Review Article - Pharmaceutical Bioprocessing (2016) Volume 4, Issue 1
Engineered nanomaterials for pharmaceutical and biomedical products new trends, benefits and opportunities
- Corresponding Author:
- Athanasios Valavanidis
Department of Chemistry, University of Athens, University Campus Zografou, 15784, Athens, Greece
E-mail: valavanidis@chem.uoa.gr
Abstract
Introduction
The technological revolution of nanotechnology
Nanotechnology has advanced very fast as the technological revolution that deals with the manipulation of matter on an atomic and molecular scale. Scientists have precisely manipulated chemical atoms and molecules for fabrication of macroscale products with a wide range of applications. These products are called engineered nanomaterials (ENMs) and have found a variety of applications in important technological fields, especially in pharmacology and biomedical products. The term nanotechnology is defined as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (1 nm=1 × 10−9 m), reflecting the fact that quantum mechanical effects are important at this quantum-realm scale [1-3].
Applications of ENMs are diverse in areas of biology, medicine, pharmacology, biomedical imaging, drug delivery, bio-molecular sensing, tissue engineering, data storage, photocatalytic pigments, cosmetics, food, etc. In the last decade the fields of nanotechnology increased substantially with “revolutionary” nanotechnological applications [4-6]. The industrial sector in the USA and the government established the National Nanotechnology Initiative (NNI, 2000) to serve as the central point of communication, cooperation, and collaboration for all Federal agencies engaged in nanotechnology research, bringing together the expertise needed to advance this broad and complex field [7].
Because of the variety of potential industrial and military applications, governments in developed countries have invested billions in nanotechnology research. The USA through NNI has invested 3.7 $ billion, the European Union countries $1.2 billion and Japan $750 million (in the period 2004-2005). In 2009, Russia announced that will channel 318 billion rubles ($10.6 billion) into development of nanotechnology by 2015 [8].
The Organization Cientifica Ltd estimated in its Annual Global Nanotechnology Research Funding report, that the world’s governments currently spend $10 billion per year, with that figure set to grow by 20% over the next three years. In 2011 it was estimated that China spent US$2.25 billion in nanotechnology research. Worldwide statistics showed that the total government funding for nanotechnology research will be $65 billion (2011), rising to $100 billion by 2014 [9]. According to a recent conference report, in the period 2009-2010 the corporations and institutional investors for nanotechnology R&D reached 9,2 and 9,7 $ billions respectively, and governments spent 8,4 and 8,2 $ billions [10].
Nanotechnology applications and new ENMs continue to evolve rapidly and the overall market for new nanoproducts is growing, along with the degree to which they are permeating our everyday lives. The Woodrow Wilson International Center for Scholars (WWICS) in the USA established a Project on Emerging Nanotechnologies (April 2005) as a partnership between the WWICS and the Pew Charitable Trusts. The Project was dedicated to helping ensure that as nanotechnologies advance, possible risks are minimized, public and consumer engagement remains strong, and the potential benefits of these new technologies are realized. The Project identified a list of more than 1,500 nano-enabled products currently on the market, reflecting a 50% increase since this list was first compiled in 2006. The list contains information on products from over 20 countries [11].
Application of nanotechnology in medical and pharmaceutical fields is fuelling hopes to significantly improve diagnosis and drug treatment of all kinds of diseases [12-14]. The nanomedicine market sector is dominated especially by drug nano-delivery methods and in vitro diagnostics represent the second leading field. Considering all patent applications in the different fields of nanomedicine, USA hold a share of 53%, Europe has 25% and Asia 12%. Biopharmaceutical and medical devices companies are well aware of the potential applications of nanotechnology to the healthcare sector, as demonstrated by the increasingly growing partnerships between these enterprises and nanomedicine startups [15]. According to a research report from the Business Communications Company (BCC), despite the catastrophic consequences of the 2008-2009 crisis on capital markets, the global nanomedicine sector, which was worth $53 billion in 2009, is projected to grow surpassing $100 billion in 2014. One of the largest segments of this market is represented by anticancer products. Valued at about $20 billion in 2009, it is expected to reach $33 billion in 2014 [16].
Nanomaterials in pharmacological and biomedical applications
ENMs and their applications to biomedicine and pharmacology has become a distinct and active area of scientific and technological developments over the last decade. Numerous advances have been achieved concerning new synthesis of nanoparticles: NPs with unique physicochemical properties, biomolecular functionalization, for detection of biomolecules and cells, applications of gold and other metals in nanoparticles for enhanced targeted action, new biomedical applications of magnetic nanomaterials, special nanoparticle- cell interactions, polymer nanoparticles for drug delivery, and nanoparticle applications in gene delivery, diagnostic imaging, molecular diagnostics and therapeutics.
Significance of nanomaterials in drug delivery
Transport barriers are vital in all biological organisms for restricting chemical molecules to pass through biological membranes and tissues towards their site of action. Inevitably, drugs in the human body face several restrictions on their journey from the time are delivered (by injection or orally) to their site of therapeutic action. Rapid filtration in the kidney and clearance via the reticulo-endothelial system is one of the important barriers in the human body. In the case of drugs substantial time is spent swimming in the bloodstream until reaching to target cells in the interior of tissues. But in front of biological tissues or cellular targets drugs meet a formidable barrier and must cross the plasma membrane. Even when they are inside the cell, the drug must escape the harsh acidic environment of endolysosomes (acidic intracellular organelles playing a key role in protein turnover and cellular homeostasis), within which biomolecular substances such as proteins and oligonucleotides may be inactivated or degraded. In addition, another barrier is the nuclear membrane (protecting the DNA and RNA of the nucleus). Also, resistance mechanisms can be encountered by drugs within pathological cells. A whole field has been developed by scientists to explore some promising ways in which nanomaterials (acting as drugs or vaccine carriers) can navigate through these barriers to reach the site of action [17].
Most of ENMs are sized from 1 to 100 nanometers (1 nm=•000000001 m or 1 × 10−9 m) which is smaller than eukaryotic or prokaryotic cells. Nanomaterials can help to bind, adsorb and carry drug molecules, probes and proteins. Also, the drug particles itself can be engineered to form nanoscale size materials that can reach inaccessible areas, such as inflamed tissues because of their enhanced permeability and retention effect. At present there are several examples of inorganic and organic nanomaterials that demonstrate unique properties, such as biocompatibility and tissue interaction for pharmacological purposes, especially effective drug delivery [18-19].
The ENMs can be designed to carry drugs that target the reticulo-endothelial cells (collective term for cells of the immune system that is comprised of macrophages and monocytes). In this way nanoparticles facilitate the passive targeting of drugs to the macrophages of liver and spleen and give the opportunity to a natural system to fight intracellular infections. Encapsulation of antimicrobial drugs in nanoparticle systems has emerged as an innovative and promising alternative that enhances therapeutic antimicrobial effectiveness. There are many examples for synthesizing nanoparticle platforms for delivering various antimicrobial drugs [20].
Carbon nanotubes as drug carriers
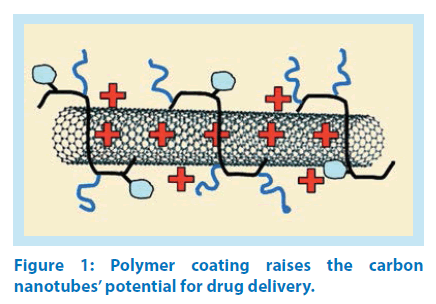
Carbon nanotubes (CNTs) are organic nanoscale molecules compatible with living organism tissues and can be used in a variety of applications as carriers of drugs in appropriate position inside the human body. CNTs have emerged as a new alternative and innovative tool for transporting and translocating therapeutic drug molecules near the target tissue. CNTs can be functionalised with bioactive peptides, proteins, nucleic acids and drugs, and used to deliver their cargos to cells and organs. Because functionalised CNTs display low toxicity and are not immunogenic, such systems hold great potential in the field of nanobiotechnology and Nanomedicine [21] Figure 1.
CNTs have gained increased attention in recent years as promising nanocarriers of drugs, owing to their high surface area, enhanced cellular uptake and the possibility to be easily conjugated with many therapeutic chemical compounds, including both small molecules and biological molecules, displaying superior efficacy, enhanced specificity and diminished side effects. Although initially CNTs had been engineered as carriers of anticancer drugs there are also emerging reports that CNTs can be used as carriers or adjunct material for the delivery of various other therapeutic substances because they offer several appealing features [22]. Scientific literature has an extensive number of research publications concerning CNT-based nano-drugs for treatment of malignant neoplasms. From the experimental observations the most significant and promising results are in the field of drug delivery of anticancer chemotherapeutics, such as doxorubicin, methotrexate, taxanes, platinum analogues, camptothecine and gemcitabine [23].
Nanoscopic dendrimers as drug vectors
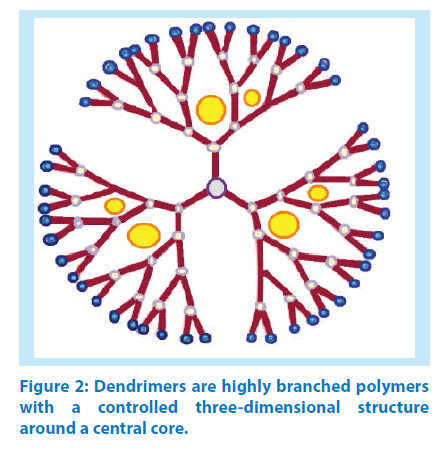
Scientists investigated new nano-scale drug carriers using dendrimers, (three-dimensional, immensely branched and organized nanoscopic macromolecules which possess low polydispersity index) that offer the possibility of increasing the therapeutic index of drug molecules. Dendrimers are able to increase the effectiveness of the drugs, lower their toxicity and achieve controlled therapeutic levels for a prolonged time. A recent overview presented approaches to the development of these novel complex nanocarriers with emphasis on those involving dendrimers and related systems [24-25] Figure 2.
Dendrimers are emerging polymeric architectures which have defined structures, versatility in drug delivery and high functionality. Dendrimers have shown potential abilities in entrapping or conjugating high molecular weight medicinal agents with hydrophilic or hydrophobic parts by host-guest interactions and covalent bonding, respectively. This is called prodrug approach, in which a medication after administration is metabolized within the human body into a pharmacologically active drug. Dendrimers show high ratio of surface groups to molecular volume, a property that made them a promising synthetic vector for gene delivery. But their use in biological systems is limited due to toxicity issues [26]. Recently, a dendrimer based prodrug has been developed for paclitaxel. This prodrug focuses on enhancement of permeability and transportation across cellular barriers. The highly functional lauryl-modified dendrimer-paclitaxel conjugates demonstrated good stability under physiological conditions and 12-fold greater permeability across Caco-2 cell and porcine brain endothelial cells monolayers than paclitaxel alone [27].
Nanomaterials for targeted cancer therapy
From the first years of nanotechnology applications in biomedicine fields it has become obvious of the potential uses in the fight against malignant neoplasms. Nanomaterials can found various applications and facilitate anticancer research, deliver anticancer agents in target tissues, improve molecular imaging of tumours, advance methods of early detection, improve prevention in the early stages of precancerous lesions, and finally make successful treatment of various types of cancer .Molecular imaging and early detection by advanced nano-techniques has the potential to help clinicians spot cancerous tumours in their earliest stages. Biomarkers with the use of nanotechnology may allow doctors to investigate cells that are undetectable through conventional imaging. For example, photoluminescent nanoparticles are able to allow cancer specialists to visually discriminate between precancerous lesions, cancerous and healthy cells. On the therapeutic level nanoscale devices can release anticancer drugs, or improve time release near or inside the tumour target site increasing substantially their therapeutic efficacy [28].
Significant synthetic advances in the last decade allowed for the preparation of engineered nanoparticles (ENPs) with highly controlled geometry, surface charge, physicochemical properties, and the decoration of their surfaces with polymers and bioactive molecules in order to improve biocompatibility and to achieve active targeting. Research in this field is stimulating the development of a diverse range of nanometer sized chemical substances that can recognize cancer tissue, enabling visualization of tumours, improve timely delivery of anticancer drugs and facilitate the destruction of tumours by different therapeutic techniques [29]. In the last decade significant synthetic advances were achieved in research laboratories (universities, and pharmaceutical companies) for the preparation of ENPs with highly controlled geometry, biocompatibility and high efficacy of targeting tumour sites. Also, research has led to the development of a diverse range of nanometersized substances enabling visualization of tumours. The next step was to improve delivery of anticancer drugs and the destruction of tumours by different therapeutic techniques. A recent review provided an overview of ENMS that have been devised for the detection and treatment of various types of cancer, as well as the emerging possibilities of nanomaterials for specific applications for targeted anticancer therapy [30].
Lessons from the 50 years’ fight against cancer (“war on cancer” National Cancer Act of 1971, USA) taught medical specialists that cancer diseases have special therapeutic problems. Cancer is a complex disease comprising of hundreds of distinct molecular diseases. Although recent research has emphasized the heterogeneity of the disease, much of malignant neoplasms’ biology remains poorly understood. Based on histopathology and molecular genetic alterations tumours are divided into different types that are essentially distinct diseases, as indicated by differences in genetic risk factors, precursor lesions, patterns of spread, and molecular events during carcinogenesis and response to chemotherapy. This broad disease profile has prompted researchers to establish the tailoring of cancer therapy, and in this direction the help of specific ENMs was very crucial. Drugs that block the growth, progression and spread of cancer by interfering with specific molecular targets (called “molecularly targeted therapies”) have been approved by FDA and are studied in a series of clinical trials (National Cancer Institute, Targeted cancer therapies, http://www.cancer. gov/about-cancer/treatment/types/targetedtherapies/ targeted-therapies-fact-sheet).
Molecularly targeted therapy can provide higher efficacy and lower toxicity than conventional chemotherapy for cancer. However, like traditional chemotherapy, molecularly-targeted cancer therapies also face the challenge of drug resistance. Combination of targeted therapies with nanotechnology approaches is according to experts a promising strategy to overcome targeted therapy drug resistance in cancer treatment [31]. The field is currently advancing along two parallel paths. Firstly, ENMs can be used to monitor molecular and microenvironment changes associated with cancer (molecular imaging). Secondly, the integration of imaging and therapeutic capabilities into single nanoparticle systems has been realized, permitting confirmation of drug delivery to tumour sites, image-guided surgery or image-guided selective tumour ablation (termed diagnostic therapy or theranostics). Despite these advances, experts consider further improvements in sensitivity and safety profiles are required [32].
The commercialization of the first-generation nanomedicine for breast cancer started few years ago. The combination of Doxil (doxorubicin HCL liposome injection) and Abraxane (paclitaxel protein-bound) for metastatic breast cancer (Clinical Trials Research Unit, West Virginia University) gave the scientific community its first lesson to understand targeted and non-targeted nanoparticles in cancer treatment under various stages of development and a platform for studying the challenges faced by nanomedicine in contemporary oncology. Pharmaceutical companies have formed partnerships to use proprietary nanoparticle technologies recognizing that nanotechnology can contribute to improve clinical outcome in cancer therapeutic agents [33].
Nanoproducts multifunctionality and molecular cancer imaging
A recent review on the advances of nanotechnology products in molecular imaging for cancer research described the numerous challenges and problems that have delayed the successful applications of ENMs in the field of biomedical imaging. ENMs with their unique optical, magnetic and chemical properties have allowed medical scientists to create imaging probes with better contrast enhancement, increased sensitivity, controlled biodistribution, better spatial and temporal information, multi-functionality and multi-modal imaging across MRI (Magnetic Resonance Imaging), PET (Positron Emission Tomography), SPECT (Single-photon Emission Computed Tomography) and ultrasound. The reviewers conclude that the clinical advantages of ENMs and benefits from their use in imaging are obvious and the lessons learned until now are promising to advance the field further for better cancer imaging that will improve prevention and therapy [34].
In recent years there are new developments in biomedical applications of multi-functional nanomaterials for multi-modal imaging and therapy of diseases. These commercial nanoproducts are polymeric, magnetic, gold and silica nanoparticles, as well as carbon materials and quantum dots (semiconductor materials, tiny particles of nanometre size). These nanoproducts have large surface area, structural diversity, multifunctionality and can circulate in the blood for long time. These properties give the nanostructured materials multimodal imaging for precise and fast diagnosis of cancer, but also can be used at the same time to deliver anticancer agents to the disease site [35].
Many research efforts took place in research laboratories all over the world to synthesize nanocrystalline particles with advanced polymer processing, coating and integrating various functionalities (different imaging modalities, targeted delivery of drugs or genes and thermal therapies). Although these nanoproducts are in the early stages of research and development into clinical products, they have shown great promise in multi-modal imaging, theranostics, and image-guided therapies [36]. Numerous papers are published on multifunctional nanoparticles carrying various functions to overcome limitations associated with conventional cancer diagnosis and therapy. For example, a review paper explored the applications of magnetic iron oxide nanoparticles with super paramagnetic properties as potential multifunctional nanoparticles for clinical translation because they have been used as magnetic resonance imaging (MRI) contrast agents. These nanoparticles can be easily tailored by including targeting moieties, fluorescence dyes, or therapeutic agents [37].
An extensive recent review describes a number of novel multi-functional nanoparticles that have attracted much attention for their ability to carry diverse functionalities to achieve effective synergistic therapeutic treatments [38].
Nanomedicine products and promising clinical applications
Scientific progress in nanomedical products is slow because involves health and safety issues as well as scrutiny by national and international agencies that regulate clinical trials of new pharmaceuticals or therapeutic methodologies. Scientists are hopeful that new developments in nanotechnology for pharmaceuticals will provide solutions to many of modern medicine’s problems. A review searching the scientific literature in 2013 found 247 nanomedicine products that are approved or in various stages of clinical study. Also, a number of nanomedicine products are already in use showing clear benefits in the treatment of human diseases. The most overwhelming trend observed in the data of this review was the large number of nanotechnological cancer treatments under development. It was found that 47% of all the confirmed in vivo products were intended for acutely life-threatening conditions (mostly advanced cancers). The majority of the cancer treatment applications identified in this study were aimed at increasing the efficacy of therapeutic delivery [39].
Other biomedical and pharmaceutical applications of novel nanoproducts are bio-molecular sensing, nanoantibiotics for infectious diseases, tissue engineering scaffolds, and immunoassays applications with quantum dots. These techniques use nanoscaled electrochemical detection, functional nanomaterial-amplified optical assays, colorimetry, fluorescence and electrochemiluminescence [40].
According to scientists the field of nanoscale size biosensors have the potential to supersede current analytical technological sensing techniques in clinical practice. Colloidal fluorescent and plasmonic ENPs produce intense responses to incident light and their presence in a target analyte gives extremely sensitive detection in solution [41]. The oscillation of electrons on the surface of metallic nanomaterials enhanced quantity of bioreceptor units at reduced volumes and even to act itself as transduction element. A number of recent studies explored the use of nano-biosensors such as gold nanoparticles, semi- conductor quantum dots, nanocarbon tubes, nanodiamonds and graphene that can amplify the absorption and scattering of light, a phenomenon known as surface plasmon resonance (SPR, absorbed light can be emitted as fluorescence light of a different wavelength). ENMs are promising candidates as biosensors due to the possibility to immobilize an enhanced quantity of bioreceptor units at reduced volumes and act itself as transduction element [42-43].
ENMs as carriers of antibiotics for infectious diseases
For decades treatment of infectious diseases with antibiotics encountered problems of microbial or bacterial resistance. Several microbes have evolved clinically significant resistance against almost every available antibiotic. The emergence of resistant and more virulent strains of bacteria has outpaced the development of new antibiotic drugs. Scientists and pharmaceutical companies have developed multidimensional strategies to combat microbial infections by modifying existing antibiotics or researching for novel substances, improving carrierdelivery systems to reduce doses, and recently developing nanomaterials as carriers of targeted antimicrobials [44].
Several classes of antimicrobial nanoparticles and nanosized carriers for antibiotics delivery in infectious sites have been tested. The results showed high efficacy in treating infectious diseases, including antibiotics resistant ones, in vitro as well as in animal models [45]. Engineered Nanoparticles (ENPs) have shown promise in treating bacterial infections, but there are significant challenges for their administration. Scientists in Harvard-MIT (Division of Health Sciences & Technology, Cambridge, MA) developed antibacterial ENPs that are suitable for systemic administration (drug-encapsulated, pH-responsive, surface charge-switching polymers) for treating bacterial infections. These ENP drug carriers were designed to shield nontarget interactions at pH 7.4 but bind avidly to bacteria in acidity, delivering drugs and mitigating in part the loss of drug activity with declining pH. According to researchers this can be a first step towards developing systemically administered drug carriers that can target and potentially treat Gram-positive, Gram-negative, or polymicrobial infections [46].
Nanotechnological applications are rapidly becoming a major driving force behind ongoing changes in the fight against resistant microbes, bacteria, viruses, etc. Despite the various problems, there is progress in the management of microbial infection, including diagnosis, antimicrobial therapy, drug delivery and vaccines in antimicrobial treatments [47]. Researchers hope that by exploring special nanoparticles (such as liposomes) which encapsulate, incorporate or even conjugate antibiotic molecules can deliver intracellularly antibiotic drugs treating more effectively infections [48].
Nanotechnology and scaffolds in tissue engineering applications
For many years of medical practices for tissue and bone engineering has developed functional substitutes for damaged tissues, bones and organs. Before the process of transplantation, cells are generally seeded on biomaterial scaffolds that recapitulate the extracellular matrix and provide cells with information that is important for tissue development. The prospect of applying nanotechnological materials (nanocomposites) for tissue engineering and extracellular matrix is considered very promising but also offers certain challenges to medical specialists. Nanomaterials exhibit unique properties that make them suitable for incorporating in tissue engineering scaffolds. At present various nanoparticles, nanoporous scaffolds, nanopatterned surfaces, nanofibers, and CNT are used for advanced fabrication of tissue engineering scaffolds and biomimetic substitutes of damaged tissues and organs. A challenge for these ENMs is the inflammatory responses they elicit in vivo [49-50].
Nanotechnological tissue-engineering scaffolds must be analogous to native extracellular matrix of tissue in both chemical composition and physical structure. Some polymeric nanofiber matrix materials are quite similar, with its nanoscaled nonwoven fibrous matrix proteins, suitable candidates for extracellular matrix-mimetic materials. Electrospinning to produce polymeric nanofibers have stimulated researchers to explore the application of nanofiber matrix as a tissue- -engineering scaffold [51].
Research showed that CNT are also attractive for use in fiber-reinforced composite materials due to their very high aspect ratio, combined with outstanding mechanical and electrical properties. Composite materials comprising a collagen matrix with embedded CNT were prepared by mixing solubilized Type I collagen with solutions of carboxylated single-walled carbon nanotubes (SWNT). Living smooth muscle cells were incorporated at the time of collagen gelation to produce cell-seeded collagen–CNT composite matrices. Such collagen–CNT composite matrices have utility as scaffolds in tissue engineering, or as components of biosensors or other medical devices [52].
Medically suitable tissue-engineered bone constructs are in great demand. Silica based mesostructured (mesoporous) nanomaterials (pore sizes in the range 2–50 nm) with surface reactive functionalities have elicited scientific interest for bone tissue engineering. Application of different mesoporous nanomaterials in the production of bone implants and bone cement give promising results and it is hoped to lead in the construction and manufacture of 3-dimensional scaffolds for bone tissues [53].
Summary of ENMs in novel pharmaceutical and biomedical products
For more than a decade the development and application of nanomaterials with pharmaceutical and biomedical properties has been advanced in a rapid pace. At present, nanoparticles, nanocapsules, micelles, microemulsions, liposomes, nanoporous materials, multilayered polymers have been synthesized and surfacefunctionalized for applications in biomedicine and pharmaceuticals. Biosensing, drug delivery, imaging, bioseparations, biocatalysis, biomolecular assembly, and molecular diagnostics are some of the applications. Table 1 contains some of the most important applications and relevant references.
| Nanomaterials | Pharmaceutical of biomedical Properties | Selected references |
|---|---|---|
| Carbon nanotubes, drug carriers and delivery | Transporting therapeutic drugs, functionalised with bioactive peptides, proteins, etc | [21,22,23] |
| Nanoscopic dendrimers | Drug vectors, nanocarriers, increased effectiveness of drugs, dendrimer prodrug for paclitaxel | [24,25,27] |
| Nanomaterials for targeted cancer therapy | Photoluminescent nanoparticles to allow visually discriminate precancerous lesions, cancerous and healthy cells | [28] |
| Nanomaterials recognising cancer tissues and drug delivery | Improving timely delivery of anticancer drugs near cancerous tissues | [29,30] |
| Nanomaterials with multifunctional molecular imaging | Controlled biodistribution, increased sensitivity | [34] |
| Multi-functional nanomaterials for multi-modal imaging | Multi-modal imaging, large surface area, structural diversity, multifunctionality, multimodal imaging, fast diagnosis (polymeric, magnetic , gold, silica, carbon tubes, quantum dots) | [35,36,37] |
| Nanomaterials as carriers of antibiotics for infectious diseases | Nanosized carriers for antibiotic drugs, delivery in infectious tissues, surface charge-switching polymers, drugs for bacterial infections | [44,46] |
| Nanomaterials as scaffolds in tissue engineering | Nanocomposites, nanoporous scaffolds, nanofibers, carbon nanotubes, biomimetic substitutes, nanoscaled nonwoven fibrous matrix proteins, silica based mesostructured nanomaterials | [49,50,51,53] |
Table 1. Nanomaterials and nanoproducts with specialized properties for pharmaceutical and biomedical applications.
Are they health risks from exposure to nanoproducts?
The rapid development of nanoproducts in medicine, pharmacology and various consumer products inevitably prompted concerns for health and safety issues by toxicologists and health agencies. The current state of knowledge is very limited and health risk assessments for the whole spectrum of nanoproducts are not available. At the same time the protection of workers in the manufacturing sector of nanomaterials and nanoproducts, health professionals in hospitals and laboratories and consumers are very important issues. The subject of health and safety problems of nanotechnological products and their applications appeared in recent publications [54-56].
WHO experts meeting (2012) on nanotechnology was convened in Bonn (Germany) to discuss nanotechnological products and risks to human health. The summary of the report from the conference stated “…The WHO Regional Office for Europe undertook a critical assessment of the current state of knowledge and the key evidence on the possible health implications of nanomaterials, with a view to identify options for risk assessment and policy formulation, and convened an expert meeting to address the issue. Current evidence is not conclusive. As complexity and uncertainty are large, risk assessment (of nanoproducts) is challenging, and formulation of evidence-based policies and regulations elusive. Innovative models and frameworks for risk assessment and risk governance are being developed and applied to organize the available evidence on biological and health effects of nanomaterials in ways to inform policy…” [57].
Another very interesting report on health risks from nanoproducts was from the RIVM (National Institute for Public Health and the Environment, Bilthoven, The Netherlands) [58]. The report contains an extensive catalogue of nanoproducts in various consumer products. The nanomaterials are mainly in the form of particles, composites, capsules, fullerenes, carbon nanotubes, coatings, nanoporous materials, quantum dots, nanofibres and nanowires. The report described in detail the toxicological problems of the various consumer products as far as health and safety is concerned. According to the report the most alarming use is nanomaterials for foodstuffs [58].
Inhalation exposure to nanomaterials is considered as an important health risk to workers, users and consumers of ENPs, especially for ENPs smaller than 100 nm diameter which can become airborne particles. These “nanostructured particles” are potentially of concern if they can deposit in the respiratory system. Some classes of nanoparticles can cause respiratory toxicity to humans [59].
Another form of exposure to ENPs is dermal penetration that gives concern to toxicologists. Intentional dermal exposure to nanoparticles may include the application of creams containing nanoparticles of TiO2 or ZnO. Non-intentional exposure could involve dermal contact with anthropomorphic substances generated during nanomaterial manufacture or combustion [60].
Despite the recent advances in toxicological assessment of nanoparticles, it is unclear whether they can penetrate the human skin and have any toxicological impact. There are concerns regarding dermal penetration including skin or other organ, cytotoxicity, accumulation, metabolism and photoactivation. For example dermal contact with nanoparticles, such as nanoscale TiO2 and ZnO (<100 nm) which are included in sunscreens can produce hydroxyl radicals (HO•). The surfaces of nanocrystals of TiO2 can generate Reactive Oxygen Species (ROS) which have oxidative potential and also can be cytotoxic [61- 62].
A study investigating epidermal and dermal penetration of human skin provided compelling evidence that nanoparticles can achieve penetration (microsphere 0.5-1.0 μm) [63] While a review on the subject of skin penetration and dermal or percutaneous absorption of metal nanoparticlers and their effect on skin (especially TiO2 and ZnO) found contradictory data [64].
Pulmonary toxicity of nanoparticles is another health and safety issue in human toxicology. Studies showed that CNT have a strong tendency to agglomerate by van der Waals forces into tattered ropes, whereas, others remain as a fine powder (much like carbon black.). Under some conditions the CNTs can reach the respiratory system and penetrate deep into the lungs. Their toxicity depends on whether they are persistent or cleared from the lung [65].
Investigation on adverse effects of single-wall carbon nanotubes (SWCNT) using a cell culture (human epidermal keratinocytes) showed that after 18 h of exposure appeared oxidative stress and cellular toxicity caused by formation of free radicals, accumulation of peroxidative products, antioxidant depletion, and loss of cell viability [66]. Also, toxicological experiments demonstrated that pharyngeal aspiration of SWCNT elicited unusual pulmonary effects in C57BL/6 mice that combined an acute inflammation with early onset yet progressive fibrosis and granulomas. A dose-dependent increase in the protein, LDH, and γ-glutamyl transferase activities in bronchoalveolar lavage were found along with accumulation of 4-hydroxynonenal (oxidative biomarker) and depletion of glutathione in lungs [67].
In vivo toxicological experiments in animals used fine-sized titanium dioxide (TiO2) particles, nanoscale rods and nanoscale dot particles for intratracheal instillation doses in rats, mice and hamsters. Results demonstrated no significant differences among any of the particle-exposed groups compared to vehicle controls with regard to inflammatory or cytotoxic lung responses at any post-exposure time periods [68-69]. A recent review collected a number toxicological study of TiO2 nanoparticles on the respiratory system and the importance of inhalation of ENPs. In the same review, dermal exposure studies (in vivo or in vitro), report that TiO2 does not penetrate the stratum corneum. In the field of nanomedicine, intravenous injection can deliver TiO2 nanoparticulate carriers directly into the human body. Upon intravenous exposure, TiO2 can induce pathological lesions of the liver, spleen, kidneys, and brain, but at high concentration exposures. The review emphasizes the lack of epidemiological data regarding adverse health effects of TiO2 nanoparticles. Studies on long-term inhalation studies in rats reported the development of lung tumours [70].
Health and safety organizations around the world have called for the responsible development of nanotechnology and commercialization of nanoproducts that do not carry risks to humans and the environment. The goals of this approach are to emphasize the importance and benefits of nanotechnology but controlling the potential adverse impacts. A primary area of concern is the potential adverse impact on workers, since they are the first people exposed to the potential hazards of nanotechnology. Research must assess and communicate hazards and risks to workers, users and consumers of nanotechnology products. Scientists argued that it is prudent to treat nanoproducts as potentially hazardous until sufficient toxicology, and exposure data are gathered for risk assessments [71].
The Environmental Protection Agency (EPA) in the U.S. has advanced a series of health and safety scientific research activities on nanotechnology products and a number of collaborative research projects with American universities and research institutes. EPA has identified manufactured nanomaterials in more than 1,300 commercial products, including medical equipment, textiles, fuel additives, cosmetics, plastics etc. Research is focusing in the most prevalent nanomaterials that may have human and environmental health and safety implications [72].
European Union’s regulatory policy for nanotechnology risks has started from 2004 aiming to tighten control and to improve regulatory adequacy and knowledge of nanotechnology risks. Although nanotechnology is among the largest EU-regulated industries and a policy domain in which EU regulatory activities continue to grow, political perspective (actors, institutions and processes) remain underexplored. The present European Union laws and regulations ensure that all applications of nanoscience and nanotechnologies meet a high level of public health, safety and environmental protection [73-74].
Conclusions
Nanotechnology has gained a great deal of public interest due to the wide range of applications of nanomaterials in many areas of human endeavours including industry, medicine and public health. In the last decade nanotechnology applications were introduced in drug delivery methods, biomedical imaging, targeted anticancer pharmaceuticals, biological sensing and antibiotic carriers. There is inevitably a heated debate among scientists for the future implications of nanomedicine and novel therapeutic agents in the prevention and cure of diseases. Also, nanotechnological applications raised concerns for health and safety issues of workers in the manufacture of nanomaterials, as well for users and consumers. Research showed that some nanomaterials have the potential for human toxicity and environmental pollution. These concerns have led to a debate among advocacy groups and governments. In the last years there were tighter national and international regulations for nanotechnology products.
References
- Wilson M, Kannangara K, Smith G, Simmons M, Raguse B. Nanotechnology: basic science and emerging technologies. Chapman and Hall/CRC, Boca Raton (2002).
- Tuan VD. Nanotechnology in biology and medicine: methods, devices, and applications. CRC Press, Boca Raton (2007).
- Sezer AD. Application of nanotechnology in drug delivery. InTech publications, Croatia(2014).
- Tiwari A, Tiwari A. Nanomaterials in drug delivery, imaging, and tissue engineering. Hobogen, John Wiley & Sons, New Jersey (2013).
- Prokopovich P. Biological pharmacological applications of nanomaterials. CRC Press, Taylor & Francis, Boca Raton (2015).
- Royal Society and Royal Academy of Engineering. Nanoscience and Nanotechnologies: Opportunities and Uncertainties. RS Policy Document 19/04, London (2013).
- National Nanotechnology Initiative. Collaborative central point of Federal Agencies in the USA, Arlington (2000).
- European Commission Research DG, Unit G4. Nanosciences and nanotechnologies. Some figures about nanotechnology R&D in Europe and beyond (2005).
- Cientifica Ltd. Global funding of nanotechnology and its impact, London
- OECD/NNI. Organization for Economic Co-operation and Development/National Nanotechnology Initiative. International Symposium on Assessing Impact of Nanotechnology, Washington DC (2012).
- The Woodrow Wilson International Center for Scholars. Project on Emerging Nanotechnologies, Washington DC (2005).
- United States Government Accountability Office (GAO) Report. Nanotechnology. Nanomaterials are widely used in commerce, but EPA faces challenges in regulating risk. GAO Publications, Washington DC (2010).
- European Commission, Research Directorate General, Directorate. Outcome of the workshops organised by the EC. Future needs and challenges for material and nanotechnology research, Brussels (2001).
- Hall JS. Nanofuture: What's next for nanotechnology. Prometheus Books, New York (2005).
- Morigi V, Tocchio A, Pellegrini CB, et al. Nanotechnology in medicine from inception to market domination. J.Drug.Deliv. 389485, 1-7 (2012).
- BCC Research. Nanotechnology in medical applications: The global market (2012).
- Hubbell JA, Chilkoti A. Nanomaterials for drug delivery. Science. 337, 303-305 (2012).
- Sezer AD. Nanotechnology and nanomaterials application of nanotechnology in drug delivery. Intech Publications, Rijeca (2014).
- Lu ZR, Sakuma S. Nanomaterials in pharmacology. Series Biomedical Sciences Pharmacology and Toxicology, Methods in Pharmacology and Toxicology. Springer, Heidelberg (2016).
- Zhang L, Pompattananangkul D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Review. Curr.Medic.Chem. 17, 585-594 (2010).
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Current.Opin.Chem.Biol. 9, 674-679 (2005).
- Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, et al. Carbon nanotubes for delivery of small molecule drugs. Advanc.Drug.Deliv.Revs. 65, 1964-2015 (2013).
- Fabbro C, Ali-Boucetta H, Ros TD, Kostarelos K, Bianco A, et al. Targeting carbon nanotubes against cancer. Chem.Commun.48, 3911-3026 (2012).
- Medina SH, El-Sayed MEH. Dendrimers as carriers for delivery o chemotherapeutic agents. Chem.Rev. 109, 3141-3157 (2009).
- Gardikis K, Micha-Screttas M, Demetzos C, Steele BR. Dendrimers and the development of new complex nanomaterials for biomedical applications. Curr.Med.Chem. 19, 4913-4928 (2012).
- Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues.J.Pharm.Bioallied.Sci. 6, 139-150 (2014).
- Teow HM, Zhou Z, Najlah M, et al. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier.Int.J.Pharm. 441, 701-711 (2013).
- National Cancer Institute. A Snapshot of Nanotechnology. What is nanotechnology? (2015).
- Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H. Nanomaterials: Applications in Cancer Imaging and Therapy. Review. Adv. Healthcare. Mater.23, H18-H40 (2011).
- Nazir S, Hussain T, Ayub A, Rashid U, MacRobert AJ. Nanomaterials in combating cancer: Therapeutic applications and developments. Review. Nanomed.Nanotechnol.Biol.Medic. 10, 19-34 (2014).
- Yan G, Jacson KS, Lara M, et al. Targeted cancer therapy; nanotechnology. Approaches for overcoming drug resistance. Curr.Medic.Chem.22, 1335-1347 (2015).
- Li C. A multifunctional targeted approach. Nat. Mater. 13: 110-115 (2014).
- Bertrand N, Wu J, Xu X, Nazila Kamaly K, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug. Deliv. Rev. 66, 2-25 (2014).
- Chapman S, Dobrovolskaia M, Farahani K, et al. Nanoparticles for cancer imaging: The good, the bad, and the promise. Nanotoday. 8, 454-460 (2013).
- Qi LJ, Chu T, Peng B, Luo J, Zhiyong FQ. Multifunctional nanostructured materials for multimodal cancer imaging and therapy. J.Nanosci.Nanotechnol. 14, 175-189 (2014).
- Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Ann.Rev.Biomed.Eng. 15, 253-282(2013).
- Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2, 3-44 (2012).
- Jia F, Liu X, Li L, et al. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. J.Control.Real. 172, 1020-1034 (2013).
- Etheridge ML, Campbell SA, Erdman AG, et al. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Medicine. 9, 1-14 (2013).
- Demchenko AP. Nanoparticles and nanocomposites for fluorescence sensing and imaging. Methods.Appl.Fluoresc. 1, 1-28 (2013).
- Philip D, Howes PD, Chandrawati R, Stevens MM. Colloidal nanoparticles as advanced biological sensors. Science. 346, 42-45 (2014).
- Holzinger M, Le Goff A, Cosnier S. Nanomaterials for biosensing applications: a review. Front.Chem. 2, 63 (2014)
- Hou W, Cronin SB. A review of surface plasmon resonance-enhanced photocatalysis. Advanc.Funct.Ma.t 23, 1612-1619 (2013).
- Sharma A, Arya DK, Dua M, Chhatwal GS, Johri AK. Nano-technology for targeted drug delivery to combat antibiotic resistance. Editorial.Expert.Opin.Drug.Deliv. 9, 1325-1332 (2012).
- Huh AJ, Kwon YJ. Nanoantibiotics: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. Control.Rel. 156, 128-145 (2011).
- Radovic-Moreno AF, Lu TK, Puscasu VA, et al. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS.Nano. 6, 4279-4287 (2012).
- Zhu X, Radovic-Moreno AF, Wu J, Langer R, Shi J. Nanomedicine in the management of microbial infection – Overview and perspectives. Nanotoday. 9, 478-498 (2014).
- Abed N, Couvreur P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int.J.Antimicrob.Agents. 43, 485-496 (2014).
- Dvir T, Timko BP, Kohane DS, Langer R. Nanotechno-logical strategies for engineering complex tissues. Nature.Nanotechnol. 6, 13-22 (2011).
- Padfmanabhan J, Kyriakides TR. Nanomaterials, inflammation and tissue engineering. Nanomedic.Nanobiotechnol. 7, 355-370 (2015).
- Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue.Engineer. 11, 101-109 (2005).
- MacDonald RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP. Collagen–carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomedical.Mat.Res.Part A. 74, 489-496 (2005).
- Shadjou N, Hasanzadeh M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: recent progress. Review. Mater.Sci.Engin. 55, 401-409 (2015).
- Hull M, Bowman D. Nanotechnology Environmental health and safety: Risks, regulation, and management. Elsevier, Amsterdam, Boston (2014).
- Asmatulu R. Nanotechnology safety. Elsevier, Amsterdam (2013).
- Malsch I, Emond C. Nanotechnology and human health. CRC Press, Baton Rouge (2014).
- World Health Organization (WHO) Regional Office for Europe. Nanotechnology and human health: Scientific evidence and risk governance. Report, WHO expert meeting, Bonn (2012).
- RIVM, National Institute for Public Health and the Environment. Exposure to nanomaterials in consumer products, Bilthoven (2009).
- Tsuji JS, Maynard AD, Howard PC, James JT, et al. Research strategies for safety evaluation of nanomaterials, Part IV: Risk assessment of nanoparticles. Toxicolog.Sci.89, 42-50 (2006).
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ.Health.Perspect. 113, 823-839 (2005).
- Uchino T, Tokunaga H, Ando M, Utsumi H. Quantitative determination of OH radical generation and its cytotoxicity induced by TiO2-UVA treatment. Toxicol.Invitro. 16, 629-635 (2002).
- Zhang AP, Sun YP. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World.J.Gastroenterol. 10, 3191-3193 (2004).
- Smijs TG, Bouwstraq JA. Focus on skin as a possible port of entry for solid nanoparticles and the toxicological impact. J.Biomed.Nanotechnol. 6: 469-484 (2010).
- Crosera M, Bovenzi M, Maina G, et al. Nanoparticle dermal absorption and toxicity: a review of the literature.Int.Arch.Occup.Environ.Health.82, 1043-1055 (2009) .
- Lam C-W, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol.Sci. 77, 126-134 (2004).
- Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J.Toxicol.Environ.Health. A 66, 1909-1926 (2003).
- Shvedova AA, Kisin ES, Mercer R, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am.J.Physiol.Lung.Cel.Mol.Physiol.289, 698-708 (2005).
- Bermudez E, Mangum JB, Wong BA, et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol.Sci.77, 347-357 (2004).
- Vlachogianni T, Fiotakis K, Loridas S, Perdicaris S, Valavanidis A. Potential toxicity and safety evaluation of nanomaterials for the respiratory system and lung cancer. Lung.Cancer.Target.Therap.4, 71-82 (2013).
- Shi H, Magaye R, Castranova V, Zhao J.Titanium dioxide nanoparticles: a review of current toxicological data. Part.Fibre.Toxicol. 10, 15 (2013).
- Schulte PA, Geraci CL, Murashov V, Kuempel ED, Zumwalde RD. Occupational safety and health criteria for responsible development of nanotechnology. J.Nanopart.Res. 16, 2153 (2014).
- EPA. Research on evaluating nanomaterials for chemical safety (2015).
- Justo-Hanani R, Dayan T. European risk governance of nanotechnology: Explaining the emerging regulatory policy. Res.Policy.44, 1527-1536 (2015).
- Mehra NK, Jain K, Jain NK. Pharmaceutical and biomedical applications of surface engineered carbon nanotubes. Drug.Discov. Today. 20(6), 750-759 (2015).