Contrast Agent Evaluation - Imaging in Medicine (2012) Volume 4, Issue 4
Safety and clinical usefulness of gadoteric acid including post-marketing surveillance
Toyohiro Ota1, Junko Kimura1 and Tsuneo Ishiguchi1*
1Department of Radiology, Aichi Medical University, 21 Nagakute-cho, Aichi-gun, Aichi prefecture 480-1195, Japan
- *Corresponding Author:
- Tsuneo Ishiguchi
Department of Radiology
Aichi Medical University, 21 Nagakute-cho Aichi-gun
Aichi prefecture 480-1195, Japan
Tel: +81 561 623 311
Fax:+81 561 633 268
E-mail: ishiguti@aichi-med-u.ac.jp
Abstract
MRI is an essential diagnostic tool in making definite diagnoses and determining therapeutic strategies. Extracellular gadolinium (Gd)-based contrast agents (GBCAs) have been widely used within clinical settings. GBCAs are chelated to improve the safety due to the potent toxicity of the Gd ion itself. Nephrogenic systemic fibrosis (NSF) has been reported in association with the use of GBCAs in patients with serious renal dysfunction. Gd-DOTA, a solution of the Gd complex containing a macrocyclic structure (gadoterate), is characterized by excellent chelate stability and low risk for NSF. Gd-DOTA has been shown to have an good tolerance overall and an excellent efficacy in clinical practice. This contrast agent is an option for risk reduction when contrast-enhanced MRI is indicated in patients with renal dysfunction.
Keywords
contrast enhancement; gadolinium; gadoteric acid; Gd-DOTA; meglumine gadoterate; MRI; ; ephrogenic systemic fibrosis
MRI, introduced in the clinical setting in the early 1980s, has become an essential diagnostic tool because it provides excellent soft tissue contrast and multiplanar observation. There are 6279 running MRI devices in Japan as of April 2011, and the number of MRI examinations performed in 2008 is estimated to be approximately 10,000,000, showing that MRI plays an important role in making definite diagnoses and determining therapeutic strategies [1,2].
For MRI, extracellular gadolinium (Gd)-based contrast agents (GBCAs) such as gadoteric acid (Gd-DOTA, Magnescope®, Guerbet Japan KK, Tokyo, Japan; Dotarem®, Guerbet, Roissy CdG, France) have been used in the clinical setting since the 1980s.
For the last 20 years, multiple articles have described the clinical benefits of extracellular GBCAs [3–7].
GBCAs are chelated to improve safety because of the potent toxicity of the Gd ion itself. These contrast agents, after intravenous administration, are nonspecifically distributed from blood to tissue extracellular fluid, and are excreted via the kidney within approximately 24 h after administration without being metabolized. Recent reports describe the onset of nephrogenic systemic fibrosis (NSF) in association with the use of extracellular GBCAs in patients with serious renal dysfunction. NSF manifests with symptoms such as skin swelling, sclerosis and pain with the onset of several days to months, sometimes several years, after the administration of Gd. In advanced cases, limb joint contracture significantly limits the activity of daily living. NSF can also cause fibrosis of internal organs which may lead to death [8,101]. NSF presumably results from the in vivo release of the Gd ion from extracellular GBCAs, and skin and tissue deposition of the free ion [9,10].
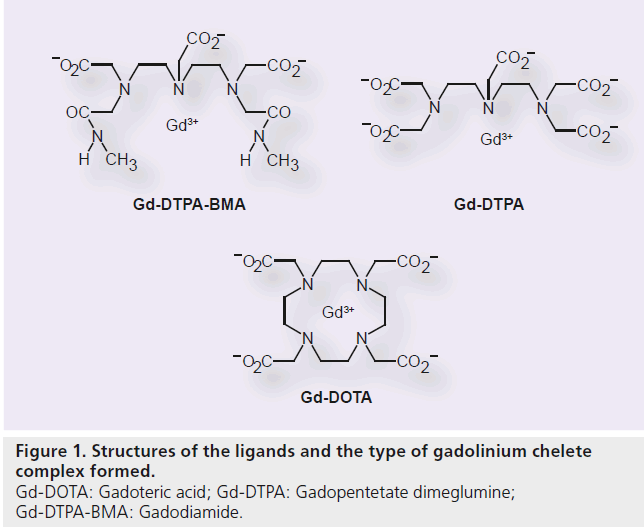
Gd-DOTA is a GBCA that has been marketed worldwide since its first approval in France in 1989. In Japan, Gd-DOTA was approved in 2000. As of 2011, this contrast agent has been registered in 75 countries and marketed in 68 countries [Guerbet, Data on File]. Gd-DOTA, a solution of the Gd complex containing a macrocyclicstructure (gadoterate), is characterized by excellent chelate stability and has a low risk of causing the onset of NSF [11].
Chemistry & physical properties of Gd-DOTA
In the development of Gd contrast agents for MRI, it is most critical to improve the stability of the chelate to prevent the release of toxic free Gd3+ ion. Gd-DOTA, with the macrocyclicstructure, has a potent binding affinity for the Gd3+ ion. Its ligand is 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid (DOTA; Figure 1). The molecular formula is C16H25GdN4O8 , and its molecular weight is 753.86 g/mol. Metal complexes are generally dissociated to metals and chelating agents upon the effect of pH. It has been confirmed that Gd-DOTA is a stable complex within the pH ranges of 4.7 to 9.7 (in vitro), requiring no stabilizer or capture agent (i.e., free ligand added to the pharmaceutical solution) to inhibit the release of the Gd3+ ion during the shelf life [102]. The osmotic pressure is 1350 mOsm/kg H2O, and the viscosity is 2.0 mPa·s at 37°C. Thermodynamic and conditional stability is an index of Gd3+ ion and ligand dissociation, while the kinetic stability indicates the dissociation rate of the Gd3+ ion and the ligand. Gd-DOTA has the highest thermodynamic stability (log10 KTHERM = 25.8), conditional stability (log10 Kcond = 18.8) and kinetic stability of all available Gd–chelate complexes (Table 1) [9,11,12–16].
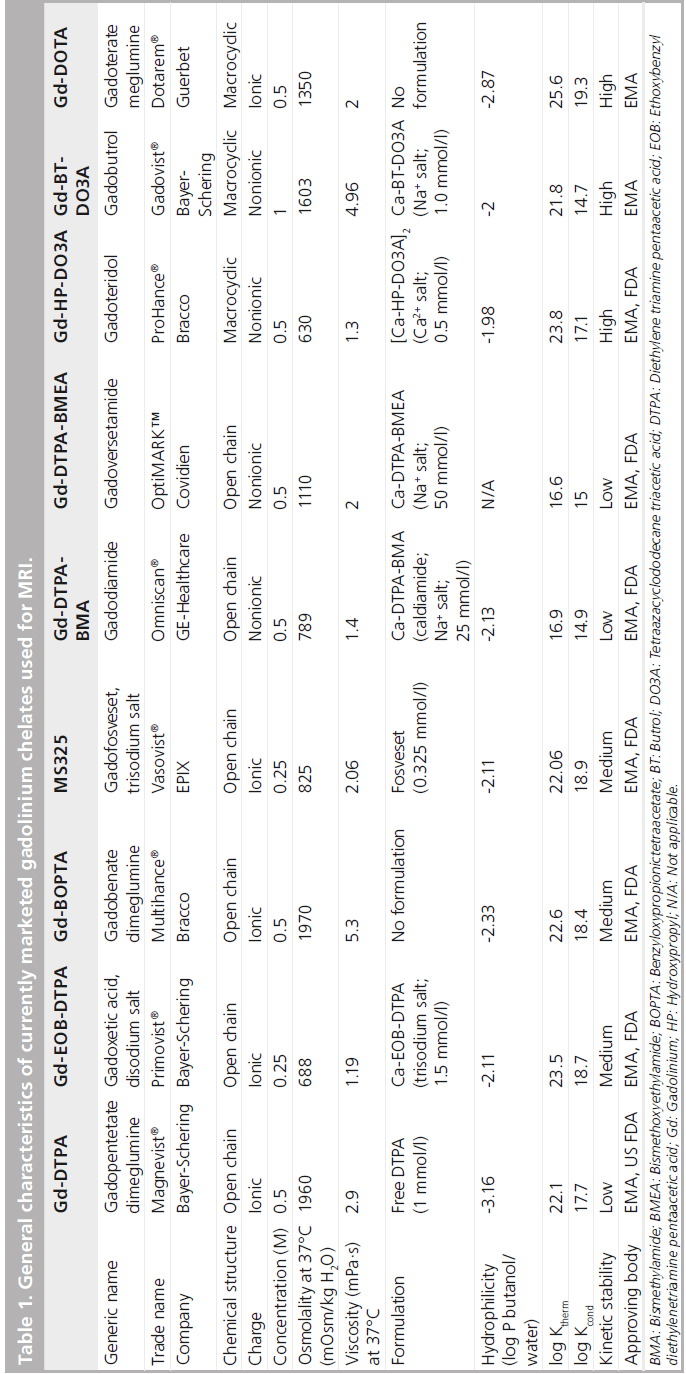
Relaxivity is the physical basis of contrast enhancement. In plasma at 37°C, T1 relaxation is 3.6 (3.4–3.8) mM-1 s-1, and T2 relaxation is 4.3(3.4–5.2) mM-1 s-1 at 1.5 T. At 3 T, T1 relaxation is 3.5 (3.3–3.7) mM-1 s-1, and T2 relaxation is 4.9(4.0–5.8) mM-1 s-1 [17].
Pharmacokinetics and pharmacodynamics of Gd-DOTA
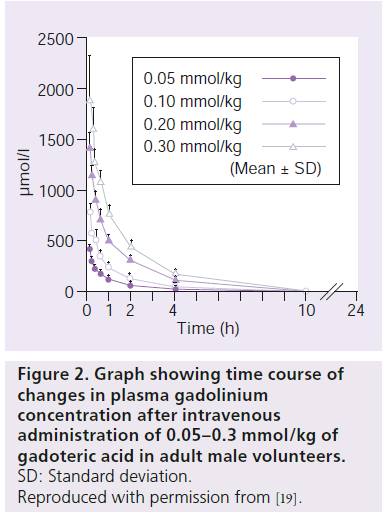
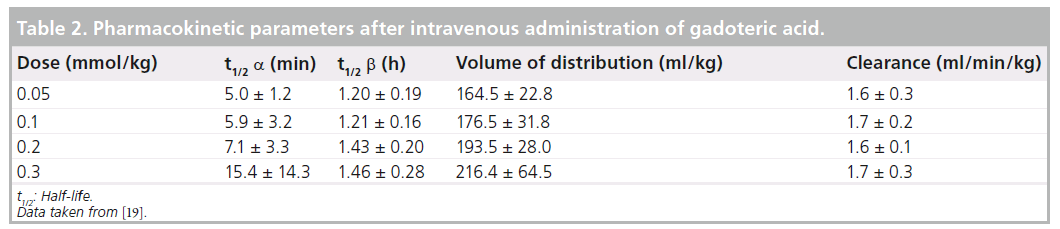
Gd-DOTA is nonspecifically distributed from blood to tissue extracellular fluid rapidly after administration. After the intravenous administration of Gd-DOTA, plasma Gd concentration showed a biphasic biexponential decay (Figure 2) [18–20]. In a Japanese Phase I study in adult male volunteers, calculating plasma pharmacokinetic parameters at the standard dose of 0.1 mmol/kg, the half-life was 5.9 ± 3.2 min in the distribution phase (a phase) and 1.21 ± 0.16 h in the elimination phase (b phase) (Table 2). The area under the blood concentration time curve (AUC) was 994.8 ± 105.7 nmol•h/ml. The cumulative urinary excretion of Gd-DOTA was 89% or higher 6 h after administration and 95% or higher 24 h after dosing. HPLC-based analysis of urinary metabolites detected only unchanged Gd-DOTA. These results indicated that Gd-DOTA, after intravenous administration, underwent no metabolism and was excreted unchanged in urine.
Figure 2.Graph showing time course of changes in plasma gadolinium concentration after intravenous administration of 0.05–0.3 mmol/kg of gadoteric acid in adult male volunteers.
The median lethal dose (LD50) in mice of Gd-DOTA is 10.6 mmol/kg and is nearly twice that of Gd-DTPA [18].
Clinical efficacy and safety of Gd-DOTA
Phase II study
A Japanese Phase II study of a GCP clinical trial in adult inpatients in whom lesions were confirmed in the brain and spinal cord, heart and thoracic region, abdominal region and limbs and MRI was required for diagnosis [21]. The dose of Gd-DOTA was set within the range where the safety of this drug was confirmed in healthy adults in the Phase I study. Taking into account the clinical dose in France, three doses of 0.05, 0.10 and 0.2 mmol/kg were established so that Gd-DOTA would be adequately safe and provide sufficient contrast enhancement.
T1- and T2-weighted MRI images were performed before contrast and T1-weighted MRI images were taken on the same sections and with the same imaging technique after administration of Gd-DOTA.
A total of 158 patients received Gd-DOTA and all were eligible for the the analysis set. They were randomized to one of the three dose groups. There were 52 patients in the 0.05 mmol/kg group, 52 patients in the 0.1 mmol/kg group and 54 patients in the 0.2 mmol/kg group. No significant difference was found in the distribution of sex, age, complications, medical history or use of prior medication among the three dose groups. Major diseases were glioma (23.6%) and meningioma (2l.8%) in the brain and spinal cord, myocardial infarction (34.1%) and lung tumor (46.3%) in the heart and thoracic region, and hepatocellular carcinoma (12.9%), renal cell carcinoma (16.1%) and uterine cervical cancer (1l.3%) in the abdominal pelvic region. Most lesions were neoplastic except for cardiac lesions.
Efficacy
In the analysis set, T1-weighted images were taken in 155 patients and dynamic imaging were performed in 70 patients. Gd-DOTA was effective in patients in whom contrast enhancement of T1-weighted images was good or excellent. The efficacy rate was 84.4% in the 0.05 mmol/kg group, 95.8% in the 0.1 mmol/kg group, and 98.0% in the 0.2 mmol/kg group, showing significant differences among the three dose groups. The χ2 test showed that the efficacy rate was significantly higher in the 0.2 mmol/kg group compared with the 0.05 mmol/kg group (p < 0.05). The U-test demonstrated that the efficacy rate was significantly higher in the 0.1 and 0.2 mmol/kg groups compared with the 0.05 mmol/kg group (p < 0.01 and p < 0.05, respectively).
Safety
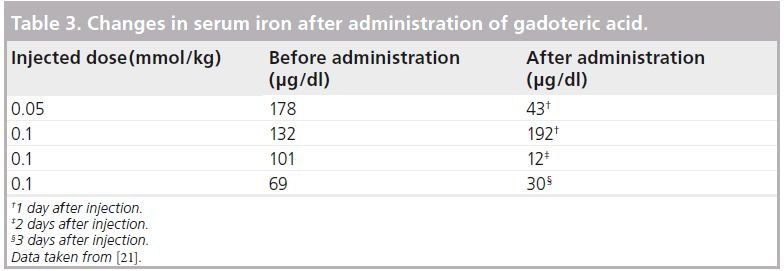
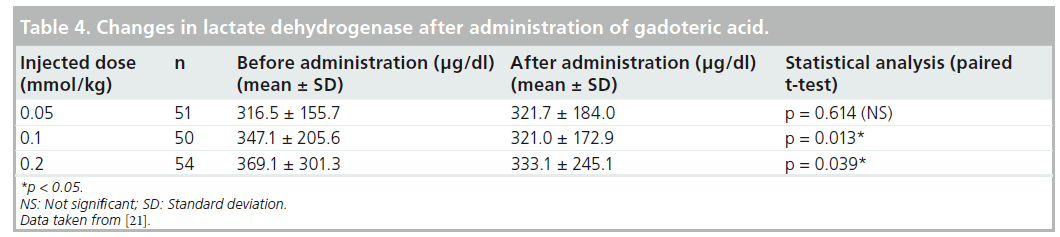
Adverse drug reactions occurred in 2 out of 158 (1.27%) patients; a distressed feeling of the chest and peculiar taste occurred in one patient. Both symptoms were mild and spontaneously resolved (within 1 min) without any treatment. There was no relationship between the dose of Gd-DOTA and onset of adverse reactions. Of 156 patients who were included in the analysis of the laboratory tests on blood (complete blood count, total bilirubin, ALT, AST, ALP, LDH, g-GTP, urea nitrogen, creatinine, electrolytes and serum iron) and on urine (proteinuria, giucosuria and urobilinogenuria), four patients experienced significant changes in serum iron with a suspected relationship with Gd-DOTA; serum iron was reduced in three of the four patients. There was no correlation with the dose of Gd-DOTA in these three patients (Table 3). No statistically significant difference was observed in changes in any laboratory parameter except for LDH between before and after administration (Table 4).
Safety rating
The safety rating was evaluated by taking into account the type, severity and duration of adverse drug reaction, and laboratory data. The safety rate per group (i.e., percentage of patients in whom Gd-DOTA was ‘safe’) was 96.2% (50 out of 52 patients), 96.2% (50 out of 52 patients) and 98.2% (53 out of 54 patients), respectively, in the 0.05, 0.1 and 0.2 mmol/kg group, showing no significant difference among the three groups. The overall safety rate was 96.8% (153 out of 158 patients).
Clinical usefulness
The clinical usefulness was evaluated based on the global consideration of ‘efficacy’ and ‘safety rating.’ The usefulness rate, in other words, the percentage of patients in whom Gd-DOTA was ‘effective’ or ‘very effective’, was 84.4% (38 out of 45 patients), 95.8% (46 out of 48 patients), and 98.0% (49 out of 50 patients), respectively, in the 0.05, 0.1 and 0.2 mmol/kg group, showing significant differences among the three groups. The U-test identified significant differences between the 0.05 and 0.1 mmol/kg groups, and between the 0.05 and 0.2 mmol/kg groups (p < 0.05).
This Phase II study examined the efficacy and safety of Gd-DOTA in MRI of cerebral and spinal cord diseases, cardiac and thoracic diseases, and abdominal and limb diseases, and the clinical optimum dose of this drug. Study results confirmed potent contrast enhancement by Gd-DOTA, and the clinical efficacy and safety of this drug. It was concluded that 0.1 mmol/kg was at least the clinical optimum dose of this drug for all these indications.
Phase III study
Results of the Phase II study confirmed contrast enhancement effect by Gd-DOTA and its safety and clinical usefulness. The clinical optimum dose of this drug was established as 0.1 mmol/kg [22–32].
In a Japanese Phase III study participating 15 institutions, the efficacy and safety of Gd-DOTA were evaluated in comparison with the control agent, gadopentetate dimeglumine (Gd-DTPA, Magnevist®, Bayer Yakuhin, Osaka, Japan; Magnevist®, Bayer HealthCare Pharmaceuticals Inc., NJ, USA), which had been widely used and was considered to be a reference product at the start of this clinical study.
This was a GCP clinical trial in adult inpatients and outpatients with confirmed or suspected lesions in the brain and spinal cord, heart and thoracic region, and abdominal region and limbs, and MRI was required for diagnosis. Outpatients were eligible if they could be followed. This was a comparative study between Gd-DOTA and Gd-DTPA (both drugs administered at 0.1 mmol/kg). Patients who underwent kidney imaging were given these at 0.05 mmol/kg. The actual dose was calculated based on body weight on the day of administration. MRI protocol was the same as in the Phase II study. Specifically, MRI was performed with the same technique as used in the daily practice at each study center. It was mandatory to take T1-weighted and T2-weighted images (or comparative images) before administration and T1-weighted images (or comparative images) after dosing. Patients underwent dynamic imaging to the furthest extent possible. T1-weighted images were taken on the same sections and with the same imaging technique before and after administration. T2-weighted images were obtained on the same sections as T1-weighted images. Cardiac MRI was conducted, in principle, by the spin echo (SE) method. The TR was the RR interval on the ECG, and only T1-weighted images were required.
Exposed films were blinded by study centers and patient numbers. The controller randomized the order of reading by the studied regions. The efficacy of the study drugs was evaluated by consensus at the image reading committee. The laboratory tests on blood (complete blood count, total bilirubin, ALT, AST, ALP, LDH, g-GTP, urea nitrogen, creatinine, electrolytes, serum iron and ferritin) and on urine (proteinuria, giucosuria and urobilinogenuria) were performed within 1 week before administration and on the next day to 3 days after dosing.
In patients with clinically significant changes, the relationship with the study drug was assessed, and the patients were followed for the course of the relevant laboratory parameter.
A total of 304 patients received the study drugs and were all eligible for the analysis set.
Efficacy
The efficacy rate, the primary efficacy end point in this study, was 92.5% (135 out of 146 patients) in the Gd-DOTA group and 95.2% (140 out of 147 patients) in the Gd-DTPA group, showing no significant difference between the two groups (χ2 test; p = 0.323). The difference in efficacy rate between the two groups was -2.8% with a 90% CI of -7.4–1.8%, confirming the equivalence of the two agents.
Safety
There were a total of 21 onsets of clinically significant changes in laboratory values in 16 patients in the Gd-DOTA group (147 patients were included in the analysis of laboratory data), and the relationship with the investigational drug was ‘probable’ or ‘related’, including increased GOT in three patients, increased serum iron in six patients, and decreased serum iron in three patients. In the Gd-DTPA group (148 patients were included in the analysis of laboratory data), there were a total of 20 onsets of clinically significant changes in laboratory values in 19 patients, and the relationship with the control drug was ‘probable’ or ‘related,’ including increased serum iron in 12 patients, decreased serum iron in four, and increased ferritin in two. There were significant differences in changes before and after administration in g-GTP, BUN and serum iron in the Gd-DTPA group (paired t-test). For serum iron, a significant difference was identified between the two groups (t-test; p = 0.010) (Table 5).
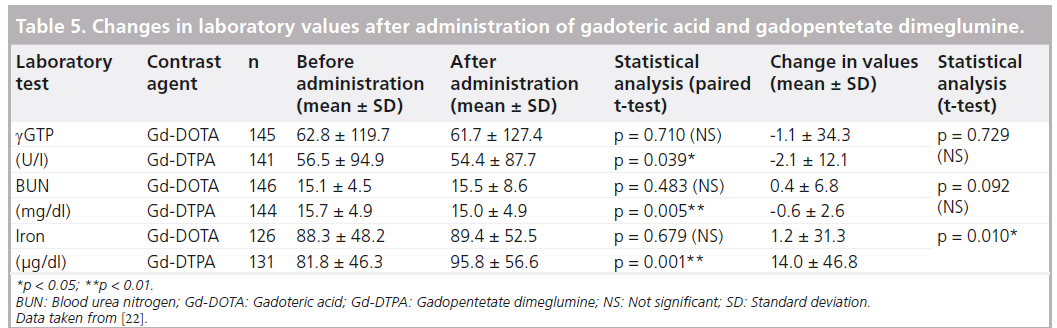
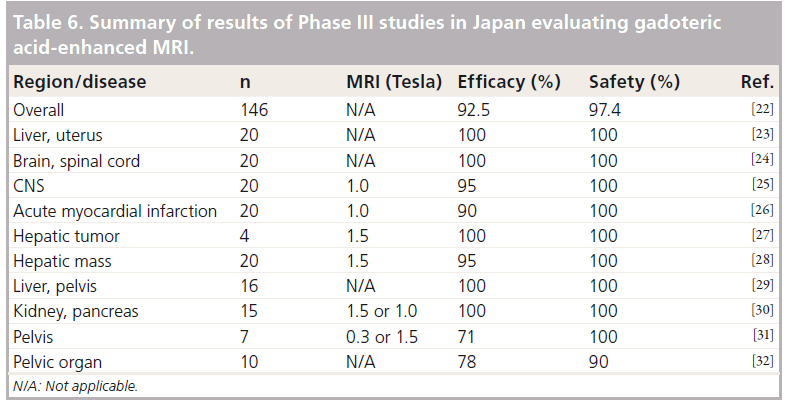
Gd-DOTA was effective and safe in the data analysis by imaged regions at each study center in this Phase III study (Table 6). Oudkerk also reported from their double-blind, randomized clinical trial comparing Gd-DTPA and Gd-DOTA that Gd-DOTA is as safe a contrast agent as Gd-DTPA and has similar diagnostic efficacy [33].
Large-scale post-marketing surveillance of Gd-DOTA
Gd-DOTA has been marketed as a contrast agent for intravenous use for more than 20 years. There were multiple post-marketing surveillance studies including many patients for the investigation of the clinical usefulness and adverse drug reactions of Gd-DOTA.
In a German multicenter study by Maurer et al., adverse events and image quality were evaluated in a total of 84,621 patients with or without risk factors (45.4% males and 54.6% females, mean age of 52.0 ± 16.9 years) [34]. One or more risk factors were found in 22.9% of the patients, for example, allergic disposition, onset of allergic reaction in the previous imaging procedure and renal impairment. Diagnosis was possible in 99.7% of the patients. Image quality was good or excellent in 97.1% of patients. Adverse events, for example, nausea and vomiting, and urticaria, occurred in 0.34% of the patients. Most of these events were mild. Significant adverse events occurred in eight of the 84,621 patients. The incidence of these events was significantly higher in patients with a history of allergy (0.62%; p < 0.001) and patients with a history of allergic reaction to contrast agents (1.23%; p < 0.001). The authors reported that there was no increase in the onset of adverse events in patients with renal disorders.
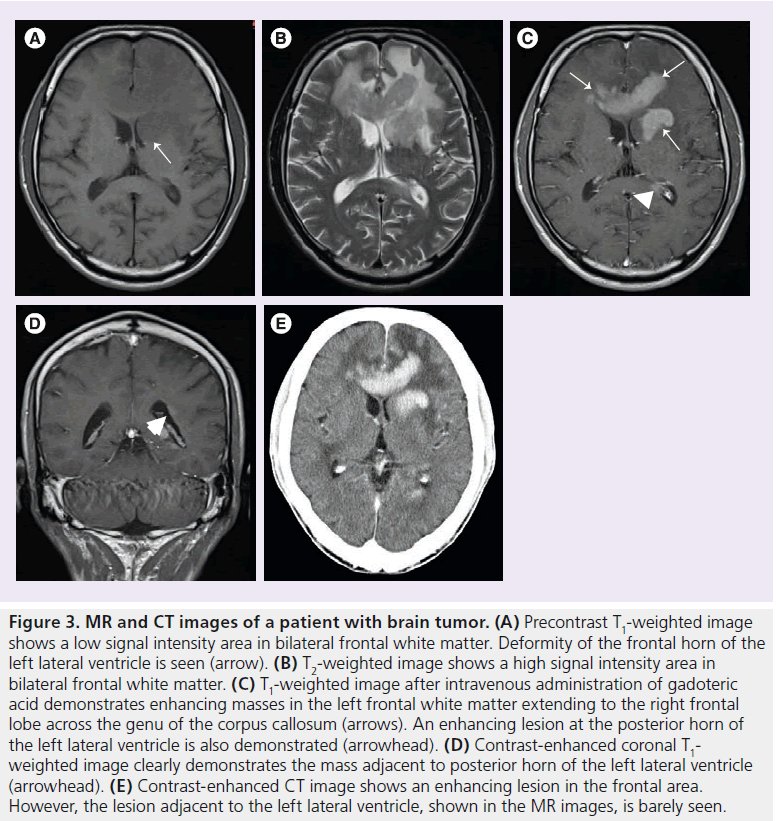
Ishiguchi et al. reported the results of a Japanese large-scale study in 3444 patients (1300 inpatients and 2144 outpatients) [35]. Gd-DOTA was ‘effective’ or ‘very effective’ in almost all patients (99.53%). There were 40 onsets of adverse events in 32 patients (incidence of all adverse events: 0.93%). Most events were gastrointestinal disorders (0.49%) and mild, with no onset of any serious event. Moderate adverse events occurred in four patients, nausea and hepatic impairment in two patients each. The authors concluded that the low incidence of adverse reactions (<1%) and the absence of serious adverse reactions reported during the survey period were consistent with a good tolerance for Gd-DOTA. The use of Gd-DOTA as an MRI-enhancing contrast medium in the clinical practice setting thus appears to be safe and effective. A representative clinical case is shown in Figure 3.
Figure 3.MR and CT images of a patient with brain tumor. (A) Precontrast T1-weighted image shows a low signal intensity area in bilateral frontal white matter. Deformity of the frontal horn of the left lateral ventricle is seen (arrow). (B) T2-weighted image shows a high signal intensity area in bilateral frontal white matter. (C) T1-weighted image after intravenous administration of gadoteric acid demonstrates enhancing masses in the left frontal white matter extending to the right frontal lobe across the genu of the corpus callosum (arrows). An enhancing lesion at the posterior horn of the left lateral ventricle is also demonstrated (arrowhead). (D) Contrast-enhanced coronal T1- weighted image clearly demonstrates the mass adjacent to posterior horn of the left lateral ventricle (arrowhead). (E) Contrast-enhanced CT image shows an enhancing lesion in the frontal area. However, the lesion adjacent to the left lateral ventricle, shown in the MR images, is barely seen
Emond et al. reported the results of the postmarketing study involving neonates and infants. One hundred and four neonates and infants, ranging in age from 3 days to 18 months, were enrolled and received one injection of Gd-DOTA (volume: 0.6–4 ml). No adverse event was reported after the Gd-DOTA injection. Image quality was rated as ‘excellent/good’ for Gd-DOTA enhanced MRI in 102 (98.0%) children. They concluded that their results suggested that immediate adverse effects were negligible following intravenous administration of Gd-DOTA in neonates and infants who were undergoing MRI on clinical indication [36].
Gd contrast agents & NSF
Many articles and warnings have recently been published concerning the association between Gd contrast agents and NSF in MRI.
In 2000, Cowper et al. reported scleromyxoedema- like cutaneous diseases in renal dialysis patients and mentioned the first case of NSF occurred in 1997 [37].
Based on a report of 25 patients experiencing NSF from the Danish Health Authority, the US FDA issued the first public health advisory in June 2006 for healthcare professionals and general public on the risk of NSF after administration of Gd contrast agents [103].
All 25 patients had serious renal dysfunction, and many of these patients underwent periodic dialysis. NSF occurred within 3 months (2 weeks to 3 months) after the dosing of Gd contrast agents, indicating for the first time the association between NSF and Gd contrast agents. This report attracted a high level of interest, leading to publications of similar announcements and reports [8,38–41,101,104].
Definition of NSF
NSF is a rare disease characterized by hyperplasia of cutaneous connective tissues. Initial symptoms are pain, pruritus, swelling and erythema, with these symptoms usually occuring in lower limbs. Thickened skin and subcutaneous tissues occurring in limbs, sometimes in the trunk, resembling a ‘woody’ texture and brawny plaques. Lesions are usually bilaterally symmetrical in limbs and the trunk, with fibrosis of internal organs, for example, muscle, diaphragm, heart, liver and lungs. Symptoms usually progress in several days to weeks, leading to contractures, such as disturbed joint mobility, and cachexia and occasional death. Experts in NSF diagnosis developed a clinicopathological diagnostic system for NSF, as described by Girardin et al. A consensus scoring system incorporating a clinical and histopathological atlas was devised to guide and standardize the evaluation and diagnosis of NSF [42].
Relationship between NSF & Gd contrast agents
The accurate mechanism of NSF onset is not yet fully understood. The following evidences have indicated the association between NSF and Gd.
There are two categories of Gd contrast agents (macrocyclic and linear ligand). Thermodynamic stability depends on the chemical structure of the Gd chelate (Table 1). In patients with renal impairment, Gd contrast agents remain in the body for long periods of time. In several studies, Gd was detected by a biopsy of skin samples from patients with impaired renal function who experienced NSF after the administration of Gd contrast agents [43,44]. It is considered from this report that the Gd ions are associated with endogenous calcium and phosphate ions, and that free Gd ions in the body deposit in the skin and tissues, leading to fibrosis [45].
In addition to the hypothesis that free Gd ion resulting from transmetallation with endogenous metal(s) might deposit in the dermis or other organs, the attraction of circulating CD34+ fibrocytes to these sites to initiate the process of fibrosis, might be another factor involved in the mechanism of NSF [9].
In addition to Gd, possible risk factors include renal disorders, inflammatory states including surgical procedures, use of erythropoietin, hypercalcemia and hyperphosphatemia. The use of contrast-enhanced MR angiography has been rapidly increasing over the years, and Gd contrast agents have been administered up to 40–60 ml, which is higher than the usual dose range. This is probably one factor leading to increased onsets of NSF [46,47].
The incidence of NSF differs among several Gd contrast agents that are currently in the market [48]. There are particularly more reports of NSF in patients treated with gadodiamide (Gd-DTPA-BMA, Onmiscan®, GE Healthcare, Lawrence, MA, USA; Omniscan, Daiichi-Snkyo Co. Ltd., Tokyo, Japan) whose thermodynamic and kinetic stabilities are low.
Regulatory positions
Based on the above since 2006, the regulatory authorities including the FDA, Medicines and Healthcare Products Regulatory Agency (MHRA), and European Medicines Agency (EMA) issued safety information, and warnings, guidelines, and manuals [103–107]. The European Society of Urogenital Radiology (ESUR) Guidelines on 2007 describes that the high-risk population includes patients with stage 4 and stage 5 (GFR < 30 ml/min) chronic kidney disease (CKD), patients on dialysis and patients with decreased renal function who have received or are waiting for liver transplantation. Based on no previous reports of NSF among patients with GFR of 60 ml/min or higher, this guideline also refers to the at-risk population including patients with stage 3 (GFR = 30–59 ml/min) CKD, and children not older than 1 year because of immature renal function. This guideline recommends that pregnant women should be regarded in a similar way to children younger than 1 year old.
The above guidelines describe that, upon the use of Gd contrast agents, the risk of NSF and the risk of not conducting Gd-based contrast imaging should be constantly compared, and that the benefits and risks of Gd-based contrast imaging should be considered with particular caution in patients with decreased renal function, patients who have undergone liver transplantation and newborns.
Recently, Wang et al. reported that among 52,954 contrast-enhanced MR examinations in conformity with their institutional restrictive GBCA guidelines, 6454 (12%) procedures were performed in patients with an estimated glomerular filtration rate of 30–59 ml/min/1.73 m2 and 36 patients with an estimated glomerular filtration rate lower than 30 ml/min/1.73 m2 underwent contrast-enhanced MRI for emergent indications. None of these patients experienced NSF [49].
The risks of NSF by various Gd contrast agents have been evaluated (Table 7). The Agency’s Committee for Medicinal Products for Human Use (CHMP) of the EMA classifies Gd contrast agents into high-, medium- and low-risk agents based on the risk of NSF [104]. Agents in the highrisk group are contraindicated in patients with severe kidney problems, patients around the time of liver transplantation and newborn babies less than 4 weeks of age. In patients with moderate kidney disorders and infants up to 1 year of age, the dose of the high-risk agents should be limited to the minimum recommended dose. It is also required that all patients should undergo a renal function test before the use of these agents.
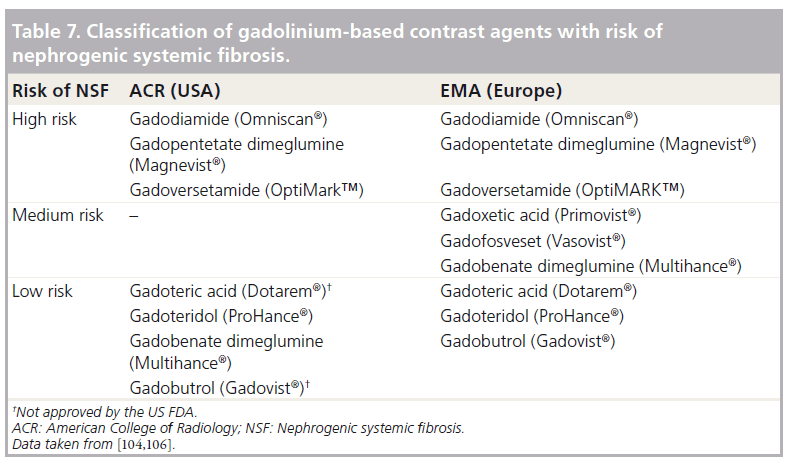
The agency advocates that the doses of medium- and low-risk agents should be limited to the minimum recommended dose in patients with severe kidney problems, patients around the time of liver transplantation, and neonates and infants up to 1 year of age, and there should be a period of at least 7 days between scans. The agency also recommends a renal function test before the use of medium- and low-risk agents in all patients.
The incidence of NSF differs among Gd contrast agents [50]. It is recommended for the reduction of NSF risk in at-risk patients that Gd contrast agents with a low risk of NSF should be used; in this context, the dose should not exceed 0.1 mmol/kg body weight. More than one dose should not be used during a scan and injections should not be repeated unless the interval between injections is at least 7 days.
Gd-DOTA & NSF
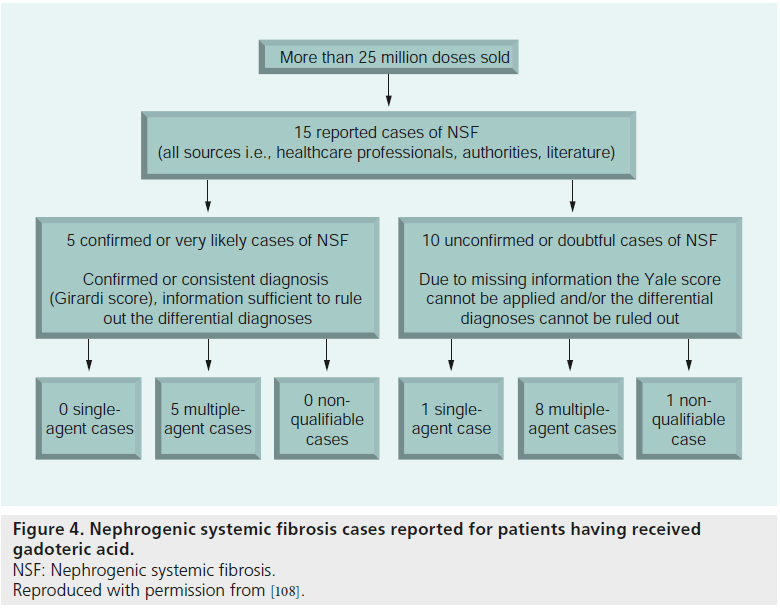
A report describes a very low incidence of NSF in patients given Gd-DOTA, which is classified in the low-risk group [108]. Among more than 25 million patients who received Gd-DOTA, 15 medically confirmed cases developed signs allowing the diagnosis of NSF. On the basis of the available information, the diagnosis of NSF is confirmed or consistent according to the Girardi score in only a third of the reported cases, and the causality of Gd-DOTA is doubtful in all cases (Figure 4). In all cases, the administration of Gd-DOTA preceded the first symptoms, except for one patient who developed NSF after injection of linear Gd chelates, the disease worsening after the patient had undergone several Gd-DOTA-enhanced MR angiography, in a context of degradation of the renal function due to graft rejection.
It is accepted that it is highly likely that Gd chelate stability is an important factor in the onset of NSF. Increased zinc excretion due to excess chelates is also regarded as an important factor in the onset of NSF. Gd chelate stability is lower for linear chelate agents, to which large amounts of excess chelates are added. By contrast, no excess chelate is added to Gd-DOTA, which has a macrocyclic molecular structure. Considering these findings, Gd-DOTA is associated with low risk of NSF.
Conclusion & future perspective
Gd-DOTA is characterized as a highly stable chelate with Gd ions. Gd-DOTA has been shown to have an overall good tolerance and an excellent efficacy in clinical practice. This contrast agent is an option for risk reduction when contrast-enhanced MRI is indicated in patients with renal dysfunction.
In the future, MRI units with higher magnetic fields (3 Tesla and more) will be popular and will make it possible to scan with higher spatial resolution in a shorter image acquisition time. CT is also a very useful diagnostic imaging modality, but the widespread use of CT will be associated with considerable radiation exposure to patients. MRI is a noninvasive imaging technique without ionizing radiation. Moreover, the Gd-based contrast agents did not seem to produce less allergic reaction than the iodine-based contrast agents used for CT. Some of the newer GBCAs, which demonstrate protein binding, are approved for use. They possess higher relaxivity, hepatobiliary excretion and prolonged residence in the bloodstream. The advantages of protein binding effects lead to either better lesion depiction and delineation and improved lesion enhancement in various applications, including in particular brain MR imaging, MR colonography and MR angiography [51–54]. Therefore, the use of MRI will be more popular as a less-invasive alternative to CT for many diseases.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: n of interest nn of considerable interest
- Sugiyama M. [Type-specific table of running MRI devices]. N. Med. Japan 38(7), 160–161 (2011).
- Yoshise S. Current situation and issues around numbers of MR units and examinations. Jap. J. Magnet. Reson. Med. 30(3), 91–99 (2010).
- Ruehm SG, Goyen M, Barkhausen J et al. Rapid magnetic resonance angiography for detection of atherosclerosis. Lancet 357(9262), 1086–1091 (2001).
- Claussen C, Laniado M, Schorner W et al. Gadolinium-DTPA in MR imaging of glioblastomas and intracranial metastases. AJNR 6, 669–674 (1985).
- Healy ME, Hesselink JR, Press GA et al. Increased detection of intracranial metastases with intravenous Gd-DTPA. Radiology 165, 619–624 (1987).
- Meaney JF, Weg JG, Chenevert TL et al. Diagnosis of pulmonary embolism with magnetic resonance angiography. N. Engl. J. Med. 336, 1422–1427 (1997).
- Semelka RC, Helmberger TK. Contrast agents for MR imaging of the liver. Radiology 218, 27–38 (2001).
- FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. US Food Drug Adm. (2010).
- Morcos SK. Nephrogenic systemic fibrosis following the administration of extracellular gadolinium based contrast agents: is the stability of the contrast agent molecule an important factor in the pathogenesis of this condition? Br. J. Radiol. 80, 73–76 (2007).
- Abraham JL, Thakral C, Skov L et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br. J. Dermatol. 158, 273–280 (2008). & Demonstrates the release of the toxic Gd3+ from gadolinium (Gd)-based contrast agents (GBCAs) in vivo, and its retention in apatite-like deposits.
- Port M, Idee JM, Medina C et al. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometal 21, 469–490 (2008). & Describes the structure–physicochemical properties relationships of marketed Gd chelates with regard to their biological consequences.
- Idee JM, Port M, Raynal I et al. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam. Clin. Pharmacol. 20, 563–576 (2006).
- Kirchin MA, Runge VM. Contrast agents for magnetic resonance imaging: safety update. Top Magn. Reson. Imaging 14, 426–435 (2003).
- Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol. Imaging 1, 128–137 (2006).
- Port M, Idee JM, Medina C et al. Stability of gadolinium chelates and their biological consequences: new data and some comments. Br. J. Radiol. 81, 258–259 (2008).
- Allard M, Doucet D, Kien P et al. Experimental study of DOTA-gadolinium: pharmacokinetics and pharmacologic properties. Invest. Radiol. 23, S271–S274 (1988).
- Rohrer M, Bauer H, Mintorovitch J et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest. Radiol. 40, 715–724 (2005).
- Bousquet JC, Saini S, Stark DD et al. Gd-DOTA: characterization of a new paramagnetic complex. Radiology 166, 693–698 (1988). & Describes the relaxivity, biodistribution and toxicity of gadoteric acid in detail.
- Matsuyama M, Ota Y, Nezu H et al. Phase I clinical study of EK-5504 (Gd-DOTA) as contrast agent for MR imaging. Med. Consul. N. Remedies 31, 513–521 (1994).
- Chachuat A, Molinier P, Bonnemain B et al. Pharmacokinetics and tolerance of Gd-DOTA (DOTAREM) in healthy volunteers and in patients with chronic renal failure. Eur. Radiol. 2, 326–329 (1992). & Describes pharmacokinetics and tolerance of gadoteric acid in detail.
- Tanimoto A, Hiramatsu K, Miyasaka K et al. [Phase II clinical study of EK-5504 (meglumine gadoterate), a MRI contrast agent with a macroring structure, in nuclear magnetic resonance computed tomography]. Med. Consult. N. Remedies 33, 571–590 (1996).
- Tanimoto A, Hiramatsu K, Miyasaka K et al. [Phase III clinical trial of meglumine gadoterate (EK-5504) contrast agent for magnetic resonance computer tomography: multicentre controlled clinical trial in comparison with gadopentetate dimeglumine]. Med. Consult. N. Remedies 33, 1367–1389 (1996).
- Akiba H, Tamagawa M, Morita K. [Phase III clinical study of MRI contrast agent EK-5504: efficacy and safety in hepatic and uterine lesions]. Med. Consul. N. Remedies 33, 21–31 (1996).
- Kawamura Y, Ishii Y. [Clinical usefulness of MRI contrast media Gd-DOTA (EK-5504)]. Med. Consult. N. Remedies 33, 625–635 (1996).
- Seo Y, Fukuoka S, Nakagawara J et al. Phase III clinical study of EK-5504 (meglumine gadoterate) as a contrast agent for MR imaging. Med. Consul. N. Remedies 33, 649–655 (1996).
- Itagane H, Haze K, Oda J et al. [Detection of acute myocardial infarction using enhanced magnetic resonance imaging with Gd-DOTA.] Med. Consul. N. Remedies 33, 657–665 (1996).
- Gabata T, Kadoya M, Takashima T. [Evaluation of effectiveness and safety of EK-5504 in cases of hepatic tumor]. Med. Consul. N. Remedies 33, 667–676 (1996).
- Sugihara S, Suto Y, Kamba M et al. [Phase III clinical study of contrast media Gd-DOTA: evaluation of effectiveness and safety for liver tumors]. Med. Consultation N. Remedies 33, 677–686 (1996).
- Ito K, Togashi K, Konishi J et al. [Phase III clinical trial of contrast media EK-5504 for MRI: evaluation in liver and pelvic region]. Med. Consult. N. Remedies 33, 705–714 (1996).
- Murakami T, Tsuda K, Kim T et al. The result of the Phase III clinical study of EK-5504 (Gd-DOTA): the Gd-DOTA enhanced magnetic resonance imaging: the evaluation of the syringe kit of EK-5504. Med. Consul. N. Remedies 33, 727–741 (1996).
- Gomi T, Hiramatsu Y, Hiramatsu K. [Phase III clinical trial of contrast media EK-5504 for MRI: application for pelvic region]. Med. Consult. N. Remedies 33, 771–775 (1996).
- Kaji Y, Sugimura K. [Phase III clinical trial of contrast media EK-5504 for MRI: application to disease of pelvic organ]. Med. Consult. N. Remedies 33, 777–784 (1996) (In Japanese).
- Oudkerk M, Sijens P, Edwin JR et al. Safety and efficacy of dotarem (Gd-DOTA) versus Magnevist (Gd-DTPA) in magnetic resonance imaging of the central nervous system. Inv. Radiol. 30, 75–78 (1995).
- Maurer M, Heine O, Wolf M et al. Tolerability and diagnostic value of gadoteric acid in the general population and in patients with risk factors: results in more than 84,000 patients. Eur. J. Radiol. 81(5), 885–890 (2012).
- Ishiguchi T, Takahashi S. Safety of gadoterate meglumine (Gd-DOTA) as a contrast agent for magnetic resonance imaging: results of a post-marketing surveillance study in Japan. Drugs R D 10(3), 133–145 (2010).
- Emond S, Brunelle F. Gd-DOTA administration at MRI in children younger than 18 months of age: immediate adverse reactions. Pediatr. Radiol. 41(11), 1401–1406.
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedemalike cutaneous diseases in renal-dialysis patients. Lancet 356, 1000–1001 (2000).
- Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): a late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging 7, 130–137 (2007).
- DeHoratius DM, Cowper SE. Nephrogenic systemic fibrosis: an emerging threat among renal patients. Semin. Dial. 19, 191–194 (2006).
- Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr. Opin. Rheumatol. 15, 785–790 (2003).
- Scheinfeld N. Nephrogenic fibrosing dermopathy: a comprehensive review for the dermatologist. Am. J. Clin. Dermatol. 7, 237–247 (2006).
- Girardi M, Kay J, Elston DM, LeBoit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J. Am. Acad. Dermatol. 65, 1095–1106 (2011).
- Abraham JL, Thakral C. Tissue distribution and kinetics of gadolinium and nephrogenic systemic fibrosis. Eur. J. Radiol. 66, 200–207 (2008).
- High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J. Am. Acad. Dermatol. 56, 21–26 (2007).
- Abraham JL, Chandrab S, Thakrala C et al. SIMS imaging of gadolinium isotopes in tissue from nephrogenic systemic fibrosis patients: release of free Gd from magnetic resonance imaging (MRI) contrast agents. Appl. Surf. Sci. 255, 1181–1184 (2008).
- Kallen AJ, Jhung MA, Cheng S et al. Gadolinium-containing magnetic resonance imaging contrast and nephrogenic systemic fibrosis: a case-control study. Am. J. Kidney Dis. 51, 966–975 (2008).
- Prince MR, Zhang HL, Roditi GH et al. Risk factors for NSF: a literature review. J. Magn. Reson. Imaging 30, 1298–1308 (2009).
- Penfield JG, Reilly RF. Nephrogenic systemic fibrosis risk: is there a difference between gadolinium-based contrast agents? Semin. Dial. 21, 129–134 (2008).
- Wang Y, Alkasab TK, Narin O et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology 260, 105–111 (2011). & Reports that no new cases of nephrogenic systemic fibrosis were identified after the adoption of restrictive Gd-based contrast agent administration guidelines.
- Perazella MA, Reilly RF. Imaging patients with kidney disease: how do we approach contrast-related toxicity ? Am. J. Med. Sci. 341, 215–221(2011).
- Cavagna FM, Maggioni F, Castelli PM et al. Gadolinium chelates with weak binding to serum proteins. Invest. Radiol. 32, 780–796 (1997).
- Knopp MV, Schoenberg SO, Rehm C et al. Assessment of gadobenate dimeglumine (Gd-BOPTA) for MR angiography: Phase I studies. Invest. Radiol. 37, 706–715 (2003).
- Knopp MV, Runge VM, Essig M et al. Primary and secondary brain tumors at MR imaging: bicentric intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology 230, 55–64 (2004).
- Knopp MV, Giesel F, Tengg-Kobligk H et al. 3D MR colonography after intravenous administration of the hepatobiliary contrast agent Gd-BOPTA: bile tagging. Eur. Radiol. Suppl. 14(Suppl. 7), O80–O83 (2004).