Review Article - Interventional Cardiology (2024)
The Role of Vitamin K2 in Cardiovascular Health
- Corresponding Author:
- Samir R. Kapadia
Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Ohio, United States of America
E-mail: kapadis@ccf.org
Received date: 29-Nov-2024, Manuscript No. FMIC-24-157910; Editor assigned: 02-Dec-2024, PreQC No. FMIC-24-157910 (PQ); Reviewed date: 16-Dec-2024, QC No. FMIC-24-157910; Revised date: 23-Dec-2024, Manuscript No. FMIC-24-157910 (R); Published date: 30-Dec-2024, DOI: 10.37532/1755- 5310.2024.16(S26).679
Abstract
Vitamin K naturally occurs as two structurally similar but functionally different vitamins: K1 and K2. Vitamin K2 activates Matrix Gla Protein (MGP) which acts as an inhibitor of vascular calcification. Vitamin K2 plays a role in cardiovascular health. It slows down the progression of coronary artery and aortic valve calcification by inhibiting vascular and valvular calcification. It also has an impact on metabolic syndrome, heart failure, microvascular function, and the progression of arterial stiffness. Vitamin K deficiency was shown to correlate with worse clinical outcomes. Additionally, vitamin K2 supplementation is safe and has been the focus of numerous studies and randomized clinical trials. While some trials have shown no significant effect of supplementation in mitigating coronary artery or valvular calcification, the overall findings remain promising. Many methods and assays to assess vitamin K status and function exist, however, in clinical practice, Protein Induced by Vitamin K Absence/antagonism (PIVKA-II) and vitamin K1 are commonly used together.
Keywords
Cardiovascular health • Metabolic syndrome • Aortic stiffness • Heart failure • Hypertension
Introduction
Vitamin K was discovered through the work of Carl Peter Henrik Dam between 1928 and 1930 [1]. He discovered that chicks fed on cholesterol and fat-free chicken feeds for more than 2-3 weeks were more likely to have a spontaneous hemorrhage [2]. He discovered a new vitamin and called it Vitamin K. In the late 1930s, Edward Albert Doisy was able to isolate vitamin K and received the Nobel Prize jointly with Dam [1]. Vitamin K is a fat-soluble vitamin that exists as 2 compounds that are structurally similar, but functionally different: Vitamin K1 (phylloquinone) and vitamin K2 (menaquinones, MKs) [3]. Vitamins K1 and K2 both have a naphthoquinone ring and a side chain of isoprenoids. The main structural difference between them is the length and saturation of the isoprenoid side chain at the 3rd carbon atom [4]. Vitamin K2 has extrahepatic activity and a longer half-life. Therefore it has an important role in activating γ-carboxyglutamate (Gla) proteins, such as Matrix Gla Protein (MGP), which is an inhibitor of vascular calcification [5]. MGP is synthesized by Vascular Smooth Muscle Cells (VSMCs). To be fully functional, MGP requires vitamin K. Several studies, including randomized clinical trials, have investigated the cardiovascular benefits of vitamin K2. This paper aims to explore the role of MGP and vitamin K2 in cardiovascular health.
Literature Review
Dietary sources of phylloquinone and menaquinones
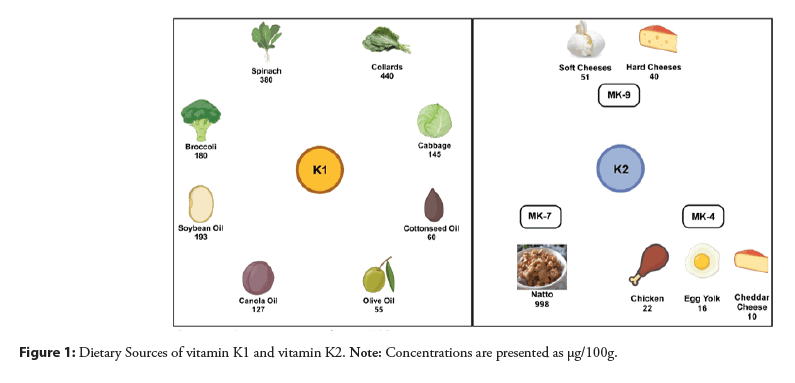
Vitamin K1 is responsible for coagulation. It is generally present in leafy green vegetables, which contribute around 60% of the total phylloquinone intake [6,7]. Leafy green vegetables with a darker color like collards have higher phylloquinone concentrations than those with lighter colors such as iceberg lettuce. Phylloquinone can also be found in plant oils such as canola, soybean, olive, and cottonseed [8]. Therefore, spreads and margarines derived from these oils are important dietary sources of phylloquinone [9,10].
Menaquinones (MKs) are characterized by the length of their isoprenoid side chain, but their origins as well as functions are not the same. Their primary origin is bacteria, except MK-4 which is formed in 2 different ways: Either via a realkylation step from menadione, or as a product of tissue-specific conversion directly from phylloquinone [8,11,12]. MK-4 formed from menadione comes from poultry products [13], and that coming from phylloquinone is found in small amounts in dairy products, and in organs such as the kidney [8]. On the other hand, MK-7 is the product of bacterial fermentation and is present in natto, a traditional Japanese soybean-based product [8]. Natto is rich in MK-7, and also contains MK-8, MK-9, and phylloquinone [8]. Figure 1 summarizes the dietary sources of vitamin K1 and K [8,9,14,15].
Matrix Gla Protein (MGP)
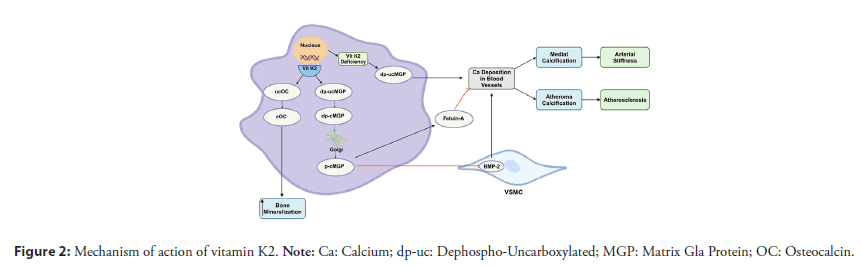
GP plays a key role in cardiovascular disease [16]. Mice who had their MGP gene knocked out were found to die prematurely due to arterial calcification and spontaneous aortic rupture [17]. MGP undergoes two post-translational modifications essential for its activation: serine phosphorylation and γ-glutamate carboxylation, the latter being a step that requires vitamin K [18]. Various forms of MGP exist, depending on its phosphorylation and carboxylation status. Since vitamin K is needed for activation of MGP, the unphosphorylated, and uncarboxylated form (dp-ucMGP) can be used as a marker of vitamin K deficiency [19,20]. The active form of MGP plays a role in preventing vascular calcification. In the absence of active MGP, VSMCs produce a matrix that favors calcium deposition, a characteristic of osteoblasts and chondrocytes [21]. Active MGP inhibits the formation of calcium crystals and modulates the transcription factors that prevent VSMCs from differentiating into cells that act similarly to osteoblasts and chondrocytes [22,23]. Active MGP is also an inhibitor of Bone Morphogenic Protein-2 (BMP-2), which induces osteogenic gene expression in VSMCs [5,23]. Moreover, it activates fetuin-A, which is an inhibitor of calcification. Figure 2 summarizes the mechanism of action of vitamin K.
Relationship of vitamin K with cardiovascular health
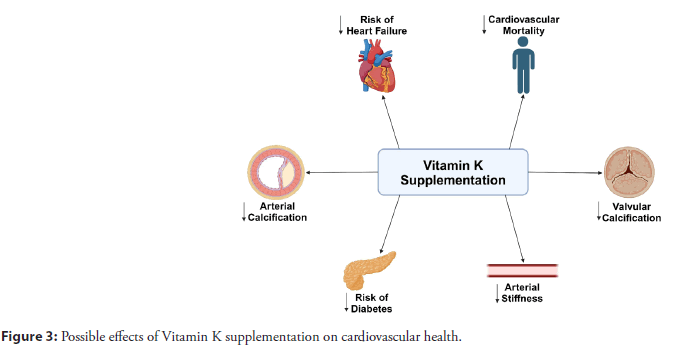
To investigate the impact of vitamin K2 on cardiovascular health, a proper assessment of vitamin K status is needed. Assays measuring MGP reflect vitamin K bioactivity over a period of weeks to months [18,24-27]. These assays rely on dual antibody ELISA to measure dephosphorylated-uncarboxylated MGP and dephosphorylated-carboxylated MGP, and mono-antibody assays measuring total uncarboxylated MGP and total dephosphorylated MGP. Table 1 summarizes those assays and their reference values. Moreover, the measurement of dp-ucMGP is used to quantify vitamin K deficiency. Figure 3 summarizes the cardiovascular benefits of vitamin K supplementation.
| Assay | dp-ucMGP | dp-cMGP | t-ucMGP | t-dpMGP |
|---|---|---|---|---|
| Method | Dual-antibody ELISA | Dual-antibody ELISA | Mono-antibody ELISA | Mono-antibody ELISA |
| Reference Values* | 447 ± 188 pM | 1763 ± 478 pM | 4704 ± 1053 nM | 14 ± 3 nM |
Note: *Reference values according to Cranenburg et al. "Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species." Thrombosis and haemostasis 104.10 (2010): 811-822. dp-ucMGP: Dephosphorylated-Uncarboxylated MGP, dp-cMGP: Dephosphorylated-Carboxylated MGP, t-ucMGP: total Uncarboxylated MGP, t-dpMGP: total Dephosphorylated MGP.
Table 1: Summarizes the method and reference values for different assays used to measure MGP.
Figure 3: Possible effects of Vitamin K supplementation on cardiovascular health.
Vascular calcification: Many studies have found that vascular calcification was associated with vitamin K deficiency. Loss-offunction mutations in the MGP gene cause systemic vascular calcification [28]. Studies have also shown that elevated dp-ucMGP levels correlate with coronary and peripheral artery calcifications, and more plaque stability [29-31]. Furthermore, Vitamin K Antagonists (VKA) was found to accelerate calcification of the coronary arteries [32-34].
Vitamin K is thought to slow the progression of Coronary Artery Calcification (CAC) [35,36]. However, few studies showed that although vitamin K2 supplementation decreased dp-ucMGP levels [37,38], it did not reduce arterial calcification [39,40]. Namely, a multicenter double-blinded randomized controlled trial with 2 years of follow-up concluded that in patients with no prior ischemic heart disease, supplementation with vitamin K2 did not slow the progression of coronary artery calcification [41]. However, this could be attributed to the short follow-up of 2 years. Longer follow-ups could show a significant reduction in mean coronary artery calcification. Moreover, the same trial found a significant reduction in the progression of coronary artery calcification in patients with CAC scores ≥ 400 Agatston Units [41].
Valvular calcification: Vitamin K2 deficiency is thought to correlate with valvular calcification. VKAs like warfarin were linked to aortic and mitral valve calcifications [42,43]. Moreover, a study comparing rivaroxaban with warfarin showed that rivaroxaban was associated with less mitral and aortic valve calcification compared to warfarin [44].
Measuring inactive forms of MGP may be useful in identifying patients at risk for progression of valvular calcification. Valvular calcification is an active process that could be modified. A study by Parker et al. found an association between serum inactive total MGP and mitral annular calcification in non-diabetic patients with CAD [45].
Studies tried to investigate the role of vitamin K2 supplementation in delaying the progression of aortic valve calcification. One randomized clinical trial found that vitamin K1 daily intake slowed the progression of aortic valve calcification [37]. On the other hand, another randomized double-blinded clinical trial concluded that in elderly men with an aortic valve calcification score >300, vitamin K2 supplementation did not affect the progression of AS [46].
Microvascular function: MGP is thought to contribute to the microvascular integrity of the heart, kidneys, and retina [47-49]. Since diastolic dysfunction can be related to inflammation of the coronary microcirculation, MGP can be involved in the disease process [50]. One study correlated dp-ucMGP with diastolic dysfunction assessed by higher Left Ventricular (LV) filling pressures and a higher E/e’ ratio [47]. The study also found a higher prevalence of dp-ucMGP in cardiac biopsies of hearts with ischemic or dilated cardiomyopathies than in normal hearts [47]. Therefore, activated MGP, with its role in preventing calcium deposition, protects the heart microcirculation and preserves LV diastolic function [3].
Markers of vitamin K deficiency are also associated with diabetes, kidney function, adiposity, and inflammation [51]. A randomized clinical trial found that low levels of carboxylated osteocalcin, indicating vitamin K deficiency, were associated with waist circumference and higher fat mass at different sites in the body [51]. More than 60% of patients with Chronic Kidney Disease (CKD) have a deficiency in vitamin K [51]. Markers of vitamin K deficiency, including elevated dp-ucMGP, were associated with a greater risk of developing advanced CKD and a lower Glomerular Filtration Rate (GFR) [52,53]. Functional vitamin K deficiency is also prevalent among kidney transplant recipients and patients on hemodialysis [54]. Although vitamin K levels improve following kidney transplantation, elevated levels of dp-ucMGP in kidney transplant recipients were associated with a greater risk of longterm mortality [55]. Some studies even suggested a correlation between MGP and serum creatinine levels in patients with CKD [56,57].
Vitamin K deficiency is also related to the microcirculation of the retina. Elevated levels of dp-ucMGP were associated with a lower retinal arteriolar diameter [58]. Retinal microvascular diameter narrowing was found to correspond to worse cardiovascular outcomes at 10 years [59].
Metabolic syndrome and diabetes: Studies have shown that supplementation with vitamin K2 deche incidence of type 2 diabetes [60]. A randomized clinical trial also showed that vitamin K2 supplementation increases insulin sensitivity [61]. Another clinical trial found that healthy postmenopausal women supplemented with vitamin K2 had a greater reduction in abdominal and visceral fat than those receiving a placebo [62].
Arterial stiffness: Markers of vitamin K deficiency including higher levels of dp-ucMGP have been correlated with aortic stiffness assessed by carotid-femoral Pulse Wave Velocity (PWV), augmentation index, and central pressure [27,63-65]. A significant reduction in arterial stiffness (by brachial-ankle PWV) was noted 3 months after switching warfarin to rivaroxaban [66]. Vitamin K1 and MK-7 were both found to decrease the arterial stiffness of the carotid artery [67]. Supplementation with MK-7 improves arterial stiffness measured by PWV in healthy patients and different subgroups [63,68-70].
Endothelial dysfunction is a predictor of worse outcomes [71]. Vitamin K2 is thought to regulate endothelial function, and some of its protective properties are due to its role in the regulation of endothelial function [72]. MGP inhibits the osteogenic properties of vascular endothelial cells in animal models [73]. Moreover, supplementation with MK-2 improves endothelial function in genetically driven mice models with hypercholesterolemia [74].
Cardiovascular outcomes and heart failure: There is a debate on whether vitamin K deficiency is associated with worse cardiovascular outcomes, with some studies correlating elevated dp-ucMGP levels with cardiovascular morbidity and mortality [16,57,75-79], and others showing no correlation [80,81].
Levels of dp-ucMGP are involved in both the systolic and diastolic functions of the heart. On the cellular level, MGP has a role in cardiac hemodynamics unrelated to calcification inhibition. MGP levels are seen during the rapid myocardial response to pressure overload [82-84], including the setting of acute myocardial infarction even befo73re left ventricular remodeling [83].
Vitamin K deficiency, suggested by elevated dp-ucMGP levels, correlates with unfavorable echocardiographic parameters in patients with heart failure and concomitant severe AS [57,76].
Therefore, dp-ucMGP can be used as a pre-procedural marker for risk assessment in patients undergoing aortic valve replacement [3,76]. In addition, levels of dp-ucMGP correlated with Left Ventricular Ejection Fraction (LVEF), N-terminal pro-brain natriuretic peptide (NTâproBNP), and mortality [46]. Elevated levels of dp-ucMGP also correlated with elevated NT-proBNP, CRP, LVEF, and diastolic dysfunction in patients with chronic heart failure [57].
Vitamin K2 has a significant role in producing mitochondrial ATP. Cardiac muscles are abundant in mitochondria and therefore, vitamin K2 can impact the function of cardiac muscles [85]. One study found that vitamin K supplementation for 8 weeks correlated with increased maximal cardiac output, stroke volume, heart rate, and decreased blood lactate during exercise [86].
Many studies explored the impact of vitamin K intake on cardiovascular outcomes. A lower incidence of CAD was found in patients with more intake of food rich in vitamin K1 [16,87]. Prospective cohort studies found that intake of vitamin K2 and not K1 decreased the incidence of severe aortic valve calcification, coronary artery disease, and mortality [35,88,89]. Another study found that patients who increased their vitamin K1 or K2 intake over time had lower mortality rates [90].
Discussion
Laboratory assessment of Vitamin K status
Different approaches are used to evaluate vitamin K status and function.
Global coagulation assays: Measurement of Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) only reveals a gross deficiency and thus should not be used [91,92]. The PT begins to rise above the reference range only when the concentration of the fully carboxylated factor II falls below ~50% [91,92].
Coagulation factor assays: If the concentration of factor V is normal, and that of factors II, VII, IX, and X is low, this suggests deficiency of a specific vitamin K-dependent clotting factor [91]. The half-lives of procoagulant Vitamin K-Dependent Proteins (VKDP) range from 6 to 50 hours, and thus, in the setting of vitamin K deficiency, the onset of abnormal coagulation is delayed [91].
Direct measurement: The most commonly used marker of vitamin K status is the direct measurement of serum K1 concentrations. Although this method can assess the levels of vitamin K in general, it does not accurately reflect the actual utilization within target tissues [91]. Vitamin K has a low endogenous concentration of around 1 part per billion and it can be measured either by liquid chromatography-mass spectrometry or high-performance liquid chromatography fluorescence detection [93,94]. Vitamin K2 can also be separated and quantified with the same chromatographic run as vitamin K1, but the clinical relevance of vitamin K2 concentrations is less well understood [91].
Other assays used clinically are those that assess the metabolites of vitamin K. High-performance liquid chromatography electrochemical detection and liquid chromatography-mass spectrometry can be used to measure levels of the 5C- and 7C-aglycone vitamin K metabolites [95,96]. The advantage of this method lies in the ability to assess the status of both vitamin K1 and K2 since they share common metabolites [91,95].
Hepatic VKDPs: Protein Induced by Vitamin K Absence/ antagonism (PIVKA-II) is the most commonly used method to evaluate vitamin K function. Multiple assays exist and they include ELISA, liquid chromatography-mass spectrometry, and automated immunoassay [97-100]. When vitamin K stores are low enough to prevent effective carboxylation of factor II, PIVKAII levels increase, even before the rise in PT [91]. Additionally, PIVKA-II can be used clinically to estimate the status of vitamin K during the preceding 3-4 days [101]. It is also used as a marker of hepatocellular carcinoma together with α-fetoprotein, to detect exposure to VKAs [102,103].
Extrahepatic VKDPs: Assessment of the utilization of vitamin K by extrahepatic tissues is possible. This is mainly done by determining the degree of carboxylation of osteocalcin and MGP [26].
As previously discussed, many methods and assays to assess vitamin K status and function exist. However, the common practice relies on using PIVKA-II and vitamin K1 in tandem [91]. Low concentrations of vitamin K1 in the serum indicate inadequate tissue stores in general. On the other hand, PIVKA-II indicates whether tissue stores are not enough for the hepatic carboxylation of factor II. When there is a low serum vitamin K1 concentration with normal PIVKA-II levels, it could be due to the susceptibility of extrahepatic to low vitamin K status [91]. Additionally, elevated PIVKA-II with normal vitamin K1 levels could be found in patients with hepatocellular carcinoma [91].
Conclusion
The role of vitamin K in cardiovascular health is an area of growing interest. While vitamin K2 is known for its role in vascular calcification, recent studies have shown an impact on heart failure, endothelial dysfunction, metabolic syndrome, and the progression of arterial stiffness. Vitamin K deficiency is associated with worse outcomes. Additionally, vitamin K2 supplementation is safe and has been the focus of numerous studies and randomized clinical trials. While some trials have shown no significant effect of supplementation in mitigating coronary artery or valvular calcification, the overall findings remain promising. Vitamin K1 levels are utilized in tandem with PIVKA-II to assess the status and function of vitamin K. Further research is needed to increase our understanding of the additional roles of vitamin K2 on cardiovascular health, and the benefits of vitamin K2 supplementation on cardiovascular outcomes.
References
- Grober U, Reichrath J, Holick MF, et al. Vitamin K: An old vitamin in a new perspective. Dermatoendocrinol. 6(1):968490 (2014).
[CrossRef] [google Scholar] [PubMed]
- Dam H. Haemorrhages in chicks reared on artificial diets: A new deficiency disease. Nature. 133(3372):909-910 (1934).
- Hariri E, Kassis N, Iskandar JP, et al. Abdelfattah O, et al. Vitamin K2-a neglected player in cardiovascular health: A narrative review. Open Heart. 8(2):001715.
[CrossRef] [Google Scholar] [PubMed]
- Suttie JW. Vitamin K in health and disease. (2009).
- Proudfoot D, Shanahan CM. Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein. Nephrology. 11(5):455-461 (2006).
[CrossRef] [Google Scholar] [PubMed]
- McKeown NM, Jacques PF, Gundberg CM, et al. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr. 132(6):1329-1334 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Thane CW, Paul AA, Bates CJ, et al. Intake and sources of phylloquinone (vitamin K1): Variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br J Nutr. 87(6):605-613 (2002).
[Crossref] [Google Scholar] [PubMed]
- Booth S. Vitamin K: Food composition and dietary intakes. Food Nutr Res. 56(1):5505 (2012).
[Crossref] [Google Scholar] [PubMed]
- Peterson JW, Muzzey KL, Haytowitz D, et al. Phylloquinone (vitamin K 1) and dihydrophylloquinone content of fats and oils. J Am Oil Chem Soc. 79:641-646 (2002).
- Piironen V, Koivu T, Tammisalo O, et al. Determination of phylloquinone in oils, margarines and butter by high-performance liquid chromatography with electrochemical detection. Food Chemistry. 59(3):473-480 (1997).
- Davidson RT, Foley AL, Engelke JA, et al. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr. 128(2):220-223 (1998).
[Crossref] [Google Scholar] [PubMed]
- Thijssen HH, Vervoort LM, Schurgers LJ, et al. Menadione is a metabolite of oral vitamin K. Br J Nutr. 95(2):260-266 (2009).
[Crossref] [Google Scholar] [PubMed]
- Elder SJ, Haytowitz DB, Howe J, et al. Vitamin K contents of meat, dairy, and fast food in the US diet. J Agric Food Chem. 54(2):463-467 (2006).
[Crossref] [Google Scholar] [PubMed]
- Damon M, Zhang NZ, Haytowitz DB, et al. Phylloquinone (vitamin K1) content of vegetables. J Food Compos Anal. 18(8):751-758 (2005).
- Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinonesx in food: Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 30(6):298-307.
- Chen HG, Sheng LT, Zhang YB, et al. Association of vitamin K with cardiovascular events and all-cause mortality: A systematic review and meta-analysis. Eur J Nutr. 58:2191-2205 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 386(6620):78-81 (1997).
[CrossRef] [Google Scholar] [PubMed]
- Stafford DW. The vitamin K cycle. J Thromb Haemost. 3(8):1873-1878.
[CrossRef] [Google Scholar] [PubMed]
- Mizuiri S, Nishizawa Y, Yamashita K, et al. Relationship of matrix Gla protein and vitamin K with vascular calcification in hemodialysis patients. Ren Fail. 41(1):770-777 (2019).
- Zwakenberg SR, Burgess S, Sluijs I, et al. Circulating phylloquinone, inactive Matrix Gla protein and coronary heart disease risk: A two-sample Mendelian Randomization study. Clin Nutr. 39(4):1131-1136 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Murshed M, Schinke T, McKee MD, et al. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 165(5):625-630 (2004).
- Price PA, Nguyen TM, Williamson MK, et al. Biochemical characterization of the serum fetuin-mineral complex. J Biol Chem. 2003278(24):22153-2260.
[Crossref] [Google Scholar] [PubMed]
- Zebboudj AF, Imura M, Bostroom K, et al. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 277(6):4388-4394 (2002).
[Crossref] [Google Scholar] [PubMed]
- Braam LA, Dissel P, Gijsbers BL, et al. Assay for human matrix gla protein in serum: Potential applications in the cardiovascular field. Arterioscler Thromb Vasc Biol. 20(5):1257-1261 (2000).
[Crossref] [Google Scholar] [PubMed]
- Cranenburg EC, Koos R, Schurgers LJ, et al. Characterisation and potential diagnostic value of circulating Matrix Gla Protein (MGP) species. Thromb Haemost. 104(10):811-822 (2010).
[Crossref] [Google Scholar] [PubMed]
- Schurgers LJ, Teunissen KJ, Knapen MH, et al. Novel conformation-specific antibodies against matrix γ-carboxyglutamic acid (Gla) protein: Undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 25(8):1629-1633 (2005).
[Crossref] [Google Scholar] [PubMed]
- Hermans MM, Vermeer C, Kooman JP, et al. Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif. 25(5-6):395-401 (2008).
[Crossref] [Google Scholar] [PubMed]
- Sun LF, Chen X. Tracheobronchial stenosis in Keutel syndrome. Indian Pediatr. 49(9) (2012).
[Crossref] [Google Scholar] [PubMed]
- Dalmeijer GW, Van der Schouw YT, Vermeer C, et al. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem. 24(4):624-628 (2013).
[Crossref] [Google Scholar] [PubMed]
- Liabeuf S, Olivier B, Vemeer C, et al. Vascular calcification in patients with type 2 diabetes: The involvement of matrix Gla protein. Cardiovasc Diabetol. 13:1-8 (2014).
[Crossref] [Google Scholar] [PubMed]
- Zwakenberg SR, Vvan der Schouw YT, Vermeer C, et al. plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology. 141(1):32-36 (2018).
[Crossref] [Google Scholar] [PubMed]
- Price PA, Faus SA, Williamson MK, et al. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 18(9):1400-1407 (1998).
[CrossRef] [Google Scholar] [PubMed]
- Rennenberg RJ, van Varik BJ, Schurgers LJ, et al. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. 115(24):5121-5123 (2010).
[Crossref] [Google Scholar] [PubMed]
- Weijs B, Blaauw Y, Rennenberg RJ, et al. Patients using vitamin K antagonists show increased levels of coronary calcification: Aan observational study in low-risk atrial fibrillation patients. Eur j heart. 32(20):2555-2562 (2011).
- Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. The J nuter. 134(11):3100-3105 (2004).
- Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 89(6):1799-1807 (2009).
[Google Scholar] [PubMed]
- De Vriese AS, Caluwe R, Pyfferoen L, et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: The Valkyrie Study. J Am Soc Nephrol. 31(1):186-196 (2020).
[Crossref] [Google Scholar] [PubMed]
- Oikonomaki T, Papasotiriou M, Ntrinias T, et al. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int Urol Nephrol. 51:2037-2044 (2019).
[Crossref] [Google Scholar] [PubMed]
- Bartstra JW, Draaisma F, Zwakenberg SR, et al. Six months vitamin K treatment does not affect systemic arterial calcification or bone mineral density in diabetes mellitus 2. Eur J Nutr. 60:1691-1699 (2021).
- Zwakenberg SR, De Jong PA, Bartstra JW, et al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: A randomized, double-blind, placebo-controlled trial. J Clin Nutr. 110(4):883-890 (2019).
- Hasific S, Oevrehus KA, Lindholt JS, et al. Effects of vitamin K2 and D supplementation on coronary artery disease in men: A RCT. JACC: Advances. 2(9):100643 (2023).
- Lerner RG, Aronow WS, Sekhri A, et al. Warfarin use and the risk of valvular calcification. J Thromb Haemost. 7(12):2023-2027 (2009).
[Crossref] [Google Scholar] [PubMed]
- Sønderskov PS, Lindholt JS, Hallas J, et al. Association of aortic valve calcification and vitamin K antagonist treatment. Eur Heart J Cardiovasc. 21(7):718-724 (2020).
[Crossref] [Google Scholar] [PubMed]
- Di Lullo L, Tripepi G, Ronco C, et al. Cardiac valve calcification and use of anticoagulants: preliminary observation of a potentially modifiable risk factor. Int J Cardiol. 278:243-249 (2019).
[Crossref] [Google Scholar] [PubMed]
- Parker BD, Schurgers LJ, Vermeer C, et al. The association of uncarboxylated matrix Gla protein with mitral annular calcification differs by diabetes status: The Heart and Soul study. Atherosclerosis. 210(1):320-325 (2010).
[Crossref] [Google Scholar] [PubMed]
- Diederichsen AC, Lindholt JS, Moller S, et al. Vitamin K2 and D in patients with aortic valve calcification: A randomized double-blinded clinical trial. Circulation. 145(18):1387-1397 (2022).
[Crossref] [Google Scholar] [PubMed]
- Wei FF, Trenson S, Monney P, et al. Epidemiological and histological findings implicate matrix Gla protein in diastolic left ventricular dysfunction. PLoS One. 13(3):0193967 (2018).
[Crossref] [Google Scholar] [PubMed]
- Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 263(23):11033-11036 (1988).
- Borras T, Smith MH, Buie LK, et al. A novel Mgp-Cre knock-in mouse reveals an anticalcification/antistiffness candidate gene in the trabecular meshwork and peripapillary scleral region. Invest Ophthalmol Vis Sci. 56(4):2203-2214 (2015).
[Crossref] [Google Scholar] [PubMed]
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 62(4):263-271 (2013).
[Crossref] [Google Scholar] [PubMed]
- Fusaro M, Gallieni M, Porta C, et al. Vitamin K effects in human health: New insights beyond bone and cardiovascular health. J Nephrol. 33:239-249 (2020).
[Crossref] [Google Scholar] [PubMed]
- Wei FF, Drummen NE, Schutte AE, et al. Vitamin K dependent protection of renal function in multi-ethnic population studies. EBioMedicine. 4:162-169 (2016).
- Wei FF, Trenson S, Thijs L, et al. Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol Dial Transplant. 33(7):1122-1128 (2018).
[Crossref] [Google Scholar] [PubMed]
- Cranenburg EC, Schurgers LJ, Uiterwijk HH, et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 82(5):605-610 (2012).
[Crossref] [Google Scholar] [PubMed]
- Keyzer CA, Vermeer C, Joosten MM, et al. Vitamin K status and mortality after kidney transplantation: A cohort study. Am J Kidney Dis. 65(3):474-483 (2015).
[Crossref] [Google Scholar] [PubMed]
- Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, et al. Plasma desphospho-uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press Res. 41(3):231-239 (2016).
[Crossref] [Google Scholar] [PubMed]
- Ueland T, Dahl CP, Gullestad L, et al Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clinical science. 121(3):119-127 (2011). [Crossref] [Google Scholar]
[PubMed]
- Wei FF, Huang QF, Zhang ZY, et al. Inactive matrix Gla protein is a novel circulating biomarker predicting retinal arteriolar narrowing in humans. Sci Rep. 8(1):15088 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: A population-based case-control study. Ophthalmology. 110(5):933-940 (2003).
[Crossref] [Google Scholar] [PubMed]
- Beulens JW, Van Der A DL, Grobbee DE, et al. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 33(8):1699-1705 (2010).
[Crossref] [Google Scholar] [PubMed]
- Choi HJ, Yu J, Choi H, et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: A placebo-controlled trial. Diabetes Care. 34(9):146-147 (2011).
[Crossref] [Google Scholar] [PubMed]
- Knapen MH, Jardon KM, Vermeer C, et al. Vitamin K-induced effects on body fat and weight: Results from a 3-year vitamin K2 intervention study. Eur J Clin Nutr. 72(1):136-141 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Mansour AG, Hariri E, Daaboul Y, et al. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients-a single-arm, single-center clinical trial. J Am Soc Hypertens. 11(9):589-597 (2017).
- Pivin E, Ponte B, Pruijm M, et al. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population–based study. Hypertension. 66(1):85-92 (2015).
- Wei FF, Thijs L, Cauwenberghs N, et al. Central hemodynamics in relation to circulating desphosphoâuncarboxylated matrix Gla protein: A population study. J Am Heart Assoc. 8(7):011960 (2019).
[Crossref] [Google Scholar] [PubMed]
- Ikari Y, Saito F, Kiyooka T, et al. Switching from Warfarin to rivaroxaban induces sufficiency of vitamin K and reduction of arterial stiffness in patients with atrial fibrillation. Heart Vessels. 35:1727-1733 (2020).
[Crossref] [Google Scholar] [PubMed]
- Braam LA, Hoeks AP, Brouns F, et al. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: A follow-up study. Thromb Haemost. 91(02):373-380 (2004).
[Crossref] [Google Scholar] [PubMed]
- Ikari Y, Torii S, Shioi A, et al. Impact of menaquinone-4 supplementation on coronary artery calcification and arterial stiffness: An open label single arm study. Nutr J. 15:1-6 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Knapen MH, Braam LA, Drummen NE, et al. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. Thromb Haemost. 113(05):1135-1144 (2015).
[Crossref] [Google Scholar] [PubMed]
- Vermeer C, Vik H. Effect of Menaquinone-7 (vitamin K2) on vascular elasticity in healthy subjects: Results from a one-year study. Vasc Dis Ther. 5:1-4 (2020).
- Della Rocca DG, Pepine CJ. Endothelium as a predictor of adverse outcomes. Clin Cardiol. 33(12):729-730 (2010).
[Crossref] [Google Scholar] [PubMed]
- Riphagen IJ, Keyzer CA, Drummen NE, et al. Prevalence and effects of functional vitamin K insufficiency: The PREVEND study. Nutrients. 9(12):1334 (2017).
[Crossref] [Google Scholar] [PubMed]
- Chlopicki S, Gryglewski RJ. Angiotensin Converting Enzyme (ACE) and HydroxyMethylGlutaryl-CoA (HMG-CoA) reductase inhibitors in the forefront of pharmacology of endothelium. Pharmacol Rep. 57:86 (2005).
[Google Scholar] [PubMed]
- Bar A, Kus K, Manterys A, et al. Vitamin K2-MK-7 improves nitric oxide-dependent endothelial function in ApoE/LDLR−/− mice. Vascul Pharmacol. 122:106581 (2019).
[Crossref] [Google Scholar] [PubMed]
- Mayer Jr O, Seidlerova J, Bruthans J, et al. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis. 235(1):162-168 (2014).
[Crossref] [Google Scholar] [PubMed]
- Ueland T, Gullestad L, Dahl CP, et al. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 268(5):483-492 (2010).
[Crossref] [Google Scholar] [PubMed]
- Van den Heuvel EG, van Schoor NM, Lips P, et al. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas. 77(2):137-141 (2014).
[Crossref] [Google Scholar] [PubMed]
- Mayer Jr O, Bruthans J, Seidlerova J, et al. The coincidence of low vitamin K status and high expression of growth differentiation factor 15 may indicate increased mortality risk in stable coronary heart disease patients. Nutr Metab Cardiovasc Dis. 31(2):540-551 (2021).
[Crossref] [Google Scholar] [PubMed]
- Mayer Jr O, Seidlerova J, Vanek J, et al. The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int J Cardiol. 203:916-922 (2016).
[Crossref] [Google Scholar] [PubMed]
- Dalmeijer GW, Van Der Schouw YT, Magdeleyns EJ, et al. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes care. 36(11):3766-3771 (2013).
[Crossref] [Google Scholar] [PubMed]
- Shea MK, Booth SL, Weiner DE, et al. Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the Health, Aging, and Body Composition Study (Health ABC). J Nutr. 147(5):888-895 (2017).
[Crossref] [Google Scholar] [PubMed]
- Hwang DM, Dempsey AA, Lee CY, et al. Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics. 66(1):1-4 (2000).
[Crossref] [Google Scholar] [PubMed]
- Mustonen E, Pohjolainen V, Aro J, et al. Upregulation of cardiac matrix Gla protein expression in response to hypertrophic stimuli. Blood Press. 18(5):286-293 (2009).
[Crossref] [Google Scholar] [PubMed]
- Rysaa J, Leskinen H, Ilves M, et al. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension. 45(5):927-933 (2005).
[Crossref] [Google Scholar] [PubMed]
- Vos M, Esposito G, Edirisinghe JN, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 336(6086):1306-1310 (2012).
[Crossref] [Google Scholar] [PubMed]
- Henning AL. Oral Consumption of Vitamin K^ sub 2^ for 8 weeks associated with increased maximal cardiac output during exercise. Altern Ther Health Med. 23(4):26 (2017).
[Google Scholar] [PubMed]
- Erkkila AT, Booth SL, Hu FB, et al. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis. 17(1):58-62 (2007).
[Crossref] [Google Scholar] [PubMed]
- Gast GC, de Roos NM, Sluijs I, et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 19(7):504-510 (2009).
[Crossref] [Google Scholar] [PubMed]
- Haugsgjerd TR, Egeland GM, Nygard OK, et al. Association of dietary vitamin K and risk of coronary heart disease in middle-age adults: The Hordaland Health Study Cohort. BMJ open. 10(5):035953 (2020).
[Crossref] [Google Scholar] [PubMed]
- Juanola-Falgarona M, Salas-Salvadó J, Martínez-González MÁ, et al. Dietary intake of vitamin K is inversely associated with mortality risk. J Nutr. 144(5):743-750 (2014).
[Crossref] [Google Scholar] [PubMed]
- Card DJ, Gorska R, Harrington DJ. Laboratory assessment of vitamin K status. J Clin Pathol. 73(2):70-75 (2020).
[Crossref] [Google Scholar] [PubMed]
- Suttie J. Vitamin K and human nutrition. (1992).
- Ducros V, Pollicand M, Laporte F, et al. Quantitative determination of plasma vitamin K1 by high-performance liquid chromatography coupled to isotope dilution tandem mass spectrometry. Anal Biochem. 401(1):7-14 (2010).
[Crossref] [Google Scholar] [PubMed]
- Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 282:408-421 (1997). Academic Press.
[Crossref] [Google Scholar] [PubMed]
- Harrington DJ, Soper R, Edwards C, et al. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J Lipid Res. 46(5):1053-1060 (2005).
[Crossref] [Google Scholar] [PubMed]
- McDonald MG, Yeung CK, Teitelbaum AM, et al. A new LC-MS assay for the quantitative analysis of vitamin K metabolites in human urine [S]. J Lipid Res.60(4):892-899 (2019).
[Crossref] [Google Scholar] [PubMed]
- Meguro T, Yamada K. A simple and rapid test for PIVKA-II in plasma. Thromb Res. 25(1-2):109-114 (1982).
[Crossref] [Google Scholar] [PubMed]
- Sohn A, Kim H, Yu SJ, et al. A quantitative analytical method for PIVKA-II using multiple reaction monitoring-mass spectrometry for early diagnosis of hepatocellular carcinoma. Anal Bioanal Chem. 409:2829-2838 (2017).
[Crossref] [Google Scholar] [PubMed]
- Ko DH, Hyun J, Kim HS, et al. Analytical and clinical performance evaluation of the Abbott architect PIVKA assay. Ann Clin Lab Sci. 48(1):75-80 (2018).
[Google Scholar] [PubMed]
- Ryu MR, Kang ES, Park HD. Performance evaluation of serum PIVKAâII measurement using HISCLâ5000 and a method comparison of HISCLâ5000, LUMIPULSE G1200, and ARCHITECT i2000. J Clin Lab Anal. 33(6):22921 (2019).
[Crossref] [Google Scholar] [PubMed]
- Motohara K, Endo F, Matsuda I, et al. Effect of vitamin K administration on acarboxy prothrombin (PIVKA-II) levels in newborns. Lancet. 326(8449):242-244 (1985).
[Crossref] [Google Scholar] [PubMed]
- Liebman HA, Furie BC, Tong MJ, et al. Des-γ-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 310(22):1427-1431 (1984).
[Crossref] [Google Scholar] [PubMed]
- Card DJ, Francis S, Deuchande K, et al. Superwarfarin poisoning and its management. BMJ Case Rep. 2014206360 (2014).
[Crossref] [Google Scholar] [PubMed]