Review Article - Imaging in Medicine (2014) Volume 6, Issue 1
FDG-PET/CT in the evaluation of paraneoplastic neurologic syndromes
Steven J Sherry1, Abdel K Tahari2, Sahar Mirpour2, Andrew Colucci1 & Rathan M Subramaniam*,1,2,3,41Department of Radiology, Boston University School of Medicine, Boston, MA, USA
2Russell H Morgan Department of Radiology & Radiologic Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
3Russell H Morgan Department of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
4Russell H Morgan Department of Otolaryngology & Head & Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- Corresponding Author:
- M Subramaniam
Department of Radiology
Boston University School of Medicine
Boston, MA, USA
Tel: +1 410 502 3956
Fax: +1 443 287 2933
E-mail: rsubram4@jhmi.edu
Abstract
Paraneoplastic neurologic syndromes (PNS) are a rare, heterogeneous group of complications associated with malignancy, unrelated to direct tumor invasion or metastasis, that may be immune-mediated. The aim of this article is to provide an overview of PNS and outline the value of 2-deoxy-2-[18F]fluoro-D-glucose PET/CT in the evaluation of patients with a PNS. 2-deoxy-2-18F-fluoro-D-glucose PET/CT is valuable to identify or exclude an occult malignancy in patients with suspected paraneoplastic syndromes.
Keywords
occult malignancy • paraneoplastic syndromes • PET/CT
Paraneoplastic neurological syndromes (PNS) are a rare, heterogeneous group of complications associated with malignancy, unrelated to direct tumor invasion or metastasis, that may be immune-mediated [1]. The development of PNS may antedate the diagnosis of cancer by several months or years [2,3]. Therefore, early localization of the initiating cancer can play a crucial role in establishing a prompt and accurate diagnosis. The aim of this article is to provide an overview of PNS and outline the value of imaging with a focus on 2-deoxy-2-18F-fluoro-dglucose (FDG) PET/CT in the management of PNS.
Overview of PNS
Pathogenesis
PNS are a collection of secondary disorders that are hypothesized to occur in patients with various forms of cancer. PNS have been documented to affect the CNS (e.g., limbic encephalitis, cerebellar degeneration, opsoclonus myoclonus and optic neuritis, among others), the peripheral nervous system (e.g., Guillain–Barré syndrome and autonomic neuropathy, among others), and the neuromuscular junction and muscle (e.g., Lambert–Eaton myasthenic syndrome, dermatomyositis and polymyositis, among others) [4].
The specific pathophysiology underlying the various subtypes of PNS is varied and poorly understood; however, autoimmune phenomena appear to be common. More specifically, antibodies [5] and T cells [6,7] responding to a cancer (often early stage or occult) are thought to develop crossreactivity. This crossreactivity can lead to an attack on homologous ectopic antigens, exclusively expressed by the central and peripheral nervous systems [8,9]. It is believed that an autoimmune response to normal self-antigens occurs because cancer cells process autoantigens in a different fashion, thereby making them reactive targets [10].
Conventional diagnostics
Owing to the heterogeneity of PNS presentations and the mild nature of early symptoms, quick and accurate diagnosis is often difficult. With this difficulty in mind, an international panel of neurologists proposed a rigorous set of clinically diagnostic criteria in 2004 [11]. By utilizing a patient’s clinical presentation, cancer workup data, and response to both immunologic and cancer therapies, these criteria try to risk-stratify patients into PNS ‘definite’ or ‘possible’ diagnostic categories.
To augment the clinical diagnosis, serum antibodies associated with PNS are routinely screened in suspected patients. Of these antibodies, some are fully characterized, and others are only partially characterized, requiring further investigation (e.g., PCA2 and antibipolar cells of the retina, among others). Antibodies that are well characterized attack antigens whose molecular identity is already known and understood. These well-characterized antibodies are specifically pathologic, and are rarely found in patients without cancer and/or PNS [12,13].
While serum antibody screens can add important clinical data to the diagnosis of PNS, there are multiple well-established caveats. First, it has been established that cancer patients without PNS may still demonstrate a positive serum antibody screen for specific PNS-involved antibodies [14,15]. Furthermore, certain antibodies are associated with multiple PNS subtypes, and certain PNS subtypes are associated with multiples types of antibodies. In addition, several PNS antibodies may occur simultaneously in the same patient (e.g., in patients with small-cell lung cancer) [16,17]. Finally, it has been shown that positive antibody screens do not always differentiate between the paraneoplastic and the nonparaneoplastic causes of their associated syndromes. For example, patients with antiamphiphysin antibodies typically have the associated stiff-person syndrome, but frequently do not have an associated cancer (and hence do not have PNS) [18,19].
In light of these various shortcomings with serum antibody screens, it is imperative to look for other, more precise methods for diagnosing patients with PNS. Lumbar puncture with the intent of screening the cerebrospinal fluid for paraneoplastic antibodies has been suggested, but in most cases, these antibodies are found in the serum as well [20]. Other efforts have looked into utilizing electrophysiology for further diagnostic characterization of PNS. However, while certain syndromes (e.g., myasthenia gravis) are associated with characteristic electrophysiological findings, this modality does not allow one to differentiate between the PNS-associated syndromes and their non-PNS counterparts.
Finally, brain MRI can aid in the diagnosis of specific PNS subtypes, such as limbic encephalitis and cerebellar degeneration [21,22]. In the majority of PNS subtypes, however, brain MRI demonstrates normal or nonspecific radiologic changes. Previous studies demonstrated that morphological cross-sectional imaging modalities, such as CT or MRI, have a low yield in the localization of cancer in cases of PNS, since lesions may be small or limited to metastatic lymph nodes [23].
Role of FDG-PET & PET/CT in PNS
In many patients, PNS is diagnosed before the discovery of a primary cancer. In these patients, PET/CT can evaluate patients with suspected PNS to detect an occult malignancy and identify metabolic brain abnormalities confirming PNS. FDG-PET/CT is useful in the diagnosis, therapy assessment and follow-up of patients with many human solid tumors [24–30]. A number of retrospective and prospective trials have agreed on the utility of FDG-PET in the setting of PNS (Table 1), and especially in the event of an unknown primary tumor [31,32] or inflammatory etiology (Figures 1–4). However, differing results have resulted in two recommendations for the application of FDG-PET in PNS: imaging when PNS is clinically suspected, or imaging only in cases of antibody-proven PNS.
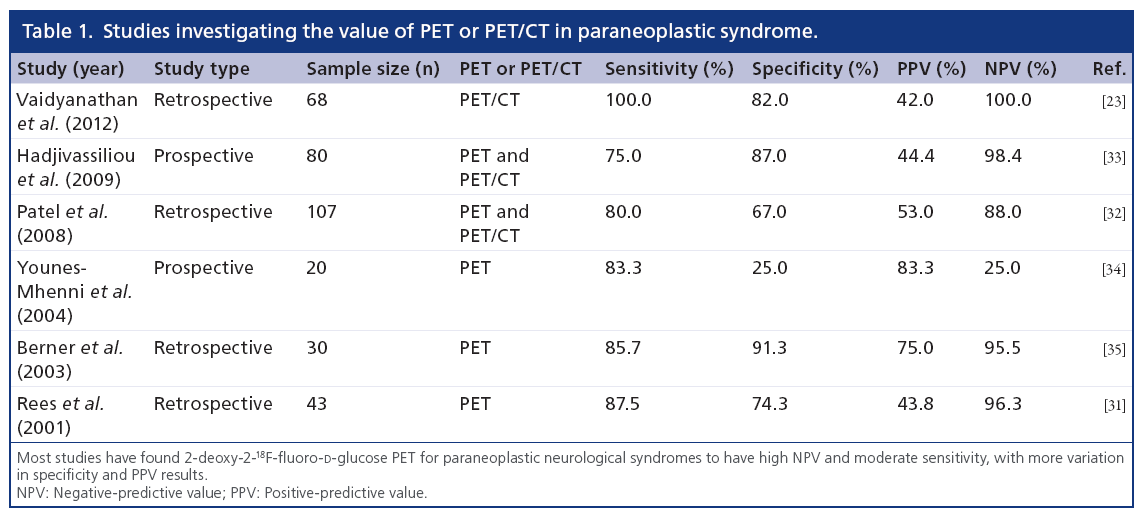
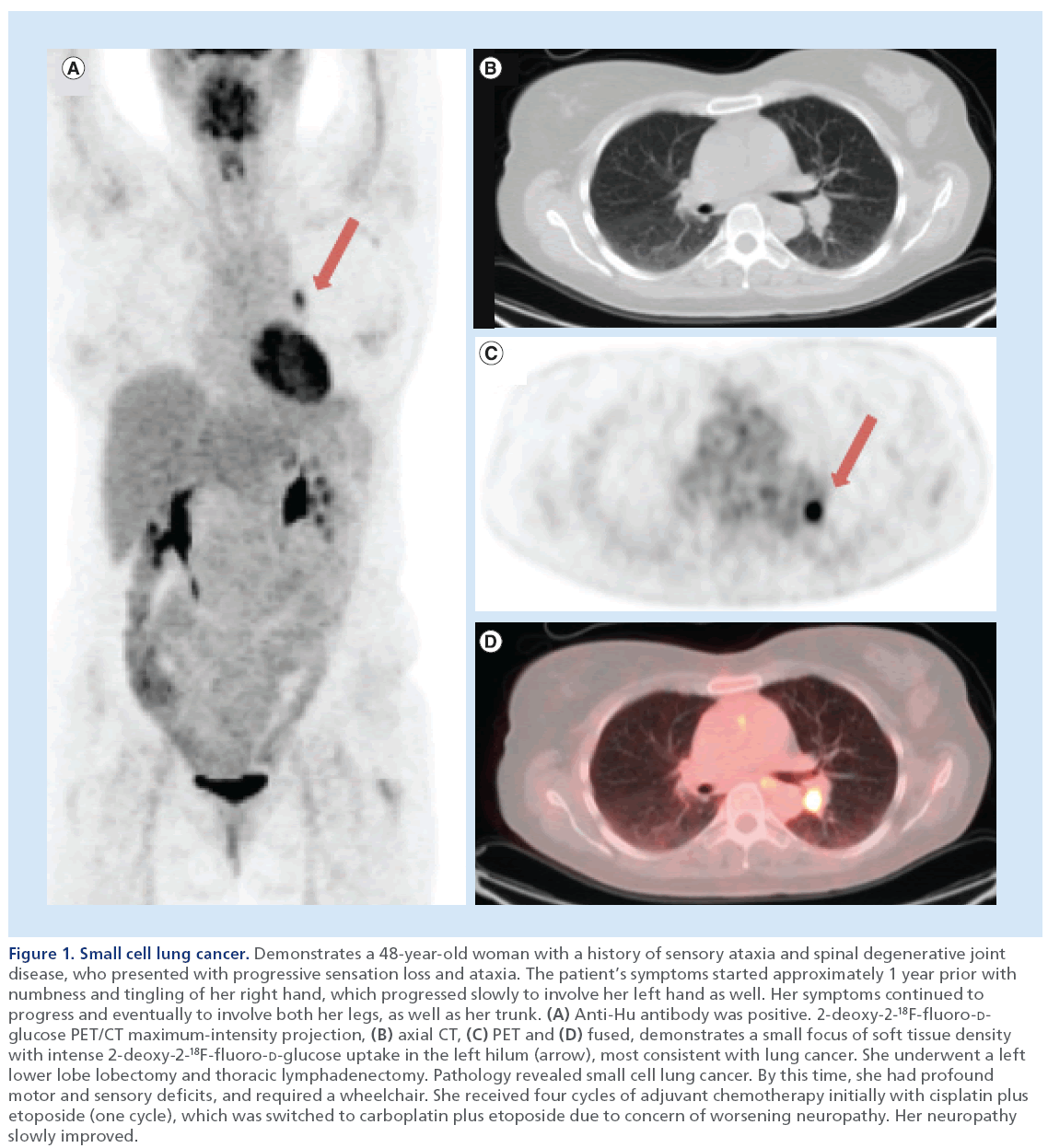
Figure 1: Small cell lung cancer. Demonstrates a 48-year-old woman with a history of sensory ataxia and spinal degenerative joint disease, who presented with progressive sensation loss and ataxia. The patient’s symptoms started approximately 1 year prior with numbness and tingling of her right hand, which progressed slowly to involve her left hand as well. Her symptoms continued to progress and eventually to involve both her legs, as well as her trunk. (A) Anti-Hu antibody was positive. 2-deoxy-2-18F-fluoro-Dglucose PET/CT maximum-intensity projection, (B) axial CT, (C) PET and (D) fused, demonstrates a small focus of soft tissue density with intense 2-deoxy-2-18F-fluoro-D-glucose uptake in the left hilum (arrow), most consistent with lung cancer. She underwent a left lower lobe lobectomy and thoracic lymphadenectomy. Pathology revealed small cell lung cancer. By this time, she had profound motor and sensory deficits, and required a wheelchair. She received four cycles of adjuvant chemotherapy initially with cisplatin plus etoposide (one cycle), which was switched to carboplatin plus etoposide due to concern of worsening neuropathy. Her neuropathy slowly improved.
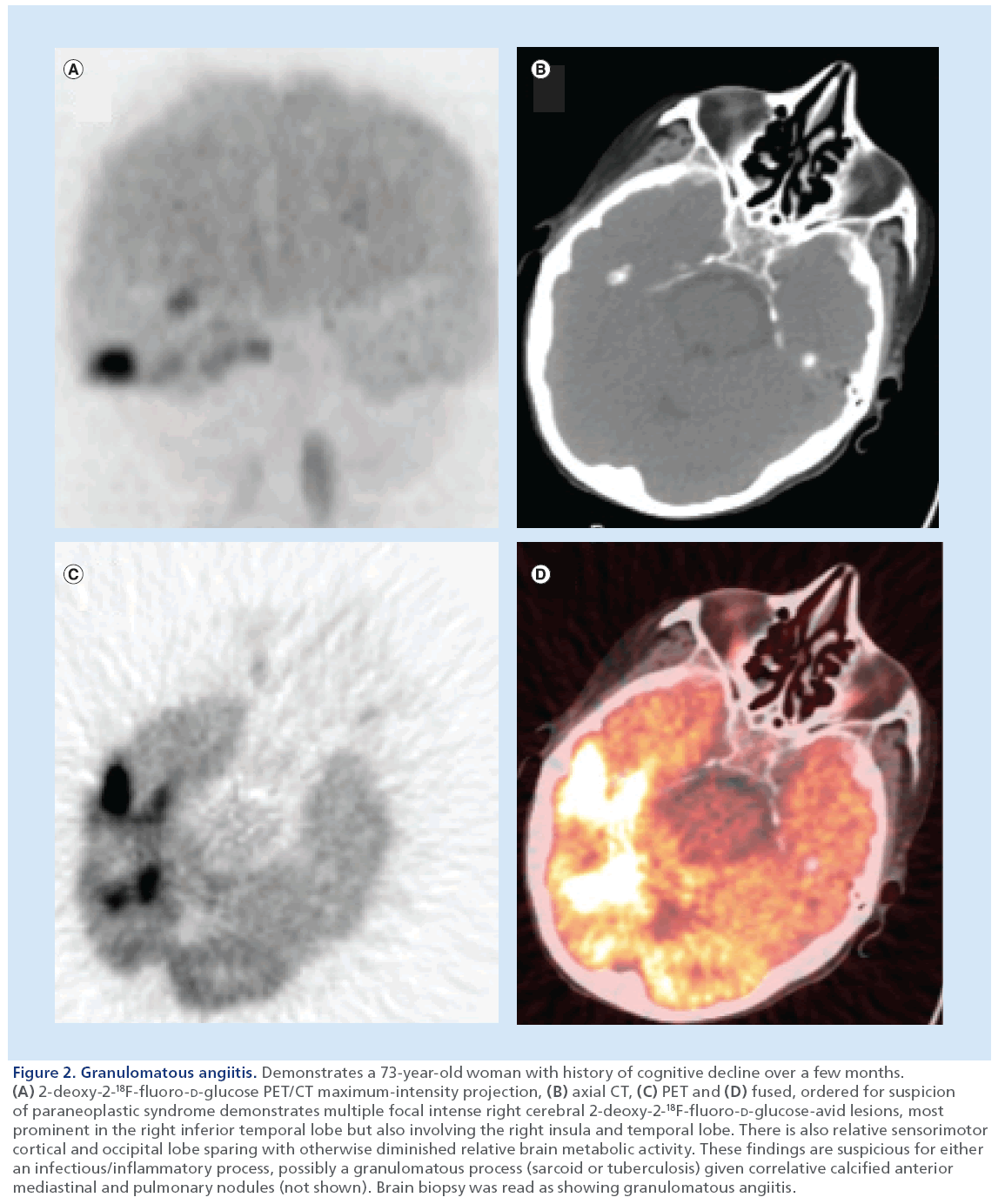
Figure 2: Granulomatous angiitis. Demonstrates a 73-year-old woman with history of cognitive decline over a few months. (A) 2-deoxy-2-18F-fluoro-D-glucose PET/CT maximum-intensity projection, (B) axial CT, (C) PET and (D) fused, ordered for suspicion of paraneoplastic syndrome demonstrates multiple focal intense right cerebral 2-deoxy-2-18F-fluoro-D-glucose-avid lesions, most prominent in the right inferior temporal lobe but also involving the right insula and temporal lobe. There is also relative sensorimotor cortical and occipital lobe sparing with otherwise diminished relative brain metabolic activity. These findings are suspicious for either an infectious/inflammatory process, possibly a granulomatous process (sarcoid or tuberculosis) given correlative calcified anterior mediastinal and pulmonary nodules (not shown). Brain biopsy was read as showing granulomatous angiitis.
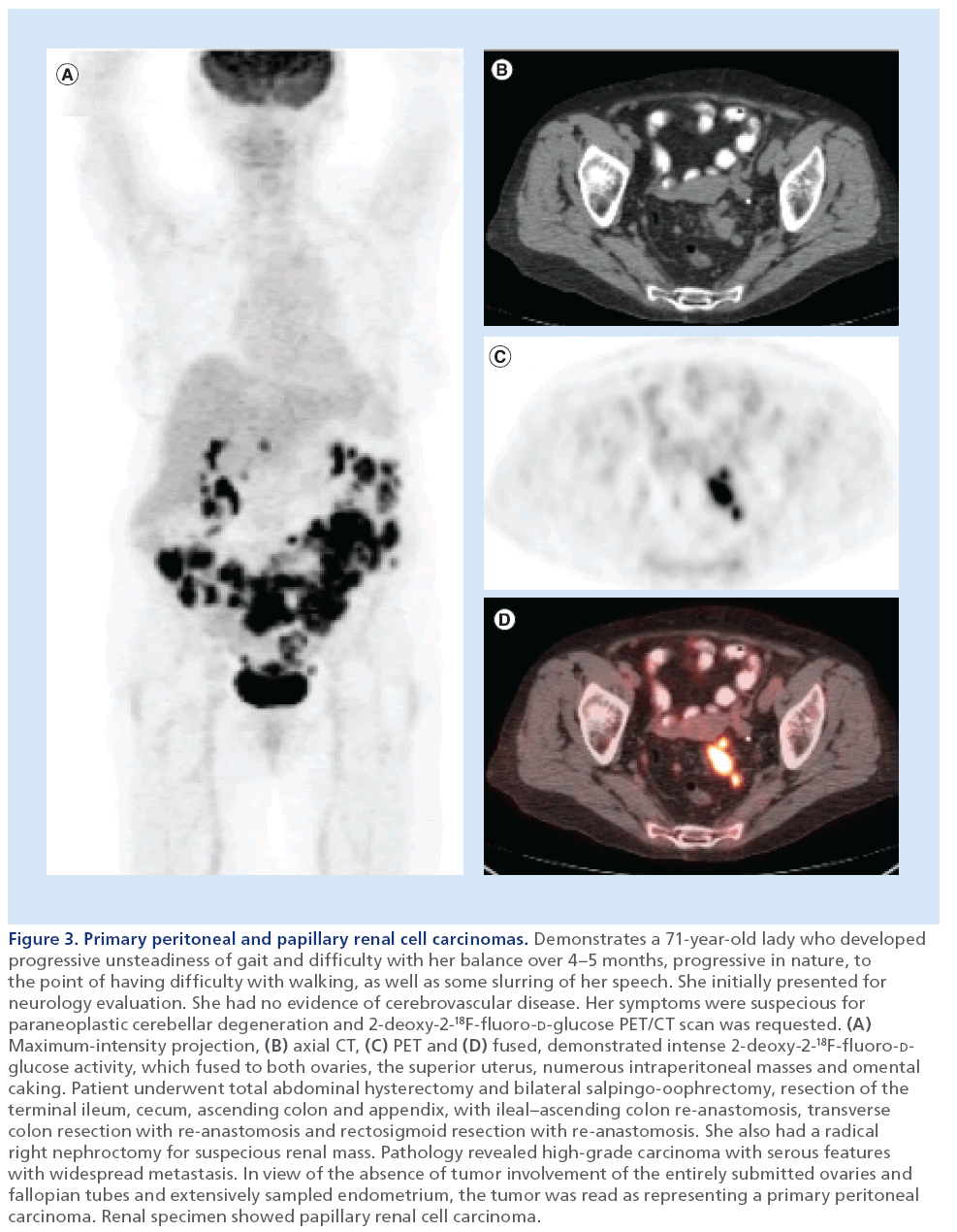
Figure 3: Primary peritoneal and papillary renal cell carcinomas. Demonstrates a 71-year-old lady who developed progressive unsteadiness of gait and difficulty with her balance over 4–5 months, progressive in nature, to the point of having difficulty with walking, as well as some slurring of her speech. She initially presented for neurology evaluation. She had no evidence of cerebrovascular disease. Her symptoms were suspecious for paraneoplastic cerebellar degeneration and 2-deoxy-2-18F-fluoro-D-glucose PET/CT scan was requested. (A) Maximum-intensity projection, (B) axial CT, (C) PET and (D) fused, demonstrated intense 2-deoxy-2-18F-fluoro-Dglucose activity, which fused to both ovaries, the superior uterus, numerous intraperitoneal masses and omental caking. Patient underwent total abdominal hysterectomy and bilateral salpingo-oophrectomy, resection of the terminal ileum, cecum, ascending colon and appendix, with ileal–ascending colon re-anastomosis, transverse colon resection with re-anastomosis and rectosigmoid resection with re-anastomosis. She also had a radical right nephroctomy for suspecious renal mass. Pathology revealed high-grade carcinoma with serous features with widespread metastasis. In view of the absence of tumor involvement of the entirely submitted ovaries and fallopian tubes and extensively sampled endometrium, the tumor was read as representing a primary peritoneal carcinoma. Renal specimen showed papillary renal cell carcinoma.
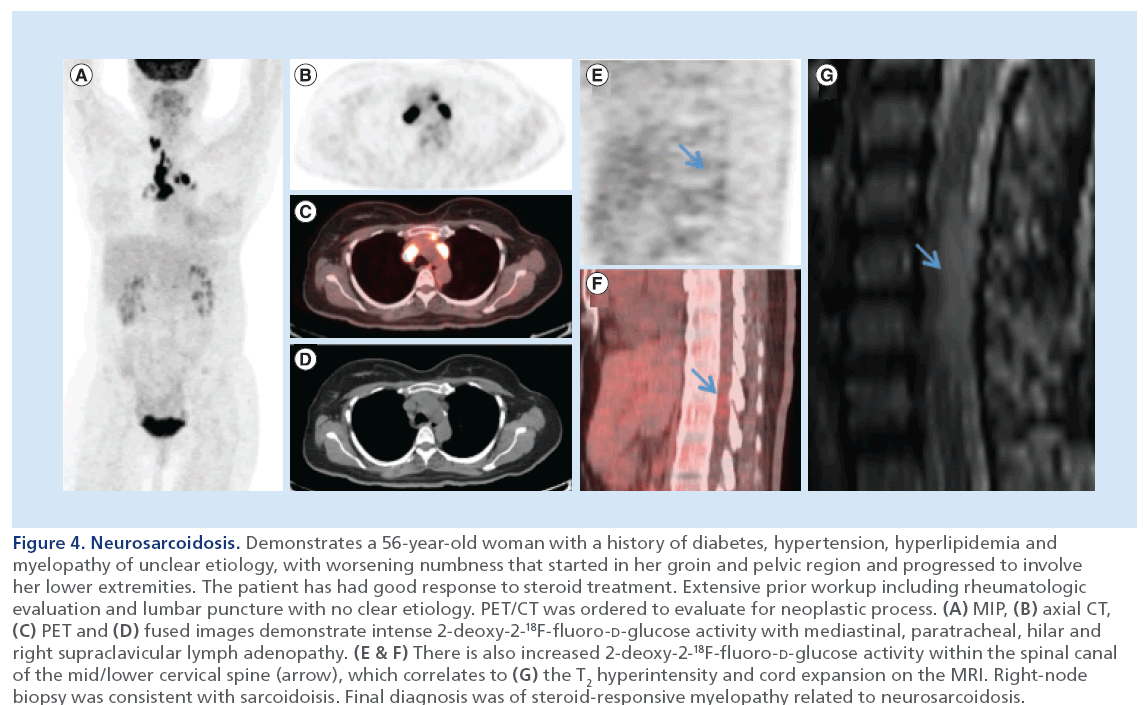
Figure 4: Neurosarcoidosis. Demonstrates a 56-year-old woman with a history of diabetes, hypertension, hyperlipidemia and myelopathy of unclear etiology, with worsening numbness that started in her groin and pelvic region and progressed to involve her lower extremities. The patient has had good response to steroid treatment. Extensive prior workup including rheumatologic evaluation and lumbar puncture with no clear etiology. PET/CT was ordered to evaluate for neoplastic process. (A) MIP, (B) axial CT, (C) PET and (D) fused images demonstrate intense 2-deoxy-2-18F-fluoro-D-glucose activity with mediastinal, paratracheal, hilar and right supraclavicular lymph adenopathy. (E & F) There is also increased 2-deoxy-2-18F-fluoro-D-glucose activity within the spinal canal of the mid/lower cervical spine (arrow), which correlates to (G) the T2 hyperintensity and cord expansion on the MRI. Right-node biopsy was consistent with sarcoidoisis. Final diagnosis was of steroid-responsive myelopathy related to neurosarcoidosis.
Rees et al. [31] retrospectively reviewed the records of 43 unselected patients with suspected PNS referred for FDG-PET scanning to determine the value of this technique when conventional imaging was negative. Of these, 16 patients had PET scans consistent with malignancy (37%) and 27 had negative scans. All but one of the patients with a negative PET result remained free of malignancy on prolonged followup. They concluded specifically that FDG-PET was useful for detecting small lesions in the setting of suspected PNS, and that false-positive or -negative studies do not occur at a clinically problematic rate.
Berner et al. found a high specificity and negativepredictive value (NPV) and determined that FDG-PET is helpful in the diagnosis of patients with suspected PNS (Figures 5 & 6) [35]. In this study, out of 30 patients with suspected PNS, seven showed malignancy (23%). Six out of the seven were positive by PET (sensitivity: 86%). PET correctly ruled out underlying malignancy in 21 out of 23 patients (specificity: 91%). McKeon et al. [36] studied 56 patients with clinically suspected PNS who underwent PET/CT after negative standard evaluations. These investigators demonstrated that the use of PET/CT increased the diagnostic yield for cancer by 18% in patients with suspected PNS for whom results of standard oncologic tests were negative.
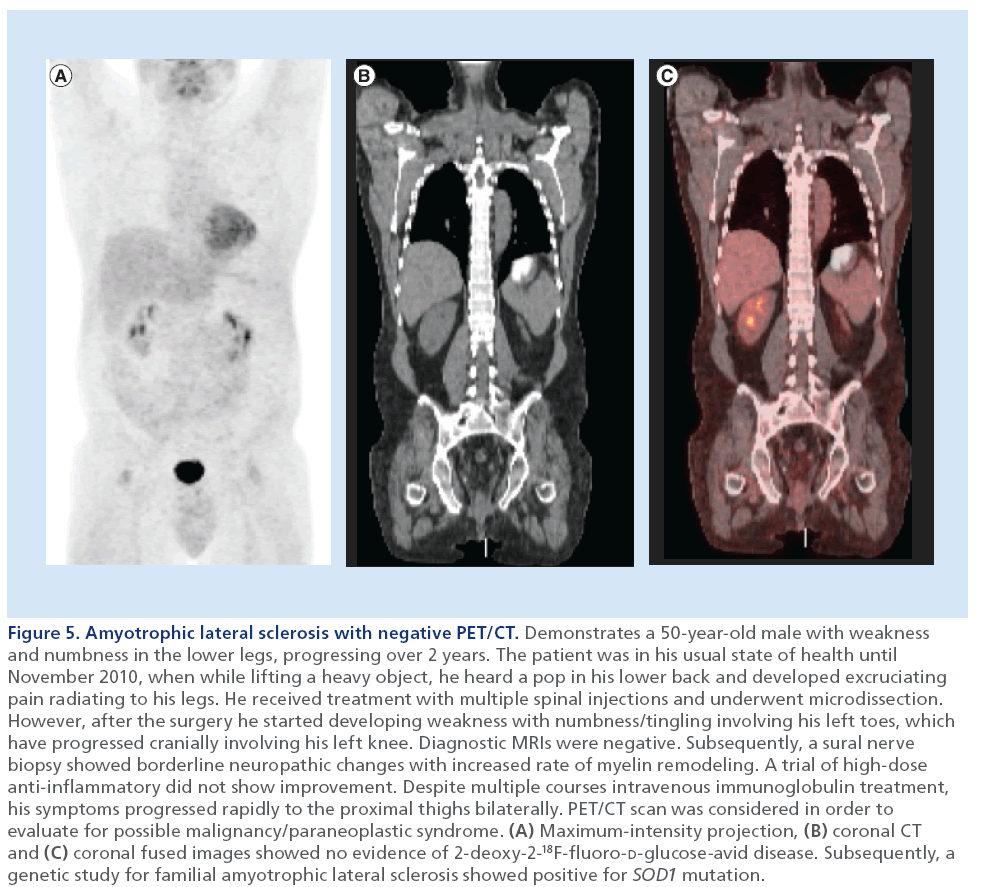
Figure 5: Amyotrophic lateral sclerosis with negative PET/CT. Demonstrates a 50-year-old male with weakness and numbness in the lower legs, progressing over 2 years. The patient was in his usual state of health until November 2010, when while lifting a heavy object, he heard a pop in his lower back and developed excruciating pain radiating to his legs. He received treatment with multiple spinal injections and underwent microdissection. However, after the surgery he started developing weakness with numbness/tingling involving his left toes, which have progressed cranially involving his left knee. Diagnostic MRIs were negative. Subsequently, a sural nerve biopsy showed borderline neuropathic changes with increased rate of myelin remodeling. A trial of high-dose anti-inflammatory did not show improvement. Despite multiple courses intravenous immunoglobulin treatment, his symptoms progressed rapidly to the proximal thighs bilaterally. PET/CT scan was considered in order to evaluate for possible malignancy/paraneoplastic syndrome. (A) Maximum-intensity projection, (B) coronal CT and (C) coronal fused images showed no evidence of 2-deoxy-2-18F-fluoro-D-glucose-avid disease. Subsequently, a genetic study for familial amyotrophic lateral sclerosis showed positive for SOD1 mutation.
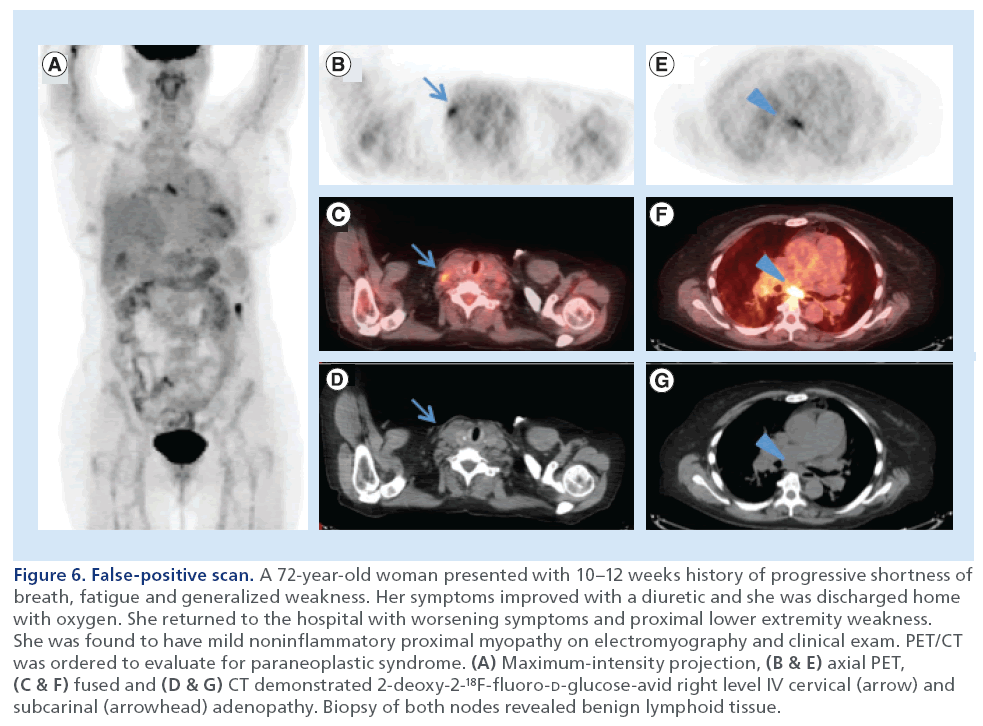
Figure 6: False-positive scan. A 72-year-old woman presented with 10–12 weeks history of progressive shortness of breath, fatigue and generalized weakness. Her symptoms improved with a diuretic and she was discharged home with oxygen. She returned to the hospital with worsening symptoms and proximal lower extremity weakness. She was found to have mild noninflammatory proximal myopathy on electromyography and clinical exam. PET/CT was ordered to evaluate for paraneoplastic syndrome. (A) Maximum-intensity projection, (B & E) axial PET, (C & F) fused and (D & G) CT demonstrated 2-deoxy-2-18F-fluoro-D-glucose-avid right level IV cervical (arrow) and subcarinal (arrowhead) adenopathy. Biopsy of both nodes revealed benign lymphoid tissue.
Linke et al. [37] showed that the integration of PET and CT scanning increases sensitivity and accuracy of tumor diagnosis. In this study, CT was positive for malignancy in three of ten patients (sensitivity of 30%), which FDG-PET detected in nine of ten (sensitivity of 90%), and the difference between the two techniques was significant (p < 0.01). The combination of both methods demonstrated a sensitivity of 100%. Nevertheless, Titulaer et al. stated a negative PET/CT does not rule out underlying cancer and repeat PET/CT scanning after a 3–6-month interval can be fruitful [38].
Patel et al. note that FDG-PET was more sensitive than CT for detecting an unknown primary tumor for patients with suspected PNS [32]. Vaidyanathan et al. showed that PET/CT is a highly sensitive and specific imaging technique in the assessment of PNS and adds confidence to clinical possibility [23]. In that retrospective study, they showed that PET/CT had 100% sensitivity, 82% specificity, 42% positivepredictive value (PPV) and 100% NPV in detecting malignant involvement of the PNS. On the other hand, clinical risk scoring on its own was 50% sensitive, 70% specific, 18% PPV and 91% NPV. On combining the two methods, the additive sensitivity was 100%, specificity was 84%, PPV was 36% and NPV was 100%.
FDG-PET useful for clinically suspected PNS for antibody-proven PNS
Younes-Mhenni et al. recommend a more restricted role for FDG-PET. Finding only moderate sensitivity and PPV, with poor specificity and NPV, they concluded that FDG-PET for PNS is only indicated for patients with well-defined PNS antibodies, in specific settings (e.g., unknown primary or difficult-tobiopsy lesions). They stated PET should be reserved for patients with well-characterized paraneoplastic antibodies when conventional imaging fails to detect primary malignancy [34].
On the contrary, Hadjivassiliou et al. found a high specificity and NPV and agreed with the utility of FDG-PET for clinically suspected PNS, without a need for positive PNS antibodies before imaging [33]. In this study, the prevalence of abnormal PET in 80 patients clinically suspected for PNS was 18 out of 80 (23%). Biopsy-proven cancer was confirmed in 44% of patients with abnormal PET as opposed to less than 2% of patients with a normal PET. According to their results, the presence of PNS antibodies should not be a prerequisite for performing a PET scan; however, the presence of PNS antibodies increases the chances of the PET scan detecting a tumor. Additionally, Matsuhisa et al. showed that PET/CT was a useful screening method for patients with clinically suspected PNS who do not demonstrate well-characterized paraneoplastic antibodies. They retrospectively studied the utility of FDGPET/ CT in 27 patients with suspected PNS and found that FDG-PET/CT detected malignancy in 19% of the cases [39].
FDG-PET & altered brain glucose metabolism:
The metabolic abnormalities depicted on PET/CT typically correlate with clinical symptoms. Both qualitative and quantitative analysis of brain glucose metabolism may be helpful in evaluating PNS, especially in patients with a normal MRI. In many patients with para neoplastic limbic encephalitis, FDG-PET shows hypermetabolism in the temporal lobes with negative findings on MRI or T2-hyperintense mesiotemporal lesions. In the literature, focal hypermetabolism on PET was attributed to seizure activity [40,41], or an underlying inflammatory process [42–44]. Patients with PNS may demonstrate abnormal hypometabolism on brain FDG-PET/CT before MRI demonstrates cerebellar atrophy [45].
Conclusion
In conclusion, FDG-PET/CT offers value in investigating suspected PNS to identify or exclude occult malignancy, with a greater likelihood of cancer detection in cases of antibody-proven PNS.
Future perspective
FDG-PET/CT is valuable in excluding occult malignancy in patients with suspected PNS. It may help in identifying the malignancy in antibody-proven PNS. The value of novel radiophramaceuticals, such as 11C-choline and 68Ga-DOTA, in identifying the occult malignancies is currently unknown in patients suspected or antibody-proven PNS and further studies will be conducted in the next few years. In addition, future novel radiopharmaceuticals targeting the immune system will help elucidate the biologic mechanisms of PNS.
Financial & competing interests disclosure
RM Subramaniam is a speaker for Eli-Lilly Amyloid Imaging and receives research support from MimVista Inc. He also received a research grant from RSNA/GE. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
- Bannas P, Weber C, Derlin T et al. 18F-FDG-PET/CT in the diagnosis of paraneoplastic neurological syndromes: a retrospective analysis. Eur. Radiol. 20, 923–930 (2010).
- Voltz R, Graus F. Diagnosis and treatment of paraneoplastic neurological disorders. Onkologie 27, 253–258 (2004).
- Graus F, Lang B, Pozo-Rosich P, Saiz A, Casamitjana R, Vincent A. P/Q type calcium-channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology 59, 764–766 (2002).
- Honnorat J, Antoine JC. Paraneoplastic neurological syndromes. Orphanet. J. Rare Dis. 2, 22 (2007).
- Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J. Neurol. 257, 509–517 (2010).
- Albert ML, Austin LM, Darnell RB. Detection and treatment of activated T cells in the cerebrospinal fluid of patients with paraneoplastic cerebellar degeneration. Ann. Neurol. 47, 9–17 (2000).
- Benyahia B, Liblau R, Merle-Beral H, Tourani JM, Dalmau J, Delattre JY. Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Ann. Neurol. 45, 162–167 (1999).
- Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 9, 275–284 (1999).
- Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/ sensory neuronopathy) antigen in human normal and tumor tissues. Am. J. Pathol. 141, 881–886 (1992).
- Savage PA, Vosseller K, Kang C et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science 319, 215–220 (2008).
- Graus F, Delattre JY, Antoine JC et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry 75, 1135–1140 (2004).
- Knudsen A, Monstad SE, Dorum A et al. Ri antibodies in patients with breast, ovarian or small cell lung cancer determined by a sensitive immunoprecipitation technique. Cancer Immunol. Immunother. 55, 1280–1284 (2006).
- McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch. Neurol. 66, 735–741 (2009).
- Drlicek M, Bianchi G, Bogliun G et al. Antibodies of the anti-Yo and anti-Ri type in the absence of paraneoplastic neurological syndromes: a long-term survey of ovarian cancer patients. J. Neurol. 244, 85–89 (1997).
- Dalmau J, Furneaux HM, Rosenblum MK, Graus F, Posner JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology 41, 1757–1764 (1991).
- Bataller L, Wade DF, Graus F, Stacey HD, Rosenfeld MR, Dalmau J. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology 62, 778–782 (2004).
- Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann. Neurol. 56, 715–719 (2004).
- Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N. Engl. J. Med. 322, 1555–1560 (1990).
- Folli F, Solimena M, Cofiell R et al. Autoantibodies to a 128-kd synaptic protein in three women with the stiff-man syndrome and breast cancer. N. Engl. J. Med. 328, 546–551 (1993).
- McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology 76, 1108–1110 (2011).
- Mason WP, Graus F, Lang B et al. Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert-Eaton myasthenic syndrome. Brain 120(Pt 8), 1279–1300 (1997).
- Dalmau J, Graus F, Villarejo A et al. Clinical analysis of anti- Ma2-associated encephalitis. Brain 127, 1831–1844 (2004).
- Vaidyanathan S, Pennington C, Ng CY, Poon FW, Han S. 18F-FDG PET-CT in the evaluation of paraneoplastic syndromes: experience at a regional oncology centre. Nucl. Med. Commun. 33, 872–880 (2012).
- Subramaniam RM, Wilcox B, Aubry MC, Jett J, Peller PJ. 18F-fluoro-2-deoxy-d-glucose positron emission tomography and positron emission tomography/computed tomography imaging of malignant pleural mesothelioma. J. Med. Imaging Radiat. Oncol. 53, 160–169; quiz 170 (2009).
- Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. Am. J. Roentgenol. 197, W260–W265 (2011).
- Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases, part 1. Am. J. Roentgenol. 197, W256–W259 (2011).
- Davison JM, Ozonoff A, Imsande HM, Grillone GA, Subramaniam RM. Squamous cell carcinoma of the palatine tonsils: FDG standardized uptake value ratio as a biomarker to differentiate tonsillar carcinoma from physiologic uptake. Radiology 255, 578–585 (2010).
- Imsande HM, Davison JM, Truong MT et al. Use of 18F-FDG PET/CT as a predictive biomarker of outcome in patients with head-and-neck non-squamous cell carcinoma. Am. J. Roentgenol. 197, 976–980 (2011).
- Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J. Nucl. Med. 53, 709–715 (2012).
- Dibble EH, Karantanis D, Mercier G, Peller PJ, Kachnic LA, Subramaniam RM. PET/CT of cancer patients: part 1, pancreatic neoplasms. Am. J. Roentgenol. 199, 952–967 (2012).
- Rees JH, Hain SF, Johnson MR et al. The role of [18F] fluoro-2-deoxyglucose-PET scanning in the diagnosis of paraneoplastic neurological disorders. Brain 124, 2223–2231 (2001).
- Patel RR, Subramaniam RM, Mandrekar JN, Hammack JE, Lowe VJ, Jett JR. Occult malignancy in patients with suspected paraneoplastic neurologic syndromes: value of positron emission tomography in diagnosis. Mayo Clin. Proc. 83, 917–922 (2008).
- Hadjivassiliou M, Alder SJ, Van Beek EJ et al. PET scan in clinically suspected paraneoplastic neurological syndromes: a 6-year prospective study in a regional neuroscience unit. Acta Neurol. Scand. 119, 186–193 (2009).
- Younes-Mhenni S, Janier MF, Cinotti L et al. FDG-PET improves tumour detection in patients with paraneoplastic neurological syndromes. Brain 127, 2331–2338 (2004).
- Berner U, Menzel C, Rinne D et al. Paraneoplastic syndromes: detection of malignant tumors using [18F] FDG-PET. Q. J. Nucl. Med. 47, 85–89 (2003).
- McKeon A, Apiwattanakul M, Lachance DH et al. Positron emission tomography-computed tomography in paraneoplastic neurologic disorders: systematic analysis and review. Arch. Neurol. 67, 322–329 (2010).
- Linke R, Schroeder M, Helmberger T, Voltz R. Antibodypositive paraneoplastic neurologic syndromes: value of CT and PET for tumor diagnosis. Neurology 63, 282–286 (2004).
- Titulaer MJ, Soffietti R, Dalmau J et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur. J. Neurol. 18, 19.e13 (2011).
- Matsuhisa A, Toriihara A, Kubota K, Makino T, Mizusawa H, Shibuya H. Utility of F-18 FDG PET/CT in screening for paraneoplastic neurological syndromes. Clin. Nucl. Med. 37, 39–43 (2012).
- Franck G, Sadzot B, Salmon E et al. [Paraneoplastic limbic encephalopathy, inappropriate ADH secretion and recurrent subclinical epileptic seizures. Clinical, anatomopathological and metabolic correlations by positron emission tomography]. Rev. Neurol. (Paris) 143, 657–669 (1987).
- Fakhoury T, Abou-Khalil B, Kessler RM. Limbic encephalitis and hyperactive foci on PET scan. Seizure 8, 427–431 (1999).
- Provenzale JM, Barboriak DP, Coleman RE. Limbic encephalitis: comparison of FDG PET and MR imaging findings. Am. J. Roentgenol. 170, 1659–1660 (1998).
- Na DL, Hahm DS, Park JM, Kim SE. Hypermetabolism of the medial temporal lobe in limbic encephalitis on 18FDGPET scan: a case report. Eur. Neurol. 45, 187–189 (2001).
- Kassubek J, Juengling FD, Nitzsche EU, Lucking CH. Limbic encephalitis investigated by 18FDG-PET and 3D MRI. J. Neuroimaging 11, 55–59 (2001).
- Basu S, Alavi A. Role of FDG-PET in the clinical management of paraneoplastic neurological syndrome: detection of the underlying malignancy and the brain PET-MRI correlates. Mol. Imaging Biol. 10, 131–137 (2008).
• Retrospective review of the database at the West of Scotland PET center from 2007 to 2010 included a total of 68 patients. A total of 18 (26%) patients had a positive PET/CT result. PET/CT was concordant with the clinical scoring in 49 (72%) patients; it upgraded the score in eight (12%) patients, and downgraded the score in 11 (16%) patients. Eight (12%) patients had confirmed malignancy. PET/CT was estimated to have 100% sensitivity, 82% specificity, 42% positive-predictive value and 100% negative-predictive value.
• Retrospective study involving 104 patients with paraneoplastic neurologic syndromes (PNS); 73 patients had at least one positive result for paraneoplastic antibody, and 31 had antibody-negative PNS. Malignancy was confirmed pathologically in ten patients, of whom eight had positive PET results. There were two cases of confirmed malignancy (fallopian tube adenocarcinoma and spindle cell uterine carcinoma), for which PET results were negative. Two patients with positive PET results declined biopsy. All patients with confirmed malignancy had positive results for at least one paraneoplastic antibody. One patient with positive results for PNS antibody and negative PET results was diagnosed as having small-cell carcinoma on a follow-up PET scan after 27 months. PET had sensitivity, specificity, positive-predictive value and negative-predictive value of 80, 67, 53 and 88%, respectively.
• Prospective study including 80 patients suspected of having paraneoplastic neurological syndromes (PNS) underwent PET. In total, 18 out of 80 (23%) were abnormal and suspicious of malignancy. The total number of definite and probable PNS with abnormal PET was 11 out of 18 (61%). The total number of definite and probable PNS with a normal PET was three out of 62 (5%). Only 50% of patients with biopsy-proven malignancy were positive for paraneoplastic antibodies. The prevalence of abnormal PET in patients presenting with classical PNS was 41% as opposed to 21% in patients with nonclassical PNS. The sensitivity and specificity of PET in diagnosing PNS was 75 and 87%, respectively.