Review Article - Imaging in Medicine (2012) Volume 4, Issue 1
MRI in ovarian cancer
Rosemarie Forstner*1, Matthias W Meissnitzer1, Alexander Schlattau1 & John A Spencer21Department of Radiology, SALK, Paracelsus Medical University, Salzburg, Müllner-Hauptstraße 48, 5020 Salzburg, Austria
2Department of Radiology, St James’s University Hospital, Beckett Street, Leeds, LS9 7TF, UK
- Corresponding Author:
- Rosemarie Forstner
Department of Radiology, SALK
Paracelsus Medical University, Salzburg
Müllner-Hauptstraße 48, 5020 Salzburg, Austria
Tel: +43 662 4482 57763
E-mail: r.forstner@salk.at
Abstract
To date MRI has primarily been used as a problem-solving modality for sonographically indeterminate adnexal masses owing to its excellent soft tissue contrast and functional properties. Qualitative diffusion-weighted imaging with visual assessment is helpful in lesion characterization and makes differentiation of solid adnexal masses more accurate. Diffusion-weighted imaging can obviate intravenous MRI contrast media, rendering this technique indispensable in cases of contraindications and in pregnancy. Staging ovarian cancer remains a CT domain. However, novel technical developments are at the brink of changing imaging and management in ovarian cancer in the years to come; MRI may shift from a problem-solving to a central management tool, possibly fulfilling a broad range of tasks from characterization, staging and even early assessment of response to treatment in the case of chemotherapy using quantitative diffusion-weighted imaging.
Keywords
diffusion-weighted MRI ▪ dynamic contrast-enhanced MRI ▪ functional imaging ▪ MRI ▪ ovarian cancer
At present, more than 75% of patients with ovarian cancer are diagnosed in an advanced tumor stage. This is mainly owing to a long period with unspecific symptoms; ovarian cancer remains the most deadly entity among all female reproductive system cancers. Overall the disease ranks fifth among the most lethal cancers in females [1].
Imaging has become a cornerstone in treatment planning for patients diagnosed with a gynecologic malignancy. Optimally based on consensus conferences imaging will assist in individualized patient management and also facilitate patient counseling [2].
Clinical findings combined with sonography (ideally with integration of tumor markers) have become central in assessing adnexal masses and based on these findings different management strategies are warranted [3].
In patients with advanced epithelial ovarian cancer (EOC) the role of imaging typically consists of noninvasive staging. In recent years triaging of patients who will benefit from primary chemotherapy has started to play an increasingly important role [4].
Furthermore, percutaneous core needle biopsy has been highlighted as a safe and valuable contribution provided by radiology in selected patients with advanced ovarian cancer [5,6].
Despite long-term utilization of pelvic MRI it is still reserved as a problem-solving modality in suspected ovarian cancer. With the introduction of functional MRI, including diffusion-weighted MRI (DWI) and dynamic contrast-enhanced (DCE) MRI, this technique holds promise to become a predictive biomarker of prognosis or treatment response in ovarian cancer [4,7,8].
DWI depicts the molecular diffusion (Brownian motion) of water protons in biological tissues [9]. It exploits the restricted diffusion within hypercellular tissues, including tumors, to provide contrast between these lesions and surrounding tissues [10]. The amount of signal loss is dependent on various factors including cellular density and the strength of the diffusion- sensitizing gradients, which is indicated by the β-value. The β-value is an index of degree of diffusion weighting and is expressed in s/mm2. DWI information can be assessed visually where hypercellular malignant tumors typically display high signal intensity (SI) on intermediate to high β-values (b = 500–1000 s/mm2) and low SI on apparent diffusion coefficient (ADC) map. Quantitative ADC assessment by calculation of ADC values seems particularly useful when combined with biochemical and morphological information for assessing effects of treatment [4].
Based on differences in contrast material uptake microvascular properties of tissues induced by tumor angiogenesis can be assessed by DCE-DWI [11]. Rapid image acquisition before, during and after intravenous contrast bolus application provides visual assessment of enhancing elements. This technique is also increasingly applied for quantitative assessment of various parameters provided by pharmacokinetic modeling [7].
In this review the role of MRI in assessing ovarian cancer, including challenges and future perspectives, will be discussed.
Ovarian cancer: general aspects
A woman’s lifetime risk of ovarian cancer is approximately 1:70 and approximately 21,550 new cases and 14,600 deaths are estimated to occur annually in the USA [1,12]. In the EU, the estimated number of newly diagnosed ovarian cancers was 43,000 per year and mortality was reported as 12 out of 100,000 per women, per year [1]. African, Asian and southern European countries have the lowest cancer rates [1]. Ovarian cancer is typically a disease of peri- and postmenopausal age. Its incidence increases with age, with the median age at diagnosis of 63 years [13]. Approximately 20% of ovarian cancers occur before the age of 40. This is particularly the case in the rare nonepithelial cancer types. Younger females are more commonly affected in hereditary ovarian cancer syndromes and in borderline tumors. The latter have the highest frequency of ovarian cancers in the age group 15–29 years [1,101]. Although a significant improvement in 5-year survival has been noted during the last three decades, ovarian cancer still has a poor overall prognosis. A 5-year survival rate has been reported as 50–90% for early stage disease (stages 1 and 2) and 21% for late-stage disease (stages 3 and 4). A review including over 5000 patients showed 5-year survival of 89, 58, 24 and 12% for stages 1, 2, 3 and 4, respectively [13].
Relevant pathology for image interpretation
Primary ovarian neoplasms comprise neoplasms of epithelial, germ-cell and sex-cord stromal origin. The most common type is EOC (85–90%), which is classified based on cellular origin into serous (60%), mucinous (5%), clear cell (10%), endometrioid (10–20%), Brenner tumor and undifferentiated cancers (1%) [14]. Furthermore, based on histopathological features and clinical behavior, epithelial tumors further subdivide into benign neoplasms, invasive (malignant) cancers and borderline tumors. The latter, also called ovarian tumors of low malignant potential, constitute approximately 10–20% of EOC. Histologically borderline tumors lack stromal invasion and are mostly confined to the ovaries (75%) [101].
Recent advances in histopathology and cytogenetics advocate a dualistic model of cancerogenesis. Type I includes low-grade serous, mucinous and endometrioid cancers and type II consists of high-grade serous, endometrioid, mixed and undifferentiated types [15]. The former seem to develop stepwise from precursor lesions (e.g., borderline tumors). By contrast, type II cancers develop de novo and are characterized by late diagnosis and unfavorable prognosis.
Ovarian cancer displays a broad range of pathomorphological features. These may range from solid, to solid and cystic, to predominantly cystic, often with serous or mucinous locules. However, in the majority of cases ovarian cancer consists of a cystic mass with solid elements.
Although no findings strongly suggestive of the different tumor types exist, some features may favor diagnosis of one subtype (e.g., papillary projections or small calcifications, so-called psammoma bodies), are found in serous ovarian cancer. This tumor type tends to be more often bilateral and is often associated with CA-125 rise [16]. Mucinous cancers are usually unilateral and CA-125 may not be markedly elevated [16].
Endometrioid and clear cell cancer are associated with endometriosis of the ovary or in the pelvis in 15–50% of cases.
Nonepithelial ovarian neoplasms are rare and include germ cell tumors and sex chord/stromal tumors. The latter comprise granulosa cell tumors, fibroma, thecoma and fibrothecoma. Germ cell tumors include mature and immature teratoma, dysgerminoma, choriocarcinoma and yolk sack tumors. In general, germ cell malignancies are extremely rare, and are primarily found in children and young adults.
A total of 5–15% of malignant ovarian masses are metastases of the ovaries mostly deriving from primaries from breast, colon or stomach cancer [17].
MRI for characterization of suspected malignant lesions
In females presenting with an adnexal mass the central role of imaging is to differentiate physiological lesions and benign tumors from malignancy [2]. Findings will essentially influence patient management, which varies from follow-up, to guidance of appropriate surgical approach (laparoscopy vs laparotomy) or referral to a dedicated cancer unit [18].
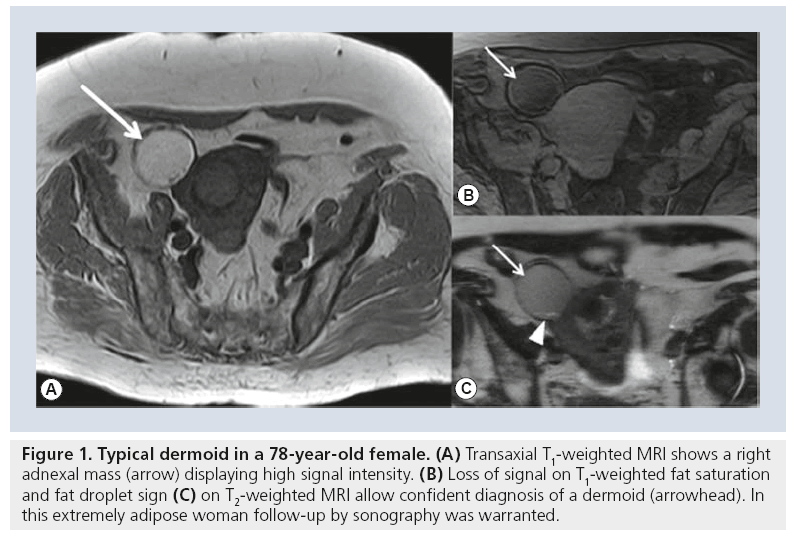
Preoperative imaging will also identify a subgroup of females with benign lesions that may warrant long-term follow-up [19]. For example, suspected pregnancy luteoma may be followed after appropriate MRI diagnosis [20]. In advanced age follow-up, rather than surgery may be a treatment option when MRI shows a benign lesion, as in benign stromal tumors or typical dermoids (Figure 1) [21].
Figure 1: Typical dermoid in a 78-year-old female. (A) Transaxial T1-weighted MRI shows a right adnexal mass (arrow) displaying high signal intensity. (B) Loss of signal on T1-weighted fat saturation and fat droplet sign (C) on T2-weighted MRI allow confident diagnosis of a dermoid (arrowhead). In this extremely adipose woman follow-up by sonography was warranted.
Ultrasonography (US) has been established as a first-line imaging modality for assessing adnexal masses with excellent performance in benign cystic adnexal masses, which account for the vast majority of all adnexal lesions.
For adnexal mass detection and characterization US shows high sensitivities (88–100%) but a wide range of specificities (39–87%). In a systematic review, including 12 studies, the accuracy of grayscale US with additional color Doppler for preoperative diagnosis of ovarian cancer yielded a sensitivity and specificity of 87 and 90%, respectively [22]. However, sonographical assessment of complex adnexal masses may be challenging, and in approximately 20% of cases lesions have to be classified as sonographically indeterminate owing to their morphology or suboptimal US. A study prospectively comparing adnexal masses in US and MRI found similar excellent sensitivities (100 vs 96.6%, respectively), but higher specificity for MRI (39.5 vs 83.7%) in discriminating malignant from benign lesions. Thus, MRI is particularly useful in indeterminate masses on US in women with a low risk of malignancy in a clinical setting [23,24]. In unequivocally malignant lesions on US the next diagnostic step should be CT staging according to European Society for Urological Radiology (ESUR) guidelines [25].
In the characterization of adnexal lesions CT is limited in the diagnosis of solid adnexal tumors and in assessment of endometriomas. However, it provides accurate diagnosis of classical dermoids and benign and malignant cystic adnexal lesions [26].
MRI provides prediction of the histologic nature of a variety of benign adnexal masses, including teratomas, cysts, endometriomas, ovarian stromal tumors containing fibrous tissue and uterine leiomyomas. It can reliably diagnose fatty and hemorrhagic lesions, which may be challenging with US. Chemical shift imaging assists in the diagnosis of scanty fatcontaining dermoids, which are typically misdiagnosed in CT and US.
Information about tissue composition and microvascularization rendered by DWI and DCE-MRI may facilitate discrimination between benign and malignant solid adnexal masses. In a study analyzing 77 complex adnexal masses, all solid lesions with low SI on DWI on high β-value images were benign [27].
While qualitative DWI with visual assessment of signal on high β-value is increasingly used for lesion characterization, the value of ADC quantification is currently limited. This is mainly owing to the broad overlap of ADC values between benign and malignant adnexal masses [28]. Pitfalls include malignant tumors with low cellular density, such as mucinous tumors, borderline tumors and solid benign tumors [10,28]. The wide ADC range is also explained by the broad histomorphologic variability in adnexal tumors and the presence of calcifications, necrosis and mucinous components. This is why in order to avoid pitfall, DWI must be analyzed in context with standard MRI sequences [10]. The risk of malignancy index incorporating CA-125, menopausal status and US findings has been used for predicting the likelihood of malignancy of an adnexal mass. A risk of malignancy index of less than 25 is associated with a 3% probability of malignancy and a risk of malignancy index of more than 250 with a 75% probability of malignancy [24,29]. A prospective study (n = 180) showed that additional specialist US and MRI serves as a useful discriminator for correct referral to an oncologic unit, with sensitivity and specificity for US of 100 and 57%, respectively, and for MRI of 92 and 86%, respectively, for malignancy [30].
MRI is limited in correctly diagnosing some rare benign adnexal tumors with solid and cystic components mimicking malignancy (e.g., carcinoids, struma ovarii, cystadenofibroma or brenner tumors) and in rare inf lammatory masses (e.g., actinomyosis) [31]. Incorrect diagnosis may also be attributed to technique, for example, if short TI inversion recovery sequences are used for fat suppression or chemical shift imaging is not performed in poor fat containing tumors [32].
Only limited data are available investigating MR spectroscopy for characterization of adnexal masses. Proton MR spectroscopy has shown potential in lesion differentiation. Owing to a variety of technical problems and overlap of 1H-MR spectral patterns for different histologic subtypes its clinical value is not yet established. Cho et al. found an intense lipid peak in malignant ovarian tumors but not in benign epithelial tumors, however, overlap with some benign teratomas was noted [33]. The absence of lactate peaks was an excellent predictor for benign adnexal tumors [4]. At 3 T a choline/creatine ratio greater than three was predictive of malignancy. By contrast, absence of choline signal or choline/creatine ratio of less than 1.5 suggested a benign tumor. The latter was found in six of seven patients with benign tumors [34].
MRI features of malignant ovarian tumors
Throughout the last two decades MRI features for the prediction of malignancy have been proposed that are analogous to those used in US and CT. Recently, a meta-analysis including 1267 ovarian masses from 18 MRI studies reported a sensitivity of 92% and specificity of 85% (area under curve = 0.95) for the detection of invasive and borderline ovarian cancer. For these tumors the pretest probability of cancer increased from 34% overall to 78% with a positive result for malignancy and decreased to 5.1% with a negative result [18].
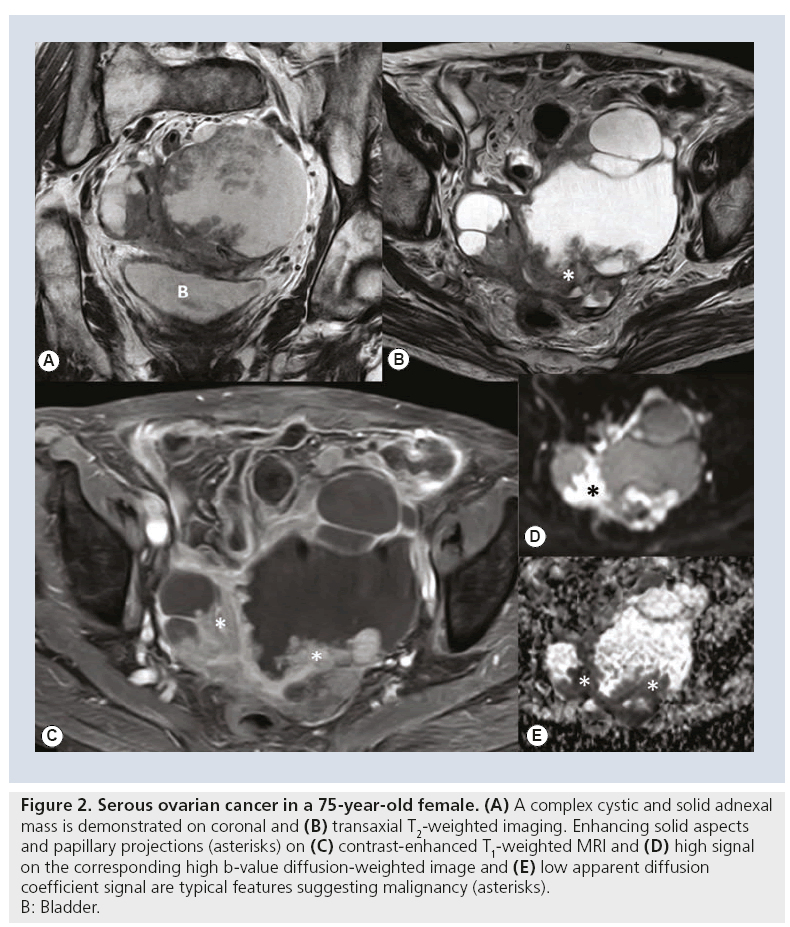
Findings suggestive of malignancy include the presence of a mass with solid enhancing or solid and cystic architecture with thick septae (>3 mm) and/or papillary projections and lesion size larger than 4 cm [26,35] (Figure 2). A diameter of more than 5–6 cm in combination with complex architecture also increases the likelihood of a malignant adnexal lesion [23]. Frequently used secondary signs include the presence of peritoneal, mesenteric or omental metastases, pelvic side wall invasion and lymphadenopathy. These signs increase the confidence in the diagnosis of malignancy.
Figure 2: Serous ovarian cancer in a 75-year-old female. (A) A complex cystic and solid adnexal
mass is demonstrated on coronal and (B) transaxial T2-weighted imaging. Enhancing solid aspects
and papillary projections (asterisks) on (C) contrast-enhanced T1-weighted MRI and (D) high signal
on the corresponding high b‑value diffusion-weighted image and (E) low apparent diffusion
coefficient signal are typical features suggesting malignancy (asterisks).
B: Bladder.
Most predictive signs of malignancy are vegetations in a cystic adnexal lesion, necrosis in a solid lesion and presence of ascites [35]. Pelvic ascites may also be found in inflammatory disease or as a physiological finding at premenopausal ages. Large amounts of ascites in combination with an adnexal mass typically indicate advanced stage ovarian cancer. Meigs syndrome consisting of a solid stromal ovarian tumor associated with ascites and pleural effusion may mimic advanced ovarian cancer.
Integration of perfusion kinetics or tissue composition may sometimes be necessary for a correct diagnosis in lesions difficult to assess with conventional MRI. Thomassin-Naggara et al. found that for combined morphologic MRI and DWI the most predictive findings of malignant masses were the presence of papillary projections (positive likelihood ratio [PLR] = 4.5), high SI on DWI at β-values of 1000 sec/mm2 within the solid component (PLR = 3.1), intermediate SI on T2-weighted imaging of the solid component (PLR = 2.2), ascites and peritoneal implants (PLR = 2), and a solid portion (PLR = 1.8) (Figure 2) [27]. In solid and complex cystic and solid adnexal tumors all malignant tumors and also some borderline tumors displayed high SI on high β-value in DWI [28]. As conventional MRI allows excellent prediction of malignancy, complementary DWI seems most beneficial in solid masses and when contrast-enhanced MRI is not feasible owing to contraindication of contrast media administration, such as allergy or renal insufficiency.
Another approach to improve characterization of adnexal masses is analysis of microvascular properties by DCE-MRI. Early enhancement patterns in 41 epithelial ovarian tumors correlated with tumor angiogenesis. Early enhancement was noted in invasive ovarian cancers and was higher than for benign (p < 0.001) and borderline tumors (p < 0.05) [11]. Another group reported the benefit of a threshold value of greater than 2.35 compared with psoas muscle acquired within the first 120 s to correctly categorize adnexal masses [36]. Moreover, information about perfusion and tissue composition can be useful for obtaining a correct diagnosis, for example in women with adnexal tumors wishing to maintain fertility [11]. Added DWI and DCE-MRI reduced the number of false positives and all false negatives could be eliminated in 87 women presenting with complex adnexal masses [11].
Malignant lesions mimicking invasive EO Cs
■ Ovarian borderline tumors
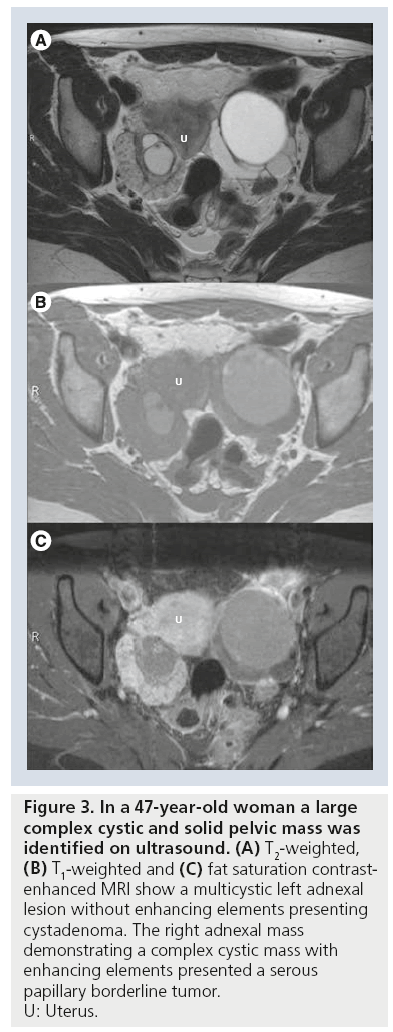
Ovarian borderline tumors, or tumors with low malignant potential, comprise up to 15–20% of all ovarian malignancies. Compared with invasive EOC they have much better survival rates with a survival in a 7-year follow-up of 92% for stage 2 and 3 disease [37]. Although reliable differentiation from invasive ovarian cancers in imaging is not possible, borderline tumors tend to present as large uni- or bi-lateral predominantly cystic ovarian tumors occurring more commonly in premenopausal women (Figure 3). Papillary projections ranging from 10 to 15 mm in size are more frequently found in borderline tumors as compared with benign and malignant epithelial ovarian tumors [38]. DCE-MRI may contribute to preoperative diagnosis of borderline tumors. Bazot et al. reported that moderate enhancement of the papillary vegetations and lack of solid elements favors diagnosis of a serous borderline tumor [38]. Mucinous borderline tumors have been described as multiseptated masses composed of locules of different contents with papillary projections that demonstrate delayed contrast medium uptake [11,38]. In a comparative study of borderline tumors the thickness of septae and size of solid components were less than in stage 1 cancers, but confident differentiation was not possible [39].
Figure 3: In a 47-year-old woman a large
complex cystic and solid pelvic mass was
identified on ultrasound. (A) T2-weighted, (B) T1-weighted and (C) fat saturation contrastenhanced
MRI show a multicystic left adnexal
lesion without enhancing elements presenting
cystadenoma. The right adnexal mass
demonstrating a complex cystic mass with
enhancing elements presented a serous
papillary borderline tumor.
U: Uterus.
Fallopian tube cancer
Histologically, in staging and in treatment fallopian tube cancer does not differ from invasive ovarian cancer. In imaging, fallopian tube cancer is difficult to diagnose preoperatively, and most advanced tubal cancers are misdiagnosed as ovarian cancer [40]. In MRI a unilateral complex cystic or solid adnexal tumor associated with hydrosalpinx should raise suspicion of fallopian tube cancer. Lymph node metastasis may be found more frequently than in ovarian cancer, and may also represent the only manifestation of dissemination [41].
Peritoneal adenocarcinomas
Peritoneal adenocarcinoma or surface papillary carcinoma of the peritoneum typically presents in a stage of advanced peritoneal dissemination. It accounts for 7–21% of ovarian carcinomas and presents similarly by age at diagnosis, histology, prognosis or response to chemotherapy as epithelial ovarian carcinomas. In MRI diffuse peritoneal spread similar to advanced ovarian cancer in combination with normal-sized ovaries is the typical finding of peritoneal adenocarcinoma, particularly with elevated CA-125 levels [42]. Image-guided percutaneus core needle biopsy allows for differentiation from other metastasizing primaries [5].
Metastases to the ovaries
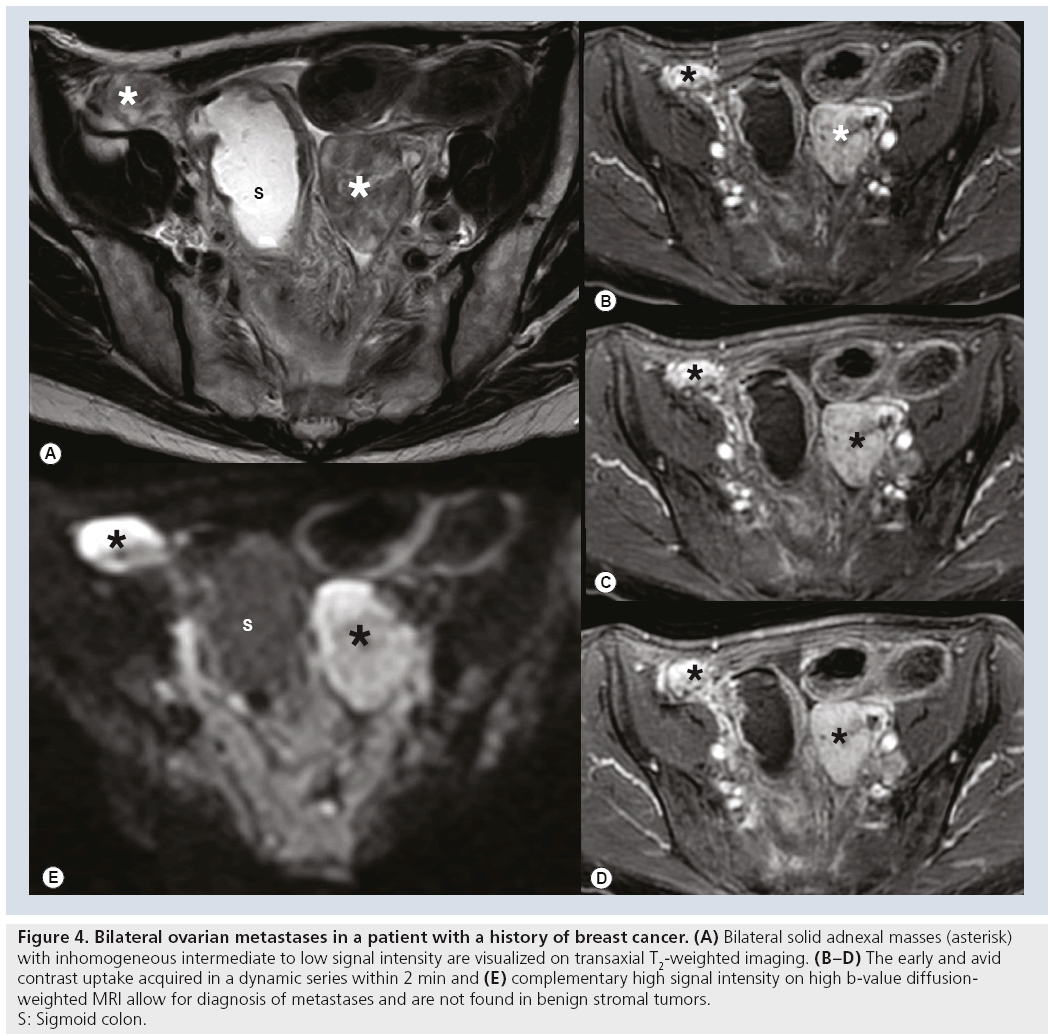
Approximately 5–15% of malignant ovarian tumors constitute metastases to the ovaries. GI tract and breast cancer are the most commonly encountered neoplasms to metastasize to the ovaries [5,43]. When ovarian metastasis displays as an inhomogeneous complex solid and cystic adnexal mass it cannot be differentiated from serous ovarian cancer in imaging [44]. History of cancer, or synchronous cancer particularly of the colon, should raise suspicion of metastasis. Approximately 50% of ovarian metastases consist of Krukenberg tumors, which are characterized by mucin-filled signet ring cells. They are typically bilateral (75%) and have smooth lobulated contours [43]. Krukenberg tumors present as solid tumors that tend to develop areas of hemorrhage and necrosis. Krukenberg tumors and solid metastases from breast cancer can be differentiated from benign solid stromal tumors, for example, fibrothecomas by DCE-MRI and by the high signal of its solid elements on DWI (Figure 4) [11].
Figure 4: Bilateral ovarian metastases in a patient with a history of breast cancer. (A) Bilateral solid adnexal masses (asterisk)
with inhomogeneous intermediate to low signal intensity are visualized on transaxial T2-weighted imaging. (B–D) The early and avid
contrast uptake acquired in a dynamic series within 2 min and (E) complementary high signal intensity on high b‑value diffusionweighted
MRI allow for diagnosis of metastases and are not found in benign stromal tumors.
S: Sigmoid colon.
Benign lesions mimicking ovarian cancer
Without appropriate clinical context differentiation of tubo–ovarian abscesses from ovarian cancer is challenging. In contrast to EOC tubo–ovarian abscess is found almost exclusively in premenopausal age. Similar to ovarian cancer it presents as unilateral or more often bilateral complex cystic adnexal masses with enhancing walls and internal septae. Uniformly thick walls of cystic adnexal lesions and signs of peritonitis, including mesh- or lace-like stranding of pelvic fat, thickening of peritoneal and of subperitoneal structures, are important discriminators from ovarian cancer. Furthermore, identification of tubal involvement (beak sign and waist sign) aids in establishing the correct diagnosis of an inflammatory entity [45].
Rare involvement of the adnexae by actinomycosis or tuberculosis may mimick ovarian cancer [46].
Staging of ovarian cancer by imaging
Traditionally, presumed ovarian cancer has been staged surgically according to the International Federation of Gynecology and Obstetrics (FIGO) or TNM system. In this procedure histopathologic diagnosis is obtained and optimal tumor debulking, the reduction of all tumor sites to a maximal diameter less than 1 cm, is attempted. Optimal cytoreduction improves the prognosis and optimizes the efficacy of adjuvant platinum-based chemotherapy [47]. However, in approximately 25% of patients with advanced ovarian cancer optimal cytoreduction cannot be achieved.
Staging by imaging is performed according to the FIGO system and is outlined in Table 1. This staging classification of ovarian cancer is based upon the classical pathways of spread encountered at the early stages of ovarian cancer including local, peritoneal and lymphatic spread. Preoperative staging by imaging has increasingly gained acceptance and has recently been incorporated into clinical practice guidelines in oncology, as findings enable more individualized treatment management [10,47,48].
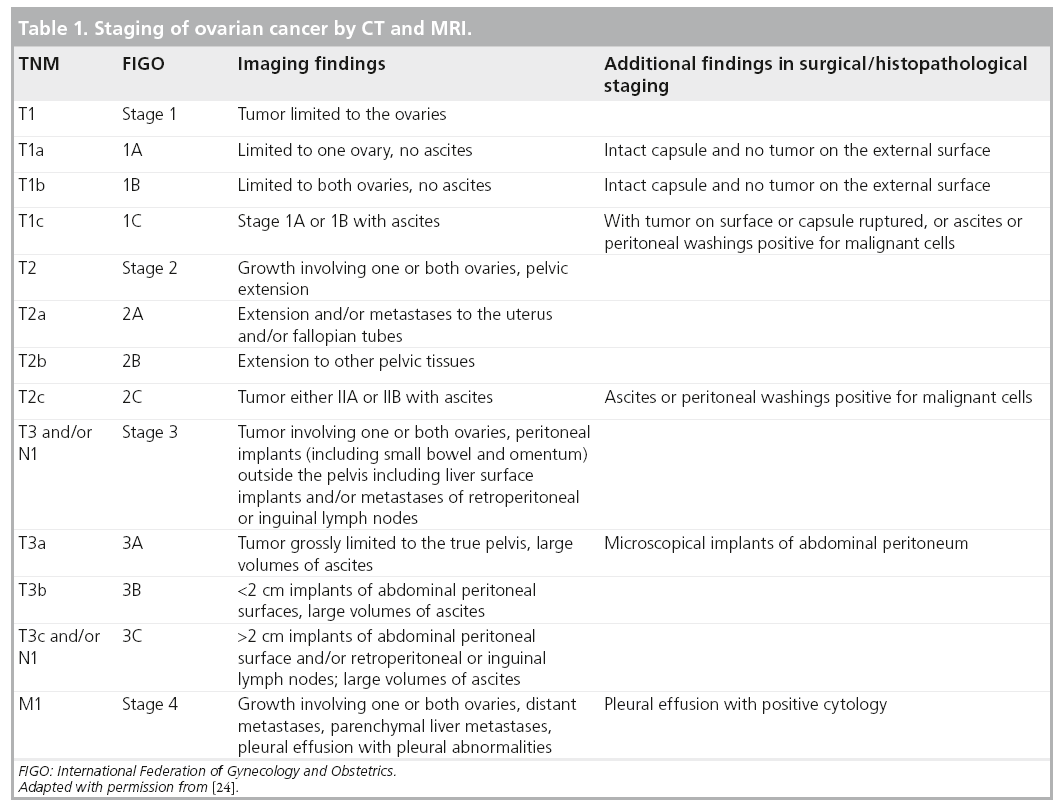
Although the established standard of care for advanced ovarian cancer remains appropriate surgical staging and cytoreduction followed by systematic chemotherapy, in patients unfit for surgery or in extreme tumor load, primary chemotherapy followed by interval debulking has become an alternative treatment option [47]. This approach has been validated by the randomized EORTC 55971 trial, which found similar overall survival but fewer complications in the group treated with neoadjuvant chemotherapy compared with those treated by standard regimen [47,48].
Before initiation of neoadjuvant chemotherapy histopathologic diagnosis must be confirmed, ideally through image-guided core needle biopsy [5].
Thus, preoperative imaging in patients with ovarian cancer plays a vital role in treatment planning and – if surgery is appropriate – in guiding optimal cytoreduction. As optimal cytoreduction depends on tumor load and site of metastases, imaging signs have been described to identify patients, in whom adequate debulking seems impossible and the neoadjuvant approach is therefore more appropriate [48,49]. These findings include sites mostly in the upper abdomen and are summarized in Box 1. However, resectability is very much a function of surgical expertise, and thus this again has to be discussed on an individual basis in a multidisciplinary setting [2,4].
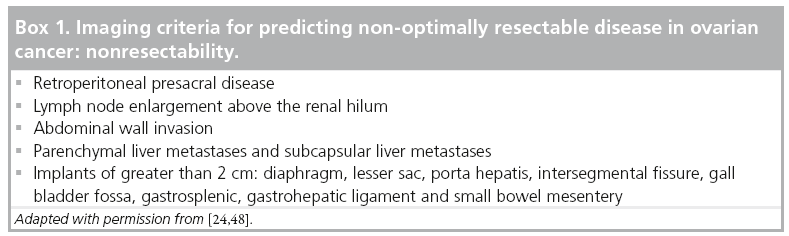
Preoperative imaging also serves as a roadmap for the surgeon to perform optimal cytoreduction. It is particularly useful to preoperatively identify lesions at sites that are difficult to assess during surgery or lesions that might be missed owing to unusual locations, and alert the surgeons to the necessity to obtain biopsies.
Typically EOC disseminates in the peritoneal cavity and peritoneal deposits are more common at sites of reduced flow of peritoneal fluid (e.g., pouch of Douglas or paracolic gutters). Other common sites include omentum, diaphragm, organ surfaces and peritoneal reflections.
Perihepatic metastases, the typical liver involvement in ovarian cancer, present stage 3 disease. They usually grow as scalloping lesions with smooth margins along the liver surface, but may sometimes (~5% of cases) invade the liver surface [49,50]. Preoperative diagnosis of invasive liver metastases has an impact on appropriate treatment planning, and usually hepatotobiliary specialist assistance will be required [50]. Liver parenchymal metastases representing hematogenous spread and stage 4 disease are extremely rare and differential diagnosis on CT images should include more likely benign liver lesions or metastases from other primaries.
The majority of patients with suspected ovarian cancer will present with the typical constellation of an adnexal mass, extensive peritoneal dissemination, ascites and CA-125 elevation. However, the clinical radiologist should also be aware of atypical manifestations of metastasizing ovarian cancer, for example, lymphadenopathy without peritoneal disease. Furthermore, 15% of patients with advanced ovarian cancer may have superior diaphragmatic lymphadenopathy, which is the principal drainage of the peritoneal cavity. In imaging, these lymph nodes present with an unusual short axis cut off for enlargement of 5 mm [51]. Tuberculosis, metastasizing tumors with a tendency of peritoneal seeding, such as gastrointestinal stromal tumors, melanoma, breast or GI tract cancers, mimic metastasizing ovarian cancer. In such equivocal cases imageguided biopsy has shown to be a safe method to distinguish between those entities.
MRI for staging ovarian cancer
Multidetector CT is the modality of choice for noninvasive staging of ovarian carcinoma, as it is widely available, reproducible and provides all relevant information for staging in a short examination time [25]. The reported accuracy for all stages ranges from 70 to 90% with an overall sensitivity in detection of peritoneal implants of 85–93% [4,51]. Although MRI performs similarly, the main reasons preventing MRI from becoming accepted as a standard modality for staging ovarian cancer includes – besides costs – technical issues, especially a much longer examination time as compared with CT. MRI has been recommended by the ESUR Female Imaging subcommittee as a primary staging tool in patients with contraindications for intravenous contrast media in CT or when radiation exposure is an issue, for example, in young females or in pregnancy. Furthermore, in many institutions staging is completed by additional abdominal coverage when pelvic MRI demonstrates an unequivocally malignant ovarian mass [25].
A multicenter trial comparing CT and MRI for staging ovarian cancer performed by the Radiology Diagnostic Oncology Group showed a similar sensitivity and performance for MRI compared with CT [52,53]. However, this study is approximately a decade old, and studies comparing recently evolved MRI and CT developments are required.
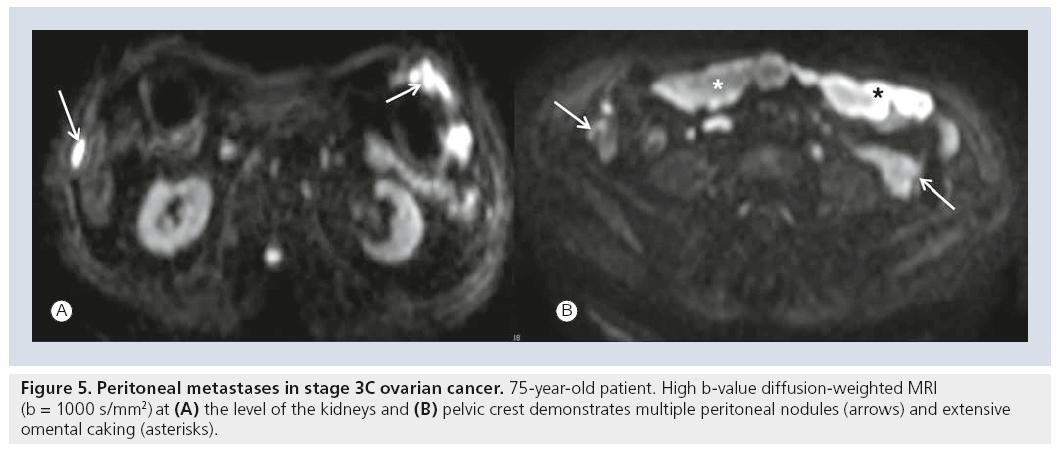
Despite technical advances CT imaging of peritoneal disease remains a problem, as peritoneal lesions will be missed owing to small size. In one study the sensitivity of CT was reduced to 25–50% for lesions with a diameter of less than 1 cm [54]. Furthermore, location of peritoneal deposits determines whether it can be identified on CT at all: deposits at peritoneal reflections, on the bowel surface or in the mesentery may be difficult to assess with CT [4]. Owing to its unique soft tissue contrast, MRI is superior to CT in the assessment of local tumor spread in the pelvis and upper abdomen. As DWI further increases tissue contrast and functional aspects it may influence future MRI staging. Peritoneal implants and omental deposits retain high SI with increasing β-values in comparison to reduced signal from surrounding ascites, bowel and fat (Figure 5) [7]. Thus, DWI may facilitate staging of ovarian cancer by improving the conspicuity in the assessment of peritoneal disease. It seems particularly useful in equivocal findings with conventional MRI sequences and increases the radiologist’s confidence in correctly identifying metastases [55].
Only a few studies have investigated DWI in peritoneal spread in gynecologic cancers. Fusion of DWI with T2-weighted imaging provided excellent sensitivity (90%) and specificity (95.5%) for evaluating the extent of peritoneal spread in ovarian cancer [56].
Low et al. found that DWI combined with contrast-enhanced MRI and oral bowel contrast with barium improved accuracy of peritoneal tumor detection on a site-per-site basis. In this study DWI was found to assist in the depiction of metastases in the mesentery, small bowel, on colonic serosal surfaces and in the pelvis [57].
Pitfalls with high SI on high β-value DWI include hypercellular tissue, such as small bowel mucosa, inflammatory processes, fluid collections, for example, lymphoceles or lesions with proteinaceous or hemorrhagic contents. However, false negatives may occur in DWI including predominately cystic lesions, calcifications and mucinous metastases [10].
Multicenter studies comparing MRI and DWI and histopathological correlation will be necessary to assess the value of this technique for staging ovarian cancer.
High sensitivity and specificity for the detection of peritoneal spread in patients with ovarian cancer has been reported for DCE MRI. Peritoneal implants are best identified on a delayed phase (<5 min). However, later diffusion of contrast media into the peritoneal cavity with opacification of ascites may be misleading [55].
Novel MRI techniques providing large volume coverage and functional MRI information include diffusion-weighted whole-body imaging with background body signal suppression, whole body DWI and integrated PET/MRI [58]. No data are available of their value in staging ovarian cancer or comparison with PET/CT for the detection of peritoneal implants or lymph node assessment. Improved detection of lymph node metastases, particularly in the upper abdomen and thorax, including the cardiophrenic and supraclavicular locations, may be clinically and prognostically relevant.
Assessment of response to chemotherapy
Traditionally treatment response in patients with ovarian cancer has been validated by serial measurements of CA-125 levels, clinical symptoms and imaging findings. CA-125 is considered the gold standard tumor marker in ovarian cancer and is used to monitor response to chemotherapy, relapse and disease progression [59].
Response Evaluation Criteria In Solid Tumors (RECIST) criteria have been established for objective assessment of treatment response in solid tumors [60]. RECIST guidelines are based on CT as a reproducible imaging technique, with MRI as the modality of second choice. In general the modalities CT and MRI are not allowed to change between baseline and followup. Unfortunately, in ovarian cancer application of RECIST is a challenge [4]. According to RECIST lesions with a diameter of less than 1 cm, which is the aim for successful cytoreduction, are excluded as target lesions. Furthermore, common findings as peritoneal metastases, ascites or pleural effusion are regarded as nonmeasurable lesions by RECIST standards.
Currently, monitoring treatment response by imaging relies mainly on change of size on serial studies. However, macromolecular and microstructural changes induced by therapy occurring at the cellular level substantially precede morphologic changes [61]. Treatment-induced cell damage is associated with increased ADC immediately after treatment. In cervical cancer the early increase of ADC after treatment seems predictive of successful cancer treatment [61–64].
In ovarian cancer preliminary results of one study (n = 20) using multiple β-values found significantly lower baseline ADC values and vascular signal fraction in peritoneal metastases than in primary tumor and in omental cake [65]. This seems to correlate with the biologic heterogeneity of ovarian cancer and may also explain mixed response patterns at different sites, for example, in primary ovarian tumors, omental cake and peritoneal implants in the same patient [10,65]. ADC values of peritoneal metastases correlated positively with the vascular fraction, which refers to a component of the DWI signal [4,55]. Kyriazi et al. investigated the value of quantitative DWI using ADC histogram analysis in assessing chemotherapy response in 42 patients. They found that DWI can aid in early monitoring of treatment efficacy in patients undergoing chemotherapy for metastatic ovarian and primary peritoneal cancer. While pretreatment ADCs were not predictive of response, early increase of ADC values (after the first and third cycle) and later decrease of skew and kurtosis of histograms were characteristic of chemotherapy response [8]. Further studies will be necessary to study reproducibility of ADC quantification and correlation with histopathology and tumor markers to validate if DWI may serve as a surrogate biomarker for advanced ovarian cancer [4,65].
Imaging recurrent ovarian cancer
Although the 5-year survival rate of women with ovarian cancer is approximately 46%, the overall survival rate has not improved significantly over the last few decades. Despite aggressive therapy the majority of women with advanced ovarian cancer will develop recurrent disease. Recurrence is usually detected by a serial rise of CA-125, which may precede clinical detection by several weeks to up to 5 months [4]. CA-125 is a marker of overall response and is neither able to differentiate between localized or diffuse disease nor is it a reliable predictor for the load of disease [10,59].
Several studies have shown the superiority of PET/CT over CT in assessing recurrence in patients with ovarian cancer [66]. PET/CT has increasingly been used for surveillance of patients treated for ovarian cancer and is most useful in patients with no evidence of recurrence in CT but rising tumor markers. The advantages of PET/CT include whole body coverage, particularly assessment of the thorax and improved diagnosis of lymph node metastases that might be missed with CT alone (e.g., in the supraclavicular region). However, microscopic peritoneal recurrence or lymph node metastases with a diameter of less than 5 mm are also beyond the detection of fludeoxyglucose PET/CT [67].
Recently, a comparative study (n = 35) of contrast-enhanced CT and PET/CT with histopathological correlation found similar performance of these modalities for site-specific and general detection of recurrent ovarian cancer [68].
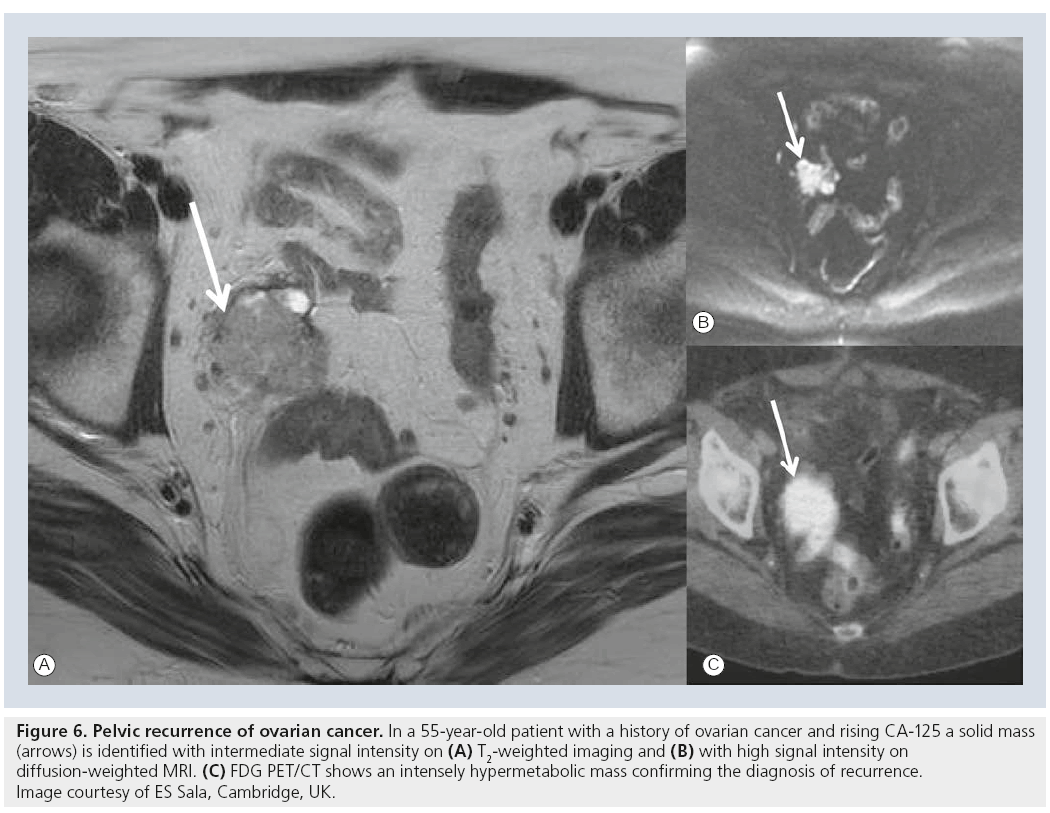
As recurrence in ovarian cancer most commonly manifests as peritoneal deposits MRI is usually not the screening modality of recurrence. In one study DCE-MRI performed excellently (sensitivity 90%, specificity 88%) in the detection of recurrent disease in patients treated for ovarian cancer and was superior to CA-125 assessment [57]. DCE-MRI would allow for quantitative analysis of kinetics, but for clinical routine it presents an as-yet unresolved technical challenge that requires high temporal resolution and large field of view coverage. Larger series will show if the novel functional MRI techniques will be able to improve the diagnostic performance of MRI compared with PET/CT and if it could be used as a potential biomarker. To date, the greatest utility of MRI is preoperative visualization of local, particularly pelvic, recurrence of ovarian cancer before secondary cytoreductive surgery (Figure 6).
Figure 6: Pelvic recurrence of ovarian cancer. In a 55-year-old patient with a history of ovarian cancer and rising CA‑125 a solid mass (arrows) is identified with intermediate signal intensity on (A) T2-weighted imaging and (B) with high signal intensity on diffusion-weighted MRI. (C) FDG PET/CT shows an intensely hypermetabolic mass confirming the diagnosis of recurrence. Image courtesy of ES Sala, Cambridge, UK.
Results from recent randomized trials may have significant implications on patients treated for ovarian cancer [48]. No benefit from early detection of relapse or early treatment of followup patients with rising tumor markers and no clinical signs of recurrence was found. Based on these data imaging of asymptomatic patients with rising CA-125 may not be justified [48]. Nevertheless, demonstration of recurrence could be clinically relevant in patient selection for appropriate surgery or radiation therapy in selected cases [4].
Future perspective & challenges
Imaging has an established role in the work-up of primary and ovarian cancer recurrence. As functional body MRI techniques are emerging from research to clinical application the future role of MRI may shift from problem solving to a central management tool in ovarian cancer.
If MRI aims to compete with CT and PET/CT for staging or surveillance of recurrence in ovarian cancer patients higher spatial and temporal resolution, including coverage of the body and thorax, is required.
Furthermore, before integration of functional imaging into clinical routine seems feasible, many obstacles have to be overcome [7,69]. DWI of the abdomen and pelvis must become a clinically robust, fast and reproducible complementary technique. Additional imaging time of approximately 15 min for abdominal and pelvic DWI series, as recently suggested for staging, will hamper its acceptance [10]. Prerequisites for successfully performing DWI include standardization of the DWI technique, administration of an antiperistaltic drug and comparison with standard sequences. To date, different β-values (values and numbers) are used and optimal thresholds for ADC quantification have not been found. Practical examination protocols with integration of DWI or multiparametric imaging for ovarian cancer will have to be validated in multicenter studies [4,10].
Special emphasis has to be placed in subspecialist training where not only understanding of radiomorphological findings but also increasingly profound knowledge of biochemistry and physics will be necessary.
Contrast-enhanced MRI is used for imaging adnexal masses and depiction of peritoneal implants. Limited data exist on quantitative analysis of DCE-MRI, which provides information on microvascular properties of tumor and surrounding tissues. Using a semiquantitative approach time intensity curves or threshold criteria can assist in the prediction of malignancy in lesions difficult to characterize with conventional MRI techniques [26,69]. Quantitative DCE-MRI is a more advanced technique where physiological parameters are extracted from a series of rapid DCE acquisitions by means of different pharmacokinetic models [69]. These provide data the microvasculature including for example, volume transfer constant, rate constant, fractional volume of extravascular extracellular space and fractional plasma volume. As novel antiangiogenetic anticancer substances are developed this MRI technique promises to play an important role in assessing ovarian cancer response to chemotherapy and may serve as an early treatment response biomarker in ovarian cancer [7,10].
Before incorporating quantitative DCE-MRI into clinical application in ovarian cancer imaging, MRI acquisition and measurement technique has to be standardized and many technical problems obviating reproducibility have to be resolved. Furthermore, in the limited studies available quantitative analysis was typically performed with in-house written software [69,70]. One preliminary study demonstrated feasibility of DCE-MRI in ovarian cancer and peritoneal deposits also on a 3 T unit [70]. Using a fast 3D sequence with a spatial resolution of 1.6 s and a coverage of ten slices, tumor-specific rapid uptake was found at approximately 5–6 s. No difference was seen for kinetic modeling results for primary or metastatic sites [70]. Areas of research include definition of the pharmacokinetic model most appropriate for ovarian cancer, analysis of tumor substructure or prediction of outcome or of treatment results [69].
Not yet solved technical problems inherent in abdominal spectroscopy include artifacts to peristalsis and breathing, limitations in accurate voxel localization and external volume suppression, field inhomogeneities, low signal-to-noise and long acquisition time [4,34]. A limited number of studies have shown the potential of 1H-MR spectroscopy for lesion differentiation in vitro and in vivo [4,33,34,71]. Its clinical utility as an adjunct to other MR techniques for assessment of adnexal lesions has yet to be investigated.
As nanoparticle technologies are under development and numerous antigens of EOC have been identified, targeted molecular imaging using MRI offers challenging fields of research in ovarian cancer [102].
A growing number of nanovectors are under development for more efficient drug delivery. Nanovectors, such as liposomes or dendrimers, serve as carriers for therapeutic agents and can be coupled with MRI contrast agents (e.g., USPIO or Gadolinium) and even combined with optical imaging [72,73]. In ovarian cancer coupling of monoclonal antibodies for in vitro and in vivo MRI with animal models are under investigation.
In the future biomarker-targeted delivery may provide selective application of therapeutics to cancer cells without collateral damage and targeted contrast agents in MRI may permit detection of smaller and earlier stage ovarian cancer and improve tumor surveillance by recognition of cancer signatures associated with the tumor microenvironment [73].
Conclusion
With today’s multidisciplinary team approach and changes in paradigm of treatment of ovarian cancer, imaging has become an integral and pivotal part of management [2,48]. The role of MRI has to be different for the patient presenting with an indeterminate adnexal mass versus the patient with clinically evident ovarian cancer confirmed by US. For the former, MRI has an established and evolving role in problem solving. Advances with integration of functional MRI including DWI and DCE-MRI promise to further improve characterization allowing confident diagnosis in the vast majority of complex adnexal masses. This is particularly useful in advanced age for avoiding unnecessary surgery, and similarly in young females with low probability of ovarian cancer and when fertility sparing surgery is desired (e.g., in borderline tumors).
For women with malignant masses CT is currently recommended for comprehensive presurgical staging. Although data suggest that MRI is at least equal, and at some sites also superior to CT, practical issues such as availability and examination time as well as limitations of spatial resolution for coverage of large fields of view hamper its routine use for staging ovarian cancer.
Before embarking on functional MRI in routine imaging the challenge is for optimization and standardization of protocols and analysis techniques [69]. Furthermore, training of radiologists has to be directed to profound understanding not only of anatomy and morphology of disease but also to knowledge about metabolic pathways, more specifically those relevant for functional imaging. Functional qualitative and quantitative properties obtained by MRI or its combination with advanced techniques, for example, nanotechnology, raise expectations that the role of MRI may shift from problem solving to a central management tool. Combined interpretation of morphological and functional imaging promises to increase performance of imaging of low-volume metastases. Quantitative MRI by DWI or DCE-MRI holds promise to become a biomarker of tumor composition, response and prognosis. However, the value of these new techniques, including comparison with PET/CT must be validated by histopathological correlation in multicenter studies.
Recent clinical developments mean that radiologists will be in greater demand at initial diagnosis, planning surgery or neoadjuvant chemotherapy, providing a tissue diagnosis and assessing response prior to interval debulking surgery. Open to debate, is the need for rigorous diagnosis of early relapse given data showing no clear survival advantage.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• • of considerable interest
- Colombo N, Peiretti M, Parma G et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guideline of diagnosis, treatment and follow-up. Ann. Oncol. 21, 23–30 (2010).
- Spencer JA. A multidisciplinary approach to ovarian cancer at diagnosis. Br. J. Radiol. 78, 94–102 (2005).
- Spencer JA, Forstner R, Cunha TM, Kinkel K. ESUR guidelines for MR imaging of the sonographically indeterminate adnexal mass: an algorithmic approach. Eur. Radiol. 20, 25–35 (2010).
- Kyriazi S, Kaye SB, deSouza NM. Imaging ovarian cancer and peritoneal metastases – current and emerging techniques. Nat. Rev. Clin. Oncol. 7, 381–393 (2010).
- Spencer JA, Swift SE, Wilkinson N, Boon AP, Lane G, Perren TJ. Peritoneal carcinomatosis: image guided peritoneal core biopsy for tumor type and patient management. Radiology 221(1), 173–177 (2001).
- Griffin N, Grant LA, Freeman S et al. Image guided biopsy in patients with suspected ovarian carcinoma: a safe and effective technique? Eur. Radiol. 19, 230–235 (2009).
- Sala E, Vargas AH, Wasserberg C, Kyriazi S, deSouza N. The role of functional MRI and PET/CT in evaluation of patients with primary and recurrent ovarian cancer. Imaging Med. 3(3), 333–343 (2011).
- Kyriazi S, Collins DJ, Messiou C et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging – value of histogram analysis of apparent diffusion coefficients. Radiology 261, 182–192 (2011).
- Bammer R. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 45(3), 169–184 (2003).
- Kyriazi S. Collins DJ, Morgan VA, Giles SL, deSouza NM. Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer. Radiographics 30(5), 1269–1285 (2010).
- Thomassin-Naggara I, Toussaint I, Perrot N et al. Characterization of complex adnexal masses: value of adding perfusion – and diffusion-weighted MR imaging to conventional MR imaging. Radiology 258(3), 793–803 (2011).
- American Cancer Society. Cancer Facts and Figures 2010. American Cancer Society, GA, USA (2010).
- Jhamb N, Lambrou N. Epidemiology and clinical presentation of ovarian cancer. In: Ovarian Cancer. Bristow R, Armstrong D (Eds). Saunders Elsevier, PA, USA, 1–16 (2010).
- Tavassoli FA, Devilee P. Pathology and Genetics of Tumours of the Breast and Female Genital System: World Health Organisation Classification. IARC Press, Lyon, France, 113–145 (2003).
- Lalwani N, Prasad SR, Vikram R et al. Histologic, molecular and cytogenetic features of ovarian cancers: implications for diagnosis and treatment. Radiographics 31, 625–646 (2011).
- Seidman JD, Russell P, Kurman RJ. Surface epithelial tumors of the ovary. In: Blaustein´s Pathology of the Female Genital Tract. Kurman RJ (Ed.). Springer, NY, USA, 791–904 (2002).
- Medeiros LR, Freitas LB, Rosa DD et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am. J. Obstet. Gynecol. 204, 67.e1–e10 (2011).
- Brown DL, Zou KH, Tempany CMC et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology 219(1), 213–218 (2001).
- Spencer JA, Ghattamaneni S. MR imaging of the sonographically indeterminate adnexal mass. Radiology 256(3), 677–694 (2010).
- Kao HW, Wu CJ, Chung KT, Wang SR, Chen CY. MR imaging of pregnancy luteoma: a case report and correlation with the clinical features. Korean J. Radiol. 6(1), 44–46 (2005).
- Thomassin-Naggara I, Cuenod CA, Darai E, Marsult C, Bazot M. Dynamic contrast-enhanced MR imaging of ovarian neoplasms: current status and future perspectives. Magn. Reson. Imag. Clin. N. Am. 16(4), 661–672 (2008).
- Medeiros LR, Rosa DD, da Rosa MI, Bozetti MC. Accuracy of CA- with color Doppler in ovarian tumor: a systematic review. Int. J. Gynecol. Cancer 19, 230–236 (2009).
- Sohaib SA, Mills TD, Sahdev A et al. The role of magnetic resonance imaging and ultrasound in patients with adnexal masses. Clin. Radiol. 60, 340–348 (2005).
- Sohaib SA, Reznek RH. MR imaging in ovarian cancer. Cancer Imaging 7, 119–129 (2007).
- Forstner R, Sala E, Kinkel K, Spencer JA. ESUR guidelines: ovarian cancer staging and follow-up. Eur. Radiol. 20(12), 2773–2780 (2010).
- Tsili AC, Tsampoulas C, Argyropoulou M et al. Comparative evaluation of multidetector CT and MR imaging in the differentiation of adnexal masses. Eur. Radiol. 18(5), 1049–1057 (2008).
- Thomassin-Naggara I, Daraï E, Cuenod CA et al. Contribution of diffusion-weighted MR imaging for predicting benignity of complex adnexal masses. Eur. Radiol. 19(6), 1544–1552 (2009).
- Takeuchi M, Matsuzaki K, Nishitani H. Diffusion-weighted magnetic resonance imaging of ovarian tumors: differentiation of benign and malignant solid components of ovarian masses. J. Comput. Assist. Tomogr. 34(2), 173–176 (2010).
- Davies AP, Jacobs I, Woolas R, Fish A, Oram D. The adnexal mass: benign or malignant? Evaluation of a risk of malignancy index. Br. J. Obstet. Gynaecol. 110, 927–931 (1993).
- van Trappen PO, Rufford BD, Mills TD et al. Differential diagnosis of adnexal masses: risk of malignancy index, ultrasonography, magnetic resonance imaging, and radioimmunoscintigraphy. Int. J. Gynecol. Cancer 17, 61–67 (2007).
- Kim KA, Park CM, Lee JH et al. Benign ovarian tumors with solid and cystic components that mimick malignancy. Am. J. Roentgenol. 182, 1259–1265 (2004).
- Froehlich JM, Metens T, Chilia B, Hauser N, Hohl MK, Kubik-Huch RA. MRI of the female pelvis: a possible pitfall in the differentiation of haemorrhagic vs. fatty lesions using fat saturated sequences with inversion recovery. Eur. J. Radiol. doi:10.1016/j.ejrad.2011.01.062 (2011) (Epub ahead of print).
- Cho SW, Cho SG, Lee JH et al. In-vivo proton magnetic resonance spectroscopy in adnexal lesions. Korean J. Radiol. 3, 105–112 (2002).
- Stanwell P, Russel P, Carter J, Pather S, Heintze S, Mountford C. Evaluation of ovarian tumors by proton magnetic resonance spectroscopy at 3 Tesla. Invest. Radiol. 43, 745–751 (2008).
- Hricak H, Chen M, Coakley FV et al. Complex adnexal masses: detection and characterization with MR imaging – multivariate analysis. Radiology 214(1), 39–46 (2000).
- Dilks P, Narayan P, Reznek R, Sahdev A, Rockall A. Can quantitative dynamic contrast-enhanced MRI independently characterize an ovarian mass. Eur. Radiol. 20, 2176–2183 (2010).
- Ozols RF, Schwartz PE, Eifel PJ. Ovarian cancer, fallopian tube carcinoma, and peritoneal carcinoma. In: Cancer. Principles and Practice of Oncology. de Vita VT Jr, Hellman S, Rosenberg SA (Eds). Lippincott Williams & Wilkins, PA, USA, 1597–1632 (2001).
- Bazot M, Nassar-Slaba J, Thomassin-Naggara I et al. MRI compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors; correlation with final histology. Eur. Radiol. 16, 2687–2699 (2006).
- DeSouza NM, O´Neill R, McIndoe GA, Dina R, Soutter WP. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage 1 disease. Am. J. Roentgenol. 184, 999–1003 (2005).
- Rezvani M, Shaaban AM. Fallopian tube disease in the nonpregnant patient. Radiographics 31, 527–548 (2008).
- Kawakami S, Togashi K, Kimura I et al. Primary malignant tumor of the fallopian tube: appearance at CT and MR imaging. Radiology 186, 503–508 (1993).
- Morita H, Aoki J, Taketomi A, Sato N, Endo K. Serous surface papillary carcinoma of the peritoneum: clinical, radiological, and pathologic findings in 11 patients. Am. J. Roentgenol. 183, 923–928 (2004).
- Choi HJ, Lee JH, Kang S et al. Contrast-enhanced CT for differentiation of ovarian metastasis from gastrointestinal tract cancer: stomach cancer versus colon cancer. Am. J. Roentgenol. 187, 741–745 (2006).
- Brown DL, Zou KH, Tempany CMC et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology 219, 213–218 (2001).
- Ghattamaneni S, Bhuskute N, Weston MJ et al. Discriminative MRI features of fallopian tube masses. Clin. Radiol. 64, 815–835 (2009).
- Kim SH, Kim SH, Yang DM et al. Unusual causes of tubo–ovarian abscess. CT and MR imaging findings. Radiographics 24, 1575–1589 (2004).
- Morgan RJ, Alvarez RD, Armstrong DK et al. Ovarian cancer. Clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 6(8), 766–794 (2008).
- Spencer JA, Perren TJ. Recent EORTC and MRCUK studies: implications for imaging ovarian cancer. Cancer Imaging 10, 135–136 (2010).
- Quayyum A, Coakley FV, Westphalen AC et al. Role of CT and MRI in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gynecol. Oncol. 96, 301–306 (2005).
- Akin O, Sala E, Chaya S et al. Perihepatic metastases from ovarian cancer: sensitivity and specificity of CT for the detection of metastases with and those without liver parenchymal invasion. Radiology 248, 511–517 (2008).
- Holloway BJ, Gore ME, Hern RP et al. The significance of paracardiac lymph node enlargement in ovarian cancer. Clin. Radiol. 52, 692–697 (1997).
- Tempany CM, Zou KH, Silverman et al. Staging of advanced ovarian cancer: comparison of imaging modalities – report from the Radiological Diagnostic Oncology Group. Radiology 215, 761–767 (2000).
- Kurtz AB, Tsimikas JV, Tempany CMC et al. Diagnosis and staging of ovarian cancer: comparative values of Doppler and conventional US, CT, and MR imaging correlated with surgery and histopathologic analysis-report of the Radiology diagnostic oncology group. Radiology 212, 19–27 (1999).
- Coakley FV, Choi PH, Gougoutas CA et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology 223(2), 495–499 (2002).
- Sala E, Rockall A, Rangarajan D, Kubik-Huch RA. The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur. J. Radiol. 76(3), 367–385 (2010).
- Fujii S, Kaneda S, Kakite S et al. Diffusionweighted imaging findings of adnexal torsion: initial results. Eur. J. Radiol. 77(2), 330–334 (2011).
- Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings – a feasibility study. Am. J. Roentgenol. 193(2), 461–470 (2009).
- Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur. Radiol. 18, 1937–1952 (2008).
- Gupta D, Lis CG. Role of CA 125 in predicting ovarian cancer survival – a review of the epidemiological literature. J. Ovarian Res. 2, 13 (2009).
- Eisenhauer E, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
- Thoeny H, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J. Magn. Reson. Imaging 32(1), 2–16 (2010).
- Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur. Radiol. 15(1), 71–78 (2005).
- Chen J, Zhang Y, Liang B, Yang Z. The utility of diffusion-weighted MR imaging in cervical cancer. Eur. Radiol. 74(3), e101–e106 (2010).
- Harry V, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol. Oncol. 111(2), 213–220 (2008).
- Sala E, Priest AN, Kataoka M et al. Apparent diffusion coefficient and vascular signal fraction measurements with magnetic resonance imaging: feasibility in metastatic ovarian cancer at 3 Tesla: technical development. Eur. Radiol. 20(2), 491–496 (2010).
- Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing ovarian carcinoma: a systematic review and meta-analysis . Eur. Radiol. 71, 164–174 (2009).
- Bristow RE, Giuntoli RL, Pannu HK et al. Combined PET/CT for detecting recurrent ovarian cancer limited to retroperitoneal lymph nodes. Gyencol. Oncol. 99(2), 294–300 (2005).
- Sala E, Kataoka M, Pandit-Taskar N et al. Recurrent ovarian cancer: use of contrastenhanced CT and PET/CT to accurately localize tumor recurrence and to predict patients’ survival. Radiology 257, 125–134 (2010).
- Punwani S. Diffusion weighted imaging of female pelvic cancers: concepts and clinical applications. Eur. Radiol. 78, 21–29 (2011).
- Priest AN, Gill AB, Kataoka M et al. Dynamic contrast enhanced MRI in ovarian cancer: initial experience at 3 T in primary and metastatic disease. Magn. Reson. Med. 63, 1044–1049 (2010).
- Massuger LF, van Vierzen PB, Engelke U, Heerschap A, Wevers R. 1H-magnetic resonance spectroscopy: a new technique to discriminate benign from malignant ovarian tumors. Cancer 82, 1726–1730 (1998).
- Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov. Today 15, 171–185 (2010).
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171 (2005).
- Chen L, Berek JS. Ovarian tumors of low malignant potential. www.uptodate.com/contents/ovarian-tumorsof- low-malignant-potential
- Leung K. Ovarian cancer antigen 183B2 monoclonal antibody conjugated to ultrasmall superparamagnetic iron oxide nanoparticles. www.ncbi.nlm.nih.gov/books/nbk53963
• • Presents imaging guidelines.
• • Review on the current and emerging techniques for imaging peritoneal metastases.
• • Demonstrated technique and value of image-guided peritoneal core biopsy.
• • Presents functional MRI techniques and PET/CT for ovarian cancer imaging.
• • Presents imaging guidelines.
• • Addresses the role of CT and MRI in predicting optimal cytoreduction for ovarian cancer.
• • Central paper for the revised version of Response Evaluation Criteria in Solid Tumors.
• • Addresses the value and limitations of diffusion-weighted MRI for female pelvic cancers.
■ Websites