Review Article - Imaging in Medicine (2011) Volume 3, Issue 5
PET/CT for the diagnosis, staging and restaging of prostate cancer
Michael Souvatzoglou†1, Florian C Gaertner1, Sarah Schwarzenboeck1, Ambros J Beer1, M Schwaiger1and Bernd J Krause1,2
1Nuklearmedizinische Klinik und Poliklinik der Technischen Universität München, Ismaninger Str. 22, 81675, München, Germany
2Klinik und Poliklinik für Nuklearmedizin, Universität Rostock, Germany
- *Corresponding Author:
- Michael Souvatzoglou
Nuklearmedizinische Klinik und Poliklinik
der Technischen Universität München
Ismaninger Str. 22, 81675, München, Germany
E-mail: msouvatz@yahoo.de
Abstract
Prostate cancer is one of the most frequent cancers in men and constitutes the third most common cause of cancer deaths. Early diagnosis of primary prostate cancer, accurate staging, as well as accurate restaging in the case of cancer recurrence after primary treatment, are important for delivering the appropriate therapy. In the last ten years, functional and molecular imaging by means of PET and PET/CT are increasingly being used for such indications. This article provides a radiolabeled tracer-based review of the diagnostic value of PET and PET/CT in primary and recurrent prostate cancer. [18F]FDG, [11C]acetate, [18F]fluoride, and [18F]- and [11C]choline are reviewed in this frame with an emphasis on the radiolabeled choline derivates. PET/CT with radioactively labeled choline derivates is a promising molecular imaging technique for restaging prostate cancer patients with biochemical recurrence after definite primary therapy. However, for the diagnosis of primary prostate cancer, choline PET/CT cannot be recommended at the present time.
Keywords
[11C]choline-PET/CT; imaging of prostate cancer; PET/CT; prostate cancer; recurrence of prostate cancer
Prostate cancer is currently the most prevalent form of cancer in men (301,500 cases, 24.1% of all incident cases) and constitutes the third most common cause of cancer deaths (10.4%) in the EU [1]. Patients suffering from prostate cancer can be categorized into three groups with respect to disease onset and course of disease; patients with primary prostate cancer who have localized disease, patients with a biochemical recurrence after primary treatment and patients with advanced stages of disease
The main modalities to diagnose prostate cancer include digital rectal examination, serum concentration of prostate specific antigen (PSA) and transrectal ultrasound (TRUS)-guided biopsies [2]. For imaging patients with prostate cancer, several conventional imaging modalities are available: TRUS, CT, MRI and bone scintigraphy. Morphological imaging techniques, such as CT, MRI and TRUS, have only shown a limited accuracy for primary diagnosis of prostate cancer, recurrent disease as well as advanced disease. Concerning diagnosis of primary prostate cancer, CT is limited as intraprostatic cancer tissue does not have a different density compared with normal prostate tissue or benign prostate pathologies. The detection of lymph node metastases is limited in morphological imaging (CT and MRI) both methods rely mainly on the size criterion; however, lymph nodes smaller than 1 cm can also bear tumor metastasis. Concerning bone metastases, CT can be used for imaging trabecular changes caused by the metastasis, however, those changes appear relatively late in the course of bone metastases. Therefore, a challenge for imaging prostate cancer is to increase the diagnostic performance. Functional and molecular imaging technologies, such as PET, compared with conventional imaging, offer the possibility to image tumor-specific processes. Combined molecular and morphological imaging results in better lesion localization and characterization, corresponding to higher diagnostic accuracy. For PET and PET/CT imaging the 18F-labeled compound 2´-[18F]f luoro-2´-deoxy-d-glucose ([18F]FDG) is the most widely used tracer in oncology. PET and PET/CT with FDG have achieved a major impact in diagnostic as well as therapeutic disease management in a number of tumor entities. For the diagnosis of prostate cancer, PET and PET/CT imaging with FDG only demonstrated a limited sensitivity [3–5]. Increased FDG uptake is regularly observed only in differentiated, aggressive and metastasized prostate carcinomas. Therefore, several PET tracers for imaging prostate cancer have been introduced and evaluated in recent years, such as f luorodihydrotestosterone, acetate, methionine and radioactively labeled choline derivates [5–11]. Among these, imaging with radioactively labeled choline derivates has gained increasing importance in prostate cancer imaging.
Imaging prostate cancer with radioactively labeled choline derivates is based on increased phosphorylcholine levels and an elevated phospholipids turnover in the tumor cells. Prostate cancer cells show an increased choline turnover compared with normal nondiseased cells, as evidenced by MR spectroscopy [12,13]. More specifically radioactively labeled choline derivates are taken up in the prostate cancer cells by means of active transport (mostly a high affinity transporter) and phosphorylated by choline kinase, representing the first step in the Kennedy cycle and thereafter are incorporated in the phospholipids membrane. Besides, an increased uptake of choline in prostate cancer cells by way of a high affinity choline transport system, key enzymes of the choline metabolism, including choline kinase, are upregulated [14,15].
In addition to molecular imaging modalities, hybrid imaging techniques have been introduced in oncologic imaging in the last decade (e.g., PET/CT and SPECT/CT), that allow generation of a synergistic diagnostic value by integrating molecular and morphological information in a nearly simultaneous imaging procedure. CT in PET/CT and SPECT/CT has revealed an added value with respect to a more precise lesion localization and characterization of lesions, resulting in a higher diagnostic accuracy.
In this article we highlight the contribution of PET/CT for the diagnosis and staging of prostate cancer with an emphasis on PET/CT imaging with radioactively labeled choline derivatives in patients with biochemical recurrence. The contribution of [18F]f luoride PET/CT concerning bone metastases is being discussed briefly. Moreover, we give an outlook on current developments in imaging prostate cancer (i.e., new imaging probes and on the hybrid MR/PET technology).
Radiotracers in primary prostate cancer
[18F]FDG in primary prostate cancer
The use of [18F]FDG in oncology is based on increased dependence of cancer for energy from glycolytic metabolism and a concomitant increase in glucose transporters in cell membranes of malignant cells. [18F]FDG imaging of prostate has shown a limited efficacy for the detection of primary and metastatic prostate cancer. Earlier studies showed little difference between FDG uptake in prostate cancer and benign prostatic tissue [16]. In another study comparing FDG PET with CT, bone scintigraphy, and clinical follow-up as reference, a total of 202 bone metastases were found in 22 untreated patients, of which only 131 were detected by FDG PET [17]. The limited efficacy of [18F]FDG PET in the detection of primary prostate cancer has been attributed to a relatively slower metabolic rate with a lower expression of glucose transport proteins, as compared with other cancers. Additionally, metabolism of [18F] FDG including renal excretion which leads to activity in the ureters and the bladder obscures the target organ (prostate) and closely related adjacent tissues (seminal vesicles), hampering the detection of prostate cancer [18]. Moreover, there is a relation between differentiation of prostate cancer and FDG uptake, as low differentiated prostate cancer cells take up FDG more avidly. This might be of diagnostic significance as low grade tumors are not clinically relevant.
[11C]acetate in primary prostate cancer
[11C]acetate uptake in tumor cells is mainly related to an enhanced lipid synthesis, reflecting the high growth activity of neoplasms [19]. However, [11C]acetate is not a cancer-specific tracer and therefore also accumulates in normal and hyperplastic prostate tissue. A semiquantitative assessment of tracer uptake in the tissue often used in PET is the standardized uptake value (SUV), defined as:
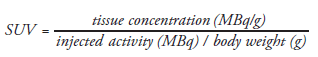
The SUV for [11C]acetate uptake was found to be higher in individuals with normal prostate tissue younger than 50 years of age than in the normal prostate of older subjects (>50 years), or those with benign prostatic hyperplasia [20]. In addition, there was no difference in prostate SUV between older subjects with normal prostate (2.3 ± 0.7) and patients with proven prostate cancer (1.9 ± 0.6).
In a comparative study evaluating 22 patients with primary prostate cancer, [11C]acetate proved to be superior to [18F]FDG [21]. A recent study accessing the utility of [11C]acetate PET/CT to image localized prostate cancer and to derive information on aggressiveness reported that the dominant lesions were not associated with prostate cancer aggressiveness as determined by histopathology, nor did PSA velocity correlate with the SUV or from the entire gland. The authors concluded that [11C]acetate PET/CT enables detection of localized prostate cancer with limited accuracy (71%) but fails to provide information on cancer aggressiveness [22].
[18F]choline & [11C]choline in primary prostate cancer
Sutinen et al. calculated kinetic parameters on the basis of graphical analysis of the dynamic uptake in the prostate within 30 min [23]. The authors reported a high correlation between the Ki‑values and SUV (r = 0.964, p = 0.0005) while there was no correlation for [11C]choline uptake in the tumor and grade of differentiation, Gleason score, volume of the prostate and PSA value. The authors demonstrated that a high [11C]choline uptake not only exists in prostate cancer but also in prostate hyperplasia, meaning there is an extensive overlap in Ki‑values and SUV values. The role of radiolabeled choline imaging for the primary detection of prostate cancer is subject to controversies. While some studies demonstrated a high sensitivity for the detection of primary prostate cancers [24–26], other studies demonstrated a lower detection rate [27–30]. For a comprehensive summary of studies on the diagnostic efficacy of [18F]choline and [11C]choline PET and PET/CT in patients with primary prostate cancer please see Table 1.
Screening for prostate cancer is possible by determining the PSA level, and was described several years ago. The gold standard for diagnosing prostate cancer is histopathological examination of prostate tissue obtained by prostate needle biopsy. However, a substantial number of men with persistently elevated PSA levels exhibit prostate needle biopsy specimens negative for prostate cancer, necessitating subsequent biopsies [31,32]. In a study evaluating the value of [18F]choline in patients with elevated PSA level and negative prostate needle biopsy, prostate cancer could be diagnosed with [18F] choline guided biopsy in 25% (five of 20) of the patients included, showing promise, as those patients had already undergone at least two previous biopsy procedures with negative results [33]. One limitation in evaluating radiolabeled choline-guided biopsies of the prostate is the biopsy procedure itself, being prone to sampling error. Additionally, prostate biopsies are usually obtained transrectally with ultrasound guidance, however a precise spatial correspondence between a focus of uptake seen on a tomographic PET/CT image and the tip of a biopsy needle, as viewed in a nontomographic 2D ultrasound image of the prostate gland, is very difficult [34].
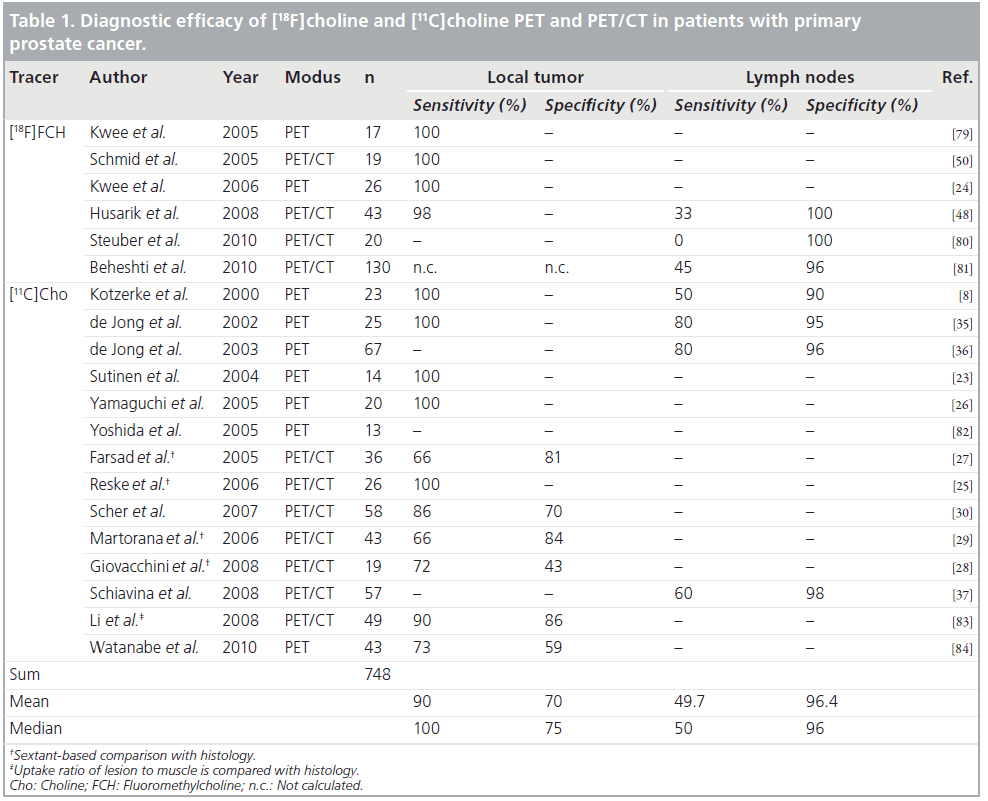
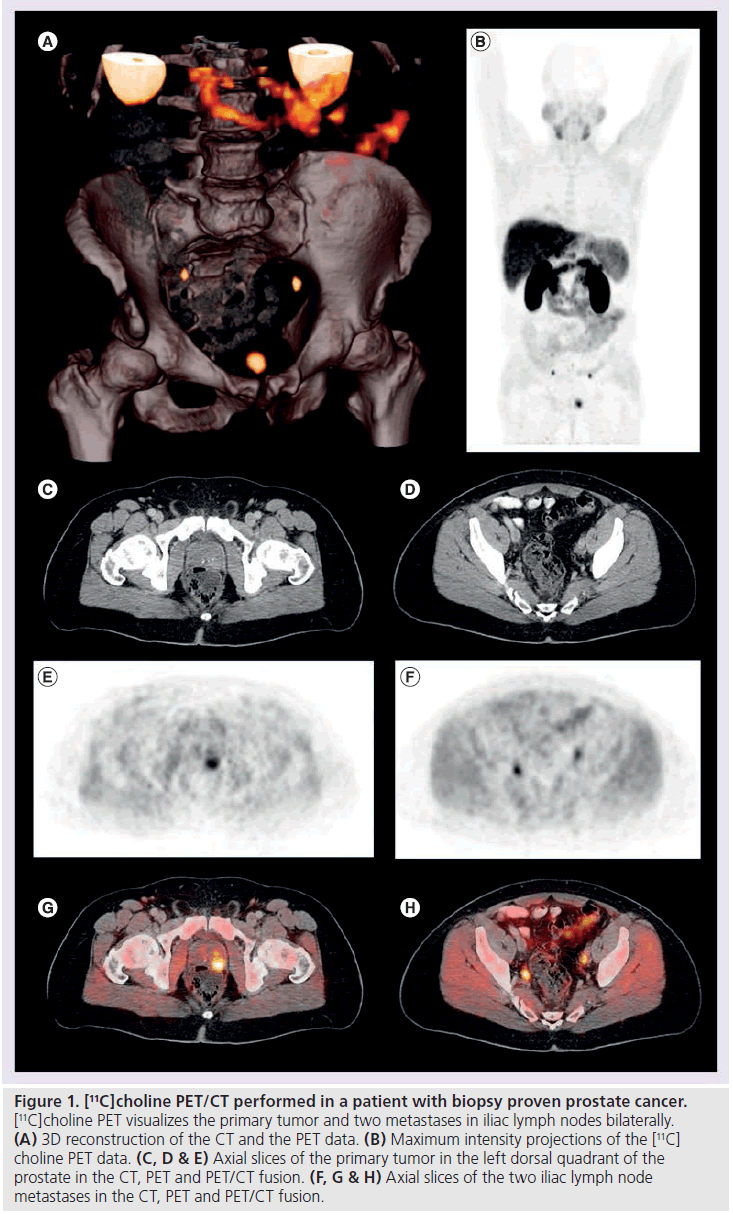
De Jong et al. demonstrated that staging for lymph node metastasis with radiolabeled choline-PET is feasible (Figure 1). [11C]choline PET identified metastatic pelvic lymph nodes with a size between 0.5 and 3 cm with a mean SUV of 4.7 (2.9–9.1). The authors report a false positive [11C]choline signal in a lymph node with inflammatory changes and 19 correct negative findings in 19 patients without lymph node metastases [35]. Kotzerke et al. found the sensitivity and specificity of [11C]choline PET to be 50 and 90%, respectively [8]. A further study by de Jong et al. explored the accuracy of [11C]choline PET in the preoperative noninvasive staging of pelvic lymph nodes in patients with prostate cancer, reporting values of 80, 96 and 93% for sensitivity, specificity and accuracy, respectively [36]. Schiavina et al. included 57 patients in a study with proven prostate cancer and an intermediate or high risk for lymph node metastases [37]. Patients underwent [11C]choline PET/CT prior to prostatectomy and extended pelvic lymph node dissection. [11C]Choline PET/CT findings were compared with two nomograms. For the detection of lymph node metastases, [11C]choline PET/CT showed a sensitivity of 60% and a specificity of 98%. There was no statistically significant difference in sensitivity and specificity between [11C]choline PET/CT and the nomograms (Briganti nomogram: 60%/ 4%; Kattan nomogram: 60%/64%). The variation of the reported sensitivities and specificities concerning nodal staging could be explained with the differences of the patient cohorts included in the respective studies (low risk vs intermediate vs high risk patients). Nomograms based on clinical data (PSA level, Gleason grade, DRE findings) provide risk stratification estimates that guide the appropriate ordering of imaging tests [38]. In this respect it is recommended that CT should be performed only in patients with a PSA level greater than 20 ng/ml, a Gleason score greater than 7 and/or clinical tumor stage T3 or higher [39]. However, lymph node staging is challenging with anatomical imaging, including conventional MRI, mainly due to the limited accuracy of morphological criteria such as size and shape [40]. Nevertheless, the current literature does not allow definite recommendations to be proposed for lymph node staging of prostate cancer with radiolabeled choline PET/CT. Recent preliminary studies evaluating functional and molecular MRI techniques for nodal staging of prostate cancer (diffusion weighted imaging, dynamic-contrastenhanced- MRI) show promising results [41,42]. However, a more detailed report on those studies is beyond the frame of this review, for more detailed information please see [40].
Summary
[18F]FDG has a limited efficacy for the detection of primary and metastatic prostate cancer. The role of [18F] and [11C] choline imaging for the primary detection of prostate cancer is controversially debated. Controversial results are reported concerning whether PET/CT can discriminate, intraprostatically, prostate cancer tissue from benign pathologies, as some studies indicate that benign prostatic hyperplasia, for example, exhibits a high tracer uptake in some cases. PET/CT might be useful for guiding biopsies in men with persistent elevated PSA values and repeated biopsies negative for prostate cancer. Furthermore, the role of PET/CT in nodal staging of primary prostate cancer is not yet clarified. It may be limited to patients grouped as high risk for metastatic disease.
Radiotracers in prostate cancer recurrence
In most patients, recurrence is suspected on the basis of an increase in the PSA level. However, treatment planning requires precise knowledge of the location and extent of the disease, and such knowledge cannot be entirely derived from biochemical data or nomograms alone. The sensitivity of CT for the detection of recurrent disease is limited, particularly when PSA levels are low [43]. The mean PSA value associated with a positive CT examination was 12.4 ng/ml and the mean PSA velocity was 30.6 ng/ml/year [44]. Radiolabeled choline PET/CT holds promise to differentiate local, regional and distant metastatic disease in recurrent prostate cancer [45–51].
[18F]FDG in prostate cancer recurrence
The limitations of [18F]FDG imaging in primary prostate cancer are similar in restaging prostate cancer when recurrence is suspected, with many studies demonstrating the limited value of [18F] FDG PET and PET/CT in this setting [3,52,53]. Schoder et al. studied [18F]FDG PET/CT for detection of disease in the setting of biochemical relapse after radical prostatectomy and demonstrated that radiopharmaceutical uptake was truly positive in 31% (28 of 91) of patients with relatively high mean PSA (9.5 ± 2.2 ng/ml). Although low PSA did not necessarily preclude [18F]FDG uptake, receiver-operating characteristic analysis determined that a PSA level of greater than 2.4 ng/ml and velocity of greater than 1.3 ng/ml/year was useful for deciding whether or not to use [18F]FDG PET/CT. They emphasized the concept of improving the likelihood of disease by incorporating aspects such as tumor burden and tumor biology combined with PSA levels [54].
Figure 1. [11C]choline PET/CT performed in a patient with biopsy proven prostate cancer. [11C]choline PET visualizes the primary tumor and two metastases in iliac lymph nodes bilaterally. (A) 3D reconstruction of the CT and the PET data. (B) Maximum intensity projections of the [11C] choline PET data. (C, D & E) Axial slices of the primary tumor in the left dorsal quadrant of the prostate in the CT, PET and PET/CT fusion. (F, G & H) Axial slices of the two iliac lymph node metastases in the CT, PET and PET/CT fusion.
[11C]acetate in prostate cancer recurrence
The value of [11C]acetate in patients with biochemical relapse of prostate cancer has been addressed in several studies [6,11,55–58]. Oyama and coworkers studied 46 patients with rising PSA after prostatectomy or primary radiation therapy [55]. All patients underwent PET with [11C]acetate and FDG (furosemide and bladder catheter for the latter). Abnormal tracer uptake, suspicious for recurrent disease, was detected in 59% of acetate studies but only in 17% of FDG studies. Using CT, bone scan, biopsy or ‘high clinical probability’ as the standard of reference, 30% of patients had their disease identified by acetate PET, whereas only 9% had disease identified by FDG PET. In this study, the probability for lesion detection was higher in patients with PSA levels greater than 3 ng/ml (59%) as compared with those with lower PSA levels (4%). In a series of 20 patients with two consecutive rising PSA measurements, [11C] acetate uptake was true-positive in 11 patients, and four patients had false-positive uptake that corresponded to other pathology [58]. Albrecht et al. evaluated [11C]acetate PET in 32 patients with evidence of biochemical relapse after initial radiation therapy (n = 15) or prostatectomy (n = 17) [6]. In the prostatectomy group, taking an arbitrary SUVmax ≥2 as the cutoff, PET showed local recurrences in 14/17 patients and two equivocal results. Distant disease was observed in six patients and an equivocal result was obtained in one. Endorectal MRI was positive in 12/12 patients. Biopsy confirmed local recurrence in six of six (100%) patients. PET was positive in five of the six patients with biopsy-proven recurrences, the result in the remaining patient being equivocal.
[18F]choline & [11C]choline in prostate cancer recurrence
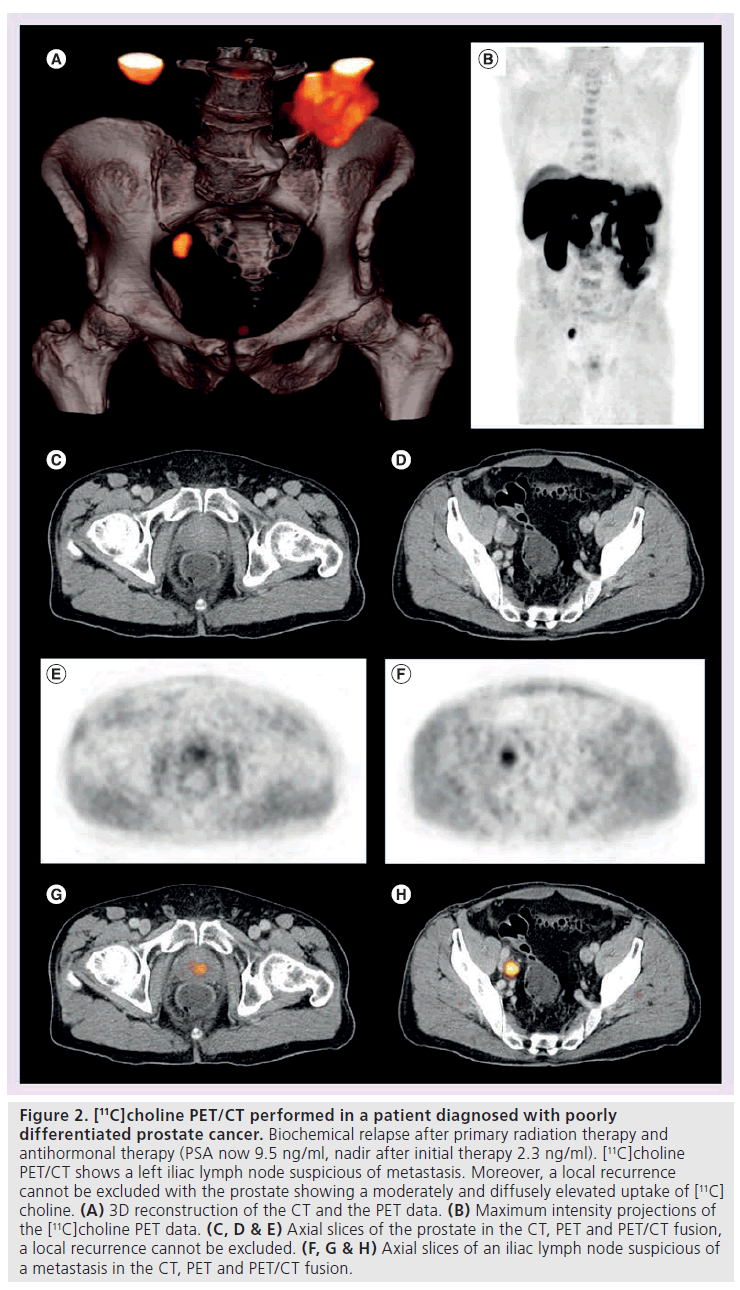
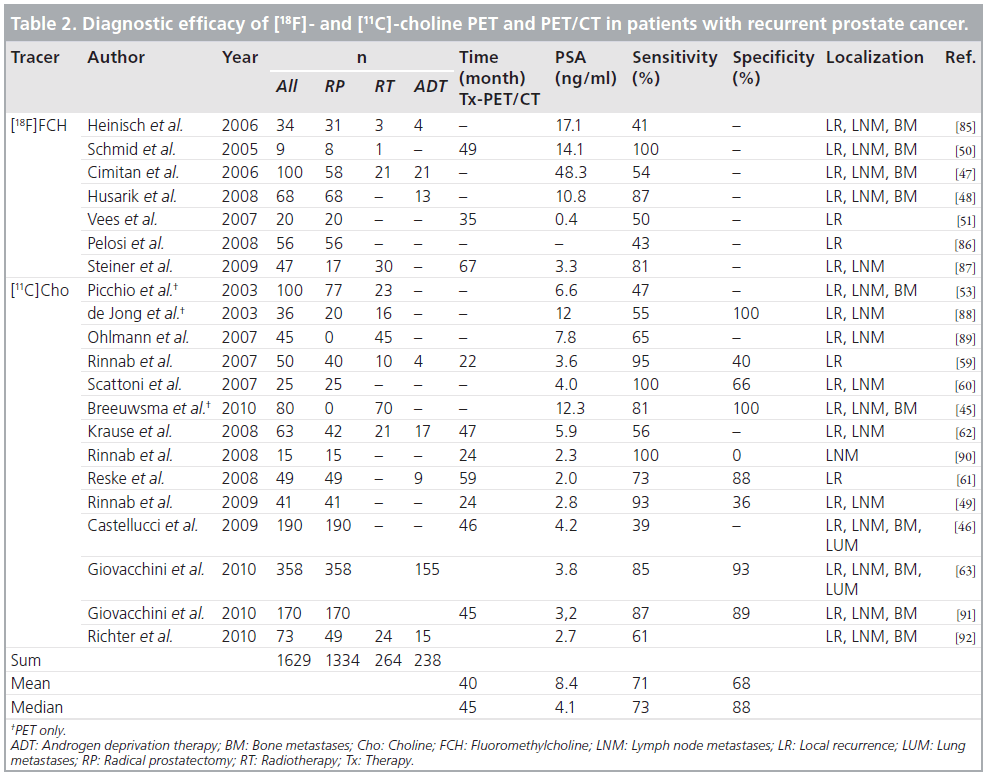
Several studies with [11C]choline PET and PET/CT reported encouraging results for the identification of recurrent prostate cancer (Figure 2). For a comprehensive summary of studies on the diagnostic efficacy of [18F]choline and [11C]choline PET and PET/CT in patients with recurrent prostate cancer please see Table 2. Picchio et al. compared [11C]choline and [18F]FDG for restaging of 100 patients with biochemical recurrence and observed at least one area of abnormal focal [11C]choline accumulation in 47% [53]. Rinnab et al. carried out a [11C]choline PET/CT study in 50 patients with biochemical recurrence, in most of whom histopathology was available [59]. The authors reported the sensitivity of [11C]choline PET/CT to be 82% when PSA levels exceeded 2.5 ng/ml, but the specificity was rather low, at 50%. Scattoni et al. evaluated the value of [11C]choline PET/CT for the diagnosis of seven lymph node metastases in 25 patients with biochemical recurrence [60]. They found a high positive predictive value even at low PSA values. The negative predictive value, however, was low; this is probably attributable to a limited capability of [11C]choline PET/CT to detect small lymph node metastases (lymph node metastases were small in a significant number of cases) and micrometastases. Reske et al. assessed the value of [11C]choline PET/CT in 49 patients with biochemical recurrence and suspicion of local recurrence [61]. A total of 36 of these patients had a biopsy-proven local recurrence and in 71% of these 36 patients, [11C]choline PET/CT correctly predicted the local recurrence. Krause et al. assessed the relationship between the detection rate of [11C]choline PET/CT and the serum PSA level in 63 patients with biochemical recurrence of prostate cancer [62]. A total of 35 (56%) of the patients showed pathological [11C]choline uptake. The detection rate of [11C]choline PET/ CT showed a relationship with the serum PSA level. Importantly, the authors found that even at PSA values less than 1.0 ng/ml, the detection efficiency of [11C]choline PET/CT was 36%. Castelluci et al. assessed the influence of total PSA, PSA velocity and PSA doubling time on the [11C]choline PET/CT detection rate in 190 prostate cancer patients with biochemical recurrence [46]. The authors confirmed a linear relation of the [11C]choline detection rate to the PSA value. In a multivariate analysis, PSA doubling time and PSA velocity were shown to be additional independent predictive factors for PET positivity. In the largest study up to now with 358 patients with biochemical recurrence (PSA >0.2 ng/ml) after radical prostatectomy, the percentage of positive scans was 19% in those with a PSA level between 0.2 and 1 ng/ml, whereas it was 46% in those with a PSA level between 1 and 3 ng/ml, and 82% in those with a PSA level higher than 3 ng/ml [63]. Therefore, a PSA value of 1 ng/ml might be a reasonable threshold when considering a choline PET/CT scan. Depending on the clinical situation and the velocity of PSA increase, scans at lower PSA levels might be indicated as well. However, as confirmation of the data by histopathology was also limited in the aforementioned study (13% histopathological correlation), definite recommendations concerning a PSA threshold for referring a patient for a choline PET/CT scan cannot yet be presented.
Figure 2. [11C]choline PET/CT performed in a patient diagnosed with poorly differentiated prostate cancer. Biochemical relapse after primary radiation therapy and antihormonal therapy (PSA now 9.5 ng/ml, nadir after initial therapy 2.3 ng/ml). [11C]choline PET/CT shows a left iliac lymph node suspicious of metastasis. Moreover, a local recurrence cannot be excluded with the prostate showing a moderately and diffusely elevated uptake of [11C] choline. (A) 3D reconstruction of the CT and the PET data. (B) Maximum intensity projections of the [11C]choline PET data. (C, D & E) Axial slices of the prostate in the CT, PET and PET/CT fusion, a local recurrence cannot be excluded. (F, G & H) Axial slices of an iliac lymph node suspicious of a metastasis in the CT, PET and PET/CT fusion.
The detection rate of an imaging method in the condition of biochemical relapse of prostate cancer in the range of low PSA values is of clinical importance as curative intended salvage radiation therapy (SRT) can be applied in this clinical situation [64]. In a recent study Castelluci et al. focused on the diagnostic performance of [11C]choline PET/ CT in 102 patients previously treated with radical prostatectomy, who presented a mild increase of PSA (<1.5 ng/ml) during follow-up. At multivariate statistical analysis only PSA doubling time and node status were shown to be significant and independent predictive factors for positive [11C]choline PET/CT. The optimal threshold for PSA doubling time established by receiver-operating characteristic analysis was 7.25 months. The authors concluded that [11C] choline could be suggested to be performed early during initial biochemical relapse in patients presenting with fast PSA kinetics [65]. Souvatzoglou et al. evaluated [11C]choline PET/CT in 37 patients referred for SRT of the prostate bed, treated initially with radical prostatectomy for prostate cancer, exhibiting biochemical relapse with a median PSA value of 0.5 ng/ml. 11/37 (30%) patients had a positive finding in the [11C]choline PET/CT, five (13%) outside of the prostatic fossa (iliac lymph nodes), implicating an extension of the planning target volume [66].
Taken together, the results of these studies demonstrate that in patients with positive [11C] choline findings, PET/CT could potentially be used to guide and individualize therapy, however, large prospective multicenter trials to corroborate the aforementioned promising data are still required.
Comparison of radiolabeled choline with radiolabeled acetate
The only study to directly compare [11C]acetate and [11C]choline with an intra-patient analysis is that by Kotzerke et al. analyzing 12 patients [67]. These subjects had various clinical indications (primary staging, increasing concentrations of PSA or follow-up) and underwent [11C]choline and [11C]acetate PET. The two examinations revealed mostly identical results, but this study, although very well conducted, was based on a small patient population.
[18F]fluoride PET & PET/CT
Bone and lymph nodes are the most common sites of metastatic deposition in prostate cancer. Patients with T3/T4 staged tumors develop bone metastases within 10 years after initial diagnosis in 12–55% of cases [68]. Approximately 50% of patients with metastatic disease die within 30 months and 80% within 5 years. Skeletal metastases occur in approximately 85% of patients dying of prostate cancer [69].
[18F]f luoride is a positron-emitting boneseeking agent introduced by Blau and coworkers in 1962 [70]. Its uptake mechanism is similar to that of [99m]Tc-methylene diphosphonate (MDP) [71]. [18F]Fluoride uptake reflects blood flow and osteoblastic activity. Schirrmeister et al. have shown in several studies the superiority of [18F]fluoride PET over [99mTc]-MDP bone scintigraphy for the detection of metastatic skeletal involvement [72]. However, [18F]fluoride is not tumor-specific: it also accumulates in benign bone abnormalities, including benign lesions that are usually not detected with [99mTc]- MDP bone scintigraphy, such as uncomplicated small cysts [73]. The use of hybrid PET/CT has significantly improved the limited specificity of [18F]fluoride PET [74]. Compared with [18F] choline PET/CT, [18F]fluoride PET/CT may be superior for the early detection of metastatic bone disease (i.e., identification of bone marrow involvement). Thus [18F]fluoride PET/CT has been found to demonstrate a higher overall sensitivity than [18F]choline PET/CT, though the difference was not statistically significant [75]. Despite its high performance, [18F]fluoride PET/CT is not widely used, possibly owing to the limited commercial availability of the tracer and the lower number of PET/CT systems compared with the number of g‑cameras.
Advanced disease
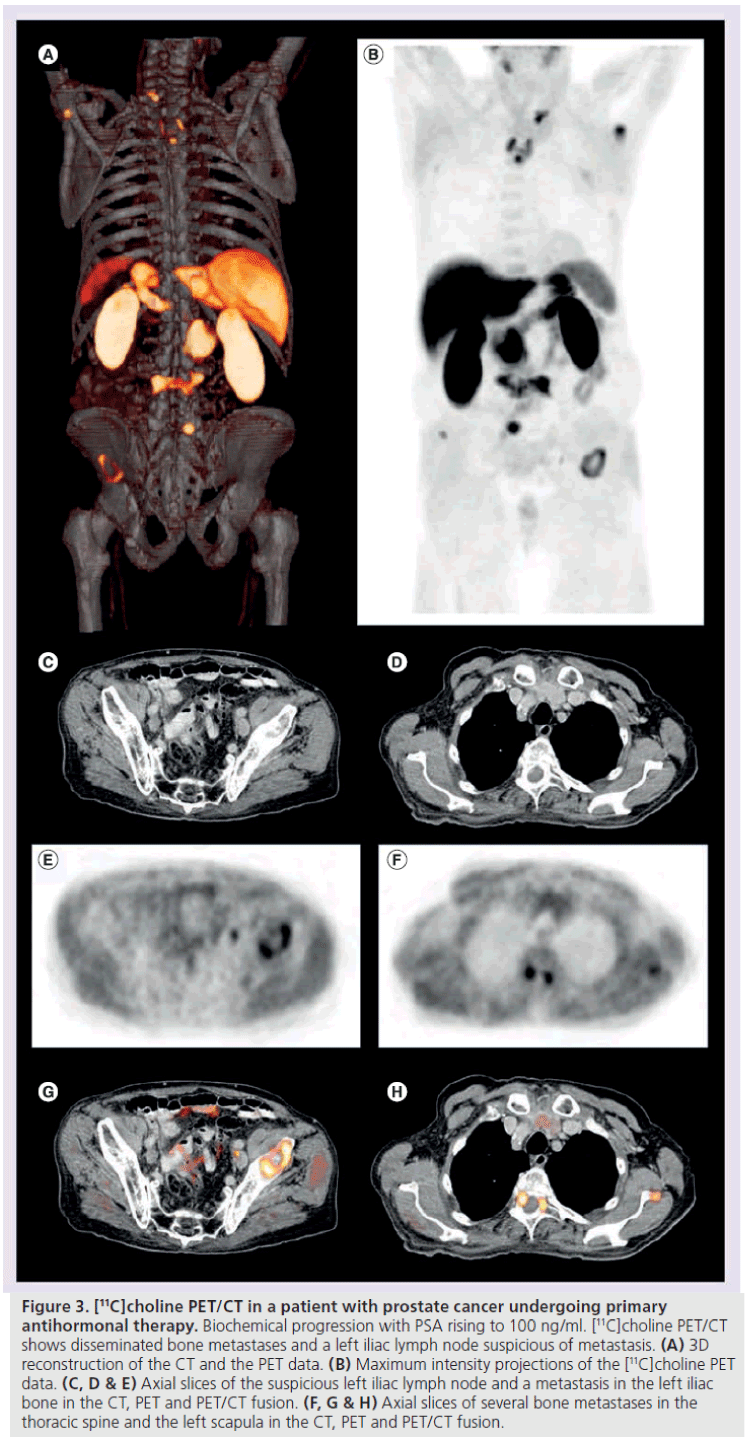
Early detection of advanced disease is of clinical importance in the disease management of patients with prostate cancer because it also implies important clinical consequences with respect to the therapy regimen. A primary goal is to detect the presence of disseminated disease as early as possible and to initiate appropriate therapy (e.g., the addition of chemotherapy). Recently, a study on the value of [11C]choline PET/CT in patients with advanced prostate cancer has been published, which demonstrated that [11C]choline PET/CT offers precise whole body restaging in a single imaging procedure, and changed disease management in 11 of 45 patients (24%) (Figure 3) [76]. Moreover, recent studies indicate that [11C]choline PET/CT has the potential to be utilized in evaluating therapy response of prostate cancer metastasis to hormonal or combined chemohormonal treatment [77,78].
Summary
The limitations of [18F]FDG imaging in primary prostate cancer are similar in restaging prostate cancer when recurrence is suspected. [18F] and [11C] choline can sensitively detect sites of recurrence in patients with biochemical relapse after definitive primary treatment, even when the PSA value is less than 1 ng/ml. There is a positive correlation between the detection rate of the scan, the PSA value and the PSA velocity. A clinical implication is that appropriate treatment could be tailored on an individualized basis according to the results of the scan, however it is not known if this would influence relapse free or overall survival. [18F]fluoride PET/CT can detect bone metastases with a high sensitivity. However, despite its high performance, [18F]fluoride PET/CT is not widely used, possibly owing to the limited commercial availability of the tracer and the lower number of PET/CT systems compared with the number of g‑cameras in which the conventional bone scintigraphy is performed. Recent studies indicate that [11C]choline PET/CT has the potential to be utilized in evaluating therapy response of prostate cancer metastasis to hormonal or combined chemohormonal treatment.
Limitations
Figure 3. [11C]choline PET/CT in a patient with prostate cancer undergoing primary antihormonal therapy. Biochemical progression with PSA rising to 100 ng/ml. [11C]choline PET/CT shows disseminated bone metastases and a left iliac lymph node suspicious of metastasis. (A) 3D reconstruction of the CT and the PET data. (B) Maximum intensity projections of the [11C]choline PET data. (C, D & E) Axial slices of the suspicious left iliac lymph node and a metastasis in the left iliac bone in the CT, PET and PET/CT fusion. (F, G & H) Axial slices of several bone metastases in the thoracic spine and the left scapula in the CT, PET and PET/CT fusion.
The studies that form the basis of this review do suffer from limitations that have to be taken into account: the patient populations were nonhomogeneous, also with regard to the primary treatment, studies are comprised of patients with a high variability in disease progression and no data are available at present that show that individualized treatment in these patients leads to improved disease-free or overall survival. Taken together, based on the currently available literature, conclusions and recommendations have to be drawn carefully. Prospective and controlled multicenter trials are necessary to further define the role of choline PET/CT in recurrent prostate cancer.
Main indications for PET/CT in prostate cancer
The application of [18F]FDG in prostate cancer is strongly limited. Among [11C]acetate and radiolabeled choline the latter are more extensively evaluated in prostate cancer. The main indication for a PET or PET/CT scan with radiolabeled choline in prostate cancer is in the evaluation of prostate cancer recurrence after primary treatment. There is a positive correlation of the scan’s detection rate with the PSA value and the PSA velocity, however, no definite threshold concerning those values for performing the scan can be defined up to date. The scan may be useful before SRT as it can provide information concerning spread of disease outside the prostate bed and, consequently, extend the planning target volume to the lymphatics in the case of such involvement. In an individualized setting PET/CT can be applied in advanced disease if information concerning the pattern of the metastatic spread is needed, or if response to treatment (e.g., chemohormonal treatment) is to be assessed. In the setting of primary prostate cancer, performing the scan can be considered in patients at high risk of metastatic disease.
Conclusion
PET/CT with radioactively labeled choline derivates is a promising molecular imaging technique for restaging prostate cancer patients with biochemical recurrence after definite primary therapy. For the diagnosis of primary prostate cancer, however, choline PET/CT cannot be recommended at the present time; choline PET/CT might be useful in patients with suspected prostate cancer with multiple negative biopsies and in patients with high-risk prostate cancer in the primary setting.
In patients with biochemical failure after primary therapy choline PET/CT has shown higher detection rates compared with other imaging modalities. The detection efficacy is linearly correlated with the PSA serum level and can be useful even at PSA serum levels as low as 0.5–1.0 ng/ml. In patients with choline positive lesions, PET/CT can have an important impact on the disease management (i.e., therapy individualization) due to the exact localization of disease and simultaneous information on potential patterns of spread of disease as a whole body imaging modality.
The first promising results on the use of choline PET/CT for therapy response assessment after anti-androgen therapy have been published. Further studies to evaluate and potentially validate choline PET/CT for therapy monitoring of patients with advanced disease receiving chemotherapy are under way.
Novel and innovative tracers, such as gastrinreleasing peptide receptor or androgen-receptor imaging tracers, hold promise to further improve the diagnostic performance of molecular imaging techniques in the diagnosis of prostate cancer. In the future, advances in hybrid imaging (i.e., PET/MR) will further increase prostate cancer imaging [40]. This hybrid technique combines the sensitive molecular signals provided by PET with the high soft tissue contrast of MRI, which – with the additional functional capabilities of MRI – might further improve imaging of prostate cancer, and finally, hopefully result in an improved patient care.
Future perspective
The field of molecular imaging of prostate cancer will evolve in several ways. Large prospective multicenter trials will have to be carried out using [11C]- and [18F]-labeled choline derivatives for imaging prostate cancer with an emphasis on recurrent disease. In these as well as in other multicenter studies the impact of imaging with multimodal techniques with respect to patient stratification on disease-free and overall survival will need to to be shown. Novel PET imaging probes will enter the arena that presumably will allow imaging of prostate cancer with higher accuracy through binding to more tumor specific targets and as a consequence higher specificity. These upcoming novel tracers also necessitate prospective multicenter trials, which should be planned early to allow for a sound prospective data base that will be necessary for the tracers to be part of clinical diagnosis in the future. The development of hybrid imaging techniques like PET/CT, SPECT/CT and more recently PET/MR bear the potential to increase the diagnostic accuracy of diagnosing disease. PET/MR combines the highly sensitive molecular target visualization provided by PET with the high soft tissue contrast and morphological and functional techniques of MRI. PET/CT with prostate cancer-specific imaging probes will need to be compared with other imaging modalities, such as diffusion weighted MRI, dynamic contrast enhanced MRI and magnetic resonance spectroscopy in a systematic prospective and multicentric way, with the perspective of an improvement of diagnosis of disease and ultimately for the benefit of the patient.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

Papers of special note have been highlighted as: * of interest
References
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol. 18(3), 581–592 (2007).
- Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J. Clin. 57(2), 90–104 (2007).
- Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18- fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur. Urol. 36(1), 31–35 (1999).
- Morris MJ, Akhurst T, Osman I et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology 59(6), 913–918 (2002).
- Nunez R, Macapinlac HA, Yeung HW et al. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. J. Nucl. Med. 43(1), 46–55 (2002).
- Albrecht S, Buchegger F, Soloviev D et al. 11C-acetate PET in the early evaluation of prostate cancer recurrence. Eur. J. Nucl. Med. Mol. Imaging 34(2), 185–196 (2007).
- Dehdashti F, Picus J, Michalski JM et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur. J. Nucl. Med. Mol. Imaging 32(3), 344–350 (2005).
- Kotzerke J, Prang J, Neumaier B et al. Experience with carbon-11 choline positron emission tomography in prostate carcinoma. Eur. J. Nucl. Med. 27(9), 1415–1419 (2000).
- Larson SM, Morris M, Gunther I et al. Tumor localization of 16b-18F-fluoro-5adihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J. Nucl. Med. 45(3), 366–373 (2004).
- Toth G, Lengyel Z, Balkay L, Salah MA, Tron L, Toth C. Detection of prostate cancer with 11C-methionine positron emission tomography. J. Urol. 173(1), 66–69; discussion 69 (2005).
- Wachter S, Tomek S, Kurtaran A et al. 11C-acetate positron emission tomography imaging and image fusion with computed tomography and magnetic resonance imaging in patients with recurrent prostate cancer. J. Clin. Oncol. 24(16), 2513–2519 (2006).
- Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Threedimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7-cm3) spatial resolution. Radiology 198(3), 795–805 (1996).
- Kurhanewicz J, Vigneron DB, Nelson SJ. Three-dimensional magnetic resonance spectroscopic imaging of brain and prostate cancer. Neoplasia 2(1–2), 166–189 (2000).
- Ramirez de Molina A, Penalva V, Lucas L, Lacal JC. Regulation of choline kinase activity by Ras proteins involves Ral-GDS and PI3K. Oncogene 21(6), 937–946 (2002).
- Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R et al. Overexpression of choline kinase is a frequent feature in human tumorderived cell lines and in lung, prostate, and colorectal human cancers. Biochem. Biophys. Res. Comm. 296(3), 580–583 (2002).
- Effert PJ, Bares R, Handt S, Wolff JM, Bull U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J. Urol. 155(3), 994–998 (1996).
- Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18] fluoro-d-glucose. Radiology 199(3), 751–756 (1996).
- Fanti S, Nanni C, Ambrosini V, Gross MD, Rubello D, Farsad M. PET in genitourinary tract cancers. Q. J. Nucl. Med. Mol. Imaging 51(3), 260–271 (2007).
- Yoshimoto M, Waki A, Yonekura Y et al. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: acetate metabolism in tumor cells. Nucl. Med. Biol. 28(2), 117–122 (2001).
- Kato T, Tsukamoto E, Kuge Y et al. Accumulation of [11C]acetate in normal prostate and benign prostatic hyperplasia: comparison with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 29(11), 1492–1495 (2002).
- Oyama N, Akino H, Kanamaru H et al. 11C-acetate PET imaging of prostate cancer. J. Nucl. Med. 43(2), 181–186 (2002).
- Jambor I, Borra R, Kemppainen J et al. Functional imaging of localized prostate cancer aggressiveness using 11C-acetate PET/ CT and 1H-MR spectroscopy. J. Nucl. Med. 51(11), 1676–1683 (2010).
- Sutinen E, Nurmi M, Roivainen A et al. Kinetics of [11C]choline uptake in prostate cancer: a PET study. Eur. J. Nucl. Med. Mol. Imaging 31(3), 317–324 (2004).
- Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J. Nucl. Med. 47(2), 262–269 (2006).
- Reske SN, Blumstein NM, Neumaier B et al. Imaging prostate cancer with 11C-choline PET/CT. J. Nucl. Med. 47(8), 1249–1254 (2006). & Examples for the debate concerning detection of primary prostate cancer with [11C]choline PET/CT.
- Yamaguchi T, Lee J, Uemura H et al. Prostate cancer: a comparative study of 11C-choline PET and MR imaging combined with proton MR spectroscopy. Eur. J. Nucl. Med. Mol. Imaging 32(7), 742–748 (2005).
- Farsad M, Schiavina R, Castellucci P et al. Detection and localization of prostate cancer: correlation of 11C-choline PET/CT with histopathologic step-section analysis. J. Nucl. Med. 46(10), 1642–1649 (2005). & Examples for the debate concerning detection of primary prostate cancer with [11C]choline PET/CT.
- Giovacchini G, Picchio M, Coradeschi E et al. [11C]choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur. J. Nucl. Med. Mol. Imaging 35(6), 1065–1073 (2008).
- Martorana G, Schiavina R, Corti B et al. 11C-choline positron emission tomography/ computerized tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J. Urol. 176(3), 954–960; discussion 960 (2006).
- Scher B, Seitz M, Albinger W et al. Value of 11C-choline PET and PET/CT in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 34(1), 45–53 (2007).
- Ellis WJ, Brawer MK. Repeat prostate needle biopsy: who needs it? J. Urol. 153(5), 1496–1498 (1995).
- Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J. Urol. 159(4), 1247–1250 (1998).
- Igerc I, Kohlfurst S, Gallowitsch HJ et al. The value of 18F-choline PET/CT in patients with elevated PSA level and negative prostate needle biopsy for localisation of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 35(5), 976–983 (2008).
- Kwee SA, DeGrado T. Prostate biopsy guided by 18F-fluorocholine PET in men with persistently elevated PSA levels. Eur. J. Nucl. Med. Mol. Imaging 35(8), 1567–1569; author reply 1570 (2008).
- de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. Visualization of prostate cancer with 11C-choline positron emission tomography. Eur. Urol. 42(1), 18–23 (2002).
- de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J. Nucl. Med. 44(3), 331–335 (2003).
- Schiavina R, Scattoni V, Castellucci P et al. 11C-choline positron emission tomography/ computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur. Urol. 54(2), 392–401 (2008).
- Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology 243(1), 28–53 (2007).
- O’Dowd GJ, Veltri RW, Orozco R, Miller MC, Oesterling JE. Update on the appropriate staging evaluation for newly diagnosed prostate cancer. J. Urol. 158(3 Pt 1), 687–698 (1997).
- Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 12(2), 181–191 (2011). & Discusses hybrid imaging of recurrent prostate cancer with a focus on MR/PET.
- Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J. Value of diffusionweighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur. J. Radiol. 72(3), 381–387 (2009).
- Eiber M, Beer AJ, Holzapfel K et al. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest. Radiol. 45(1), 15–23 (2010).
- Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin. Oncol. R. Coll. Radiol. 22(1), 46–55 (2010).
- Johnstone PA, Tarman GJ, Riffenburgh R, Rohde DC, Puckett ML, Kane CJ. Yield of imaging and scintigraphy assessing biochemical failure in prostate cancer patients. Urol. Oncol. 3(4), 108–112 (1997).
- Breeuwsma AJ, Pruim J, van den Bergh AC et al. Detection of local, regional, and distant recurrence in patients with PSA relapse after external-beam radiotherapy using 11C-choline positron emission tomography. Int. J. Radiat. Oncol. Biol. Phys. 77(1), 160–164 (2010).
- Castellucci P, Fuccio C, Nanni C et al. Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J. Nucl. Med. 50(9), 1394–1400 (2009). & Evaluates [11C]choline PET/CT in prostate cancer recurrence.
- Cimitan M, Bortolus R, Morassut S et al. [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur. J. Nucl. Med. Mol. Imaging 33(12), 1387–1398 (2006).
- Husarik DB, Miralbell R, Dubs M et al. Evaluation of [18F]-choline PET/CT for staging and restaging of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 35(2), 253–263 (2008).
- Rinnab L, Simon J, Hautmann RE et al. [11C] choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J. Urol. 27(5), 619–625 (2009).
- Schmid DT, John H, Zweifel R et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology 235(2), 623–628 (2005).
- Vees H, Buchegger F, Albrecht S et al. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/ml) after radical prostatectomy. BJU Int. 99(6), 1415–1420 (2007).
- Chang CH, Wu HC, Tsai JJ, Shen YY, Changlai SP, Kao A. Detecting metastatic pelvic lymph nodes by 18F-2-deoxyglucose positron emission tomography in patients with prostate-specific antigen relapse after treatment for localized prostate cancer. Urol. Int. 70(4), 311–315 (2003).
- Picchio M, Messa C, Landoni C et al. Value of [11C]choline-positron emission tomography for re-staging prostate cancer: a comparison with [18F]fluorodeoxyglucose-positron emission tomography. J. Urol. 169(4), 1337–1340 (2003). & Demonstrates the limited diagnostic accuracy of 2´-[18F]fluoro-2´-deoxy-dglucose- PET compared with [11C]choline PET.
- Schoder H, Herrmann K, Gonen M et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin. Cancer Res. 11(13), 4761–4769 (2005).
- Oyama N, Miller TR, Dehdashti F et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J. Nucl. Med. 44(4), 549–555 (2003).
- Fricke E, Machtens S, Hofmann M et al. Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 30(4), 607–611 (2003).
- Kotzerke J, Volkmer BG, Neumaier B, Gschwend JE, Hautmann RE, Reske SN. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 29(10), 1380–1384 (2002).
- Sandblom G, Sorensen J, Lundin N, Haggman M, Malmstrom PU. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology 67(5), 996–1000 (2006).
- Rinnab L, Mottaghy FM, Blumstein NM et al. Evaluation of [11C]-choline positronemission/ computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int. 100(4), 786–793 (2007).
- Scattoni V, Picchio M, Suardi N et al. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur. Urol. 52(2), 423–429 (2007).
- Reske SN, Blumstein NM, Glatting G. [11C] choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 35(1), 9–17 (2008).
- Krause BJ, Souvatzoglou M, Tuncel M et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA value in patients with biochemical recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 35(1), 18–23 (2008).
- Giovacchini G, Picchio M, Coradeschi E et al. Predictive factors of [11C]choline PET/ CT in patients with biochemical failure after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 37(2), 301–309 (2010). & Evaluates [11C]choline PET/CT in prostate cancer recurrence.
- Stephenson AJ, Scardino PT, Kattan MW et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 25(15), 2035–2041 (2007).
- Castellucci P, Fuccio C, Rubello D et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur. J. Nucl. Med. Mol. Imaging 38(1), 55–63 (2011). & Evaluating [11C]choline PET/CT in prostate cancer recurrence.
- Souvatzoglou M, Krause BJ, Purschel A et al. Influence of 11C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother. Oncol. 99(2), 193–200 (2011).
- Kotzerke J, Volkmer BG, Glatting G et al. Intraindividual comparison of [11C]acetate and [11C]choline PET for detection of metastases of prostate cancer. Nuklearmedizin 42(1), 25–30 (2003).
- Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA 277(6), 467–471 (1997).
- Wall HV. Nuclear Medicine in Clinical Diagnosis and Treatment. Second Edition. Murray IPC, Ell PJ (Eds). Churchill Livingstone, volume 2, 1176 (1998).
- Blau M, Nagler W, Bender MA. Fluorine-18: a new isotope for bone scanning. J. Nucl. Med. 3, 332–334 (1962).
- Even-Sapir E, Mishani E, Flusser G, Metser U. 18F-Fluoride positron emission tomography and positron emission tomography/computed tomography. Semin. Nucl. Med. 37(6), 462–469 (2007).
- Schirrmeister H, Guhlmann A, Elsner K et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J. Nucl. Med. 40(10), 1623–1629 (1999).
- Even-Sapir E, Metser U, Flusser G et al. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J. Nucl. Med. 45(2), 272–278 (2004).
- Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-ofview SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J. Nucl. Med. 47(2), 287–297 (2006).
- Beheshti M, Vali R, Waldenberger P et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur. J. Nucl. Med. Mol. Imaging 35(10), 1766–1774 (2008).
- Tuncel M, Souvatzoglou M, Herrmann K et al. [11C]Choline positron emission tomography/computed tomography for staging and restaging of patients with advanced prostate cancer. Nucl. Med. Biol. 35(6), 689–695 (2008).
- De Waele A, Van Binnebeek S, Mottaghy FM. Response assessment of hormonal therapy in prostate cancer by [11C] Choline PET/CT. Clin. Nucl. Med. 35(9), 701–703 (2010).
- Krause BJ, Souvatzoglou M, Herrmann K et al. [11C]Choline as pharmacodynamic marker for therapy response assessment in a prostate cancer xenograft model. Eur. J. Nucl. Med. Mol. Imaging 37(10), 1861–1868 (2010).
- Kwee SA, Coel MN, Lim J, Ko JP. Prostate cancer localization with 18fluorine fluorocholine positron emission tomography. J. Urol. 173(1), 252–255 (2005).
- Steuber T, Schlomm T, Heinzer H et al. [F(18)]-fluoroethylcholine combined in-line PET-CT scan for detection of lymph-node metastasis in high risk prostate cancer patients prior to radical prostatectomy: preliminary results from a prospective histology-based study. Eur. J. Cancer 46(2), 449–455 (2010).
- Beheshti M, Imamovic L, Broinger G et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology 254(3), 925–933 (2010).
- Yoshida S, Nakagomi K, Goto S, Futatsubashi M, Torizuka T. 11C-choline positron emission tomography in prostate cancer: primary staging and recurrent site staging. Urol. Int. 74(3), 214–220 (2005).
- Li X, Liu Q, Wang M et al. C-11 choline PET/CT imaging for differentiating malignant from benign prostate lesions. Clin. Nucl. Med. 33(10), 671–676 (2008).
- Watanabe H, Kanematsu M, Kondo H et al. Preoperative detection of prostate cancer: a comparison with 11C-choline PET, 18F-fluorodeoxyglucose PET and MR imaging. J. Magn. Reson. Imaging 31(5), 1151–1156 (2010).
- Heinisch M, Dirisamer A, Loidl W et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA <5 ng/ml? Mol. Imaging Biol. 8(1), 43–48 (2006).
- Pelosi E, Arena V, Skanjeti A et al. Role of whole-body 18F-choline PET/CT in disease detection in patients with biochemical relapse after radical treatment for prostate cancer. Radiol. Med. 113(6), 895–904 (2008).
- Steiner C, Vees H, Zaidi H et al. Three-phase 18F-fluorocholine PET/CT in the evaluation of prostate cancer recurrence. Nuklearmedizin 48(1), 1–9; quiz N2-3 (2009).
- de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur. Urol. 44(1), 32–38; discussion 38–39 (2003).
- Ohlmann C, Pfister D, Thüer D, Wille S, Engelmann UH, Heidenreich A. 11C-Colinepositron emission tomography/computerized tomography (C-PET/CT) for tumor localization of locally recurrent prostate cancer after radiation therapy. Eur. Urol. (Suppl. 6), 229 (2007).
- Rinnab L, Mottaghy FM, Simon J et al. [11C] Choline PET/CT for targeted salvage lymph node dissection in patients with biochemical recurrence after primary curative therapy for prostate cancer. Preliminary results of a prospective study. Urol. Int. 81(2), 191–197 (2008).
- Giovacchini G, Picchio M, Scattoni V et al. PSA doubling time for prediction of [11C] choline PET/CT findings in prostate cancer patients with biochemical failure after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 37(6), 1106–1116 (2010). & Evaluates [11C]choline PET/CT in prostate cancer recurrence.
- Richter JA, Rodriguez M, Rioja J et al. Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol. Imaging Biol. 12(2), 210–217 (2010).