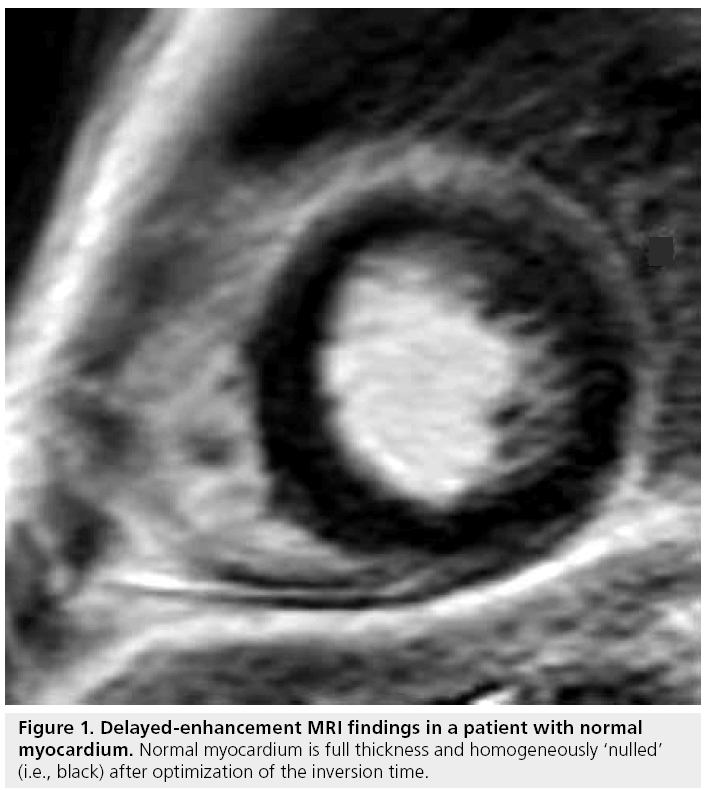
Review Article - Imaging in Medicine (2010) Volume 2, Issue 3
Delayed-enhancement cardiac MRI in the evaluation of cardiomyopathies
Thananya Boonyasiranant1 & Scott D Flamm†1Division of Cardiology, Department of Internal Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand
- Corresponding Author:
- Scott D Flamm
Cardiovascular Imaging Laboratory
Imaging Institute & Cardiovascular Medicine
Heart & Vascular Institute, Cleveland Clinic, Cleveland, OH, USA
Tel: +1 216 444 2740
Fax: +1 216 636 0822
E-mail: flamms@ccf.org
Abstract
Cardiomyopathies are a diverse group of disorders with etiologies ranging from ischemic to infiltrative. Clinical differentiation may be complex, but diagnosis is essential as complications include congestive heart failure and sudden death. Recent developments in cardiac MRI, particularly the implementation of delayed-enhancement imaging has introduced a novel approach to noninvasive tissue characterization that can more accurately differentiate the spectrum of cardiomyopathies. This article summarizes the comprehensive diagnostic abilities of cardiac MRI in defining cardiac anatomy and physiology and, more importantly, identifying pathologic tissues and differentiating those with substrates amenable to therapy. The value of delayed-enhancement imaging in prognostics will also be presented.
Keywords
cardiomyopathy ▪ infiltrative disorders ▪ ischemic heart disease ▪ MRI ▪ noninvasive imaging
Cardiomyopathy is an important entity in the cardiovascular disease spectrum, with etiologies including coronary artery disease, valvular heart disease, intrinsic and infiltrative myocardial diseases and hypertension. Cardiomyopathy, particularly when leading to congestive heart failure, contributes to considerable mortality, morbidity and substantial economic burden throughout the world. An early diagnosis of a specific cardiomyopathy’s underlying etiology may be important, as some etiologies necessitate specific treatment and may be readily treatable.
Noninvasive imaging is playing an evolving role in establishing specific diagnoses, identifying etiologies, providing prognostic information and even assessing potential therapeutic effect. Cardiovascular magnetic resonance (CMR) is a particularly promising modality for the imaging of cardiomyopathy, given the spectrum of information obtained in a single examination. CMR has the ability to provide accurate and highly reproducible quantification of biventricular systolic function and mass without geometric assumption, assessment of regional wall motion or myocardial perfusion. This article, however, will focus on CMR’s ability to characterize tissue, including myocardial viability and the presence of interstitial fibrosis via the delayed-enhancement (DE) technique.
DE technique
The classically described segmented k-space, inversion-recovery gradient-echo sequence following administration of gadolinium-based contrast media (the DE MRI technique [DE-MRI]) is predicated on the concept that infarcted myocardium accumulates a greater amount of gadolinium than surrounding noninfarcted tissue and becomes ‘hyperenhanced’ or bright in appearance. Two mechanisms are believed to explain the increased accumulation of gadolinium in regions of infarction: a differential wash-in and wash-out rate of gadolinium and a different volume of distribution between viable and nonviable tissue. Normal myocardium is believed to have a relatively rapid wash-in and wash-out rate, whereas a slower, more delayed, wash-in and wash-out rate is observed in the infarcted, nonviable tissue as a result of impaired coronary and microvascular blood supply. Altered volumes of distribution also play a role. Gadolinium chelates, used clinically (with the exception of the recently approved gadofosveset trisodium [1]), are extracellular, interstitial agents that rapidly distribute from the intravascular space and equilibrate with the interstitial space; these agents are typically excluded from the intracellular space. In normal myocytes, gadolinium is precluded from the intracellular space by intact sarcolemmal membranes and, thus, only approximately 15–20% of a given volume of myocardium is available for gadolinium accumulation. In acute myocardial injury, the sarcolemmal membrane integrity is disrupted and the intracellular space effectively becomes additional interstitial space. The interstitial space increases to approximately 75% of a given volume, thereby permitting increased accumulation of gadolinium. In the setting of chronic myocardial infarction, the intracellular space is relatively small as a result of the fibrosis and collagen replacement, and the available interstitial space similarly expands the volume of distribution [2].
An increased amount of gadolinium chelate per unit volume shortens T1, hence, increases signal intensity on a T1-weighted image. The image acquisition is typically performed 10–20 min after intravenous administration of gadolinium chelate (usually 0.10–0.20 mmol/kg) to allow physiologic wash-out of contrast in normal myocardium. In order to maximize the contrast between normal myocardium and infarcted myocardium, the inversion time is optimized to null the signal from normal myocardium (Figure 1) [3]. The standard segmented k-space, inversion-recovery gradient-echo sequence technique requires optimized timing of scan acquisition relative to the volume of contrast administered, along with an appropriate selection of inversion time to null normal remote myocardium [4]. The same is true for variations on the technique, such as single-shot techniques that acquire each image in a single heartbeat (albeit at lower spatial and temporal resolution) [5] and inversion-recovery steady-state free-precession approaches. A recently introduced modification of the technique, called phase-sensitive inversion recovery, corrects for variations in the inversion-recovery time and allows greater flexibility in imaging [6,7]. While there are differences between and among the various DE-MRI techniques, when applied correctly they provide similar information regarding the extent and degree of fibrosis present. There are also multiple advantages of the DE-MRI technique: in particular, it is straightforward to implement and does not require physiologic or pharmacologic stress.
Accuracy of DE-MRI
Comparison to histopathology
The close correlation between DE-MRI and histopathology-determined infarct size has been demonstrated in multiple studies. Results consistently demonstrate the near-identical quantification of infarct size in both acute and chronic myocardial infarct settings [8–10].
Comparison to other noninvasive modalities
Precise differentiation between viable and nonviable myocardium has a direct impact on clinical decision-making in the setting of cardiac dysfunction. Several noninvasive modalities have been proposed for the evaluation of myocardial viability, including DE-MRI, SPECT, PET, dobutamine stress echocardiography and CT.
SPECT defines infarcted myocardium based on regional discrepancies in radiotracer uptake as a result of impaired mitochondrial function (sestamibi SPECT) or impaired cellular membrane integrity (thallium SPECT). DE-MRI was superior to SPECT in multiple head-tohead studies [11,12]. These studies have demonstrated that DE-MRI and SPECT detect transmural infarct (>50% transmurality) at similar rates, while DE-MRI detects subendocardial infarcts (<50% transmurality) missed by SPECT. The explanation for this discrepancy appears to reflect the superior spatial resolution of DE-MRI compared with SPECT.
In PET, infarcted myocardium is identified by impaired metabolic function, manifested as matched defects of reduced myocardial blood flow (13N-ammonia) and metabolism (18F-fluodeoxyglucose). Previous studies have demonstrated that DE-MRI detects subendocardial infarcts not seen by PET, similar to the findings compared with SPECT; specifically, PET missed 36–55% of subendocardial infarcts identified by DE-MRI [13,14].
Low-dose dobutamine stress echocardiography determines myocardial viability based on inotropic response of hypocontractile segments. One study compared DE-MRI with dobutamine stress echocardiography and revealed that few segments with transmural or nearly transmural infarct by DE-MRI had contractile reserve by dobutamine stress echocardiography, while half of the segments found to be fully viable by DE-MRI demonstrated absence of contractile reserve [15]. The possible mechanisms explaining these discrepancies are tethering of viable segments to infarcted segments, myocyte adaptation that impairs dobutamine response and absent coronary flow reserve in the chronically hypoperfused region of viable but dysfunctional myocardium.
CT assesses myocardial viability based on relative tissue contrast concentrations on delayed postcontrast imaging similar to DE-MRI. However, DE-MRI provides approximately a three- to five-fold higher difference in image intensity between viable and infarcted myocardium [16,17]. To date, there are only limited data in humans comparing DE-MRI versus postcontrast CT (using a 16-detector CT scanner). These data reveal close agreement in overall infarct size, but disagreement in degree of infarct transmurality [17]. A later study in a pig model used a 64-detector CT scanner for delayed postcontrast CT images and DE-MRI with histopathology as the gold standard. This study revealed close correlation with histopathology for the detection of ischemic irreversible myocardial damage for both delayed postcontrast CT and DE-MRI [16]. While CT has potential to determine myocardial viability with delayed postcontrast images, its clinical applicability remains limited as a result of the not insignificant radiation dose and need for potentially nephrotoxic contrast agent.
Pattern of DE
Ischemic cardiomyopathy
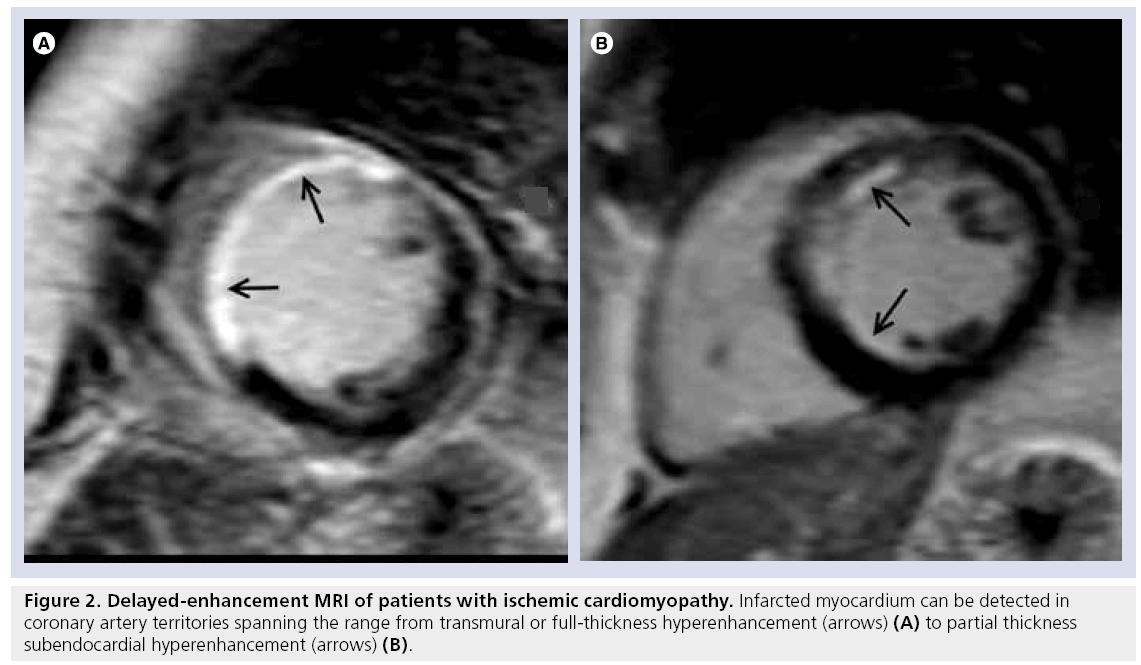
Ischemic cardiomyopathy is characterized by a scar that typically involves the subendocardial layer, and in more severe ischemic damage can have a more complete gradient of transmurality, becoming a full-thickness scar (Table 1). This pattern is secondary to the wavefront phenomenon of ischemic cell death [18] and characteristically occurs in the distribution corresponding to coronary artery territories (Figure 2).
Figure 2: Delayed-enhancement MRI of patients with ischemic cardiomyopathy. Infarcted myocardium can be detected in coronary artery territories spanning the range from transmural or full-thickness hyperenhancement (arrows) (A) to partial thickness subendocardial hyperenhancement (arrows) (B).
Delayed-enhancement MRI is a robust, noninvasive technique for the assessment of viable myocardium owing to its excellent spatial resolution. The traditional myocardial viability techniques of SPECT and PET have been demonstrated to be comparable to DE-MRI for the evaluation of a transmural scar, although, as noted previously, inferior to DE-MRI in the setting of a subendocardial scar [11,13]. The ability to differentiate between subendocardial and transmural scars has an important clinical impact as the degree of transmurality has a direct correlation with the extent of irreversible damage and an inverse relationship with recovery of contractile function after revascularization [19,20]. The predictive value of DE-MRI remains even after adjustment for traditional well-known prognosticators of allcause mortality or cardiac transplantation [21]. Furthermore, the extent of infarct is a better predictor of inducible ventricular tachycardia than the classically used left ventricular ejection fraction [22].
In addition to the benefits derived from localization and quantification of chronically damaged nonviable myocardium, DE-MRI can identify acutely irreversibly damaged myocardium and directly identify areas of no-reflow with microvascular obstruction. Microvascular obstruction is seen on DE-MRI as a central black area within a region of hyperenhancement. The core of the infarcted tissue remains dark as neither blood nor gadolinium chelate is able to penetrate the area of microvascular obstruction owing to extensive myocyte necrosis and localized edema compressing supplying feeding vessels [23]. Recognition of microvascular obstruction is becoming increasingly important as a potent predictor of adverse ventricular remodeling and cardiovascular outcomes [24,25].
Nonischemic cardiomyopathy
Although various classification schemes of cardiomyopathy have been proposed, including classifications based on genetic interrogation, this article will follow the categories advocated by the WHO owing to its broad-based acceptance and practical application to clinical practice.
Dilated cardiomyopathy
Discrimination between ischemic and nonischemic etiology of cardiomyopathy is critical as there are significant disparities in therapeutic options and prognosis. The gold standard for this differentiation remains invasive coronary angiography, with the demonstration of significant epicardial coronary stenosis indicating ischemic etiology. Nevertheless, the presence of normal coronary arteries may be insufficient to exclude ischemic etiology as some cases, although felt to be a small minority, may be caused by spontaneous recanalization of a previously occluded vessel, embolization with subsequent lysis or vasospasm. The evolving role of DE-MRI to distinguish between these separate categories is based on a high sensitivity of 81–100% to detect hyperenhancement in an ischemic pattern along a coronary artery territory in concert with impaired left ventricular function [26,27].
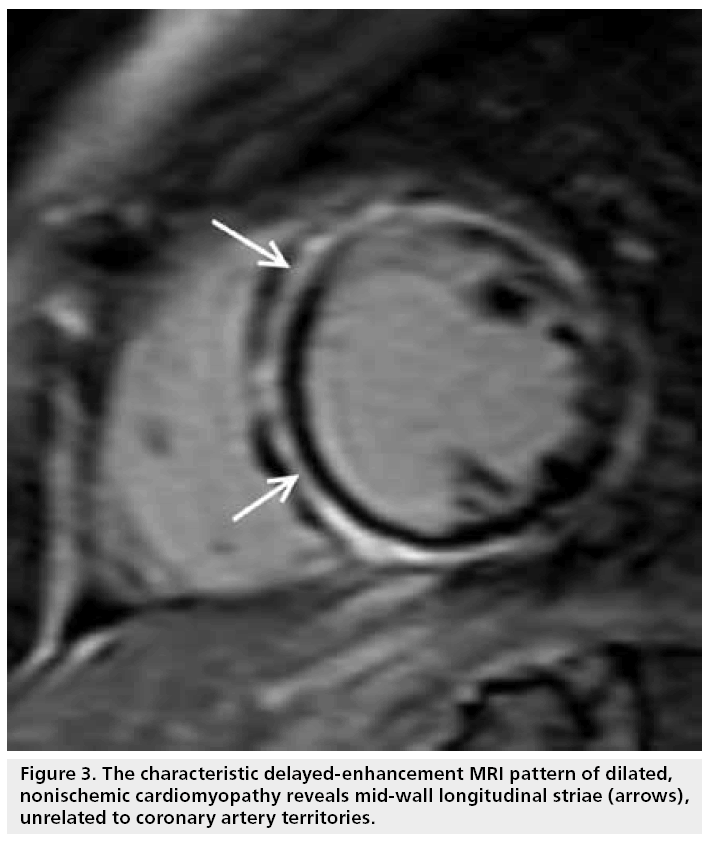
By contrast, hyperenhancement is only present in 10–26% of patients with nonischemic, dilated cardiomyopathy, and the majority have absence of hyperenhancement [26–28]. Furthermore, the pattern of hyperenhancement is distinctly different: mid-wall longitudinal striae are seen, typically in the basal septum, and are not related to coronary territories (Figure 3). This characteristic finding has been correlated to focal fibrosis from autopsy study [29].
Figure 3: The characteristic delayed-enhancement MRI pattern of dilated, nonischemic cardiomyopathy reveals mid-wall longitudinal striae (arrows), unrelated to coronary artery territories.
Similar to data from ischemic cardiomyopathy, the presence of hyperenhancement in dilated cardiomyopathy also serves as an independent predictor of all-cause mortality, hospitalization and inducible ventricular arrhythmia, even after adjustment for left ventricular ejection fraction [30–32]. The existence of hyperenhancement contributes to an eightfold higher composite end point of heart failure hospitalization, implantable defibrillator shocks and cardiac mortality. In at least one study, DE-MRI has been successfully used to identify the arrhythmogenic substrate and, thereby, guide electrophysical mapping and ablation [33].
Hypertrophic cardiomyopathy
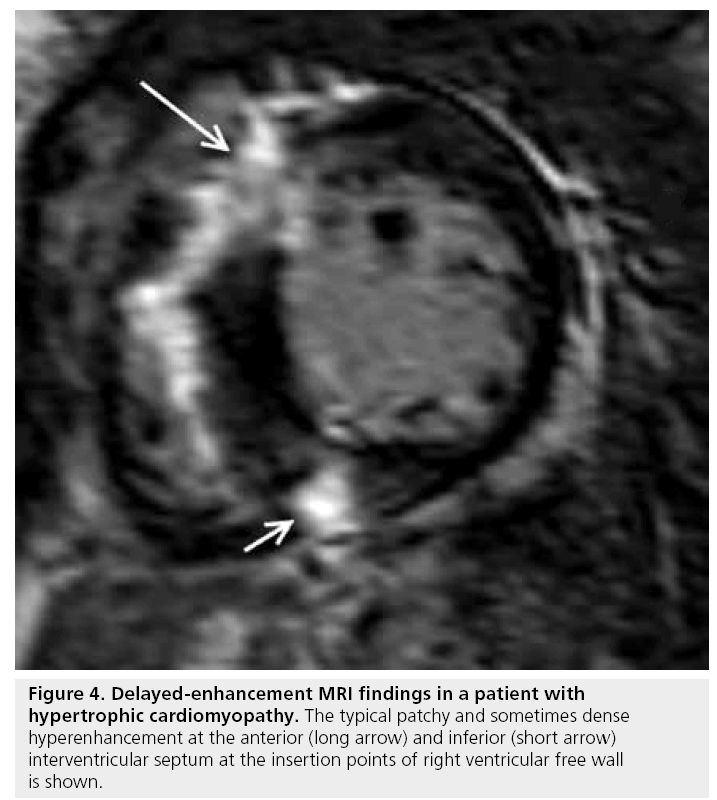
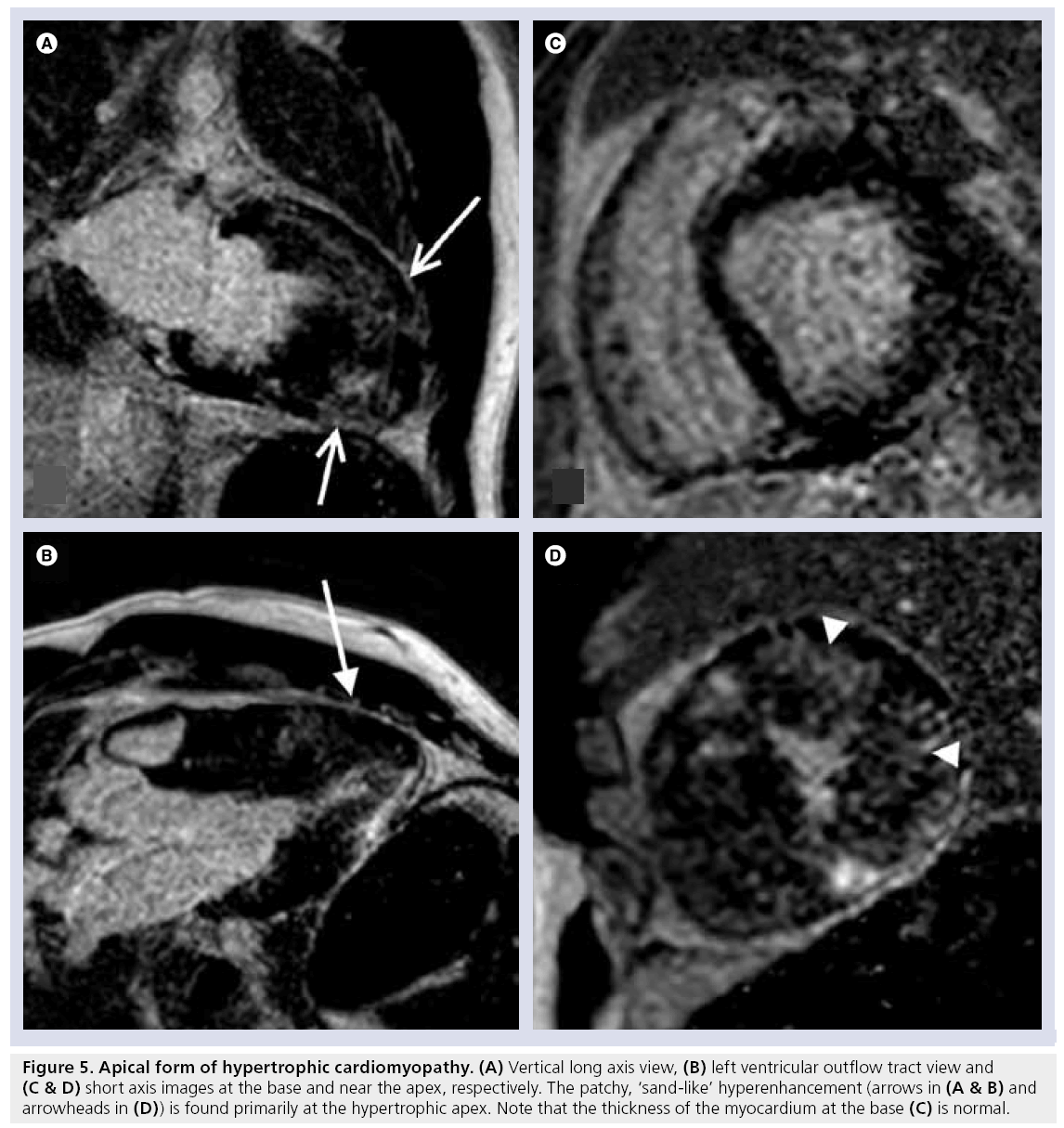
Cardiac MRI has a spectrum of tools available for the assessment of hypertrophic cardiomyopathy (HCM), including the pattern and magnitude of myocardial hypertrophy, which may be particularly valuable in regions that are difficult to visualize by transthoracic echocardiography (e.g., the apex and inferolateral walls), accurate determination of left ventricular mass and the identification of papillary muscle abnormalities. In addition, DE-MRI has proved particularly adept at identifying the presence of myocardial interstitial fibrosis. The characteristic pattern of hyperenhancement is patchy, mid-wall, and commonly involves the interventricular septum at the anterior and inferior insertion points of the right ventricular free wall and, in more severe forms, can extend into the more apical portions of the ventricular myocardium (Figures 4 & 5) [34,35]. The relationship between the presence of hyperenhancement and increased fibrosis and collagen components has been demonstrated histologically [36]. The existence and magnitude of hyperenhancement generally correlates with the degree of wall thickness, nonetheless, it may be found in areas with normal wall thickness [37]. The ability of DE-MRI to identify interstitial fibrosis even in regions of normal wall thickness is particularly advantageous as the degree of fibrosis is related to the risk of ventricular arrhythmias in HCM [38].
Figure 5: Apical form of hypertrophic cardiomyopathy. (A) Vertical long axis view, (B) left ventricular outflow tract view and (C & D) short axis images at the base and near the apex, respectively. The patchy, ‘sand-like’ hyperenhancement (arrows in (A & B) and arrowheads in (D)) is found primarily at the hypertrophic apex. Note that the thickness of the myocardium at the base (C) is normal.
Restrictive cardiomyopathy Amyloidosis
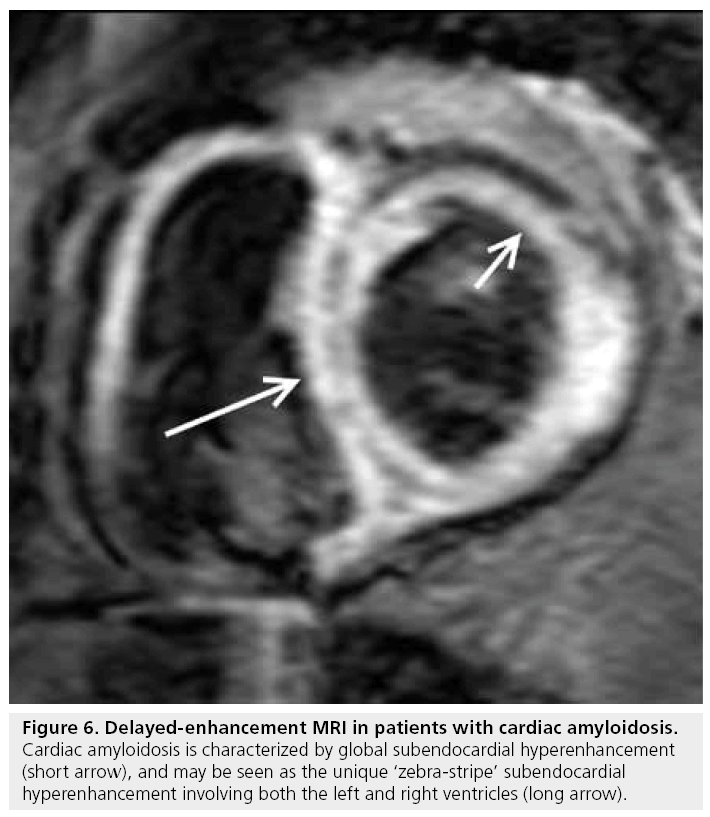
Cardiac involvement is common in patients with systemic amyloidosis. Furthermore, cardiac mortality is the most common cause of death with a median survival time of less than 6 months [39]. The hyperenhancement pattern demonstrated by DE-MRI is global diffuse or patchy, but may have a ‘zebra-stripe’ pattern of subendocardial hyperenhancement of the left and right ventricles, with sparing of the mid-wall of the interventricular septum (Figure 6) [39,40]. Using endomyocardial biopsy as a gold standard, typical DE-MRI hyperenhancement patterns have a strong correlation with cardiac involvement (80% sensitivity and 94% specificity of typical DE-MRI findings), and are distinctly different from those seen in other restrictive cardiomyopathies and HCM [41]. On the basis of histopathologic correlation, the hyperenhancement probably represents interstitial expansion from amyloid protein deposition.
Figure 6: Delayed-enhancement MRI in patients with cardiac amyloidosis. Cardiac amyloidosis is characterized by global subendocardial hyperenhancement (short arrow), and may be seen as the unique ‘zebra-stripe’ subendocardial hyperenhancement involving both the left and right ventricles (long arrow).
Another unique feature of DE-MRI in cardiac amyloidosis is an atypically dark appearance of the blood pool, apparently reflecting fast blood pool depletion from high myocardial uptake [39].
From a prognostic perspective, Ruberg et al. noted that the presence of hyperenhancement on DE-MRI correlates with the level of B-type natriuretic peptide in patients with light-chain amyolidosis, but did not report a relationship with survival [42]. By contrast, Austin et al. studied 47 patients with biopsy-proven amyloidosis and found that only DE-MRI was associated with worsening heart failure and worsened survival [43].
Sarcoidosis
Cardiac involvement in sarcoidosis remains challenging as a substantial proportion of patients have no clinical manifestations, thereby creating difficulties in early diagnosis. Nonetheless, it is important to attempt early and precise diagnosis as affected patients can develop progressive heart failure and sudden cardiac death from arrhythmias, which can be prevented by optimized therapy [44].
Endomyocardial biopsy is considered the gold standard in diagnosis, but naturally imposes a not insignificant risk and may be insensitive owing to the patchy nature of infiltration. Echocardiography may be ineffective in the absence of left ventricular wall-motion abnormalities, which may only present later in the disease state, whereas CMR offers the benefit of tissue characterization by DE-MRI revealing granulomatous inf iltrates in the myocardium. The noncaseating granulomas create an increase in extracellular space and, therefore, result in focal hyperenhancement. Interestingly, the hyperenhancement may be seen in patients without left ventricular dilation or focal wall-motion abnormality, suggesting that DE-MRI may be beneficial in the diagnosis in the early, asymptomatic stage of disease.
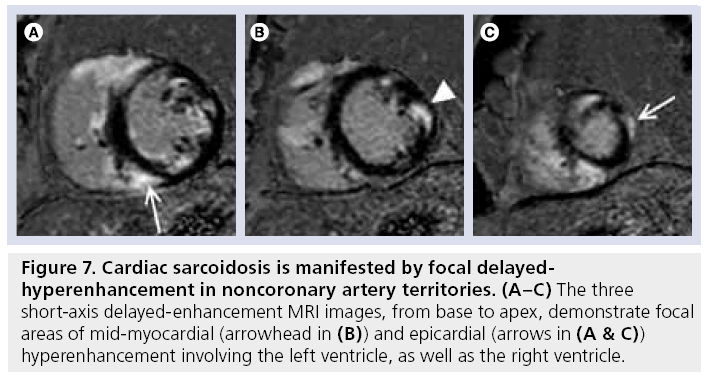
The characteristic DE-MRI pattern is usually focal areas of mid-wall or subepicardial hyperenhancement, frequently in the anteroseptal and inferolateral walls (Figure 7). Two recent studies have demonstrated that approximately a quarter of patients with biopsy-proven sarcoidosis, but no known cardiac involvement or symptoms have cardiac infiltrates on DE-MRI [45,46]. One of these studies used the Japanese Ministry of Health guideline as a gold standard with DE-MRI demonstrating 100% sensitivity and 78% specificity [45].
Figure 7: Cardiac sarcoidosis is manifested by focal delayedhyperenhancement in noncoronary artery territories. (A–C) The three short-axis delayed-enhancement MRI images, from base to apex, demonstrate focal areas of mid-myocardial (arrowhead in (B)) and epicardial (arrows in (A & C)) hyperenhancement involving the left ventricle, as well as the right ventricle.
To date, there is little prognostic data available, aside from a single study by Patel et al., where myocardial damage detected by DE-CMR appeared to be associated with future adverse events, including cardiac death; however, the number of events was small, thus limiting extrapolation [47]. The extent and intensity of hyperenhancement may be used to evaluate response to steroid treatment as shown in one recent follow-up study [48].
Anderson–Fabry disease
Anderson–Fabry disease is amenable to therapy using enzyme replacement, which can regress myocardial hypertrophy and improve cardiac function [49]. Thus, early diagnosis is particularly important in an effort to limit deleterious complications and improve outcomes.
The typical DE-MRI features involve subepicardial hyperenhancement with a predilection to the basal inferolateral region [50]. Histopathologic data demonstrate a close correlation between DE-MRI hyperenhancement and replacement fibrosis [51].
Anderson–Fabry disease is similar to other entities with increased myocardial thickness, such as HCM and cardiac amyloidoses, but can be differentiated by the unique patterns of hyperenhancement by DE-MRI.
Endomyocardial fibrosis
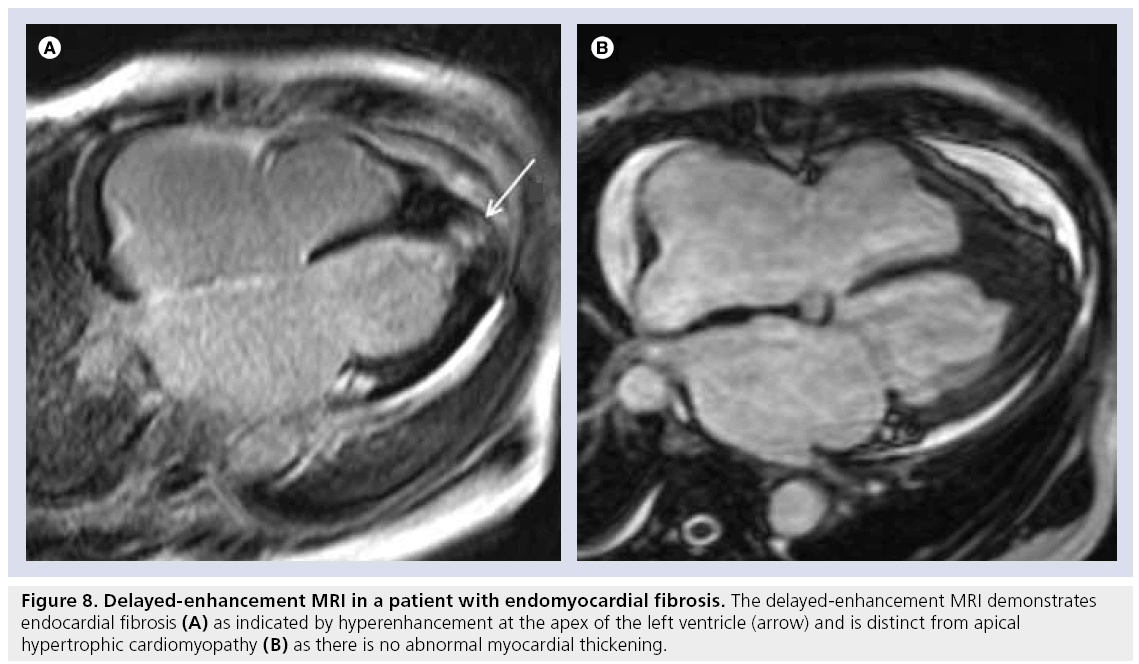
In endomyocardial fibrosis, left ventricular wall thickening occurs as a result of fibrous replacement and thrombus formation. In some situations, it may be difficult to differentiate endomyocardial fibrosis from forms of myocardial hypertrophy, such as the apical variety of HCM. However, DE-MRI can reveal a ‘rind’ of endocardial fibrosis (a thickened rim of hyperenhancement along the endocardial border) frequently accompanied by adjacent thrombus at the apex (Figure 8). DE-MRI is also adept at identifying intracavitary thrombus, which can then be treated with anticoagulation therapy.
Figure 8: Delayed-enhancement MRI in a patient with endomyocardial fibrosis. The delayed-enhancement MRI demonstrates endocardial fibrosis (A) as indicated by hyperenhancement at the apex of the left ventricle (arrow) and is distinct from apical hypertrophic cardiomyopathy (B) as there is no abnormal myocardial thickening.
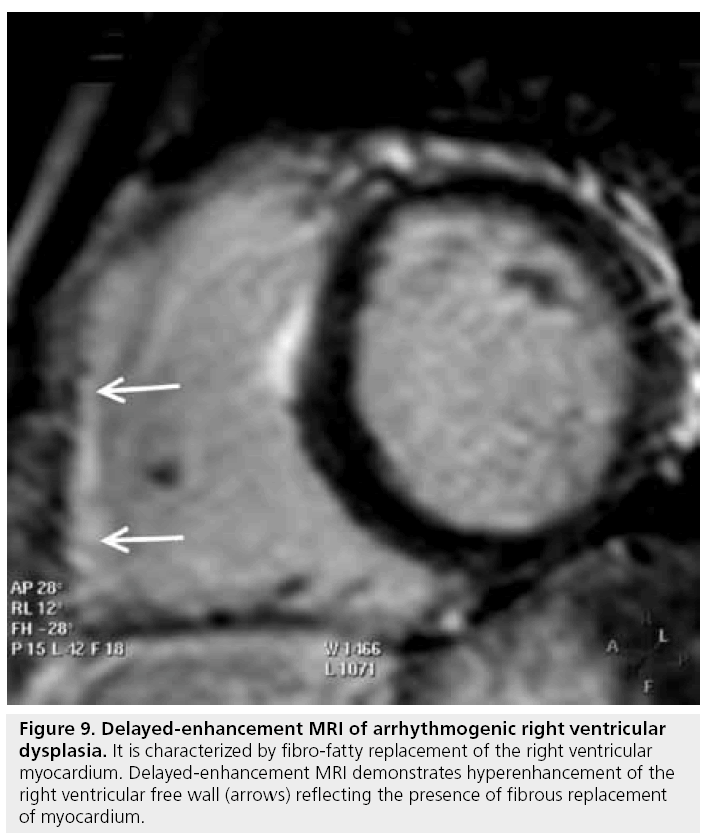
Arrhythmogenic right ventricular dysplasia
The diagnosis of arrhythmogenic right ventricular dysplasia (ARVD) is still challenging, despite a multidisciplinary workup approach based on the Task Force criteria [52]. The disease process is morphologically and functionally characterized by right ventricular dilation and dysfunction, focal aneurysms and fibro-fatty replacement of the myocardium, sometimes with concomitant involvement of the left ventricle.
While endomyocardial biopsy remains the gold standard and Task Force criteria requirement for identification of myocardial fibro-fatty replacement, CMR’s ability to characterize tissue, both fatty tissue using spin-echo image sequences and fibrous tissue with DE-MRI, presents an emerging and unique opportunity for noninvasive diagnosis of ARVD. The use of DE-MRI may be particularly advantageous as the identification of fibrous tissue is proposed to be more diagnostic than the presence of fat alone, and fibrous tissue replacement has been documented to be more arrhythmogenic than fatty tissue replacement [53]. In a recent study, fibrous replacement identified by DE-MRI was common in the right ventricle of patients with ARVD, while fatty infiltration was rare (Figure 9) [54]. Furthermore, the presence of hyperenhancement by DE-MRI and right ventricular dysfunction has been linked to inducible ventricular tachycardia in a recent study [55]. Unfortunately, there is no classic pattern of DE yet established or included in Task Force criteria.
Figure 9: Delayed-enhancement MRI of arrhythmogenic right ventricular dysplasia. It is characterized by fibro-fatty replacement of the right ventricular myocardium. Delayed-enhancement MRI demonstrates hyperenhancement of the right ventricular free wall (arrows) reflecting the presence of fibrous replacement of myocardium.
To date, data suggest that regional wallmotion abnormalities remain the principal imaging criterion for the diagnosis of ARVD [56]. It is important to reiterate that the accuracy and inter-observer variability of CMR in this difficult diagnosis are insufficient for CMR to be the sole investigation in the diagnostic workup, but CMR remains a critical final component in this evaluation [57].
Left ventricular noncompaction
Left ventricular noncompaction is an unusual congenital cardiomyopathy secondary to developmental arrest of compaction of the fetal loose, myocardial fiber meshwork and, in the isolated form more typically seen in adults, is characterized by severe left ventricular dysfunction, arrhythmias and thromboembolic events. Imaging diagnoses have been predicated on an exaggerated ratio of noncompact hyper-trabeculated to compact myocardium, yet the diagnostic criteria remain insufficiently well established [58]. Based on cine functional imaging, a ratio of the noncompacted to compacted layer of myocardium of greater than 2.3, or when the percentage of the mass of left ventricle trabeculations compared with global left ventricle mass is greater than 20%, has been used in two recent studies with reasonable accuracy [59,60]. DE-MRI is also useful in demonstrating the fibrosis that occurs subendocardially and in the deep trabecular recesses of the noncompacted layer [61]. In addition, an initial correlation has been established between the degree of DE and impairment of left ventricular function [59]. The condition is uncommon enough that no prognostic data relative to DE-MRI is available to date.
Takotsubo cardiomyopathy
This recently defined cardiomyopathy is characterized by transient regional wall-motion abnormalities without significant coronary artery obstruction, typically occurs in middle-aged to elderly females and is often associated with emotional or other stress. The presentation is similar to that of acute myocardial infarction with electrocardiographic and enzymatic abnormalities present. However, the wall-motion abnormalities usually involve the entire apical half of the left ventricle in what is considered a noncoronary artery distribution. CMR has revealed the regional dysfunction patterns and, perhaps more importantly, DE-MRI has demonstrated the lack of DE indicating that there is no detectable, irreversible myocardial damage within the limits of spatial resolution of the technique [62– 64]. Similar to left ventricular noncompaction there are no prognostic data relative to DE-MRI presently available.
Clinical implications
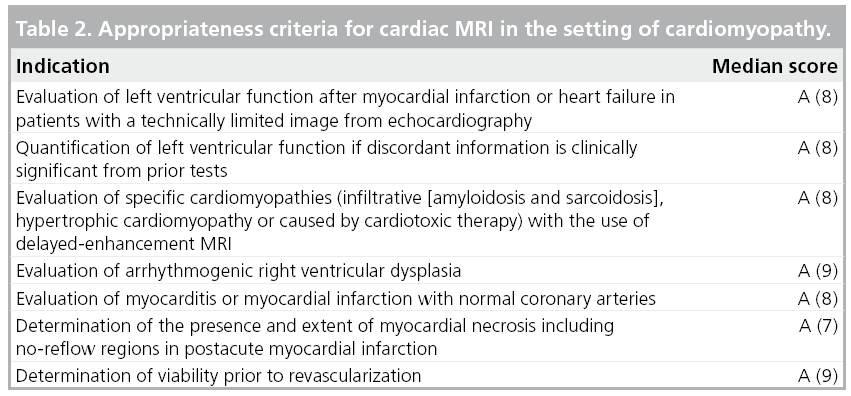
Cardiac MRI can be considered as a pivotal noninvasive modality for the evaluation of cardiomyopathy, encompassing diagnostic, therapeutic and prognostic perspectives. The roles of CMR have been established in the 2006 ACCF/ ACR/SCCT/SCMR/ASNC/NASCI/SCAI/ SIR Appropriateness criteria for cardiac CT and cardiac MRI (Table 2) [65].
Diagnostic perspective
The ability of DE-MRI to differentiate between ischemic and nonischemic etiologies of cardiomyopathy can further reduce the small, but not insignificant risk of invasive coronary angiography in a substantial portion of patients with systolic dysfunction but normal coronary arteries. Furthermore, DE-MRI can differentiate some specific etiologies of nonischemic cardiomyopathy, thereby, potentially avoiding the risk of endomyocardial biopsy.
Therapeutic perspective
In patients with ischemic cardiomyopathy, the discrimination between viable and nonviable myocardium is crucial for therapeutic decisions centered on the benefit or lack of revascularization. The diminished likelihood of functional recovery in the setting of transmurally or neartransmurally infarcted myocardium will help preclude those patients from the higher risk of surgery in left ventricular dysfunction without likelihood of significant benefit. In nonischemic cardiomyopathies, less data are available; however, the spatial extent and amount of delayed-hyperenhancement was used to evaluate response to steroid treatment in one recent follow-up study in patients with sarcoidosis [48]. More data are certain to follow on other etiologic entities.
Prognostic perspective
Cardiac MRI has demonstrated a potent prognostic role in the majority of cardiomyopathies investigated, where the presence and extent of hyperenhancement correlates closely with increased hospitalization rates, arrhythmias, higher mortality and worse overall outcomes. The predictive value of DE-MRI remains even after adjustment for traditional well-known prognosticators of all-cause mortality or hospitalization in both ischemic and dilated cardiomyopathy [66]. Furthermore, the extent of infarct is a better predictor of inducible ventricular arrhythmia in ischemic, dilated and HCM, although it has not yet been established as one of the criteria for internal cardiovertor-defibrillator placement [22,31,32,38,67].
Among the uncommon cardiomyopathies, such as amyloidosis or sarcoidosis, the prognostic significance has been investigated in a few small studies [47,68,69].
Future perspective
Volumetric scanning
Delayed-enhancement MRI has relied primarily on qualitative assessment of the degree of hyperenhancement within the myocardium for both ischemic or nonischemic cardiomyopathies. The majority of imaging is performed using 2D techniques that require manual or semiquantitative summing of the areas of DE on acquired slices throughout the ventricle. Despite solid diagnostic and prognostic data using these approaches, multiple investigators have documented the potential errors as a result of slice misregistration, gaps between slices and other mathematical assumptions.
Advances in CMR hardware and software, including improved imaging at 3 T field strength are leading to true, 3D volumetric imaging of the left ventricle. In addition, high-field-strength imaging (i.e., 3.0 T) has the benefit of an increased signal-to-noise ratio and contrast sensitivity, as compared with 1.5 T scanners [70]. Such data sets will eliminate the intrinsic, small but real errors of current approaches and provide more comprehensive information regarding the ventricle. As a further benefit, imaging may be simpler (by eliminating or reducing complex planning approaches) as well as faster, thereby facilitating throughput, increasing efficiency and enhancing the patient experience with cardiac MRI.
Quantification
There are currently varied approaches to determining the absolute amount of fibrosis – manifested by delayed-hyperenhancement – within the myocardium. Fortunately, owing to the intrinsic robustness of DE-MRI data, the values obtained by different approaches to semi-quantification or quantification are similar; however, there remains a lack of standardization. Regions of hyperenhancement are commonly defined by determining the standard deviation of the signal within normal remote myocardium and setting threshold values above the mean signal level of normal remote myocardium. Investigators have used thresholds ranging from two standard deviations up to six standard deviations above normal remote myocardium. For ischemic cardiomyopathy this approach has worked well with only minor differences in quantitative values despite a range of thresholds.
This is considerably more complicated and perhaps more important in the nonischemic and infiltrative cardiomyopathies where interstitial fibrosis and smaller areas of replacement fibrosis occur. The hyperenhancement is more diffuse and small areas of hyperenhancement are interspersed between islands of normal myocardial cells. As a result, instead of bright hyperenhancement voxels, the myocardium may be gray because of averaging between normal dark myocardium and bright fibrosis, all of which are below the limits of spatial resolution for individual differentiation. To correctly quantitate the interstitial fibrosis present a combination of even further improvements in spatial resolution (which are already beyond alternative noninvasive imaging techniques) are required, as well as novel approaches using phase-sensitive reconstruction techniques, gradations of inversion times and other, yet to be developed, tools.
Novel contrast agents
The majority of gadolinium-chelate contrast agents currently used for DE-MRI are considered interstitial, nonintravascular agents. These agents have been responsible for the important development of tissue characterization capable by CMR, and will likely remain the primary workhorse in CMR DE imaging of cardiomyopathies. Nonetheless, newer contrast agents are imminent, including those that are intravascular [71], or borne via nanoparticles [72] or specific ligands [73]. Theoretically, an intravascular gadoliniumchelate contrast agent could have similar abilities for enhancing areas of irreversible myocardial damage compared with the current interstitial agents. While the distribution into the interstitial space is less, the greater degree of relaxivity or ‘brightness’ from the contrast agent could compensate. A potential concern is that the intravascular contrast agents may in actuality have a lower degree of relative myocardial enhancement compared with the interstitial agents, although only limited investigation has been performed to date. Other agents with a greater ability for metabolic characterization, and amenable to MRI are in development, but not yet available for clinical testing [74].
Integrated prognostication
In the coming genomic medical revolution, there will be increasing trends toward detecting disease in the earliest, presymptomatic stages. CMR already includes functional, morphologic, hemodynamic and tissue characterization among its abilities, and each of these components will only continue to increase in the speed with which it can be acquired, as well as the sophistication of its discriminatory abilities. Providing a more comprehensive integration of these components, along with genomic information and risk profiles should result in greater precision for individual patient prognosis. The role of CMR in the evaluation of early pathophysiologic changes in patients at risk of cardiomyopathy will remain challenging, but the promise of precision diagnoses, leading to earlier intervention, may limit congestive heart failure and cardiac death to substantially smaller fractions of cardiomyopathy patients.
Conclusion
The cardiomyopathies constitute a group of particularly challenging diagnoses with a diverse set of investigational and therapeutic strategies. Comprehensive care not only requires knowledge of cardiac anatomy and physiology, but also characterization of pathologic tissues and an understanding of modifiable substrates (i.e., those amenable to therapy). CMR represents a single, comprehensive modality with the ability to address most, and in many cases, all of these aspects. Specifically, DE-MRI can provide valuable data regarding tissue characterization and continues to provide data for further defining prognosis. Continued application of CMR and, in particular, DE-MRI in the cardiomyopathies promises further improvements in diagnosis and therapeutic outcomes.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• • of considerable interest
- Bremerich J, Bilecen D, Reimer P: MR angiography with blood pool contrast agents. Eur. Radiol. 17(12), 3017–3024 (2007).
- Weinsaft JW, Klem I, Judd RM: MRI for the assessment of myocardial viability. Magn. Reson. Imaging Clin. N. Am. 15(4), 505–525 v–vi (2007).
- Wagner A, Mahrholdt H, Thomson L et al.: Effects of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J. Am. Coll. Cardiol. 47(10), 2027–2033 (2006).
- Mahrholdt H, Wagner A, Holly TA et al.: Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 106(18), 2322–2327 (2002).
- Sievers B, Elliott MD, Hurwitz LM et al.: Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrastenhancement cardiovascular magnetic resonance. Circulation 115(2), 236–244 (2007).
- Huber AM, Schoenberg SO, Hayes C et al.: Phase-sensitive inversion-recovery MR imaging in the detection of myocardial infarction. Radiology 237(3), 854–860 (2005).
- Kellman P, Arai AE, McVeigh ER, Aletras AH: Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn. Reson. Med. 47(2), 372–383 (2002).
- Kim RJ, Fieno DS, Parrish TB et al.: Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100(19), 1992–2002 (1999).
- Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM: Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J. Am. Coll. Cardiol. 36(6), 1985–1991 (2000).
- Amado LC, Gerber BL, Gupta SN et al.: Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J. Am. Coll. Cardiol. 44(12), 2383–2389 (2004).
- Wagner A, Mahrholdt H, Holly TA et al.: Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 361(9355), 374–379 (2003).
- Lee VS, Resnick D, Tiu SS et al.: MR imaging evaluation of myocardial viability in the setting of equivocal SPECT results with (99m)Tc sestamibi. Radiology 230(1), 191–197 (2004).
- Klein C, Nekolla SG, Bengel FM et al.: Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 105(2), 162–167 (2002).
- Kuhl HP, Beek AM, van der Weerdt AP et al.: Myocardial viability in chronic ischemic heart disease: comparison of contrast-enhanced magnetic resonance imaging with 18F-fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 41(8), 1341–1348 (2003).
- Nelson C, McCrohon J, Khafagi F, Rose S, Leano R, Marwick TH: Impact of scar thickness on the assessment of viability using dobutamine echocardiography and thallium single-photon emission computed tomography: a comparison with contrastenhanced magnetic resonance imaging. J. Am. Coll. Cardiol. 43(7), 1248–1256 (2004).
- Baks T, Cademartiri F, Moelker AD et al.: Multislice computed tomography and magnetic resonance imaging for the assessment of reperfused acute myocardial infarction. J. Am. Coll. Cardiol. 48(1), 144–152 (2006).
- Mahnken AH, Koos R, Katoh M et al.: Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J. Am. Coll. Cardiol. 45(12), 2042–2047 (2005).
- Reimer KA, Lowe JE, Rasmussen MM, Jennings RB: The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 56(5), 786–794 (1977).
- Kim RJ, Wu E, Rafael A et al.: The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 343(20), 1445–1453 (2000).
- Selvanayagam JB, Kardos A, Francis JM et al.: Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation 110(12), 1535–1541 (2004).
- Cheong BY, Muthupillai R, Wilson JM et al.: Prognostic significance of delayedenhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation 120(21), 2069–2076 (2009).
- Bello D, Fieno DS, Kim RJ et al.: Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J. Am. Coll. Cardiol. 45(7), 1104–1108 (2005).
- Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S: The role of cardiovascular magnetic resonance imaging in heart failure. J. Am. Coll. Cardiol. 54(15), 1407–1424 (2009).
- Nijveldt R, Beek AM, Hirsch A et al.: Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J. Am. Coll. Cardiol. 52(3), 181–189 (2008).
- Nijveldt R, van der Vleuten, Hirsch A et al.: Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc. Imaging 2(10), 1187–1194 (2009).
- McCrohon JA, Moon JC, Prasad SK et al.: Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 108(1), 54–59 (2003).
- Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA: Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 45(5), 743–748 (2005).
- Casolo G, Minneci S, Manta R et al.: Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: diagnostic accuracy of late gadolinium enhancement. Am. Heart J. 151(1), 101–108 (2006).
- Roberts WC, Siegel RJ, McManus BM: Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am. J. Cardiol. 60(16), 1340–1355 (1987).
- Wu KC, Weiss RG, Thiemann DR et al.: Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 51(25), 2414–2421 (2008).
- Assomull RG, Prasad SK, Lyne J et al.: Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 48(10), 1977–1985 (2006).
- Nazarian S, Bluemke DA, Lardo AC et al.: Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation 112(18), 2821–2825 (2005).
- Bogun FM, Desjardins B, Good E et al.: Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J. Am. Coll. Cardiol. 53(13), 1138–1145 (2009).
- Rudolph A, Abdel-Aty H, Bohl S et al.: Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J. Am. Coll. Cardiol. 53(3), 284–291 (2009).
- Yamada M, Teraoka K, Kawade M, Hirano M, Yamashina A: Frequency and distribution of late gadolinium enhancement in magnetic resonance imaging of patients with apical hypertrophic cardiomyopathy and patients with asymmetrical hypertrophic cardiomyopathy: a comparative study. Int. J. Cardiovasc. Imaging 25(Suppl. 1), 131–138 (2009).
- Moon JC, Reed E, Sheppard MN et al.: The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 43(12), 2260–2264 (2004).
- Rickers C, Wilke NM, Jerosch-Herold M et al.: Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 112(6), 855–861 (2005).
- Adabag AS, Maron BJ, Appelbaum E et al.: Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 51(14), 1369–1374 (2008).
- Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S: Evaluation and management of the cardiac amyloidosis. J. Am. Coll. Cardiol. 50(22), 2101–2110 (2007).
- Maceira AM, Joshi J, Prasad SK et al.: Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 111(2), 186–193 (2005).
- Vogelsberg H, Mahrholdt H, Deluigi CC et al.: Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared with endomyocardial biopsy. J. Am. Coll. Cardiol. 51(10), 1022–1030 (2008).
- Ruberg FL, Appelbaum E, Davidoff R et al.: Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am. J. Cardiol. 103(4), 544–549 (2009).
- Austin BA, Tang WH, Rodriguez ER et al.: Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc. Imaging 2(12), 1369–1377 (2009).
- Sharma S: Cardiac imaging in myocardial sarcoidosis and other cardiomyopathies. Curr. Opin. Pulm. Med. 15(5), 507–512 (2009).
- Smedema JP, Snoep G, van Kroonenburgh MP et al.: Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J. Am. Coll. Cardiol. 45(10), 1683–1690 (2005).
- Cheong BY, Muthupillai R, Nemeth MA et al.: The utility of delayed-enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy-proven systemic sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 26(1), 39–46 (2009).
- Patel MR, Cawley PJ, Heitner JF et al.: Detection of myocardial damage in patients with sarcoidosis. Circulation 120(20), 1969–1977 (2009).
- Schulz-Menger J, Wassmuth R, Abdel-Aty H et al.: Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 92(3), 399–400 (2006).
- Hughes DA, Elliott PM, Shah J et al.: Effects of enzyme replacement therapy on the cardiomyopathy of Anderson–Fabry disease: a randomised, double-blind, placebocontrolled clinical trial of agalsidase a. Heart 94(2), 153–158 (2008).
- Moon JC, Sachdev B, Elkington AG et al.: Gadolinium enhanced cardiovascular magnetic resonance in Anderson–Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur. Heart J. 24(23), 2151–2155 (2003).
- Moon JC, Sheppard M, Reed E, Lee P, Elliott PM, Pennell DJ: The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson–Fabry disease. J. Cardiovasc. Magn. Reson. 8(3), 479–482 (2006).
- McKenna WJ, Thiene G, Nava A et al.: Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 71(3), 215–218 (1994).
- Burke AP, Farb A, Tashko G, Virmani R: Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation 97(16), 1571–1580 (1998).
- Pfluger HB, Phrommintikul A, Mariani JA, Cherayath JG, Taylor AJ: Utility of myocardial fibrosis and fatty infiltration detected by cardiac magnetic resonance imaging in the diagnosis of arrhythmogenic right ventricular dysplasia – a single centre experience. Heart Lung Circ. 17(6), 478–483 (2008).
- Tandri H, Saranathan M, Rodriguez ER et al.: Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J. Am. Coll. Cardiol. 45(1), 98–103 (2005).
- Tandri H, Macedo R, Calkins H et al.: Role of magnetic resonance imaging in arrhythmogenic right ventricular dysplasia: insights from the North American arrhythmogenic right ventricular dysplasia (ARVD/C) study. Am. Heart J. 155(1), 147–153 (2008).
- Germans T, van Rossum AC: The use of cardiac magnetic resonance imaging to determine the aetiology of left ventricular disease and cardiomyopathy. Heart 94(4), 510–518 (2008).
- Kohli SK, Pantazis AA, Shah JS et al.: Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur. Heart J. 29(1), 89–95 (2008).
- Petersen SE, Selvanayagam JB, Wiesmann F et al.: Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 46(1), 101–105 (2005).
- Jacquier A, Thuny F, Jop B et al.: Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 31(9), 1098–1104 (2010).
- Ivan D, Flamm SD, Abrams J, Kindo M, Heck K, Frazier OH: Isolated ventricular non-compaction in adults with idiopathic cardiomyopathy: cardiac magnetic resonance and pathologic characterization of the anomaly. J. Heart Lung Transplant. 24(6), 781–786 (2005).
- Sharkey SW, Windenburg DC, Lesser JR et al.: Natural history and expansive clinical profile of stress (Takotsubo) cardiomyopathy. J. Am. Coll. Cardiol. 55(4), 333–341 (2010).
- Eitel I, Behrendt F, Schindler K et al.: Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur. Heart J. 29(21), 2651–2659 (2008).
- Mitchell JH, Hadden TB, Wilson JM, Achari A, Muthupillai R, Flamm SD: Clinical features and usefulness of cardiac magnetic resonance imaging in assessing myocardial viability and prognosis in Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome). Am. J. Cardiol. 100(2), 296–301 (2007).
- Hendel RC, Patel MR, Kramer CM et al.: ACCF/ACR/SCCT/SCMR/ASNC/NASCI/ SCAI/SIR 2006 Appropriateness Criteria for Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group. J. Am. Coll. Radiol. 3(10), 751–771 (2006).
- Cheong BY, Muthupillai R, Wilson JM et al.: Prognostic significance of delayedenhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation 120(21), 2069–2076 (2009).
- Wu KC, Weiss RG, Thiemann DR et al.: Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 51(25), 2414–2421 (2008).
- Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ: Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J. Cardiovasc. Magn. Reson. 10(1), 54 (2008).
- Ruberg FL, Appelbaum E, Davidoff R et al.: Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am. J. Cardiol. 103(4), 544–549 (2009).
- Klumpp BD, Sandstede J, Lodemann KP et al.: Intraindividual comparison of myocardial delayed enhancement MR imaging using gadobenate dimeglumine at 1.5 T and 3 T. Eur. Radiol. 19(5), 1124–1131 (2009).
- Tang L, Merkle N, Schar M et al.: Volumetargeted and whole-heart coronary magnetic resonance angiography using an intravascular contrast agent. J. Magn. Reson. Imaging 30(5), 1191–1196 (2009).
- Caruthers SD, Cyrus T, Winter PM, Wickline SA, Lanza GM: Anti-angiogenic perfluorocarbon nanoparticles for diagnosis and treatment of atherosclerosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 1(3), 311–323 (2009).
- Pan D, Senpan A, Caruthers SD et al.: Sensitive and efficient detection of thrombus with fibrin-specific manganese nanocolloids. Chem. Commun. (Camb.) (22), 3234–3236 (2009).
- Pan D, Lanza GM, Wickline SA, Caruthers SD: Nanomedicine: perspective and promises with ligand-directed molecular imaging. Eur. J. Radiol. 70(2), 274–285 (2009).
• • Considered a seminal paper as it was the first to describe the delayed-enhancement (DE)-MRI technique.
• • This was the first comparison of the DE-MRI technique to PET, and demonstrated DE-MRI’s superiority over PET, which has been considered the gold standard in myocardial viability assessment.
• • Considered a seminal paper for being the first clinical trial to assess the utility of DE-MRI in patients with myocardial dysfunction prior to elective revascularization.
• • Largest study establishing the prognostic value of DE in patients with or without left ventricular dysfunction.