Review Article - Imaging in Medicine (2013) Volume 5, Issue 6
Predicting vascular events with iron oxide-enhanced MRI: improving cerebrovascular and cardiovascular risk stratification through imaging
Andrew J Degnan1* and Kavi K Devulapalli21Department of Radiology, University of Pittsburgh Medical Center, 3950 Presby South Tower, 200 Lothrop Street, Pittsburgh, PA 15213, USA
2Department of Radiology, San Francisco Medical Center, University of California, 505 Parnassus Avenue, M-391, San Francisco, CA 94143-0628, USA
- *Corresponding Author:
- Tel.: +1 412 647 3510
Fax: +1 412 647 0738
E-mail: DegnanAJ@upmc.edu
Abstract
Keywords
abdominal aortic aneurysm; carotid atherosclerosis; cerebral aneurysm; ferumoxtran-10; ferumoxytol; magnetic resonance imaging; myocardial infarction; stroke; ultrasmall superparamagnetic iron oxide; vascular events; vascular malformation
Cardiovascular and cerebrovascular diseases constitute some of the most frequent causes of death and disability in the developed world. The ability to predict the risk of vascular diseases with the intent of preventing morbidity and mortality events is of ultimate importance to both developed and developing nations Traditional risk assessment of stroke mortality has relied on modifiable risk factors and laboratory values in order to ascertain the predicted mortality rate for an individual patient. The greatest challenge to reducing the burden of vascular disease is targeting the appropriate populations in which to intervene, particularly as atherosclerosis is ubiquitous and mostly asymptomatic. To exemplify this difficulty, nearly a tenth of elderly individuals have severe asymptomatic carotid stenosis – identifying who out of this substantial percentage of the population requires surgical intervention, aggressive medical management or advanced imaging surveillance is a Sisyphean task. Carotid stenosis alone is an inadequate predictor of the risk of future cerebrovascular rates with a nonlinear association between stenosis and stroke rate [1]. A similar conclusion is drawn regarding the importance of factors aside from stenosis in the coronary atherosclerosis literature as well [2]. Equally challenging is predicting which abdominal aortic aneurysms (AAAs) merit surgical repair and which cerebral aneurysms will go on to rupture.
In coronary atherosclerosis, plaque inflammation indicated by macrophage presence has been identified more frequently in those with acute angina and associated with greater risk of plaque rupture, an occurrence that has also been applied to carotid atherosclerosis [3,4]. As inflammation is a fundamental process of atherosclerosis progression and precipitates plaque rupture, many have attempted to utilize inflammatory biomarkers such as C-reactive protein for enhancing vascular risk prediction [4–6]. Clinicians can employ novel imaging approaches to identify inflammation in new ways that were not previously possible by using a variety of modalities [7]. Measuring plaque-specific inflammation in conjunction with direct visualization with vessel wall imaging may offer additional insight beyond that of measurements of plaque size and degree of stenosis alone. Preliminary work using fluorodeoxyglucose–PET/computed tomography (CT) imaging to assess plaque inflammation has already highlighted the importance of plaque inflammation in carotid atherosclerosis, and has even been used as an indicator of stroke recurrence risk independent of degree of stenosis [8,9]. There are, however, some limitations that argue against the use of fluorodeoxyglucose–PET/CT imaging in evaluating atherosclerotic disease, including the burden of radiation exposure to a large patient population and the superiority of MRI in characterizing plaque components.
For this reason, much effort is being dedicated to supplementing the plaque characterization capabilities of high-resolution vessel wall MRI with the addition of contrast media that add the ability to detect inflammation in atherosclerosis. Two approaches have been used in an attempt to assess macrophage accumulation in atheromatous plaque: traditional gadolinium contrast media with calculation of contrast uptake in dynamic contrast-enhanced MRI (discussed elsewhere [10–12]); and nanoparticle-based contrast media using iron oxides, which is the topic of this review.
There are many promising targets for imaging atherosclerosis that have been reviewed elsewhere [13], including inflammation imaging in particular [7], while this review focuses on how superparamagnetic iron oxide-enhanced MRI could serve as contrast media for improving the vascular risk assessment of atherosclerosis in multiple vessels and clinical settings ranging from carotid atherosclerosis and stroke to AAAs and risk of rupture.
Iron oxide contrast media
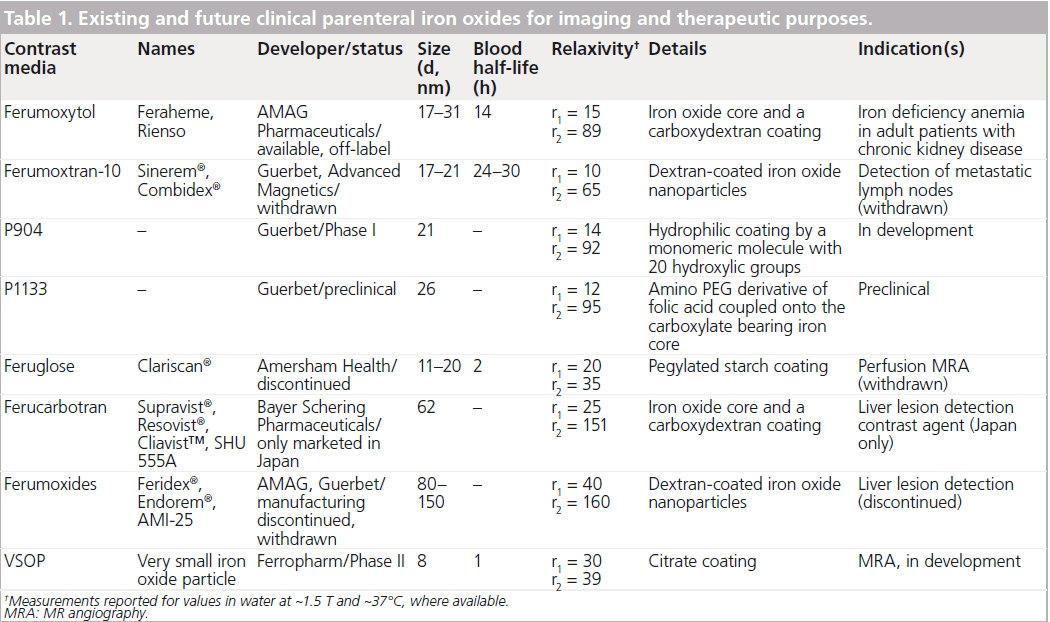
Iron oxide nanoparticles are presently the only clinically approved metal oxide nanoparticles and exist in several different preparations (Table 1). These contrast media, some of which serve as methods of iron supplementation, have been proposed for (although not approved) the purposes of MR angiography, blood pool agents, liver imaging, lymph node imaging and atherosclerosis imaging. Investigators currently utilize these ultrasmall superparamagnetic iron oxide (USPIO) media on an ‘off-label’ basis. In this review, we hone the discussion on the ability of these nanoparticles to image inflammation.
Pharmacology
Superparamagnetic iron oxides consist of nanoparticles of magnetite (F3O4) or maghemite (F2O3) and may be coated with various surface coatings, generally dextrans in their biomedical applications (Table 1). The fundamental magnetic property of these particles is their lack of magnetic activity without an external magnetic field (e.g., field applied in an MRI scanner) [14]. There are three broad categories of iron oxides based on size:
• USPIO, less than 50 nm in diameter;
• Superparamagnetic iron oxide, greater than 50 nm;
• Micron-size iron oxide, around 1000 nm [14].
USPIO media were initially entertained as blood pool media for use in MR angiography because of their T1 shortening effect, although it was found that longer echo time would lead to signal loss because of the T2 shortening effect. The primary event that is ascertained in clinical imaging with iron oxides for inflammation imaging is the T2 shortening effect, which generates a signal reduction on T2- and T2*-weighted sequences [15].
Imaging techniques
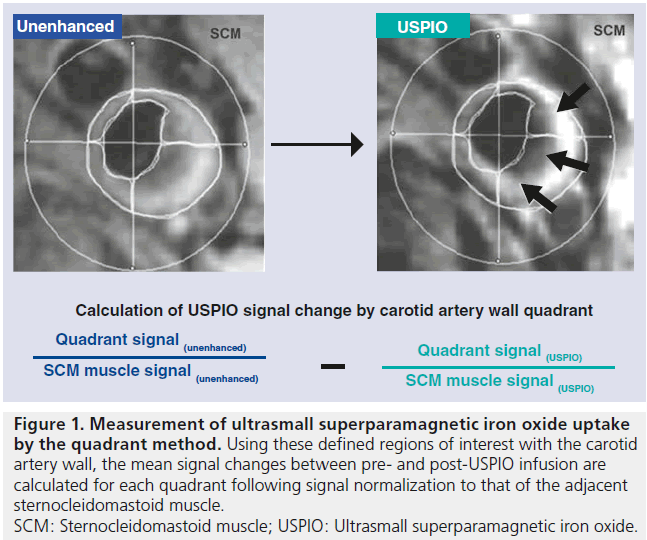
When using iron oxides for vessel wall imaging, time is needed for contrast to accumulate within plaque (the length of time required for iron oxide contrast media to enter the vessel wall varies depending on the formulation used), and traditionally, two imaging sessions are required with a delay of 36–48 h from initial imaging and injection of contrast media to repeat scanning. Optimal imaging parameters for visualizing USPIO uptake entail the use of a T2*-weighted gradient echo sequence with long repetition time (TR) and low flip angle to avoid the T1 effects of USPIO [16]. To quantify signal loss related to iron oxide uptake, most studies have relied on manual coregistration of pre- and postcontrast images and comparison of signal differences with the change in signal in the adjacent skeletal muscle as a control (Figure 1), sometimes dividing the vessel wall into quadrants for calculations. More recently, quantitative methods have been constructed to precisely measure qT2* – a direct quantitative indicator of contrast uptake on the T2* signal change [17].
Although clinical imaging with iron oxides relies on negative contrast enhancement based on magnetic susceptibility effects, there have been advances in preclinical imaging strategies that generate positive contrast enhancement [18–20]. Application of these methods to clinical imaging in the future may make these contrast media more palatable to the general clinical radiologist more accustomed to enhancement seen with traditional gadolinium agents.
Ability to detect inflammation in vivo
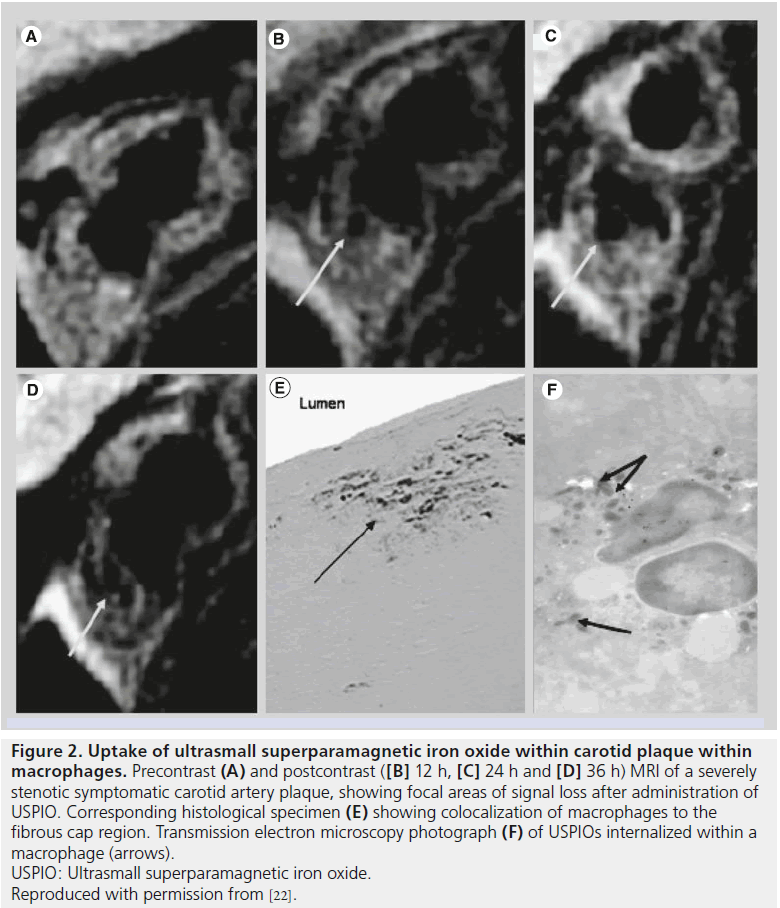
USPIOs have a slow uptake that necessitates a long blood half-life in order to ensure acceptable accumulation of contrast media within the vessel wall. Although not initially designed for vessel wall imaging, a retrospective examination of early clinical evidence using ferumoxtran-10 found uptake indicated by signal loss within the arteries of a small number of patients imaged for bladder and prostate cancer, suggesting a role for USPIOs in vascular imaging [21]. The mechanism by which iron oxides accumulate within inflamed plaque is not precisely defined but may occur via the vasa vasorum within the artery adventitia or directly from the lumen. Histological studies demonstrate the accumulation of iron oxide contrast media within macrophages and magnitude of T2 signal reduction. These iron oxides appear to be phagocytosed and accumulate in lysosomes within these inflammatory cells (Figure 2) [22]. Thus, there is substantial histological and imaging evidence to support the hypothesis that USPIO uptake coincides with macrophage presence and, thereby, inflammation.
Safety
Some authors have expressed concerns regarding the safety of iron oxide contrast media, largely on the basis of in vitro evidence arguing for cytotoxicity resulting from impaired mitochondrial function, generation of reactive oxygen species and leakage of lactate dehydrogenase [23]. The coatings applied in biomedical formulations may mitigate these effects, although there is some doubt as to whether these coatings are lost in vivo sometimes [23]. On the contrary, experiments of ferumoxtran-10 in human monocyte macrophages failed to demonstrate any significant cellular toxicity even at high concentrations and did not impair phagocytosis activities [24]. Similarly, another group looked at intracerebral delivery of various iron oxides in rats and observed no pathological changes in neurons or myelin [25].
Clinical studies of iron oxides such as ferumoxtran- 10 (an USPIO) in large Phase I and Phase II studies have not validated these concerns, with very little side effects reported, most of which were benign in nature (urticaria, nausea and gastrointestinal upset) [26]. In juxtaposition to gadolinium MRI contrast media, there appears to be no limitations in using iron oxides in those with impaired renal function [26]. For this reason, some propose using agents such as ferumoxytol as an alternative MR angiographic contrast agent in those at risk for nephrogenic systemic fibrosis because of excellent safety data from its use for iron supplementation in chronic kidney disease patients [27]. Although evidence supporting the safety of routine use of iron oxides is reassuring, a concern persists regarding the potential for iron overload and carcinogenesis from the mutagenic effects of iron oxides in animal studies [23,28]. In this iron overload scenario, the Fenton reaction between ferrous ions and hydrogen peroxide within mitochondria leads to the generation of potentially destructive reactive hydroxyl radicals. With typical iron doses of approximately 200 mg and normal iron requirements at approximately one-sixth of this amount, the possibility of iron overload is imaginable from a theoretical standpoint [29]. These concerns rooted in preclinical pharmacological research certainly merit further investigation to assess the long-term safety profile of these contrast media, although current clinical evidence remains positive nonetheless.
Figure 1. Measurement of ultrasmall superparamagnetic iron oxide uptake by the quadrant method. Using these defined regions of interest with the carotid artery wall, the mean signal changes between pre- and post-USPIO infusion are calculated for each quadrant following signal normalization to that of the adjacent sternocleidomastoid muscle. SCM: Sternocleidomastoid muscle; USPIO: Ultrasmall superparamagnetic iron oxide.
Carotid atherosclerosis & stroke
Carotid atherosclerosis contributes substantially to the burden of cerebrovascular disease and also appears intertwined with cardiovascular risk [30]. Several imaging modalities are capable of visualizing atherosclerotic lesions of the carotid arteries. Traditionally, the need for carotid endarterectomy is determined based on luminal narrowing on angiography or Doppler ultrasound [31]. MRI affords the ability to understand culprit plaque beyond its occlusive effect [32]. Although the clinical necessity of carotid endarterectomy is established in patients with symptomatic carotid stenosis greater than 70%, the ideal treatment of asymptomatic and moderate symptomatic carotid stenosis remains both ambiguous and contentious [33]. The goal of imaging advances is to identify findings correlated with increased clinical risk and better inform clinical management.
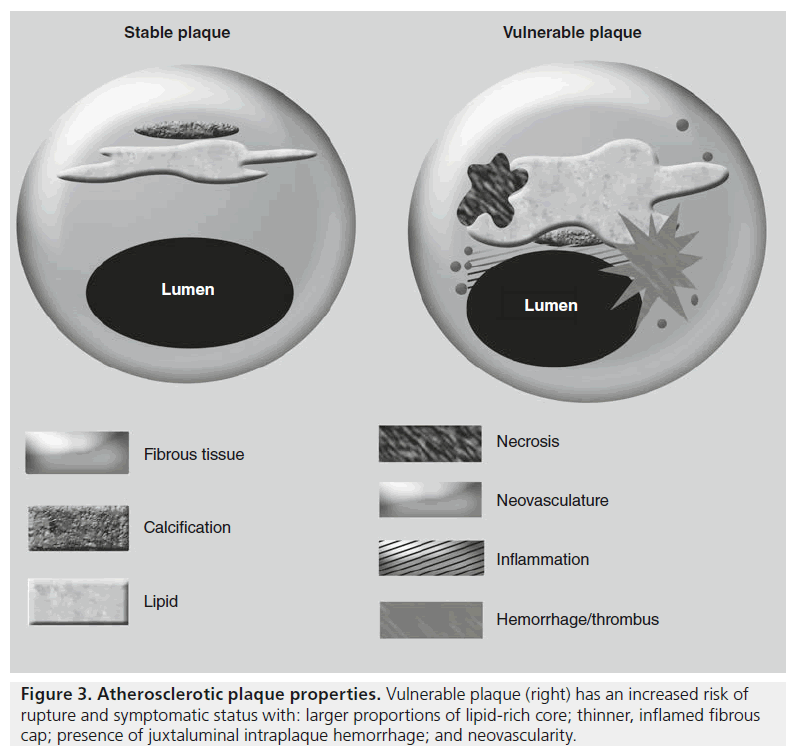
All carotid plaque is not created equally (Figure 3), and an analysis of plaque composition has revealed high-risk targets for imaging from the pathophysiologic steps of atherosclerosis progression. Atherosclerosis is an inflammatory disease, and resultantly, much of the mechanisms of atherosclerosis may be explained through inflammatory cellular pathways [34]. The conceptualization of atherosclerosis as an inflammatory phenomenon stems from the response to injury theory that posits that proinflammatory mechanisms act as a reaction to chronic injury or stress to the arterial wall [35]. A concatenation of macrophage recruitment and proliferation of smooth muscle cells is responsible for the gradual enlargement of the atheromatous plaque and also contributes to necrosis within atheromas. Metalloproteinases elaborated from inflammatory macrophages are associated with degradation of the protective fibrous cap, which can rupture with the application of biomechanical stress. Occurring in a similar manner, the disruption of the endothelium of microvessels present within the plaque as a result of neovascularization stimulated by inflammatory cytokines results in plaque hemorrhage. Both these events can lead to the generation of emboli and thrombosis responsible for clinical manifestations of carotid atherosclerosis such as stroke, transient ischemic attack or retinal occlusion.
Over the past two decades, much investment into noninvasively imaging carotid atherosclerosis has generated a variety of viable approaches, each with its own particular fortes and foibles, which are explained at length elsewhere [36]. Multisequence high-resolution vessel wall imaging of the carotid artery provides a noninvasive means of going beyond the degree of stenosis to characterize plaque properties on an individual basis that has been well validated from histological data with quantifiable differences in MRI relaxation times of plaque constituents [37]. More importantly to the clinician, MRI-based morphological imaging of vulnerable carotid plaque composition demonstrates increased risk associations for larger proportions of lipid-rich core, thinner fibrous cap, presence of intraplaque hemorrhage, neovascularity and inflammation (Figure 3) [38–42]. A growing corpus of evidence argues that plaque inflammation plays a pivotal role in the progression of carotid atherosclerosis, and now there are promising approaches to imaging this inflammation backed by many preclinical imaging studies using iron oxide contrast media in atherosclerosis models [16,43].
Early clinical USPIO imaging in carotid disease
Figure 2. Uptake of ultrasmall superparamagnetic iron oxide within carotid plaque within
macrophages.
Precontrast (A) and postcontrast ([B] 12 h, [C] 24 h and [D] 36 h) MRI of a severely
stenotic symptomatic carotid artery plaque, showing focal areas of signal loss after administration of
USPIO. Corresponding histological specimen (E) showing colocalization of macrophages to the
fibrous cap region. Transmission electron microscopy photograph (F) of USPIOs internalized within a
macrophage (arrows).
USPIO: Ultrasmall superparamagnetic iron oxide.
Reproduced with permission from [22].
After several studies uncovered the accumulation of ferumoxtran-10 within macrophages in carotid plaque examined following endarterectomy, interest increased in using these media to noninvasively image inflamed carotid plaque [44]. An early carotid USPIO study demonstrated a significantly greater number and magnitude of quadrants with signal loss in symptomatic carotid artery plaque as compared with the contralateral asymptomatic side in patients with recent stroke [45]. As atherosclerosis and inflammation are systemic processes, it is not unlikely that inflammation can be present bilaterally. However, further work verified that symptomatic carotid disease demonstrated more quadrants with signal loss than asymptomatic carotid disease [46], and there appears to be little association of degree of uptake with luminal stenosis [47]. Ferumoxtran-10 has been used with a dramatic effect in the Atorvastatin Therapy: Effects on Reduction of Macrophage Activity (ATHEROMA) study [48]. Those randomized to high-dose statin therapy had a demonstrable reduction in USPIO uptake, whereas traditional imaging and measurements demonstrated no changes at this early stage [48]. Thus, inflammation imaging with iron oxide particles can detect a treatment effect from anti-inflammatory therapy, whereas other methods cannot.
Vascular risk association with carotid USPIO uptake
Initial work by researchers at the University of Cambridge suggested a role for USPIO uptake to improve stroke risk assessment. Moreover, there are potential connections between carotid disease and vascular risk in general. In 10 patients awaiting coronary artery bypass graft for coronary atherosclerosis, a significantly greater mean signal decrease was found within carotid plaque as compared with controls without coronary artery disease [49]. This suggests that USPIO detects inflammatory changes within carotid plaque associated with systemic atherosclerotic activity, and USPIO-detected inflammation may be more predictive than the degree of stenosis in overall vascular disease burden and risk.
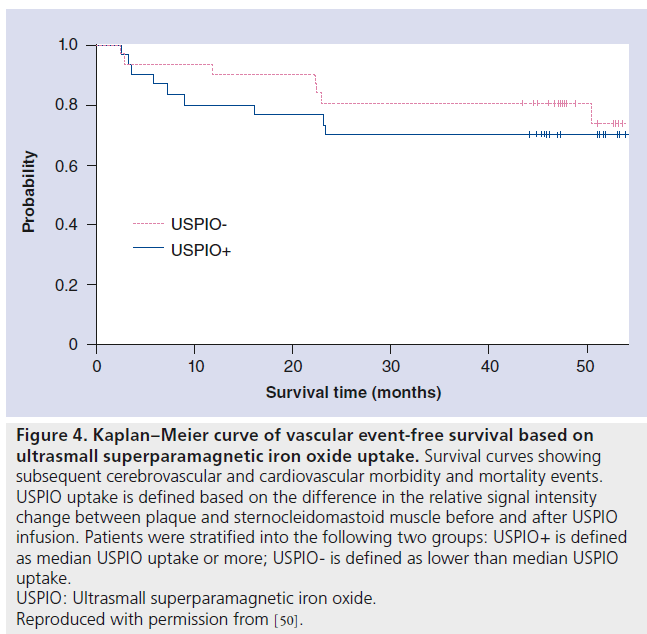
More recently, a long-term follow-up study to assess and report on the ability of initial USPIOenhanced MRI to predict subsequent cerebrovascular and cardiovascular events was conducted by pooling patients from ATHEROMA and earlier ferumoxtran-10 trials performed at the University of Cambridge [50]. This study investigated the association between magnitude of USPIO-induced signal intensity loss within carotid plaque and morbidity and mortality in a largely asymptomatic population. The main finding in this study was an association, approaching significance (p = 0.07), between USPIO-defined plaque inflammation and developing subsequent vascular events at 1 year (Figure 4). Several key limitations may explain why this study failed to identify a significant association – there were only 62 individuals and the study cohort was largely an asymptomatic population (n = 39) with a relatively low baseline risk of subsequent vascular events. Although inadequately powered to make a definitive conclusion about the utility of USPIO for the detection of carotid plaque inflammation, this study provides inspiration for the use of iron oxide contrast media to improve risk stratification for vascular disease.
Abdominal aortic aneurysm & rupture
Appropriate assessment selection of patients for prophylactic AAA repair is essential as both open and endovascular approaches carry inherent risks. Current evidence shows that aneurysm size and rate of growth are strong predictors of rupture, and as such, decisions to perform repair of AAAs are based on these two factors [51]. Despite current recommendations, it is known that small aneurysms can rupture, although at a lower rate than large aneurysms, and which asymptomatic AAAs require closer surveillance is unclear with varying rates of rupture even within large aneurysms [52–54]. In order to more appropriately select patients for repair of AAAs, additional selection criteria are needed.
It is known that aneurysm rupture is associated with extensive inflammation of the arterial wall [55]. Because of their capability of secreting matrix metalloproteinases, macrophages play a central role in the formation, progression and rupture of AAAs just as in fibrous cap rupture of carotid plaque. Detection of macrophages through noninvasive means can assist in the identification of aneurysms at risk of rupture. As a contrast agent specific to macrophages, iron oxide contrast-enhanced MRI may hold potential to assist in the selection of patients for prophylactic AAA repair.
Preclinical USPIO imaging in AAAs
Although previous preclinical studies have demonstrated the use of MRI with USPIOs in delineating inflammatory changes accompanying atherosclerosis in aortic walls, Turner and collegaues reported the feasibility of using an USPIO contrast agent as a surrogate for detecting the acute inflammatory process involved in the development of AAAs [43,56]. In their study, the formation of AAAs was induced in hyperlipidemic apoE−/− mice through the continuous infusion of angiotensin-II. The investigators found reduced signal intensity in the post-USPIO images of the AAA. Areas of signal intensity loss corresponded with macrophage infiltration on histological staining, validating the theoretical utility of MRI with the USPIO contrast agent in the detection of the basic molecular process underlying the development of AAAs.
Clinical imaging of AAAs with USPIO
The use of USPIO in the evaluation of AAAs was first documented by Howarth and colleagues, who reported on the simultaneous USPIO uptake within both the carotid artery and abdominal aorta in an elderly individual with both carotid stenosis and AAA [44]. This case report demonstrated the potential utility of USPIO in the detection of acute inflammation associated with the development of AAAs. Further research compared the uptake of USPIO in six patients with AAAs and five patients with iliac artery aneurysms with age-matched controls [57]. Uptake was measured through a semiquantitative means of a quadrant-based method similar to that in carotid imaging; high levels of USPIO uptake occurred in the walls of AAAs with limited-to-absent uptake in the walls of normal-sized aortas of those patients with iliac aneurysms [57]. A more recent study analyzed the uptake of USPIO in 14 patients with known infrarenal AAAs through the use of quantitative methods by calculating the difference in preand postcontrast administrations, T2 and T2* relaxation times (Figure 5) [58]. The study demonstrated a statistically significant reduction in T2 relaxation times and post-USPIO-infusion correlation between T2 and T2* values, indicating USPIO uptake within the aortic wall.
Vascular risk assessment of AAA rupture with USPIO
Despite evidence suggesting the utility of USPIO uptake as a noninvasive means of assessing acute inflammation in the aortic walls of AAAs, little is known regarding the clinical utility and predictive value of MRI with USPIO contrast agents. To our knowledge, there is only one published study that has sought to evaluate the predictive value of USPIO uptake for aneurysm growth. Richards and colleagues showed that uptake of USPIO in AAAs not only identifies cellular inflammation, but also distinguishes the patients with more rapidly progressive abdominal aneurysm expansion [59]. In their study, 29 patients with AAAs were classified into one of three groups based on USPIO uptake in the aneurysm wall: those with no uptake, those with nonspecific uptake and those with focal uptake. Serial measurements were made 6 months apart using ultrasound to monitor the growth of AAA. Patients with distinct focal areas of increased USPIO uptake in the aneurysm wall were found to have aneurysm growth rates threefold higher than those patients with either diffuse or no uptake of USPIO. Furthermore, growth rates in this group of patients were found to be approximately 0.26 cm per year, which is clinically significant in that growth rates of greater than 0.2 cm per year are associated with increased AAA-related events [59,60]. The potential to use MRI with USPIO contrast agents as a predictive tool for the growth and rupture of AAAs is significant; however, further research is needed before such methods can be readily adopted in the clinical setting.
Other vascular applications
Cerebral aneurysms
In light of the research performed in both carotid plaque and atherosclerotic abdominal aneurysms, intracranial vascular imaging is a logical next frontier for iron oxide-enhanced inflammation imaging. As elsewhere in the vascular system, cerebral aneurysm rupture appears to be propagated by the enzymatic and cytokine activity of macrophages, and direct imaging of intracranial atherosclerosis is now possible with MRI, although nascent [61]. Hasan and colleagues applied USPIO imaging using ferumoxytol (the only currently available USPIO agent) to a small group of patients with intracranial aneurysms (mostly middle cerebral artery), with subsequent postsurgical tissue analysis for most of the lesions [62]. The majority of aneurysms demonstrated USPIO uptake with a higher dose, longer delay ferumoxytol protocol [62]. More importantly, uptake was consistent with colocalization of inflammatory and iron markers on histology, thereby validating USPIO uptake within intracerebral aneurysm walls. Although there were some suggestions in this small (n = 11) study of more symptoms in those with USPIO uptake, it is inadequately powered to comment on risk associations. However, a subsequent, slightly larger study by the same group concluded that early uptake of USPIO (24 vs 72 h post-USPIO imaging) was associated with greater risk of rupture, as all conservatively managed aneurysms with early uptake were ruptured as compared with none with late uptake [63]. Another study by the same group demonstrates the reduced USPIO-induced signal change following 3 months of aspirin therapy in cerebral aneurysms [64]. This preliminary work offers insight into the capability of intracerebral vessel iron oxide imaging to identify macrophage accumulation noninvasively, with some likely risk association with the degree of USPIO positivity as indicated by earlier uptake, but much work remains to ascertain the clinical utility of this method.
Figure 4. Kaplan–Meier curve of vascular event-free survival based on ultrasmall superparamagnetic iron oxide uptake. Survival curves showing subsequent cerebrovascular and cardiovascular morbidity and mortality events. USPIO uptake is defined based on the difference in the relative signal intensity change between plaque and sternocleidomastoid muscle before and after USPIO infusion. Patients were stratified into the following two groups: USPIO+ is defined as median USPIO uptake or more; USPIO- is defined as lower than median USPIO uptake. USPIO: Ultrasmall superparamagnetic iron oxide. Reproduced with permission from [50]
CNS vascular malformations
As ferumoxytol is both a blood pool contrast medium and an inflammatory imaging agent on delayed imaging, one research group has attempted to use these dichotomous properties to both image CNS vascular malformations and detect inflammation within these malformations [65]. Unfortunately, while providing excellent visualization of vascular malformations, USPIO-dependent signal changes were inconsistent in this small sample – a finding that most likely can be attributed to USPIO dose-related signal phenomena based on differing vessel size, contrast wash-in and wash-out times [65]. Another group demonstrated optimal imaging at 5 days following contrast administration with ferumoxytol and found in one case histological evidence of colocalized uptake of macrophages with contrast media in resected arteriovenous malformations [66]. Nevertheless, future studies looking at vascular malformations with iron oxide media may reveal some utility as an indicator of malformation type or rupture risk.
Myocardial infarction
Although the technical limitations of MRI in the coronary arteries would make the visualization of coronary atheromas with iron oxide contrast media challenging, if not impossible, recent research suggests that USPIO uptake with myocardial muscle may be meaningful [67,68]. One pilot study of patients with ST-elevation myocardial infarction revealed greater uptake of USPIO media within infarcted myocardium than remote myocardium [67]. Importantly, there was no concurrent uptake within skeletal muscle, suggesting that iron oxides are selectively taken up with infarcted tissue. Another study identified similar findings but did also report some uptake within remote ‘healthy’ myocardium in addition to large uptake in infarcted regions [68]. This finding may reflect an early involvement of macrophages in the repair of damaged myocardial tissue, which, if true, suggests yet another indication for iron oxide imaging to visualize inflammatory activity within the myocardium. Inflammation within tissue hampers healing, and thus, this information could provide a target for an intervention with guided drug delivery. This application could be extended beyond the setting of myocardial infarction and could offer an indication of myocardial disease that could be factored into risk assessment following myocardial injury.
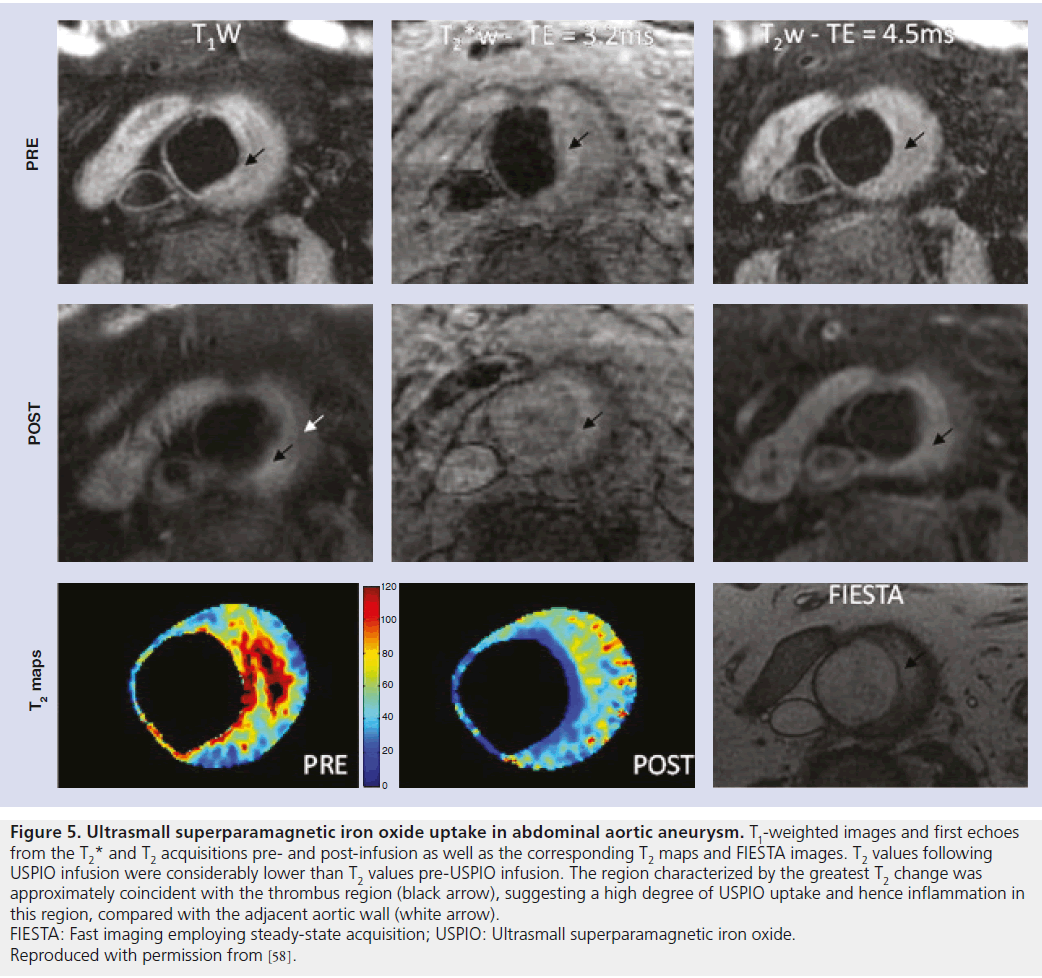
Figure 5. Ultrasmall superparamagnetic iron oxide uptake in abdominal aortic aneurysm. T1-weighted images and first echoes from the T2* and T2 acquisitions pre- and post-infusion as well as the corresponding T2 maps and FIESTA images. T2 values following USPIO infusion were considerably lower than T2 values pre-USPIO infusion. The region characterized by the greatest T2 change was approximately coincident with the thrombus region (black arrow), suggesting a high degree of USPIO uptake and hence inflammation in this region, compared with the adjacent aortic wall (white arrow). FIESTA: Fast imaging employing steady-state acquisition; USPIO: Ultrasmall superparamagnetic iron oxide. Reproduced with permission from [58].
Conclusion
Noninvasive vascular MRI offers promise for prognostic evaluation, treatment selection and assessment of therapeutic outcomes in atherosclerosis and aneurysms. The addition of iron oxide contrast media may aid in the detection of inflammation that can potentially augment risk stratification beyond plaque component identif ication and stenosis measurements. USPIO-based imaging methods show promise in highlighting the presence of inflammation within carotid atherosclerotic plaques. Although there is no statistically significant association between the quantitative USPIOinduced signal change and myocardial infarction, stroke, transient ischemic attack or death in a recent retrospective follow-up study, the finding of an association near statistical significance suggests that future studies with more patients and perhaps better USPIO media may aid in the assessment of risk in patients with as yet asymptomatic carotid disease. This imaging modality may offer hope for stratifying asymptomatic patients and determining the balance between therapeutic options for these particular patients whose risk of clinical events is poorly understood. However, challenges in terms of marketing approval and clinical availability are presently substantial barriers to widespread application.
Newer research utilizing iron oxide contrast media in imaging the abdominal aorta suggests the possible feasibility of such methods to assess inflammation in aneurysms prone to rupture [58], although determinations of risk association have not yet been performed. Should USPIO uptake prove to confer increased risk within AAAs, these methods could be used to promote surgical intervention in high-risk, inflamed aortas more susceptible to rupture despite not meeting traditional luminal measurements. Other applications of iron oxide imaging in vascular disease are possible, with preliminary work examining cerebral aneurysms, suggesting an increased risk of rupture with early iron oxide uptake and vascular malformations as well as myocardial infarction. These fields are all in the earliest stages of clinical application and require vetting to demonstrate their clinical utility.
Key limitations to widespread application of MRI-based vascular imaging
Although this review has focused on the benefits of vascular imaging using MRI and USPIO media in particular, these methods are not without substantial drawbacks. The foremost limitation is the lack of regulatory approval for USPIO preparations as MR contrast media, which precludes their use outside of ‘off-label’ investigational use. There is presently no evidence to suggest that any new USPIO media are likely to be approved specifically for MR contrast purposes in the near future. Obvious limitations of MRI-based methods include higher cost, long acquisition times, susceptibility to an artifact from a variety of sources including motion and metal, and contraindication in certain groups of patients with medical devices and hardware. Iron oxide imaging methods present even further technical challenges that restrict their use to academic medical centers with dedicated research support. USPIO imaging for measurement of contrast uptake in carotid plaque traditionally requires two separate imaging visits spaced 2−3 days apart. Newer methods involving quantitative T2* eliminate the need for a pre-USPIO acquisition; however, a prescan USPIO infusion visit is still required to allow for adequate circulation time [17]. The greatest consideration out of these caveats is the practical consideration of cost to society. The economic burden of atherosclerosis is substantial and considerable direct financial consequences result following the diagnosis of atherosclerosis [69]. The addition of advanced testing to clinical practice will undoubtedly be challenged by payers and must be justified in the era of a cost-conscious practice of medicine with greater access to and demands on healthcare. Therefore, to continue to use advanced imaging such as USPIO-based MRI, researchers must design prospective studies to directly address the question of how the information garnered from these imaging methods can affect clinical outcomes and potentially reduce cost. It may be possible that a lack of USPIO uptake in a small aneurysm or plaque may confer a much lower risk of rupture and this information could influence clinical decision-making to avert expensive, risky surgical intervention. At present, the effect of this information on clinical outcomes remains unclear, as these USPIO-enhanced imaging applications are in the early stages of clinical application.
Future perspective
As more research interest is directed toward cardiovascular and cerebrovascular risk assessment, more prospective studies will guide future advances in vascular imaging, directing clinical practice toward those novel imaging methods demonstrating the greatest promise and practicality. Synergy between imaging research and pharmaceutical development will facilitate the development of theranostics – imaging agents simultaneously capable of delivering directed therapies. With advances in contrast media applications, it may be possible to offer individuals truly personalized medical care that focuses on halting the progression of vascular disease in its earliest stages. There is already excellent preclinical work to suggest a role for combined diagnostic and therapeutic imaging strategies.
Just as important as the development of new contrast agents is the careful, rational application of technology to clinical practice in a way that minimizes risks and maximizes patient outcomes. While some efforts are presently directed at radioiodinated contrast media such as N1177 (a CT contrast medium that has affinity for macrophages), it is unlikely that such approaches, which confer radiation exposure, will be deemed clinically acceptable in the near future. Resultantly, it is imaginable that MRI-based modalities will supplant nuclear medicine methods including PET/CT on the basis of iatrogenic cancer risk reduction. MRI will undoubtedly stand out as an imaging modality of choice based on merits of its noninvasive nature, high sensitivity on an anatomic basis and molecular target level, and amenability to customizable, targeted contrast media. Ultrasound approaches employing microbubble contrast for the detection of highrisk neovascularization within atherosclerotic plaque (reviewed elsewhere [36]) may similarly find a prominent role in imaging vascular conditions, especially in light of its affordability and noninvasive nature. MRI, however, still retains key advantages in molecular imaging, reliability and plaque characterization.
Iron oxides are ideal candidates for imaging and theranostic platforms because of their biocompatibility, nanoscale size and suitability for molecular targeting. This review demonstrates that iron oxides already have inspired clinical applications in vascular imaging of inflammation for stroke, myocardial infarction and aneurysm rupture. Further improvements in design of iron contrast media can bolster the uptake of USPIO specifically by macrophages, ameliorating signal-to-noise ratio in inflammation imaging [70]; several groups have already begun preclinical work targeting USPIOs and MPIOs to specific molecular targets of inflammation and thrombosis including selectins, cell adhesion molecules and activated platelets (reviewed elsewhere [36]). The future will hopefully see the collaboration of multiple disciplines to fulfill the promise of theranostics and individualized medicine for patients with preclinical vascular disease. However, before such advances can occur, regulatory guidance must be established for a medium with combined therapeutic and diagnostic purposes [14]; moreover, individualized approaches to treatment must be steeled against cost-containment healthcare policies with proof of their effectiveness. Perhaps, if these practical considerations are addressed adequately, iron oxide-based contrast media may secure a niche within clinical imaging of vascular disease. Functionalization of USPIOs with anti-inflammatory medications will be one addition to a growing armamentarium of theranostic agents to provide targeted treatment of infarcted myocardium, inflamed atheromatous plaque or aneurysms. With these improvements, surgical intervention for vascular disease may be selectively employed and unnecessary operative risk mitigated in individuals with low vascular risk.
In addition, other uses outside of vascular imaging are being examined, including improvement of multiple sclerosis plaque detection [71] and inf lammation following stroke [72]. Expansion of iron oxide media to applications aside from vascular imaging alone will enable for greater economic viability for manufacturers and encourage further product development. Innovative work is already progressing in transforming USPIOs into molecular imaging probes with the capability of iron oxides to be incorporated into liposomes [73]. Newer research in the preclinical stage with a citrate-coated very small iron oxide particle in hyperlipidemic rabbits may facilitate shorter infusion to imaging intervals, but much further investigation is required prior to clinical evaluation [74]. Molecular imaging probe strategies tailor contrast media for use in tumor imaging, atherosclerosis imaging and drug delivery. Patients can expect to benefit directly from advances in the development of iron oxide-based contrast imaging, and the hope is that these contrast media will progress from being rusty to refined.
These findings encourage a novel use for USPIO contrast media and could affect clinical decision-making in the future with further prospective risk assessment-based studies. New insights into iron oxide contrast media, in particular molecular-targeted agents and theranostic approaches, could improve risk stratification and help address the growing burden of vascular disease.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert t-estimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Acknowledgements
AJD would like to thank Jonathan H. Gillard of the University of Cambridge for his mentorship.
References
Papers of special note have been highlighted as:
*of interest
** of considerable interest
- Inzitari D, Eliasziw M, Gates P et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 342(23), 1693–1700 (2000).
- Glaudemans AW, Slart RH, Bozzao A et al. Molecular imaging in atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 37(12), 2381–2397 (2010).
- Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation 90(2), 775–778 (1994).
- Erbel C, Dengler TJ, Wangler S et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 106(1), 125–134 (2011).
- Schaub N, Reichlin T, Meune C et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin. Chem. 58(1), 246–256 (2012).
- Emerging Risk Factors Collaboration, Kaptoge S, Angelantonio E, Di Lowe G et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375(9709), 132–140 (2010).
- Camici PG, Rimoldi OE, Gaemperli O, Libby P. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur. Heart J. 33(11), 1309–1317 (2012).
- Figueroa AL, Subramanian SS, Cury RC et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, zlaque morphology, and histopathology. Circ. Cardiovasc. Imaging 5(1), 69–77 (2012).
- Marnane M, Merwick A, Sheehan OC et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann. Neurol. 71(5), 709–718 (2012).
- Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 241(2), 459–468 (2006).
- Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn. Reson. Med. 59(3), 507–514 (2008).
- Chen H, Cai J, Zhao X et al. Localized measurement of atherosclerotic plaque inflammatory burden with dynamic contrast-enhanced MRI. Magn. Reson. Med. 64(2), 567–573 (2010).
- Young VEL, Degnan AJ, Gillard JH. Advances in contrast media for vascular imaging of atherosclerosis. Imaging Med. 3(3), 353–366 (2011). & A broad overview of advances in atherosclerosis imaging approaches including molecular-targeted approaches.
- Corot C, Warlin D. Superparamagnetic iron oxide nanoparticles for MRI: contrast media pharmaceutical company R&D perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. doi:10.1002/wnan.1225 (2013) (Epub ahead of print). & Considerations for developing iron oxide media that are viable from a production and marketing standpoint are explored. Also provides a summation of the challenges facing the development of contrast media and the future of theranostics.
- Bjornerud A, Johansson LO, Briley-Saebo K, Ahlstrom HK. Assessment of T1 and T2* effects in vivo and ex vivo using iron oxide nanoparticles in steady state – dependence on blood volume and water exchange. Magn. Reson. Med. 47(3), 461–471 (2002).
- Tang TY, Muller KH, Graves MJ et al. Iron oxide particles for atheroma imaging. Arterioscler. Thromb. Vasc. Biol. 29(7), 1001–1008 (2009).
- Patterson AJ, Tang TY, Graves MJ, Muller KH, Gillard JH. In vivo carotid plaque MRI using quantitative T2* measurements with ultrasmall superparamagnetic iron oxide particles: a dose–response study to statin therapy. NMR Biomed. 24(1), 89–95 (2011). & The possibility of using only positron oxide imaging to obtain quantitative T2* values to indicate uptake is demonstrated. Such a method could allow for easier acceptance of iron oxide imaging by eliminating the baseline imaging session, thereby reducing cost.
- Mani V, Briley-Saebo KC, Hyafil F, Fayad ZA. Feasibility of in vivo identification of endogenous ferritin with positive contrast MRI in rabbit carotid crush injury using GRASP. Magn. Reson. Med. 56(5), 1096–1106 (2006).
- Mani V, Briley-Saebo KC, Itskovich VV, Samber DD, Fayad ZA. Gradient echo acquisition for superparamagnetic particles with positive contrast (GRASP): sequence characterization in membrane and glass superparamagnetic iron oxide phantoms at 1.5T and 3T. Magn. Reson. Med. 55(1), 126–135 (2006).
- Makowski MR, Varma G, Wiethoff AJ et aplaque progression in ApoE-/- mice using susceptibility gradient mapping. Circ. Cardiovasc. Imaging 4(3), 295–303 (2011).
- Schmitz SA, Taupitz M, Wagner S, Wolf KJ, Beyersdorff D, Hamm B. Magnetic resonance imaging of atherosclerotic plaques using superparamagnetic iron oxide particles. J. Magn. Reson. Imaging 14(4), 355–361 (2001).
- U-King-Im JM, Tang T, Moustafa RR, Baron JC, Warburton EA, Gillard JH. Imaging the cellular biology of the carotid plaque. Int. J. Stroke 2(2), 85–96 (2007).
- Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano. Rev. 1 (2010).
- Muller K, Skepper JN, Posfai M et al. Effect of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitro. Biomaterials 28(9), 1629–1642 (2007).
- Muldoon LL, Sandor M, Pinkston KE, Neuwelt EA. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery 57(4), 785–796; discussion 785–796 (2005).
- Bernd H, Kerviler E, De Gaillard S, Bonnemain B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest. Radiol. 44(6), 336–342 (2009).
- Neuwelt EA Hamilton BE Varallyay CG et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 75(5), 465–474 (2009).
- Bourrinet P, Bengele HH, Bonnemain B et al. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest. Radiol. 41(3), 313–324 (2006).
- Winer JL, Liu CY, Apuzzo ML. The use of nanoparticles as contrast media in neuroimaging: a statement on toxicity. World Neurosurg. 78(6), 709–711 (2012).
- Johnsen SH, Mathiesen EB, Joakimsen O et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke 38(11), 2873–2880 (2007).
- Koga M, Kimura K, Minematsu K, Yamaguchi T. Diagnosis of internal carotid artery stenosis greater than 70% with power Doppler duplex sonography. AJNR Am. J. Neuroradiol. 22(2), 413–417 (2001).
- Gillard JH. Advances in atheroma imaging in the carotid. Cerebrovasc. Dis. 24(Suppl. 1), 40–48 (2007).
- Raman G, Moorthy D, Hadar N et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Ann. Intern. Med. 158(9), 676–685 (2013).
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362(6423), 801–809 (1993).
- van der Wal AC, Koch KT, Piek JJ, de Boer OJ, Becker AE. Inflammation in atherosclerotic plaques: a clinically crucial event. Fibrinolysis Proteolysis 11(Suppl. 1), 125–128 (1997).
- Degnan AJ, Young VE, Gillard JH. Advances in noninvasive imaging for evaluating clinical risk and guiding therapy in carotid atherosclerosis. Expert Rev. Cardiovasc. Ther. 10(1), 37–53 (2012).
- Degnan AJ, Young VE, Tang TY et al. Ex vivo study of carotid endarterectomy specimens: quantitative relaxation times within atherosclerotic plaque tissues. Magn. Reson. Imaging 30(7), 1017–1021(2012).
- Gao P, Chen ZQ, Bao YH, Jiao LQ, Ling F. Correlation between carotid intraplaque hemorrhage and clinical symptoms: systematic review of observational studies. Stroke 38(8), 2382–2390 (2007).
- Hosseini AA, Kandiyil N, Macsweeney ST, Altaf N, Auer DP. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann. Neurol. 73(6), 774–784 (2013).
- Kwee RM, Truijman MT, van Oostenbrugge RJ et al. Longitudinal MRI study on the natural history of carotid artery plaques in symptomatic patients. PloS One 7(7), e42472 (2012).
- Mauriello A, Sangiorgi GM, Virmani R et al. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis 208(2), 572–580 (2010).
- Turc G, Oppenheim C, Naggara O et al. Relationships between recent intraplaque hemorrhage and stroke risk factors in patients with carotid stenosis: the HIRISC study. Arterioscler. Thromb. Vasc. Biol. 32(2), 492–499 (2012).
- Hyafil F, Laissy JP, Mazighi M et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 26(1), 176–181 (2006).
- Howarth SP, Tang TY, Graves MJ et al. Non-invasive MR imaging of inflammation in a patient with both asymptomatic carotid atheroma and an abdominal aortic aneurysm: a case report. Ann. Surg. Innov. Res. 1, 4 (2007).
- Tang T, Howarth SP, Miller SR et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 37(9), 2266–2270 (2006).
- Howarth SP, Tang TY, Trivedi R et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur. J. Radiol. 70(3), 555–560 (2009).
- Tang TY, Howarth SP, Miller SR et al. Correlation of carotid atheromatous plaque inflammation using USPIO-enhanced MR imaging with degree of luminal stenosis. Stroke 39(7), 2144–2147 (2008).
- Tang TY, Howarth SP, Miller SR et al. The ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 53(22), 2039–2050 (2009).
- Tang TY, Howarth SP, Miller SR et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques in patients with asymptomatic carotid stenosis undergoing coronary artery bypass grafting: an ultrasmall superparamagnetic iron oxide enhanced magnetic resonance study. Eur. J. Vasc. Endovasc. Surg. 35(4), 392–398 (2008).
- Degnan AJ, Patterson AJ, Tang TY, Howarth SP, Gillard JH. Evaluation of ultrasmall superparamagnetic iron oxide-enhanced MRI of carotid atherosclerosis to assess risk of cerebrovascular and cardiovascular events: follow-up of the ATHEROMA trial. Cerebrovasc. Dis. 34(2), 169–173 (2012). & Follow-up data for the ATHEROMA trial and other patients with carotid atherosclerosis imaged using ferumoxtran-10 were examined. There was an association between ultrasmall superparamagnetic iron oxide (USPIO)-induced signal change and risk of cardiovascular and cerebrovascular events at 1 year that approached but did not reach significance (p = 0.07).
- Chaikof EL, Brewster DC, Dalman RL et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J. Vasc. Surg. 50(Suppl. 4), S2–S49 (2009).
- Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst. Rev. 3, CD001835 (2012).
- Powell JT, Sweeting MJ, Brown LC, Gotensparre SM, Fowkes FG, Thompson SG. Systematic review and meta-analysis of growth rates of small abdominal aortic aneurysms. Br. J. Surg. 98(5), 609–618 (2011).
- Powell JT, Brown LC, Greenhalgh RM, Thompson SG. The rupture rate of large abdominal aortic aneurysms: is this modified by anatomical suitability for endovascular repair? Ann. Surg. 247(1), 173–179 (2008).
- Wilson WR, Anderton M, Schwalbe EC et al. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation 113(3), 438–445 (2006).
- Turner GH, Olzinski AR, Bernard RE et al. In vivo serial assessment of aortic aneurysmformation in apolipoprotein E-deficient mice via MRI. Circ. Cardiovasc. Imaging 1(3), 220–226(2008).
- Truijers M, Futterer JJ, Takahashi S, Heesakkers RA, Blankensteijn JD, Barentsz JO. In vivo imaging of the aneurysm wall with MRI and a macrophage-specific contrast agent. AJR Am. J. Roentgenol. 193(5), W437–W441 (2009).
- Sadat U, Taviani V, Patterson AJ et al. Ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging of abdominal aortic aneurysms – a feasibility study. Eur. J. Vasc. Endovasc. Surg. 41(2), 167–174 (2011).
- Richards JM, Semple SI, MacGillivray TJ et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ. Cardiovasc. Imaging 4(3), 274–281 (2011). &` These researchers looked at abdominal aortic aneurysms with iron oxide-based MRI and reported a threefold higher aneurysm growth rate in those with distinct signal loss. This finding occurred even though comparators had similar aneurysm dimensions.
- Thompson AR, Cooper JA, Ashton HA, Hafez H. Growth rates of small abdominal aortic aneurysms correlate with clinical events. Br. J. Surg. 97(1), 37–44 (2010).
- Degnan AJ, Gallagher G, Teng Z, Lu J, Liu Q, Gillard JH. MR angiography and imaging for the evaluation of middle cerebral artery atherosclerotic disease. AJNR Am. J. Neuroradiol. 33(8), 1427–1435 (2012).
- Hasan DM, Mahaney KB, Magnotta VA. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler. Thromb. Vasc. Biol. 32(4), 1032–1038(2012).
- Hasan D, Chalouhi N, Jabbour P et al. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke 43(12), 3258–3265 (2012). nn Ferumoxytol imaging of intracranial vessels was used. The first to demonstrate evidence to suggest that early USPIO uptake (at 24 h of postinfusion imaging) is potentially associated with greater risk of cerebral aneurysm rupture. Though the study was small (n = 30 intracerebral aneurysms), it encourages further investigation into cerebral aneurysms with USPIO.
- Hasan DM, Chalouhi N, Jabbour P et al. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J. Am. Heart Assoc. 2(1), e000019 (2013).
- Dosa E, Tuladhar S, Muldoon LL, Hamilton BE, Rooney WD, Neuwelt EA. MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke 42(6), 1581–1588 (2011).
- Hasan DM, Amans M, Tihan T et al. Ferumoxytol-enhanced MRI to image inflammation within human brain arteriovenous malformations: a pilot investigation. Transl. Stroke Res. 3(Suppl. 1), 166–173 (2012).
- Alam SR, Shah AS, Richards J et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ. Cardiovasc. Imaging 5(5), 559–565 (2012).
- Yilmaz A, Dengler MA, van der Kuip H et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 34(6), 462–475 (2013).
- Ohsfeldt RL, Gandhi SK, Fox KM, Bullano MF, Davidson M. Medical and cost burden of atherosclerosis among patients treated in routine clinical practice. J. Med. Econ. 13(3), 500–507 (2010).
- Saito S, Tsugeno M, Koto D et al. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int. J. Nanomedicine 7, 5415–5421 (2012).
- Tourdias T, Roggerone S, Filippi M et al. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology 264(1), 225–233 (2012).
- Deddens LH, Van Tilborg GA, Mulder WJ, Vries HE, De Dijkhuizen RM. Imaging neuroinflammation after stroke: current status of cellular and molecular MRI strategies. Cerebrovasc. Dis. 33(4), 392–402 (2012).
- Frascione D, Diwoky C, Almer G et al. Ultrasmall superparamagnetic iron oxide (USPIO)-based liposomes as magnetic resonance imaging probes. Int. J. Nanomedicine 7, 2349–2359 (2012).
- Wagner S, Schnorr J, Ludwig A. Contrastenhanced MR imaging of atherosclerosis using citrate-coated superparamagnetic iron oxide nanoparticles: calcifying microvesicles as imaging target for plaque characterization. Int. J. Nanomedicine 8, 767–779 (2013).