Review Article - Diabetes Management (2017) Volume 7, Issue 3
Promising hope, treatment of diabetes with a stem cell
- *Corresponding Author:
- Amira Ragab El Barky
Chemistry Department
Biochemistry Division, Tanta University
Tanta, Egypt
E-mail: amiramaram52@yahoo.com
Abstract
Diabetes mellitus represents a serious global health problem. It is a widespread disease that’s affecting many millions of people worldwide. One of the main effects is the decrease in β-cell mass, which is ubiquitous in most of all patients with type 1 diabetes. The management of diabetes without any side effects is still a challenge. Stem cell therapy holds a great promise for the repair of injured tissues and organs. It is one of the most promising therapies for diabetes mellitus. So, this article review aimed to educate the role of MSCS in diabetes.
Graphical Abstract
Keywords
diabetes, stem cell, mesenchymal stem cell
Abbreviations
DM: Diabetes Mellitus, IPCs: Insulin-Producing Cells, STZ: Streptozotocin, TBARS: Thiobarbituric Acid Reactive Substances, MSCs: Mesenchymal Stem Cell
Introduction
Diabetes is a global health matter which characteristic of hyperglycemia, which prompts the reproduction of free radicals, hence oxidative stress occur [1]. DM generated from the destruction of the pancreatic beta cell which causes decrease insulin secretion, defects of insulin receptor and hence affects their action or it arises from a combination of them [2]. The chronic hyperglycemia, which arises according to diabetes, can cause severe damage, dysfunction and failure of various organs [3]. It is expected that by the year 2035, there will be numerous people that gain diabetes which will jump more than 592 million [4]. Diabetes is implicated in numerous complications, for example, retinopathy, neuropathy, kidney disease and peripheral vascular disease [5]. Different pathogenic processes are implicated in the advancement of diabetes, the t processes differ from complete destruction of the pancreatic β-cell to the oddity of insulin actions [2,3].
Stem cell therapy holds a great promise for the repair of injured tissues and organs. It is one of the most promising therapies for diabetes mellitus [6]. Transplanted islet tissue very carefully mimics the physiology of the wasted islets and patients no longer need daily insulin injections. This review article aims to elucidate the potential role of Mesenchymal Stem Cell (MSCs) in controlling damages of vital tissues in diabetes.
Mesenchymal Stem Cell (MSCs)
Stem cells are unspecialized cells that have the ability of self-renovation and differentiation in response to the suitable signal [7]. MSCs are bone marrow cells, different from the hematopoietic stem cells, which possess an extensive proliferative and capability to differentiate into many different cell types, including osteocytes, adipocytes, chondrocytes, myocytes, cardiomyocytes, and neurons [8]. The greatest number of MSCs is found in neonates than it is reduced during the lifespan to about one-half at the age of 80 [9].
Stem cells and diabetes mellitus
Insulin therapy has extremely improved the quality of life in patients with diabetes, especially in individuals with type 1 diabetes. However, the method is inaccurate and does not completely control the minute-to-minute variation in systemic blood glucose [10]. Because of these shortcomings, research has been directed towards establishing cells based therapies that circumvent the need for exogenous insulin delivery by conventional injection or most modern pump technology [11,12].
Stem cells generate unbelievable interest in repairing failing tissues and organs [12]. The stem cell remedy has become a good concept to create insulin-producing cells for patients with type I [13]. Langerhans islets have their own glucose sensor, produce and release insulin in response to glucose, preserve normoglycaemia and function. Therefore, β-cell replacement by islet cell transplantation can prevent diabetes. The two types of clinical islet cell transplants which have been completed include allogeneic islet cell transplantation for the remedy of type 1 diabetes and autologous islet cell transplantation for the protection of surgical diabetes after a total pancreatectomy. Allogeneic islet cell transplantation has several hurdles such as unstable outcomes of islet isolation. The side effects which may arise from immunosuppressant and demand to multiple people for transplant the pancreas make it very difficult [14].
Mesenchymal stem cells will grow and differentiate according to their environment. In vivo, when injected into the pancreas, it is predictable that MSCs can differentiate to create pancreatic cells with exocrine and endocrine functions. Thus, transplantation of MSCs from bone marrow stem cells can repair the pancreas in its role to provide paracrine effects and other cell differentiation effects [15]. Bone marrow stem cells can differentiate into cells that can secrete insulin in-vitro [16,17], but Bartholomew et al. were not able to confirm this finding.
The pancreas is an organ that has limited ability to proliferate [18]. However, these MSCs cells can proliferate and differentiate in-vitro [19]. The use of growth factors in differentiation processes has been studied. Most of the growth factors are pleiotropic and changes motility, proliferation, morphogenesis and survival of cells [20].
Much different design has been utilized to stimulate the differentiation of Insulin Producing Cells (IPCs) from stem cells in-vitro. These designs have participated in the differentiation procedure by using special medium, a variety of induction and different growth factors, such as vitamin derivatives for example, nicotinamide [21], GLP-1, which is an anti-diabetic agent in β-cell expansion, function, propagation and neogenesis [22], and nourished ES cells with all-trans retinoic acid [23], betacellulin, activin A [24].
In T1DM rats, MSC was capable of differentiating to create pancreatic insulin-producing cells, releasing insulin and improving diabetic symptoms [25,26].
Those insulin-producing pancreatic beta cells express the multiple genes that related to the development or function of pancreatic beta cells, including high expression of pancreatic and duodenal homeobox 1, insulin, and glucagon [26], and were capable of releasing insulin in a glucose-dependent manner that led to refinement diabetic state in STZ-induced diabetes in nude mice [26]. Transplantation of MSCs into STZ-induced diabetes in C57Bl/6 mice prompts achieving normal blood glucose level and prevents glycosuria. This was accompanied by improved renal function tests and pancreatic histology of regeneration of normal beta-pancreatic islets [27]. In diabetic NOD mice, the injection of MSC has the ability to reduce the diabetogenic T cells to penetrate pancreatic beta cell islets and thus preventing the β-cell destruction [28].
There is a mutual action of MSC’s in cotransplantation with pancreatic islets which resulted in amended graft morphology and improved revascularization indicating that possible trophic factors secreted by MSCs are helping islet engraftment [29]. Furthermore, multiple intravenous (IV) injection of MSCs to a rodent model of T2DM resulted in normal blood glucose levels, which kept stable for approximately more than two months after infusion. Moreover, serum insulin and C-peptide levels were restored back to normal levels after MSC injection and pancreatic islets were repaired [30].
Previous and recent studies on stem cells treatment
On a study by Aziz et al. [31] they derived MSCs from the bone marrow of male albino rats, they characterized MSCs morphologically and by CD29 marker and they infused MSCs (5 × 106 cell/rat) into STZ-induced diabetes female rats. Diabetic female rats which received MSCs displayed a significant decrease in serum glucose levels and a significantly increased serum insulin levels as compared with the STZ non-treated group. Moreover, cardiovascular performance was also improved in the STZ/ MSC group as compared with the STZ nontreated group [31]. Moreover, they stated that MSCs improve the kidney function and concluded that MSC are capable of improving the kidney function and regenerating kidney tissues in diabetic nephropathy rats most probably through their paracrine action via different growth factors such as VEGF, TGFβ & TNFα and anti-apoptotic action via bcl2 and Bax genes (Table 1) [32].
| Source | Dosage | Experimental models of diabetes | Route of administration | Diabetic history | After stem cell infusions | Reference |
|---|---|---|---|---|---|---|
| Adipose Tissue Derived Stem Cells (ADSCs) | 3 × 106 autologous ADSCs in 0.5 mL PBS | Type II, female sprague-dawley rats (8 weeks old) | Tail vein injection, Detrusor at the time of repeat laparotomy | Diabetic animal group had modest reduction of plasma insulin, significantly higher plasma glucose level, and more dyslipidemia relative to the control group. | Improved voiding function was noted in ADSCs-treated rats as compared with phosphate-buffered saline-treated rats. Though some ADSCs differentiated into smooth muscle cells, paracrine pathway seems to play the main role in this process, thus resulting in the reduction of apoptosis and preservation of ‘‘suburothelial capillaries network. | [68] |
| Bone marrow mesenchymal stem cells, pancreatic cells | • 200,000 MSC cells. • 200,000 Pancreatic Cells (PSCs) |
Wistar rats | Intraperitoneally | Blood glucose levels (fasting and 2 h post prandial) were significantly increased after aloxan injection. On the other hand both C-peptide and insulin levels were significantly decreased. | Plasma glucose level (fasting and 2 h post prandial) was significantly decreased after transplantation of MSCs and PSCs. Moreover, C peptide and insulin level was significantly increasing after transplantation of MSCs and PSCs | [15] |
| • Bone marrow mesenchymal stem cells • Insulin Producing Cells (IPCS) |
1 × 105 MSCs cell/rat 1 × 105 IPCs cell/rat |
Type I, Wistar rats | Tail vein injection | There was an incredible increase in the serum glucose levels and a significant decrease in the serum insulin level in the STZ-induced diabetes groups as compared to the control normal group. | MSCs treated group significantly reduced elevated serum glucose level. Also, both MSCs and IPCs treated group showed a significant increase in serum insulin levels after six weeks of the experiment as compared to the STZ-induced diabetes group but it didn't reach the normal value as compared to the control normal group. | [6] |
| Human umbilical cord blood stem cells (hUCBSCs) | 1.5 × 107 cell | Patients were type II diabetes complicated with impotencies | Corpus cavernosa | All had normal laboratory findings except diabetes mellitus related one | The blood glucose levels were lowered at second weeks and needed to reduce the medication doses from one month in insulin and three months in hypoglycemic agent | [69] |
| Bone marrow- derived mesenchymal stem cells | 106cells per rat | Type1, Diabetic nephropathy female albino rats | Intravenous injection in rat tail vein | Diabetic nephropathy with blood glucose >200 mg/dl. | MSC therapy significantly improved 24 h urinary albumin, serum urea, creatinine and glucose levels. | [32] |
| Human bone marrow-derived mesenchymal stem cells | • 1 million undifferentiated MSCs • 1,000 IPC clusters |
Diabetic nude mice | Renal subcapsular space | The diabetic mice had blood glucose levels exceeded 16.5 mmol/L. | The blood glucose levels of the diabetic mice implanted with the differentiated clusters of cells were normalized within few days, whereas those receiving no cells or undifferentiated cells remained hyperglycemic. | [37] |
Table 1. Sources and dosage of stem cell.
Human cord blood, which derived from healthy donors and all of them were checked for any pathogenic antigen antibodies and only that are free were used for isolating human cord blood-derived stem cells CB-SCs. They concluded that T1D patients have realized an improvement of metabolic control and also, showed a significant reduction in an autoimmunity which lasts several months after a single treatment and stated that the improvement may be achieved with more than one dose. Notably, reversal of autoimmunity, leads to regeneration of islet β-cells and improvement of metabolic control in long-standing T1D subjects [33].
MSC was generated from 10-gram adipose tissue [34]. MSC was harvested on day 10 after MSCs culturing, then they differentiated to create pancreatic Insulin Secreting Cells (ISC) on day 14, they quantified by using a hemocytometer and tested for sterility, viability and insulin secreting markers such as Pax-6, Ipf-1, and Isl-1 by immunofluorescence. The prepared inoculum was then mixed with Hematopoietic Stem Cells (HSC) generated from 100-ml cultured BM which was aspirated on day 9 [35]. A combined cell inoculum of the ISC and HSC was infused as described previously into the portal circulation, thymus and into the subcutaneous tissue [35]. Ten patients suffering from diabetes with mean age 20.2, suffering from diabetes, approximately 8.1 years ago were subjected to MSCs infusion, the results showed that MSCs treatment have not any side effects to all of diabetic patients. All of the patients had improvement in C-peptide, Hb1Ac, blood sugar levels and exogenous insulin demand. After 3 months of MSCs infusions, GAD antibodies were analyzed and it showed a significant reduction [36].
In a study by Gabr et al. [37], Bone Marrow Aspirates (BMA) were collected in an anticoagulant heparin falcon tube from six consenting donors (Table 1). The BMA were diluted 1:1 with low glucose Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum. After 3 days, the non-adherent cells were removed. The remaining adherent MSCs was cultured to 80% confluence before passaging with trypsin. At passage 3, MSCs were differentiated to create pancreatic insulin-producing cells. The ability of differentiated cells was established normoglycaemia in diabetic nude mice was examined by their implantation in the renal sub capsular space. The sera of diabetic mice that treated with IPCs contained human insulin and c-peptide but measly levels of mouse insulin. BM-MSCs from diabetic and nondiabetic human subjects could be differentiated without genetic manipulation to form IPCs that, when transplanted, could maintain euglycemia in diabetic mice for 3 months [37].
EL Barky et al. [6] derived MSCs from bone marrow of white male albino rats. MSCs were distinguished morphologically and by CD –ve 34 and CD +ve 105. They were then differentiated into IPCs and both of them were infused into the independently tail vein of STZ-induced diabetes in rats (Table 1). They reported that both MSC and IPCs therapy significantly improved the body weight, S. Insulin, α-amylase, adiponectin, creatinine, total cholesterol, TAG, IL-6, TNF-α and liver L-MDA and glycogen levels in STZ-induced diabetes model and concluded stem cells, which have the ability to differentiate into insulin-producing cells (IPCs), would provide a potentially free source of islet cells for transplantation and mitigate the major limitations of availability and allogeneic rejection. Therefore, stem cell therapy is becoming the most favorable therapy for DM [6].
Mechanism action of MSCs in diabetes
Diabetes Mellitus (DM) is metabolic, endocrine disorder worldwide; it is usually accompanied by diverse complications such as, retinopathy, neuropathy, nephropathy and cardiovascular disease [38]. It is a major public health problem throughout the world and is the leading cause of global mortality [39].
Type-I is an autoimmune disorder with complete destruction of the pancreatic β-cells so it characterized by insulin deficiency and requires insulin injection for its treatment [2,3] whereas Type-II is insulin resistance and it can manage by the administration of synthetic drugs [40].
Both Type-I and Type-II diabetes are considerable public health concern with numerous complications, leading to a constant increase in treatment costs [41]. Several distinct types of diabetes mellitus exist and are caused by a complex interaction of genetics and environmental factors [42].
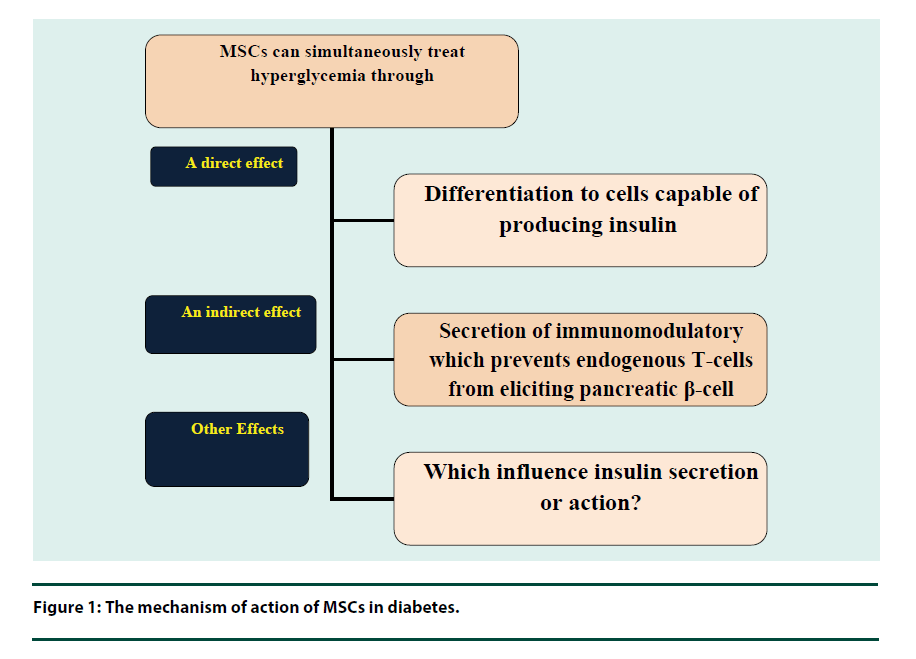
Oxidative stress results in macro and micro vascular complications exerting toxicities on different vital organs in the patient with diabetes [43]. Oxidative stress is produced by the formation of advanced glycation end product which has a strong role with the diabetic complications [44]. Free radicals resulted in oxidative stressmediated injury and enhanced program β-cell death [45]. It also reacts with polyunsaturated fatty acids, which exist in the membrane and cause lipid peroxidation [46]. The high level of lipid peroxidation marker, Thiobarbituric Acid Reactive Substances (TBARS), in the diabetes is conceded is an indication of the deficiency of the antioxidant [47]. MSC has a beneficial effect on glycemic via a direct differentiation of cells able to producing insulin, or through an indirect effect on secretion of immune modulators, which prevent endogenous T cells from destruction pancreatic β-cell, or other which still unknown factors, that have an effect on insulin secretion or action (Figure 1) [48].
MSCs were able to differentiate to create pancreatic insulin producing cells in STZ-T1DM animal models, secreting insulin and relief, diabetic complication, These insulin-producing cells express multiple genes such as duodenal home box 1, insulin, and glucagon and were able to secrete insulin that responsible for decrease glucose level and hence ameliorate diabetes in STZ- nude mice [49].
Some trophic factors, such as vascular endothelial growth factor [50], ciliary neurotropic factor, Von Willebrand factor [51], and Il-6 [52], can be released by MSCs and have the ability to prolong islets’ life. Scuteri [53] confirmed that MSCs can provide long survival for Langerhans islets and they also endow in creating Pdx1. Therefore, the utilization of stem cells is becoming the most promising therapy for DM [54].
Bone marrow mesenchymal stem cells (BM-MSCs) can be utilized to originate insulin-producing cells [55]. BM-MSCs have a special interest because patients with diabetes mellitus who incidentally receive a bone marrow transfusion can improve β-cell function [56]. Also, it’s lowering apoptotic factors, such as IL- 1β so; it can lower apoptosis [57]. Mesenchymal stem cells have an immunosuppressive effect [58], anti-inflammatory and immunomodulatory characteristics [59].
Chen et al. [60] stated that MSCs could successfully differentiate in vitro to create pancreatic β-like cells. These cells were morphologically identical to the pancreatic beta cells. Moreover, they could also transcript, translate and excrete insulin. Mesenchymal stem cells can be converted to create pancreatic insulinproducing cells and their potential therapeutic remedy for diabetes builds on the differentiation capacity [61]. When BMSCs at 3rd passage 70%-80% confluent, they were differentiated to create pancreatic insulin producing cells by using cockatiel of a growth factor for example, activin A, β-fibroblast and epidermal growth factor, 2- mercaptoethanol, nicotinamide and high glucose. Processing BMSCs with insulin-promoting factors such as nicotinamide, high glucose induction and growth factors such as activin A and GLP-1 can convert them to pancreatic beta cells and produce insulin. Nicotinamide, which is a poly (ADP-ribose) synthetase inhibitor, is a well-known inducer to differentiate stem cells into pancreatic beta cells and can protect cells from glucotoxicity-induced by exposure to high glucose [62]. Moreover, Tang et al. [62] theorized high glucose concentration is a strong inducer of pancreatic islet differentiation. But Sun et al. [61] stated that using media with high glucose concentration only cannot differentiate bone marrow MSCs to create insulin producing cells. Activin A, which is a member of the transforming growth factor-beta super-family, can adjust neogenesis of β-cells in rats and stimulate ESCs to create insulin producing cells [63-65].
Neshati et al. [64] reported that MSCs can be differentiated to create pancreatic beta cells by using media contain high glucose concentration, nicotinamide and 2-mercaptoethanol. Furthermore, Mohamed et al. [65] reported that low glucose medium concentrations can use for cell expansion and for differentiation to create pancreatic insulin producing cells. The medium was replaced with high glucose, which considers the first step of differentiation processes. The second step of differentiation MSCS to create pancreatic insulin producing cells is done through treating both β-mercaptoethanol and nicotinamide as promoting factors [66,67].
Conclusion
This review article has displayed some of the main roles of MSCs as a novel therapy for diabetes. Stem cell therapy has generated incredible interest for failing tissues, organs repair and regeneration. Bone marrow Mesenchymal Stem Cells (MSCs) can be used to create pancreatic beta cells, thus patients no longer require multiple daily insulin injections. Transplantation of MSCs could reform the pancreas in its role to provide paracrine effects and other cell differentiation effects.
Highlights
• Diabetes mellitus is a metabolic disorder, affecting many millions of people worldwide.
• DM generated from the destruction of the pancreatic beta cell or insulin resistance.
• Stem cell therapy holds a great promise for the repair of injured tissues and organs.
• Stem cell is one of the most promising therapies for diabetes mellitus.
Transplanted islet tissue very carefully mimics the physiology of the wasted islets, and patients no longer need daily insulin injections.
References
- El Barky AR, Hussein SA, Alm-Eldeen AA et al. Anti-diabetic activity of Holothuria thomasi saponin. Biomed. Pharmacother. 84(1), 1472–1487 (2016).
- El Barky AR, Ali EMM, Mohamed TM. Marine sea cucumber saponin and diabetes. Austin. Pancreat. Disord. 1(1), 1–7 (2017).
- El Barky AR, Hussein SA, Alm-Eldeen AA et al. Saponins and their potential role in diabetes mellitus. Diabetes Manag. 7(1), 148–158 (2017).
- Sheweita SA, Mashaly S, Newairy AA et al. Changes in oxidative stress and antioxidant enzyme activities in streptozotocin-induced diabetes mellitus in rats: role of Alhagi maurorum extracts. Oxidative Med. Cell. Longevity. 2016(2016), 1–8 (2016).
- Chehade JM, Mooradian, AD. A rational approach to drug therapy of type 2 diabetes mellitus. Drugs. 60(1), 95–113 (2000).
- El Barky AR, Ezz AA, Alm-Eldeen AA et al. Can stem cells ameliorate the pancreatic damage induced by streptozotocin in rats? Can. J. Diabetes. 41(4), 1–5 (2017).
- Windley W, Teixeira F, Levin L et al. Disinfection of immature teeth with a triple antibiotic paste. J. Endod. 31(1), 439–443 (2005).
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical Applications. Folia Histochemica Et Cytobiologica; 44(4), 215–230 (2006).
- Fibbe WE, Noort WA. Mesenchymal stem cells and hematopoietic stem cells transplantation. Ann. N. Y. Acad. Sci. 996(12), 235–244 (2003).
- Guo T, Hebrok M. Stem cells to pancreatic β-cells: new sources for diabetes cell therapy. Endocrine Rev. 30(1), 214–227 (2009).
- Cohen ND, Shaw JE. Diabetes: advances in treatment. Intern. Med. J. 37(2), 383–388, (2007).
- Kaur H, Bhaskar N, Ishaq S et al. Stem Cells: Source for diabetes cell therapy. J. Diabetol. 3(3), 1–9 (2012).
- Mccall, MD, Toso C, Baetge EE et al. Are stem cells a cure for diabetes? Clin. Sci. 118(1), 87–97 (2010).
- Matsumoto S. Clinical allogeneic and autologous islet cell transplantation: update. Diabetes Metab. J. 35(1), 199–206 (2011).
- Armand P, Fedik AR, Wibisono S et al. Autologus MSC bone marrow stem cell and allogenic pancreatic stem cell for repair of beta pancreatic cell in experimental diabetes mellitus. Afr. J. Intern. Med. 1(1), 10–16 (2012).
- Sotiropoulou PA, Perez SA, Salagianni M et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 24(1), 462–471 (2006).
- Baksh D, Tuan RS. Canonical and noncanonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J. Cell Physiol. 212(3), 817–826 (2007).
- Soria B, Skoudy A, Martin F. From stem cell to beta cell: new strategies in cell therapy of diabetes mellitus. Diabetologia. 44(1), 407–415 (2001).
- Demeterco C, Beatttie GM, Dib SA. A role for Activin A and Beta Cellulin in human fetal pancreatic cell differentiation and growth. J. Clin. Endocrinol. Metab. 85(1), 3892–3897 (2000).
- Tariq M, Masoud MS, Mehmood A et al. Stromal cell derived factor-1alpha protects stem cell derived insulin-producing cells from glucotoxicity under high glucose conditions in-vitro and ameliorates drug induced diabetes in rats. J. Transl. Med. 11(1), 115 (2013).
- Otonkoski T, Beattie GM, Mally MI et al. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J. Clin. Invest. 92(3), 1459–1466 (1993).
- Egan JM, Bulotta A, Hui H et al. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab. Res. Rev. 19(6), 115–123 (2003).
- Micallef SJ, Janes ME, Knezevic K et al. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 54(1), 301–305 (2005).
- Shi Y, Hou L, Tang F et al. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 23(5), 656–662 (2005).
- Dong QY, Chen L, Gao GQ et al. Allogeneic diabetic mesenchymal stem cells transplantation in streptozotocin-induced diabetic rat. Clin. Invest. Med. 31(6), E328–E337 (2008).
- Xie QP, Huang H, Xu B et al. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation. 77(1), 483–491 (2009).
- Ezquer FE, Ezquer ME, Parrau DB et al. Systemic administration of multipotent mesenchymal stromal cells revert hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol. Blood Marrow Transplant. 14(1), 631–640 (2008).
- Madec AM, Mallone R, Afonso G et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 52(4), 1391–1399 (2009).
- Ito T, Itakura S, Todorov I et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 89(5), 1438–1445 (2010).
- Hao H, Liu J, Shen J et al. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverses hyperglycemia in experimental type2 diabetes rats. Biochem. Biophys. Res. Commun. 436(1), 418–423 (2013).
- Abdel Aziz MT, El-Asmar MF, Haidara M et al. Effect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic rats. Med. Sci. Monit. 14(11), BR249-BR255 (2008).
- Abdel Aziz MT, Wassef MA, Ahmed HH et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol. Metabol. Syndr. 6(2), 34 (2014).
- Zhao Y, Jiang Z, Zhao T et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC. Med. 10(2), 3 (2012).
- Dave SD, Vanikar AV, Trivedi HL. Ex vivo generation of glucose sensitive insulin secreting mesenchymal stem cells derived from human adipose tissue. Indian J. Endocr. Metab. 16(1), S65–S69 (2012).
- Vanikar AV, Dave SD, Thakkar UG et al. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010(2010), 1–5 (2010).
- Dave SD, Vanikar AV, Trivedi HL et al. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin. Exp. Med. 15(2), 41–45 (2015).
- Gabr MM, Zakaria MM, Refaie AF et al. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 22(3), 133–145 (2013).
- Ebrahimi E, Shirali S, Talaei R. the protective effect of marigold hydroalcoholic extract in stz-induced diabetic rats: evaluation of cardiac and pancreatic biomarkers in the serum. J. Bot. 2016(2016), 1–6 (2016).
- Herman, WH, Zimmet P. Type 2 diabetes: an epidemic requiring global attention and urgent action. Diabetes Care. 35(5), 943– 4 (2012).
- Piero NM, Murugi NJ, Okoth OR et al. Prevention of type I diabetes mellitus: The role of immune interventions. J. Clin.Cell. Immunol. S2(1), 1–6 (2012).
- Xi Y, Bu S. Stem cells therapy in diabetes mellitus. J. Stem Cell Res. Ther. 4(5), 1–6 (2014).
- Chitra V, Varma PV, Raju MV et al. Study of antidiabetic and free radical scavenging activity of the seed extract of Strychnos nuxvomica. Int. J. Pharma. Pharm. Sci. 2(1), 106–110 (2010).
- Andreassi MG, Barale R, Iozzoand P et al. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis. 26(1), 77–83 (2011).
- Villeneuve LM, Natarajan R. The role of epigenetics in the pathology of diabetic complications. Am. J. Physiol. Renal. Physiol. 299(1), F14–F25 (2010).
- Rother KI. Diabetes treatment–bridging the divide. N. Engl. J. Med. 356(6), 1499–1501 (2007).
- Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN. Oncol. 2012(2012), 1–21 (2012).
- Erejuwa OO, Sulaiman SA, Abdul Wahab MS et al. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin induced diabetic rats. Int. J. Mol. Sci. 11(1), 2056–2066 (2010).
- Davey GC, Patil SB, O’Loughlin A et al. Mesenchymal stem cell-based treatment for microvascular and secondary complications of Diabetes mellitus. Front. Endocrinol. 5(1), 86 (2014).
- Zanini C, Bruno S, Mandili G et al. Differentiation of mesenchymal stem cells derived from pancreatic islets and bone marrow into islet-like cell phenotype. PLoS ONE. 6(12), e28175 (2011).
- Figliuzzi M, Cornolti R, Perico N et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant. Proc. 41(3), 1797–1800 (2009).
- Rossignol J, Boyer C, Lévèque X et al. Mesenchymal stem cell transplantation and DMEM administration in a 3NP rat model of Huntington's disease: morphological and behavioral outcomes. Behav. Brain. Res. 217(2), 369–378 (2011).
- Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J. Gastroenterol. 10(20), 3016–3020 (2004).
- Scuteri A, Donzelli E, Rodriguez-Menendez V et al. A double mechanism for the mesenchymal stem cells' positive effect on pancreatic islets. PLoS ONE. 9(1), e84309 (2014).
- Mishra PK, Singh SR, Joshua IG et al. Stem cells as a therapeutic target for diabetes. Front. Biosci. 1(15), 461–477 (2010).
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 35(3), e00191 (2015).
- Kim JW, Luo JZ, Luo LG. Islet Transplantation: Potential Role of Stem Cells. Insights in Stem Cells. 2(1), 1–3 (2016).
- Redfield RR, Rickels, MR Naji A et al. Pancreas Transplantation in the Modern Era. Gastroenterol. Clin. North Am. 45(2), 145–166 (2016).
- Mabed M, Shahin M. Mesenchymal stem cell-based therapy for the treatment of type 1 diabetes mellitus. Curr. Stem Cell Res. Ther. 7(1), 179–190 (2012).
- Ryan JM, Barry FP, Murphy JM et al. Mesenchymal 9 stem cells avoid allogeneic rejection. J Inflamm (Lond). 2(1), 1–8 (2005).
- Volarevic V, Al-Qahtani A, Arsenijevic N et al. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 43(3), 255–263 (2010).
- Sun Y, Chen L, Hou XG et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin. Med. J. 120(3), 771–776 (2007).
- Tang DQ, Cao LZ, Burkhardt BR et al. In vivo and in vitro characterization of insulin- producing cells obtained from murine bone marrow. Diabetes. 53(2), 1721–1732 (2004).
- Li L, Yi M, Seno M et al. Activin A and betacellulin: effect on regeneration of pancreatic beta-cells in neonatal streptozotocin-treated rats. Diabetes. 53(6), 608–615 (2004).
- Neshati Z, Matin MM, Bahrami AR et al. Differentiation of mesenchymal stem cells to insulin-producing cells and their impact on type 1 diabetic rats. J. Physiol. Biochem. 66(2), 181–187 (2010).
- Mohamed AA, Saad MM, Abdeen SH et al. Generation of insulin producing cells using mesenchymal stem cells derived from bone marrow of new-zealand white rabbits. Can. J. Clin. Nutrition. 1(1), 47–66 (2013).
- Zhang H, Qiu X, Shindel AW et al. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type ii diabetic rat model. Stem Cells Dev. 21(9), 1391–1400 (2012).
- Bahk JY, Han H, Lee YS. Stem cell treatment for complicated diabetes. Int. J. Stem. Cells. 1(1), 91–95 (2008).