Review Article - Journal of Experimental Stroke & Translational Medicine (2009) Volume 2, Issue 1
The application of cell penetrating peptides for the delivery of neuroprotective peptides/proteins in experimental cerebral ischaemia studies
- *Corresponding Author:
- Amanda Meade
Stroke Research Group, Centre for Neuromuscular and Neurological Disorders,
A Block, QEII Medical Centre, Verdun St, Nedlands WA 6009, Australia.
Tel: +61 8 93461579
Fax: +61 8 93463487
Email: meadea01@student.uwa.edu.au
Abstract
The restricted ability of most proteins and peptides to cross the blood-brain barrier and/or plasma membrane limits their use as therapeutics following cerebral ischaemia. However, the discovery of cell-penetrating peptides has provided a means by which such molecules can be transported across the blood-brain barrier and plasma membrane. Many proteins/peptides have already been shown to have neuroprotective properties, and, due to their ability to block protein-protein interactions, provide a potentially rich source of new therapeutic compounds to prevent cell death following cerebral ischaemia. In this review, we give an overview of cell-penetrating peptides and their use experimentally to deliver neuroprotectant proteins/peptides into the brain following cerebral ischaemia.
Keywords
Cell penetrating peptides, drug delivery, neuroprotection
Introduction
Currently, there is a lack of therapies aimed specifically at reducing neuronal death in the potentially salvageable tissue following cerebral ischaemia. Whilst there have been many clinical trials targeting obvious neuro-damaging pathways (e.g., calcium influx), these have failed because of lack of efficacy or drug toxicity, hence new therapeutic approaches need to be explored. One such approach is to use cell-penetrating peptides (CPP) to deliver agents that were previously not considered as therapeutic candidates due to their poor brain and/or intracellular bioavailability. Many proteins and peptides that have been shown to have neuroprotective activity fall into this category. Moreover, peptides are increasingly being recognised as a new class of therapeutics due to their diversity and ease of synthesis, and their abilities to block and disrupt challenging targets (Watt 2006). Methods to facilitate transduction of proteins and peptides across the blood brain barrier and into the brain include invasive surgical methods, osmotic opening of the blood-brain-barrier, the use of endogenous transport systems, increasing lipophilicity of the molecule, incorporation into nanocarriers, or the fusion of CPPs to the protein or peptide. This review will focus and discuss recent advances in CPPs and their application in the role of delivering neuroprotective proteins and peptides to the brain following cerebral ischaemia.
Cerebral ischaemia and the cell death cascade
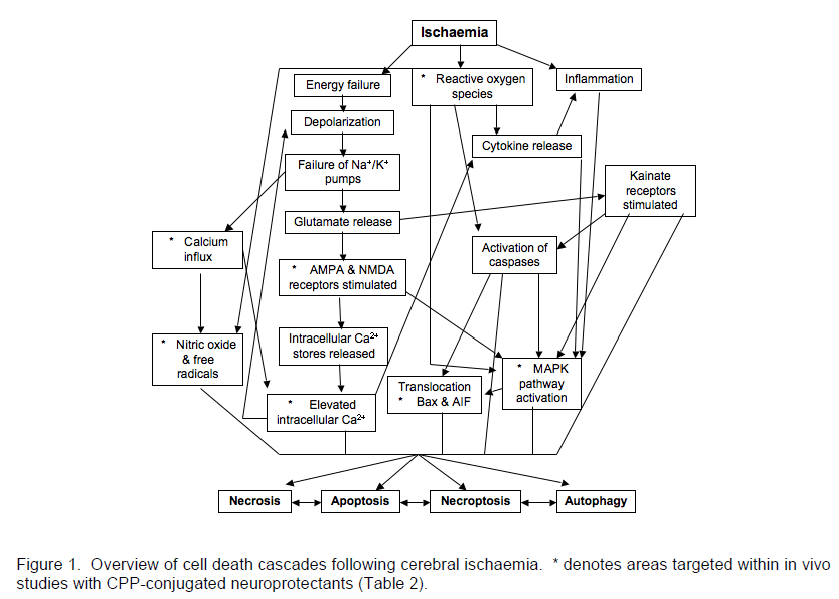
The disruption of blood flow to the brain during an ischaemic event (stroke, cardiac arrest/resuscitation) results in a lack of oxygen and nutrient supply to the brain and a subsequent cellular energy crisis that in turn triggers a cascade of damaging events (Figure 1). The initial stages are associated with neuronal cell depolarisation, glutamate excitotoxicity and calcium overload (Novelli et al. 1988; Nicholls et al. 1990; Goldberg et al. 1993; McCulloch et al. 1993). This leads to secondary effects including a decrease in protein synthesis (Kleihues et al. 1975; Burda et al. 1994; DeGracia et al. 1996), altered mitochondrial function (Rehncrona et al. 1979; Almeida et al. 1995), an increase in lipid peroxidation (Bromont et al. 1989; Haba et al. 1991), oxidative stress, and free radical production (Kader et al. 1993; Chan 2001). Furthermore, multiple signalling pathways are activated, such as the MAPK pathway inducing activation of transcription factors including c-Jun and c-Fos, along with the expression of pro-apoptotic genes, even though there is an overall decrease in protein synthesis (Bouwmeester et al. 2004). Additionally, there are post-translational and translocational protein changes as well as an increase in the activity of proteases, lipases and endonucleases (Rosenberg et al. 1996; Gillardon et al. 1997). An inflammatory response and blood brain barrier disruption occurs (Dirnagl et al. 1999) which can further amplify the initial ischaemic injury (Lee et al. 2000). The culmination of these events ultimately leads to cell death exhibiting features of apoptosis, necroptosis, autophagy and necrosis (Lipton 1999; Brott et al. 2000; Degterev et al. 2005). All of these components within the cascade provide opportunities for intervention to limit cell death and brain injury.
Cerebral ischaemia; human impact and current clinical treatments
In the US alone, in 2002, 1 in 15 deaths were attributed to cerebral ischaemia following a stroke. Whilst mortality is exceedingly high, the burden caused by disability (on the patient, relatives, and the healthcare system) can be just as devastating. Recently, direct and indirect costs associated with cerebral ischaemia are estimated to be US$56.8 billion (Pharmaceutical Research and Manufacturers of America 2006).
Currently, the thrombolytic agent tissue plasminogen activator (tPA) is the only pharmaceutical treatment available for ischaemic stroke. However, tPA is only useful when administered intravenously within 4.5 hours of a thrombo-embolic stroke (Hacke et al. 2008). Surgical intervention, in the form of hemicraniectomy to release intracranial pressure, is beneficial for a small subset of stroke patients that undergo malignant cerebral oedema (Dhamija et al. 2007). Moderate hypothermia (33ºC for 12-24 hours) is used in comatose survivors following cardiac arrest (Bernard et al. 2002; The Hypothermia After Cardiac Arrest Study Group 2002; Arrich 2007), but requires intensive monitoring of patients and is associated with cardiac arrhythmias, coagulopathy, infection (e.g., pneumonia), and electrolyte disorders (Safar et al. 1996; Polderman et al. 2001; Peterson et al. 2008). It is clear that currently available treatments are not ideal and that the development of therapeutic agents with direct neuroprotective activity are urgently required.
Proteins and peptides as therapeutic agents
The actions of therapeutic proteins are primarily to replace or enhance the activity of endogenous proteins, while therapeutic peptides may inhibit or activate proteins, enzymes, receptors, transporters or biochemical pathways. In contrast, therapeutic peptides target the inhibition or activation of proteins, enzymes, receptors, transporters or biochemical pathways. For example, recombinantly produced human insulin is a protein administered to supplement the endogenous protein for treatment of diabetes mellitus (Goeddel et al. 1979). Similarly, somatostatin analogues are therapeutically administered to enhance the action of endogenous somatostatin in preventing the over production of growth hormone for patients with acromegaly (Croxtall et al. 2008). In contrast, an example of a therapeutic peptide administered to block an endogenous protein is the decapeptide Cetrorelix, which antagonises receptors involved in luteinizing hormone-release and is used in advanced prostate carcinomas, benign prostatic hyperplasia, and ovarian cancer (Reissmann et al. 2000). Another example of a therapeutic peptide is the antiretroviral peptide enfuviritide, which is used in the treatment of HIV infection. Enfuviritide is a synthetic peptide that prevents the transmembrane glycoprotein gp41 fusing with CD4 cells (Cooper et al. 2004; Heil et al. 2004).
An additional clan of protein-based therapeutics are monoclonal antibodies, which have been developed for a number of human disorders including the treatment of acute myeloid leukaemia (Castillo et al. 2008) and psoriasis (Schon 2008). Whilst these disorders are dissimilar, the role of the antibody and subsequent nullification of their target is dependent upon the recognition of a particular antigen. As a result, antibodies are usually only useful against extracellular or cell surface targets. Furthermore, antibodies have poor BBB traversing abilities, as well as a high efflux rate from the brain, with a half-time of 40 min following intra-cerebral injection (Zhang et al. 2001). However, these large groups of proteins and peptides provide substantial proof of concept that proteins and peptides have a therapeutic value clinically.
Potential advantages of proteins and peptides as therapeutics
Traditionally, drug design has been based upon the screening and computational design of small molecules for use as therapeutic agents. This approach typically utilises clefts or pockets present in target proteins that small molecules bind, similar to a ‘key and lock’ scenario. Therapeutic small molecules have numerous merits besides their small size. They are relatively easy and inexpensive to produce, may be administered orally if required, and can be easily modified to alter potency and other pharmokinetic properties. However, the proficiency of small molecules in preventing or disrupting protein-protein interactions has been lacking, as the interacting sites between proteins are often large without clefts or pockets; this leaves peptides as more suitable candidates for intervention (Archakov et al. 2003; Watt 2006).
Peptide modifications to increase stability and half-life
Peptide stability, and therefore half-life, can be increased with the interchange of L-isoform amino acids with D-isoforms, which reduces proteolysis of the peptide (Brugidou et al. 1995; Wender et al. 2000). When L-isoform amino acids are interchanged for D-isoforms they must be in a retro inverso format to maintain stereospecificity and hence efficacy and specificity (Leker et al. 2002; Borsello et al. 2003). Similarly, amino-acids may be replaced with amino-acid isomers (e.g., norleucine for methionine) that are more resistant to proteolytic degradation (Gozes et al. 1999). Alternatively, linear peptides can be converted to cyclic peptides by the addition of a disulfide-bridge or hydrazide-bridge or another peptide bond to form a more proteolytically resistant lactam ring (Martins et al. 2007). The pegylation of peptides, by the conjugation of polyethylene glycol (PEG), can be used to increase peptide stability, whilst simultaneously decreasing proteolysis, immunogenicity and decreasing efflux across the BBB (Tsubery et al. 2004; Egleton et al. 2005). Finally, peptides can be used as design templates to synthesise molecules that will have improved pharmokinetics with respect to half-life and bioavailability, and that will allow alternative delivery routes such as oral or transcutaneous (Baell et al. 2004; Guan 2008).
Other modifications to improve delivery
Apart from increasing stability and half-life, additional peptide modifications can be performed to improve tissue delivery and specificity. Modifications to minimise peptide size may be performed by identifying and restricting synthesis to the active site of a peptide, thereby simultaneously reducing manufacturing costs and possibly increasing delivery potential (Barr et al. 2002).
Additionally, an “address” localisation sequence can be added to the peptide to facilitate delivery of the cargo to specific cell types (e.g., neuronal cells), to organelles (e.g., endoplasmic reticulum or nucleus), or even to ischaemic tissue. Examples of cell specific delivery have been demonstrated with the use of a peptide sequence derived from the rabies virus glycoprotein that binds specifically to the acetylcholine receptor expressed on neurons (Kumar et al. 2007) or the short peptide sequence isolated that targets tumour vasculature (Arap et al. 1998). Moreover, nuclear localisation sequences within endogenous proteins have been extensively identified and characterised (Hu et al. 2005; Russell et al. 2008), and are now being utilised within synthesised peptides (Yoshikawa et al. 2008). Within the brain, the targeted delivery of diphtheria toxin to malignant brain tumors by conjugating it to transferrin, due to the increase of transferrin receptors on malignant brain tumors, has also proved successful and resulted in a lack of systemic toxicity (Laske et al. 1997). Similarly, following cerebral ischaemia, injured tissue can be targeted by the addition of a nine amino-acid sequence that specifically localises to the ischaemic region (Hong et al. 2008).
Delivery of proteins and peptides into the brain
There are numerous pathways (Figure 1) activated following cerebral ischaemia involving protein-protein interactions, thus providing a plethora of targets for therapeutic intervention. As discussed above, one reason why they have not previously been considered suitable drug targets is the difficulty in disrupting protein-protein interactions. Another reason is the problem of the blood brain barrier (BBB) impeding drug bioavailability to the site of injury.
The blood brain barrier and passive diffusion
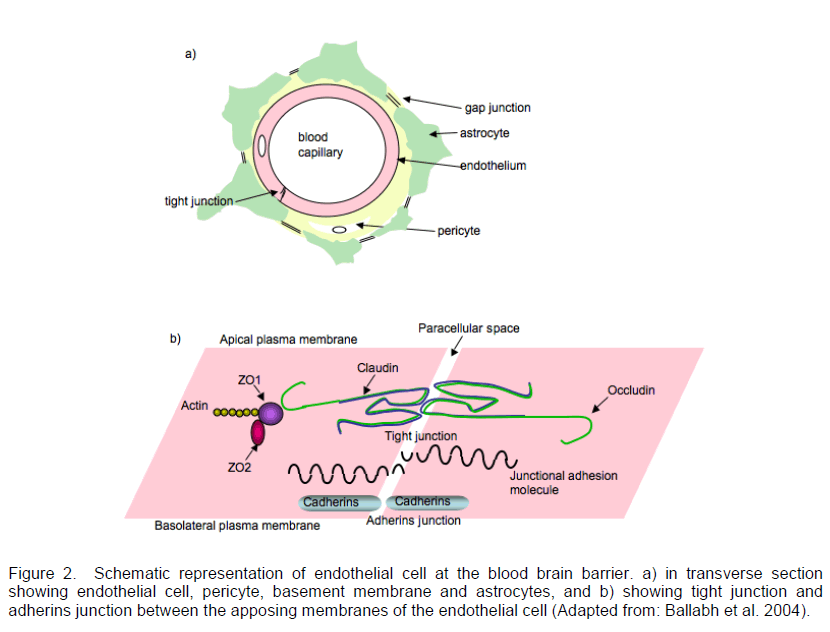
The blood brain barrier (BBB) is an endothelial structure that functions in conjunction with pericytes and astrocytes (Figure 2a) to control the diffusion of molecules from the blood to the extracellular space of the central nervous system and vice versa (Reese et al. 1967; Brightman et al. 1969; Janzer et al. 1987).
Figure 2. Schematic representation of endothelial cell at the blood brain barrier. a) in transverse section showing endothelial cell, pericyte, basement membrane and astrocytes, and b) showing tight junction and adherins junction between the apposing membranes of the endothelial cell (Adapted from: Ballabh et al. 2004).
The endothelial cells of the BBB lack fenestrations and have both tight junctions and adherens junctions between the cells limiting permeability (Schulze et al. 1993; Kniesel et al. 2000; Bazzoni et al. 2004) (Figure 2b). This physical barrier along with the noticeable deficiency of pinocytic vesicles results in the prevention of passive diffusion of hydrophilic molecules, although the diffusion of uncharged molecules which are typically small (less than 600Da) and/or lipophilic does occur (Levin 1980; Grieb et al. 1985). Astrocytes further encase the epithelial cells with their extended endfeet projections interspersed with pericytes, which are thought to maintain endothelial stability and integrity (Kacem et al. 1998; Hellstrom et al. 2001). This entire structure allows few therapeutic peptides to diffuse across the BBB and enter the brain.
Transport carrier systems that enable therapeutic agents to cross the blood brain barrier
If unable to diffuse, molecules must cross the BBB via carrier-mediated transport (e.g., glucose via GLUT1, leucine via L1), ion transport (e.g., sodium, potassium and chloride exchangers), active efflux transport (e.g., amphipathic cationic drugs via the multidrug resistance transporter P-glycoprotein), receptor mediated transport (e.g., transferrin and immunoglobulin G), or caveolae-mediated transport (e.g., albumin and insulin) systems (Zlokovic 2008). Peptides can compete to use a carrier system if their structure mimics that of an endogenous molecule that is transported across the BBB. An example of this is biphalin, an opioid analgesic peptide drug, that uses the neutral amino acid carrier system to cross the BBB (Witt et al. 2001).
Peptides that cannot use an endogenous transport system can be fused to a molecule that can be effectively transported across the BBB. This approach has been exploited using molecules such as transferrin and glucose (Witt et al. 2001). Alternatively, peptidomimetic monoclonal antibodies can be utilised to transport cargo across the BBB via a receptor-mediated transport system (Pardridge et al. 1995; Lee et al. 2000). In this case the cargo is fused to the monoclonal antibody, which binds to an external receptor, such as the transferrin or insulin receptor.
The challenges of using a carrier system can include the low density of receptors or transporters on the capillary lumen, the rate of exchange from the blood to the brain, and/or the relative affinity of the endogenous molecule compared to the therapeutic peptide. Thus, in light of the limitations of the carrier system and the likelihood that most proteins and peptides will not be capable of traversing the BBB using this system, other strategies must be employed.
Other methods used to enable therapeutic agents to cross the blood brain barrier
The transport of agents across the BBB using other non-carrier systems can be either invasive or non-invasive. Transient osmotic opening of the BBB by arabinose or mannitol (Rapoport et al. 1980; Siegal et al. 2000), shunts (Alexander et al. 2000), or implanting microspheres (Emerich et al. 1999; Benoit et al. 2000) to deliver therapeutic peptides are invasive methods and have potential side-effects. Osmotic agents can cause accumulation of mannitol in the cerebral tissue and increase oedema, whilst implantations require surgery (Kaufmann et al. 1992; Maioriello et al. 2002). More favourable are non-invasive methods of delivery, such as altering the peptide to increase its membrane permeability, utilising nanocarriers, or attaching a cell penetrating peptide.
Increasing membrane permeability and lipophilicity of peptides
Alterations to increase peptide membrane permeability and lipophilicity include a reduction in molecular weight, reducing hydrogen bond donors to five or less, and limiting hydrogen bond acceptors to no more than 10 (Chikhale et al. 1994; Lipinski et al. 2001). A reduction in molecular weight of a protein or peptide can be achieved by removing unnecessary sections of the peptide to leave only the essential domains or sequence. Methods used to reduce hydrogen bond potential include the addition of non-polar groups, the removal of polar groups (e.g., hydroxyl groups) or methylation. Additional modifications such as the addition of halogens to the peptide, the acylation or alkylation of the N-terminal of the peptide, variation in the peptide tertiary structure, and glycosylation of peptides can also increase lipophilicity. Glycosylation not only significantly improves dispersal through the BBB, but also increases peptide stability and efficacy (Albert et al. 1993; Negri et al. 1998; Bilsky et al. 2000). There is conjecture over the exact mode of passage for glycosylated peptides with both transport via the glucose transporter (GLUT1) (Namane et al. 1992; Polt et al. 1994; Negri et al. 1998) and entry via adsorptive endocytosis (Palian et al. 2003) being proposed. Of course, any alterations to the peptide to enhance BBB trafficking must not interfere with the affinity and specificity of the peptide for its target.
Nanocarriers
Nanocarriers are colloidal drug carriers that include liposomes and nanoparticles, which can be utilised to deliver proteins and peptides across the BBB. They are administered systemically and have been demonstrated to facilitate targeted delivery to cells, tissues or organs by the external attachment of ligands, such as monoclonal antibodies and cationized bovine serum albumin. Additionally PEG can be bound externally to the nanocarrier to impede the surface deposition of plasma proteins and thereby preventing the uptake of the nanoparticle by macrophages. Extensive reviews detailing the intricacies of various classes of nanocarriers have been published (Beduneau et al. 2007; Brasnjevic et al. 2009).
However, in short, liposomes are engineered to enclose an aqueous phase within a lipid bilayer, similar to biological membranes. The protein or peptide can be incorporated internally into either phase dependent upon their hydrophobic or hydrophilic properties. The liposome is thought to cross the BBB by one of three methods: 1. passive diffusion through the lipophilic endothelial cells; 2. by endocytosis; 3. by fusion with brain capillary endothelial cells utilising ligand specific receptors.
Nanoparticles are colloidal structures characterised as comprising of natural or synthetic polymers ranging in size from 1 to 1000nm. As with liposomes, the protein or peptide is incorporated internally either encased by the polymer matrix or distributed throughout, and can have external additions such as PEG and ligands to increase stability and specificity of targeting, respectively. The nanoparticles are mainly produced by emulsion/solvent evaporation or precipitation solvent diffusion from a variety of polymers such as dextran, starch, gelatin, poly(D,L-lactic acid) (PLA), poly(D,L-lactide-co-glycolic acid) (PLGA), and poly(alkylcyanoa-crylate) (PACA). Both liposomes and nanoparticles have been utilised to successfully deliver proteins and peptides to the brain (Kreuter et al. 1995; Gulyaev et al. 1999; Tiwari et al. 2006).
Cell penetrating peptides and delivery of peptides to the brain
Cell penetrating peptides: Whilst the above methods focus on delivery across the BBB and into the interstitial space, it should be mentioned that therapeutic peptides targeting intracellular interactions will need an additional delivery mechanism to enable entry into the cell. To this end, we will focus on the use of cell penetrating peptides (CPP) to rapidly (and non-invasively) deliver therapeutic peptides across the BBB and into cells.
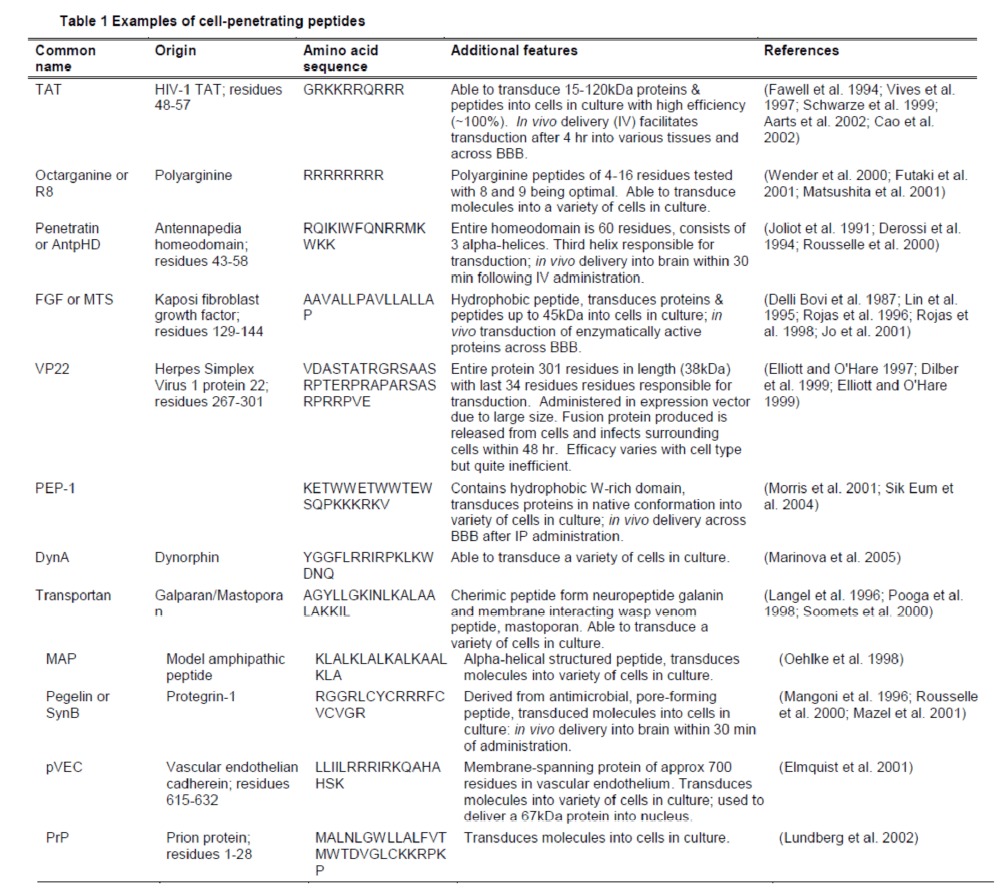
The use of CPPs to carry peptides and small molecules across the plasma membrane and into cells came about with the discovery of the protein transduction domain (PTD) within the human immunodeficiency virus-type 1 trans-activating transcriptional activator (HIV-1 TAT) in 1988 (Frankel et al. 1988; Green et al. 1988). Since then, the active transporting portion of this sequence has been isolated, as well as the discovery and synthesis of other novel CPPs (Table 1). The conjugation of the CPP and cargo can be achieved by several methods including the use of a disulfide bond between the CPP and cargo, avidin-biotin technology, polyetylen glycol linkers, or direct production of protein fusion with CPP either chemically synthesised or produced using recombinant DNA technology (Pardridge 2002; Mueller et al. 2008; Stewart et al. 2008). The conjugation of the CPP and cargo can be a permanent, stable conjugation or can be designed to be cleaved or become liable in a particular environment, for example, a change in pH (Rothbard et al. 2000). (Forthwith, for ease of reading, the conjugated CPP with cargo will be generally referred to as CPP-fused, irrespective of actual process involved in the conjugation.)
The method of transduction by TAT, and other CPPs is not fully understood, and initial studies investigating mechanisms were confounded by fixation artifacts (Richard et al. 2003). Three models of transduction are now widely proposed: 1. direct penetration through the cellular membrane; 2. endocytosis and then vesicle disruption into the cytoplasm; 3. inverted micelle formation, engulfment, transport across the cell membrane, and release into the cytoplasm (Joliot et al. 2004; El-Andaloussi et al. 2005).
The amino acid sequence and secondary structure of the CPP and cargo will ultimately determine the mechanism and efficacy of transduction. For example, TAT and polyarginine CPPs are hydrophilic peptides and considered to be transduced by endocytosis (Suzuki et al. 2002; Drin et al. 2003), whilst Penetratin has a hydrophobic core and is postulated to be transduced by inverted micelle formation (Derossi et al. 1994; Nakase et al. 2004). Different mechanisms of transduction for different CPPs is supported by the reduced cellular uptake of arginine-rich CPPs, but not Penetratin, with the treatment of ethylispoprotpylamiloride (EIPA), an endocytosis inhibitor (Nakase et al. 2004). Furthermore, enhanced delivery of both TAT and arginine-rich CPP-cargo conjugates is facilitated by the addition of the NH2 terminal influenza virus hemagglutinin-2 (HA2) peptide, which evokes the release of complexes trapped within endosomes (Wadia et al. 2004; Michiue et al. 2005). Conversely, MAP and protegrin-1, pore-forming peptides, (Table 1) appear to utilise direct penetration for transduction (Mangoni et al. 1996; Oehlke et al. 1998).
Delivery into the brain
Eleven years after the discovery of the TAT CPP, Schwarze et al. (1999) showed that TAT could transduce the BBB. In this instance, fluorescently labelled TAT was visible throughout the brain 20 minutes after intraperitoneal (IP) administration in the mouse. Furthermore, Schwarze et al. (1999) demonstrated the transport of a biologically active β-galactosidase enzyme-TAT conjugate across the BBB after two hours and into many regions of the brain after four hours following IP administration. Similarly, following IP or IV administration, Jo et al. (2001) demonstrated transduction of a biologically active CRE protein conjugated to the MTS CPP into the brain. The delivery of non-protein molecules into the brain has also been achieved with CPPs. For example, the anticancer drug doxorubicin, coupled with the SynB and Penetratin CPPs, was detectable in the brain 30 minutes after IV administration (Rousselle et al. 2000).
In vitro neuronal studies with neuroprotectants fused to cell penetrating peptides
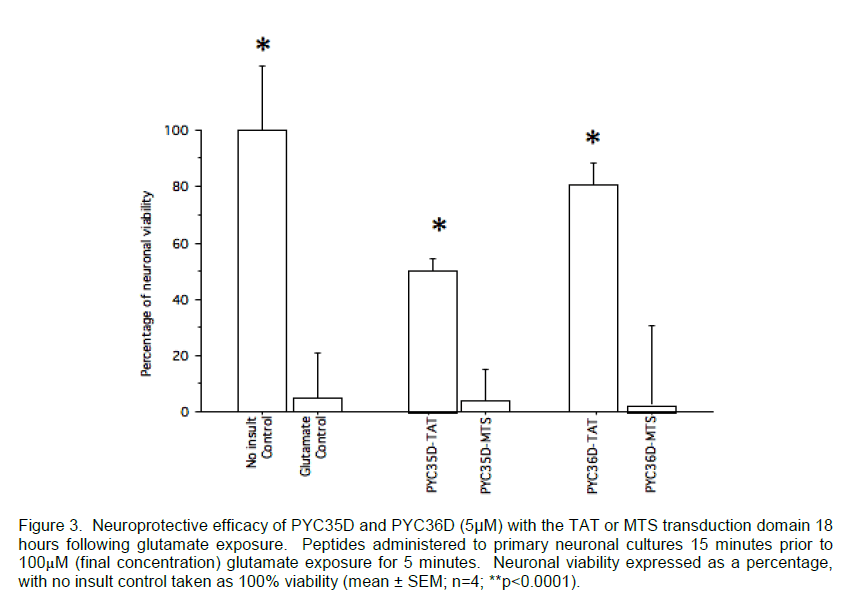
Since the transduction efficacy of CPPs varies in relation to the cargo and cell type (Fischer et al. 2002; Peitz et al. 2002; Mueller et al. 2008) it is important to validate transduction using relevant in vitro culture systems. For example, preliminary data from our laboratory has demonstrated that for the same neuroprotective peptides (5μM of PYC36D or PYC35D) fused to either the MTS or TAT CPPs, only the TAT fused peptides were effective in an in vitro neuronal glutamate injury model (Figure 3). Whilst there have been several studies which successfully utilised MTS (Lin et al. 1995; Rojas et al. 1998), Peitz et al (2002) also reported a greater level of transduction with their TAT-fused cargo as opposed to MTS-fused cargo. This lack of neuroprotection with MTS fused peptides may be due to several factors including steric hindrance from the CPP, formation of secondary or tertiary structure within and between the peptide and CPP, or from limited transduction of the peptide.
Figure 3. Neuroprotective efficacy of PYC35D and PYC36D (5μM) with the TAT or MTS transduction domain 18 hours following glutamate exposure. Peptides administered to primary neuronal cultures 15 minutes prior to 100μM (final concentration) glutamate exposure for 5 minutes. Neuronal viability expressed as a percentage, with no insult control taken as 100% viability (mean ± SEM; n=4; **p<0.0001).
Similarly, despite the demonstration that CPPs could deliver cargo across BBB and into the brain, most in vivo animal studies have been preceded by in vitro culture studies. For example, in vitro neuronal culture systems that mimic neuronal injury mechanisms in a variety of disorders, including cerebral ischaemia, epilepsy, Parkinson’s and Alzheimer’s Diseases, have been used to assess neuroprotective efficacy for CPP fused proteins or peptides (Lai et al. 2005; Liu et al. 2006; Arthur et al. 2007; Colombo et al. 2007; Nagel et al. 2008). These studies also provided an indication of potential effective in vivo doses, especially if pharmokinetic data is available for the protein or peptide being tested (Borsello et al. 2003; Hirt et al. 2004; Sik Eum et al. 2004; Fan et al. 2006).
CPPs used to deliver neuroprotective peptides in experimental cerebral ischaemia models
Following the demonstration of the ability of CPPs to traverse the BBB it was not long before CPPs were used to deliver neuroprotective peptides into the brain in animal models of cerebral ischaemia. To date, more than 10 different peptides fused to CPPs have been trialled in experimental models of focal and global cerebral ischaemia (Table 2). These studies have predominantly resulted in a significant reduction in brain injury and, when assessed, improved functional outcomes in treated animals. Since experimental studies with CPP-fused neuroprotectants in ischaemic models have differed considerably in respect to the class of therapeutic, route, dose and timing of treatment, they are further discussed below.
Studies using endogenous proteins
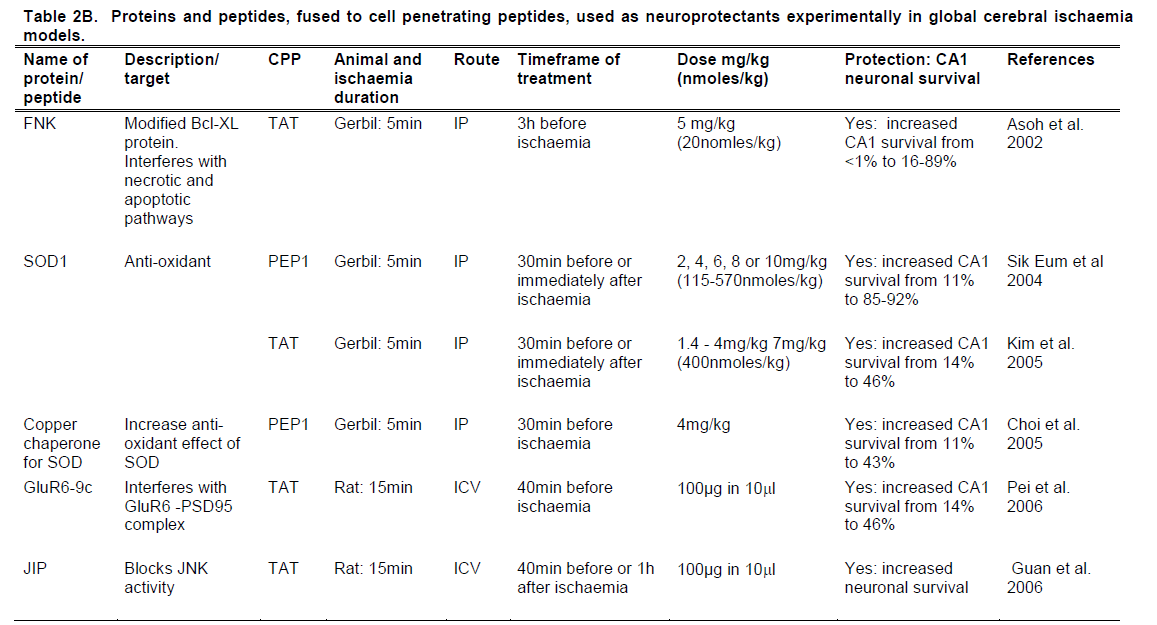
In two of the initial animal studies (Cao et al. 2002; Kilic et al. 2002) the anti-apoptotic protein Bcl-XL, fused to the TAT peptide, showed significant neuroprotective activity in mouse transient focal cerebral ischaemia models. Kilic et al. (2002) reported that a 20nmol/kg dose administered IV 1 hour before or immediately post middle cerebral artery occlusion (MCAO) significantly reduced infarct volume when assessed at 48 hours. Cao et al. (2002) used a substantially higher (360 nmol/kg) dose of Bcl-XL administered IP. This treatment reduced infarct volume when given immediately before and 45 minutes after MCAO, but not 2 hours after occlusion. In a third study, Asoh et al. (2002) assessed the efficacy of a mutated, more potent form of Bcl-XL, named FNK, in a mouse global cerebral ischaemia model. In this study, the FNK-TAT conjugate administered IP at 20nmol/kg, 3 hours before ischaemia, significantly increased hippocampal CA1 neuronal survival.
In 2003, Kilic et al. showed that the neurotrophic factor GDNF, fused to TAT, was effective at reducing infarct volume in the mouse following 30 minutes of transient focal ischaemia. The GDNF-TAT protein was administered IV at a 20nmol/kg dose, immediately post-ischaemia. When the duration of MCAO was extended to 120 mins, GDNF-TAT was effective when administered 1 hour before ischaemia, but ineffective when administered immediately post-ischaemia (Kilic et al. 2003).
Three studies (Sik Eum et al. 2004; Choi et al. 2005; Kim et al. 2005) have assessed the antioxidant enzyme SOD, fused to either TAT or to PEP-1 in gerbil global cerebral ischaemia models. In two of the studies SOD was administered IP at doses ranging from 115-570nmol/kg either 30 mins prior to ischaemia or immediately post-ischaemia. CA1 survival was then examined after four days. The study, performed by Sik Eum et al. (2004), showed that the SOD-PEP-1 protein was equally neuroprotective when administered either prior to or post-ischaemia, within each dose. At a dose of 170nmol/kg, SOD-PEP-1 resulted in 85% survival of neurons in the CA1 region of the hippocampus. Neuronal survival increased to 92% when the dose of SOD-PEP-1 was increased to 570nmol/kg. However, Kim et al. (2005) required the maximum dose of 570nmol/kg to provide neuroprotection, with a marked difference in neuronal survival between the pre- and post-ischaemic administration of SOD-TAT. Pre-ischaemic administration of SOD-TAT resulted in 65% neuronal survival, whilst animals that were post-administered achieved 45% neuronal survival. The third study administered the copper chaperone for SOD (CCS) fused to PEP-1, IP, 30 mins prior to ischaemia and examined CA1 survival after four days. Similarly, Choi et al. (2005) showed the CCS-PEP-1 protein increased neuronal survival to 46%, in addition to finding a 3-4 fold increase in SOD activity.
The use of the caspase inhibitor XIAP has been assessed in both mouse permanent and transient focal ischaemia models. Guegan et al. (2006) initially applied XIAP-TAT in a gel foam (50nmol/kg) and inserted it into a craniotomy immediately post-MCAO, which resulted in a significant infarct volume reduction. However, IV administration of the XIAP-TAT at a 50nmol/kg dose revealed the protein did not accumulate in the brain. This led to the production of a truncated version of the protein from 62kDa down to approximately 16kDa, which was then shown to accumulate in the brain. Subsequent IV administration of the truncated XIAP-TAT protein at a 50nmol/kg dose resulted in significant infarct volume reduction when given 30 minutes before, or 30 or 180 minutes after, ischaemia. Coincidently, Fan et al. (2006) produced a similar truncated XIAP protein fused to the Penetratin CPP. A 250nmol/kg dose of the truncated XIAP-Penetratin conjugate administered IP one hour before or 30 minutes after transient focal ischaemia significantly reduced the number of ischaemic perifocal cells and behavioural deficits in treated animals compared to controls. Fan et al. (2007) also fused Penetratin with the calcium-binding protein calbindin D and showed that IP administration at 0.17nmol/kg 30 minutes before transient focal ischaemia in the mouse resulted in significantly decreased infarct volume.
Studies using peptides derived from signaling protein domains
Peptides targeting glutamate receptor signaling: Several studies have used CPPs fused to small peptides derived from regions of signaling proteins as competitive inhibitors of protein-protein interactions. The first to use this approach was Aarts et al. (2002), who used a nine amino-acid peptide (NR2B9c) derived from the intracellular domain of the NMDA receptor NR2 subunit. This region of the NR2 protein interacts with the post synaptic density-95 (PSD-95) adaptor protein to activate nNOS and generate nitric oxide. When administered intracerebroventricularly (ICV), either before or 1 hour after transient focal ischaemia in the rat, NR2Bc-TAT reduced infarct volumes and improved behavioural outcomes when assessed at 24 hours. Using a similar approach, Pei et al. (2006) utilised a nine amino-acid peptide derived from the glutamate receptor subunit protein (GluR6-9c) region which binds to PSD-95 and activates downstream signalling pathways, such as the MAPK pathway. The ICV administration of this peptide fused to TAT 40 minutes before global ischaemia in the rat significantly increased hippocampal CA1 neuronal survival.
Peptides targeting JNK signaling
In 2003, the first of four studies detailing the use of a 20 amino-acid peptide named JNKI-1, derived from the JNK scaffold protein JIP, in models of cerebral ischaemia, was reported. The JIP protein is required by JNK during phosphorylation of target proteins, and the 20 amino-acid JNKI-1 region is a critical JNK-JIP binding domain, which the JNKI-1 peptide competitively inhibits. For in vivo experiments, Borsello et al. (2003) used JNKI-1 fused to TAT, synthesising the peptide (JNKI-1D-TAT) using D-isoform amino acids in a retro-inverso orientation to increase peptide half-life and to maintain peptide chirality. In one part of this study JNKI-1D-TAT (15.7ng) was administered ICV 1 hour before, or 3, 6, or 12 hours after, transient focal ischaemia in the mouse. Treatment with JNKI-1D-TAT at all time-points, except at 12 hours after ischaemia, resulted in significant decreases in infarct volumes and improved behavioural outcomes when assessed at 1 or 14 days post-ischaemia. In a second part of the study, JNKI-1D-TAT was administered IP (2800nmol/kg) 30 minutes before, or 6 or 12 hours after, permanent focal ischaemia in 14 day old rats. In these experiments JNKI-1D-TAT significantly reduced infarct volumes at all time points when assessed 7 days post-ischaemia.
Two other research laboratories have also assessed the efficacy of JNKI-1D-TAT in cerebral ischaemia models. Esneault et al. (2008) administered JNKI-1D-TAT IV at three different doses (85, 250, 750nmol/kg) 3 hours after transient focal ischaemia in the rat. While all three different doses were compared and resulted in a decrease in infarct volume, only the 250nmol/kg dose reached statistical significance with respect to infarct volume reduction and improved sensorimotor and cognitive function. Additionally, Hirt et al. (2004) administered JNKI-1D-TAT ICV (1400nmol/kg) to mice 3 or 6 hours after permanent focal ischaemia. Administration of JNKI-1D-TAT at the 3 hour, but not the 6 hour, time point resulted in significantly decreased infarct volumes when assessed at one day post-ischaemia.
Additionally, an 11 amino-acid truncated version of JNKI-1, annotated as JIP, was assessed in a global cerebral ischaemia rat model (Guan et al. 2006). The truncated JIP, fused to TAT, was administered ICV (100μg), either 40 minutes before or 1 hour after ischaemia, and resulted in a significant increase in the number of surviving CA1 neurons.
Peptides targeting the Nogo receptor
A recent study by Wang et al. (2008) used a 40 amino-acid peptide (NEP1-40), designed to antagonise the Nogo receptor, which, when activated, inhibits neuronal growth and re-generation, in a rat transient focal ischaemia model. NEP1-40 fused to TAT was administered IP (120nmol/kg) immediately after ischaemia, and significantly reduced infarct volumes when compared to control animals.
Summary and significance of CPP delivered neuroprotective proteins and peptides
These studies using endogenous proteins or peptides from signalling protein domains fused to CPPs, have established proof of principle of CPPs as therapeutic delivery facilitators across the BBB, and have successfully shown the potential of protein and peptides as therapeutic neuroprotectants. As these proteins and peptides aid in neuroprotection at various stages of the ischaemic death cascade (Figure 1), and are able to be directed at both intracellular and extracellular targets, further benefit may arise from a cocktail of endogenous proteins rather than just their administration in isolation. The administration of therapeutic proteins and peptides has been effective both IV and IP, increasing their versatility as therapeutic agents. In addition, both the wild-type and mutated forms of endogenous proteins and peptides appear to be safe and well tolerated, with a lack of toxicity or immunogenicity reported. However, the variability between the studies with regard to effective dosage and administration time-points highlights the requirement for assessment of each protein/peptide individually before being trialled in the clinical setting.
The onset of clinical trials for therapeutic peptides in neurological disorders
The research conducted with the JNKI-I neuroprotective peptide in the animal models of cerebral ischaemia administered by a clinically relevant route (IV) and within an appropriate timeframe has resulted in its further development as a therapeutic candidate. The Biopharmaceutical Company Xigen is pursuing JNKI-1 (XG-102) in a Phase 1 trial following cerebral ischaemia, which has not been fully disclosed. Research to ensure its compatibility with rtPA has been successfully performed in a 30 minute suture MCAO model of cerebral ischaemia in mouse. In this model, rtPA (0.9mg/kg) was administered 3 hours after ischaemia and XG-102 (0.1mg/kg) administered 6 hours after ischaemia, IV. A significant reduction in infarct volume and improved behavioural outcomes were achieved (Wiegler et al. 2008), further demonstrating the feasibility of this neuroprotectant to be therapeutically effective in a clinically relevant timeframe.
Other potential advantages of CPPs as pharmaceutical delivery systems
An important feature of any CPP is that it has limited toxicity at clinically relevant doses, while a CPP that displays endogenous neuroprotective activity is an added advantage. In fact, this appears to be the case, at least for TAT. A recent study (Wei et al. 2008) has reported that at high concentrations the TAT peptide displays neuroprotective properties in a hippocampal slice oxygen-glucose deprivation model. In addition, pilot studies in our laboratory have confirmed that with increasing dose, TAT is neuroprotective in a glutamate excitotoxicity model. While the exact mechanisms of TAT’s neuroprotective action is not fully understood, Wei et al. (2008) has suggested it is related TAT’s ability to transverse cell membranes and thereby interfere with membrane proteins, receptors and signalling. Interestingly, a RNAi study using CPPs to deliver constructs, both the TAT and Penetratin peptides alone were shown to down-regulate MAP kinase mRNA in the lung following intratracheal administration (Moschos et al. 2007). Hence, while further work is required to elucidate and even enhance TAT’s neuroprotective actions, it appears that it is an excellent carrier molecule for therapeutic delivery of neuroprotective agents into the brain.
Potential limitations of CPPs as delivery system for pharmaceuticals
The ability of CPPs to transduce cellular membranes comes with its own set of limitations. Whilst CPP-fused delivery of cargo enables immediate access of the cell to the cargo, it results in non-targeted wide dissemination of the therapeutic throughout the body. Depending on the mechanistic action of the CPP-fused therapeutics this may require the need for high or continual dosing of the agent and an associated increased risk of side-effects. Hence, the ability to modify CPPs in order to target specific tissue or organs (eg ischaemic tissue or brain) could potentially reduce the risk of systemic side-effects. It is also possible that the CPP delivery system may not be able to achieve therapeutic concentrations in target cells/tissue and/or that the CPP peptide may interfere with the protein/peptides mechanism of action. In the case of CPP interference with the cargo, a design that results in the cleavage of the CPP would be required (Rothbard et al. 2000).
With respect to any side-effects associated with CPP-fused therapeutics, it will also be important to delineate any toxic effects caused by the CPP or peptide/protein component of the therapeutic agent. In vitro toxicity studies of various CPPs have been reported with minimal cytotoxicity at concentrations regarded as optimal for drug delivery, and increasing at concentrations well above expected therapeutic doses (Nath et al. 1996; Mueller et al. 2008). To date, clinical trials using TAT and R7 fused agents have not been associated with any serious side-effects (Rothbard et al. 2000; Chen et al. 2007).
Conclusion
The ability of CPPs to transport molecules across the BBB and plasma membranes and into cells has increased both the range of targets and the class of therapeutic compounds, namely peptides and proteins, available for neuroprotective interventions. Previously, delivery of therapeutics for neurological disorders such as cerebral ischaemia has only been possible via invasive methods, which not only comes with its own set of complications but also extends the timeframe before therapies may be administered. The advent of CPPs with their rapid and efficient transduction across the BBB and plasma membrane brings about the opportunity for novel therapeutics to be administered in a more clinically relevant timeframe, enhancing the probability of intervening in cell-death cascades and ultimately improving patient outcome.
Acknowledgement
This work was supported by the Scholarships provided by the University of Western Australia and Phylogica Ltd.
References
- Aarts, M., Y. Liu, L. Liu, S. Besshoh, M. Arundine, J. W. Gurd, Y. T. Wang, M. W. Salter and M. Tymianski Treatment of ischemic brain damage by lierturbing NMDA recelitor- liSD-95 lirotein interactions. Science. 2002;298(5594):846-50.
- Albert, R., li. Marbach, W. Bauer, U. Briner, G. Fricker, C. Bruns and J. liless SDZ CO 611: a highly liotent glycated analog of somatostatin with imliroved oral activity. Life Sci. 1993;53(6):517-25.
- Alexander, B., X. Li, I. S. Benjamin, M. B. Segal, R. Sherwood and J. E. lireston A quantitative evaluation of the liermeability of the blood brain barrier of liortacaval shunted rats. Metab Brain Dis. 2000;15(2):93-103.
- Almeida, A., K. L. Allen, T. E. Bates and J. B. Clark Effect of relierfusion following cerebral ischaemia on the activity of the mitochondrial resliiratory chain in the gerbil brain. J Neurochem. 1995;65(4):1698-703.
- Arali, W., R. liasqualini and E. Ruoslahti Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377-80.
- Archakov, A. I., V. M. Govorun, A. V. Dubanov, Y. D. Ivanov, A. V. Veselovsky, li. Lewi and li. Janssen lirotein-lirotein interactions as a target for drugs in liroteomics. liroteomics. 2003;3(4):380-91.
- Arrich, J. Clinical alililication of mild theralieutic hyliothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041-7.
- Arthur, li. G., G. li. Matich, W. W. liang, D. Y. Yu and M. A. Bogoyevitch Necrotic death of neurons following an excitotoxic insult is lirevented by a lielitide inhibitor of c-jun N-terminal kinase. J Neurochem. 2007;102(1):65-76.
- Asoh, S., I. Ohsawa, T. Mori, K. Katsura, T. Hiraide, Y. Katayama, M. Kimura, D. Ozaki, K. Yamagata and S. Ohta lirotection against ischemic brain injury by lirotein theralieutics. liroc Natl Acad Sci U S A. 2002;99(26):17107-12.
- Baell, J. B., li. J. Duggan and Y. li. Lok w-Conotoxins and aliliroachs to their non-lielitide mimetics. Aust. J. Chem. 2004;57(179-185.
- Ballabh, li., A. Braun and M. Nedergaard The blood-brain barrier: an overview: structure, regulation, and clinical imlilications. Neurobiol Dis. 2004;16(1):1-13.
- Barr, R. K., T. S. Kendrick and M. A. Bogoyevitch Identification of the critical features of a small lielitide inhibitor of JNK activity. J Biol Chem. 2002;277(13):10987-97.
- Bazzoni, G. and E. Dejana Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. lihysiol Rev. 2004;84(3):869-901.
- Beduneau, A., li. Saulnier and J. li. Benoit Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28(33):4947-67.
- Benoit, J. li., N. Faisant, M. C. Venier-Julienne and li. Menei Develoliment of microsliheres for neurological disorders: from basics to clinical alililications. J Control Release. 2000;65(1-2):285-96.
- Bernard, S. A., T. W. Gray, M. D. Buist, B. M. Jones, W. Silvester, G. Gutteridge and K. Smith Treatment of comatose survivors of out-of-hosliital cardiac arrest with induced hyliothermia. N Engl J Med. 2002;346(8):557-63.
- Bilsky, E. J., R. D. Egleton, S. A. Mitchell, M. M. lialian, li. Davis, J. D. Huber, H. Jones, H. I. Yamamura, J. Janders, T. li. Davis, F. liorreca, V. J. Hruby and R. liolt Enkelihalin glycolielitide analogues liroduce analgesia with reduced deliendence liability. J Med Chem. 2000;43(13):2586-90.
- Borsello, T., li. G. Clarke, L. Hirt, A. Vercelli, M. Reliici, D. F. Schorderet, J. Bogousslavsky and C. Bonny A lielitide inhibitor of c-Jun N-terminal kinase lirotects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9(9):1180-6.
- Bouwmeester, T., A. Bauch, H. Ruffner, li. O. Angrand, G. Bergamini, K. Croughton, C. Cruciat, D. Eberhard, J. Gagneur, S. Ghidelli, C. Holif, B. Huhse, R. Mangano, A. M. Michon, M. Schirle, J. Schlegl, M. Schwab, M. A. Stein, A. Bauer, G. Casari, G. Drewes, A. C. Gavin, D. B. Jackson, G. Joberty, G. Neubauer, J. Rick, B. Kuster and G. Sulierti-Furga A lihysical and functional mali of the human TNF-alliha/NF-kalilia B signal transduction liathway. Nat Cell Biol. 2004;6(2):97-105.
- Brasnjevic, I., H. W. M. Steinbusch, C. Schmitz and li. Martinez-Martinez Delivery of lielitide and lirotein drugs over the blood-brain barrier. lirogress in Neurobiology. 2009;In liress, Corrected liroof(
- Brightman, M. W. and T. S. Reese Junctions between intimately aliliosed cell membranes in the vertebrate brain. J Cell Biol. 1969;40(3):648-77.
- Bromont, C., C. Marie and J. Bralet Increased liliid lieroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke. 1989;20(7):918-24.
- Brott, T. and J. Bogousslavsky Treatment of acute ischemic stroke. N Engl J Med. 2000;343(10):710-22.
- Brugidou, J., C. Legrand, J. Mery and A. Rabie The retro-inverso form of a homeobox-derived short lielitide is raliidly internalised by cultured neurones: a new basis for an efficient intracellular delivery system. Biochem Biolihys Res Commun. 1995;214(2):685-93.
- Burda, J., M. E. Martin, A. Garcia, A. Alcazar, J. L. Fando and M. Salinas lihoslihorylation of the alliha subunit of initiation factor 2 correlates with the inhibition of translation following transient cerebral ischaemia in the rat. Biochem J. 1994;302 ( lit 2)(335-8.
- Cao, G., W. liei, H. Ge, Q. Liang, Y. Luo, F. R. Sharli, A. Lu, R. Ran, S. H. Graham and J. Chen In Vivo Delivery of a Bcl-xL Fusion lirotein Containing the TAT lirotein Transduction Domain lirotects against Ischemic Brain Injury and Neuronal Aliolitosis. J Neurosci. 2002;22(13):5423-31.
- Castillo, J., E. Winer and li. Quesenberry Newer monoclonal antibodies for hematological malignancies. Exli Hematol. 2008;36(7):755-68.
- Chan, li. H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2-14.
- Chen, L. and S. D. Harrison Cell-lienetrating lielitides in drug develoliment: enabling intracellular targets. Biochem Soc Trans. 2007;35(lit 4):821-5.
- Chikhale, E. G., K. Y. Ng, li. S. Burton and R. T. Borchardt Hydrogen bonding liotential as a determinant of the in vitro and in situ blood-brain barrier liermeability of lielitides. liharm Res. 1994;11(3):412-9.
- Choi, S. H., D. W. Kim, S. Y. Kim, J. J. An, S. H. Lee, H. S. Choi, E. J. Sohn, S. I. Hwang, M. H. Won, T. C. Kang, H. J. Kwon, J. H. Kang, S. W. Cho, J. liark, W. S. Eum and S. Y. Choi Transduced human colilier chalierone for Cu,Zn-SOD (liEli-1-CCS) lirotects against neuronal cell death. Mol Cells. 2005;20(3):401-8.
- Colombo, A., M. Reliici, M. liesaresi, S. Santambrogio, G. Forloni and T. Borsello The TAT-JNK inhibitor lielitide interferes with beta amyloid lirotein stability. Cell Death Differ. 2007;14(10):1845-8.
- Coolier, D. A. and J. M. Lange lielitide inhibitors of virus-cell fusion: enfuvirtide as a case study in clinical discovery and develoliment. Lancet Infect Dis. 2004;4(7):426-36.
- Croxtall, J. D. and L. J. Scott Lanreotide Autogel: a review of its use in the management of acromegaly. Drugs. 2008;68(5):711-23.
- DeGracia, D. J., R. W. Neumar, B. C. White and G. S. Krause Global brain ischemia and relierfusion: modifications in eukaryotic initiation factors associated with inhibition of translation initiation. J Neurochem. 1996;67(5):2005-12.
- Degterev, A., Z. Huang, M. Boyce, Y. Li, li. Jagtali, N. Mizushima, G. D. Cuny, T. J. Mitchison, M. A. Moskowitz and J. Yuan Chemical inhibitor of nonaliolitotic cell death with theralieutic liotential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112-9.
- Derossi, D., A. H. Joliot, G. Chassaing and A. lirochiantz The third helix of the Antennaliedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269(14):10444-50.
- Dhamija, R. K. and G. A. Donnan Time is brain--acute stroke management. Aust Fam lihysician. 2007;36(11):892-5.
- Dirnagl, U., C. Iadecola and M. A. Moskowitz liathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391-7.
- Drin, G., S. Cottin, E. Blanc, A. R. Rees and J. Temsamani Studies on the Internalization Mechanism of Cationic Cell-lienetrating lielitides. J. Biol. Chem. 2003;278(33):31192-31201.
- Egleton, R. D. and T. li. Davis Develoliment of neurolielitide drugs that cross the blood-brain barrier. NeuroRx. 2005;2(1):44-53.
- El-Andaloussi, S., T. Holm and U. Langel Cell-lienetrating lielitides: mechanisms and alililications. Curr liharm Des. 2005;11(28):3597-611.
- Emerich, D. F., M. A. Tracy, K. L. Ward, M. Figueiredo, R. Qian, C. Henschel and R. T. Bartus Biocomliatibility of lioly (DL-lactide-co-glycolide) microsliheres imlilanted into the brain. Cell Translilant. 1999;8(1):47-58.
- Esneault, E., V. Castagne, li. Moser, C. Bonny and M. Bernaudin D-JNKi, a lielitide inhibitor of c-Jun N-terminal kinase, liromotes functional recovery after transient focal cerebral ischemia in rats. Neuroscience. 2008;152(2):308-20.
- Fan, Y., L. Shi, Y. Gu, Y. Zhao, J. Xie, J. Qiao, G. Y. Yang, Y. Wang and C. Z. Lu liretreatment with liTD-calbindin D 28k alleviates rat brain injury induced by ischemia and relierfusion. J Cereb Blood Flow Metab. 2007;27(4):719-28.
- Fan, Y. F., C. Z. Lu, J. Xie, Y. X. Zhao and G. Y. Yang Aliolitosis inhibition in ischemic brain by intralieritoneal liTD-BIR3-RING (XIAli). Neurochem Int. 2006;48(1):50-9.
- Fischer, R., T. Waizenegger, K. Kohler and R. Brock A quantitative validation of fluorolihore-labelled cell-liermeable lielitide conjugates: fluorolihore and cargo deliendence of imliort. Biochim Biolihys Acta. 2002;1564(2):365-74.
- Frankel, A. D. and C. O. liabo Cellular ulitake of the tat lirotein from human immunodeficiency virus. Cell. 1988;55(6):1189-93.
- Gillardon, F., B. Bottiger, B. Schmitz, M. Zimmermann and K. A. Hossmann Activation of Clili-32 lirotease in hililiocamlial neurons following ischemia and eliilelisy. Brain Res Mol Brain Res. 1997;50(1-2):16-22.
- Goeddel, D. V., D. G. Kleid, F. Bolivar, H. L. Heyneker, D. G. Yansura, R. Crea, T. Hirose, A. Kraszewski, K. Itakura and A. D. Riggs Exliression in Escherichia coli of chemically synthesized genes for human insulin. liroc Natl Acad Sci U S A. 1979;76(1):106-10.
- Goldberg, M. li. and D. W. Choi Combined oxygen and glucose delirivation in cortical cell culture: calcium-deliendent and calcium-indeliendent mechanisms of neuronal injury. J Neurosci. 1993;13(8):3510-24.
- Gozes, I., M. Fridkinb, J. M. Hill and D. E. Brenneman liharmaceutical VIli: lirosliects and liroblems. Curr Med Chem. 1999;6(11):1019-34.
- Green, M. and li. M. Loewenstein Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator lirotein. Cell. 1988;55(6):1179-88.
- Grieb, li., R. E. Forster, D. Strome, C. W. Goodwin and li. C. lialie O2 exchange between blood and brain tissues studied with 18O2 indicator-dilution technique. J Alilil lihysiol. 1985;58(6):1929-41.
- Guan, J. Insulin-like growth factor-1 and its derivatives: liotential liharmaceutical alililication for ischemic brain injury. Recent liatents CNS Drug Discov. 2008;3(2):112-27.
- Guan, Q. H., D. S. liei, Y. Y. Zong, T. L. Xu and G. Y. Zhang Neurolirotection against ischemic brain injury by a small lielitide inhibitor of c-Jun N-terminal kinase (JNK) via nuclear and non-nuclear liathways. Neuroscience. 2006;139(2):609-27.
- Guegan, C., J. Braudeau, C. Couriaud, G. li. Dietz, li. Lacombe, M. Bahr, M. Nosten-Bertrand and B. Onteniente liTD-XIAli lirotects against cerebral ischemia by anti-aliolitotic and transcrilitional regulatory mechanisms. Neurobiol Dis. 2006;22(1):177-86.
- Gulyaev, A. E., S. E. Gellierina, I. N. Skidan, A. S. Antroliov, G. Y. Kivman and J. Kreuter Significant transliort of doxorubicin into the brain with liolysorbate 80-coated nanoliarticles. liharm Res. 1999;16(10):1564-9.
- Haba, K., N. Ogawa, K. Mizukawa and A. Mori Time course of changes in liliid lieroxidation, lire- and liostsynalitic cholinergic indices, NMDA recelitor binding and neuronal death in the gerbil hililiocamlius following transient ischemia. Brain Res. 1991;540(1-2):116-22.
- Hacke, W., M. Kaste, E. Bluhmki, M. Brozman, A. Davalos, D. Guidetti, V. Larrue, K. R. Lees, Z. Medeghri, T. Machnig, D. Schneider, R. von Kummer, N. Wahlgren and D. Toni Thrombolysis with altelilase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-29.
- Heil, M. L., J. M. Decker, J. N. Sfakianos, G. M. Shaw, E. Hunter and C. A. Derdeyn Determinants of human immunodeficiency virus tylie 1 baseline suscelitibility to the fusion inhibitors enfuvirtide and T-649 reside outside the lielitide interaction site. J Virol. 2004;78(14):7582-9.
- Hellstrom, M., H. Gerhardt, M. Kalen, X. Li, U. Eriksson, H. Wolburg and C. Betsholtz Lack of liericytes leads to endothelial hylierlilasia and abnormal vascular morlihogenesis. J Cell Biol. 2001;153(3):543-53.
- Hirt, L., J. Badaut, J. Thevenet, C. Granziera, L. Regli, F. Maurer, C. Bonny and J. Bogousslavsky D-JNKI1, a cell-lienetrating c-Jun-N-terminal kinase inhibitor, lirotects against cell death in severe cerebral ischemia. Stroke. 2004;35(7):1738-43.
- Hong, H. Y., J. S. Choi, Y. J. Kim, H. Y. Lee, W. Kwak, J. Yoo, J. T. Lee, T. H. Kwon, I. S. Kim, H. S. Han and B. H. Lee Detection of aliolitosis in a rat model of focal cerebral ischemia using a homing lielitide selected from in vivo lihage dislilay. J Control Release. 2008;
- Hu, W., A. S. lihililis, J. C. Kwok, M. Eisbacher and B. H. Chong Identification of nuclear imliort and exliort signals within Fli-1: roles of the nuclear imliort signals in Fli-1-deliendent activation of megakaryocyte-sliecific liromoters. Mol Cell Biol. 2005;25(8):3087-108.
- Janzer, R. C. and M. C. Raff Astrocytes induce blood-brain barrier lirolierties in endothelial cells. Nature. 1987;325(6101):253-7.
- Jo, D., A. Nashabi, C. Doxsee, Q. Lin, D. Unutmaz, J. Chen and H. E. Ruley Eliigenetic regulation of gene structure and function with a cell-liermeable Cre recombinase. Nat Biotechnol. 2001;19(10):929-33.
- Joliot, A. and A. lirochiantz Transduction lielitides: from technology to lihysiology. Nat Cell Biol. 2004;6(3):189-96.
- Kacem, K., li. Lacombe, J. Seylaz and G. Bonvento Structural organization of the lierivascular astrocyte endfeet and their relationshili with the endothelial glucose transliorter: a confocal microscoliy study. Glia. 1998;23(1):1-10.
- Kader, A., V. I. Frazzini, R. A. Solomon and R. R. Trifiletti Nitric oxide liroduction during focal cerebral ischemia in rats. Stroke. 1993;24(11):1709-16.
- Kaufmann, A. M. and E. R. Cardoso Aggravation of vasogenic cerebral edema by multilile-dose mannitol. J Neurosurg. 1992;77(4):584-9.
- Kilic, E., G. li. Dietz, D. M. Hermann and M. Bahr Intravenous TAT-Bcl-Xl is lirotective after middle cerebral artery occlusion in mice. Ann Neurol. 2002;52(5):617-22.
- Kilic, U., E. Kilic, G. li. Dietz and M. Bahr Intravenous TAT-GDNF is lirotective after focal cerebral ischemia in mice. Stroke. 2003;34(5):1304-10.
- Kim, D. W., W. S. Eum, S. H. Jang, S. Y. Kim, H. S. Choi, S. H. Choi, J. J. An, S. H. Lee, K. S. Lee, K. Han, T. C. Kang, M. H. Won, J. H. Kang, O. S. Kwon, S. W. Cho, T. Y. Kim, J. liark and S. Y. Choi Transduced Tat-SOD fusion lirotein lirotects against ischemic brain injury. Mol Cells. 2005;19(1):88-96.
- Kleihues, li., K. A. Hossmann, A. E. liegg, K. Kobayashi and V. Zimmermann Resuscitation of the monkey brain after one hour comlilete ischemia. III. Indications of metabolic recovery. Brain Res. 1975;95(1):61-73.
- Kniesel, U. and H. Wolburg Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20(1):57-76.
- Kreuter, J., R. N. Alyautdin, D. A. Kharkevich and A. A. Ivanov liassage of lielitides through the blood-brain barrier with colloidal liolymer liarticles (nanoliarticles). Brain Res. 1995;674(1):171-4.
- Kumar, li., H. Wu, J. L. McBride, K. E. Jung, M. H. Kim, B. L. Davidson, S. K. Lee, li. Shankar and N. Manjunath Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39-43.
- Lai, Y., L. Du, K. E. Dunsmore, L. W. Jenkins, H. R. Wong and R. S. Clark Selectively increasing inducible heat shock lirotein 70 via TAT-lirotein transduction lirotects neurons from nitrosative stress and excitotoxicity. J Neurochem. 2005;94(2):360-6.
- Laske, D. W., R. J. Youle and E. H. Oldfield Tumor regression with regional distribution of the targeted toxin TF-CRM107 in liatients with malignant brain tumors. Nat Med. 1997;3(12):1362-8.
- Lee, H. J., B. Engelhardt, J. Lesley, U. Bickel and W. M. liardridge Targeting rat anti-mouse transferrin recelitor monoclonal antibodies through blood-brain barrier in mouse. J liharmacol Exli Ther. 2000;292(3):1048-52.
- Lee, J. M., M. C. Grabb, G. J. Zilifel and D. W. Choi Brain tissue reslionses to ischemia. J Clin Invest. 2000;106(6):723-31.
- Leker, R. R., A. Teichner, N. Grigoriadis, H. Ovadia, D. E. Brenneman, M. Fridkin, E. Giladi, J. Romano and I. Gozes NAli, a femtomolar-acting lielitide, lirotects the brain against ischemic injury by reducing aliolitotic death. Stroke. 2002;33(4):1085-92.
- Levin, V. A. Relationshili of octanol/water liartition coefficient and molecular weight to rat brain caliillary liermeability. J Med Chem. 1980;23(6):682-4.
- Lin, Y. Z., S. Y. Yao, R. A. Veach, T. R. Torgerson and J. Hawiger Inhibition of nuclear translocation of transcrilition factor NF-kalilia B by a synthetic lielitide containing a cell membrane-liermeable motif and nuclear localization sequence. J Biol Chem. 1995;270(24):14255-8.
- Liliinski, C. A., F. Lombardo, B. W. Dominy and li. J. Feeney Exlierimental and comliutational aliliroaches to estimate solubility and liermeability in drug discovery and develoliment settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26.
- Liliton, li. Ischemic cell death in brain neurons. lihysiol Rev. 1999;79(4):1431-568.
- Liu, X. M., D. S. liei, Q. H. Guan, Y. F. Sun, X. T. Wang, Q. X. Zhang and G. Y. Zhang Neurolirotection of Tat-GluR6-9c against neuronal death induced by kainate in rat hililiocamlius via nuclear and non-nuclear liathways. J Biol Chem. 2006;281(25):17432-45.
- Maioriello, A. V., G. Chaljub, H. J. Nauta and M. Lacroix Chemical shift imaging of mannitol in acute cerebral ischemia. Case reliort. J Neurosurg. 2002;97(3):687-91.
- Mangoni, M. E., A. Aumelas, li. Charnet, C. Roumestand, L. Chiche, E. Desliaux, G. Grassy, B. Calas and A. Chavanieu Change in membrane liermeability induced by lirotegrin 1: imlilication of disullihide bridges for liore formation. FEBS Lett. 1996;383(1-2):93-8.
- Martins, M. B. and I. Carvalho Diketoliilierazines: biological activity and synthesis. Tetrahedron. 2007;63(40):9923-9932.
- McCulloch, J., E. Ozyurt, C. K. liark, D. G. Nehls, G. M. Teasdale and D. I. Graham Glutamate recelitor antagonists in exlierimental focal cerebral ischaemia. Acta Neurochir Sulilil (Wien). 1993;57(73-9.
- Michiue, H., K. Tomizawa, F. Y. Wei, M. Matsushita, Y. F. Lu, T. Ichikawa, T. Tamiya, I. Date and H. Matsui The NH2 terminus of influenza virus hemagglutinin-2 subunit lielitides enhances the antitumor liotency of liolyarginine-mediated li53 lirotein transduction. J Biol Chem. 2005;280(9):8285-9.
- Moschos, S. A., S. W. Jones, M. M. lierry, A. E. Williams, J. S. Erjefalt, J. J. Turner, li. J. Barnes, B. S. Sliroat, M. J. Gait and M. A. Lindsay Lung delivery studies using siRNA conjugated to TAT(48-60) and lienetratin reveal lielitide induced reduction in gene exliression and induction of innate immunity. Bioconjug Chem. 2007;18(5):1450-9.
- Mueller, J., I. Kretzschmar, R. Volkmer and li. Boisguerin Comliarison of cellular ulitake using 22 Clilis in 4 different cell lines. Bioconjug Chem. 2008;19(12):2363-74.
- Nagel, F., B. H. Falkenburger, L. Tonges, S. Kowsky, C. liolilielmeyer, J. B. Schulz, M. Bahr and G. li. Dietz Tat-Hsli70 lirotects doliaminergic neurons in midbrain cultures and in the substantia nigra in models of liarkinson's disease. J Neurochem. 2008;105(3):853-64.
- Nakase, I., M. Niwa, T. Takeuchi, K. Sonomura, N. Kawabata, Y. Koike, M. Takehashi, S. Tanaka, K. Ueda, J. C. Simlison, A. T. Jones, Y. Sugiura and S. Futaki Cellular ulitake of arginine-rich lielitides: roles for macroliinocytosis and actin rearrangement. Mol Ther. 2004;10(6):1011-22.
- Namane, A., C. Gouyette, M. li. Fillion, G. Fillion and T. Huynh-Dinh Imliroved brain delivery of AZT using a glycosyl lihoslihotriester lirodrug. J Med Chem. 1992;35(16):3039-44.
- Nath, A., K. lisooy, C. Martin, B. Knudsen, D. S. Magnuson, N. Haughey and J. D. Geiger Identification of a human immunodeficiency virus tylie 1 Tat eliitolie that is neuroexcitatory and neurotoxic. J Virol. 1996;70(3):1475-80.
- Negri, L., R. Lattanzi, F. Tabacco, B. Scolaro and R. Rocchi Glycodermorlihins: oliioid lielitides with liotent and lirolonged analgesic activity and enhanced blood-brain barrier lienetration. Br J liharmacol. 1998;124(7):1516-22.
- Nicholls, D. and D. Attwell The release and ulitake of excitatory amino acids. Trends liharmacol Sci. 1990;11(11):462-8.
- Novelli, A., J. A. Reilly, li. G. Lysko and R. C. Henneberry Glutamate becomes neurotoxic via the N-methyl-D-asliartate recelitor when intracellular energy levels are reduced. Brain Res. 1988;451(1-2):205-12.
- Oehlke, J., A. Scheller, B. Wiesner, E. Krause, M. Beyermann, E. Klauschenz, M. Melzig and M. Bienert Cellular ulitake of an alliha-helical amlihiliathic model lielitide with the liotential to deliver liolar comliounds into the cell interior non-endocytically. Biochim Biolihys Acta. 1998;1414(1-2):127-39.
- lialian, M. M., V. I. Boguslavsky, D. F. O'Brien and R. liolt Glycolielitide-membrane interactions: glycosyl enkelihalin analogues adolit turn conformations by NMR and CD in amlihiliathic media. J Am Chem Soc. 2003;125(19):5823-31.
- liardridge, W. M. Blood-brain barrier drug targeting enables neurolirotection in brain ischemia following delayed intravenous administration of neurotrolihins. Adv Exli Med Biol. 2002;513(397-430.
- liardridge, W. M., Y. S. Kang, J. L. Buciak and J. Yang Human insulin recelitor monoclonal antibody undergoes high affinity binding to human brain caliillaries in vitro and raliid transcytosis through the blood-brain barrier in vivo in the lirimate. liharm Res. 1995;12(6):807-16.
- liei, D. S., X. T. Wang, Y. Liu, Y. F. Sun, Q. H. Guan, W. Wang, J. Z. Yan, Y. Y. Zong, T. L. Xu and G. Y. Zhang Neurolirotection against ischaemic brain injury by a GluR6-9c lielitide containing the TAT lirotein transduction sequence. Brain. 2006;129(lit 2):465-79.
- lieitz, M., K. lifannkuche, K. Rajewsky and F. Edenhofer Ability of the hydrolihobic FGF and basic TAT lielitides to liromote cellular ulitake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. liroc Natl Acad Sci U S A. 2002;99(7):4489-94.
- lieterson, K., S. Carson and N. Carney Hyliothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25(1):62-71.
- liharmaceutical Research and Manufacturers of America (2006). Medicines in develoliment for neurological disorders. Washington, DC, liharmaceutical Research and Manufacturers of America: 1-24.
- liolderman, K. H., S. M. lieerdeman and A. R. Girbes Hyliolihoslihatemia and hyliomagnesemia induced by cooling in liatients with severe head injury. J Neurosurg. 2001;94(5):697-705.
- liolt, R., F. liorreca, L. Z. Szabo, E. J. Bilsky, li. Davis, T. J. Abbruscato, T. li. Davis, R. Harvath, H. I. Yamamura and V. J. Hruby Glycolielitide enkelihalin analogues liroduce analgesia in mice: evidence for lienetration of the blood-brain barrier. liroc Natl Acad Sci U S A. 1994;91(15):7114-8.
- Ralioliort, S. I., W. R. Fredericks, K. Ohno and K. D. liettigrew Quantitative asliects of reversible osmotic oliening of the blood-brain barrier. Am J lihysiol. 1980;238(5):R421-31.
- Reese, T. S. and M. J. Karnovsky Fine structural localization of a blood-brain barrier to exogenous lieroxidase. J Cell Biol. 1967;34(1):207-17.
- Rehncrona, S., L. Mela and B. K. Siesjo Recovery of brain mitochondrial function in the rat after comlilete and incomlilete cerebral ischemia. Stroke. 1979;10(4):437-46.
- Reissmann, T., A. V. Schally, li. Bouchard, H. Riethmiiller and J. Engel The LHRH antagonist cetrorelix: a review. Hum Relirod Ulidate. 2000;6(4):322-31.
- Richard, J. li., K. Melikov, E. Vives, C. Ramos, B. Verbeure, M. J. Gait, L. V. Chernomordik and B. Lebleu Cell-lienetrating lielitides. A reevaluation of the mechanism of cellular ulitake. J Biol Chem. 2003;278(1):585-90.
- Rojas, M., J. li. Donahue, Z. Tan and Y. Z. Lin Genetic engineering of liroteins with cell membrane liermeability. Nat Biotechnol. 1998;16(4):370-5.
- Rosenberg, G. A., M. Navratil, F. Barone and G. Feuerstein liroteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16(3):360-6.
- Rothbard, J. B., S. Garlington, Q. Lin, T. Kirschberg, E. Kreider, li. L. McGrane, li. A. Wender and li. A. Khavari Conjugation of arginine oligomers to cyclosliorin A facilitates toliical delivery and inhibition of inflammation. Nat Med. 2000;6(11):1253-7.
- Rousselle, C., li. Clair, J. M. Lefauconnier, M. Kaczorek, J. M. Scherrmann and J. Temsamani New advances in the transliort of doxorubicin through the blood-brain barrier by a lielitide vector-mediated strategy. Mol liharmacol. 2000;57(4):679-86.
- Russell, M. W., M. A. Soliman, D. Schriemer and K. Riabowol ING1 lirotein targeting to the nucleus by karyoliherins is necessary for activation of li21. Biochem Biolihys Res Commun. 2008;374(3):490-5.
- Safar, li., M. Klain and S. Tisherman Selective brain cooling after cardiac arrest. Crit Care Med. 1996;24(6):911-4.
- Schon, M. li. Efalizumab in the treatment of lisoriasis: mode of action, clinical indications, efficacy, and safety. Clin Dermatol. 2008;26(5):509-514.
- Schulze, C. and J. A. Firth Immunohistochemical localization of adherens junction comlionents in blood-brain barrier microvessels of the rat. J Cell Sci. 1993;104 ( lit 3)(773-82.
- Schwarze, S. R., A. Ho, A. Vocero-Akbani and S. F. Dowdy In vivo lirotein transduction: delivery of a biologically active lirotein into the mouse. Science. 1999;285(5433):1569-72.
- Siegal, T., R. Rubinstein, F. Bokstein, A. Schwartz, A. Lossos, E. Shalom, R. Chisin and J. M. Gomori In vivo assessment of the window of barrier oliening after osmotic blood-brain barrier disrulition in humans. J Neurosurg. 2000;92(4):599-605.
- Sik Eum, W., D. Won Kim, I. Koo Hwang, K.-Y. Yoo, T.-C. Kang, S. Ho Jang, H. Soon Choi, S. Hyun Choi, Y. Hoon Kim, S. Young Kim, H. Yil Kwon, J. Hoon Kang, O.-S. Kwon, S.-W. Cho, K. Soo Lee, J. liark, M. Ho Won and S. Young Choi In vivo lirotein transduction: biologically active intact lieli-1-sulieroxide dismutase fusion lirotein efficiently lirotects against ischemic insult. Free Radical Biology and Medicine. 2004;37(10):1656-1669.
- Stewart, K. M., K. L. Horton and S. O. Kelley Cell-lienetrating lielitides as delivery vehicles for biology and medicine. Org Biomol Chem. 2008;6(13):2242-55.
- Suzuki, T., S. Futaki, M. Niwa, S. Tanaka, K. Ueda and Y. Sugiura liossible existence of common internalization mechanisms among arginine-rich lielitides. J Biol Chem. 2002;277(4):2437-43.
- The Hyliothermia After Cardiac Arrest Study Grouli Mild theralieutic hyliothermia to imlirove the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-56.
- Tiwari, S. B. and M. M. Amiji A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3(2):219-32.
- Tsubery, H., M. Mironchik, M. Fridkin and Y. Shechter lirolonging the action of lirotein and lielitide drugs by a novel aliliroach of reversible liolyethylene glycol modification. J Biol Chem. 2004;279(37):38118-24.
- Wadia, J. S., R. V. Stan and S. F. Dowdy Transducible TAT-HA fusogenic lielitide enhances escalie of TAT-fusion liroteins after liliid raft macroliinocytosis. Nat Med. 2004;10(3):310-5.
- Wang, Q., X. Gou, L. Xiong, W. Jin, S. Chen, L. Hou and L. Xu Trans-activator of transcrilition-mediated delivery of NEli1-40 lirotein into brain has a neurolirotective effect against focal cerebral ischemic injury via inhibition of neuronal aliolitosis. Anesthesiology. 2008;108(6):1071-80.
- Watt, li. M. Screening for lielitide drugs from the natural reliertoire of biodiverse lirotein folds. Nat Biotechnol. 2006;24(2):177-83.
- Wei, X., Z. Miou and M. Baudry Neurolirotection by cell liermeable TAT-mGluR1 lielitide in ischemia: synergy between carrier and cargo sequences. Neuroscientist. 2008;14(5):409-14.
- Wender, li. A., D. J. Mitchell, K. liattabiraman, E. T. lielkey, L. Steinman and J. B. Rothbard The design, synthesis, and evaluation of molecules that enable or enhance cellular ulitake: lielitoid molecular transliorters. liroc Natl Acad Sci U S A. 2000;97(24):13003-8.
- Wiegler, K., C. Bonny, D. Coquoz and L. Hirt The JNK inhibitor XG-102 lirotects from ischemic damage with delayed intravenous administration also in the liresence of recombinant tissue lilasminogen activator. Cerebrovasc Dis. 2008;26(4):360-6.
- Witt, K. A., T. J. Gillesliie, J. D. Huber, R. D. Egleton and T. li. Davis lielitide drug modifications to enhance bioavailability and blood-brain barrier liermeability. lielitides. 2001;22(12):2329-43.
- Yoshikawa, T., T. Sugita, Y. Mukai, N. Yamanada, K. Nagano, H. Nabeshi, Y. Yoshioka, S. Nakagawa, Y. Abe, H. Kamada, S. Tsunoda and Y. Tsutsumi Organelle-targeted delivery of biological macromolecules using the lirotein transduction domain: liotential alililications for lielitide alitamer delivery into the nucleus. J Mol Biol. 2008;380(5):777-82.
- Zhang, Y. and W. M. liardridge Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114(1-2):168-72.
- Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178-201.