. Reson. Imaging 12(5), 790–794 (2000).
- Skinner MP, Yuan C, Mitsumori L et al. Serial magnetic resonance imaging of experimental atherosclerosis detects lesion fine structure, progression and complications in vivo. Nat. Med. 1(1), 69–73 (1995). & Initial demonstration of the feasibility to image exeprimental atherosclerosis in rabbits in vivo.
- Trouard TP, Altbach MI, Hunter GC, Eskelson CD, Gmitro AF. MRI and NMR spectroscopy of the lipids of atherosclerotic plaque in rabbits and humans. Magn. Reson. Med. 38(1), 19–26 (1997).
- McConnell MV, Aikawa M, Maier SE, Ganz P, Libby P, Lee RT. MRI of rabbit atherosclerosis in response to dietary cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 19(8), 1956–1959 (1999).
- Johnstone MT, Botnar RM, Perez AS et al. In vivo magnetic resonance imaging of experimental thrombosis in a rabbit model. Arterioscler. Thromb. Vasc. Biol. 21(9), 1556–1560 (2001). & In vivo identification of thrombosis associated with plaque disruption in rabbits.
- Helft G, Worthley SG, Fuster V et al. Atherosclerotic aortic component quantification by noninvasive magnetic resonance imaging: an in vivo study in rabbits. J. Am. Coll. Cardiol. 37(4), 1149–1154 (2001).
- Helft G, Worthley SG, Fuster V et al. Progression and regression of atherosclerotic lesions – monitoring with serial noninvasive magnetic resonance imaging. Circulation 105(8), 993–998 (2002).
- Ronald JA, Walcarius R, Robinson JF, Hegele RA, Rutt BK, Rogers KA. MRI of early- and late-stage arterial remodeling in a low-level cholesterol-fed rabbit model of atherosclerosis. J. Magn. Reson. Imaging 26(4), 1010–1019 (2007).
- Ma X, Zhao Q, Zhao L et al. In vivo MR imaging of plaque disruption and thrombus formation in an atherosclerotic rabbit model. Int. J. Cardiovasc. Imaging doi:10.1007/ s11239-006-7861-x (2011) (Epub ahead of print).
- Yuan C, Skinner MP, Kaneko E et al. Magnetic resonance imaging to study lesions of atherosclerosis in the hyperlipidemic rabbit aorta. Magn. Reson. Imaging 14(1), 93–102 (1996).
- Worthley SG, Helft G, Fuster V et al. Serial in vivo MRI documents arterial remodeling in experimental atherosclerosis. Circulation 101(6), 586–589 (2000).
- Sharma R, Singh RB. MRI of coronary artery atherosclerosis in rabbits: histopathology–MRI correlation and atheroma characterization. Thromb. J. 2(1), 5 (2004).
- Worthley SG, Helft G, Fuster V et al. Noninvasive in vivo magnetic resonance imaging of experimental coronary artery lesions in a porcine model. Circulation 101(25), 2956–2961 (2000).
- Toussaint JF, Southern JF, Fuster V, Kantor HL. T2-weighted contrast for NMR characterization of human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15(10), 1533–1542 (1995).
- Shinnar M, Fallon JT, Wehrli S et al. The diagnostic accuracy of ex vivo MRI for human atherosclerotic plaque characterization. Arterioscler. Thromb. Vasc. Biol. 19(11), 2756–2761 (1999).
- Serfaty JM, Chaabane L, Tabib A, Chevallier JM, Briguet A, Douek PC. Atherosclerotic plaques: classification and characterization with T2-weighted high-spatial-resolution MR imaging – an in vitro study. Radiology 219(2), 403–410 (2001).
- Morrisett J, Vick W, Sharma R et al. Discrimination of components in atherosclerotic plaques from human carotid endarterectomy specimens by magnetic resonance imaging ex vivo. Magn. Reson. Imaging 21(5), 465–474 (2003).
- Rogers WJ, Prichard JW, Hu YL et al. Characterization of signal properties in atherosclerotic plaque components by intravascular MRI. Arterioscler. Thromb. Vasc. Biol. 20(7), 1824–1830 (2000).
- Ronen RR, Clarke SE, Hammond RR, Rutt BK. Carotid plaque classification: defining the certainty with which plaque components can be differentiated. Magn. Reson. Med. 57(5), 874–880 (2007).
- Yuan C, Beach KW, Smith LH Jr, Hatsukami TS. Measurement of atherosclerotic carotid plaque size in vivo using high resolution magnetic resonance imaging. Circulation 98(24), 2666–2671 (1998).
- Saam T, Ferguson MS, Yarnykh VL et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler. Thromb. Vasc. Biol. 25(1), 234–239 (2005).
- Yuan C, Mitsumori LM, Ferguson MS et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 104(17), 2051–2056 (2001). & The application of the multicontrast MRI approach to identify different plaque components.
- Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 106(11), 1368–1373 (2002).
- Mitsumori LM, Hatsukami TS, Ferguson MS, Kerwin WS, Cai J, Yuan C. In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques. J. Magn. Reson. Imaging 17(4), 410–420 (2003).
- Kampschulte A, Ferguson MS, Kerwin WS et al. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 110(20), 3239–3244 (2004).
- Chu B, Kampschulte A, Ferguson MS et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35(5), 1079–1084 (2004).
- Saam T, Cai J, Ma L et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 240(2), 464–472 (2006).
- Toussaint JF, Southern JF, Fuster V, Kantor HL. Water diffusion properties of human atherosclerosis and thrombosis measured by pulse field gradient nuclear magnetic resonance. Arterioscler. Thromb. Vasc. Biol. 17(3), 542–546 (1997).
- Qiao Y, Ronen I, Viereck J, Ruberg FL, Hamilton JA. Identification of atherosclerotic lipid deposits by diffusion-weighted imaging. Arterioscler. Thromb. Vasc. Biol. 27(6), 1440–1446 (2007).
- Pachot-Clouard M, Vaufrey F, Darrasse L, Toussainti JF. Magnetization transfer characteristics in atherosclerotic plaque components assessed by adapted binomial preparation pulses. Magma 7(1), 9–15 (1998).
- Wasserman BA, Smith WI, Trout HH 3rd, Cannon RO 3rd, Balaban RS, Arai AE. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging initial results. Radiology 223(2), 566–573 (2002).
- Kramer CM, Cerilli LA, Hagspiel K, Dimaria JM, Epstein FH, Kern JA. Magnetic resonance imaging identifies the fibrous cap in atherosclerotic abdominal aortic aneurysm. Circulation 109(8), 1016–1021 (2004).
- Cai J, Hatsukami TS, Ferguson MS et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 112(22), 3437–3444 (2005).
- Maintz D, Ozgun M, Hoffmeier A et al. Selective coronary artery plaque visualization and differentiation by contrast-enhanced inversion prepared MRI. Eur. Heart J. 27(14), 1732–1736 (2006).
- Yeon SB, Sabir A, Clouse M et al. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. J. Am. Coll. Cardiol. 50(5), 441–447 (2007).
- Ibrahim T, Makowski MR, Jankauskas A et al. Serial contrast-enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc. Imaging 2(5), 580–588 (2009).
- Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. A fast 3D approach for coronary MRA. J. Magn. Reson. Imaging 10(5), 821–825 (1999).
- Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation 99(24), 3139–3148 (1999). nn Application of advanced pulse sequences for coronary MR angiography.
- Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Breathhold threedimensional coronary magnetic resonance angiography using real-time navigator technology. J. Cardiovasc. Magn. Reson. 1(3), 233–238 (1999).
- Stuber M, Botnar RM, Danias PG et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J. Am. Coll. Cardiol. 34(2), 524–531 (1999). & Application of advanced pulse sequences for coronary MR angiography.
- Katoh M, Spuentrup E, Stuber M et al. Inversion prepared coronary MR angiography: direct visualization of coronary blood flow. Rofo 177(2), 173–178 (2005). & Application of advanced pulse sequences.
- Kim WY, Astrup AS, Stuber M et al. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in Type 1 diabetes with and without diabetic nephropathy. Circulation 115(2), 228–235 (2007).
- Pedersen SF, Thrysoe SA, Paaske WP et al. CMR assessment of endothelial damage and angiogenesis in porcine coronary arteries using gadofosveset. J. Cardiovasc. Magn. Reson. 13, 10 (2011).
- Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ. Res. 96(3), 327–336 (2005).
- Nahrendorf M, Jaffer FA, Kelly KA et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114(14), 1504–1511 (2006).
- Mcateer MA, Schneider JE, Ali ZA et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler. Thromb. Vasc. Biol. 28(1), 77–83 (2008).
- van Bochove GS, Paulis LE, Segers D et al. Contrast enhancement by differently sized paramagnetic MRI contrast agents in mice with two phenotypes of atherosclerotic plaque. Contrast Media Mol. Imaging 6(1), 35–45 (2011).
- Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 103(3), 415–422 (2001).
- Yancy AD, Olzinski AR, Hu TC et al. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: critical determinants of atherosclerotic plaque labeling. J. Magn. Reson. Imaging 21(4), 432–442 (2005).
- Hyafil F, Laissy JP, Mazighi M et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 26(1), 176–181 (2006).
- Herborn CU, Vogt FM, Lauenstein TC et al. Magnetic resonance imaging of experimental atherosclerotic plaque: comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 24(2), 388–393 (2006).
- Sigovan M, Boussel L, Sulaiman A et al. Rapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal model. Radiology 252(2), 401–409 (2009).
- Durand E, Raynaud JS, Bruneval P et al. Magnetic resonance imaging of ruptured plaques in the rabbit with ultrasmall superparamagnetic particles of iron oxide. J. Vasc. Res. 44(2), 119–128 (2007).
- Korosoglou G, Weiss RG, Kedziorek DA et al. Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using ‘positive contrast’ magnetic resonance imaging. J. Am. Coll. Cardiol. 52(6), 483–491 (2008).
- Morishige K, Kacher DF, Libby P et al. High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation 122(17), 1707–1715 (2010).
- Smith BR, Heverhagen J, Knopp M et al. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed. Microdev. 9(5), 719–727 (2007).
- Schmitz SA, Winterhalter S, Schiffler S et al. USPIO-enhanced direct MR imaging of thrombus: preclinical evaluation in rabbits. Radiology 221(1), 237–243 (2001).
- Makowski MR, Varma G, Wiethoff AJ et al. Noninvasive assessment of atherosclerotic plaque progression in ApoE-/- mice using susceptibility gradient mapping. Circ. Cardiovasc. Imaging 4(3), 295–303 (2011).
- Kooi ME, Cappendijk VC, Cleutjens KB et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107(19), 2453–2458 (2003).
- Trivedi RA, U-King-Im JM, Graves MJ, Kirkpatrick PJ, Gillard JH. Noninvasive imaging of carotid plaque inflammation. Neurology 63(1), 187–188 (2004).
- Trivedi RA, U-King-Im JM, Graves MJ et al. MRI-derived measurements of fibrous-cap and lipid-core thickness: the potential for identifying vulnerable carotid plaques in vivo. Neuroradiology 46(9), 738–743 (2004).
- Trivedi RA, Mallawarachi C, U-King-Im JM et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler. Thromb. Vasc. Biol. 26(7), 1601–1606 (2006).
- Tang T, Howarth SP, Miller SR et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 37(9), 2266–2270 (2006).
- Tang TY, Howarth SP, Miller SR et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques contralateral to symptomatic carotid stenosis: an ultra small superparamagnetic iron oxide enhanced magnetic resonance study. J. Neurol. Neurosurg. Psychiat. 78(12), 1337–1343 (2007).
- Howarth SP, Tang TY, Trivedi R et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur. J. Radiol. 70(3), 555–560 (2009).
- Amirbekian V, Lipinski MJ, Briley-Saebo KC et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl Acad. Sci. USA 104(3), 961–966 (2007).
- Mulder WJ, Strijkers GJ, Briley-Saboe KC et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn. Reson. Med. 58(6), 1164–1170 (2007).
- Te Boekhorst BC, Bovens SM, Rodrigues-Feo J et al. Characterization and in vitro and in vivo testing of CB2-receptor- and NGAL-targeted paramagnetic micelles for molecular MRI of vulnerable atherosclerotic plaque. Mol. Imaging Biol. 12(6), 635–651 (2010).
- Te Boekhorst BC, Bovens SM, Hellings WE et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristics. Cardiovasc. Res. 89(3), 680–688 (2011).
- Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 126(50), 16316–16317 (2004).
- Cormode DP, Chandrasekar R, Delshad A et al. Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconjug. Chem. 20(5), 937–943 (2009).
- Yamakoshi Y, Qiao H, Lowell AN et al. LDL-based nanoparticles for contrast enhanced MRI of atheroplaques in mouse models. Chem. Commun. (Camb.) 47(31), 8835–8837 (2011).
- Li D, Patel AR, Klibanov AL et al. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ. Cardiovasc. Imaging 3(4), 464–472 (2010).
- Briley-Saebo KC, Shaw PX, Mulder WJ et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation 117(25), 3206–3215 (2008).
- Aoki S, Aoki K, Ohsawa S, Nakajima H, Kumagai H, Araki T. Dynamic MR imaging of the carotid wall. J. Magn. Reson. Imaging 9(3), 420–427 (1999).
- Kerwin W, Hooker A, Spilker M et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 107(6), 851–856 (2003). & Showed that dynamic contrast-enhanced MRI provides an indication of the extent of neovasculature within carotid atherosclerotic plaque.
- Lin W, Abendschein DR, Haacke EM. Contrast-enhanced magnetic resonance angiography of carotid arterial wall in pigs. J. Magn. Reson. Imaging 7(1), 183–190 (1997).
- Yuan C, Kerwin WS, Ferguson MS et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J. Magn. Reson. Imaging 15(1), 62–67 (2002).
- Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 241(2), 459–468 (2006).
- Calcagno C, Cornily JC, Hyafil F et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler. Thromb. Vasc. Biol. 28(7), 1311–1317 (2008).
- Steen H, Lima JA, Chatterjee S et al. High-resolution three-dimensional aortic magnetic resonance angiography and quantitative vessel wall characterization of different atherosclerotic stages in a rabbit model. Invest. Radiol. 42(9), 614–621 (2007).
- Phinikaridou A, Ruberg FL, Hallock KJ et al. In vivo detection of vulnerable atherosclerotic plaque by MRI in a rabbit model. Circ. Cardiovasc. Imaging 3(3), 323–332 (2009).
- Winter PM, Morawski AM, Caruthers SD et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with a(v) b3-integrin-targeted nanoparticles. Circulation 108(18), 2270–2274 (2003).
- Winter PM, Neubauer AM, Caruthers SD et al. Endothelial a(v)b3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26(9), 2103–2109 (2006).
- Lobbes MB, Heeneman S, Passos VL et al. Gadofosveset-enhanced magnetic resonance imaging of human carotid atherosclerotic plaques: a proof-of-concept study. Investigat. Radiol. 45(5), 275–281 (2010).
- Lobbes MB, Miserus RJ, Heeneman S et al. Atherosclerosis: contrast-enhanced MR imaging of vessel wall in rabbit model – comparison of gadofosveset and gadopentetate dimeglumine. Radiology 250(3), 682–691 (2009).
- Gomori JM, Grossman RI, Goldberg HI, Zimmerman RA, Bilaniuk LT. Intracranial hematomas: imaging by high-field MR. Radiology 157(1), 87–93 (1985).
- Clark RA, Watanabe AT, Bradley WG Jr, Roberts JD. Acute hematomas: effects of deoxygenation, hematocrit, and fibrin-clot formation and retraction on T2 shortening. Radiology 175(1), 201–206 (1990).
- Moody AR, Pollock JG, O’Connor AR, Bagnall M. Lower-limb deep venous thrombosis: direct MR imaging of the thrombus. Radiology 209(2), 349–355 (1998).
- Rapoport S, Sostman HD, Pope C, Camputaro CM, Holcomb W, Gore JC. Venous clots: evaluation with MR imaging. Radiology 162(2), 527–530 (1987).
- Moody AR, Murphy RE, Morgan PS et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 107(24), 3047–3052 (2003).
- Corti R, Osende JI, Fayad ZA et al. In vivo noninvasive detection and age definition of arterial thrombus by MRI. J. Am. Coll. Cardiol. 39(8), 1366–1373 (2002). & Direct thrombus detection by MRI due to the short T1 relaxation time of methemoglobin.
- Viereck J, Ruberg FL, Qiao Y et al. MRI of atherothrombosis associated with plaque rupture. Arterioscler. Thromb. Vasc. Biol. 25(1), 240–245 (2005).
- Botnar RM, Perez AS, Witte S et al. In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation 109(16), 2023–2029 (2004). & In vivo application of a fibrin-targeted contrast agent to visualize thrombosis associated with plaque disruption in a rabbit model.
- Winter PM, Cai K, Chen J et al. Targeted PARACEST nanoparticle contrast agent for the detection of fibrin. Magn. Reson. Med. 56(6), 1384–1388 (2006).
- Flacke S, Fischer S, Scott MJ et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation 104(11), 1280–1285 (2001).
- Johansson LO, Bjornerud A, Ahlstrom HK, Ladd DL, Fujii DK. A targeted contrast agent for magnetic resonance imaging of thrombus: implications of spatial resolution. J. Magn. Reson. Imaging 13(4), 615–618 (2001).
- Botnar RM, Buecker A, Wiethoff AJ et al. In vivo magnetic resonance imaging of coronary thrombosis using a fibrin-binding molecular magnetic resonance contrast agent. Circulation 110(11), 1463–1466 (2004).
- Spuentrup E, Buecker A, Katoh M et al. Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation 111(11), 1377–1382 (2005).
- Spuentrup E, Fausten B, Kinzel S et al. Molecular magnetic resonance imaging of atrial clots in a swine model. Circulation 112(3), 396–399 (2005).
- Spuentrup E, Katoh M, Wiethoff AJ et al. Molecular magnetic resonance imaging of pulmonary emboli with a fibrin-specific contrast agent. Am. J. Respir. Crit. Care Med. 172(4), 494–500 (2005).
- Stracke CP, Katoh M, Wiethoff AJ, Parsons EC, Spangenberg P, Spuntrup E. Molecular MRI of cerebral venous sinus thrombosis using a new fibrin-specific MR contrast agent. Stroke 38(5), 1476–1481 (2007).
- Sirol M, Aguinaldo JG, Graham PB et al. Fibrin-targeted contrast agent for improvement of in vivo acute thrombus detection with magnetic resonance imaging. Atherosclerosis 182(1), 79–85 (2005).
- Spuentrup E, Botnar RM, Wiethoff AJ et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: initial results in patients. Eur. Radiol. 18(9), 1995–2005 (2008).
- Johansson LO, Bjornerud A, Ahlstrom HK, Ladd DL, Fujii DK. A targeted contrast agent for magnetic resonance imaging of thrombus: implications of spatial resolution. J. Magn. Reson. Imaging 13(4), 615–618 (2001).
- Klink A, Lancelot E, Ballet S et al. Magnetic resonance molecular imaging of thrombosis in an arachidonic acid mouse model using an activated platelet targeted probe. Arterioscler. Thromb. Vasc. Biol. 30(3), 403–410 (2010).
- Miserus RJ, Herias MV, Prinzen L et al. Molecular MRI of early thrombus formation using a bimodal a2-antiplasmin-based contrast agent. JACC Cardiovasc. Imaging 2(8), 987–996 (2009).
- Makowski MR, Wiethoff AJ, Blume U et al. Assessment of atherosclerotic plaque burden with an elastin-specific magnetic resonance contrast agent. Nat. Med. 17(3), 383–388 (2011). & In vivo application of a new MRI contrast agent that binds specifically to elastin to assess plaque progression.
- von Bary C, Makowski M, Preissel A et al. MRI of coronary wall remodeling in a swine model of coronary injury using an elastin-binding contrast agent. Circ. Cardiovasc. Imaging 4(2), 147–155 (2011).
- Lancelot E, Amirbekian V, Brigger I et al. Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler. Thromb. Vasc. Biol. 28(3), 425–432 (2008).
- Ronald JA, Chen JW, Chen Y et al. Enzymesensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation 120(7), 592–599 (2009).
- van Tilborg GA, Vucic E, Strijkers GJ et al. Annexin A5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug. Chem. 21(10), 1794–1803 (2010).
- Crawford T, Levene CI. Medial thinning in atheroma. J. Pathol. Bacteriol. 66(1), 19–23 (1953).
- Bond MG, Adams MR, Bullock BC. Complicating factors in evaluating coronary artery atherosclerosis. Artery 9(1), 21–29 (1981).
- Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316(22), 1371–1375 (1987). & Showed that human coronary arteries enlarge in relation to plaque area and that luminal stenosis may be delayed until the lesion occupies 40% of the internal elastic lamina area.
- Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation 106(3), 296–299 (2002). & Showed that coronary MRI can be used to detect a Glagov type of arterial remodeling in vivo.
- Miao C, Chen S, Macedo R et al. Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (Multi- Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 53(18), 1708–1715 (2009).
- Tang TY, Howarth SP, Miller SR et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 53(22), 2039–2050 (2009).
- Vymazal J, Spuentrup E, Cardenas-Molina G et al. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a Phase 2 clinical study of feasibility. Investigat. Radiol. 44(11), 697–704 (2009).
- Buxton DB, Antman M, Danthi N et al. Report of the National Heart, Lung, and Blood Institute working group on the translation of cardiovascular molecular imaging. Circulation 123(19), 2157–2163 (2011).
- Stone GW, Maehara A, Lansky AJ et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364(3), 226–235 (2011).
- Calvert PA, Obaid DR, O’Sullivan M et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in vulnerable atherosclerosis) study. J. Am. Coll. Cardiol. Imaging 4(8), 894–901 (2011).
- Paigen B, Holmes PA, Mitchell D, Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis 64(2–3), 215–221 (1987).
- Stewart-Phillips JL, Lough J. Pathology of atherosclerosis in cholesterol-fed, susceptible mice. Atherosclerosis 90(2–3), 211–218 (1991).
- Purcell-Huynh DA, Farese RV Jr, Johnson DF et al. Transgenic mice expressing high levels of human apolipoprotein B develop severe atherosclerotic lesions in response to a high-fat diet. J. Clin. Invest. 95(5), 2246–2257 (1995).
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258(5081), 468–471 (1992).
- Plump AS, Smith JD, Hayek T et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71(2), 343–353 (1992).
- Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 14(1), 141–147 (1994).
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14(1), 133–140 (1994).
- Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler. Thromb. Vasc. Biol. 20(12), 2587–2592 (2000).
- Cheng C, Tempel D, Van Haperen R et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J. Clin. Investigat. 117(3), 616–626 (2007).
- Ding SF, Ni M, Liu XL et al. A causal relationship between shear stress and atherosclerotic lesions in apolipoprotein E knockout mice assessed by ultrasound biomicroscopy. Am. J. Physiol. Heart Circ. Physiol. 298(6), H2121–H2129 (2010).
- Lutgens E, Daemen M, Kockx M et al. Atherosclerosis in APOE*3-leiden transgenic mice: from proliferative to atheromatous stage. Circulation 99(2), 276–283 (1999).
- Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 93(5), 1885–1893 (1994).
- Powell-Braxton L, Veniant M, Latvala RD et al. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat. Med. 4(8), 934–938 (1998).
- Sanan DA, Newland DL, Tao R et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc. Natl Acad. Sci. USA 95(8), 4544–4549 (1998).
- Masucci-Magoulas L, Goldberg IJ, Bisgaier CL et al. A mouse model with features of familial combined hyperlipidemia. Science 275(5298), 391–394 (1997).
- Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in ‘knockout’ mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl Acad. Sci. USA 91(10), 4431–4435 (1994).
- Van Herck JL, De Meyer GR, Martinet W et al. Impaired fibrillin-1 function promotes features of plaque instability in apolipoprotein E-deficient mice. Circulation 120(24), 2478–2487 (2009).
- Lemaitre V, O’Byrne TK, Borczuk AC, Okada Y, Tall AR, D’Armiento J. ApoE knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J. Clin. Invest. 107(10), 1227–1234 (2001).
- Kuhlencordt PJ, Gyurko R, Han F et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104(4), 448–454 (2001).
- Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation 103(25), 3099–3104 (2001).
- Welch CL, Sun Y, Arey BJ et al. Spontaneous atherothrombosis and medial degradation in ApoE-/-, Npc1-/- mice. Circulation 116(21), 2444–2452 (2007).
- Page IH, Brown HB. Induced hypercholesterolemia and atherogenesis. Circulation 6(5), 681–687 (1952).
- Malinow MR, Hojman D, Pellegrino A. Different methods for the experimental production of generalized atherosclerosis in the rat. Acta Cardiol. 9(5), 480–499 (1954).
- Constantinides P. Experimental Atherosclerosis. Elsevier Publishing Co., NY, USA (1965). 187 Constantinides P, Gutmann-Auersperg N, Hospes D. Acceleration of intimal atherogenesis through prior medial injury. AMA Arch. Pathol. 66(3), 247–254 (1958).
- Constantinides P, Booth J, Carlson G. Production of advanced cholesterol atherosclerosis in the rabbit. Arch. Pathol. 70, 80–92 (1960).
- Weidinger FF, McLenachan JM, Cybulsky MI et al. Hypercholesterolemia enhances macrophage recruitment and dysfunction of regenerated endothelium after balloon injury of the rabbit iliac artery. Circulation 84(2), 755–767 (1991).
- Abela GS, Picon PD, Friedl SE et al. Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation 91(3), 776–784 (1995).
- Phinikaridou A, Hallock KJ, Qiao Y, Hamilton JA. A robust rabbit model of human atherosclerosis and atherothrombosis. J. Lipid Res. 50(5), 787–797 (2009).
- Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis 36(2), 261–268 (1980).
- Havel RJ, Kita T, Kotite L et al. Concentration and composition of lipoproteins in blood plasma of the WHHL rabbit. An animal model of human familial hypercholesterolemia. Arteriosclerosis 2(6), 467–474 (1982).
- Kita T, Goldstein JL, Brown MS, Watanabe Y, Hornick CA, Havel RJ. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc. Natl Acad. Sci. USA 79(11), 3623–3627 (1982).
- Buja LM, Kita T, Goldstein JL, Watanabe Y, Brown MS. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis 3(1), 87–101 (1983).
- Seddon AM, Woolf N, La Ville A et al. Hereditary hyperlipidemia and atherosclerosis in the rabbit due to overproduction of lipoproteins. II. Preliminary report of arterial pathology. Arteriosclerosis 7(2), 113–124 (1987).
- Fox R. Handbook on Genetically Standardized JAX Rabbit. Jackson Laboratory, Bar Harbor, ME, USA (1975).
- Overturf ML, Smith SA, Hewett-Emmett D et al. Development and partial metabolic characterization of a dietary cholesterolresistant colony of rabbits. J. Lipid Res. 30(2), 263–273 (1989).
- Brousseau ME, Hoeg JM. Transgenic rabbits as models for atherosclerosis research. J. Lipid Res. 40(3), 365–375 (1999).
- Tjwa M, Carmeliet P, Moons L. Novel transgenic rabbit model sheds light on the puzzling role of matrix metalloproteinase-12 in atherosclerosis. Circulation 113(16), 1929–1932 (2006).
- Ratcliffe HL, Luginbuhl H. The domestic pig: a model for experimental atherosclerosis. Atherosclerosis 13(1), 133–136 (1971).
- Lee KT, Lee WM. Advanced coronary atherosclerosis in swine produced by combination of balloon-catheter injury and cholesterol feeding. In: Atherosclerosis: Metabolic, Morphologic and Clinical Aspects. Manning GW, Haust MD (Eds). Plenum Press, NY, USA, 597–602 (1977).
- Cevallos WH, Holmes WL, Myers RN, Smink RD. Swine in atherosclerosis research – development of an experimental animal model and study of the effect of dietary fats on cholesterol metabolism. Atherosclerosis 34(3), 303–317 (1979).
- Reitman JS, Mahley RW, Fry DL. Yucatan miniature swine as a model for diet-induced atherosclerosis. Atherosclerosis 43(1), 119–132 (1982).
- Phillips RW, Westmoreland N, Panepinto L, Case GL. Dietary effects on metabolism of Yucatan miniature swine selected for low and high glucose utilization. J. Nutr. 112(1), 104–111 (1982).
- Phillips RW, Panepinto LM, Spangler R, Westmoreland N. Yucatan miniature swine as a model for the study of human diabetes mellitus. Diabetes 31(Suppl. 1 Pt 2), S30–S36 (1982).
- Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50(7), 1654–1665 (2001).
- Rapacz J, Hasler-Rapacz J, Taylor KM, Checovich WJ, Attie AD. Lipoprotein mutations in pigs are associated with elevated plasma cholesterol and atherosclerosis. Science 234(4783), 1573–1577 (1986).
- Hasler-Rapacz J, Ellegren H, Fridolfsson AK et al. Identification of a mutation in the low density lipoprotein receptor gene associated with recessive familial hypercholesterolemia in swine. Am. J. Med. Genet. 76(5), 379–386 (1998).
- Prescott MF, McBride CH, Hasler-Rapacz J, Von Linden J, Rapacz J. Development of complex atherosclerotic lesions in pigs with inherited hyper-LDL cholesterolemia bearing mutant alleles for apolipoprotein B. Am. J. Pathol. 139(1), 139–147 (1991).
- Thim T, Hagensen MK, Drouet L et al. Familial hypercholesterolaemic downsized pig with human-like coronary atherosclerosis: a model for preclinical studies. Eur. Intervent. 6(2), 261–268 (2010).
- Steiner A, Kendall FE. Atherosclerosis and arteriosclerosis in dogs following ingestion of cholesterol and thiouracil. Arch. Pathol. (Chic.) 42(4), 433–444 (1946).
- Luzio NR, O’Neal RM. The rapid development of arterial lesions in dogs fed an infarct-producing diet. Exp. Mol. Pathol. 1, 122–132 (1962).
- Chaabane L, Pellet N, Bourdillon MC et al. Contrast enhancement in atherosclerosis development in a mouse model: in vivo results at 2 Tesla. Magma 17(3–6), 188–195 (2004).
- Alsaid H, Sabbah M, Bendahmane Z et al. High-resolution contrast-enhanced MRI of atherosclerosis with digital cardiac and respiratory gating in mice. Magn. Reson. Med. 58(6), 1157–1163 (2007).
- Hockings PD, Roberts T, Galloway GJ et al. Repeated three-dimensional magnetic resonance imaging of atherosclerosis development in innominate arteries of low-density lipoprotein receptor-knockout mice. Circulation 106(13), 1716–1721 (2002).
- Meding J, Urich M, Licha K et al. Magnetic resonance imaging of atherosclerosis by targeting extracellular matrix deposition with Gadofluorine M. Contrast Media Mol. Imaging 2(3), 120–129 (2007).
- Barkhausen J, Ebert W, Heyer C, Debatin JF, Weinmann HJ. Detection of atherosclerotic plaque with gadofluorine-enhanced magnetic resonance imaging. Circulation 108(5), 605–609 (2003).
- Sirol M, Itskovich VV, Mani V et al. Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation 109(23), 2890–2896 (2004).
- Sirol M, Moreno PR, Purushothaman KR et al. Increased neovascularization in advanced lipid-rich atherosclerotic lesions detected by gadofluorine-M-enhanced MRI: implications for plaque vulnerability. Circ. Cardiovasc. Imaging 2(5), 391–396 (2009).
- Ronald JA, Chen Y, Belisle AJL et al. Comparison of gadofluorine-M and Gd-DTPA for noninvasive staging of atherosclerotic plaque stability using MRI. Circ. Cardiovasc. Imaging 2(3), 226–234 (2009).
- Sirol M, Fuster V, Badimon JJ et al. Chronic thrombus detection with in vivo magnetic resonance imaging and a fibrin-targeted contrast agent. Circulation 112(11), 1594–1600 (2005).
- Hyafil F, Vucic E, Cornily JC et al. Monitoring of arterial wall remodelling in atherosclerotic rabbits with a magnetic resonance imaging contrast agent binding to matrix metalloproteinases. Eur. Heart J. 32(12), 1561–1571 (2011).
- Cornily JC, Hyafil F, Calcagno C et al. Evaluation of neovessels in atherosclerotic plaques of rabbits using an albumin-binding intravascular contrast agent and MRI. J. Magn. Reson. Imaging 27(6), 1406–1411 (2008).
- Brushett C, Qiu B, Atalar E, Yang X. High-resolution MRI of deep-seated atherosclerotic arteries using motexafin gadolinium. J. Magn. Reson. Imaging 27(1), 246–250 (2008).
- Toussaint JF, Lamuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 94(5), 932–938 (1996).
- Yuan C, Zhang SX, Polissar NL et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 105(2), 181–185 (2002).
- Yuan C, Zhao XQ, Hatsukami TS. Quantitative evaluation of carotid atherosclerotic plaques by magnetic resonance imaging. Curr. Atheroscler. Rep. 4(5), 351–357 (2002).
- Zhang S, Hatsukami TS, Polissar NL, Han C, Yuan C. Comparison of carotid vessel wall area measurements using three different contrast-weighted black blood MR imaging techniques. Magn. Reson. Imaging 19(6), 795–802 (2001).
- Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 102(9), 959–964 (2000).
- Ota H, Yu W, Underhill HR et al. Hemorrhage and large lipid-rich necrotic cores are independently associated with thin or ruptured fibrous caps: an in vivo 3T MRI study. Arterioscler. Thromb. Vasc. Biol. 29(10), 1696–1701 (2009).
- Saam T, Yuan C, Chu B et al. Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis 194(2), e34–e42 (2007).
- Takaya N, Yuan C, Chu B et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a highresolution magnetic resonance imaging study. Circulation 111(21), 2768–2775 (2005).
- Takaya N, Yuan C, Chu B et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI – initial results. Stroke 37(3), 818–823 (2006).
- Saam T, Underhill HR, Chu B et al. Prevalence of American Heart Association type 6 carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J. Am. Coll. Cardiol. 51(10), 1014–1021 (2008).
- Underhill HR, Yuan C, Yarnykh VL et al. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. Am. J. Neuroradiol. 31(3), 487–493 (2010). & Prospective investigation of asymptomatic individuals with 50–79% stenosis; provided evidence that the size of the lipid-rich necrotic core may govern the risk of future surface disruption.
- Wasserman BA, Astor BC, Sharrett AR, Swingen C, Catellier D. MRI measurements of carotid plaque in the atherosclerosis risk in communities (ARIC) study: methods, reliability and descriptive statistics. J. Magn. Reson. Imaging 31(2), 406–415 (2010).
- Astor BC, Sharrett AR, Coresh J, Chambless LE, Wasserman BA. Remodeling of carotid arteries detected with MR imaging: atherosclerosis risk in communities carotid MRI study. Radiology 256(3), 879–886 (2010).
- Wasserman BA, Wityk RJ, Trout HH 3rd, Virmani R. Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke 36(11), 2504–2513 (2005).
- Wasserman BA, Casal SG, Astor BC, Aletras AH, Arai AE. Wash-in kinetics for gadolinium-enhanced magnetic resonance imaging of carotid atheroma. J. Magn. Reson. Imaging 21(1), 91–95 (2005).
- Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 241(2), 459–468 (2006).
- Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn. Reson. Med. 59(3), 507–514 (2008).
- Kerwin WS, Zhao X, Yuan C, Hatsukami TS, Maravilla KR, Underhill HR. Contrast-enhanced MRI of carotid atherosclerosis: dependence on contrast agent. J. Magn. Reson. Imaging 30(1), 35–40 (2009).
- Underhill HR, Yuan C, Yarnykh VL et al. Arterial remodeling in [corrected] subclinical carotid artery disease. JACC Cardiovasc. Imaging 2(12), 1381–1389 (2009).
- Murphy RE, Moody AR, Morgan PS et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation 107(24), 3053–3058 (2003).
- Fayad ZA, Nahar T, Fallon JT et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation 101(21), 2503–2509 (2000).
- Summers RM, Andrasko-Bourgeois J, Feuerstein IM et al. Evaluation of the aortic root by MRI: insights from patients with homozygous familial hypercholesterolemia. Circulation 98(6), 509–518 (1998).
- Jaffer FA, O’Donnell CJ, Larson MG et al. Age and sex distribution of subclinical aortic atherosclerosis: a magnetic resonance imaging examination of the Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 22(5), 849–854 (2002).
- Taniguchi H, Momiyama Y, Fayad ZA et al. In vivo magnetic resonance evaluation of associations between aortic atherosclerosis and both risk factors and coronary artery disease in patients referred for coronary angiography. Am. Heart J. 148(1), 137–143 (2004).
- Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation 102(21), 2582–2587 (2000).
- Botnar RM, Kim WY, Bornert P, Stuber M, Spuentrup E, Manning WJ. 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magn. Reson. Med. 46(5), 848–854 (2001). & Application of advanced pulse sequences for coronary vessel wall MRI.
- Botnar RM, Stuber M, Lamerichs R et al. Initial experiences with in vivo right coronary artery human MR vessel wall imaging at 3 tesla. J. Cardiovasc. Magn. Reson. 5(4), 589–594 (2003). & Application of advanced pulse sequences for coronary vessel wall MRI.
- Katoh M, Spuentrup E, Buecker A, Manning WJ, Gunther RW, Botnar RM. MR coronary vessel wall imaging: comparison between radial and spiral k-space sampling. J. Magn. Reson. Imaging 23(5), 757-762 (2006).
- Katoh M, Spuentrup E, Buecker A et al. MRI of coronary vessel walls using radial k-space sampling and steady-state free precession imaging. Am. J. Roentgenol. 186(6 Suppl. 2), S401–S406 (2006).
- Jansen CH, Perera D, Makowski MR et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 124(4), 416-424 (2011).
- Stuber M, Botnar RM, Danias PG et al. Contrast agent-enhanced, free-breathing, three-dimensional coronary magnetic resonance angiography. J. Magn. Reson. Imaging 10(5), 790-799 (1999).