Research Article - Journal of Experimental Stroke & Translational Medicine (2018) Volume 11, Issue 1
Pharmacological Modulation of Autophagy for Neuroprotection in Ischaemic Stroke
- *Corresponding Author:
- Dr Ruoli Chen
School of Pharmacy, Institution for Science and Technology in Medicine, Keele University
Staffordshire ST5 5BG, UK
Tel: 01782733849
E-mail: r.chen@keele.ac.uk
Article Citation: Alex Gunn, Ayesha Singh, Aipo Diao, Ruoli Chen. Pharmacological Modulation of Autophagy for Neuroprotection in Ischaemic Stroke. J Exp Stroke Transl Med. 2018 December. Online access at www.jestm.com
Abstract
Autophagy is a highly regulated, catabolic process, through which macromolecules such as damaged organelles and proteins are degraded and recycled to maintain cellular homeostasis. Various studies have shown the role of autophagy activation in the brain cells such as astrocytes, microglia, neurons and capillary endothelial cells upon an ischaemic insult. The underlying mechanism and the role of autophagy in ischaemic stroke, however, are yet to be fully elucidated. Recent studies have suggested that insufficient or excessive autophagy results in nerve damage and cell death whereas mild/moderate autophagy has a neuroprotective effect. It has been proposed that autophagy may be a therapeutic target in stroke treatment; however, there is a lot of debate as to whether induction or diminution of autophagy plays a role in neuronal survival after cerebral ischaemia and indicate that it has a dual role depending on the time of induction of autophagy. This review has summarized the role of autophagy in ischaemic stroke and explored effects of pharmacological autophagy modulators in ischaemic stroke treatment. Further studies are needed for translating the potential therapeutic approach in stroke treatment aiming at characterizing the timing, amount and specificity of the autophagy modulation.
Keywords
Ischaemia, Stroke, Neuroplasticity, Autophagy, Neuroprotective
Introduction
A stroke is defined as the sudden onset of focal neurological deficit, and is a major cause of morbidity and mortality around the world with around 17 million incidences of first-time stroke reported in 2010 (Mozaffarian et al., 2016). In 2013, stroke was the second leading cause of death worldwide accounting for 12% of all deaths and it was the third leading cause of disability (Mozaffarian et al., 2016). In the UK, stroke is a major cause of disability as about two-thirds of stroke-survivors are discharged with a disability. Around 140,000 strokes occur in the UK each year and there are nearly 60,000 deaths due to stroke every year in the UK (Stroke Association UK, 2017). Stroke leads to an increase in economic burden, costing the NHS UK about £9 billion per annum (Stroke Association UK, 2017). Stroke can be classified into haemorrhagic or ischaemic stroke (Plaza-Zabala et al., 2017). 85% of all strokes are ischemic; resulting from an occlusion of the cerebral artery leading to the deprivation of oxygen and nutrients in a brain region (Wang et al., 2015). As a result, ischaemic stroke results in neuronal injury and death that can lead to severe disability and neurological impairment. Haemorrhagic strokes are less common and are defined as a rupture of blood vessels within the brain, leading to either an intraparenchymal, subarachnoid or intraventricular bleed (Li H et al., 2018).
In ischemic stroke, neuronal damage results from multiple forms of cell death, such as necrosis, apoptosis and autophagy, and various studies have identified links between the three. Autophagy and apoptosis are affected by the regulatory pathway phosphatidylinositol 3-phosphate (PI3P)/Akt. Apoptosis is suppressed by the PI3K signalling of downstream proteins, including mTOR (mechanistic target of rapamycin) a key regulator of autophagy (Li H et al., 2018). Beclin binding proteins (Bcl-2) is a protein found in mitochondria that is primarily anti-apoptotic and shown to inhibit cell death. Bcl-2 has also been identified as an anti-autophagic signalling molecule, through sustaining Beclin 1. Some members of the Bcl-2 family are prodeath proteins believed to result in disruption of the cell membrane that causes mitochondrial death. It is theorised that neuroprotection may be afforded by the inhibition of prodeath Bcl-2 proteins and the promotion of prosurvival proteins. However, studies have shown that the knockdown of these proteins was not capable of providing sufficient neuroprotection (Vosler et al., 2009). Beclin 1 may also be cleaved by caspases in apoptosis, so the pro-autophagic activity of Beclin is destroyed. Additionally, this cleavage results in amplification of pro-apoptotic signalling, induced widespread cell death, showing again that there is a lot of crosstalk between apoptosis and autophagy signalling (Kang et al., 2011).
Recently, studies have found autophagy to be a key regulatory factor in ischemic stroke, unveiling a new range of potential therapeutic targets for neuroprotection (Papadakis et al., 2013). Nevertheless, the full mechanism has not yet been construed, hence, there is still controversy over whether autophagy is neuroprotective or pro-death under hypoxia (Wang et al., 2018a). The aim of this review article is to elucidate the role of autophagy in ischaemic stroke and explore pharmacological modulation of autophagy as therapeutic use for neuroprotection in ischaemic stroke.
Autophagy and its Regulators
Autophagy is a well-conserved mechanism that is activated in response to nutrient deprivation and is a highly regulated process for degradation of macromolecules via the lysosomal system (Wang P et al., 2012). There are three kinds of autophagy: microautophagy, chaperone-mediated autophagy and macroautophagy which is being the most well studied (Wang et al., 2018a). One function of autophagy is to promote cell survival either by eradicating the cells of damaged organelles or toxic pathogens or by regenerating metabolites for energy and growth during nutrient-deprivation (Kim et al., 2018). However, it is important to consider that excess and prolonged autophagy may also promote cell death by excessive self-digestion and degradation of essential cellular component (Wang P et al., 2012). Until recent studies, it was believed that autophagy was a non-selective process, however, there is a high level of selectivity involve. Autophagic receptors recognise specific cargo, making degradation selective of specific substrates (Wang et al., 2018a). A variation of autophagic receptors is required to attain selectivity, by acting as a bridge between autophagosome and substrate (Plaza-Zabala et al., 2017).
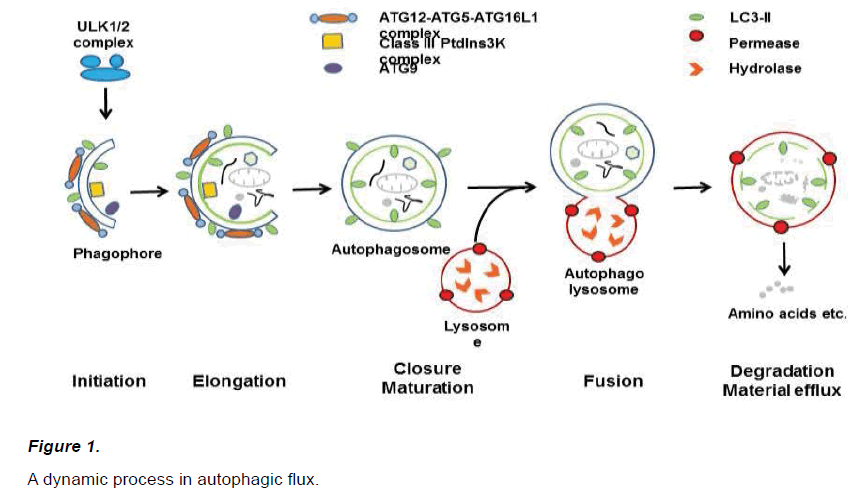
Autophagy is regulated by a complex network of interaction between varieties of molecules. There are more than 30 ATG (autophagyrelated genes) proteins that have been identified in yeast, and the majority of which are conserved in mammals (Wang et al., 2018a). As shown in (Figure 1), initially, phagophore are formed, which elongate to form autophagosomes. An autophagosome is a double-membrane vesicle that envelops unnecessary or dysfunctional proteins, before fusing with a lysosome to form an autolysosome, allowing cellular components to be digested (Chu CT, 2018).
Nucleation of the phagophore occurs following induction by the ULK1/2 complex. Elongation of the phagophore is aided by the ATG12– ATG5-ATG16L1 complex, the class III PtdIns3K complex, LC3-II, and ATG9. Eventually, the expanding membrane closes around its cargo to form an autophagosome which is a double-membrane vesicle that envelops unnecessary macromolecules, and LC3-II is cleaved from the outer membrane of this structure. The outer membrane of the autophagosome will then fuse with the lysosomal membrane to form an autolysosome to allow digestion of the cellular material. The contents of the autolysosome are then degraded and exported back into the cytoplasm for reuse by the cell.
The signalling involved with autophagy is complex and vast, with many contributing and interlinking regulatory proteins. The main regulator of autophagy is PI3K/AKT signalling pathway, which may enhance cell survival. In this pathway, Akt phosphorylates mTOR to suppress autophagy (Li H et al., 2018). There are two main proteins that sense external stimuli for autophagy, AMPK (Adenine monophosphateactivated protein kinase) and mTORC1 (mammalian target of rapamycin complex 1). mTOR is one of the downstream proteins affected by ischemia and has an important role in determining a cells death or survival response (Perez-Alvarez et al., 2018). This gives mTOR great potential as a target for improving response to ischaemic stroke. mTOR is composed of several domains; C-terminal FAT domain (FATC), C-terminal kinase domain (KD), rapamycin binding domain (FRB), transactivation /transformation- associated domain (FAT) and an N-terminal domain. mTOR has two forms (mTORc1 and mTORc2) with differing rapamycin sensitivities dependant on FKBP12 binding. Kinase activity of mTORc1 is blocked be FKBP12, whereas mTORc2 has no affinity for the FKBP12-rapamycin complex. mTORc1 is considered to be more tightly regulated than mTORc2 and is most involved in autophagy control, although mTORc2 is less well characterised (Perez-Alvarez et al., 2018). mTOR and AMPK work in tandem to closely regulate several factors responsible for initiation of autophagy, such as ULK1, VPS34 and autophagy/Beclin 1 regulator 1 (AMBRA1) (Kim and Guan, 2015). AMPK is a nutrient sensor and mediator of autophagy in response to ischemia (Ma et al., 2015). When levels of AMP are increased (such as in ischaemic conditions where energy is limited) AMPK is activated by phosphorylation. This inhibits mTORC1, resulting in activation of autophagy, as mTORc1 has an inhibitory effect on autophagy through several regulatory factors (Yang L et al., 2017; Li H et al., 2018; Perez-Alvarez et al., 2018). By inhibition of mTORc1, ULK-1 is activated and beclin-1 complex recruited to catalyse the formation of PI3P. PI3P is crucial for the formation of the phagophore (Plaza-Zabala et al., 2017). It has been reported that direct activation of AMPK increases ischemic preconditioning neuroprotection and that AMPK signalling during ischemia has a neuroprotective role, through facilitating autophagy. There is, however, some concern over the effect that AMPK has on fatty acid metabolism and glucose oxidation (Ma et al., 2015; Wang et al., 2018). mTOR has been found to have a strong inverse relationship with autophagy, as in any conditions where there is lack of nutrients or increased cell stress, autophagy is induced and mTOR is inhibited (Kim and Guan, 2015). mTOR has been found to maintain the expression of essential factors during hypoxic stress, by cap-independent translational control of HIF-1α, VEGF and IGF2 that occurs without phosphorylation of mTOR (Perez-Alvarez et al., 2018). It can be so deduced that mTOR is a key factor that affects a body's endogenous response to hypoxic stress. Furthermore, the critical role of mTORc1 in the regulation of autophagy is given more significance, by the knowledge that mTORc1 is unregulated in later stages of autophagy (Kim and Guan, 2015). In some studies, the role of mTOR has been shown to be neuroprotective in ischemia (Papadakis et al., 2013; Wu et al., 2017), however, inhibition of mTOR also promoted protective mechanisms making this a contentious issue (Perez-Alvarez et al., 2018). Activation of mTORc1, through the silencing of TSC-1, was found to aggravate neuronal injury through the inhibition of autophagy. On the other hand, downregulation of TSC-1 can prevent overexpression of autophagy and block autophagic flux, thereby promoting cell survival (Plaza-Zabala et al., 2017).
Hypoxia-inducible factor (HIF) is a crucial transcription factor in the endogenous response to hypoxic stress and is involved in the upregulation of hundreds of human genes that code for various cellular processes such as angiogenesis, erythropoiesis, cell proliferation, vasomotor control, bronchodilation and energy metabolism (Chan et al., 2016). HIF- transcription complex is heterodimeric consisting of α and β subunit. The HIF-α subunit is regulated by oxygen-dependent enzyme propyl-4-hydroxylase (PHDs) (Chen et al., 2012). Under normoxia proline residue in HIF-1α is hydroxylated by PHD allowing it to bind to VHL (von Hippel-Lindau) resulting in polyubiquitination and proteasomal degradation. This leads to polyubiquitination of HIF-1α, exposing it to proteasomal degradation (Tanimoto K, 2000). During hypoxic conditions, PHD activity is inhibited resulting into stabilisation and accumulation of HIF-1α which dimerizes with HIF-1β in the nucleus forming HIF-1 transcription factor that binds to hypoxiaresponse element (HRE), targeting genes that code for various cellular processes such glucose transporter-1, phosphofructokinase, aldolase, pyruvate kinase, lactate dehydrogenase and erythropoietin (Kenneth and Rocha., 2008). To date, there has been research conducted to implicate both beneficial and adverse effects of the promotion of HIF-1 during ischaemia. Despite the above indicated beneficial effects of neuroprotection, some studies have shown that HIF-1 may mediate apoptosis during hypoxia and could result in increased blood bran barrier (BBB) permeability and oedema ( Zhang et al., 2016).
Recent focus has shifted to studying the role of HIF-1α regulated Bnip3 (Bcl2/adenovirus e1b-interacting protein 3) induced autophagy or apoptosis (Niu et al., 2018). Bnip3 may induce autophagy through dissociating beclin 1 (crucial for phagophore induction) from Bcl-2 by competitively binding Bcl-2 or by inhibition of Rheb and mTOR (Li H et al., 2018). This leads to upregulation of mitophagy and removal of damaged mitochondria, thereby reducing reactive oxygen species (ROS) produced during hypoxia (Gong et al., 2014). HIF-1α may also stabilise p53 thereby affecting autophagy (Li H et al., 2018). When looking within mitochondria, mitochondrial ROS results in a HIF-1α induced mitophagy that reduces mitochondria mass, O2 demand and generation of ROS (Forrester et al., 2018). It is possible that this mechanism may be able to improve the lifespan of cells during hypoxic stress. Due to the inhibition of mitophagy causing increased neuronal injury, it has been established that mitophagy plays an important role in neuroprotection under cerebral ischaemia (Wang P et al., 2012). Overproduction of ROS in the mitochondria during ischaemia results in damage to proteins, nucleic acids and lipids that eventually cause cellular injury and death (Wang P et al., 2012). The production of ROS during hypoxia (Chen RL et al., 2018), drives the production of HIF-1α mediated GLUT-1 and triggers glycolysis (Forrester et al., 2018). This shifts the cell from performing oxygen dependent oxidative phosphorylation to the oxygen-independent glycolysis, enabling continued ATP production during ischemia. ROS may also regulate the activity of AMPK, through alteration in the cells AMP/ATP ratio to favour AMPK activity. Hence, autophagic activity may be controlled by ROS (Forrester et al., 2018).
HIF-1α is just one of several factors involved in the transcription of apelin (a key regulator of autophagy) and its receptor. During hypoxia, HIF-1α binds the HRE of APLNR genes to upregulate apelin and APLNR expression. Therefore, under hypoxic conditions expression of apelin is increased (Wu et al., 2017). Although, the expression of apelin has been found to be promoted in the early stages of hypoxia, in the later stages the expression is reduced. This is thought to be regulated by several pathways including endoplasmic reticulum stress and the activation of STAT3 that occurs in the reperfusion phase of ischemic stroke. This variation of apelin expression at different times in ischemia may affect when the promotion of apelin could be therapeutically beneficial (Wu et al., 2017). Apelin is thought to improve the response to ischemic stroke, through reduced oxidative stress, reduced inflammation, and inhibition of apoptosis and regulation of autophagy (Wu et al., 2017). Apelin has a dual regulatory effect on autophagy that is believed to moderate it to stay within a favourable range. During ischemia, apelin promotes autophagy through the AMPK-mTORULK1 pathway to have a protective effect. Whereas in reperfusion, apelin prevents excessive autophagy through the Akt-Bcl2- Beclin1 pathway (Ma et al, 2015). This makes apelin a crucial regulator in the endogenous control of autophagy levels and an interesting potential target for preconditioning in ischemic stroke.
Autophagy in Ischaemic Stroke
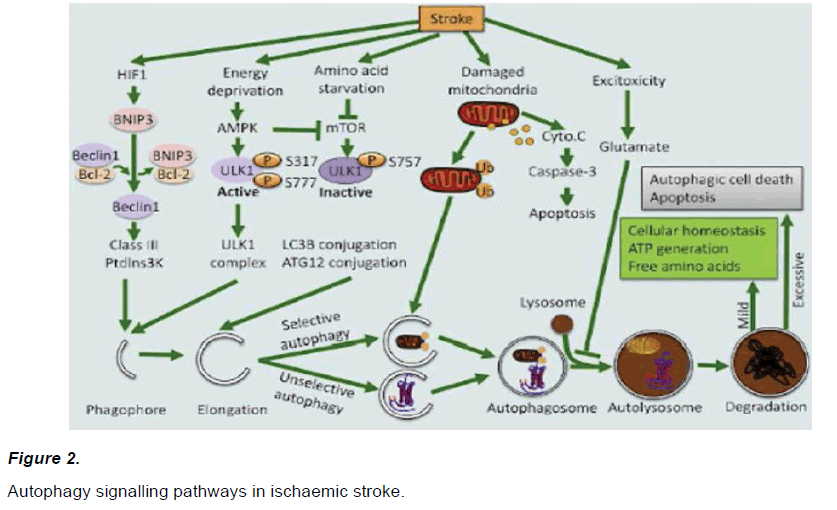
The role of autophagy during ischaemia has been controversial. While some studies indicated that autophagy induces cell death during hypoxia (Azad et al, 2008; Zhang et al, 2009), studies have also reported that autophagy induced cell survival (Zhang et al, 2017). Studies have suggested that autophagy may protect neurons through degradation of damaged organelles, preventing apoptosis or delaying ionic imbalance to stop ensuing necrosis (Wei et al., 2012). Recent studies demonstrating autophagy to have a neuroprotective role in both oxygen-glucose deprivation (OGD) models in vitro and ischemic model in vivo (Wang et al., 2018a). Autophagy has been found to have a protective role in reperfusion post-ischaemic injury, acting as a pathological self-defence mechanism. This may partly be due to the role of mitophagy (the recognition and removal of dysfunctional mitochondria through autophagy-mediated degradation) in preventing downstream apoptosis (Yang L et al., 2017; Plaza-Zabala et al., 2017; Li H et al., 2018). Studies have suggested that the detrimental effects of autophagy may be the result of the inhibition of Bcl-2 during Beclin 1 mediated autophagy (Wei et al., 2012; Plaza-Zabala et al., 2017). Previously studies suggested that BNIP3 plays a detrimental effect during autophagy, however, more recent studies suggest that BNIP3 plays an important role in neuroprotection induced by ischaemic preconditioning (Yan et al, 2013). Some theories underlying Bnip3 induced autophagy during ischaemia have been proposed, none of which have been accepted yet and further studies are still required in the area (Zhang et al, 2017). Based on recent studies, putative autophagy signalling pathways during ischaemia have emerged (Figure 2). Studies suggest that HIF-1 promotes expression of Bnip3 which can compete with Bcl-2 and promote beclin-1 release. Studies have also suggested that AMPK activation and mTOR inhibition plays an important role in activating autophagy, which then results in subsequent autophagosome and autolysosome formation resulting degradation of target molecules e.g. damaged mitochondria (Zhang et al., 2017).
Figure 2: Autophagy signalling pathways in ischaemic stroke.
Hypoxia could activate HIF, a key transcription factors of Bcl-2 and BNIP3. BNIP3 can compete with beclin-1 by binding to Bcl-2 thereby releasing beclin-1 to trigger autophagy. In addition, energy deprivation and amino acid starvation can activate AMPK and inhibit mTOR, respectively. Activated AMPK promotes phosphorylation of ULK1 resulting in its activation. mTOR inhibition suppresses the phosphorylation of Ulk1 facilitating the interaction between Ulk1 and AMPK. Moreover, activation of AMPK could inhibit the activity of mTOR. Both class III PtdIns3 K complex and ULK1 complex are essential for phagophore formation. Moreover, two ubiquitin-like conjugation systems, namely Atg12-Atg5 conjugation and LC3-phosphatidylethanolamine (PE) conjugation, are involved in phagophore elongation. In tandem, the formation of autophagosomes and autolysosome captures unselective or selective targets, such as damaged mitochondria, for degradation. Mild autophagy leads to ATP generation and free amino acid release, which are beneficial for cellular homeostasis. In contrast, excessive autophagy promotes autophagic cell death or apoptosis.
Mild autophagy resulting in cellular homeostasis and ATP generation in neuronal cells is generally considered neuroprotective, while excessive autophagy results in apoptotic/autophagic cell death (Wang et al., 2018a). Overall, it is believed that under certain conditions (e.g. tissue development and impairment of apoptosis) autophagy does mediate cell death, but in general the impact of autophagy on cell death is not substantial (Plaza-Zabala et al., 2017). Recently it has been suggesting that saving neurones from cell death is not sufficient in stroke treatment, the survival of all cell types in the neurovascular unit is crucial to maintaining neurological function. This means treatment needs to be multimodal, to ensure the survival and functioning of glia, endothelial and oligodendrocytes (Moskowitz et al., 2010).
Astrocytes are the most abundant cell type within the brain and severe many crucial functions. Autophagy was induced in astrocytes both in vitro and in vivo (Zhang et al., 2010; Qin et al., 2010), and inhibition of autophagy with 3-MA or bafilomycin A1 significantly attenuated death of astrocyte following OGD (Qin et al., 2010). A stronger autophagy under starvation conditions in primary sheep astrocytes than neurons was seen (Mura et al., 2014). Thrombin injected in caudate promoted autophagic vacuoles formation in astrocyte but not neurons (Hu et al., 2016). Gabryel et al., (2014) demonstrated that AMP-activated protein kinase was involved in induction of protective autophagy in primary rat astrocytes following OGD. Conversely, cocaine caused type II programmed cell death through autophagy in astrocytes (Cao et al., 2016).
Microglia are the primary regulators of inflammation in response to damage within the brain as well as being responsible for the phagocytosis of central nervous system debris. Phagocytosis differs from autophagy, in that it only takes place in immune cells (e.g. macrophages, microglia, dendritic cells and neutrophils) although it has recently been identified that there is much functional overlap between the two pathways. Induction of autophagy (by rapamycin or nutrient deprivation) has been found to reduce phagocytosis in macrophages, suggesting there is an inverse relationship. However, phagocytosis is not dependant on autophagy, as knockdown of autophagic genes does not inhibit phagocytic response, rather the lack of ATG-7 increases phagocytic reuptake through increased scavenger receptors on the surface (Plaza-Zabala et al., 2017). Although the primary role of microglia is to promote functional recovery of cells and neutralize harm under stress, similarly to autophagy, if this neuroprotective mechanism is sustained by unresolved damage, it may in fact, cause neurotoxicity (Plaza-Zabala et al., 2017). HIF-1α induction may cause autophagic cell death of microglia during hypoxia (Li H et al., 2018). Microglia has been demonstrated to contribute to all phases of ischaemic strokes. During ischemia, microglia abnormally phagocytoses live neurons and release of pro-inflammatory mediators that increase neuronal injury. Whereas, in the later stages of stroke microglia can promote repair through factors such as BDNF and GDNF (Moskowitz et al., 2010; Plaza-Zabal et al., 2017). In microglia, autophagy is a key for promoting cell function and survival, however, dysregulation of autophagy may be responsible for phagocytosis and inflammation. Autophagic proteins regulate phagocytosis through expression of engulfment receptors and production of autophagic enzymes that aid phagocytic degradation. It has also been established that the inflammatory response of microglia is under autophagic control. Recent data showing autophagy to negatively regulate inflammasomes in microglia (Plaza-Zabala et al., 2017).
The BBB is formed by brain capillary endothelial cells, astrocytes and pericytes. Engelhardt et al., (2015) performed a comparative study on these cells individually under hypoxia stress and found the endothelial cells had the highest responsiveness and sensitivity to hypoxia and was not able to precede autophagy compared to astrocytes and pericytes. Autophagy activation in endothelial cells in the ischaemic region was detected in mice after unilateral common artery occlusion (Adhami et al., 2006), as well as in rats following MCAO (Li et al., 2014). Rapamycin increased autophagy after OGD and reperfusion, reduced OGD and reperfusion induced cell death while 3-MA caused an opposite effect (Li et al., 2014). It was identified that where endothelial damage to the BBB has resulted from accumulation of impaired organelles and lipid droplets, autophagy was of benefit to their removal (Kim et al., 2018). In agreement, Chloroquine inhibits autophagy in rat brain endothelial cells following MCAO and significantly enhanced BBB permeability and brain oedema (Fang et al., 2015).
Pharmacological Modulation of Autophagy in Ischaemic Stroke
Inhibitors of autophagy
2-methoxyestradiol (2-ME2)
2-methoxyestradiol is a natural metabolite of estradiol, widely used as an HIF-1α inhibitor. 2-ME2 was tested in rat models of global cerebral ischaemia in which lower survival rate was seen (Zhou et al, 2008). However, a study by Xin et al., (2011) confirmed that autophagy participated in post-ischaemic neuronal injury and demonstrated that 2-ME2 exhibited a protective effect against ischaemia/ reperfusion injury induced neuronal injury. During reperfusion, the expression of Beclin 1 and LC3-II significantly reduced indicating a decrease in autophagy activation.
3-methyladenine (3-MA)
3-methyladenine is a well-known selective inhibitor of autophagy and PIK3. This was established by the reduction in levels of LC3-II and Beclin 1. Inhibition of autophagy by 3-MA has been shown to reduce neuronal cell death, infarct size and brain edema in vivo. A study showed that autophagy was activated in permanent MCAO model in rats and administration of 3-MA resulted in the reduction of infarct volume, brain edema and motor deficits (Wen et al., 2008). Inhibition of autophagy also prevented pyramidal neuron death after ischaemia (Li H et al., 2018). Administration of 3-MA to primary neurons and SH-SY5Y cell line subjected to OGD showed a reduction in cell death in comparison to control (Wang et al., 2018b). Additionally, in neonatal cerebral ischaemia, 3-MA was found to reduce injury even when administered 4 hours after ischaemia (Wei et al., 2016). In contrast, a study by Wang et al., (2018b) showed an increase in apoptosis in cells subjected to 3-MA after in both OGD and MCAO experiments. More recently, studies have suggested dual roles of autophagy inhibitor 3-MA in different stages of ischaemia. Prior to reperfusion, 3-MA triggered a higher rate of neuronal death, however, 48-72 h after reperfusion, 3-MA significantly protected neurons from death (Li H et al., 2018). 3-MA was seen to suppress neuroprotection induced by ischaemic preconditioning however, when administered before reperfusion 3-MA significantly reduced infarct size and reduced brain edema (Wang et al, 2018b). This suggests that inhibition of autophagy could play a protective role during reperfusion, as autophagy might involve in post ischaemic injuries (Xin et al., 2011). Variations in results seen between studies may also be attributed to a different effect seen dependent on the dose or route of administration used and may to be related to the accuracy of the experimental model (Wang et al., 2018b). Despite its vast use as an autophagy inhibitor; 3-MA is limited by its lack of specificity. Not only does 3-MA inhibit PI3K to thereby restrict autophagy, but it also inhibits PIK3. Hence, results cannot be simply attributed to an inhibition of autophagy. Moreover, some studies have found that 3-MA may have a stimulatory effect on autophagy if suboptimal levels are administered long-term (Li H et al., 2018).
Bafilomycin A1
Bafilomycin A1 is a specific inhibitor of vacuolar-type H+-ATPase that results in acidification of lysosomes, blocking lysosomal degradation and inhibiting autophagic flux (Plaza-Zabala et al., 2017). A study by Zhang et al (2013) revealed that autophagy was activated in cortical neurons during ischaemic-reperfusion, in both mice models of global cerebral ischaemia and OGD model. Inhibitions of autophagy by Bafilomycin A1 reinforced brain and cell injury during reperfusion. The protective role of autophagy during reperfusion may be attributable to mitophagy-related mitochondrial clearance, which may involve PARK2. However, the off-target effects of bafilomycin on mitochondria may make the effects.
Chloroquine
Chloroquine is a widely used inhibitor of autophagy, due to the alteration of lysosomal pH. Protonation of chloroquine once inside the lysosome traps it inside, so the pH is altered, inhibiting autophagic flux (Redmann et al., 2017). A study by Buckley et al, (2014) showed that chloroquine treatment reduced lesion size and improved neurological score in embolic clot MCAO models of rat, but didn’t increase survival. The study suggested autophagic induction by rapamycin was more preferential and chloroquine effect may have been by offtarget effects of chloroquine on apoptosis.
Silibinin
Silibinin is a naturally derived antioxidant, found to reduce neuronal injury after hypoxia. In permanent MCAO models of rat, Silibinin significantly alleviated neurological deficit, reduced infarct volume and suppressed brain edema (Wang L et al, 2012). It was found that the mechanism by which Silibinin achieved its neuroprotective effect through phosphorylation of PI3K/Akt-1/mTOR. Silibinin has also been found to play a role in inhibition of apoptosis and autophagy (Li Y et al, 2018).
Selenium
Selenium is currently under clinical trials for its potential benefits in ischemic stroke. Selenium pre-treatment protected neurons against ischemic damage by reducing oxidative damage, restoring mitochondrial activity and stimulating mitochondrial biogenesis. Selenium also normalized ischaemia induced activation of Beclin-1 and LC3-II (Mehta et al., 2012).
Ganglioside GM1
Ganglioside GM1, an important component of lipid raft, is particularly abundant in the brain. GM1 significantly reduced infarction volume and neuro-behavioural deficit after MCAO in rats, accompanying with reduced activation of autophagy compared to the vehicle-treated rats (Li et al., 2016).
Ginsenoside
Ginsenoside inhibits autophagy shown by downregulation of LC3-II and beclin-1 (Chen Z et al., 2010). Under hypoxic stress, Ginsenoside has been found to reduce neuronal injury and prevention of apoptosis plays an important role. HIF-1α has been linked to the signalling involved in anti-apoptotic effects of Ginsenoside. Both levels of HIF-1α and VEGF are upregulated and levels sustained longer in cells treated with Ginsenoside. Whereas, caspase-3 is downregulated (Tang et al., 2016). Recent studies suggested that Ginsenoside inhibits autophagy by blocking the autophagosome-lysosome fusion process by raising lysosomal pH and attenuating lysosomal cathepsin activity, resulting in the accumulation of the autophagosome marker LC3-II (Zheng et al., 2016).
Fingolimod (FTY720)
Fingolimod is an agonist of sphingosine-1 phosphate receptors and an approved oral treatment for multiple sclerosis. There is growing evidence that Fingolimod can reduce brain injury and enhance functional recovery following ischaemic stroke. A study by Li et al., (2017) suggested that Fingolimod effectively decreases neuronal autophagy through the mTOR/p70S6K pathways and attenuate ischaemic brain injury in mice.
L-3-n-Butylphthalide (L-NBP)
L-NBP is a clinically approved drug for treating ischaemic stroke in China (Abdoulaye and Guo, 2016). In cerebral ischemia, L-NBP improved neurological functions and reduced brain injuries in mice with repeated cerebral ischaemia/reperfusion by activating Akt/mTOR signalling resulting in inhibition of neuronal apoptosis and autophagy (Xu et al., 2017).
Promoters of Autophagy
Rapamycin
Rapamycin is a known autophagy inducer. Rapamycin positively modulates autophagy through the inhibition of mTOR - an inhibitory autophagic regulator (Plaza-Zabala et al., 2017; Li H et al., 2018). Rapamycin has also resulted in mitophagy activation, leading to mitochondrial dysfunction in an ischemic model. This was demonstrated by an increased expression of LC3-II and Beclin 1 in mitochondria (Tang et al., 2016; Li H et al., 2018). Induction of autophagy by Rapamycin shows autophagy to have a protective role under OGD conditions (Kim et al., 2018). The neuroprotective effects of rapamycin have been identified in various MCAO models with outcomes showing reductions in infarct size and improved neurological outcome. Studies have suggested that both preconditioning and post conditioning of rats subjected to MCAO with rapamycin improved neurological function, decreased infarct volume by decreasing apoptotic neurons and activating autophagy (Zeng et al., 2017; Li H et al., 2018). Studies have suggested that pre-treatment with rapamycin mimicked hyperbaric oxygen preconditioning (Li H et al., 2018). However, a study also suggested that administration of Rapamycin at the onset of reperfusion might attenuate the neuroprotective effect of ischemic post conditioning (Li H et al., 2018). There is, however, some variation in results seen when different doses and routes of administration are used in these models (Wang et al., 2018a).
Lithium chloride
Lithium chloride is an autophagy inducer that has been found to partly block the accumulation of p62 under hypoxic stress and hence reduce neuronal death (Wei et al., 2012).
Bexarotene and rosiglitazone
Bexarotene (LGD1069) is a selective retinoid X receptor (RXR) agonist currently used in treating cutaneous T-cell lymphoma. Acute administration of Bexarotene significantly reduced infarct volume and neurological deficits in mice after the MCAO. These neuroprotective effects are dependent on the activation of RXR/PPARγ (peroxisome proliferator-activated receptor γ) (Certo et al., 2015). PPAR controls the cells cytoprotective stress responses and can aid in the restoration of cellular homeostasis, hence promotion may improve the chances of cell survival (Cai et al., 2018). Rosiglitazone, an agonist of PPARγ, also has been found to have a neuroprotective effect through the inhibition of apoptosis and autophagy. Inhibition of apoptosis was demonstrated by the decreased expression of caspase 3 and increased expression of anti-apoptotic factor Bcl-2. Rosiglitazone also reduced both LC3 and Beclin 1; two indicators of autophagy, suggesting that neuroprotection may be the result of preventing overactivation of autophagy after injury (Yao et al., 2015). In microglia, Rosiglitazone treatment reduced the production of inflammatory markers (Luo et al., 2006).
Melatonin
Melatonin is a natural product of the pineal gland and is an antioxidant that has been identified as a potential inducer of autophagy. A number of studies demonstrated that melatonin has neuroprotective effects in experimental models of ischaemic stroke via multiple effects (Feng et al., 2017; Lin et al., 2018; Rancan et al., 2018). In OGD conditions, melatonin was found to promote cell survival, however, this effect was inhibited by administration of 3-MA, suggesting cell survival through autophagy (Zheng et al., 2014). In rats, melatonin ameliorated brain injuries, improved neurological score following MCAO. The neuroprotective effects were found to be through targeting the autophagy pathway by inhibiting expression of Beclin-1 and conversion of LC3, as well as activating the PI3K/Akt pro-survival pathway (Zheng et al., 2014).
Visfatin
Visfatin, an enzyme in NAD (+) biosynthesis promoted neuronal survival during cerebral ischaemia by induction of autophagy. A study suggested that Visfatin promotes neuronal survival through inducing autophagy via regulating TSC2-mTOR-S6K1 signalling pathway in a SIRT1-dependent manner during cerebral ischemia (Wang P et al., 2012).
Metformin
Recent studies have suggested that Metformin preconditioning induced autophagy by activation of AMPK, an enzyme that coordinates control of cell growth and autophagy. Metformin can be used to induce autophagy in an AMPK-dependent manner. In permanent MCAO mice, prolonged pre-treatment to 7 days of metformin (10 mg/kg) significantly ameliorated brain infarct, neurological scores and cell apoptosis. Shorter (3 days or 1 day) or without pre-treatment of metformin was not effective, suggesting a pre-treatment time window (Deng et al., 2016). In transient MCAO mice, metformin showed no neuroprotection even with pre-treatment (Deng et al., 2016). Chronic administration of metformin for 6 weeks had better neurological function and reduces infarct size post MCAO (Zhu et al., 2015). Contrary to other studies into the timing of autophagy, metformin was also found to significantly improve outcome even when given post-experimental stroke (Jia et al., 2015).
Spermidine
Spermidine is a natural polyamine presented widely in mammalian cells and has implicated to extend the lifespan of several organisms by induction of autophagy. It is suggested that Spermidine restores autophagic function through the inhibition of caspase 3 cleavage of Beclin-1 retaining Beclin-1 mediated autophagy. Studies have suggested that Spermidine plays a neuroprotective role in both acute ischaemia and during ischaemia/reperfusion (Yang Y et al., 2017).
Therapeutic Strategies and Challenges
Autophagy may be thought of as a neuronal ‘quality control’ process, and could functions to prevent rather than promote cell death (Dhaliwal et al., 2017). Applying autophagy promoters, such as rapamycin, metformin, spermidine, in preclinical stroke models consistently generate neuroprotective effects (Zeng et al., 2017; Kim et al., 2018; Deng et al., 2016; Zhu et al., 2015; Jia et al., 2015; Yang Y et al., 2017). Rapamycin is the only agent to induce autophagy chronically currently; however the “off target” effects make rapamycin unfeasible for patients vulnerable for stroke (Rubinsztein et al., 2012). There is no report of adverse effects associated with autophagy induction in in vivo, while constitutive autophagy induction may not be necessary from a therapeutic view (Rubinsztein et al., 2012). Intermittent up regulation of autophagy might be effective and associated with less adverse effects in patients (Ravikumar et al., 2006). Perez-Alvarez et al. (2018) suggested that promotion of autophagy only had a beneficial effect on outcome if it occurs before or during the ischemia. Conversely, autophagy inhibitors, such as 2-ME2 and 3-MA, worsen the ischaemic stroke outcomes (Zhou et al., 2008; Wang et al., 2018b). Nevertheless, some autophagy inhibitors such as bafilomycin A1, choloroquine, selenium, are neuroprotective towards ischemic stroke, probably due to “off-target” effects (Wang L et al., 2012; Zhang et al., 2013; Buchley et al., 2014; Redmann et al., 2017). Some studies have found that both 2-ME2 and 3MA could induce neuroprotection in preclinical ischemic stroke models when given during reperfusion as autophagy might participate in post ischemia injury (Xin et al., 2011; Wen et al., 2008; Wei et al., 2016).
Since stroke is difficult to be cured, prevention by tackling risk factors associated with stroke is advocated. Stroke occurs mostly in the elderly, partially due to ageing related changes in the brain (Chen RL et al., 2010). Autophagy is essential for keeping brain homeostasis during ageing. Reduction in autophagy can be seen by increasing mTOR activity and down regulation of ATG, reduced autophagic flux and impaired lysosome clearance, also affects microglia, increasing the pro-inflammatory response (Plaza-Zabala et al., 2017). This leads to accumulation of misfolded and dysfunctional proteins in the form of aggregates, leading to neurodegenerative diseases. Hence, there is potential for induction of autophagy being beneficial in removing these proteins to delay brain ageing and prevent occurrence of neurodegerative diseases (Perez-Alvarez et al., 2018). Diabetes is associated with higher incidence and worsen outcomes of stroke (Tuttolomondo et al., 2015), while obesity is a risk factor for stroke but is associated with reduced mortality and morbidity in stroke patients, so called ‘obesity paradox’ in stroke (Haley and Lawrence, 2016). In both obesity and diabetes, the mitochondria are overloaded metabolically, causing mitochondrial damage. Mitophagy removes dysfunctional mitochondria and eliminates this vicious cycle of oxidative stress and mitochondrial damage, and thus counteracts pathogenic processes. Autophagy also mediates exercise-induced increases in muscle glucose uptake and protects β cells against ER stress in diabetogenic conditions, and appears to be an attractive target for therapeutic interventions against obesity and diabetes (Sarparanta et al., 2017).
Although diabetes, obesity, hypertension are common in stroke, stroke incidence and subtype distribution are different among ethnicities (Feigin et al., 2014; White et al., 2005). A recent genome-wide association study was conducted on small-vessel occlusion stroke patients and discovered 3 SNPs clustered at 3p25.3 in ATG7 (encoding Autophagy Related 7), which has been implicated in autophagy (Lee et al., 2017). Nutritional genomics which allow nutritional interventions to enable gene expression, together with mitophagy, could help maintain neuron lifespan in chronic metabolic and neurodegenerative diseases (Martins IJ, 2016, 2018).
Conclusion
Although autophagy can be considered an endogenous neuroprotective pathway in response to ischemia, it has been identified that if promoted for prolonged periods or in excess it can be detrimental to neuronal survival. This is a commonly seen U- shaped response to stress (Chen G et al., 2018; Perez-Alvarez et al., 2018). Both the timing and the amount of autophagy are the key to whether this process is neuroprotective or pro-cell death. Future investigations on specific inducer of autophagy (particularly the inducer of mitophagy) would be invaluable, and could aid in discovering how promoters/inhibitors of autophagy could be used to regulate autophagy to reduce infarct size in ischemic stroke.
Competing Interests’s Statement
None.
Acknowledgments
This work was supported by research grants received from the Wellcome Trust (200633/z/16/z) and (211970/Z/18/Z).
References
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, et al. (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169: 566-583.
- Azad MB, Chen Y, Henson ES, Cizeau J, Mcmillan-Ward E, et al. (2008) Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4: 195-204.
- Buckley KM, Hess DL, Sazonova IY, Periyasamy-Thandavan S, Barrett JR, et al. (2014) Rapamycin up-regulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL and embolic MCAO murine models of stroke. Exp Transl Stroke Med 6: 8.
- Cai W, Yang T, Liu H , Han L, Zhang K, et al. (2018) Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair. Prog Neurobiol 163: 27-58.
- Cao L, Walker MP, Vaidya NK, Fu M, Kumar S, et al. (2016) Cocaine-mediated autophagy in astrocytes involves sigma 1 Receptor PI3K mTOR Atg5/7 Beclin-1 and induces type ii programed cell death. Mol Neurobiol 53: 4417-4430.
- Certo M, Endo Y, Ohta K, Sakurada S, Bagetta G, et al. (2015) Activation of RXR/PPARγ underlies neuroprotection by bexarotene in ischemic stroke. Pharmacol Res 102: 298-307.
- Chan M, James H, Martym C, Scholfied J, Ratcliffe P (2016) Pharmacological target of HIF hydroxylases-A new field in medicine development. Mol Aspects Med 47: 54-75.
- Chen G, Leak R, Sun Q, Zhang J, Chen J (2018) Neurobiology of stroke: Research progress and perspectives. Prog Neurobiol 163: 1-4.
- Chen RL, Balami J, Esiri M, Chen LH, Buchan AM (2010) Ischaemic stroke in the elderly: An overview of evidence. Nat Rev Neurology 6: 256-265
- Chen RL, Nagel S, Papadakis M, Bishop T, Pollard P, et al. (2012) Roles of individual prolyl-4-hydroxylases subtypes (PHD1-3) in cerebral ischaemia: insights from genetically modified mice. J Physiol 590: 4079-4091.
- Chen RL, Lai UH, Zhu LL, Singh A, Ahmed M, et al. (2018) Reactive oxygen species (ROS) formation in the brain at different oxygen Levels: role of hypoxia inducible factors. Frontiers cell Dev Biology6: 132.
- Chen Z, Lu T, Yue X, Wei N, Jiang Y, et al. (2010) Neuroprotective effect of ginsenoside Rb1 on glutamate-induced neurotoxicity: With emphasis on autophagy. Neurosci Lett 482: 264-268.
- Chu CT (2018). Mechanisms of selective autophagy and mitophagy: Implications for neurodegenerative diseases. Neurobiol Dis 9961: 30275-30284.
- Deng T, Zheng YR, Hou WW, Yuan Y, Shen Z, et al. (2016) Pre-stroke metformin treatment is neuroprotective involving AMPK reduction. Neurochem Res. 41: 2719-2727.
- Dhaliwal J, Trinkle-Mulcahy L, Lagace D (2017) Autophagy and adult neurogenesis: Discoveries made half a century ago yet in their infancy of being connected. Brain Plasticity 3: 99-110.
- Engelhardt S, Huang SF, Patkar S, Gassmann M, Ogunshola OO (2015). Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: a comparative study. Fluids Barriers CNS 12: 4.
- Fang L
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, et al. (2014) Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 383: 245-254.
- Feng D, Wang B, Wang L, Abraham N, Tao K, et al. (2017) Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res 62: e12395.
- Forrester S , Kikuchi D , Hernandes M , Xu Q, Griendling K (2018). Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122: 877-902.
- Gabryel B, Kost A, Kasprowska D, Liber S, Machnik G, et al. (2016) FTY720-induced enhancement of autophagy protects cells from FTY720 cytotoxicity in colorectal cancer. Oncol Rep 35: 2833-2842.
- Gong G, Hu L, Liu Y, Bai S, Dai X, et al. (2014) Upregulation of HIF-1α protein induces mitochondrial autophagy in primary cortical cell cultures through the inhibition of the mTOR pathway. Int J Mol Med 34: 1133-1140.
- Haley MJ, Lawrence CB (2017) Obesity and stroke: Can we translate from rodents to patients. Curr 13: 352-369.
- Hu S, Wu G, Ding X, Zhang Y (2016) Thrombin preferentially induces autophagy in glia cells in the rat central nervous system. Neuro sci Lett 630: 53-58.
- Jia J, Cheng J, Ni J, Zhen X (2015) Neuropharmacological actions of metformin in stroke. Current Neuropharmacol 13: 389-394.
- Kang R, Zeh H, LotzeS M, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death & Differentiation 18: 571-580.
- Kenneth NS, Rocha S (2008) Regulation of gene expression by hypoxia. J Biochemical 414: 19.
- Mosteller M, Condreay LD, Harris EC, Ambery C, Beerahee M, et al. (2014) Exploring the roles of UGT1A1 and UGT1A3 in oral clearance of GSK2190915, a 5-lipoxygenase-activating protein inhibitor. Pharmacogenet Genomics 24: 618-21.
- Kim K, Shin D, Kim J, Shin Y, Rajanikant G, et al. (2018) Role of autophagy in endothelial damage and blood–brain barrier disruption in ischemic stroke. Stroke 49: 1571-1579.
- Kim Y, Guan K (2015) mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125: 25-32.
- Lee TH, Ko TM, Chen CH, Chang YJ, Lu LS, et al. (2017) A genome-wide association study links small-vessel ischemic stroke to autophagy. Sci Rep 7: 15229.
- Li H, Gao A, Feng D, Wang Y, Zhang L, et al. (2014) Evaluation of the protective potential of brain microvascular endothelial cell autophagy on blood-brain barrier integrity during experimental cerebral ischemia-reperfusion injury. Transl Stroke Res 5: 618-26.
- Li H, Wu J, Shen H, Yao X, Liu C, et al. (2018) Autophagy in hemorrhagic stroke: Mechanisms and clinical implications. Prog Neurobiol 163: 79-97.
- Li L
- Li X, Wang M, Qin C, Fan W, Tian D, Liu J. (2017) Fingolimod suppresses neuronal autophagy through the Mtor/P70s6k pathway and alleviates ischemic brain damage in mice. Plos One 12: E0188748.
- Li Y, Li Y, Yang L, Zhang K, Zheng K, et al. (2018) Silibinin exerts antidepressant effects by improving neurogenesis through BDNF/TrkB pathway. Behav Brain Res 348: 184-191.
- Lin YW, Chen TY, Hung CY, Tai SH, Huang SY, et al. (2018) Melatonin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. Int J Mol Med 42: 182-192.
- Ma S, Wang Y, Chen Y, Cao F (2006) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochimica ET Biophysica Acta (BBA) 1852: 271-276.
- Martins IJ (2016) Early diagnosis of neuron mitochondrial dysfunction may reverse global metabolic and neurodegenerative disease. GJMR 2: 1-8.
- Wu CX, Liu R, Gao M, Zhao G, Wu S, et al. (2013) Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 546: 57-62.
- Mehta SL, Kumari S, Mendelev N, Li A (2012) Selenium preserves mitochondrial function stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci 13: 79.
- Moskowitz M, Lo E, Iadecola C (2010) The science of stroke: Mechanisms in search of treatments. Neuron 68: 161.
- Mozaffarian D (2016). Executive summary: Heart disease and stroke statistics-2016 update: A report from the American heart association. Circ 133: 447-454.
- Mura E, Lepore G, Zedda M, Giua S, Farina V (2014) Sheep primary astrocytes under starvation conditions express higher amount of LC3 II autophagy marker than neurons. Arch Ital Biol 152: 47-56.
- Niu G, Zhu D, Zhang X, Wang J, Zhao Y, et al. (2018) Role of hypoxia-inducible factors 1α (HIF1α) in SH-SY5Y cell autophagy induced by oxygen-glucose deprivation. Med Sci Monit 24: 2758-2766.
- Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, et al. (2013) Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med 19: 351-357.
- Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology mechanism and regulation. Antioxid Redox Signal 20: 460-73.
- Perez-Alvarez M, Villa Gonzalez M, Benito-Cuesta I, Wandosell F (2018) Role of mTORC1 Controlling Proteostasis after Brain Ischemia. Frontiers in Neuroscience 12.
- Plaza-Zabala A, Sierra-Torre V, Sierra A (2017) Autophagy and microglia: Novel partners in neurodegeneration and ageing. Int J Mol Sci 18: 598.
- Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, et al. (2010) Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy 6: 738-53.
- Rancan L, Paredes SD, García C, González P, Rodríguez-Bobada C, et al. (2018) Comparison of the effect of melatonin treatment before and after brain ischemic injury in the inflammatory and apoptotic response in aged rats. Int J Mol Sci 19.
- Ravi kumar B, Berger Z, Vacher C, O'Kane CJ, Rubinsztein DC (2006) Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet 15: 1209-16.
- Redmann M, Benavides G, Berryhill T, Wani W, Ouyang X, et al. (2017) Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biology 11: 73-81.
- Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11: 709-30.
- Sarparanta J, García-Macia M, Singh R (2017) Autophagy and mitochondria in obesity and type 2 diabetes. Curr Diabetes Rev 13: 352-369.
- Stroke association (2017) State of the nation-Stroke statistics 2017. United Kingdom: Stroke Association.
- Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, et al. (2012) Neuroprotection for ischaemic stroke: Translation from the bench to the bedside. Int J Stroke 7: 407-418.
- Tang B, Wang D, Li M, Wu Q, Yang Q, et al. (2016) An in vivo study of hypoxia-inducible factor-1α signaling in ginsenoside Rg1-mediated brain repair after hypoxia/ischemia brain injury. Ped. Res 81: 120-126.
- Tanimoto K (2000) Mechanism of regulation of the hypoxia-inducible factor-1alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J 19: 4298-4309.
- Tuttolomondo A, Maida C, Maugeri R, Iacopino G, Pinto A (2015) Relationship between diabetes and ischemic stroke: Analysis of diabetes- related risk factors for stroke and of specific patterns of stroke associated with diabetes mellitus. J Diabetes Metab 6: 5.
- Vosler P, Graham S, Wechsler L, Chen J (2009) Mitochondrial targets for stroke: Focusing basic science research toward development of clinically translatable therapeutics. Stroke 40: 3149-3155.
- Wang L, Wang C, Wang Z, Zhang X, Zhang X, et al. (2012) Protection by silibinin against experimental ischemic stroke: Up-regulated pakt, pmtor Hif-1α And Bcl-2 down-regulated bax, nf-Κb Expression. Neurosci Lett 529: 45-50.
- Wang P, Guan Y, Du H, Zhai Q, Su D, et al. (2012) Induction of autophagy contributes to the Neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 8: 77-87.
- Wang P, Shao B, Deng Z, Chen S, Yue Z, et al. (2018) Autophagy in ischemic stroke. Prog Neurobiol 163: 98-117.
- Wang P, Xie R, Cheng M, Sapolsky R, Ji X, et al. (2018) The mTOR cell signaling pathway is crucial to the long-term protective effects of ischemic post conditioning against stroke. Neurosci Lett 676: 58-65.
- Wang Y, Reis C, Applegate R, Stier G, Martin R, et al. (2015) Ischemic conditioning-induced endogenous brain protection: Applications pre-per-or post-stroke. Exp Neurol 272: 26-40.
- Wei K, Wang P, Miao C (2012) A double-edged sword with therapeutic potential: An updated role of autophagy in ischemic cerebral injury. CNS Neuroscience & Therapeutics 18: 879-886.
- Wei X, Zhou Z, Li L, Gu J, Wang C, et al. (2016) Intrathecal injection of 3-methyladenine reduces neuronal damage and promotes functional recovery autophagy attenuation after spinal cord ischemia/reperfusion injury in rats. Biol Pharmaceutics Bull 39: 665-673.
- White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, et al. (2005) Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111: 1327-31.
- Wu Y, Wang X, Zhou X, Cheng B, Li G, et al. (2017) Temporal expression of apelin/apelin receptor in ischemic stroke and its therapeutic potential. Frontiers Mol Neurosci 10.
- Xin X, Pan J, Wang X, Ma J, Ding J, et al. (2011) 2-Methoxyestradiol attenuates autophagy activation after global ischemia. Can J Neurol Sci 38: 631-638.
- Xu J, Huai Y, Meng N, Dong Y, Liu Z, et al. (2017) L-3-n-butylphthalide activates Akt/mTOR signaling inhibits neuronal apoptosis and autophagy and improves cognitive impairment in mice with repeated cerebral ischemia-reperfusion injury. Neurochem Res 42: 2968-2981.
- Yan Wj, Dong Hl, Xiong LZ (2013) The protective roles of autophagy in ischemic preconditioning. Acta Pharmacol Sin 34: 636-643.
- Yang L, Wang H, Shen Q, Feng L, Jin H (2017) Long non-coding RNAs involved in autophagy regulation. Cell Death & Dis 8: e3073.
- Yang Y, Chen S, Zhang Y, Lin X, Song Y, et al. (2017) Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death & Dis 8: e2738.
- Yao J, Zheng K, Zhang X (2015) Rosiglitazone exerts neuroprotective effects via the suppression of neuronal autophagy and apoptosis in the cortex following traumatic brain injury. Mol Med Rep 12: 6591-6597.
- Zhang J, Ney P (2009) Role of Bnip3 and Nix in cell death, autophagy, and Mitophagy. Cell Death & Differentiation 16: 939-946.
- Zhang X, Deguchi K, Yamashita T, Ohta Y, Shang J, et al. (2010) Temporal and spatial differences of multiple protein expression in the ischemic penumbra after transient mcao in rats. Brain Research 1343: 143-152.
- Zhang X, Yan H, Yuan Y, Gao J, Shen Z, et al. (2013) cerebral ischemia-reperfusion induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9: 1321-33.
- Zhang Z, Yan J, Shi H (2016) role of hypoxia inducible factor 1 in hyperglycaemia-exacerbated blood-brain barrier disruption in ischemic stroke. Neurobiol Dis 95: 82-92.
- Zhang ZH, Wu QY, Zheng R, Chen C, Chen Yet al. (2017) Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an alzheimer's disease mouse model. J Neurosci 37: 2449-2462.
- Zheng K, Li Y, Wang S, Wang X, Liao C, et al. (2016). Inhibition of autophagosome-lysosome fusion by ginsenoside ro via the esr2-ncf1-ros pathway sensitizes esophageal cancer cells to 5-fluorouracil-induced cell death via the chek1-mediated dna damage checkpoint. Autophagy 12: 1593-1613.
- Zheng Y, Hou J, Liu J, Yao M, Li L, et al. (2014) Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J. Pharmacol Sci 124: 354-64.
- Zhou D, Matchett G, Jadhav V, Dach N, Zhang J (2008) The effect of 2-methoxyestradiol a HIF-1αinhibitor in global cerebral ischemia in rats. Neurol Res 30: 268-271.
- Zhu XC, Jiang T, Zhang QQ, Cao L, Tan MS, et al. (2015) Chronic metformin preconditioning provides neuroprotection via suppression of nf-κb-mediated inflammatory pathway in rats with permanent cerebral ischemia. Mol Neurobiol 52: 375-85.