Review Article - Imaging in Medicine (2011) Volume 3, Issue 2
Imaging of traumatic brain injury: current and future
Yoshimi Anzai†1 and Satoshi Minoshima11Department of Radiology, University of Washington, 1959 NE Pacific Street, Seattle, WA 98195-7115, USA
- *Corresponding Author:
- Yoshimi Anzai
Department of Radiology, University of Washington
1959 NE Pacific Street, Seattle, WA 98195-7115, USA
Tel.: +1 206 598 5130
Fax: +1 206 598 8475
E-mail: anzai@uw.ed
Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability among young adults. CT remains the imaging modality of choice in the acute setting, in order to triage patients requiring emergent surgical intervention or conservative medical management. The vast majority of TBI patients, however, are categorized as mild TBI or concussion. Some patients with mild TBI or concussion have a wide variety of neurological and psychological symptoms where CT and MR remain normal. Advanced imaging techniques, such as diffusion tensor imaging, PET, SPECT or functional MRI, have potential to demonstrate abnormalities that are not detected on morphological imaging studies. Future research is needed to validate new imaging techniques and determine which imaging technique most accurately reveals abnormalities that correlate with functional deficits.
Keywords
concussion; diffusion tensor imaging; imaging; magnetic resonance; prediction; SPECT; trauma; traumatic brain injury
Epidemiology
Traumatic brain injury (TBI) is a form of brain damage caused by external mechanical force, such as rapid acceleration or deceleration injury, direct impact, blast waves or penetration by a projectile, leading to temporary or permanent impairments, functional disability or psychosocial maladjustment. TBI is typically classified as mild, moderate or severe based on the Glasgow Coma Scale (GCS), which grades a person’s level of consciousness based on verbal, motor and eyeopening reactions to stimuli. GCS correlates well with outcomes of TBI patients. Generally, a GCS of 13 or above is considered mild, 9 to 12 is moderate and 8 or below is severe. Patients with mild TBI may present with a brief change in mental status or loss of consciousness. Patients with severe TBI may have an extended period of unconsciousness or amnesia after injury. The degree of disability correlates with the severity of head trauma. Approximately 80–85% of TBI are concussions or other forms of mild TBI. Post-traumatic seizure disorders are more common in patients with severe TBI. Men are twice as likely as women to sustain TBI. The two age groups at highest risk for TBI are 0–4‑year-olds and 15–19‑year-olds. In fact, TBI is a leading cause of death among young adults. Falls are the leading cause of TBI, particularly in children under 4 years of age and elderly people over the age of 75 years. The rate of motor vehicle crashrelated TBI and sport- or recreation-related TBI is highest among adolescents and young adults [101].
Traumatic brain injury is a major health and socioeconomic problem throughout the world. The CDC reports 1.7 million people sustain a TBI each year in the USA [102]. Among them, approximately 1.4 million are treated and released from emergency rooms, 275,000 are hospitalized and 52,000 die from TBI (CDC: National Center for Injury Prevention and Control) [101]. These numbers do not include those patients with TBI who did not seek medical attention. In 2000, direct medical costs and indirect costs, such as loss of productivity related to TBI, are estimated to be $60 billion per year in the USA.
The CDC estimates that at least 5.3 million Americans (~2% of the US population) have a long-term or life-long disability as a result of TBI [1,102]. Symptoms related to chronic TBI are relatively broad and affect cognition, sensation, language or emotion. Repeated TBI occurring over an extended period of time can result in cumulative neurological deficits. Due to the improved diagnosis and management of TBI, the death rate related to TBI has declined. As a result, the number of people living with disabilities that result from TBI has increased.
Pathophysiology of TBI
Damage caused by TBI is divided into primary TBI and secondary TBI. Primary injuries are directly related to the contact force at the moment of injury. The primary injury generally includes skull fractures, epidural and subdural hematomas (SDHs), traumatic subarachnoid hemorrhage, brain contusion and diffuse axonal injury. Traumatic injury involving cervical vessels often leads to infarction due to traumatic dissection or thromboembolic complications.
Secondary injury is a cascade of events triggered by the initial injury that occurs in the minutes to days following the primary trauma and may continue for a long time. Secondary brain injury includes cerebral edema, infarction/ ischemia and brain herniation. Under normal circumstance, cerebral blood flow is relatively constant due to protective autoregulation. Outside of the limits of autoregulation, or when autoregulation fails in some cases of head trauma, raising mean arterial pressure will raise cerebral perfusion pressure (CPP), and increasing intracranial pressure (ICP) will lower CPP. Brain contusion or hematoma results in cerebral edema or increased ICP. As ICP increases, CPP decreases. When CPP falls below 50 mmHg, the brain becomes ischemic. This further increases ICP, leading to cerebral hypoxia, ischemia, cerebral edema and brain herniation. Recent investigations suggest aggressive maintenance of CPP, in conjunction with controlling ICP, leads to a better outcome [2,3].
Excitatory neurotransmitters, including glutamate and aspartate, are often significantly elevated after a TBI. These excitatory neurotransmitters lead to the influx of chloride and sodium ions into neurons and dysfunction of mitochondria, causing further cell damage and cerebral swelling. Excitatory neurotransmitters also increase an influx of calcium, which are closely related to delayed cellular damage and degradation of axonal transport. Other biochemical mechanisms affecting secondary TBI include: an increase in extracellular potassium, leading to edema; an increase in cytokines, contributing to inflammation; and a decrease in intracellular magnesium, contributing to further calcium influx.
In addition to increased ICP and excitatory neurotransmitter, cerebral edema in some cases can be due to post-traumatic hyperemia due to a loss of normal cerebral autoregulation. Dysautoregulation can result from hypoxemia and hypercapnia, both of which lead to vasodilatation. This mechanism is more common in pediatric and young adult populations. As a result of these cascades of events, CPP is reduced and infarction results. When cerebral edema is severe enough, it causes brain herniation. Types of brain herniation will be discussed later.
Types of TBI
Primary injury Extra-axial hemorrhage: epidural hematoma, SDH & subarachnoid hemorrhage Epidural hematoma
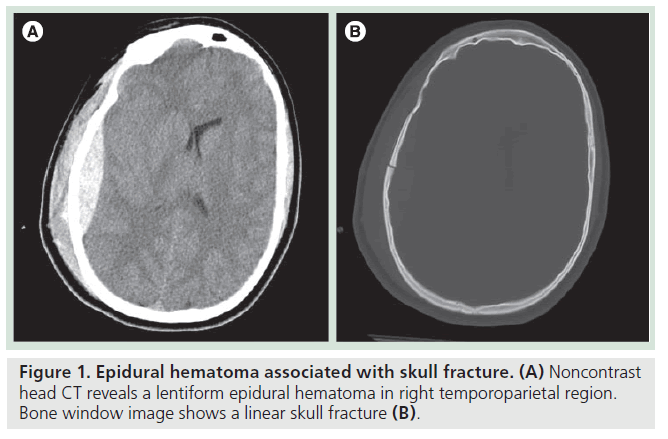
The epidural space is a potential space between the inner table of the calvarium and the dura. In a normal circumstance, no epidural space is present because of firm attachment of the outer dural layer to the inner table of the skull. Epidural hematoma (EDH) often develops from injury to the middle meningeal artery or its branches from the skull fracture and, therefore, approximately 75% of EDH is seen in the temporoparietal location (Figure 1). The expanding hematoma strips the outer layer of dura from the skull, but is confined to the epidural space, typically resulting in a biconvex shape. Venous EDH, on the other hand, is a result of bleeding from meningeal and diploic veins or from dural sinuses into the epidural spaces. Supratentorial venous EDH is commonly associated with a tear along the sphenoparietal sinus or superior sagittal sinus.
Epidural hematoma is more common than SDH in the posterior fossa and is frequently venous in its origin. The posterior fossa EDH involves the torcular herophili and the transverse sinus. Presence of air also indicates a site of fracture. It is extremely rare for EDH to cross the suture line, as the periostial layer of the dura adheres tightly to the cranial suture. Another unique feature of EDH is that it can extend above and below the tentorium, unlike SDH. Typically, extra-axial hematoma appears homogeneously hyperdense on noncontrast CT. Heterogeneous attenuation of EDH is indicative of active bleeding. Although rapidly expanding EDH needs emergency evacuation, isolated EDH is often associated with favorable clinical outcomes regardless of its size [4,5], in part due to its less frequent association with parenchymal injury.
Subdural hematoma
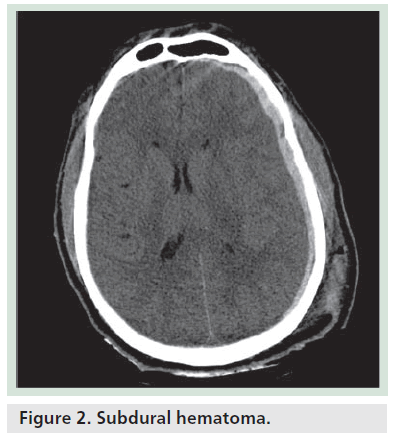
Subdural hematoma is a hemorrhagic collection between the meningeal layer of dura and arachnoid. More than half of SDH is traumatic, although spontaneous SDH does frequently occur, particularly in elderly patients or patients with coagulopathy. SDH is due to injury of a superficial cortical vein. As SDH accumulates, it pushes arachnoid away and stretches the bridging veins, resulting in further venous rupture and accumulation of SDH. As the brain becomes atrophied, the subdural space enlarges and stretches the bridging veins further, thus SDH is more common in the elderly and is reported to account for as many as 40–60% cases of patients with TBI. Bilateral SDH is commonly seen in elderly patients with underlying coagulopathy. Approximately 10% of SDH is associated with EDH [6]. The initial scan immediately after trauma may not show a small SDH due to tamponade effect, but it may become readily apparent after evacuation of a larger contralateral SDH. SDH is often crescentic in shape, does not cross the midline and extends along the dural reflection (Figure 2). Unlike EDH, however, it does cross the suture line. The majority of SDH is homogeneously hyperdense on noncontrast CT; some SDH may appear isodense to gray matter. These ‘isodense’ SDH can be seen in patients with severe anemia, disseminated intravascular coagulation or with an associated arachnoid tear causing CSF to leak into the subdural space from the subarachnoid space. Heterogeneous SDH can be seen in the setting of active bleeding. SDH with hematocrit/ hemoglobin levels is also seen in patients with coagulopathy or acute bleeding superimposed on chronic SDH, so-called acute-on-chronic SDH.
The common locations of SDH are cerebral convexity, parafalcine and interhemispheric, occasionally extending along the tentorium cerebri. Posterior fossa SDH is rare and more common in children than adults. SDH can be located at the site of injury or the opposite side of direct impact (coup and contra coup injuries). SDH can be simple or complicated depending on the absence or presence of underlying brain injury or cerebral edema. Complicated SDH associated with underlying cerebral edema has been reported to have 89% mortality compared with 20% mortality for simple SDH [7].
Subarachnoid hemorrhage
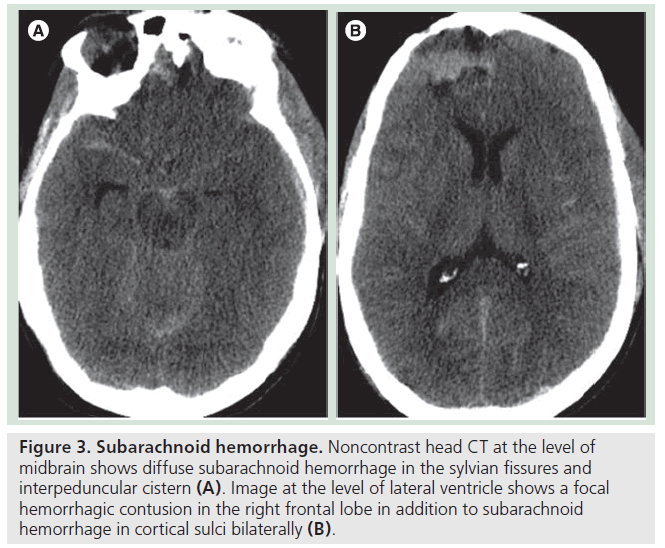
Traumatic subarachnoid hemorrhage (tSAH) is a fairly common finding in patients with head trauma. The hemorrhage is from thin layers of cortical veins passing through subarachnoid space. It is commonly seen beneath a skull fracture or near a brain contusion, most likely representing direct injury of surface vessels. The interpedincular cistern is one of the most common locations for tSAH (Figure 3). Although tSAH is fairly common and was thought to be insignificant in the past, recent studies indicate that the presence of tSAH can predict outcomes of patients. A study from the European Brain Injury Consortium strongly indicated a statistically significant association between the presence of tSAH and poor outcomes. As opposed to 41% of patients without tSAH achieving the level of good recovery, only 15% of patients with tSAH showed good recovery after trauma. Patients with tSAH exhibited lower GCS scores at the time of admission. tSAH is more common in elderly patients than younger patients. After adjusting for admission GCS and age, the presence of tSAH remained a significant predictor for unfavorable outcomes (odds ratio: 2.49; 95% CI: 1.74–3.55; p < 0.001) [8].
Subarachnoid hemorrhage is readily detected on either CT or magnetic resonance (MR). A study comparing multidetector-row CT and 1.5 T MR to forensic-pathological findings showed an accuracy, sensitivity and specificity of 89, 82 and 92% using CT; and 90, 83 and 94% using MRI, respectively. MRI using T1, T2 and T2GRE was more sensitive than CT in the detection of SAH (p = 0.001), whereas there was no significant difference in the detection of epidural and subdural hemorrhages (p = 0.248 and 0.104, respectively) [9].
Figure 3.Subarachnoid hemorrhage. Noncontrast head CT at the level of midbrain shows diffuse subarachnoid hemorrhage in the sylvian fissures and interpeduncular cistern (A). Image at the level of lateral ventricle shows a focal hemorrhagic contusion in the right frontal lobe in addition to subarachnoid hemorrhage in cortical sulci bilaterally (B).
One of the most common complications related to SAH is hydrocephalus. In the acute phase, hydrocephalus could be due to inflammatory arachnoiditis or intraventricular hemorrhage. Chronic hydrocephalus is believed to be due to reduction of CSF absorption from the occlusion of arachnoid villi. If a patient reaches a stable clinical condition and then deteriorates, development of hydrocephalus should be suspected.
Another complication of SAH is superficial siderosis, which is a hemosiderin deposition in the leptomeninges. On MR, superficial siderosis appears as a thin layer of signal loss on T2 weighted images along the basilar cistern outlining the brain stem or cerebellar folia and cranial nerve structures (Figure 4). This often results from a recurrent SAH, although a single episode of SAH could cause superficial siderosis. Patients with superficial siderosis often present with hearing loss or cerebellar ataxia. Susceptibilityweighted images (SWI) or T2* gradient echo (GRE) images are most sensitive for detection of superficial siderosis [10].
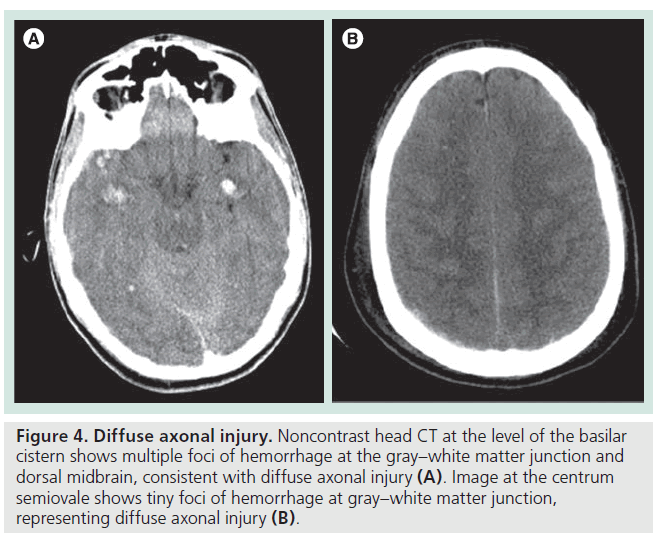
Figure 4.Diffuse axonal injury. Noncontrast head CT at the level of the basilar cistern shows multiple foci of hemorrhage at the gray–white matter junction and dorsal midbrain, consistent with diffuse axonal injury (A). Image at the centrum semiovale shows tiny foci of hemorrhage at gray–white matter junction, representing diffuse axonal injury (B).
Injury to brain parenchyma: brain contusion, diffuse axonal injury & brain herniation Brain contusion
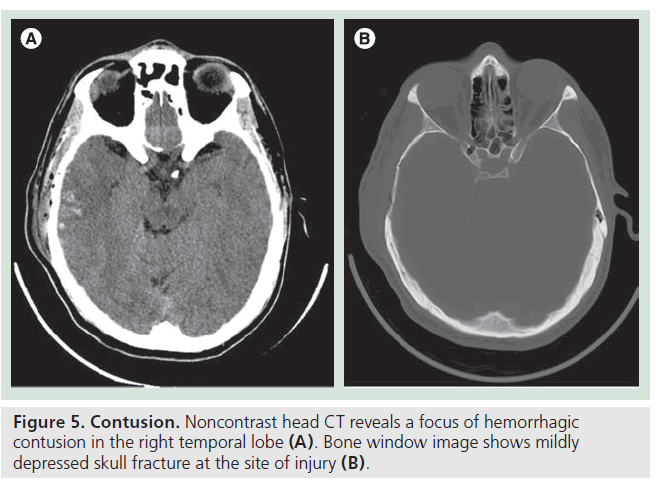
Cerebral contusion or hematoma could be due to direct coup or contra coup injury. Since contusion is caused by the brain being compressed against the rough surface of the skull, for example anterior or middle cranial fossa, the base of frontal lobes and anterior and inferior temporal lobes are the most common sites for brain contusion (Figure 5). Generally, contusions can be nonhemorrhagic, microhemorrhagic or grossly hemorrhagic, in which case it is considered to be a hematoma. Contusion involves gray matter as well as white matter, one of the features differentiating it from diffuse axonal injury (DAI). Another important clinical observation is that mass effect or edema surrounding an area of contusion increases within a few days after trauma, presumably related to vasogenic or cytotoxic edema. Follow-up imaging studies as well as close monitoring of ICP is required for patients with cerebral contusion. Rapid increase in mass effect can be observed within the first 12 h in some patients with severe cerebral contusion. One of the hypotheses to explain this phenomenon (other than vasogenic or cytotoxic edema) is hyperosmolality in the center of the contusion, generating osmotic gradient from the center to periphery, leading to accumulation of water inside the contusion [11]. There is no effective medical treatment for traumatic brain contusion or hematoma. Surgical evacuation is performed for a large hematoma causing significant mass effect or herniation. Presence of tSAH and the volume of brain contusion are both reported to be associated with poor clinical outcomes [12,13].
Diffuse axonal injury
Diffuse axonal injury is a widespread extensive white matter shearing injury caused by torsional shearing forces during a trauma. It is one of the major causes of unconciousness and persistent vegetative state after TBI. It frequently occurs after motor vehicle accidents due to accelerating and decelerating injuries, and also as a result of child abuse. There are certain anatomical areas that are vulnerable to DAI. Areas of different tissue density and rigidity interface are susceptible for shearing injury. These include the subcortical gray–white matter junction, the corpus callosum, the internal capsule and the brain stem (Figure 4). An autopsy study has demonstrated that long tract fibers, such as medial longitudinal fasciculus, medial lemniscus, superior cerebellar peduncle and corticospinal tracts, are more susceptible to DAI [14].
Axonal injury is thought to be due to stretching of axons causing physical disruption and cytoskeletal misalignment. This interferes with normal axonal transport, a key mechanism of delivering mitochondria, lipids, synaptic vesicles and proteins to and from a neuronal cell body. Axons are 1000- or 10000-times the length of the cell body. Since axons depend on axoplasmic transport for vital proteins and materials, injury to axons, such as DAI, leads to degeneration of distal axons, termed Wallerian degeneration. This takes place within a few days after trauma. Dysfunction of axonal transport is also related to neuronal deaths, leading to development of neurodegenerative disease. Patients with DAI often later present with cognitive dysfunction, suggesting that the injury of axonal transport contributes to one of the underlying mechanisms. The extent of DAI is reported to correlate well with prognosis as well as functional outcomes [15,16].
Diffuse axonal injury is classified based on severity: grade I: widespread axonal damage with no focal abnormality; grade II: grade I plus focal abnormality, especially corpus callosum; and grade III: grade I plus grade II, associated with damage in the brain stem [17].
CT scans often underestimate the extent of DAI, because the vast majority of DAI lesions are microscopic injuries and may not be grossly hemorrhagic. MR, particularly GRE or SWIs, are promising imaging techniques that can reveal the extent of abnormality correlating with prognosis. Recently, diffusion tensor imaging (DTI) has been a focus of imaging research, as DTI is a powerful in vivo imaging tool to assess white matter integrity.
Brain herniation
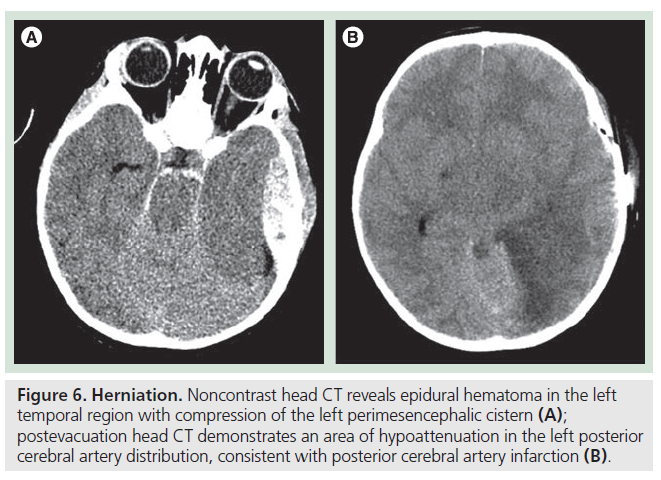
As a result of these various traumatic brain injuries, increasing edema and mass effect, if severe enough, causes brain herniation. There are several types of brain herniation observed in the trauma setting. The most common type is subfalcine herniation, where the cingulate gyrus of the frontal lobe is displaced underneath the falx cerebri when an expanding hematoma causes a substantial shift of midline structures. Branches of anterior cerebral artery are occasionally compressed by the subfalcine herniation, leading to infarction of anterior cerebral artery distribution. Uncal herniation involves the displacement of the medial temporal lobe, particularly the uncus and hippocampal gyrus over the ipsilateral edge of the tentorium cerebri. This type of herniation often results in compression of the third cranial nerve as well as the distal posterior cerebral artery (Figure 6). When uncal herniation is severe enough to displace and often rotate the midbrain, contralateral cerebral peduncle is compressed against the free edge of the tentorium, resulting in motor impairment of the ipsilateral body. This leads to false localizing sign clinically (right side lesion causes right side weakness, instead of left side weakness) and is termed Kernohan’s notch. The most severe herniation in the trauma setting is descending transtentorial herniation, which is the downward displacement of the basal nuclei and cerebral hemisphere through the tentorial notch. This happens in the setting of severe diffuse cerebral edema. On CT, lack of visualization of basilar cistern and effacement of perimesencephalic cistern is a critical finding. Duret hemorrhage, seen in the severe form of descending transtentorial herniation, is a brain stem (often pons) hemorrhage resulting from stretching and lacerating injury of pontine perforating vessels of the basilar artery. Severe TBI in the posterior fossa could lead to cerebellar tonsilar herniation, which is characterized by the cerebellar tonsils descending through the foramen magnum and compressing the medulla, leading to bradycardia and/or respiratory arrest.
Vascular injury
Vascular injury related to trauma has substantial consequence and implications for brain damage, either by ischemic infarction from flow limiting dissection or by thromboembolic stroke from mural thrombus at the site of dissection. Vascular injury was found to occur in approximately 1% of patients with trauma [6].
In the past, catheter angiogram was performed to address vascular dissection, which is an invasive procedure with 1–2% risk of having stroke related to angiogram. With recent advancement of multidetector row CT scan technology, the quality of CT angiography (CTA) images has markedly improved in recent years.
CTA is the best modality to evaluate the extent of vascular injury in the setting of acute trauma. CTA allows a rapid assessment of vascular narrowing and mural thrombus as well as the presence of pseudoaneurysm [18]. In addition, CTA reveals the relationship between vascular injury and fractures involving cervical spine and skull base. One of the shortcomings of CTA is the assessment of vascular injury near the skull base, or evaluation of densely calcified artery. Some researchers have reported that use of dual energy improves visualization of vascular structure near the skull base or assessment of stenosis at the site of calcification [19–21]. MR angiography is another modality used to assess vascular dissection, particularly when a patient presents with neurological symptoms or progression that requires brain MRI for stroke evaluation. Proton density images clearly show the presence of intimal thrombus as an area of hyperintensity.
The Biffl classification of vascular injury is commonly used to grade traumatic vascular dissection, as follows [22]:
• Grade I – intimal irregularity with less than 25% narrowing
• Grade II – dissection with more than 25% luminal narrowing
• Grade III – pseudoaneurysm
• Grade IV – occlusion
• Grade V – transection with extravasation
The prevalence of stroke associated with blunt carotid artery injury increases with the injury grade, with prevalence of 3, 11, 33, 44 and 100% associated with injury grades, I, II, III, IV and V, respectively. Early recognition of vascular injury and anticoagulation reduces the incidence of significant stroke. If the patient sustains traumatic injury to other parts of the body, precluding anticoagulation and transcranial Doppler examination shows substantial emboli, emergency stenting can be performed in the acute traumatic setting. Other than stroke symptoms, patients with carotid artery dissection/injury may present with an enlarging neck hematoma, unexplained neurological symptoms or Horner syndrome [6]. The majority of vertebral artery dissection is associated with cervical spine fracture. When cervical spine fracture involves transverse foramina or traumatic spondylolisthesis more than 5 mm potentially increasing stretching injury, CTA is indicated in order to assess the presence of vertebral artery dissection [18].
Although arteriogram is the gold standard for diagnosis of vascular injury, CTA or MR angiography can be safely used to diagnose vascular injury. Recent advancement of multidetector row CT scans and improved multiplanar reformat imaging techniques allow CTA to accurately detect significant vascular injury. CTA may have a limited sensitivity for Biffl Grade I injury, which probably does not adversely affect management of vascular injury.
Diagnostic imaging studies for TBI
As we discussed above, CT remains a vital tool in the assessment of patients with TBI in the acute setting, as it quickly provides critical information that directly impacts management of acute TBI patients. CT reveals a hematoma, midline shift, compression of ventricles, hydrocephalus and depressed fractures that necessitate surgical intervention. CT allows us to triage patients who needs surgical intervention versus conservative care.
Diagnostic accuracy of CT for TBI is difficult to assess as surgery or biopsy is not always performed for TBI, other than for evacuation of large hematomas. Imaging findings of CT are often compared with MRI as a gold standard or correlated with 6 months’ clinical outcome.
MRI is superior to CT in terms of sensitivity to detect subtle abnormalities and address injury in the posterior fossa and brainstem. MR is indicated for patients with neurological symptoms not explained by CT abnormality or mild TBI patients with persistent symptoms. A study comparing head CT performed within 24 h and 3T MR performed within 2 weeks after a mild TBI event in 36 patients found that MR detected intraparenchymal abnormalities in 75% of patients with mild TBI, a much higher rate than CT, predominantly consisting of traumatic axonal injury and cerebral contusions [23]. Another prospective study trying to define the role of MR in the acute trauma setting enrolled 123 patients with TBI. They reported that in 82 (67%) patients, the findings of CT and MRI were identical. In the remaining 41 patients, MR demonstrated subtle additional TBI findings, but this information did not affect clinical management. They concluded that head CT is the only imaging test necessary in the first 48 h after TBI [24].
When the initial head CT shows abnormality related to TBI, such as tSAH, parenchymal contusion or extra-axial hemorrhage, how often do we need to image in order to assess the stability of findings? Most case series investigating the role of serial head CT in TBI patients have suggested that they only occasionally provide valuable information. A decision analytic model indicated that awaiting clinical deterioration in a young patient with intracranial injury at the initial abnormal scan is not cost effective compared with routine follow-up head CT [25]. A prospective observational study addressing the natural course of hemorrhage and traumatic intracranial hematoma showed that increased traumatic intracranial hematoma occurs earlier in the post-traumatic course (within the first 24 h) [26]. Therefore, when the initial CT scan shows abnormality (e.g., hematoma or tSAH), follow-up head CT is recommended to assure stability of the abnormality. Clinical prediction rules might be helpful to define which patients need serial follow-up imaging and how often we should image.
Sensitivity and specificity of CT is lower than MRI with GRE or SWI. When head CT is normal, yet there is discrepancy between imaging findings and clinical symptoms, one should consider obtaining brain MRI for accurate assessment of TBI. Typical MRI protocols for TBI include T1-weighted sagittal images, coronal fluid attenuated inversion recovery (FLAIR) images, T2-weighted axial images, coronal GRE or SWI images and diffusion-weighted images (DWI). No intravenous contrast is necessary for assessment of TBI on MR.
The role of advanced imaging & future research direction
There has been an increase in research interests and necessity to address patients with mild TBI or concussion. As stated previously, the vast majority of TBI patients suffer from mild TBI or so-called concussion. Imaging using conventional CT or MR neither visualizes any structural abnormality nor predicts neurocognitive and functional outcomes of mild TBI patients [23]. It has been hypothesized that postconcussion symptoms are caused by microstructural damage of the brain due to sheering injury of deep white matter.
Diffusion tensor imaging promises to reveal traumatic axonal injury secondary to stretching or shearing forces. DTI provide a quantitative measure of the bulk motion of water molecular diffusion and is sensitive to spatial orientation of fiber tracts. Mean diffusivity (MD) is an index of the rate of diffusion averaged over all directions, also known as ADC. Fractional anisotropy (FA) is a measure of directionality of diffusion, ranging from 0 to 1. Axial diffusivity is the degree of diffusion along the fiber orientation; as opposed to radial diffusivity, which is the degree of diffusion orthogonal to the fiber orientation. When axons are injured, this will restrict axial diffusivity and possibly increase radial diffusivity, thus leading to decreased FA and increased MD. DTI is sensitive to detect subtle axonal injuries that are invisible on conventional MRI [27–29]. DTI detecting decreased FA values predicts future neurobehavioral outcomes of TBI patients [30–33]. Niogi et al. found the positive correlation between attention control and FA in left hemisphere anterior corona radiata, as well as memory performance and FA in the uncinate fasciculus [34].
There have been contradictory results of DTI in the setting of acute trauma. Some reported restricted diffusion (decreased MD) and increased FA in the acute stage of TBI [35,36] presumably related to increased water movement into the intracellular space, leading to cytotoxic edema, whereas others reported a reduction of FA and increased diffusivity [37,38].
Figure 6.Herniation. Noncontrast head CT reveals epidural hematoma in the left temporal region with compression of the left perimesencephalic cistern (A); postevacuation head CT demonstrates an area of hypoattenuation in the left posterior cerebral artery distribution, consistent with posterior cerebral artery infarction (B).
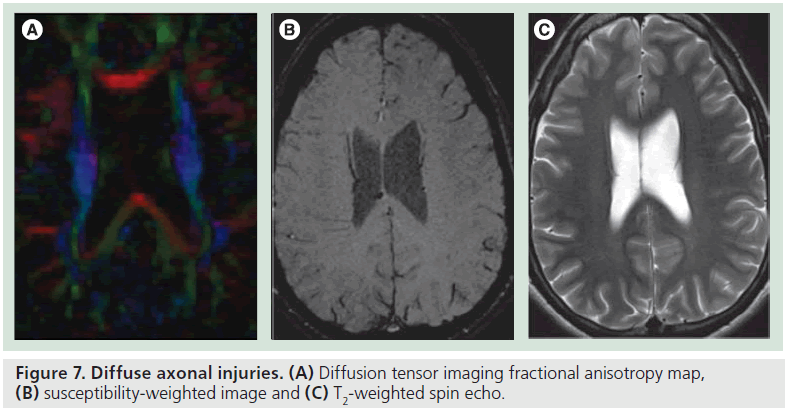
A recent report investigating DTI and magnetoencephalography (MEG) demonstrated that DTI or MEG detected abnormalities in patients with mild TBI and normal brain MR [39]. DTI reveals an area of reduced FA corresponding to subtle TBI, often invisible on conventional brain MR (Figure 7). It was reported that DTI or MEG is more sensitive than conventional MRI, but the specificity of these findings are yet to be determined. Moreover, the ability of these advanced imaging techniques to predict neuropsychological outcomes needs to be investigated in longitudinal prospective studies.
Previous functional MRI studies investigating mild TBI patients have shown altered patterns of activation during the performance of working memory tasks, consistent with reports of both increased activation in the dorsolateral prefrontal cortex and increased activation in the posterior parietal area, which is more apparent with increased task difficulty. This is consistent with the aggravation of postconcussion symptoms in demanding environments or situations at work or school. In addition, more dispersed activation and the recruitment of contralateral cerebral hemisphere during a paced auditory serial addition task indicates some brain plasticity counterbalance functional deficit. Further research is needed in order to verify functional MRI findings and establish the use of such information in the post-traumatic rehabilitation program.
Functional imaging studies using SPECT or PET have been used to evaluate patients with mild TBI where CT or MRI showed no detectable abnormalities [40,41]. Hypoperfusion on SPECT has been documented in medial temporal lobe and frontal lobe in 90% of 20 patients who had persistent neuropsychological symptoms after concussion. A FDG-PET study in 54 TBI patients demonstrated an initial brief response of hyperglycolysis followed by a relatively prolonged period of metabolic depression [42]. Correlation with neuropsychological tests and functional imaging study, however, remain variable, particularly in patients with mild TBI.
A brain SPECT study has also been used to monitor the neuronal fatigue among chronic TBI patients using the Paced Auditory Serial Addition Test (PASAT). They discovered that patients with mild TBI showed a larger area of supratentorial activation during the PASAT test, but a smaller area of cerebellar activation compared with healthly controls, suggestive of frontocerebellar dissociation, a potential explanation for cognitive fatigue in the chronic recovery phase of mild TBI [43].
One of the areas in which functional imaging studies may play a role is the assessment of the neurological rehabilitation program. Lewis et al. performed SPECT imaging for patients with chronic TBI who underwent the Holistic Approach to NeuroDevelopment and Learning Efficiency (HANDLE) and evaluated their brain perfusion patterns. The goal of HANDLE was to improve neurological and behavioral function by teaching TBI patients a series of physical and mental activities without drugs. They found increased cerebral blood flow in vermis and cerebellar hemisphere and anterior cingulate gyrus several months after the intervention, suggestive of involvement of cerebellum in the functional recovery after TBI [44].
Figure 7.Diffuse axonal injuries. (A) Diffusion tensor imaging fractional anisotropy map, (B) susceptibility-weighted image and (C) T2-weighted spin echo.
Intensive research is currently in progress for a new type of TBI among Iraq war Veterans suffering from postconcussive symptoms after blast exposure. Peskind et al. found a regional hypometabolism in cerebellum, vermis, pons and medial temporal lobe [45] using FDG-PET imaging. Further research on chronic TBI using functional imaging studies is warranted.
Role of imaging in the prediction of injury or outcome in TBI
As discussed in this article, imaging plays a major role for TBI patients, from triaging patients in the acute setting to functional abnormality in mild TBI or concussion patients where CT or MR is negative, as well as evaluation of neuropsychological rehabilitation in chronic TBI patients. However, caution is required in order to clarify who needs imaging among patients with head trauma, given ionizing radiation risk and overall medical costs involved. It is estimated that CT scans contribute approximately 45% of the US population’s collective radiation dose from all medical x-ray examinations. This is particularly serious among pediatric trauma patients. Even though radiation exposure is a concern for both adults and children, children are more sensitive to radiation than adults and have a longer life expectancy, leading to a larger window of opportunity for radiation-related illness. When the pediatric patient needs head CT after trauma, pediatric head CT protocol endorsed by many professional organizations (American College of Radiology, Society of Pediatric Radiology, American Academy of Pediatrics and American Academy of Family Physicians) is strongly encouraged [103].
Prediction rules for mild TBI: who needs to be imaged?
There have been several prediction rules for mild TBI patients, addressing who needs to be imaged or who would benefit from a head CT scan. Recently, discussion focus has shifted toward ‘who can safely avoid head CT’ without adverse consequences.
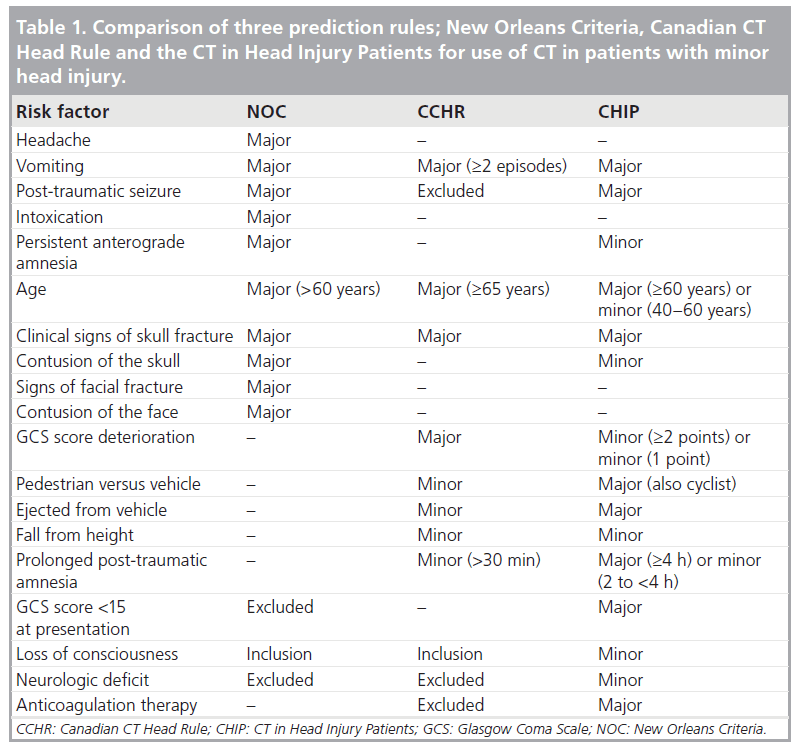
If a patient presents with moderate-to-severe head injury, head CT is indicated to assess the extent of injury and the need for surgical intervention. For patients with mild TBI (GCS <13 or higher), it is critically important to define a group of patients with TBI without missing a patient with significant TBI that necessitates surgical intervention. There are a few prediction rules for mild TBI. The most widely cited prediction rules include the New Orleans Criteria (NOC) [46], the Canadian Head CT Rule (CCHR) [47] and the CT in Head Injury Patients (CHIP) [48].
Clinical symptoms and risk factors commonly used in the clinical prediction rules are: age, headache, vomiting, intoxication, amnesia, post-traumatic seizure, signs of skull fracture, GCS score and GCS score deterioration after trauma. The primary outcome of interest is head CT abnormality and the secondary outcome is neurosurgical intervention. The summary of prediction rules is shown in Table 1. These prediction rules have different specificity; however, sensitivity for detecting lesions requiring neurosurgical intervention is 100%. Specificity for NOC, CCHR and CHIP are 24, 32 and 23%, respectively. A study comparing NOC and CCHR reported that both NOC and CCHR are highly sensitive for predicting neurosurgical intervention; however, CCHR is more specific than NOC [49]. One should keep in mind that a prediction rule can be used only as a decisionsupport system to complement clinical judgment and is not meant to replace clinical judgment.
A study focusing on pediatric head trauma in 2043 children [50,51] revealed that abnormal mental status, clinical signs of skull fracture, history of vomiting, scalp hematoma or headache identified 99% (95% CI: 94–100) of those with TBI on CT scan and 100% (95% CI: 97–100) of those with TBI requiring acute intervention. Of the 304 (24%) children undergoing CT who had none of these predictors, only 1 (0.3%; 95% CI: 0–1.8) had TBI on CT and that patient was discharged from the emergency department (ED) without complications. However, a prediction rule can be used only as a part of the decision support, as it can only complement, not replace, clinical judgment. Clearly there are medical–legal implications for not imaging trauma patients among ED physicians. If clinical suspicion is high enough, head CT is indicated regardless of the prediction rule. These prediction rules need to be validated in a separate cohort of pediatric head trauma populations, as radiation to children has substantially negative biological effects compared with that to adults.
Prediction of clinical outcomes based on CT
Another prediction rule is to predict clinical outcomes based on the initial head CT for patients with moderate-to-severe TBI. The classification of TBI into mild, moderate and severe is generally based on clinical evaluation using GCS. The increased use of early sedation, intubation and ventilation in moderate-to-severe TBI patients has decreased the value of the GCS score in evaluation. Alternatively, TBI is classified based on structural abnormalities on CT or MRI. The Marshall CT classification has been developed based on the investigation of 2269 patients to assess prediction of clinical outcomes after TBI [52]. The model discriminated well in the development population (AUC: 0.78–0.80) as well as in the external validity study (AUC: 0.83–0.89). CT findings included in the Marshall CT classification are: presence or absence of mass (hematoma) lesion; midline shift more than 5 mm; compressed or absent cistern; and presence or absence of evacuated mass lesions. Another more recent outcome prediction scale is the Rotterdam Score [53], developed by modification of the Marshall CT classification, focusing on CT criteria, including degree of compression of basilar cistern (normal: 0; partially effaced: 1; completely absent: 2), midline shift (less than 5 mm: 0; more than 5 mm: 1), tSAH or intraventricular hemorrhage (absent: 0; present: 1) and EDH (present: 0; absent: 1). Interestingly, the presence of EDH is protective, meaning patients with EDH have better clinical outcomes compared with those who did not have EDH. By adding one more point, the worst score is 6 and the best score is 1. The Rotterdam score is extremely easy to use and has a high degree of prediction of 6‑month mortality. The 6‑month mortality for scores 1 and 2 are 0 and 6.8% and that for scores 5 and 6 are 53 and 61%, respectively. The Rotterdam score improved the diagnostic accuracy of Marshall CT classification from AUC 0.67 to 0.71. The outcome prediction has an important implication for physicians and neurosurgeons working in the ED or with trauma patients when they are faced with patients’ family members for discussing prognosis and potential surgical intervention.
Future perspective of imaging in TBI
Standardization of trauma head CT interpretation & follow-up
As head CT provides pivotal information for patients with moderate and severe head trauma, more standardization of reporting and scoring of severity of TBI are expected. Instead of narrative descriptions of findings, the CT report will become more standardized and structured, facilitating effective communication to physicians and neurosurgeons working in the ED. Wide implementation of the Rotterdam Score, modified from original Marshall classification, can be valuable in order to provide an estimate of 6‑month mortality for severe TBI patients.
Another problem in current practice is the lack of a follow-up imaging policy for patients with positive TBI findings. How often do we need to scan these patients with intracranial hemorrhage in order to detect early expansion of hematoma or progression of cerebral edema? Heavy sedation and intubation of these severe TBI patients makes clinical assessment less valuable, leading to almost routine head CT for neurointensive care unit patients. There are substantial variations of practice in this regard. Certain guidelines would be beneficial in order to comply with judicious use of radiation and target medical imaging resources to those who benefit from them.
Advanced MR application for mild TBI/concussion patients
Conventional CT and MR are not sensitive enough to detect subtle brain abnormality among patients with mild TBI or postconcussion with psychological and cognitive impairment. They have a wide variety of somatic, psychological and cognitive, and emotional symptoms, such as cognitive fatigue, and memory and attention deficits. The presence of these symptoms for more than 3 months after trauma is considered as postconcussion syndrome. We need to develop objective imaging tests that allow us to visualize brain injury, not only by group analysis with control, but at an individual patient level. DTI is a promising technique that allows assessment of white matter integrity by measuring fractional anisotropy or mean diffusivity.
Certain anatomical locations that are commonly abnormal on DTI in the trauma setting include uncinate, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, internal capsule and corpus callosum. These findings support the notion of microstructual injury in white matter, potentially disrupting axonal transport in postconcussion syndrome patients. Future research is needed in order to address the impact of axonal transport injury on development of neurodegenerative disease.
Development of imaging techniques to evaluate therapeutic efficacy of preclinical drugs
Beyond the acute phase of TBI management, there is no effective treatment to promote functional recovery for patients with TBI [54]. The most prevalent and disturbing aspects of chronic TBI among survivors are cognitive deficits and somatosensory dysfunction. TBI is also considered an epigenetic risk factor for Alzheimer disease [55]. Any treatment to facilitate functional recovery would be of great clinical and socioeconomic benefits. Many neuroprotective agents, stem cells or bone marrow stromal cells treatment, promotion of angiogenesis, neurogenesis and synaptogenesis have been tested, yet much work needs to be completed before observing the improved clinical outcomes.
Imaging is a powerful tool to visualize inside human body/brain and in vivo monitoring of pharmakokinetics. Future emphasis of imaging research in trauma patients should have a strong connection with advancement of treatment for TBI patients. Early use of imaging in preclinical trials would benefit efficient use of this resource among pharmaceutical industries.

Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Langlois JA, Rutland-Brown W, Wald MM: The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 (2006).
- Kelly DF, Martin NA, Kordestani R et al.: Cerebral blood flow as a predictor of outcome following traumatic brain injury. J. Neurosurg. 86, 633–641 (1997).
- Young JS, Blow O, Turrentine F, Claridge JA, Schulman A: Is there an upper limit of intracranial pressure in patients with severe head injury if cerebral perfusion pressure is maintained? Neurosurg. Focus 15, E2 (2003).
- Bor-Seng-Shu E, Aguiar PH, de Almeida Leme RJ, Mandel M, Andrade AF, Marino R Jr: Epidural hematomas of the posterior cranial fossa. Neurosurg. Focus 16, ECP1 (2004).
- Gerlach R, Dittrich S, Schneider W, Ackermann H, Seifert V, Kieslich M: Traumatic epidural hematomas in children and adolescents: outcome analysis in 39 consecutive unselected cases. Pediatr. Emerg. Care 25, 164–169 (2009).
- Nedeltchev K, Baumgartner RW: Traumatic cervical artery dissection. Front Neurol. Neurosci. 20, 54–63 (2005).
- Nagurney JT, Borczuk P, Thomas SH: Elderly patients with closed head trauma after a fall: mechanisms and outcomes. J. Emerg. Med. 16, 709–713 (1998).
- Servadei F, Murray GD, Teasdale GM et al.: Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery 50, 261–267; discussion 267–269 (2002).
- Anon J, Remonda L, Spreng A et al.: Traumatic extra-axial hemorrhage: correlation of postmortem MSCT, MRI, and forensicpathological findings. J. Magn. Reson. Imaging 28, 823–836 (2008).
- Thomas B, Somasundaram S, Thamburaj K et al.: Clinical applications of susceptibility weighted MR imaging of the brain – a pictorial review. Neuroradiology 50, 105–116 (2008).
- Kawamata T, Mori T, Sato S, Katayama Y: Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg. Focus 22(5), E5 (2007).
- Chieregato A, Fainardi E, Morselli‑Labate AM et al.: Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery 56, 671–680 (2005).
- Ono J, Yamaura A, Kubota M, Okimura Y, Isobe K: Outcome prediction in severe head injury: analyses of clinical prognostic factors. J. Clin. Neurosci. 8, 120–123 (2001).
- Shukla D, Mahadevan A, Sastry KV, Shankar SK: Pathology of post traumatic brainstem and hypothalamic injuries. Clin. Neuropathol. 26, 197–209 (2007).
- Wang H, Duan G, Zhang J, Zhou D: Clinical studies on diffuse axonal injury in patients with severe closed head injury. Chin. Med. J. 111, 59–62 (1998).
- Jackson JC, Obremskey W, Bauer R et al.: Long-term cognitive, emotional, and functional outcomes in trauma intensive care unit survivors without intracranial hemorrhage. J. Trauma 62, 80–88 (2007).
- Bigler ED, Clark E, Farmer J: Traumatic brain injury: 1990s update – introduction to the special series. J. Learn. Disabil. 29, 512–513 (1996).
- Sliker CW: Blunt cerebrovascular injuries: imaging with multidetector CT angiography. Radiographics 28, 1689–1708 (2008).
- Zhang LJ, Wu SY, Poon CS et al.: Automatic bone removal dual-energy CT angiography for the evaluation of intracranial aneurysms. J. Comput. Assist. Tomogr. 34, 816–824.
- Korn A, Bender B, Thomas C et al.: Dual energy CTA of the carotid bifurcation: advantage of plaque subtraction for assessment of grade of the stenosis and morphology. Eur. J. Radiol. DOI:10.1016/j. ejrad.2010.08.028 (2010) (Epub ahead of print).
- Uotani K, Watanabe Y, Higashi M et al.: Dual-energy CT head bone and hard plaque removal for quantification of calcified carotid stenosis: utility and comparison with digital subtraction angiography. Eur. Radiol. 19, 2060–2065 (2009).
- Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM: Blunt carotid arterial injuries: implications of a new grading scale. J. Trauma 47, 845–853 (1999).
- Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P: Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J. Neurotrauma 25, 1049–1056 (2008).
- Manolakaki D, Velmahos GC, Spaniolas K, de Moya M, Alam HB: Early magnetic resonance imaging is unnecessary in patients with traumatic brain injury. J. Trauma 66, 1008–1012 (2009).
- Stein SC, Fabbri A, Servadei F: Routine serial computed tomographic scans in mild traumatic brain injury: when are they cost-effective? J. Trauma 65, 66–72 (2008).
- Narayan RK, Maas AI, Servadei F, Skolnick BE, Tillinger MN, Marshall LF: Progression of traumatic intracerebral hemorrhage: a prospective observational study. J. Neurotrauma 25, 629–639 (2008).
- Niogi SN, Mukherjee P, Ghajar J et al.: Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 29, 967–973 (2008).
- Kou Z, Wu Z, Tong KA et al.: The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J. Head Trauma Rehabil. 25, 267–282.
- Kumar R, Gupta RK, Husain M et al.: Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 23, 675–685 (2009).
- Levin HS, Wilde EA, Chu Z et al.: Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 23, 197–208 (2008).
- Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M: Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 22, 115–122 (2008).
- Tollard E, Galanaud D, Perlbarg V et al.: Experience of diffusion tensor imaging and 1H spectroscopy for outcome prediction in severe traumatic brain injury: preliminary results. Crit. Care Med. 37, 1448–1455 (2009).
- Wozniak JR, Krach L, Ward E et al.: Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 22, 555–568 (2007).
- Niogi SN, Mukherjee P, Ghajar J et al.: Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131, 3209–3221 (2008).
- Wilde EA, McCauley SR, Hunter JV et al.: Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 (2008).
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D: Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma 24, 1447–1459 (2007).
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME: Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 23, 794–802 (2002).
- Inglese M, Makani S, Johnson G et al.: Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 103, 298–303 (2005).
- Huang M, Theilmann RJ, Robb A et al.: Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 26(8), 1213–1226 (2009).
- Umile EM, Sandel ME, Alavi A, Terry CM, Plotkin RC: Dynamic imaging in mild traumatic brain injury: support for the theory of medial temporal vulnerability. Arch. Phys. Med. Rehabil. 83, 1506–1513 (2002).
- Nedd K, Sfakianakis G, Ganz W et al.: 99mTc-HMPAO SPECT of the brain in mild to moderate traumatic brain injury patients: compared with CT – a prospective study. Brain Inj. 7, 469–479 (1993).
- Bergsneider M, Hovda DA, McArthur DL et al.: Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J. Head Trauma Rehabil. 16, 135–148 (2001).
- Hattori N, Swan M, Stobbe GA et al.: Differential SPECT activation patterns associated with PASAT performance may indicate frontocerebellar functional dissociation in chronic mild traumatic brain injury. J. Nucl. Med. 50, 1054–1061 (2009).
- Lewis DH, Bluestone JP, Savina M, Zoller WH, Meshberg EB, Minoshima S: Imaging cerebral activity in recovery from chronic traumatic brain injury: a preliminary report. J. Neuroimaging 16, 272–277 (2006).
- Peskind ER, Petrie EC, Cross DJ et al.: Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage 54(Suppl. 1), S75–S82 (2010).
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM: Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 343, 100–105 (2000).
- Stiell IG, Wells GA, Vandemheen K et al.: The Canadian CT Head Rule for patients with minor head injury. Lancet 357, 1391–1396 (2001).
- Smits M, Dippel DW, Steyerberg EW et al.: Predicting intracranial traumatic findings on computed tomography in patients with minor head injury: the CHIP prediction rule. Ann. Intern. Med. 146, 397–405 (2007).
- Stiell IG, Clement CM, Rowe BH et al.: Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA 294, 1511–1518 (2005).
- Maguire JL, Boutis K, Uleryk EM, Laupacis A, Parkin PC: Should a head-injured child receive a head CT scan? A systematic review of clinical prediction rules. Pediatrics 124, E145–E154 (2009).
- Palchak MJ, Holmes JF, Vance CW et al.: A decision rule for identifying children at low risk for brain injuries after blunt head trauma. Ann. Emerg. Med. 42, 492–506 (2003).
- Marshall LF, Marshall SB, Klauber MR et al.: The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9(Suppl. 1), S287–S292 (1992).
- Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW: Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57, 1173–1182 (2005).
- Xiong Y, Mahmood A, Chopp M: Emerging treatments for traumatic brain injury. Expert Opin. Emerg. Drugs 14, 67–84 (2009). 55 Schofield PW, Tang M, Marder K et al.: Alzheimer’s disease after remote head injury: an incidence study. J. Neurol. Neurosurg. Psychiatry 62, 119–124 (1997).
Websites
- Injury Prevention & Control: Traumatic Brain Injury www.cdc.gov/TraumaticBrainInjury/index. html
- National Institute of Neurological Disorders and Stroke; Geiger W, Damasio H: Traumatic brain injury: hope through research (2002) www.ninds.nih.gov/disorders/tbi/tbi.htm
- The Alliance For Radation Safety In Pediatric Imaging www.imagegently.org