Perspective - Imaging in Medicine (2014) Volume 6, Issue 1
Theranostic MRI: the future for Type 1 diabetes management?
Ping Wang1 & Anna Moore*,11Molecular Imaging Laboratory, MGH/MIT/HMS Athinoula A Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02129, USA
- Corresponding Author:
- Anna Moore
Molecular Imaging Laboratory
MGH/MIT/HMS Athinoula A Martinos Center for Biomedical Imaging
Department of Radiology, Massachusetts General Hospital
Harvard Medical School, Boston, MA 02129, USA
E-mail: amoore@helix.mgh.harvard.edu
Abstract
Diabetes mellitus is the most common metabolic disorder worldwide. Type 1 diabetes mellitus results from the lack of insulin production induced by autoimmune destruction of pancreatic β-cells. Theranostic MRI is an emerging field that uses nanometerscale materials to provide diagnostic insight with simultaneous treatment. These nanoparticle platforms can accommodate targeting ligands, therapeutic moieties, along with complementary imaging modalities for targeted therapeutic goals, while achieving highly accurate multimodality imaging. In this review, we summarize various types of nanocarriers that have been explored for theranostic imaging. We also outline recent progress in theranostic MRI for Type 1 diabetes management. Finally, we discuss future considerations and opportunities afforded by theranostic imaging as a new platform in this field.
Keywords
diabetes • islet transplantation • molecular imaging • MRI
Type 1 diabetes (T1D), which accounts for an estimated 5–10% of diabetic Americans, is a chronic and potentially disabling disease that represents a major public health and clinical concern. T1D is an autoimmune disease in which CD4+ and CD8+ T cells infiltrate the islets of Langerhans, resulting in β-cell destruction, leaving patients dependent upon exogenous insulin for survival [1]. Despite advances in diabetes technologies and therapeutics, the majority of T1D patients do not reach the recommended glycemic goals and remain at high risk for developing microvascular complications [2]. Insulin has been proven to control hyperglycemia and delay progression of some complications [3]. However, a clear need exists to develop novel treatments that can either affect the underlying pathophysiology of the disease and/or help patients with established disease to improve glycemic control [4]. science, synthetic chemistry and molecular imaging.
The term ‘theranostic’ is defined as a material that combines the modalities of therapy and diagnostic imaging [5]. In contrast to the development and use of separate materials for these two objectives, theranostics combine these features into one ‘package’, which has the potential to overcome undesirable differences in biodistribution and selectivity that currently exist between distinct imaging and therapeutic agents [6–10].
MRI does not utilize ionizing radiation, has tomographic capabilities, can deliver the highest resolution images in vivo and has unlimited depth penetration [11]. The contrast used for MRI originates from local variations of water protons and the chemically bound state of proton concentrations in tissues [12]. Two relaxation time constants, including spin–lattice relaxation (T1) and spin–spin relaxation (T2), associated with the time decay of proton magnetization back to equilibrium following a radio-frequency pulse, are used to measure intrinsic tissue variations that generate anatomical contrast [13]. Since MRI is not sensitive enough for early diagnosis, development of the novel MRI agents for achieving higher sensitivity is equally important. Magnetic nanoparticles are among the most widely utilized contrast agents for MRI. Not only can magnetic nanoparticles be functional as imaging tools, but they can also be useful as a therapeutic moiety carrier and an actuator for controlled drug release, which potentially makes MRI one of the most important modalities for the application of theranostic imaging [14].
During recent years, considerable efforts have been devoted to the development of the theranostic MRI for T1D and islet transplantation. This current article summarizes the latest developments in various theranostic MRI probes designed for T1D treatment and management.
Theranostic nanocarriers as smart actuators for MRI
Multidisciplinary field of theranostic imaging combines molecular biology, synthetic chemistry and nanotechnology. Nanoparticles used for imaging are generally categorized as organic and inorganic materials. Organic nanoparticles include polymers, polymeric micelles and dendrimers. They are developed primarily for drug delivery [14]. Compared with organic nanoparticles, inorganic platforms posses more diverse and distinct physical features contingent to their size and composition. Of particular significance is the use of magnetic nanoparticles for theranostic MRI. The ease of the synthesis of magnetic nanoparticles and subsequent surface modifications to introduce additional therapeutic and targeting functionalities has enabled these systems to be employed as a smart platform for the MRI. The composition and size of these nanometer -sized materials enable multiple components to be carried. Various cell-specific imaging, targeting and therapeutic moieties can be incorporated into a single magnetic nanoparticle that is designed for theranostic MRI [15,16].
Imaging reagents used for theranostic MRI
The ideal theranostic MRI probes should be able to:
• Selectively accumulate in target tissues or cells;
• Effectively deliver therapeutic moieties;
• Provide morphological and functional information of the area;
• Biodegrade with safe byproducts [7].
Although no probe has achieved all of the above criteria, some of them possess one or more features. To date, the probes applied in theranostic MRI include iron oxide nanoparticles, gadolinium (Gd)-loaded nanoparticles and manganese (Mn)-based nanoparticles.
Iron oxide nanoparticles for theranostic MRI
Superparamagnetic iron oxide (SPIO) nanoparticles used for theranostic MRI are usually composed of three components:
• A biodegradable superparamagnetic iron core for MRI;
• A polymer surface coating, that not only serves as a protective layer, but also as a functional layer decorated with various linkers;
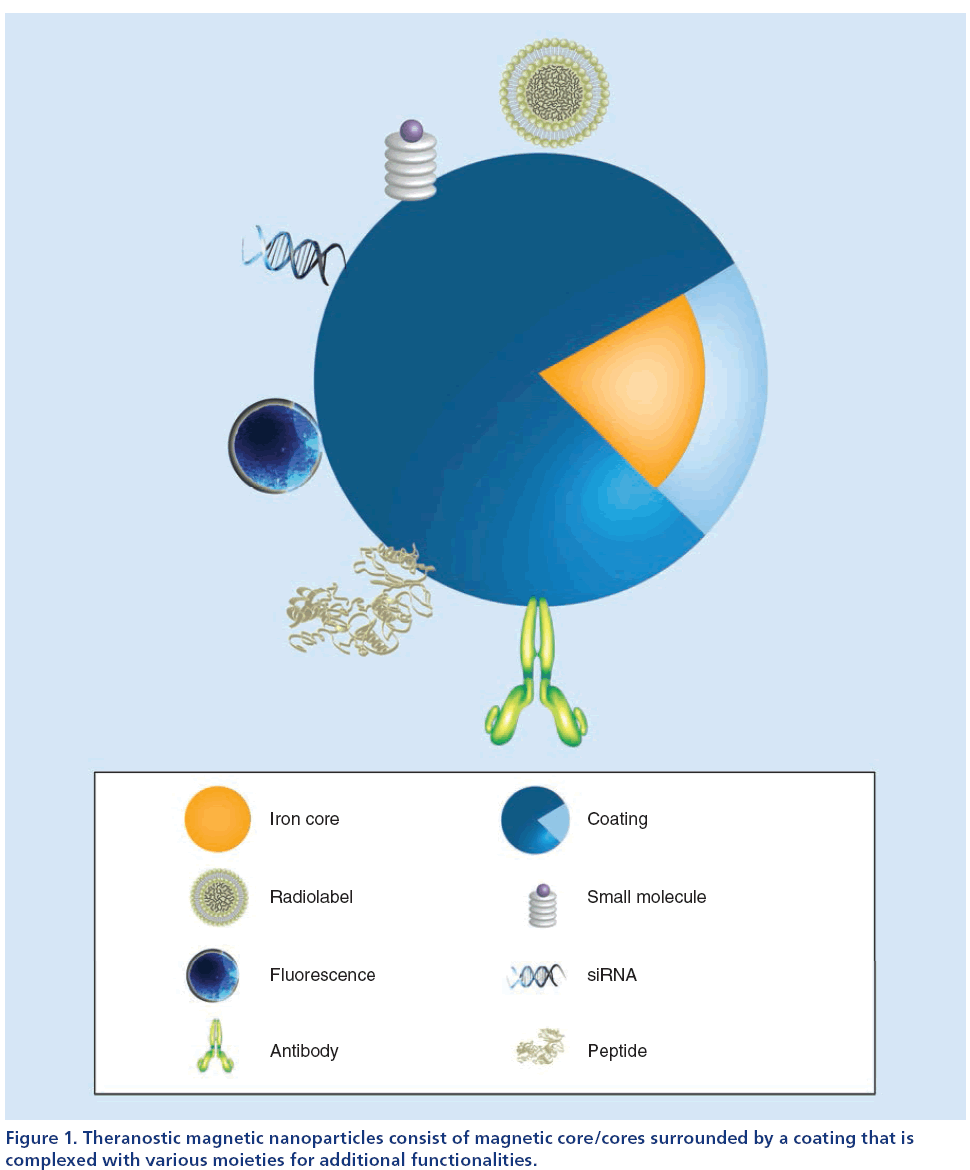
• Various functional moieties attached to the coating that serve as targeting macromolecules, therapeutic payloads or additional imaging tags (Figure 1) [14,17].
Figure 1: Theranostic magnetic nanoparticles consist of magnetic core/cores surrounded by a coating that is complexed with various moieties for additional functionalities.
The iron oxide core affects the transverse (spin–spin) relaxation time of protons in nearby tissues. It decreases the T2 relaxation time, which can be efficiently visualized as a darkening of the tissues on T2-weighted MRIs. There have been several coating materials utilized for stabilizing the particles, controlling their size and transforming them into multifunctional nanodrugs, such as polyethylene glycol (PEG), dextran, silica, polyethylenimine, polyvinylpyrrolidone, fatty acids, polypeptides, chitosan and gelatin, among others [17]. Iron oxide nanoparticles are clinically approved under many brand names including Resovist® (Schering AG, Germany), Ferumoxtran-10 (Sinerem®, Guerbet, France; Combidex® Advanced Magnetics Inc., MA, USA), Gastromark® (AMAG Pharmaceuticals, Inc., MA, USA) and Feridex® (AMAG Pharmaceuticals, Inc., MA, USA).
Iron oxide magnetic nanoparticles are useful for visualizing the location and trafficking of therapeutic moieties in vivo. It is also possible to encapsulate these nanoparticles with drugs in a nanogel for monitoring drug release in the target areas. Zhang et al. developed multifunctional and degradable nanogels encapsulating both model drug (fluorescently labeled dextran) and iron oxide nanoparticles by polymerizing zwitterionic monomers with a disulfide crosslinker. This theranostic nanogel exhibited targeted delivery to human umbilical vein endothelial cells. Once the nanogels enter the reducing intracellular zone, the disulfide bonds are cleaved thus releasing the sample drug, which could be imaged by MRI [18].
Renal excretion represents an important route of elimination for circulating SPIO nanoparticles. Studies have demonstrated that SPIO nanopaticles with dextran coating underwent nearly 25% elimination via the urine and feces over a period of 19 days in animals [19]. The internalized iron oxide nanoparticles get degraded at low lyzosomal pH, releasing free Fe(III) into the cytoplasm. The released iron is stored in the body reserves with the help of iron-regulating proteins such as ferritin and hemosiderin [20]. Particle size, surface coating and surface charge are major determinants of the biodistribution and pharmacokinetics of SPIO nanoparticles [21].
SPIO nanoparticles, as a negative contrast agent, has certain shortcomings. T2- and T2*-weighted images generally provide lower resolution compared with T1-weighted images. In some cases, owing to their negative contrast, it is difficult to distinguish iron oxide nanoparticles from other hypointensities in the MRI, which may arise from vascular hemorrhage or iron-rich tissues, such as the spleen and liver [22,23].
Gd-loaded nanoparticles for theranostic MRI
Gd-based contrast agents are paramagnetic contrast agents that mainly reduce T1 relaxation time resulting in positive (brighter) contrast in T1-weighted images [24]. The most commonly used Gd-based agents are usually obtained by compexation of the Gd3+ ion with chelates, such as diethylene-triamine-pentaacetic acid, 1,4,7,10-tetraazacyclo odecane-1,4,7,10-tetraacetic acid and di-pyridoxyl-di-phosphate [25,26]. Gd complexes can also be conjugated to different enzymes or ligands for theranostic MRI. Major classes of Gdloaded nanoparticles include lipid-based nanoparticles, polymeric nanoparticles, micelles, dendrimers and Gd–silica nanoparticles [9].
Tse et al. prepared Gd and europium-doped silicate nanoparticles that act as bimodal imaging agents for MRI and luminescence. The longitudinal relaxivity and transverse relaxivity, a measure of MRI contrast agent efficiency, were up to four-times higher than that of the clinically employed OmniscanTM (gadodiamide; GE Healthcare Inc., WI, USA). In addition, these mesoporous nanoparticles have the potential to serve as controlled release matrices for theranostic imaging [27].
Gd-based contrast agents have also been applied for encapsulation for cell tracking and theranostic MRI. Arifin et al. introduced a biohybrid theranostic agent, in which Gd-based nanoparticles served as trimodal diagnostic markers for cell transplantation monitoring. The grafts were successfully localized with T1- weighted MRI. By placing the contrast agent formulation in the extracellular space of the hydrogel, large amounts of contrast agents were incorporated with negligible toxicity. Besides the diagnostic function, the encapsulation also protected the grafts from immune rejection [28,29].
A potential issue in this approach is that free Gd ions are cytotoxic and could be retained in the liver, spleen and other organs. Gd-based contrast agents have been associated with nephrogenic systemic fibrosis in patients with severe renal diseases [30].
Mn-based nanoparticles for theranostic imaging
Mn has paramagnetic properties with five unpaired electrons that permit a high spin number, long electronic relaxation times and labile water exchange [31]. Mn-based contrast agents can be classified into two types: small molecule agents and macromolecular agents. Small molecule agents are, similar to Gd chelates, complexes of Mn2+ ions with chelates such as di-pyridoxyl-di-phosphate or diethylene-triaminepentaacetic acid. Macromolecular agents consist of Mn oxides such as MnO, MnO2 and Mn3O4[32]. These oxides can be formulated into nanoparticles, which could be functionalized for theranostic purposes [26,33].
Bae et al. synthesized multifunctional hollow MnO nanoparticles by a bioinspired surface functionalization approach, using 3,4-dihydroxy-l-phenylalanine (DOPA) as an adhesive moiety, for targeted delivery of therapeutic gene silencing and simultaneous MRI monitoring [34]. Howell et al. reported the design and synthesis of multifunctional lipid–micellar nanoparticles containing MnO for theranostic MRI. Oleic acid-coated MnO nanoparticles were encapsulated in micelles composed of polyethylene glycol, phosphatidylethanolamine, cholesteryl 3β-N-(di-methyl-aminoethyl)- carbamate hydrochloride and dioleoyl-phosphatidylethanolamine. The particles were loaded with plasmid and could be efficiently taken up by target cells in vivo. These results demonstrate that Mn-based nanoparticles are capable of theranostic MRI [31].
Although these proof-of-concept studies demonstrate the exciting advances in preparing Mn-based nanoparticles, some issues including cytotoxicity still need to be considered. Studies show that the brain is vulnerable to Mn exposure. Symptoms comparable to the characteristics of Parkinson’s disease have been reported [33].
Therapeutic & target moieties for theranostic MRI
Advances in nanoparticles technology have created new paradigms for theranostics imaging. Simultaneous- targeted imaging and therapy are made possible by attaching a variety of imaging and therapeutic components [14]. Nanoparticle vehicles can accommodate a wide variety of drug candidates that have been shelved owing to solubility or pharmacokinetic reasons. In addition to small molecules, other moieties such as siRNAs, DNAs and peptides can effectively be delivered via these carrier platforms [14,16].
siRNAs
siRNAs are short double-stranded RNA containing 19-23 nucleotides. They can suppress a target complementary gene at the post-transcriptional mRNA level by means of RNAi, which is a promising therapeutic tool for a variety of diseases including diabetes. So far, the delivery of naked siRNAs is hindered by the nonspecific distribution, poor cellular uptake and the failure of endosomal escape [35]. Nanoparticles have several favorable attributes such as uniform size, superior biocompatibility and facile surface modification that qualify them as candidates for siRNA in vivo delivery tools. At the same time, magnetic nanoparticles can serve as a real-time imaging agents for monitoring siRNA transport into the cell and subsequent release at the target site. For in vivo delivery, siRNA is conjugated to the nanoparticles via disulfide linkage that can be cleaved enzymatically for its facile release. Subsequent endocytosed nanoparticles escape from the endosome, glutathione breaks the disulfide bond to release the siRNA for the inhibition of protein expression [36].
DNA
Biological molecules such as DNA represent the genetic material of most organisms and organelles. Most hereditary information is encoded in the chemical language of DNA and reproduced in most cells of living organisms [37]. The double-stranded helical structure of DNA is a key to its use in applications of replacing a mutated gene causing diseases with a healthy copy of the same gene.
Bhakta et al. developed Gd oxide-doped silica nanoparticles (50 nm), the surface of which was functionalized to anchor DNA. The surface of the nanoparticle was modified by 3-aminopropyltrimethoxysilane, which allowed for electrostatic binding of DNA. The plasmid DNA held over the surface of the nanoparticle was firmly immobilized and protected from DNase attack. These particles are paramagnetic with high transfection efficiencies in cells in vitro [38].
Chen et al. developed a nanoplatform that effectively transports plasmid DNA into T cells by attaching a T-cell specific ligand, the CD3 single-chain antibody, to the ends of PEG-grafted polyethylenimine. This polymer was first complexed with SPIO nanoparticles and was subsequently used to condense plasmid DNA into nanoparticles. The reporter gene assay demonstrated that the nanoplatform functionalized with a targeting ligand, produced a 16-fold increase in gene transfection in a T-lymphocyte cell line. In addition, MRI successfully visualized this targeting event in cell culture [39].
As a versatile gene vector, minicircle DNA (mcDNA) has a great potential for gene therapy. However, some serious challenges remains, such as effective delivery of mcDNA into targeted cells/tissues and noninvasive monitoring of mcDNA delivery. Wan et al. developed an MRI visible gene delivery system with a core consisting of SPIO nanocrystals and a shell made out of biodegradable stearic acid modified with lowmolecular- weight polyethyleneimine via self-assembly. Furthermore, the nanoparticle could effectively bind with mcDNA and protect it from enzymatic degradation [40].
miRNA
miRNAs are small noncoding RNA molecules consisting of 21–23 nucleotides that regulate gene expression by targeting mRNAs for either cleavage or translational repression [41]. They have been shown to play important roles in a broad range of biological processes including development, cellular differentiation, proliferation and apoptosis by association with the 3´ untranslated region of target mRNAs [42–44]. It is now becoming clear that miRNAs make an important contribution to alterations in gene expression observed in dysfunctional β-cells and are likely to be involved in the development of T1D [45]. On the other hand, miRNAs could play an important role in T1D therapy. For example, Cantaluppi et al. [46] reported that microvesicles released from endothelial progenitor cells could enhance human islet vascularization by carrying proangiogenic miR-126 and miR-296 targeting islet endothelium. The development of theranostic imaging techniques could assist in delivering of miRNA mimics or anti-miRNA nucleotides to correct the level of key miRNAs under diabetic conditions and lead to new strategies for treating T1D [47].
Schade et al. developed a technique to efficiently deliver miRNA into bone marrow-derived human mesenchymal stem cells with the help of a magnetic nonviral vector based on cationic polymer polyethylenimine bound to SPIO nanoparticles. This study shows high potential of theranostic imaging in stem cells transplantation for diabetes treatment [48].
Here, we summarize contrast agents and therapeutic moieties that appeared to be promising for the theranostic imaging of T1D. These tools have to be tested further for T1D specific pathological targets.
T1D pathological targets for theranostic MRI
T1D is an autoimmune disease characterized by complex lymphocytic infiltrate accompanied by a range of alterations in the microvasculature, culminating in specific destruction of insulin-producing β-cells [49]. Microvasculature leakage, mononuclear cell infiltration of the pancreatic islets and β-cell destruction are the pathological hallmarks of T1D [50–52]. New strategies, involving theranostic imaging, have been designed to target these biomarkers for the purpose of prevention and therapy.
Imaging of vasculature changes during the progression of inflammation
The progression of inflammation is associated with changes in pancreatic islet vasculature and subsequent vasculature dysfunction. At sites of inflammation, blood vessels become ‘leaky’ and allow large molecules to extravasate through the walls of the damaged vessel into the surrounding tissue. This process of passive accumulation of nanoparticles at sites through leaky vasculature, is known as the enhanced permeability and retention effect, which is sufficient to favorably alter biodistribution in order to improve the diagnostic and therapeutic efficacy [16]. Vascular leakage could be utilized for delivering therapeutic and imaging agents to the islets. Our group demonstrated that this property of islet vasculature could be exploited for delivery of protected graft copolymer (PGC) labeled with Gd diethlene-trimine-pentaacetic acid and fluorescein, a T1 contrast blood pool agent. in vivo MRI was used to demonstrate accumulation of this agent in the areas of leaky vasculature in the islets of streptozotocin (STZ)-induced diabetic mice [53]. Subsequently, the same contrast agent was utilized to assess changes in islet vasculature in a spontaneous T1D animal model. It was demonstrated that PGC labeled with Gd diethlene-trimine-pentaacetic acid and fluorescein, could highlight changes in vascular permeability and blood volume of diseased pancreatic islets [54].
For therapeutic applications, Castillo et al. utilized fatty acid-containing PGC for delivery of GLP-1 to pancreatic β-cells for treatment of Type 2 diabetes [55]. GLP-1 is a hormone that inhibits β-cell apoptosis, restores glucose sensitivity and stimulates β-cell proliferation and differentiation [56]. Native GLP-1, which is cleaved by DPP-4, has a very short blood half-life. In the above study, PGC was used to stabilize GLP-1, prolonging its blood half-life and, ultimately, delivering it to pancreatic islets. in vivo data proved higher efficacy of PGC-GLP-1 in maintaining blood glucose levels in diabetic male Zucker fatty rats compared with exendin-4 [55]. This study indicates theranostic potential of a long circulating blood pool agent for diabetes treatment.
Superparamagnetic T2 contrast agents have also been exploited for visualization of the changes in the microvasculature that invariably accompany inflammation. SPIO nanoparticles were used for detection of vascular leakage in association with insulitis in murine models of T1D, permitting noninvasive visualization of the inflammatory lesions in real time [57,58]. Pushing this work forward to clinic translation, Gaglia et al. reported on the development of a MRI method to visualize active insulitis in T1D patients. Ten patients and 12 healthy controls received T2 contrast agent SPIO (ferumoxtran-10) at a dose of 2.6 mg of iron/kg of bodyweight over a period of 30 min. MRI analysis of the pancreas on three consecutive slices demonstrated lower T2 relaxation time within the pancreas at 48 h, suggesting higher retention of the probe in the patient compared to control subjects. They also found that the patient had more heterogeneity in T2 throughout the pancreas with a greater change in T2 in the head and body compared with the tail of the pancreas [59]. The authors believe that these SPIO particles could migrate from the leaky vessels into the surrounding tissue, where they are phagocytized by inflammatory cells [59]. The road to the above studies was paved in earlier publications describing labeling of these inflammatory cells with magnetic nanoparticles for the purpose of detection of early insulitis [60,61]. These studies are discussed in more detail below.
Theranostic imaging of the lymphocyte infiltration during the progression of inflammation
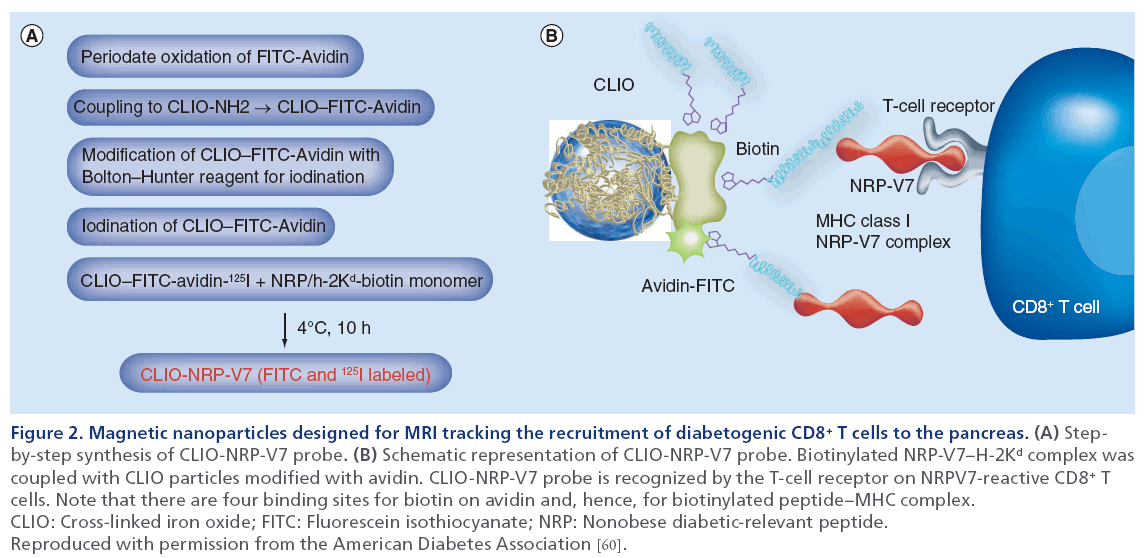
Chronic infiltration of islets by autoreactive T cells, results in the destruction of insulin-producing β-cells, leading to the onset of T1D. Therefore, most therapeutic strategies target lymphocyte populations responsible for this destruction [62]. Development of noninvasive imaging techniques for tracking infiltrating T cells in vivo could aid in early diagnosis and treatment of diabetes [10]. Several studies have already demonstrated the potential of in vivo MRI for tracking labeled cytotoxic T lymphocytes infiltrating pancreatic islet in different animal models (Figure 2) [60,61,63,64].
Figure 2: Magnetic nanoparticles designed for MRI tracking the recruitment of diabetogenic CD8+ T cells to the pancreas. (A) Stepby- step synthesis of CLIO-NRP-V7 probe. (B) Schematic representation of CLIO-NRP-V7 probe. Biotinylated NRP-V7–H-2Kd complex was coupled with CLIO particles modified with avidin. CLIO-NRP-V7 probe is recognized by the T-cell receptor on NRPV7-reactive CD8+ T cells. Note that there are four binding sites for biotin on avidin and, hence, for biotinylated peptide–MHC complex. CLIO: Cross-linked iron oxide; FITC: Fluorescein isothiocyanate; NRP: Nonobese diabetic-relevant peptide. Reproduced with permission from the American Diabetes Association [60].
To selectively image T cells involved in autoantigen recognition, antigen-specific SPIO nanoparticles were synthesized by Medarova et al. [65]. They consisted of iron oxide nanoparticles conjugated through avidin– biotin linkage to a complex of MHC with NRP-V7 peptide (a high-avidity mimotope of IGRP) [49,66]). Subsequent to intravenous injection in nonobese diabetic (NOD) mice of different age, the target-specific nanoparticles recognized H-2Kd-restricted CD8+ T cells infiltrating pancreatic islets. As determined by MRI analysis of pancreas-associated T2 relaxation times, accumulation of the nanoparticles in the pancreata was age-dependent, correlated well with the progression of insulitis and provided quantitative information on the infiltration of CD8+ T cells carrying TCR specific for NRP-V7 peptides. This study indicated that this approach could serve as a surrogate marker for selecting T1D patients who might benefit from immunosuppressive therapy at the early stage of the disease. Having proved accumulation of target-specific nanoparticles in antigen-specific T-cells, we subsequently investigated the utility of these complexes for theranostic purposes. Studies in NOD mice demonstrated that accumulation of nanoparticles coated with disease-relevant peptide–MHC complexes led to protection from and reversal of diabetes in these animals by expanding (in an epitope-specific manner) a subset of antigen-experienced autoreactive CD8+ cells (Tregs) that suppressed the activation and recruitment of noncognate specificities to islets [67]. This study demonstrated that target-specific nanoparticles have the potential to become powerful theranostic tools for diabetes treatment [67].
Development of theranostic probes for targeting endogenous pancreatic β-cells
The availability of β-cell-specific imaging agents is of critical importance for advancement of our understanding and treatment of T1D [68]. To date, approaches that have been explored for developing β-cell-specific MRI probes included Mn-enhanced MRI [69], zinc-responsive T1 agent [70] and GLP-1 receptor (GLP-1R)-targeting iron oxide nanoparticles [71]. Among these probes, GLP-1R-targeting nanoparticles seem to be one of the candidates for theranostic MRI of endogenous islets.
Exploiting the fact that GLP-1R is highly expressed on β-cells [72], Zhang et al. developed targeted SPIO nanoparticles using GLP-1 analog exendin-4 as a ligand. The results demonstrate that exendin-4 conjugated nanoparticles specifically bind to and internalized by GLP-1R-expressing β-cell line [71]. Notably, systemic delivery of exendin-4-loaded magnetic nanoparticles in nude mice bearing insulinomas, led to generation of a strong MRI contrast. While these results opened the door for theranostic MRI of the native islets, they were limited to imaging insulinomas. Clearly, more studies are required to prove that imaging of endogenous islets would be possible by targeting GLP-1R, considering that this receptor is also expressed in other tissues [73]. Keeping in mind that GLP-1 and its analogs have been used for treatment of both Type 1 and 2 diabetes [74,75], the potential of this theranostic approach cannot be underestimated.
Overall, the field of theranostic MRI of pancreatic islets has undergone a significant surge; however, it still suffers from limited resolution of the imaging modality and lower specificity of the probes targeting β-cells. At present, single-cell resolution cannot yet be achieved in order to enable differentiation between scattered islets, single β-cells or surrounding tissue. Imaging biomarkers should adhere to several strict requirements. The successful candidate:
• Should be expressed specifically by β-cells and not by any other pancreatic cells;
• Should be expressed in a sufficient number of copies to be available for an imaging probe;
• Tissues surrounding the pancreas should be devoid of this marker in order to avoid high background signal from these organs during imaging [76].
In an extensive review on the etiology, immunology and therapeutic strategies of T1D published in 2011 [77], it was stated that monoclonal IgM antibody IC2 [76], which specifically binds to the surface of β-cells, might be the only reliable marker for noninvasive imaging and quantification of native β-cells [77,78]. In line with previous work carried out by Kavishawar et al., we have recently successfully identified the IC2 antigen [79], which revealed itself in a form of cholesterolstabilized sphingomyelin patches on β-cells. These findings have significant implications for developing theranostic strategies for targeting endogenous β-cells.
Theranostic MRI in islet transplantation
Islet transplantation has emerged as one of the most promising therapeutic approaches for T1D treatment during the recent decade [80]. The Edmonton protocol significantly improves the short-term rate of success of islet transplantation, with an 80% insulin independence being at 1-year postallergenic islet transplantation [81]. Unfortunately, this rate decreases dramatically to 10% by 5 years after transplantation. Studies identified that a number of immunological and nonimmunological factors contribute to islet graft loss after transplantation [82,83].
The factors that underlie the deterioration of islet-graft function include allogeneic immune response [84], recurrence of autoimmunity [85], instant blood-mediated inflammatory reaction, hypoxia-induced cell apoptosis, and nonspecific inflammation [83]. Theranostic MRI is a promising approach that enables tracking of transplanted islets and detection of the islet graft injury combined with the means of graft protection.
Theranostic MRI for graft rescue
Beyond being functionalized as a tool for diagnostic imaging of islet grafts, contrast agents can also deliver therapeutic moieties. siRNAs are small molecules that are capable of selectively silencing the expression of any gene of choice with single nucleotide specificity [86]. Taking advantage of the propensity of pancreatic islets to avidly take up dextran-coated SPIOs, Medarova et al. designed a probe that consisted of a dextran-coated iron oxide core, conjugated to siRNA. The utility of this approach was investigated by delivering siRNA-nanoparticle probes, which target genes implicated in apoptosis, to islets prior to islet transplantation. As proof-of-concept, our studies firstly demonstrated that siRNA-tagged iron nanoparticles could accumulate in pancreatic islets in quantities sufficient for detection by MRI in vitro and for silencing target genes (GFP was used as a model gene) [87]. More recent studies by Wang et al. used a theranostic nanoparticle probe to target the apoptotic-related gene caspase-3 in islets prior to transplantation. MRI showed improved survival among protected islets compared with islets in the control group [88]. We then further boosted islet survival by using a theranostic nanoparticle probe, to silence MHC class 1 molecule β-2 microglobulin, a protein associated with the histocompatibility complex that is involved in T-cell recognition of β-cells. Silencing β-2 microglobulin enabled islet protection from immune rejection caused by adoptively transferred splenocytes. MRI analysis of graft volume revealed that, as expected, volumes of both experimental and control grafts decreased post T-cell transfer. However, the rate of graft volume decrease in the experimental group was significantly lower than in the control group, consequently resulting in a delay in hyperglycemia caused by T-cell challenge. As such, the mean time for developing diabetes in the control group was 6.5 ± 4.5 days, whereas in the experimental group it was delayed by up to 23.8 ± 4.8 days [89]. Theranostic probes used in these studies successfully served as an imaging reporter for monitoring graft fate and as a vehicle for therapeutic delivery [88,89]. These studies have laid the groundwork for future clinical translation, in which genes responsible for islet damage can be targeted by delivering a cocktail of nanoparticles, or nanoparticles decorated with various sets of siRNAs, in order to improve graft outcome.
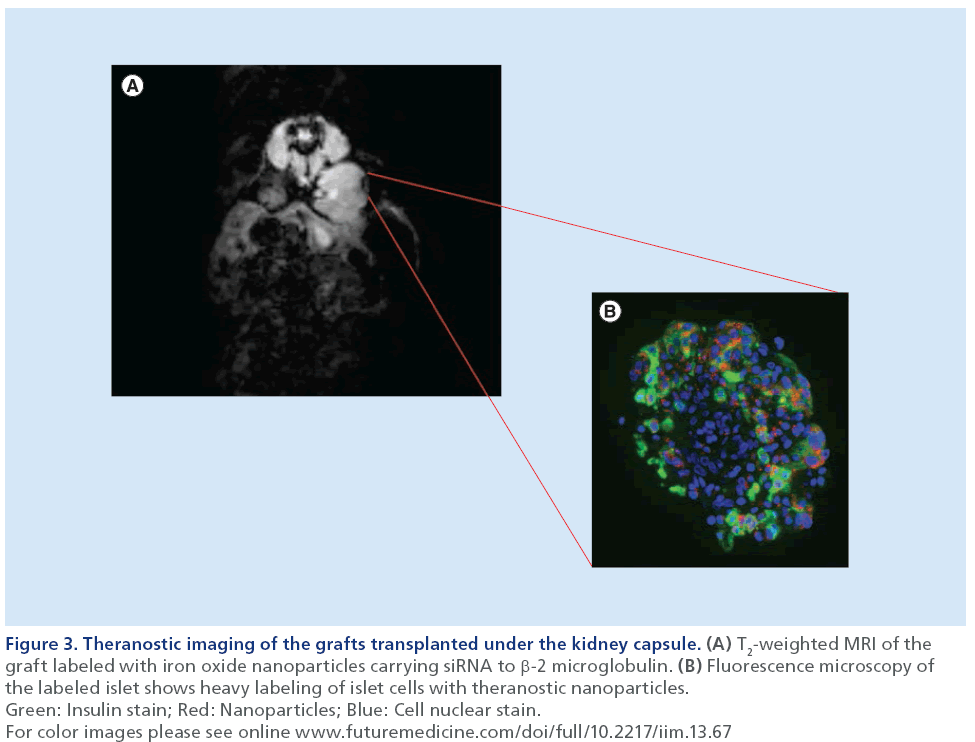
Furthermore, the inherent imaging capabilities of this approach permit the noninvasive tracking of the contrast agent conjugated to therapeutic moiety and its relationship to graft fate (Figure 3) [90,91]. Conceivably, this technique represents a valuable tool, not only for monitoring the disease onset and progression, but also for assessing the delivery of therapy using imaging-guided paradigms for future studies on T1D [92].
Figure 3: Theranostic imaging of the grafts transplanted under the kidney capsule. (A) T2-weighted MRI of the graft labeled with iron oxide nanoparticles carrying siRNA to β-2 microglobulin. (B) Fluorescence microscopy of the labeled islet shows heavy labeling of islet cells with theranostic nanoparticles. Green: Insulin stain; Red: Nanoparticles; Blue: Cell nuclear stain. For color images please see online www.futuremedicine.com/doi/full/10.2217/iim.13.67
Theranostic imaging of encapsulated islet grafts
Another theranostic MRI strategy for transplanted islets is graft encapsulation with magnetocapsules. Barnett et al. developed SPIO-labeled alginate magnetocapsules for monitoring and immunoprotection of islet grafts [93]. These magnetocapsules proved their functionality by restoring normoglycemia in STZinduced diabetic mice and in diabetic swine transplanted with human islets. In addition, MRI provided the ability to monitor distribution and localization of transplanted pancreatic islets over time in vivo in real time. Later, Barnett et al. included perfluorocarbon emulsions detectable by 19F-MRI and ultrasound, into alginate islet microcapsules with encapsulated islets. The results showed that perfluorocarbons did not alter the permeability of the capsules or affect islet function [94]. Arifin et al. encapsulated human pancreatic islets with Gd chelate-loaded magnetocapsules. Grafts were functional in vivo and normoglycemia was sustained for at least 6 weeks without the use of immunosuppressive drugs [28]. These studies demonstrated exceptional theranostic capabilities of magnetocapsules for protecting transplanted grafts from immune rejection and for tracking islets with noninvasive imaging [95,96].
Early detection of immune rejection in transplanted islets
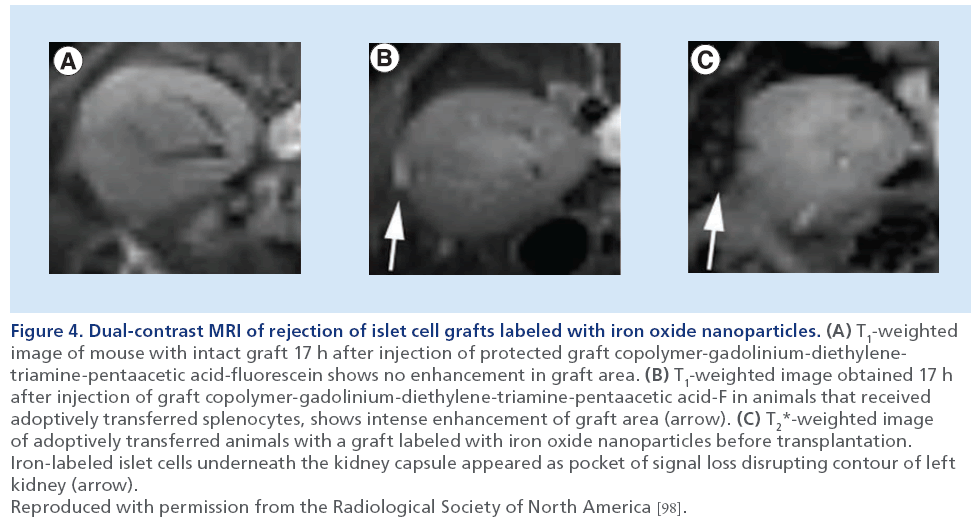
Damage, due to immunologic factors after transplantation, is one of the main reasons for islet graft failure in diabetic patients. Immune rejection contributes to long-term losses of the islet grafts. Current immune monitoring methods usually identify graft dysfunction when it is too late to take action [97]. A reliable monitoring tool is required in order to allow for earlier detection of harmful events. More importantly, detection of immune-mediated rejection at an early stage would facilitate prompt intervention. The studies by Wang et al. recently reported on an application of dual contrast-enhanced MRI for monitoring immune rejection in transplanted islet grafts at an early stage. In our studies, NOD/severe combined immunodeficiency mice were transplanted with SPIO-labeled human islets under the kidney capsule. To induce immune rejection, these mice were adoptively transferred with splenocytes from NOD diabetic mice. To detect immune attack, we utilized protected graft copolymer labeled with Gd diethlene-trimine-pentaacetic acid and fluorescein (PGC-Gd-DTPA-F), which accumulates in the sites of inflammation characterized by enhanced permeability and retention effect. MRI results demonstrated significantly greater accumulation of PGC-Gd-DTPA-F in the graft area after adoptive transfer compared with that before the transfer. Graft area was identified by the signal loss due to the presence of iron oxide-labeled islets. These results were confirmed by histological staining, which showed notable leakage of the contrast agent into the islet cell interstitium. These results demonstrated that PGC-Gd-DTPA-F-enhanced MRI allows for in vivo assessment of vascular damage of the graft due to immune rejection (Figure 4) [98,99]. Considering PGC as a versatile carrier for in vivo drug delivery [100], this platform could be used for theranostic MRI of early detection and treatment of immune rejection of islet grafts in the future.
Figure 4: Dual-contrast MRI of rejection of islet cell grafts labeled with iron oxide nanoparticles. (A) T1-weighted image of mouse with intact graft 17 h after injection of protected graft copolymer-gadolinium-diethylenetriamine- pentaacetic acid-fluorescein shows no enhancement in graft area. (B) T1-weighted image obtained 17 h after injection of graft copolymer-gadolinium-diethylene-triamine-pentaacetic acid-F in animals that received adoptively transferred splenocytes, shows intense enhancement of graft area (arrow). (C) T2*-weighted image of adoptively transferred animals with a graft labeled with iron oxide nanoparticles before transplantation. Iron-labeled islet cells underneath the kidney capsule appeared as pocket of signal loss disrupting contour of left kidney (arrow). Reproduced with permission from the Radiological Society of North America [98].
Outlook & perspective
Multimodality theranostic imaging for T1D
To date, no single imaging modality can fulfill all the requirements of specifically imaging β-cell mass and monitoring transplanted islets or inflammatory process involved in insulitis in vivo. Each imaging modality has its advantages and limitations in terms of sensitivity, tissue penetration, spatial resolution and clinical potential [13]. MRI has high spatial resolution and the highest soft-tissue contrast; however, it gives little functional and quantitative information [92]. By contrast, nuclear imaging including PET and singlephoton emission CT can give information regarding the physiological status of the particular target organ. Disadvantages of nuclear imaging include low spatial resolution and tracers’ short half-life time [68,101]. With rapid development of imaging facilities and multifunctional probes, combining complementary imaging modalities seems to be the solution of choice. Combined and simultaneous PET/MRI could provide the exact anatomical localization and the quantification information of the target organs in humans. With this new bimodal approach novel functional– anatomical and multiparametric applications become feasible that can be expected to deliver information beyond that accessible by separately applied modalities. Although the two technologies were initially regarded as inherently incompatible, different solutions have been developed and implemented to realize PET/MRI instruments for both small-animal and human-bimodal imaging [102]. In addition, novel SPIO probes conjugated with radioactive tracers has been developed [103].
in vivo imaging of stem-cell transplantation for diabetes
In T1D patients, the loss of only one cell type provides a unique opportunity as only this cell type and not a whole organ has to be generated or transplanted. In addition, the critical function of β-cells is to release insulin directly into the bloodstream, a function they can fulfill even when not placed into their original location. These aspects specific to diabetes suggest that patients suffering from T1D would be good candidates for stem-cell replacement strategies [104].
Stem cell-based therapies continue to show great promise for treatment of T1D, and significant progress has brought these therapies closer to a clinical reality through research efforts [105]. Pluripotent stem cells have the potential to differentiate into specialized cells of all three primary germ layers. Embryonic stem (ES) cells [106], induced pluripotent stem cells [107–109] and adipose tissue-derived stem cells [110–112] have to be tested for generating insulin producing cells that could potentially be used to treat T1D. Mesenchymal stromal cells, derived from bone marrow or other sources, have been utilized to improve engraftment of pancreatic islets by suppressing inflammatory damage and immune-mediated rejection [113–116].
For clinical translation, encouraging results were obtained in a small number of patients with early-onset disease who received autologous hematopoietic stem cell transplantation [117–121]. However, the results demonstrated that T1D patients responded differently to stem cell transplantation [119]. Therefore, in vivo imaging is urgently needed in this field for monitoring the survival and investigating the differentiation process of the transplanted stemss cells in real time. Recently, bioluminescence imaging (BLI) [122] was utilized for monitoring ES cell survival and differentiation into insulin-producing cells in a diabetic animal model. Raikwar et al. generated a double transgenic mouse ES cell line ectopically expressing Pdx1-Aequorea coerulescens GFP (AcGFP) fusion protein, and rat insulin promoter (RIP)-driven luciferase reporter. Real-time noninvasive BLI was used to monitor cell fate and function after transplantation. The authors speculated that pancreatic endoderm-like cells (PELCs) migrated into the STZ-damaged pancreas and differentiated into IPCs in vivo [123–125]. The differentiation of double transgenic ES cells transplanted under the renal capsule or systemically infused could be imaged by BLI as early as day 3 and until day 35 post-transplantation [123–125]. Considering that BLI is not an applicable clinical modality, MRI might be more advantageous for application in this field [23]. Magnetic nanoparticles represent a versatile platform suitable for theranostics imaging, precise drug-controlled release [126–128] and cell signaling control [36,129]. Similar to pancreatic islets, stem cells can be prelabeled with the above mentioned bifunctional nanoparticles for theranostic MRI. We are confident that theranostic MRI will play an important role for stem cell transplantation therapies for T1D in the future.
Conclusion
By combining diagnosis, therapy and targeting in one platform, theranostic imaging possesses the potential to revolutionize the arena of healthcare [35]. Although there is an agreement on the potential that the theranostic holds, significant challenges remain and these key issues need to be addressed before clinical translation of these platforms. After systemic injection, nanoparticles will be taken up by reticuloendothelial system, in which probes are rapidly shuttled out of the circulation to nontarget organs such as liver, spleen and bone marrow. Therefore, the first challenge hampering theranostic MRI is that it is crucial to maximize the interaction of theranostic probes with the target tissues and to minimize the off-target uptake by other organs [16]. Another important issue is the difference in dose and circulation time between a therapeutic drug and an imaging agent. Imaging requires a higher signal for the area of interest compared with the surrounding tissue. Accordingly, most imaging probes need to be quickly cleared from the blood. However, a therapeutic drug usually is designed to have longer circulation times for adequate uptake by target tissues. We believe multimodality imaging platforms will play significant role in resolving these problems.
Future perspective
Although still in early phases of development, theranostic imaging represents promising new directions for clinical translation [16]. Considering the current developmental stage of theranostic MRI, it is still too early to predict its success; however, rapid advances in this field have promising potential for T1D management. Multifunctional theranostic imaging may indeed revolutionize drug delivery process and dramatically alter modern medicine in the future towards personalized medicine [16].
Financial & competing interests disclosure
The authors would like to acknowledge the following support for some of the studies cited in this manuscript: JDRF grant award 37-2009-30 to A Moore, RO1DK080784 to A Moore, RO1DK078615 to A Moore and R24DK096465-01 to A Moore. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Church EJ. Imaging diabetes. Radiol. Technol. 80(4), 340–360 (2009).
- Wood JR, Miller KM, Maahs DM et al. Most youth with Type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care 36(7), 2035–2037 (2013).
- White NH, Sun W, Cleary PA et al. Effect of prior intensive therapy in Type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes 59(5), 1244–1253 (2010).
- Pettus J, Hirsch I, Edelman S. GLP-1 agonists in Type 1 diabetes. Clin. Immunol. 149(3), 317–323 (2013).
- Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug. Chem. 22(10), 1879–1903 (2011).
- Janib SM, Moses AS, Mackay JA. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 62(11), 1052–1063 (2010).
- Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 44(10), 1050–1060 (2011).
- Ma X, Zhao Y, Liang XJ. Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc. Chem. Res. 44(10), 1114–1122 (2011).
- Liu Y, Zhang N. Gadolinium loaded nanoparticles in theranostic magnetic resonance imaging. Biomaterials 33(21), 5363–5375 (2012).
- Wang P, Moore A. Theranostic magnetic resonance imaging of Type 1 diabetes and pancreatic islet transplantation. Quant. Imaging Med. Surg. 2(3), 151–162 (2012).
- Wang P, Medarova Z, Moore A. Molecular imaging: a promising tool to monitor islet transplantation. J. Transplant. 2011, 202915 (2011).
- Lauterbur PC. Image formation by induced local interactions. Examples employing nuclear magnetic resonance. 1973. Clin. Orthop. Relat. Res. 244, 3–6 (1989).
- Wang P, Moore A. Molecular imaging of stem cell transplantation for neurodegenerative diseases. Curr. Pharm. Des. 18(28), 4426–4440 (2012).
- Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 44(10), 863–874 (2011).
- Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew. Chem. Int. Ed. Engl. 48(23), 4174–4179 (2009).
- Lee DY, Li KC. Molecular theranostics: a primer for the imaging professional. AJR Am. J. Roentgenol. 197(2), 318–324 (2011).
- Yigit MV, Moore A, Medarova Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharm. Res. 29(5), 1180–1188 (2012).
- Zhang L, Xue H, Cao Z, Keefe A, Wang J, Jiang S. Multifunctional and degradable zwitterionic nanogels for targeted delivery, enhanced MR imaging, reductionsensitive drug release, and renal clearance. Biomaterials 32(20), 4604–4608 (2011).
- Weissleder R, Stark DD, Engelstad BL et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR Am. J. Roentgenol. 152(1), 167–173 (1989).
- Gu J, Xu H, Han Y et al. The internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. Sci. China. Life Sci. 54(9), 793–805 (2011).
- Wahajuddin,Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int. J. Nanomedicine 7, 3445–3471 (2012).
- Muja N, Bulte JW. Magnetic resonance imaging of cells in experimental disease models. Prog. Nucl. Magn. Reson. Spectrosc. 55(1), 61–77 (2009).
- Wang P, Moore A. Molecular imaging of stem cell transplantation for neurodegenerative diseases. Curr. Pharm. Des. 18(28), 4426–4440 (2012).
- Baumann D, Rudin M. Quantitative assessment of rat kidney function by measuring the clearance of the contrast agent Gd(DOTA) using dynamic MRI. Magn. Reson. Imaging 18(5), 587–595 (2000).
- Ai H. Layer-by-layer capsules for magnetic resonance imaging and drug delivery. Adv. Drug. Deliv. Rev. 63(9), 772–788 (2011).
- Rumenapp C, Gleich B, Haase A. Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm. Res. 29(5), 1165–1179 (2012).
- Tse NM, Kennedy DF, Kirby N et al. Mesoporous europogadolinosilicate nanoparticles as bimodal medical imaging agents and a potential theranostic platform. Adv. Healthc. Mater. 2(6), 836–845 (2013).
- Arifin DR, Long CM, Gilad AA et al. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 260(3), 790–798 (2011).
- Arifin DR, Kedziorek DA, Fu Y et al. Microencapsulated cell tracking. NMR Biomed. 26(7), 850–859 (2013).
- Grobner T. Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 21(4), 1104–1108 (2006).
- Howell M, Mallela J, Wang C et al. Manganese-loaded lipid-micellar theranostics for simultaneous drug and gene delivery to lungs. J. Control. Release 167(2), 210–218 (2013).
- Kim T, Momin E, Choi J et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adiposederived mesenchymal stem cells. J. Am. Chem. Soc. 133(9), 2955–2961 (2011).
- Pan D, Caruthers SD, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Revisiting an old friend: manganese-based MRI contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 3(2), 162–173 (2011).
- Bae KH, Lee K, Kim C, Park TG. Surface functionalized hollow manganese oxide nanoparticles for cancer targeted siRNA delivery and magnetic resonance imaging. Biomaterials 32(1), 176–184 (2011).
- Prabhu P, Patravale V. The upcoming field of theranostic nanomedicine: an overview. J. Biomed. Nanotechnol. 8(6), 859–882 (2012).
- Lee JH, Kim ES, Cho MH et al. Artificial control of cell signaling and growth by magnetic nanoparticles. Angew. Chem. Int. Ed. Engl. 49(33), 5698–5702 (2010).
- Abu-Salah KM, Ansari AA, Alrokayan SA. DNA-based applications in nanobiotechnology. J. Biomed. Biotechnol. 2010, 715295 (2010).
- Bhakta G, Sharma RK, Gupta N, Cool S, Nurcombe V, Maitra A. Multifunctional silica nanoparticles with potentials of imaging and gene delivery. Nanomedicine 7(4), 472–479 (2011).
- Chen G, Chen W, Wu Z et al. MRI-visible polymeric vector bearing CD3 single chain antibody for gene delivery to T cells for immunosuppression. Biomaterials 30(10), 1962–1970 (2009).
- Wan Q, Xie L, Gao L et al. Self-assembled magnetic theranostic nanoparticles for highly sensitive MRI of minicircle DNA delivery. Nanoscale 5(2), 744–752 (2013).
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466(7308), 835–840 (2010).
- Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
- Krek A, Grun D, Poy MN et al. Combinatorial microRNA target predictions. Nat. Genet. 37(5), 495–500 (2005).
- Lewis B, Burge C, Bartel D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 (2005).
- Roggli E, Gattesco S, Caille D et al. Changes in microRNA expression contribute to pancreatic beta-cell dysfunction in prediabetic NOD mice. Diabetes 61(7), 1742–1751 (2012).
- Cantaluppi V, Biancone L, Figliolini F et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 21(6), 1305–1320 (2012).
- Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNA-related disease? Transl. Res. 157(4), 253–264 (2011).
- Schade A, Delyagina E, Scharfenberg D et al. Innovative strategy for microRNA delivery in human mesenchymal stem cells via magnetic nanoparticles. Int. J. Mol. Sci. 14(6), 10710–10726 (2013).
- Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406(6797), 739–742 (2000).
- Di Gialleonardo V, De Vries EF, Di Girolamo M, Quintero AM, Dierckx RA, Signore A. Imaging of beta-cell mass and insulitis in insulin-dependent (Type 1) diabetes mellitus. Endocr. Rev. 33(6), 892–919 (2012).
- Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human Type 1 diabetes. Clin. Exp. Immunol. 155(2), 173–181 (2009).
- Spencer J, Peakman M. Post-mortem analysis of islet pathology in Type 1 diabetes illuminates the life and death of the beta cell. Clin. Exp. Immunol. 155(2), 125–127 (2009).
- Medarova Z, Castillo G, Dai G, Bolotin E, Bogdanov A, Moore A. Noninvasive magnetic resonance imaging of microvascular changes in Type 1 diabetes. Diabetes 56(11), 2677–2682 (2007).
- Medarova Z, Greiner DL, Ifediba M et al. Imaging the pancreatic vasculature in diabetes odels. Diabetes Metab. Res. Rev. 27(8), 767–772 (2011).
- Castillo GM, Reichstetter S, Bolotin EM. Extending residence time and stability of peptides by protected graft copolymer (PGC) excipient: GLP-1 example. Pharm. Res. 29(1), 306–318 (2012).
- Vilsboll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes. Metab. 11(Suppl. 3), 11–18 (2009).
- Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in Type 1 diabetes. Proc. Natl Acad. Sci. USA 101(34), 12634–12639 (2004).
- Turvey SE, Swart E, Denis MC et al. Noninvasive imaging of pancreatic inflammation and its reversal in Type 1 diabetes. J. Clin. Invest. 115(9), 2454–2461 (2005).
- Gaglia JL, Guimaraes AR, Harisinghani M et al. Noninvasive imaging of pancreatic islet inflammation in Type 1A diabetes patients. J. Clin. Invest. 121(1), 442–445 (2011).
- Moore A, Grimm J, Han B, Santamaria P. Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes 53(6), 1459–1466 (2004).
- Moore A, Sun PZ, Cory D, Hogemann D, Weissleder R, Lipes MA. MRI of insulitis in autoimmune diabetes. Magn. Reson. Med. 47(4), 751–758 (2002).
- Lernmark A, Larsson HE. Immune therapy in Type 1 diabetes mellitus. Nat. Rev. Endocrinol. 9(2), 92–103 (2013).
- Billotey C, Aspord C, Beuf O et al. T-cell homing to the pancreas in autoimmune mouse models of diabetes: in vivo MR imaging. Radiology 236(2), 579–587 (2005).
- Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 58(4), 725–734 (2007).
- Medarova Z, Tsai S, Evgenov N, Santamaria P, Moore A. in vivo imaging of a diabetogenic CD8+ T cell response during Type 1 diabetes progression. Magn. Reson. Med. 59(4), 712–720 (2008).
- Amrani A, Serra P, Yamanouchi J et al. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J. Immunol. 167(2), 655–666 (2001).
- Tsai S, Shameli A, Yamanouchi J et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32(4), 568–580 (2010).
- Arifin DR, Bulte JW. Imaging of pancreatic islet cells. Diabetes Metab. Res. Rev. 27(8), 761–766 (2011).
- Antkowiak PF, Stevens BK, Nunemaker CS, Mcduffie M, Epstein FH. Manganese-enhanced magnetic resonance imaging detects declining pancreatic beta-cell mass in a cyclophosphamide-accelerated mouse model of Type 1 diabetes. Diabetes 62(1), 44–48 (2013).
- Lubag AJ, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Noninvasive MRI of beta-cell function using a Zn2+- responsive contrast agent. Proc. Natl Acad. Sci. USA 108(45), 18400–18405 (2011).
- Zhang B, Yang B, Zhai C, Jiang B, Wu Y. The role of exendin-4-conjugated superparamagnetic iron oxide nanoparticles in beta-cell-targeted MRI. Biomaterials 34(23), 5843–5852 (2013).
- Tornehave D, Kristensen P, Romer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J. Histochem. Cytochem. 56(9), 841–851 (2008).
- Cline GW, Zhao X, Jakowski AB, Soeller WC, Treadway JL. Islet-selectivity of G-protein coupled receptor ligands evaluated for PET imaging of pancreatic beta-cell mass. Biochem. Biophys. Res. Commun. 412(3), 413–418 (2011).
- Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in Type 1 diabetic patients with and without residual beta-cell function. Diabetes 60(5), 1599–1607 (2011).
- Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in Type 1 diabetic patients with and without residual beta-cell function. Diabetes Care 34(7), 1463–1468 (2011).
- Moore A, Bonner-Weir S, Weissleder R. Noninvasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes 50(10), 2231–2236 (2001).
- Van Belle TL, Coppieters KT, Von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol. Rev. 91(1), 79–118 (2011).
- Malaisse WJ, Maedler K. Imaging of the beta-cells of the islets of Langerhans. Diabetes Res. Clin. Pract. 98(1), 11–18 (2012).
- Kavishwar A, Medarova Z, Moore A. Unique sphingomyelin patches are targets of a beta-cell-specific antibody. J. Lipid. Res. 52(9), 1660–1671 (2011).
- He SR, Mai G, Lu YR, Chen YN, Zhang S, Cheng JQ. Monitoring of clinical islet transplantation. Chin. Med. J. (Engl.) 126(3), 578–585 (2013).
- Agarwal A, Brayman KL. Update on islet cell transplantation for Type 1 diabetes. Semin. Intervent. Radiol. 29(2), 90–98 (2012).
- Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J. Leukoc. Biol. 77(5), 587–597 (2005).
- Shapiro AM. Islet transplantation in Type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev. Diabet. Stud. 9(4), 385–406 (2012).
- Rickels MR, Kamoun M, Kearns J, Markmann JF, Naji A. Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity. J. Clin. Endocrinol. Metab. 92(7), 2410–2414 (2007).
- Braghi S, Bonifacio E, Secchi A, Di Carlo V, Pozza G, Bosi E. Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with Type 1 diabetes. Diabetes 49(2), 218–224 (2000).
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411(6836), 494–498 (2001).
- Medarova Z, Kumar M, Ng SW et al. Multifunctional magnetic nanocarriers for image-tagged SiRNA delivery to intact pancreatic islets. Transplantation 86(9), 1170–1177 (2008).
- Wang P, Yigit MV, Medarova Z et al. Combined small interfering RNA therapy and in vivo magnetic resonance imaging in islet transplantation. Diabetes 60(2), 565–571 (2011).
- Wang P, Yigit MV, Ran C et al. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes 61(12), 3247–3254 (2012).
- Gotthardt M. A therapeutic insight in beta-cell imaging? Diabetes 60(2), 381–382 (2011).
- Bulte JW. The magnetic appeal of silencing theranostics. Diabetes 61(12), 3068–3069 (2012).
- Medarova Z, Moore A. MRI as a tool to monitor islet transplantation. Nat. Rev. Endocrinol. 5(8), 444–452 (2009).
- Barnett BP, Arepally A, Karmarkar PV et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat. Med. 13(8), 986–991 (2007).
- Barnett BP, Ruiz-Cabello J, Hota P et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 258(1), 182–191 (2011).
- Cormode DP, Mulder WJ, Fayad ZA. Science to practice: versatile method to track transplanted encapsulated islet cells with multiple imaging modalities. Radiology 258(1), 1–2 (2011).
- Kiessling FM. Science to practice: are theranostic agents with encapsulated cells the key for diabetes therapy? Radiology 260(3), 613–615 (2011).
- Lacotte S, Berney T, Shapiro AJ, Toso C. Immune monitoring of pancreatic islet graft: towards a better understanding, detection and treatment of harmful events. Expert Opin. Biol. Ther. 11(1), 55–66 (2011).
- Wang P, Schuetz C, Ross A, Dai G, Markmann JF, Moore A. Immune rejection after pancreatic islet cell transplantation: in vivo dual contrast-enhanced MR imaging in a mouse model. Radiology 266(3), 822–830 (2013).
- Clement O. Science to practice: dual contrast-enhanced MR imaging to monitor for rejection of pancreatic islet transplantation? Radiology 266(3), 693–694 (2013).
- Bogdanov AA Jr, Mazzanti M, Castillo G, Bolotin E. Protected graft copolymer (PGC) in imaging and therapy: a platform for the delivery of covalently and non-covalently bound drugs. Theranostics 2(6), 553–576 (2012).
- Andralojc K, Srinivas M, Brom M et al. Obstacles on the way to the clinical visualisation of beta cells: looking for the Aeneas of molecular imaging to navigate between Scylla and Charybdis. Diabetologia 55(5), 1247–1257 (2012).
- Herzog H, Van Den Hoff J. Combined PET/MR systems: an overview and comparison of currently available options. Q. J. Nucl. Med. Mol. Imaging 56(3), 247–267 (2012).
- Choi JS, Park JC, Nah H et al. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 47(33), 6259–6262 (2008).
- Hebrok M. Generating beta cells from stem cells-the story so far. Cold Spring Harb. Perspect. Med. 2(6), a007674 (2012).
- Wen Y, Chen B, Ildstad ST. Stem cell-based strategies for the treatment of Type 1 diabetes mellitus. Expert Opin. Biol. Ther. 11(1), 41–53 (2011).
- Mao GH, Chen GA, Bai HY, Song TR, Wang YX. The reversal of hyperglycaemia in diabetic mice using PLGA scaffolds seeded with islet-like cells derived from human embryonic stem cells. Biomaterials 30(9), 1706–1714 (2009).
- Maehr R, Chen S, Snitow M et al. Generation of pluripotent stem cells from patients with Type 1 diabetes. Proc. Natl Acad. Sci. USA 106(37), 15768–15773 (2009).
- Zhang D, Jiang W, Liu M et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell. Res. 19(4), 429–438 (2009).
- Alipio Z, Liao W, Roemer EJ et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc. Natl Acad. Sci. USA 107(30), 13426–13431 (2010).
- Zhang S, Dai H, Wan N, Moore Y, Dai Z. Promoting long-term survival of insulin-producing cell grafts that differentiate from adipose tissue-derived stem cells to cure Type 1 diabetes. PLoS ONE 6(12), e29706 (2011).
- Chandra V, Swetha G, Muthyala S et al. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS ONE 6(6), e20615 (2011).
- Jeon K, Lim H, Kim JH et al. Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a Type 1 diabetes mouse model. Stem Cells Dev. 21(14), 2642–2655 (2012).
- Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58(8), 1797–1806 (2009).
- Yeung TY, Seeberger KL, Kin T et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS ONE 7(5), e38189 (2012).
- King CC. Mesenchymal stem cells: protectors of islets? Regen. Med. 8(1), 20–21 (2013).
- Hematti P, Kim J, Stein AP, Kaufman D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev. (Orlando) 27(1), 21–29 (2013).
- Voltarelli JC, Couri CE, Stracieri AB et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed Type 1 diabetes mellitus. JAMA 297(14), 1568–1576 (2007).
- Couri CE, Oliveira MC, Stracieri AB et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed Type 1 diabetes mellitus. JAMA 301(15), 1573–1579 (2009).
- Zhang X, Ye L, Hu J et al. Acute response of peripheral blood cell to autologous hematopoietic stem cell transplantation in Type 1 diabetic patient. PLoS ONE 7(2), e31887 (2012).
- Safety and efficacy study of autologous stem cell transplantation for early onset Type I diabetes mellitus. http://clinicaltrials.gov/show/NCT00315133
- Autologous hematopoietic stem cell transplantation for early onset Type 1 diabetes. http://clinicaltrials.gov/show/NCT00807651
- Contag CH, Spilman SD, Contag PR et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem. Photobiol. 66(4), 523–531 (1997).
- Raikwar SP, Zavazava N. Real-time non-invasive imaging of ES cell-derived insulin producing cells. Methods Mol. Biol. 590, 317–334 (2009).
- Raikwar SP, Zavazava N. Spontaneous in vivo differentiation of embryonic stem cell-derived pancreatic endoderm-like cells corrects hyperglycemia in diabetic mice. Transplantation 91(1), 11–20 (2011).
- Raikwar SP, Zavazava N. PDX1-engineered embryonic stem cell-derived insulin producing cells regulate hyperglycemia in diabetic mice. Transplant Res. 1(1), 19 (2012).
- Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Magneticsensitive silica nanospheres for controlled drug release. Langmuir 24(1), 239–244 (2008).
- Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Stimuliresponsive controlled drug release from magnetic-sensitive silica nanospheres. J. Nanosci. Nanotechnol. 9(2), 866–870 (2009).
- Thomas CR, Ferris DP, Lee JH et al. Noninvasive remotecontrolled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 132(31), 10623–10625 (2010).
- Mannix RJ, Kumar S, Cassiola F et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol. 3(1), 36–40 (2008).
• • Normoglycemia was sustained for at least 6 weeks without the use of immunosuppressive drugs after transplanted with encapsulated mouse β-cells. Gadolinium– gold microcapsules could be visualized with MRI, microcomputed tomography and ultrasound in vivo.
• • Using a long circulating paramagnetic contrast agent, this study demonstrated that changes in vascular permeability associated with early stages of diabetes could be monitored noninvasively by MRI. These properties of the altered islet vasculature could be exploited for the diagnosis and treatment of the disease.
• • Demonstrated that inflammation of pancreatic islets by autoreactive T cells could be detected in real-time by MRI. The labeled T cell could be potentially used for visualizing the presence of ongoing autoimmune responses and further for the drug delivery.
• • Proved that iron oxide nanoparticles coated with human diabetes-relevant pHLA complexes could restore normoglycemia in a humanized model of diabetes. These observations expose a paradigm for future theranostic imaging of diabetes.
• • Proved that the theranostic imaging probe, which targets the apoptosis gene, could result in significantly better survival of transplanted islets, which could be monitored by in vivo MRI.
• This study developed a dual-purpose theranostic siRNA magnetic nanoparticle probe that targeted a key component of the MHC I complex (β-2 microglobulin) and protected islet graft from immune rejection.
• The findings of this study proved that magnetocapsuled β cells were functional in vivo and restored normal glycemia in streptozotocin-induced diabetic mice and human islets in swine. In addition, this theranostic magnetocapsule can protect human islets graft in a large-animal model.
• This study utilized dual contrast-enhanced MRI to detect the immune rejection after islet transplantation. This approach could be potentially applied for theranostic imaging for islet graft rescue.