Review Article - Interventional Cardiology (2023)
Apolipoprotein E key to inflammation and cholesterol efflux in atherosclerosis
- Corresponding Author:
- Anastasia V. Poznyak Institute for Atherosclerosis Research, Osennyaya 4-1-207, 121609 Moscow, Russia, E-mail: tehhy_85@mail.ru
- Alexander N. Orekhov Institute for Atherosclerosis Research, Osennyaya 4-1-207, 121609 Moscow, Russia, E-mail: a.h.opexob@gmail.com
Received date: 03-Feb-2023, Manuscript No. FMIC-23-88524; Editor assigned: 06-Feb-2023, PreQC No. FMIC-23-88524 (PQ); Reviewed date: 20-Feb-2023, QC No. FMIC-23-88524; Revised date: 27-Feb-2023, Manuscript No. FMIC-23-88524 (R); Published date: 06-Mar-2023, DOI: 10.37532/1755- 5310.2023.15(S15).368
Abstract
Lipid metabolism is an often-underestimated aspect of health and its maintenance. However, lipids and their derivatives are involved in many key processes in the human body. ApoE is one of the important participants in lipid homeostasis. One of its remarkable properties is the ability to resist the development of atherosclerosis. Recently, data began to appear on various mechanisms that can mediate the anti- atherosclerotic effects of ApoE, including blocking the formation of foam cells and inhibiting increased hematopoiesis, among others. This suggests ample opportunities for the use of ApoE as an anti-atherosclerotic agent. In our review, we focused on the relationship of ApoE with inflammation and cholesterol in the context of atherogenesis. We also considered the possibilities of therapeutic application.
Keywords
Apolipoproteins E • Cardiovascular diseases • Apoproteins • Lipids • Atherosclerosis • Apolipoproteins• Cholesterol
Abbrevations
ApoE: Apolipoprotein E; BBB: Blood-Brain Barrier; LDL: Low- Density Lipoprotein; AD: Alzheimer’s Disease; CSF: Cerebrospinal Fluid; CNS: Central Nervous System; ApoA: Apolipoprotein A; ABCA1: Member 1 of human transporter sub-family ABCA; mRNA: messenger Ribonucleic Acid; HDL: High- Density Lipoprotein; CETP: Cholesteryl Ester Transfer Protein; AcLDL: Acetylated Low-Density Lipoprotein; IFNγ: Interferon Gamma; AEM-2: Apolipoprotein E Mimetic Peptide-2; ApoB: Apolipoprotein B; ApoCiii: Apolipoprotein C-III; LRP1: Low Density lipoprotein receptor-related Protein 1; CVD: Cardiovascular Disease; Lp(a): Lipoprotein a; HSPG: Heparan Sulfate ProteoGlycan; SMCs: Smooth Muscle Cells; ECs: Endothelial Cells; VLDL: Very- Low-Density Lipoprotein; DHA: Docosahexaenoic acid; EPA: Eicosapentaenoic Acid; EpK: apoE mimetic peptide
Introduction
Cholesterol homeostasis and ApoE
Brain cholesterol homeostasis can be independent of the peripheral one, as it may be synthesized in the brain. This is supported by the fact that dietary cholesterol levels seem to have almost no effect on cholesterol synthesis or metabolism in the brain but have a pronounced effect on the de novo synthesis of peripheral cholesterol [1]. Additionally, most studies show that plasma lipoproteins cannot go through the Blood-Brain Barrier (BBB), though there is one study that has demonstrated that LDL can cross the BBB via receptor-mediated transcytosis [2].
Patients with Alzheimer’s disease have higher brain cholesterol flow even in the early stages of dementia. Compared to the control group, levels of 24S-hydroxycholesterol, a more soluble cholesterol form, are elevated in both plasma and CSF of AD patients, although the reason for it is not known [3]. Patients with AD appear to respond positively to cholesterol-lowering treatment, which highlights the importance of cholesterol metabolism in AD even while cholesterol-lowering drugs may not affect the brain’s cholesterol levels [4].
It is known that ApoE’s role in normal cholesterol metabolism is more important than that of other proteins, e.g., multiple ApoE isoforms interactions with specific lipoprotein receptors lead to noticeably diverse effects on cholesterol metabolism. ApoE seems to be essential for lipid homeostasis and cholesterol mobilization in the CNS. CNS ApoE levels, distribution, and ApoE allele status may probably have an influence on AD risk through cholesterol metabolism fluctuations in CNS [5].
Literature Review
Cholesterol efflux and ApoE
The efflux of cholesterol can be carried out in four pathways. ABCA1-mediated efflux includes the cell membrane cholesterol and phospholipid transfer to lipid-poor acceptors. Of those acceptors, ApoA-I is the most researched, though other amphipathic apoproteins like ApoE can act as acceptors, too [6]. That was shown with human ApoE isoforms such as ApoE2, ApoE3, and ApoE4 incubated with HeLa cells expressing ABCA1 and J774 cells. ApoE structure is arranged as a C-terminal (residues 201–299) domain that comprises several amphipathic α-helices and an N-terminal (residues 1–191) domain that comprises a four-helical bundle [7]. The C-terminal domain of ApoE3 doubles the intact ApoE3 outflow capacity. The C-terminal domain is responsible for ApoE’s capacity to induce cholesterol efflux was verified with the use of chimeric molecules, including the interchange of ApoA-I and ApoE C-terminal and N-terminal domains. The reciprocal chimera or the intact apoproteins are less efficient as a cholesterol acceptor from J774 cells than the chimeric molecule composed of the ApoE C-terminal domain and the ApoA-I N-terminal domain (ApoA-1 (1–180)/ApoE (201–299)) [8,9]. Though J774 cells cannot express ApoE, controlled by the cytomegalovirus promoter, those cells can express human ApoE3. This allows us to assess possible differences between the action of endogenous and exogenous ApoE on cellular cholesterol homeostasis and efflux of cholesterol, while the cholesterol loading of macrophages has the interfering effect of the sterol-mediated upregulation of ApoE gene expression [10]. Mazzone, et al., using J774+E cells, pointed out the endogenous ApoE high efficiency in promoting sterol efflux compared to that of the exogenous ApoE [11]. The ApoE endogenous expression leads to the secreted ApoE association with the plasma membrane and its binding to membrane lipids, proteoglycans, and the LDL receptor on the cell surface. Decreased association of ApoE with these molecules on the cell surface results in the reduction of cholesterol efflux [12,13]. These procedures do not influence the exogenous ApoE-mediated enhanced sterol efflux on control J774 cells since this cell surface association is not identified with exogenous ApoE. It is of interest that ABCA1 does not influence the increase of efflux observed with J774+E cells. Besides J774 cells, also a murine cell line RAW ± E and mouse peritoneal macrophages from wild-type and Apoe−/− mice have been noticed not to have such an effect [14].
Cholesterol loading in the human macrophage THP-1 cells leads to an increase in ApoE mRNA by about 15 times. As was foreseeable, the LDL receptor and ApoE are mutually regulated by cholesterol loading. On the contrary, ApoE expression upregulation upon loading with cholesterol is not demonstrated in human monocyte-derived macrophages. Although, even with no exogenous sterol acceptor present, those cells can efflux cholesterol upon loading. Notwithstanding, this efflux is abolished by antibody-mediated ApoE removal and thus is ApoE-dependent [15, 16].
There are three main isoforms of human ApoE. Compared to ApoE3, the most common isoform, ApoE2 and ApoE4, each has one amino acid substitution at residues 158 and 112, respectively. The ApoE4 affinity is a little higher than that of ApoE3, and ApoE2 LDL receptor binding capacity is reduced [17]. ApoE2 was demonstrated to have the most effect on cholesterol efflux than ApoE3 and ApoE4, using monocyte-derived cholesterol-loaded macrophages taken from individuals homozygous for the three isoforms. Although, the exogenous human ApoE isoforms do not differ in ability to induce cholesterol efflux from cells [18].
Secreted ApoE interaction with the LDL receptor on the cell surface is shown using peritoneal macrophages obtained from ApoE gene replacement mice, which instead of the murine ApoE gene, express the human ApoE isoforms. Depending on the ApoE isoform’s ability to interact with the receptor, both sterol efflux and ApoE secretion into the medium change as a result of the LDL receptor upregulation by simvastatin therapy [19].
Thereby, sterol efflux and secretion of ApoE2 are unaffected by the increase of LDL receptor expression, while cholesterol efflux and secretion of ApoE4 are reduced. The differences in the receptor binding affinity of isoforms are mirrored by differences in ApoE isoforms mediated efflux of cholesterol. In accordance with this, the enhanced LDL receptor expression in mice with ApoE4 gene replacement lowers the efflux of cholesterol from peritoneal macrophages [20]. The bone marrow transplantation from those mice into Ldlr−/− mice results in increased atherosclerosis. These effects of increased LDL receptor expression in vitro and in vivo are not seen in mice with ApoE3 gene replacement [21].
The reverse cholesterol transport beyond the surface of macrophage cells if affected by ApoE, e.g., ApoE that was secreted by macrophages can bind to human HDL2. The HDL2 can increase in size without CETP present, which makes it possible to transfer a large load of cholesterol to the liver to secrete cholesterol into bile. The HDL extension can be avoided if the ApoE is removed [22].
The plasma of genetically diverse mouse strains was used to assess different serum or plasma fractions acceptor quality. To obtain serum HDL, the ApoB-containing lipoproteins needed to be removed. The Heinecke laboratory used cAMP-stimulated J774 cells and BHK cells with inducible ABCA1 expression to assess cholesterol efflux [23]. Macrophage cholesterol efflux capacity is little in serum HDL from Apoa1−/− and Apoe−/− mice. Efflux capacity in serum HDL from mice deficient in both proteins is very poor. Still, wild-type HDL serum and serum HDL isolated from Apoe−/− mice have the analogous capacity, and therefore ApoA-I mainly determines ABCA1-specific efflux. In addition, serum HDL from five diverse inbred mouse strains was used in similar tests. These strains differ in apoprotein content and HDL size [24]. There was a remarkable discovery that cholesterol efflux capacity from macrophages and ApoE levels on HDL correlate inversely. Truly, Apoe−/− serum HDL normalized for HDL particle concentration makes a better acceptor than serum HDL of wild type. ApoE on HDL could be a negative cholesterol efflux regulator, as these studies suggest [25].
Reverse cholesterol transport and ApoE
The above data mostly describes experiments that concentrate narrowly on ApoE and the efflux of cholesterol from cultured macrophages. Only a few studies have investigated the macrophages and systemic ApoE effect on reverse cholesterol transport in vivo. One study focused on a comparison between macrophage-to-feces reverse cholesterol transport in Apoe−/− mice that received an injection of cholesterol-loaded Apoe−/− peritoneal macrophages and in wild-type (ApoE-expressing) mice that received an injection of cholesterol loaded wild-type peritoneal macrophages. While Apoe−/− mice showed reduced reverse cholesterol transport, they also had lower HDL levels and higher plasma lipid levels than the wild-type mice, which is confusing for interpretation [26]. But then again, with wild-type mice used as recipients to get similar plasma lipids and lipoproteins levels, mice that have gotten Apoe−/− macrophages demonstrated a reduction of reverse cholesterol transport in comparison to mice that have gotten wild-type macrophage injections. Via injecting the wild-type cholesterol-loaded macrophages into Apoe−/− and wild-type recipients, the role of systemic ApoE’s effect on reverse cholesterol transport was excluded [27]. These studies display the macrophage ApoE’s ability to facilitate reverse cholesterol transport in vivo. It is not known if it is true for macrophages in the artery wall, as this conclusion has been reached using injections of peritoneal macrophages.
Late experiments in vivo suggest ApoE can interact with other cellular functions to affect cholesterol macrophage homeostasis [28, 29]. Becker and colleagues reported a “macrophage sterol responsive network,” which showed various proteins been upregulated or downregulated in cholesterol-loaded peritoneal macrophages derived from Ldlr−/− mice fed a Western-type diet [30]. Cholesterol and its esters are accumulated in these cells, but they also show a significant decrease in the expression of the ApoE protein, despite what has been known of macrophages incubated with AcLDL in culture. The basis of these different phenotypes in culture in vitro and in vivo concerning the expression of ApoE is yet unclear [31]. Reardon, et al. to find mechanisms explaining the higher risk of atherosclerosis in type 2 diabetes, have expanded their studies to macrophages in insulin-resistant and obese mice [32]. Insulin resistance induction in Western-diet-fed Ldlr−/− mice uncovered the significant role of IFNγ in mediating increased atherosclerosis, a decrease in the secretion of ApoE from macrophages, and increased accumulation of cholesterol by peritoneal macrophages. Experiments in hyperlipidemic Ldlr−/− mice that were fed a high-cholesterol low-fat diet not inducing insulin resistance did not show such an impact. The IFNγ effect on the macrophage ApoE phenotype applies to other macrophages, too, such as murine arterial macrophages. In this model, the reduction of macrophage ApoE secretion effect on reverse cholesterol transport has not been displayed yet.
ApoE in inflammation
Inflammation can be affected by ApoE. Sudden suppression of ApoE in mice rapidly influences B-cell and T-cell activity and germ centers. B-cell activity suppression improves humoral responses to oxidatively modified lipoproteins, which are of importance in atherosclerosis, and other self-antigens. In animal models, plasma cholesterol was reduced by the ApoE mimetic peptide AEM-2, which also ameliorated mitochondrial function in human macrophages studied on a model of stimulation with lipopolysaccharide [33].
ApoE can be located in atherosclerotic lesions and may suppress the classical complement cascade activation, while ApoE can associate only with activated C1q. Factor H inhibits another pathway of complement activation in plasma, as it may bind lipoprotein-associated or non-lipid ApoE. Cholesterol efflux is induced, and proinflammatory cytokine release from macrophage-foam cells is inhibited by these complexes. ApoE can also stimulate the polarization of macrophages into the anti-inflammatory M2 phenotype, aside from stimulation of cholesterol efflux from them [34,35].
Physiologic and pathologic body processes may be affected by microRNAs. In animals, the effects that lipoprotein-associated ApoE has on miRNA may affect anti-inflammatory and anti-atherosclerotic activity. The response in miR146a macrophage is different in the transgenic mice nervous system. The action of two variants of the miR146c that is found in atherosclerotic lesions is increased by ApoE4 [36].
Endocrine or paracrine effects can be caused by exosomes, membrane-coated physiologically secreted structures that contain intracellular material, via their proteins, lipids, and carbohydrates of microRNA. There was no effect indicated of ApoE4 expressed in mice at an early stage of their development, but the exosome production was impaired with age [37].
Apolipoprotein E and clearance of lipoproteins
The clearance of ApoB-containing lipoprotein residues is mediated by ApoE. Nascent chylomicrons and VDLD are rapidly modified in the circulation, assimilate, exchange apoproteins, and undergo lipolysis. They may also change with environmental and genetic conditions. ApoCiii inhibits lipolysis and lipoprotein clearance, while ApoE stimulates clearance when not interfered with by ApoCiii. While small residues of lipoproteins are better excreted by the liver, they can migrate to other tissues and cause inflammation with their products of lipid peroxidation. In ApoCiii deficiency, increased lipolysis is atheroprotective and lowers levels of both residual lipoproteins and triglyceride-rich lipoproteins. Residual lipoproteins may represent a substantial part of the remaining risk after LDL concentration is controlled [38].
ApoE is a ligand for a family of 7 related receptors and associates with HSPG, and unlike ApoB, which acts as a ligand only on LDL, ApoE is a functional ligand across the entire range of receptors, while LDL Receptor-related Protein 1 (LRP1) is particularly essential in the liver. Insulin phosphorylates the intracellular C-terminus to release LRP1 from intracellular retention to relocate to the cell membrane and control LRP1. As a result, the clearance of residual lipoprotein is increased [39].
One of the key HSPG for clearance of residual lipoprotein is syndecan 1. Its action may be impaired by different interferences, such as disruption of glucosamine N- deacetylase-N-sulfotransferase 1, uronyl 2-O- sulfotransferase, heparan sulfate copolymerase, glucosaminyl 6-O-sulfotransferase 2, except for glucosaminyl 6-O-sulfotransferase 1 which does not interfere with residual absorption. The CD36 scavenger receptor is another contributor to the clearance of chylomicron residues. Lipoproteins can also integrate their lipid contents directly into cell membranes in addition to receptor-mediated uptake [40,41].
While lipoprotein residues are degraded in the endosomal system after internalization, ApoE is excreted from the liver and not cleaved. The uninjured vascular endothelium cannot be passed through by large triglyceride-rich lipoproteins, although small LDL and residues may penetrate the vessel wall. In mouse vessels, residual LDL and lipoproteins undergo transcytosis and produce HDL particles containing ApoE and smaller particles containing ApoB. Endothelial lipase can modify such particles so that they may influence atherosclerosis [42].
ApoE and cardiovascular risk
ApoE levels are affected by multiple risk factors for cardiovascular disease. Routine ApoE genotyping or determination of its levels, while needs further study, could upgrade risk assessment [43].
Isoforms of ApoE affect the risk of cardiovascular disease from the first days of life, including breastfeeding response. In childhood, the ApoE2 lipid-lowering effect appears with increased HDL and decreased LDL levels. The ApoE4 isoform may indicate the risk of cardiovascular disease in diabetes mellitus. Also, it is associated with higher thickness of the carotid intima-media complex and increased levels of ApoB, Lp(a), and LDL cholesterol [44].
Hypertriglyceridemia, abdominal obesity, insulin resistance, and low HDL cholesterol characterize metabolic syndrome, which is associated with elevated levels of ApoE. Premature damage to the coronary arteries is associated with more ApoE-rich lipoproteins, increased triglyceride levels, decreased HDL-cholesterol levels, smaller LDL particles, and increased lipoprotein(a) concentrations, regardless of isoforms of ApoE [45].
In epidemiological studies, the effect of common variants on CVD risk through minor lipid profile changes is clearly shown. Although, more serious and pathological effects may also occur. Dysbetalipoproteinaemia begins in postmenopausal women and in middle-aged men. However, with more serious metabolic stress, an earlier onset is possible [46].
With dominantly inherited forms of ApoE2 penetrance is high and is low with homozygosity. Lp glomerulopathy is due to thrombi and aggregation of lipoprotein and is mostly associated with a dysbetalipoproteinemia phenotype. Pathological change in ApoE bears a resemblance to membranous nephropathy. Dysbetalipoproteinaemia may be dominated by hypertriglyceridemia, but there were also reported some other mutations associated with hypertriglyceridemia, including natural variants that decrease or increase the concentration of LDL compared to wild type. One mutation causes LDL hypercholesterolemia by the dominant hereditary type. Such hypercholesterolemia can be diagnosed as familial hypercholesterolemia. While some of the mutations may reduce the AD risk, other mutations could lead to cognitive deterioration [47,48].
Apolipoprotein E in multifactorial atherosclerosis
ApoE affects cholesterol levels, which take part in the risk of atherosclerosis in patients without severe lipid disorders along with other factors, and more information on the specific ApoE variants effects may ameliorate risk assessment, considering the potential for antagonistic pleiotropy. When variants in a gene promote health and development and maintain reasonable function but under stress, such as aging, and others, lead to significant favorable or detrimental differences, antagonistic pleiotropy may be applied [49]. ApoE4 carriers already show decreased concentrations of HDL cholesterol in childhood, which indicates risk. ApoE4 in neonates and infants can raise morbidity and mortality from traumatic brain injury and other stressors. In neonates and infants, ApoE4 carriers may have increased morbidity and mortality from stressors such as traumatic brain injury [50].
ApoE is clearly a major risk factor for cognitive disorders and atherosclerosis, at least partially by increasing the concentration of LDL, although the composition may also vary. Thereby, the complications associated with atherosclerosis and cognitive deterioration are very important for life expectancy. ApoE4 carriers have higher, and ApoE2 carriers have lower levels of the atherogenic ceramide species, Cer (d18:1/16:0) [51].
ApoE4 is invariably associated with manifest and subclinical atherosclerosis. Consequently, in patients with ApoE4, the risk increases by 46% compared to individuals with ApoE3, while it decreases by 26% with ApoE2. In elderly Europeans, ApoE4 is associated with a higher risk. Oddly enough, this does not happen in patients of Mongolian origin. Allelic frequencies for ε2, ε3, and ε4 were 7.9%, 78.6%, and 13.5% respectively. From ApoE2 homozygosity to ApoE4 homozygosity, there was indicated an increase in LDL and total cholesterol. In the ApoE2 group, HDL cholesterol and triglyceride levels were higher [52].
Dementia was a considerable contributor to the increase in mortality in ApoE4 carriers. ApoE4 allele atherogenic nature had a chances ratio of 1.34 relative to the ApoE3 allele, while the ApoE2 allele atheroprotective nature ratio was 0.82. After the coronary artery evaluated the risk of cardiovascular disease, the presence of one ApoE2 allele is beneficial. Variants in ApoB have been reported to promote atherosclerosis, while variants in ApoCiii have proved to protect from atherogenesis. In the Amish community, variants in both ApoB and ApoCiii had founder effects. The intima-media thickness of the carotid arteries is bigger in ApoE4 carriers and even larger in concomitant diabetes patients [53].
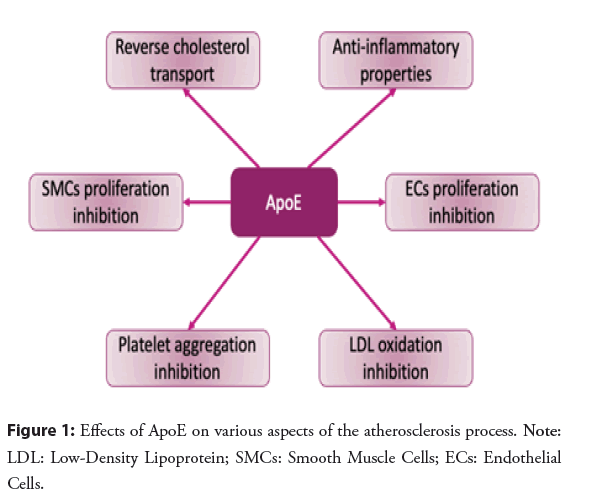
The interconnection of intima-media thickness of the carotid arteries and ApoE4 was not affected in Black individuals despite their higher levels of LDL cholesterol and prevalence of ApoE4. The ApoE2 gene did not show any CVD protection function in patients with diabetes, whereas ApoE4 further contributed to the disease. The development of dysbetalipoproteinemia with loss of cardiovascular protection is accelerated by diabetes [54] (Figure 1).
Discussion
Atherosclerosis of major extracranial arteries with hyperlipidemia may result in cerebrovascular damage, while some conditions may also damage intracranial arteries. Pathogenesis of Alzheimer’s dementia involves ApoE4 and is frequently associated with cerebral amyloid angiopathy. Normally, small arteries are affected by cerebral amyloid angiopathy, which leads to side deposits, repeating small hemorrhages, and gradual cognitive decline. Intracranial hemorrhage is followed by slower recovery in patients with ApoE4 but not in cases of ischemic stroke [55,56].
The known trends in polymorphisms of ApoE are supported by the UK Biobank study and other major studies, which can also identify other associations. Dysbetalipoproteinemia is possibly implicated in predisposition to ApoE2 homozygous peripheral vascular disease. Moreover, arterial aneurysm, thromboembolism, hallux valgus, and peptic ulcer are also associated with this genotype. It is noteworthy that ApoE4 shows a protection function against such conditions as gall bladder and liver disease, chronic obstructive pulmonary disease, diabetes type 2, and obesity [57].
Genotyping might be a more useful method in clinical practice than the determination of ApoE concentration in plasma. Plasma ApoE level is typically between 35 and 70 mg/l; in the cerebrospinal fluid, it is approximately 12 times lower. The isoform, triglyceride-rich lipoproteins, their HDL subgroups, and residues affect the concentration of ApoE, which is differently distributed among lipoproteins and changes with diet. CV risk is affected by triglyceride-rich lipoproteins and their residues through augmentation of apoproteins E, Cii, and Ciii [58]. In patients of middle age, ApoE levels have not been associated with CVD despite concentration-genotype association. ApoE levels did not influence cardiovascular risk in the ApoE3/3 group. According to a large Danish study spanning 25 years, decreased ApoE levels were related to death from dementia, and increased levels were related to death from cancer and cardiovascular disease. The highest septile compared to the fifth septile gave an odds ratio of 1.28 for CVD, while comparing the lowest septile with the fifth septile gave an odds ratio for dementia of 1.44. Polymorphism progression from ApoE2/2 to ApoE4/4 was related to increased total cholesterol, LDL cholesterol, and ApoB. Levels of ApoE were reduced in the same order as well as did ApoAi and HDL cholesterol. Calculated residual cholesterol and plasma triglyceride association in the ApoE isoform sequence was U-shaped, whereas Lp (a) was the highest in ApoE3/3 carriers. There was a large difference in the risk of CV mortality in ApoE2 homozygotes. The presence of undiagnosed patients with dysbetalipoproteinemia is a possible reason for the said difference [59].
Therapeutic applications
ApoE can give opportunities for new types of therapy for neurological dysfunction and atherosclerosis and offer other new applications.
Retinoic acid stimulates an increase in the expression of ApoE in activated macrophages, which leads to a neuroprotective effect [60].
ApoE expression increase may also be caused by Probucol, an antioxidant with a moderate lipid-modifying effect in plasma, which has a beneficial effect on AD manifestation. The ApoE expression in macrophages is increased by glucocorticoid hormones [61]. “Structural correctors,” or high-affinity small molecule compounds, may beneficially alter the maturation of ApoE4 [62].
Changing ApoE isoforms
ApoE mutations may not interfere with health for many years. Lifestyle, fibrates, and statins may treat attributable dyslipidemia effectively. However, this new kind of therapy can be helpful for individuals with lipoprotein glomerulopathy or dysbetalipoproteinemia, provided that gene editing becomes a safe clinical practice. If the BBB cannot be crossed, AD prevention through gene editing will be quite a difficult task. Transition to the ApoE2 state might lead to a highly atherogenic state unless the dysbetalipoproteinemia pathogenesis is completely clear and predictable [63,64].
In theory, dysbetalipoproteinemia might respond to continual (recombinant) ApoE3 infusions. The administration of plasma from ApoE3 donors leading to pancreatitis in acute severe dyslipidemia has not been described. Cysteamine and other aminothiols were used to alter ApoE and transform ApoE2 to make it more functional. Some ApoE4 has been formed after cysteamine therapy in patients with ApoE3 homozygosity [65,66].
Specific applications of ApoE
Anantharamaiah and colleagues and White and colleagues reported ApoE-like proteins’ ability to alter atherosclerosis, inflammation, and lipoprotein profile and affect cell metabolism, which eventually impacts neurological function [67,68].
Cholesterol efflux from cells is enhanced by the ApoE mimetic peptide EpK, which demonstrated a favorable effect on atherosclerosis in animal models. Lipoprotein clearance via HSPG is improved by peptide Ac-hE18-NH 2, which comprises most of the ApoE from the LDL receptor binding domain to the carboxy terminus. Protection against atherosclerosis may be reached with a decrease in the concentration of lipoprotein by hEp, a peptide expressed in the liver. Inter alia, hEp also prevents the binding of the hepatitis C virus [69].
The blood-brain barrier may be protected in case of a trauma or subarachnoid hemorrhage as well as the metabolic activity in the nervous system may be retained by ApoE-mimetic peptides, which also could find use in mitochondrial diseases. Solid lipid nanoparticles could be coated with ApoE at about 120–160 nm size, similar to residual lipoproteins. That would enable the transcellular transport by clathrin-mediated endocytosis through the BBB and help deliver medicaments to the brain. Incidentally, infused into the lungs covalently linked ApoE peptides on solid lipid nanoparticles were delivered to the brain successfully [70].
ApoE in personalized medicine
Treatment in precision or personalized medicine is individualized based on the information about genetic variants and their impact on risk and response to intervention or sequelae. Even though ApoE is not the main cardiovascular disease, dyslipidemia, and neurodegenerative state risk factor, information about the ApoE status can be useful in clinical practice in such cases [71].
High-fat diets increase plasma cholesterol, and that effect was used successfully in ApoE4 patients to choose healthier diets. Usually, ApoE2-carrying individuals have moderately raised concentrations of triglyceride, and switching to low-fat diets had a significant decrease in plasma cholesterol and triglyceride. Individuals with ApoE4 homozygosity experienced a decrease in LDL concentration, and those with ApoE2 alleles had both triglyceride and LDL reduced. Progress in the plasma lipid profile after exercise was also better in ApoE2 patients. In ApoE3/3 individuals, exercise did not affect the lipid profile, VLDL particles were diminished, and small LDL levels were improved [72].
In the brain, the DHA biosynthesis may not be adequate to demands. ApoE4 carriers have reduced plasma DHA absorption into the brain, and thereby, greater nutritional supplements would be useful, albeit they have similar levels of DHA. For ApoE4 carriers, it may be necessary to reduce the consumption of alcohol. In the case of atherogenic levels of lipoprotein, ApoE4 contributes to the n-3 fatty acids LDL-increasing effects [73]. Response to medication may be affected by ApoE genotype, e.g., in patients with the ApoE2 allele Atorvastatin had the best effect in increasing HDL cholesterol. The most effective in ApoE2 patients and the least effective in ApoE4 patients were fibrates, which may effectively decrease the triglyceride, ApoE, and ApoB levels. Triglyceride levels can be lowered by large doses of n-3 polyunsaturated fatty acids. 4.9 g per day for DHA and 4.8 g per day for EPA lowered the ApoE level by approximately 15%. In patients with metabolic syndrome, ApoE levels may be reduced by 25% by extended-release niacin [74].
Neoplasms spread and growth can be affected by ApoE, which is expressed and secreted by liposarcoma. The oral squamous carcinoma invasion is associated with the expression of ApoE. Exosomes carrying ApoE are secreted by macrophages in gastric carcinoma, which can stimulate their spread. ApoE is an autonomous breast cancer predictive factor with adverse concentrations of more than 43 mg/L [75].
ApoE in the management of dyslipidaemia and atherosclerosis
Mild dyslipidemia responds to life patterns and medications. All the risk factors should be controlled due to atherosclerosis’s multifactorial nature. In many cases of LDL hypercholesterolemia, statins, recommended in current guidelines, are sufficient. But there is a need for further research in the field of hypertriglyceridemia, inflammation, and immune responses. Even though dysbetalipoproteinemia is a more serious condition, it typically may be controlled in the same way. In the case of dysbetalipoproteinemia, dyslipidemia may be better controlled by gene editing, ApoE chemical modification, the presence of ApoE, or peptides stimulating residual clearance [76]. Therapeutic steps, making more ApoE and its peptides available, may be applied, even though the neurological disease associated with ApoE4 pathogenesis may differ from that of atherosclerosis. This can be used to modulate macrophage response and to raise ApoE lipidation. A better protection against atherosclerosis may be given by reconstituted HDL if it contains ApoE and not ApoAi due to its ability to bind to a wider range of receptors [77].
For dysbetalipoproteinemia to be treated most effectively, more thorough detection and study of this atherogenic condition are required, including extra attention to residual lipoproteins clearance. In patients with dysbetalipoproteinemia, fibrates ameliorated the excursion of postprandial triglyceride. In the mouse model, the plasma cholesterol levels were decreased, and inflammation was reduced by myristyl-hE18A-NH, a peptide similar to the LDL receptor binding region of ApoE with a lipid binding domain to bind to lipoproteins. Mutations associated with the replacement of arginine with cysteine may have a better function with aminothiols, which provide a charged protein fragment [78].
Conclusion
Although ApoE is not a major risk factor for cardiovascular disease, dyslipidemia, and neurodegenerative conditions, information on ApoE status may be useful in clinical practice in such cases. ApoE-like proteins are able to alter the profile of atherosclerosis, inflammation, and lipoproteins, as well as influence cellular metabolism, which ultimately leads to an effect on neurological function. Cholesterol efflux from cells is enhanced by the ApoE mimetic peptide EpK, which has shown positive effects on atherosclerosis in animal models. Due to the known anti-atherosclerotic actions of ApoE, this lipoprotein clearly deserves attention as part of a therapeutic strategy for the treatment of atherosclerosis.
Author Contributions
Writing-original draft preparation, A.V.P.; writing-review and editing, V.N.S., I.I.E., I.I.N., N.A.G., A.N.O.
Funding
This research was funded by Russian Science Foundation, grant number 22-65-00005.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin U, Park SJ, Park SM, et al. Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp Neurobiol. 28(5): 554-567 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Brankatschk M, Eaton S. Lipoprotein particles cross the blood-brain barrier in drosophila. J Neurosci. 30(31): 10441-10447 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Gamba P, Giannelli S, Staurenghi E, et al. The Controversial role of 24-S-hydroxycholesterol in Alzheimer's disease. Antioxidants (Basel). 10(5): 740 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Barone E, Di Domenico F, Butterfield DA, et al. Statins more than cholesterol lowering agents in Alzheimer disease: Their pleiotropic functions as potential therapeutic targets. Biochem Pharmacol. 88(4): 605-616 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Huang Y, Mahley RW. Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis. 72 Pt A: 3-12 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Getz GS, Reardon CA. Apoprotein e and reverse cholesterol transport. Int J Mol Sci. 19(11): 3479 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Tran TN, Kosaraju MG, Tamamizu-Kato S, et al. Acrolein modification impairs key functional features of rat apolipoprotein E: Identification of modified sites by mass spectrometry. Biochemistry. 53(2): 361-375 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Cun W, Jiang J, Luo G, et al. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol. 84(21): 11532-11541(2010)
[CrossRef] [Google Scholar] [PubMed]
- Vedhachalam C, Narayanaswami V, Neto N, et al. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 46(10): 2583-2593 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Crouchet E, Lefèvre M, Verrier ER, et al. Extracellular lipid-free apolipoprotein E inhibits HCV replication and induces ABCG1-dependent cholesterol efflux. Gut. 66(5): 896-907 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Mazzone T, Pustelnikas L, Reardon CA, et al. Post-translational regulation of macrophage apoprotein E production. J Biol Chem. 267(2): 1081-1087 (1992).
[Google Scholar] [PubMed]
- Husain MA, Laurent B, Plourde M, et al. APOE and Alzheimer’s disease: From lipid transport to physiopathology and therapeutics. Front Neurosci. 15: 630502 (2021).
- Sainger R, Grau JB, Poggio P, et al. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers. 17(2): 111-118 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Aissa BM, Lewandowski CT, Ratia KM, et al. Discovery of nonlipogenic ABCA1 inducing compounds with potential in Alzheimer’s disease and type 2 diabetes. ACS Pharmacol Transl Sci. 4(1): 143-154 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Getz GS, Reardon CA. Apoproteins E, A-I, and SAA in macrophage pathobiology related to atherogenesis. Front Pharmacol. 10: 536 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Lupoli R, Vaccaro A, Ambrosino P, et al. Impact of Vitamin D deficiency on subclinical carotid atherosclerosis: A pooled analysis of cohort studies. J Clin Endocrinol Metab. 102(7): 2146-2153 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Frieden C, Garai K. Structural differences between apoE3 and apoE4 may be useful in developing therapeutic agents for Alzheimer's disease. Proc Natl Acad Sci USA. 109(23): 8913-8918 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Alagarsamy J, Jaeschke A, Hui DY, et al. Apolipoprotein e in cardiometabolic and neurological health and diseases. Int J Mol Sci. 23(17): 9892 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Lucic D, Huang ZH, Gu de S, et al. Regulation of macrophage apoE secretion and sterol efflux by the LDL receptor. J Lipid Res. 48(2): 366-372 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Johnson LA, Olsen RH, Merkens LS, et al. Apolipoprotein E-low density lipoprotein receptor interaction affects spatial memory retention and brain ApoE levels in an isoform-dependent manner. Neurobiol Dis. 64: 150-162 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Fazio S, Major AS, Swift LL, et al. Increased atherosclerosis in LDL receptor-null mice lacking ACAT1 in macrophages. J Clin Invest. 107(2): 163-171 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Ouimet M, Barrett TJ, Fisher EA, et al. HDL and reverse cholesterol transport. Circ Res. 124(10): 1505-1518 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Sontag TJ, Carnemolla R, Vaisar T, et al. Naturally occurring variant of mouse apolipoprotein A-I alters the lipid and HDL association properties of the protein. J Lipid Res. 53(5): 951-963 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Pamir N, Hutchins PM, Ronsein GE, et al. Plasminogen promotes cholesterol efflux by the ABCA1 pathway. JCI Insight. 2(15): e92176 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Adorni MP, Ronda N, Bernini F, et al. High density lipoprotein cholesterol efflux capacity and atherosclerosis in cardiovascular disease: Pathophysiological aspects and pharmacological perspectives. Cells. 10(3): 574 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Annema W, Dikkers A, Freark de Boer J, et al. ApoE promotes hepatic selective uptake but not RCT due to increased ABCA1-mediated cholesterol efflux to plasma. J Lipid Res. 53(5): 929-940 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Feig JE, Parathath S, Rong JX, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 123(9): 989-998 (2011).
[CrossRef] [Google Scholar] [PubMed]
- de Chaves EP, Narayanaswami V. Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol. 3(5): 505-530 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Sainger R, Grau JB, Branchetti E, et al. Comparison of transesophageal echocardiographic analysis and circulating biomarker expression profile in calcific aortic valve disease. J Heart Valve Dis. 22(2): 156-165 (2013).
[Google Scholar] [PubMed]
- Becker L, Gharib SA, Irwin AD, et al. A macrophage sterol-responsive network linked to atherogenesis. Cell Metab. 11(2): 125-135 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Lappalainen J, Yeung N, Nguyen SD, et al. Cholesterol loading suppresses the atheroinflammatory gene polarization of human macrophages induced by colony stimulating factors. Sci Rep. 11(1): 4923 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Reardon CA, Lingaraju A, Schoenfelt KQ, et al. Obesity and insulin resistance promote atherosclerosis through an ifnγ-regulated macrophage protein network. Cell Rep. 23(10): 3021-3030 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Bouchareychas L, Raffai RL. Apolipoprotein e and atherosclerosis: From lipoprotein metabolism to microrna control of inflammation. J Cardiovasc Dev Dis. 5(2): 30 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Yin C, Ackermann S, Ma Z, et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med. 25(3): 496-506 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Branchetti E, Bavaria JE, Grau JB, et al. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. (10): 2349-2357 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Teter B, LaDu MJ, Sullivan PM, et al. Apolipoprotein E isotype-dependent modulation of microRNA-146a in plasma and brain. Neuroreport. 27(11): 791-795 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Zhang Y, Yu M, Tian W, et al. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 49(1): 3-13 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Sacks FM. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr Opin Lipidol. 26(1): 56-63 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Laatsch A, Panteli M, Sornsakrin M, et al. Low density lipoprotein receptor-related protein 1 dependent endosomal trapping and recycling of apolipoprotein E. PLoS One. 7(1): e29385 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Stanford KI, Bishop JR, Foley EM, et al. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 119(11): 3236-3245 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Poggio P, Songia P, Cavallotti L, et al. PCSK9 involvement in aortic valve calcification. J Am Coll Cardiol. 72(24): 3225-3227 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Jawi MM, Frohlich J, Chan SY, et al. Lipoprotein(a) the insurgent: A new insight into the structure, function, metabolism, pathogenicity, and medications affecting lipoprotein(a) molecule. J Lipids. 2020: 3491764 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, et al. Plasma levels of apolipoprotein E, APOE genotype, and all-cause and cause-specific mortality in 105 949 individuals from a white general population cohort. Eur Heart J. 40(33): 2813-2824 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Jasienska G, Ellison PT, Galbarczyk A, et al. Apolipoprotein E (ApoE) polymorphism is related to differences in potential fertility in women: A case of antagonistic pleiotropy? Proc Biol Sci. 282(1803): 20142395 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Limonova AS, Ershova AI, Meshkov AN, et al. Case report: Hypertriglyceridemia and premature atherosclerosis in a patient with apolipoprotein e gene ε2ε1 genotype. Front Cardiovasc Med. 7: 585779 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Likozar AR, Zavrtanik M, Šebeštjen M, et al. Lipoprotein(a) in atherosclerosis: From pathophysiology to clinical relevance and treatment options. Ann Med. 52(5): 162-177 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Saito T, Matsunaga A, Fukunaga M, et al. Apolipoprotein E-related glomerular disorders. Kidney Int. 97(2): 279-288 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Myasoedova VA, Ravani AL, Frigerio B, et al. Novel pharmacological targets for calcific aortic valve disease: Prevention and treatments. Pharmacol Res. 136: 74-82 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Garcia AR, Finch C, Gatz M, et al. APOE4 is associated with elevated blood lipids and lower levels of innate immune biomarkers in a tropical Amerindian subsistence population. Elife. 10: e68231 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Reuter-Rice K, Regier M, Bennett E, et al. The effect of the relationship of APOE polymorphisms and cerebral vasospasm on functional outcomes in children with traumatic brain injury. Biol Res Nurs. 20(5): 566-576 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Martínez-Martínez AB, Torres-Perez E, Devanney N, et al. Beyond the CNS: The many peripheral roles of APOE. Neurobiol Dis. 138: 104809 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Emrani S, Arain HA, DeMarshall C, et al. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer's disease: A systematic review. Alzheimers Res Ther. 12(1): 141(2020).
[CrossRef] [Google Scholar] [PubMed]
- Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 11(1): 667 (2020).
- Sriprasert I, Mack WJ, Hodis HN, et al. Effect of apoe4 genotype on the association between metabolic phenotype and subclinical atherosclerosis in postmenopausal women. Am J Cardiol. 124(7): 1031-1037 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Duong MT, Nasrallah IM, Wolk DA, et al. Cholesterol, atherosclerosis, and APOE in Vascular Contributions to Cognitive Impairment and Dementia (VCID): Potential mechanisms and therapy. Front Aging Neurosci. 13: 647990 (2021).
- Poggio P, Branchetti E, Grau JB, et al. Osteopontin-CD44v6 interaction mediates calcium deposition via phospho-Akt in valve interstitial cells from patients with noncalcified aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 34(9): 2086-2094 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Karjalainen JP, Mononen N, Hutri-Kähönen N, et al. The effect of apolipoprotein E polymorphism on serum metabolome-a population-based 10-year follow-up study. Sci Rep. 9(1): 458 (2019).
- Yamazaki Y, Zhao N, Caulfield TR, et al. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat Rev Neurol. 15(9): 501-518 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Ozen E, Mihaylova RG, Lord NJ, et al. Association between APOE genotype with body composition and cardiovascular disease risk markers is modulated by BMI in healthy adults: Findings from the bodycon study. Int J Mol Sci. 23(17): 9766 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Clemens V, Regen F, Le Bret N, et al. Retinoic acid enhances apolipoprotein e synthesis in human macrophages. J Alzheimers Dis. 61(4): 1295-1300 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Yamazaki Y, Painter MM, Bu G, et al. Apolipoprotein e as a therapeutic target in Alzheimer’s disease: A review of basic research and clinical evidence. CNS Drugs. 30(9): 773-789 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Williams T, Borchelt DR, Chakrabarty P, et al. Therapeutic approaches targeting apolipoprotein E function in Alzheimer’s disease. Mol Neurodegener. 15(1): 8 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Brisson D, Ledoux K, Bossé Y, et al. Effect of apolipoprotein E, peroxisome proliferator-activated receptor alpha and lipoprotein lipase gene mutations on the ability of fenofibrate to improve lipid profiles and reach clinical guideline targets among hypertriglyceridemic patients. Pharmacogenetics. 12(4): 313-320 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Zanobini M, Loardi C, Poggio P, et al. The impact of pericardial approach and myocardial protection onto postoperative right ventricle function reduction. J Cardiothorac Surg. 13(1): 55 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Kemp SB, Carpenter ES, Steele NG, et al. Apolipoprotein e promotes immune suppression in pancreatic cancer through nf-κb-mediated production of CXCL1. Cancer Res. 81(16): 4305-4318 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Momkute L, Vilkeviciute A, Gedvilaite G, et al. Association of APOE serum levels and APOE ε2, ε3, and ε4 alleles with optic neuritis. Genes (Basel). 13(7): 1188 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Anantharamaiah GM, Garber DW, Goldberg D, et al. Novel fatty acyl apoE mimetic peptides have increased potency to reduce plasma cholesterol in mice and macaques. J Lipid Res. 59(11): 2075-2083 (2018).
[CrossRef] [Google Scholar] [PubMed]
- White Z, Milad N, Sellers SL, et al. Effect of dysferlin deficiency on atherosclerosis and plasma lipoprotein composition under normal and hyperlipidemic conditions. Front Physiol. 12: 675322 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Amar MJ, D'Souza W, Turner S, et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther. 334(2): 634-641 (2010).
- Topal GR, Mészáros M, Porkoláb G, et al. ApoE-targeting increases the transfer of solid lipid nanoparticles with donepezil cargo across a culture model of the blood-brain barrier. Pharmaceutics. 13(1): 38 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Freudenberg-Hua Y, Li W, Davies P, et al. The role of genetics in advancing precision medicine for Alzheimer’s disease-a narrative review. Front Med (Lausanne). 5: 108 (2018).
- Fote GM, Geller NR, Reyes-Ortiz AM, et al. Ascoping review of dietary factors conferring risk or protection for cognitive decline in apoe ε4 carriers. J Nutr Health Aging. 25(10): 1167-1178 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Heath RJ, Wood TR. Why have the benefits of DHA not been borne out in the treatment and prevention of Alzheimer’s disease? A narrative review focused on DHA metabolism and adipose tissue. Int J Mol Sci. 22(21): 11826 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Santos-Ferreira C, Baptista R, Oliveira-Santos M, et al. Apolipoprotein e2 genotype is associated with a 2-fold increase in the incidence of type 2 diabetes mellitus: Results from a long-term observational study. J Lipids. 2019: 1698610 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Paskeh MDA, Entezari M, Mirzaei S, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 15(1): 83 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Linton MF, Yancey PG, Davies SS, et al. The role of lipids and lipoproteins in atherosclerosis. (2019).
- Chernick D, Ortiz-Valle S, Jeong A, et al. Peripheral versus central nervous system APOE in Alzheimer's disease: Interplay across the blood-brain barrier. Neurosci Lett. 708: 134306 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Kei A, Miltiadous G, Bairaktari E, et al. Dysbetalipoproteinemia: Two cases report and a diagnostic algorithm. World J Clin Cases. 3(4): 371-376 (2015).
[CrossRef] [Google Scholar] [PubMed]