Review Article - Imaging in Medicine (2010) Volume 2, Issue 2
Multifunctional gadolinium oxide nanoparticles: towards image-guided therapy
Stéphane Roux, Anne-Charlotte Faure, Céline Mandon, Sandrine Dufort, Charlotte Rivière, Jean-Luc Bridot, Brice Mutelet, Christophe A Marquette, Véronique Josserand, Géraldine Le Duc, Alain Le Pape, Claire Billotey, Marc Janier, Jean-Luc Coll, Pascal Perriat & Olivier Tillement†Laboratoire de Physico-Chimie des Matériaux Luminescents, UMR 5620 CNRS, Université Claude Bernard Lyon 1, 69622 Villeurbanne Cedex, France
- Corresponding Author:
- Olivier Tillement
Laboratoire de Physico-Chimie des Matériaux Luminescents
UMR 5620 CNRS, Université Claude Bernard Lyon 1
69622 Villeurbanne Cedex, France
Tel: +33 472 431 200
Fax: +33 472 431 233
E-mail: tillement@pcml.univ-lyon1.fr
Abstract
Owing to their multifunctional character, nanoparticles appear well suited for combining sensing, imaging and therapy. Nanoparticles composed of a gadolinium oxide core and a polysiloxane shell were designed for the detection of biomolecules, fluorescence and MRI, and for cancer therapy. Each component (gadolinium oxide core and polysiloxane shell) of these nanoparticles plays a crucial role and enables the resulting nanoparticles to emit intense but transient light and/or long-lived and highly photostable light, to enhance the contrast of magnetic resonance images, to improve the colloidal stability, to specifically interact with biomolecules, to absorb x‑ray photons and to capture thermal neutrons. The hybrid gadolinium oxide nanoparticles exhibit a great potential for sensing applications and for image-guided therapy.
Keywords
biomolecule detection ▪ fluorescence imaging ▪ gadolinium oxide ▪ MRI ▪ nanoparticle ▪ neutron capture ▪ radiotherapy
The recent and tremendous progression in the field of nanotechnology has opened the door to nanomedicine, which appears well-suited for personalized therapy [1–3]. Personalized therapy carries the promise of decisive improvements in medicine, since treatment will be adapted to each patient. However, the development of new tools is required for tailoring efficient therapeutic protocols. These rest on early diagnosis, the delivery of therapeutic agents in the correct location (diseased tissues) and at the correct time to enhance the therapeutic effects and, at the same time, preserve the surrounding healthy tissues. Alongside the important development of the chemistry of the dendrimers [4], the intense and creative research devoted to nanotechnology has led, among others, to the development of multifunctional nanoparticles that exhibit attractive behavior for personalized therapy. First, the composition and the shape of the nanoparticles can be engineered to combine imaging and therapy [5,6]. Furthermore, the multifunctional character of the nanoparticles can be accurately tuned to suit the therapeutic protocol for each case [5,6]. This issue was recently addressed by interesting reviews highlighting the potential of multifunctional nanoparticles for nanomedicine and, especially, personalized therapy [1–3].
Owing to their physical properties that allow the combination of imaging and remotecontrolled therapeutic effect, iron oxide- and gold-based nanoparticles were described in a huge number of papers as interesting candidates for biomedical applications [7–9].
Superparamagnetic iron oxide nanoparticles are indeed well known as mediators for inductive hyperthermia (i.e., hyperthermia is generated in tissues containing magnetic particles by the application of an alternating magnetic field) [10–11] and as negative contrast agents for MRI [12]. The strong absorption of x-ray photons of gold element can be exploited for x-ray imaging [13,14] and for enhancing the dose effect of the radiotherapy [15,16]. The dose-enhancement will lead to the administration of a lower dose for the same effect on the diseased tissues, but with a better preservation of healthy surrounding tissues. Owing to their design, oxide nanoparticles coated by a gold shell, gold nanorod or nanocages exhibit an additional therapeutic ability. The absorption of near-infrared photons renders them cytotoxic via the photothermal effect [17–20]. In contrast to the x-ray photons, the penetration depth of near-infrared photons in biological tissues does not exceed 2 cm. For this reason, the photothermal effect obtained by the absorption of gold nanostructures is limited to superficial tissues. Furthermore, gold nanoparticles can be functionalized by gadolinium chelates for a follow-up by MRI, which is a safer medical imaging technique than x-ray imaging [21,22].
Despite their great variety of properties that can be exploited for the purpose of combining imaging and therapy, studies on lanthanidebased nanoparticles for image-guided therapy are performed less frequently. Besides the paramagnetic character of gadolinium ions (Gd3+), which can be advantageously exploited for MRI [23,24], the strong propensity of the Gd element to absorb x-ray photons (owing to the relatively high atomic number of 57) and the high neutron capture cross-section of two gadolinium isotopes (155,157Gd) [25–29] render Gd3+-based compounds very attractive, since they can combine medical imaging (MRI) and two different therapeutic protocols to fighting cancer (radiotherapy and neutron-capture therapy). Gd3+ chelates with low molecular weight (Gd-diethylenetriaminepentaacetic acid [DTPA], Gd-1,4,7,10-tetraazacyclododecane- N,N´,N´´,N´´´-tetraacetic acid) are extensively applied as positive contrast agents for MRI. Owing to their small molecular size, these contrast agents are, unfortunately, cleared too quickly [30]. Many Gd3+-based nanoparticles were, therefore, designed to improve the contrast enhancement, the biodistribution and the pharmacokinetics. In addition, this strategy afforded the opportunity to elaborate multifunctional contrast agents since they are obtained by the assembly of various building blocks.
The resulting contrast agents exhibit high molecular relaxivity (i.e., the relaxivity per contrast agent) since each particle carries a large amount of Gd3+. Moreover, the ionic relaxivity of Gd3+ (i.e., the relaxivity per Gd3+) increases when particulate contrast agents are obtained by the covalent grafting of gadolinium chelate onto the nanostructure. The immobilization of the gadolinium chelates leads to the increase of the rotational correlation time due to the decrease of the rotational motion of the grafted gadolinium chelate [31]. The other important difference between gadolinium chelates with low molecular weight and particulate contrast agents lies in their behavior in the bloodstream. Nanosized contrast agents are better suited to differentiate various tissues than small molecules. They are sufficiently large to be retained in vessels with a normal endothelial barrier, while they can escape from the bloodstream when the endothelial barrier is altered. Such a characteristic allows the passive accumulation of nanoparticles by the enhanced permeability and retention effect in solid tumors or inflamed tissues since they are irrigated by leaky microvasculature [32,33].
By applying multiple methods for the elaboration of nanoparticles, a great variety of positive contrast agents for MRI were yielded. Most of the nanoparticles are rendered paramagnetic by the presence of gadolinium chelates, which can be grafted to polymers [34,35], self-assembled peptide amphiphiles [36,37], viral capsids [38], dendrimers [4,39,40], entrapped in liposomes [41], micelles [42,43], polymeric nanoparticles [44,45], fullerenes [46], carbon nanotubes [47], clays [48] or mesoporous silica nanoparticles [49], and immobilized on high-density lipoprotein nanoparticles [50,51], quantum dots [52,53], lipid particles [54] or on gold nanoparticles [21,22]. In comparison to the gadolinium chelates with low molecular weight, paramagnetic nanoparticles exhibit a larger size, a higher content in Gd3+ ions and a longer circulation time. As a result, an increase in the contrast enhancement is observed. The condensation of a high number of Gd3+ chelates to macromolecules or nanostructures improves the positive contrast of MRI as each resulting contrast agent exhibits a higher active element (Gd3+) content than do molecular contrast agents with low molecular weight and a slower rotational motion [31]. These contrast agents, therefore, exert a stronger influence on the relaxation of water protons owing to the increase in ion molar relaxivity and in molecular relaxivity. In the case of gadolinium chelates encapsulated liposomes, the increase is partly due to the long circulation time [55]. Furthermore, some of them were easily functionalized by small organic molecules such as biotargeting groups or fluorescent dyes. These functionalizations may confer additional attractive features (luminescent properties and/or cell targeting specificity) on the particles [4,34,38–41,50–54].
Despite their strong potential for biomedical applications, the crystalline nanoparticles based on inorganic gadolinium, such as gadolinium oxide [56–58], gadolinium fluoride [59,60], and gadolinium carbonate [61,62], have only recently been evaluated, whereas they appear very well suited for improving the efficiency of imaging and therapy (neutrontherapy and microbeam radiation therapy [63]) owing to the large amount of Gd3+ ions per particle as compared with gadolinium chelates, which contain only one Gd3+ per molecule. Moreover, gadolinium oxide constitutes an excellent host matrix for luminescent rare earth (RE) ions. Since crystalline gadolinium oxide is transparent, doping with either terbium, europium or neodymium ions renders the material luminescent.
Pioneering studies devoted to gadolinium oxide nanoparticles were performed by Roberts and Watkin [56,58]. They revealed the potential for gadolinium oxide nanoparticles to be used as contrast agents for MRI. However, these preliminary works suffered from the lack of well-defined hydrophilic gadolinium oxide nanoparticles. The exploitation of the attractive characteristics of gadolinium oxide nanoparticles for MRI became conceivable only when a reproducible and efficient synthesis protocol of naked gadolinium oxide nanoparticles and their functionalization were developed. In this article, the strategy explored by Tillement’s research group for developing biological sensors and contrast agents combining imaging and therapy from multifunctional gadolinium oxide nanoparticles is presented.
Synthesis of hybrid gadolinium oxide nanoparticles
To observe the promising properties of gadolinium, the synthesis of hybrid nanoparticles composed of gadolinium oxide core embedded in a polysiloxane shell was envisaged. This first required perfecting a reproducible and reliable synthesis of gadolinium oxide nanoparticles [64,65]. Gadolinium oxide nanoparticles were obtained by applying a modified polyol route. The alkaline hydrolysis of gadolinium chloride salt in a high boiling diol, the diethylene glycol, led to the formation of nanosized crystalline gadolinium oxide nanoparticules (core diameter: 2.2 nm, hydrodynamic diameter [Dh]: 3.3 ± 1.8 nm). The solvent diethylene glycol not only has a high boiling point, which allows synthesis to be carried out at relatively high temperature, but also ensures the control of the growth of the nanoparticles and prevents their agglomeration, since diethylene glycol molecules are able to absorb onto the nanoparticles through the coordination of Gd3+. Larger core sizes (Dh: 5.2 ± 1.2 nm, 8.9 ± 2.1 nm) can be yielded from colloids containing the 2.2 nm sized nanoparticles that act as nucleation sites [66]. In contrast to most of the lanthanide elements, Gd3+ ions are not fluorescent in the visible domain of light. However, the nanosized core (Dh: 3.3 nm) can be doped by luminescent RE ions that give the resulting particle a highly photostable luminescence, characterized by narrow emission bands [64,65,67–69]. The alkaline hydrolysis of Gd3+ chloride is, therefore, carried out in the presence of a small amount of luminescent lanthanide chloride salts. The second important finding lies in the functionalization of these cores. The strategy developed by Tillement’s research group rests on the encapsulation of the gadolinium oxide cores in a polysiloxane shell that was obtained by the sequential hydrolysis–condensation of a mixture of aminopropyltriethoxysilane (APTES) and tetraethyl orthosilicate (molar APTES/tetraethyl orthosilicate = 0.6) [70]. The thickness of the polysiloxane shell, which is determined by the number of precursors (APTES and tetraethyl orthosilicate), can be tuned between 2 and 18 nm [66,70,71]. For in vivo applications (cell labeling, in vivo imaging and therapy), the polysiloxane shell is fixed at approximately 2 nm whereas it is set between 2 and 18 nm for sensing applications. This functionalization mode provides several important advantages. The most striking feature of the polysiloxane shell results from its chemical nature, which can provide new properties to the particles. The polysiloxane shell facilitates the functionalization of the particles, which rests on the presence of amine functions in the polysiloxane shell owing to the use of APTES for the formation of the shell. These amine functions can act as grafting sites for the covalent immobilization of molecules bearing a reactive group that are able to condense with amine functions (carboxylic acid, NHS ester, iso[thio]cyanate groups). The functionalization of the nanoparticles can be achieved in two distinct steps, before and after the hydrolysis– condensation of polysiloxane shell precursors. The functionalization performed before and after the hydrolysis (i.e., before and after the formation of the polysiloxane shell) allows incorporation of different types of molecules without prejudicial competition. In this way, many organic dyes (fluorescein isothiocyanate [FITC], rhodamine B isothiocyanate, cyanine 5-NHS Cy5-NHS) can be covalently bound to the inner part of the polysiloxane shell whereas the outer part of the polysiloxane shell can be derivatized by biotargeting groups, such as biotin, SAV, nucleic acids and by hydrophilic molecules (poly[ethylene glycol] [PEG], DTPA moieties) [70,71]. Since a high colloidal stability in biological fluids is required, this implies a controlled postfunctionalization of the hybrid gadolinium oxide nanoparticles by highly hydrophilic molecules (PEG or DTPA). The derivatization of the outer part of the polysiloxane shell with short PEG chains, carrying a carboxylic acid group at each end (M = 250 gmol-1), and with DTPA is reflected by an increase of the Dh (10–12 nm) [72].
The polysiloxane shell ensures the protection of the core and favors dispersion of the nanoparticles in aqueous solution without loss of luminescence of RE ions [70]. The protective effect of the shell was validated by the preservation of the inorganic luminescence in acidic media when the cores are doped by Tb3+ ions. The polysiloxane shell exerts another positive influence on the luminescence of RE ions present in the cores, since its presence induces the replacement of RE-OH bonds, which behave as quenchers for the luminescence by RE-O-Si bonds and an energy transfer from polysiloxane shell to luminescent centers [70,73]. The encapsulation of luminescent RE-doped gadolinium oxide cores yielded nano-objects that are more fluorescent than the uncoated gadolinium oxide cores. This phenomenon was also observed when the gadolinium oxide nanoparticles doped by Tb3+ ions are intimately associated to gold nanoparticles that act as antenna. These antenna, which absorb the excitation light and transfer the energy to the luminescent ions, induce a strong exaltation of the emitted light [74].
Doubly luminescent nanoprobes
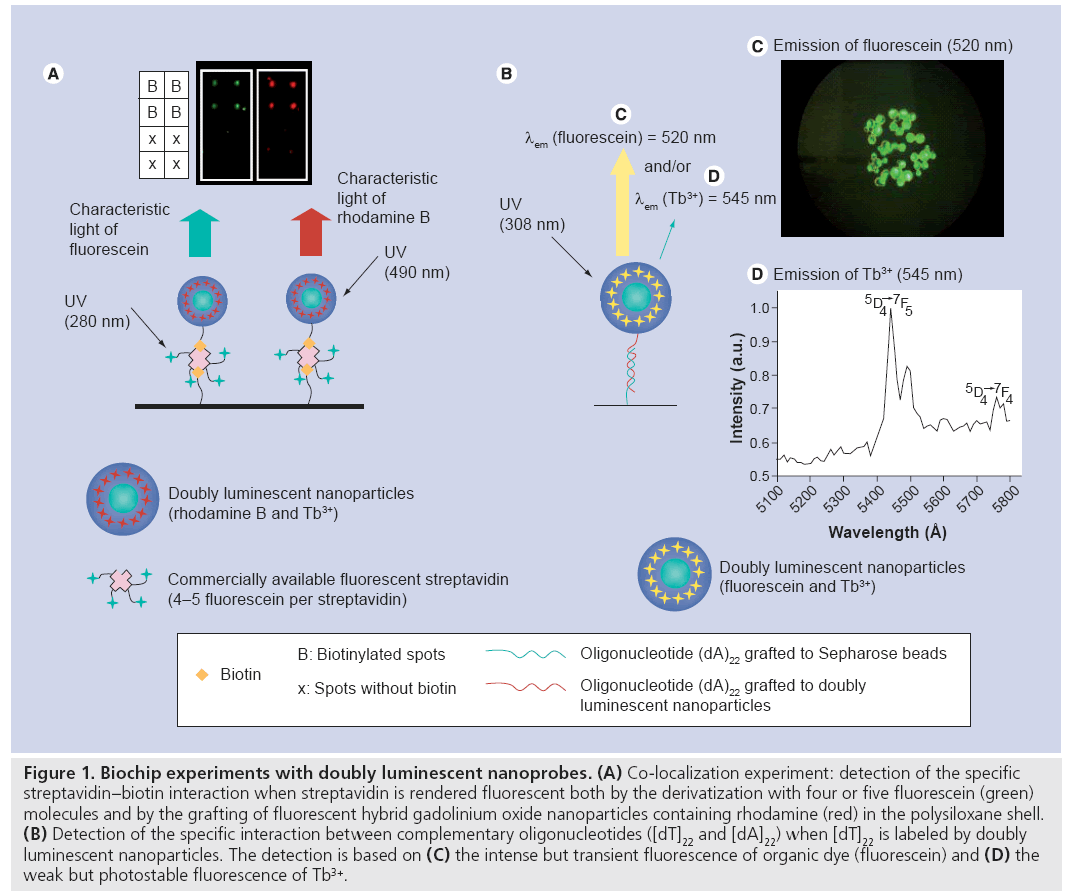
The potential of hybrid gadolinium oxide nanoparticles as markers was evaluated by an experiment based on the co-localization of fluorescent nanoparticles and SAV, which is well known for its specific and quasi-irreversible interaction with biotine (Figure 1A) [71]. The colocalization experiment was based on the use of a biochip with four biotinylated spots, four spots without biotin and of commercially available fluorescein conjugated SAV (FITC-SAV, λem = 512 nm). The latter was bound to the hybrid gadolinium oxide nanoparticles whose inner part of the polysiloxane shell is functionalized by rhodamine B (λem = 605 nm). As a result, the labeling of FITC-SAV by hybrid gadolinium oxide can be detected both by the green light emitted by fluorescein (bound to SAV) and by the red light emitted by rhodamine (entrapped in the polysiloxane shell of the nanoparticles). After incubation of the biochip in the colloid containing FITC-SAVlabeled fluorescent nanoparticles, the biochip is observed at two different wavelengths. At 280 nm, green light characteristic of fluorescein is emitted from the four biotinylated spots, whereas red light characteristic of rhodamine B is emitted from these spots when they are exposed to the monochromatic light whose wavelength is 490 nm. Furthermore, no light is emitted from the spots devoid of biotin whatever the wavelengths. The fact that both green and red lights are emitted from the same spots of the biochip (but at different excitation wavelengths) indicates that FITC-SAV and fluorescent gadolinium oxide nanoparticles are located at the same spots. From this experiment, it can be deduced that the grafting of SAV onto the nanoparticles was successfully performed and that the specific interaction of SAV with biotin is preserved when SAV is labeled by nanoparticles. The immobilization of SAV can be carried out after surface derivatization of nanoparticles with either biotine (owing to the specific noncovalent interaction between SAV and biotin) [67] or with a rigid spacer phenylene diisothiocyante, whose two isothiocyanante ends allow covalent coupling with one of the amino groups of SAV and of the polysiloxane shell [71]. Since no alteration of the behavior of labeled SAV was observed, multifunctional gadolinium oxide nanoparticles appear to be efficient fluorescent markers for sensing application based on the specific and quasi-irreversible interaction between biotine and SAV, which is largely exploited for the detection assays.
Figure 1: Biochip experiments with doubly luminescent nanoprobes. (A) Co-localization experiment: detection of the specific streptavidin–biotin interaction when streptavidin is rendered fluorescent both by the derivatization with four or five fluorescein (green) molecules and by the grafting of fluorescent hybrid gadolinium oxide nanoparticles containing rhodamine (red) in the polysiloxane shell. (B) Detection of the specific interaction between complementary oligonucleotides ([dT]22 and [dA]22) when [dT]22 is labeled by doubly luminescent nanoparticles. The detection is based on (C) the intense but transient fluorescence of organic dye (fluorescein) and (D) the weak but photostable fluorescence of Tb3+.
Owing to the high content of organic dyes (fluorescein, rhodamine or cyanine 5) whose fluorescence is very intense (high quantum yield) but transient (very short lifetime; 4 ns for FITC and 3.6–4.1 ns for FITC in polysiloxane shell [75]), a lowering of the detection threshold was noticed compared with commercially available fluorescent markers [70]. Indeed, the presence of a large amount of organic dyes entrapped inside each particle, in other words labeling of biomolecules by a multitude of fluorophores, facilitates detection with classical devices owing to signal amplification. Since the light emitted by organic dyes present in the polysiloxane shell of the hybrid gadolinium oxide nanoparticles is very intense, the light emission from Tb3+ ions present in the oxide core is masked. However, the intense light of the organic dyes vanishes quickly under exposure to light owing to their weak photostability. By contrast, luminescent RE ions are highly photostable and have a long luminescence lifetime following excitation (1.4 ms for Tb3+). Figure 1B demonstrates that the hybridization of two complementary oligonucleotides can be easily detected by intense but transient light emitted from FITC and covalently bound to the inner part of the polysiloxane shell, but also by the photostable light of Tb3+ ions in the core despite the low intensity. Indeed Sepharose™ beads, which are coated by (dA)22 strands, become strongly fluorescent when they are incubated with the complementary (dT)22 oligonucleotides labeled with doubly fluorescent nanoparticles. The intense fluorescence is assigned to fluorescein derivatives that are covalently bound to the inner part of the polysiloxane shell since the fluorescence was getting less and less intense during the exposure to the excitation light. After the complete photobleaching of the fluorescein (Figure 1C), the light emitted by Tb3+ ions can be detected (Figure 1D). The specific interaction of biomolecules can, therefore, be detected by the intense but transient light emitted from fluorescein and also by the weak but photostable light from Tb3+ ions. The possibility to detect the specific interaction of biomolecules by different techniques improves the reliability of the experiments. These bioconjugated, doubly luminescent nanoparticles composed of a gadolinium core doped by luminescent Tb3+ ions and a fluorescent polysiloxane shell, appear as very efficient nanoprobes for the specific detection of biomolecules [70,76]. Furthermore, long-term studies and time-resolved luminescence of biomolecules labeled by doubly luminescent nanoparticles can be undertaken due to the presence of Tb3+ ions in the oxide matrix, which give a long-lived and photostable luminescence signal.
Hybrid gadolinium oxide nanoparticles combining imaging & therapy
The main interest of these nanoparticles lies in the presence of gadolinium element. In vitro experiments demonstrated that these nanoparticles exert a significant influence on the relaxation-time of water protons, which reflects the positive contrast enhancement that is more pronounced than the one induced by Gd-DTPA, a widely used molecular contrast agent for clinical diagnosis (8.8 vs 4.1 s-1mM-1 per Gd3+ and 3700 vs 4.1 s-1mM-1 per contrast agent) [66]. This contrast enhancement associated to the fluorescence of near-infrared organic dyes that are covalently bound to the inner part of the polysiloxane shell make these particles very attractive for medical imaging [66] provided that the biodistribution and pharmacokinetics of these hybrid gadolinium oxide nanoparticles are well controlled. Nevertheless, two isotopes of gadolinium (155Gd and 157Gd) are characterized by a huge neutron capture cross-section 66- (for 157Gd) and 16- (for 155Gd) times higher than the one of 10boron, which is the most widely used for the elaboration of N-chlorotaurine agents [25,77]. Gadolinium oxide nanoparticles appear to be promising substitutes for clinically approved N-chlorotaurine agents that are based on 10boron. Finally, the relatively high atomic number of gadolinium is very interesting for radiosensitizer applications [63]. Thanks to the presence of Gd3+ in the oxide core, the application of these hybrid nanoparticles is not restricted to the fluorescence-based detection assay of biomolecules. The combination, in the same nanoparticle of dual modality of imaging (fluorescence imaging resulting from the entrapment of organic dyes in the polysiloxane shell and MRI, owing to the presence of Gd3+) and of remotely controlled therapeutic activity of gadolinium element, opens the door to image-guided therapy. In other words, the tumoricide effect of hybrid gadolinium oxide nanoparticles can only be initiated by an external physical stimulus (x-ray or thermal neutron beam) when the accumulation of nanoparticles in the diseased tissue is sufficiently different as compared with the surrounding healthy tissues, which is certified by fluorescence imaging and MRI. This strategy should improve the tumor destruction and also better preserve healthy tissue. A better therapeutic efficiency is, therefore, expected, provided that the nanoparticles are sufficiently small to escape from the leaky microvasculature of malignant tumors (upper limit of the pore size 12 nm) [78]. To reveal the potential of hybrid gadolinium oxide nanoparticles for image-guided therapy, experiments on cells and on small animals were carried out.
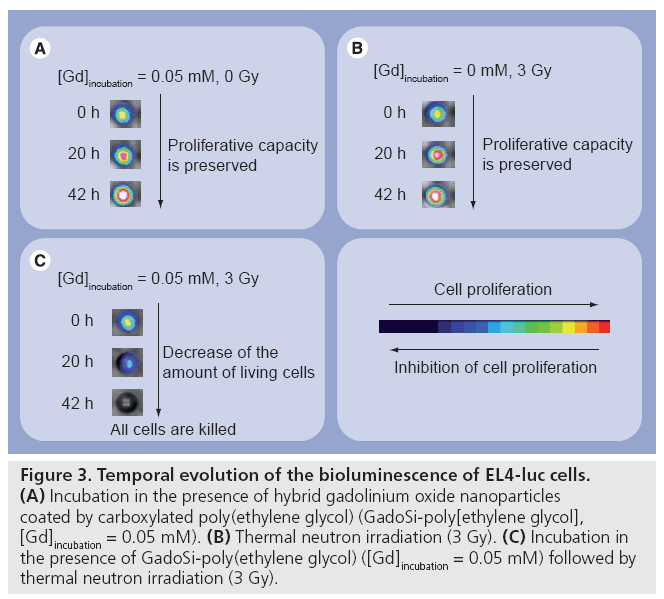
Poly(ethylene glycol)-ylated hybrid gadolinium oxide nanoparticles can be internalized in various cells when the particles are mixed with cells for at least 10 min [79]. The uptake of the nanoparticles was revealed by fluorescence imaging and by MRI (Figure 2). After 1 h incubation, cells became highly fluorescent. As seen in Figure 2, various organic dyes (rhodamine B isothiocyanate and FITC) can be entrapped in the polysiloxane shell of the particles. As compared with unloaded cells (i.e., cells that were never in contact with the nanoparticles), cells that were incubated with fluorescent and paramagnetic gadolinium oxide nanoparticles exhibited an enhancement of the positive contrast of T1-weighted MR images. Since fluorescence imaging shows the accumulation of dye-labeled gadolinium oxide nanoparticles in the cytoplasm of the cells (Figure 2), this enhancement can be assigned to the presence of the nanoparticles. Owing to a higher sensitivity, quicker acquisition and easier handling, fluorescence imaging is a better-suited tool than MRI is for monitoring the uptake from cells. However, the possibility to track the labeled cells by MRI is attractive when cells are used as cargo for the vectorization of nanoparticles to diseased tissues. Viability tests (using MTT assay-proliferation kit) demonstrated that the mortality of cells (human fibroblast and MCF7 cells) after incubation was in the same range as cells that were not in contact with the nanoparticles (control) [79]. Furthermore, the presence of hybrid gadolinium oxide nanoparticles in the cells does not alter the proliferative capacity. Figure 3A depicts the evolution of the bioluminescence of murine lymphoma cells (EL4-luc) after the internalization of hybrid gadolinium oxide nanoparticles. The bioluminescence of EL4-luc that resulted from the transfection by the luciferase coding gene, reflects the metabolic activity of the cells. The increase in bioluminescence over time of hybrid oxide nanoparticle-loaded EL4-luc cells indicates that the cells proliferate. Furthermore, this evolution is very similar to the one observed for negative control. The proliferative capacity of EL4-luc cells was preserved despite the uptake of the nanoparticles. The preliminary studies performed on cells demonstrated that nanoparticles composed of a paramagnetic gadolinium oxide core and a fluorescent and hydrophilic polysiloxane shell are not cytotoxic and do not alter the metabolic activity and the proliferative capacity of cells. However, the activation of these biocompatible nanoparticles by harmless thermal neutron beam induced a killing effect (Figure 3C) whereas the uptake of these nanoparticles in EL4-luc cells and the irradiation of unlabeled cells are harmless (Figures 3A & 3B). Indeed, the administration of a 3 Gy dose to labeled EL4-luc cells induces the decrease of bioluminescence intensity (Figure 3C), whereas the irradiation of unlabeled cells with the same dose or the labeling of the cells with the same [Gd]incubation does not affect the proliferative capacity (i.e., the bioluminescence intensity increases in both cases) (Figures 3A & B). The application of the hybrid gadolinium oxide nanoparticles can, therefore, be envisaged for neutron capture therapy since cytotoxicity is activated by a harmless neutron beam [80]. This preliminary study performed on cells clearly demonstrates the potential of hybrid gadolinium oxide nanoparticles for image-guided therapy. However, this attractive feature must be validated by experiments on small animals.
Figure 2: Fluorescence imaging and MRI of fibroblast and MCF7 cells. (A) Confocal laser scanning microscopic images of cells after 1 h incubation at 37°C with hybrid gadolinium oxide nanoparticles containing either fluorescein (green) or rhodamine (red) as organic dyes in the polysiloxane shell ([Gd]incubation = 0.2 mM). The cells are stained by the uptake of the nanoparticles. Particles are located exclusively in the cytoplasm and stay excluded from the nucleus. (B) T1-weighted images of agar gel-containing cells. Magnetic resonance images of the cells incubated with hybrid gadolinium oxide nanoparticles ([Gd]incubation = 0.3 mM) exhibit brighter contrast than negative control does ([Gd]incubation = 0.0 mM).
Figure 3: Temporal evolution of the bioluminescence of EL4-luc cells. (A) Incubation in the presence of hybrid gadolinium oxide nanoparticles coated by carboxylated poly(ethylene glycol) (GadoSi-poly[ethylene glycol], [Gd]incubation = 0.05 mM). (B) Thermal neutron irradiation (3 Gy). (C) Incubation in the presence of GadoSi-poly(ethylene glycol) ([Gd]incubation = 0.05 mM) followed by thermal neutron irradiation (3 Gy).
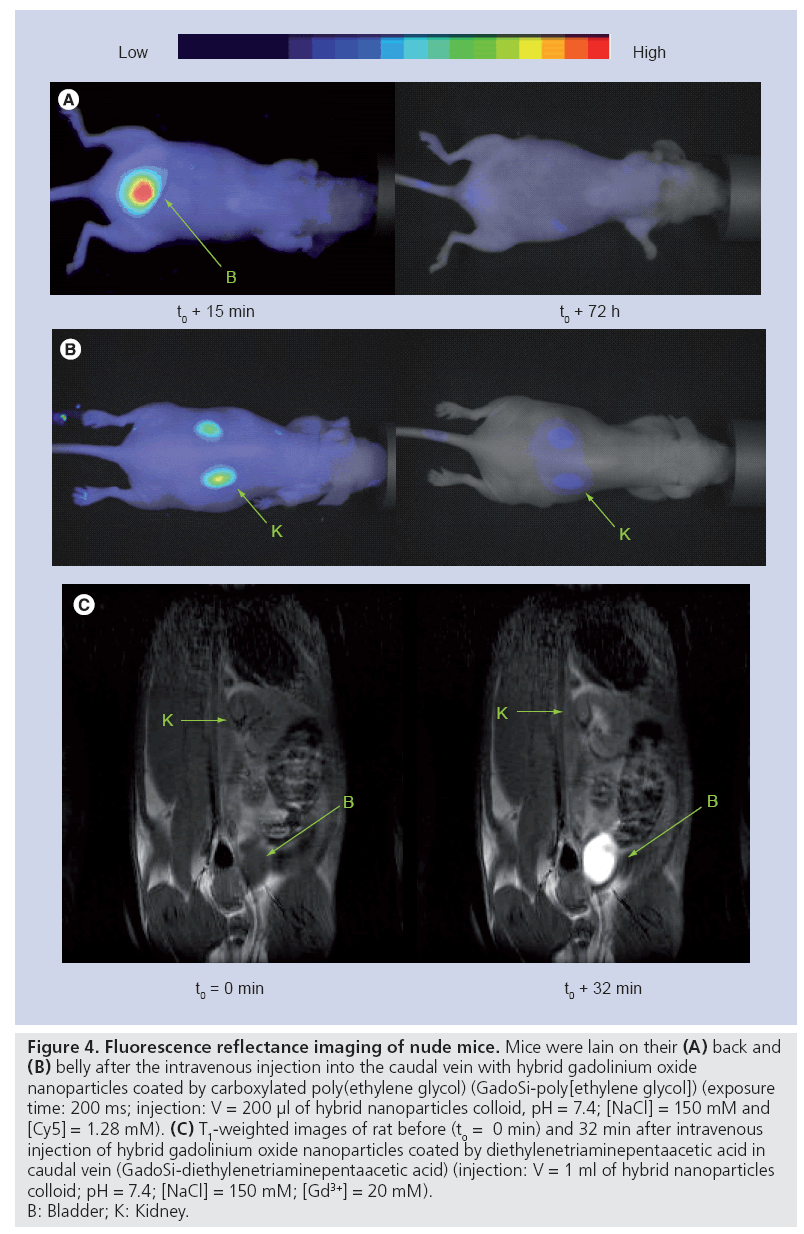
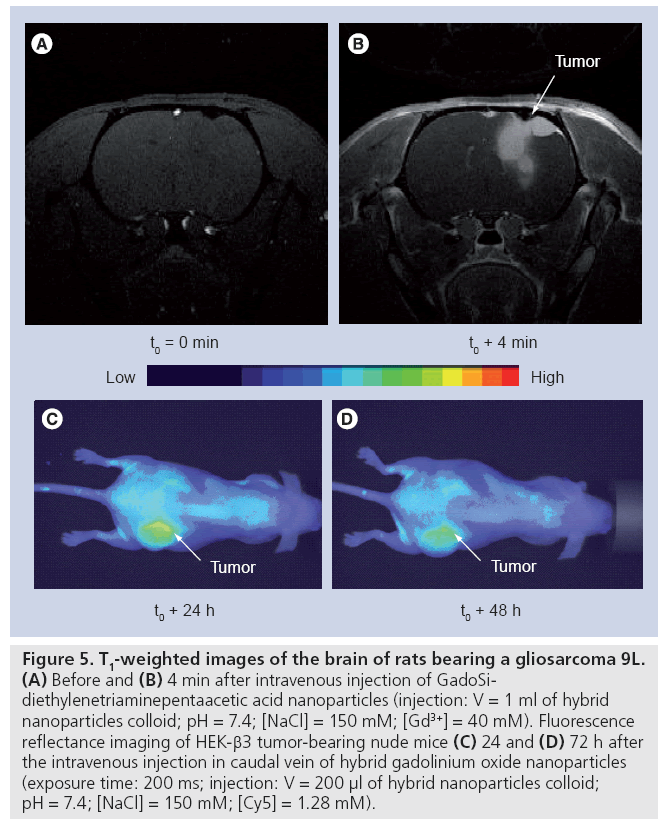
Prior to evaluating the therapeutic activity of these particles on small animals, the influence of the postfunctionalization of the hybrid gadolinium oxide nanoparticles by hydrophilic molecules on the biodistribution and the pharmacokinetics was investigated as the potential of therapeutic agents can be seriously affected by an undesirable accumulation in healthy tissue or by a too quick or too slow removal from living bodies. In a previous study, we demonstrated that the biodistribution and the retention time depend on the length of PEG chains and of the terminal end group [72]. The derivatization of gadolinium oxide-fluorescent polysiloxane coreshell nanoparticles by small carboxylated PEG (PEG-COOH; molecular weight = 250 gmol-1) or DTPA (GadoSiPEG and GadoSiDTPA, respectively; Dh: 10–12 nm) prevents undesirable accumulations in the liver, spleen and lungs. The follow-up by MRI and fluorescence imaging after intravenous injection of these nanoparticles reveals that they circulate freely in the bloodstream before elimination by renal excretion (Figure 4). However, the intravenous injection of these particles to gliosarcomebearing rats led to a positive contrast enhancement of MR images in the periphery of the tumor (Figures 5A & B) [101]. The enhancement of contrast, which reflects the presence of the nanoparticles in the tumor zone, is observed for at least 30 min after the intravenous injection (Figure 5B). In the case of mice bearing a HEK-b3 tumor, such a passive accumulation is observed by fluorescence imaging (Figures 5C & D) 24 and 72 h after the intravenous injection of hybrid gadolinium oxide nanoparticles. The tumor zone remains more fluorescent than the surrounding tissue. If the contrast enhancement can be explained by a denser vasculature around the tumor, the remanence of the contrast probably results from the passive accumulation in the tumor that is based on the enhanced permeability and retention effect caused by the increased capillary permeability in the tumor vasculature. Such a passive accumulation is possible owing to the small size of the nanoparticles (Dh: 10–12 nm). The observation is, therefore, consistent with the size of the pores of the leaky microvasculature of malignant tumors [78,81].
Figure 4: Fluorescence reflectance imaging of nude mice. Mice were lain on their (A) back and (B) belly after the intravenous injection into the caudal vein with hybrid gadolinium oxide nanoparticles coated by carboxylated poly(ethylene glycol) (GadoSi-poly[ethylene glycol]) (exposure time: 200 ms; injection: V = 200 μl of hybrid nanoparticles colloid, pH = 7.4; [NaCl] = 150 mM and [Cy5] = 1.28 mM). (C) T1-weighted images of rat before (to = 0 min) and 32 min after intravenous injection of hybrid gadolinium oxide nanoparticles coated by diethylenetriaminepentaacetic acid in caudal vein (GadoSi-diethylenetriaminepentaacetic acid) (injection: V = 1 ml of hybrid nanoparticles colloid; pH = 7.4; [NaCl] = 150 mM; [Gd3+] = 20 mM). B: Bladder; K: Kidney.
Figure 5: T1-weighted images of the brain of rats bearing a gliosarcoma 9L. (A) Before and (B) 4 min after intravenous injection of GadoSidiethylenetriaminepentaacetic acid nanoparticles (injection: V = 1 ml of hybrid nanoparticles colloid; pH = 7.4; [NaCl] = 150 mM; [Gd3+] = 40 mM). Fluorescence reflectance imaging of HEK-β3 tumor-bearing nude mice (C) 24 and (D) 72 h after the intravenous injection in caudal vein of hybrid gadolinium oxide nanoparticles (exposure time: 200 ms; injection: V = 200 μl of hybrid nanoparticles colloid; pH = 7.4; [NaCl] = 150 mM; [Cy5] = 1.28 mM).
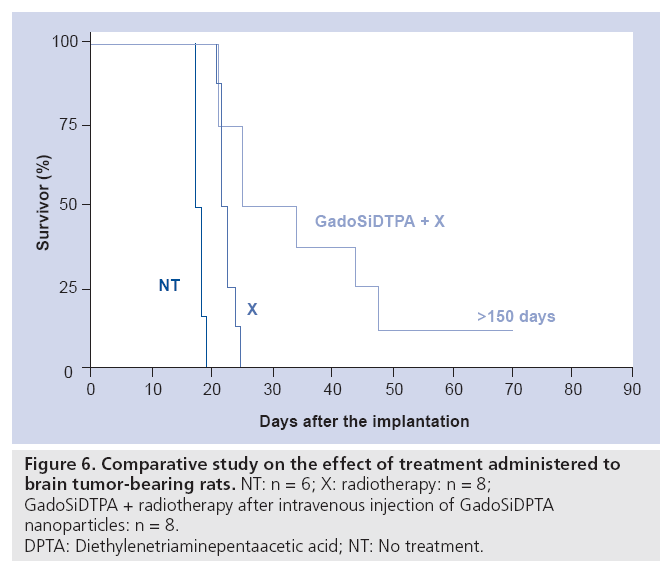
The strong and persistent contrast enhancement of the tumor observed in MR images (Figure 5B) allows an obvious delineation of the tumor and of healthy tissue. The passive accumulation of hybrid gadolinium oxide in the tumor was exploited for radiotherapy. The irradiation of tumor-bearing rats with x-ray microbeam 20 min after GadoSiDTPA injection led to a longer survival of the rats (Figure 6). As compared with the median and mean survival time of nontreated rats (n = 6), the rats that received GadoSiDTPA nanoparticles and were submitted to x-ray beam (n = 8) exhibit higher medians (29.5 vs 17.5 days) and mean survival times (36.75 ± 17.5 days vs 17.66 ± 0.33 days). Furthermore, among diseased rats that were treated by radiotherapy after intravenous injection of GadoSiDTPA nanoparticles there is one long-term survivor. Therefore, hybrid gadolinium oxide nanoparticles can act both as contrast agents for in vivo imaging (fluorescence imaging and MRI) and agents for cancer therapy whose therapeutic activity is remotely controlled by x-ray (radiotherapy) or neutron beams (neutron capture therapy).
Figure 6: Comparative study on the effect of treatment administered to
brain tumor-bearing rats. NT: n = 6; X: radiotherapy: n = 8;
GadoSiDTPA + radiotherapy after intravenous injection of GadoSiDPTA
nanoparticles: n = 8.
DPTA: Diethylenetriaminepentaacetic acid; NT: No treatment.
Conclusion
The studies on hybrid gadolinium oxide nanoparticles (i.e., nanoparticles with a gadolinium oxide core encapsulated in a fluorescent and hydrophilic polysiloxane shell) revealed that these nanoparticles exhibit promising potential for sensor applications and image-guided therapy. As demonstrated, the gadolinium oxide core gives the nanoparticles paramagnetic properties and a strong propensity to absorb x-ray photons and thermal neutrons that can be exploited for MRI, radiotherapy and neutron capture therapy, provided that a very high colloidal stability in biological fluids is ensured and that the biodistribution is accurately controlled. The encapsulation of the cores in a polysiloxane shell provides the opportunity to precisely tune the behavior of the resulting MRI contrast and therapeutic agents since it contains amino groups. The latter facilitates the derivatization of the nanoparticles by highly hydrophilic molecules and bioconjugation. Furthermore, the core-shell structure of these hybrid nanoparticles allows the combination of two complementary fluorescence modes: the intense but transient fluorescence of the organic dyes covalently bound to the polysiloxane shell, and the long-lived and photostable luminescence of RE ions, such as terbium and europium. As a result, paramagnetic gadolinium oxide core encapsulated in a fluorescent and hydrophilic shell provides two classes of nanoparticles: doubly luminescent nanoparticles for more reliable sensing applications and nanoparticles for image-guided therapy. Experiments on lymphoma cells and on tumor-bearing rats demonstrated that the hybrid gadolinium oxide nanoparticles are rendered cytotoxic and tumoricidal after the interaction with an external physical stimulus (x-ray or neutron beam). This remotely controlled therapeutic activity makes the nanoparticles containing gadolinium very attractive, since the treatment can be initiated only when the particles are preferentially located in the diseased zone. Therefore, the possibility to follow-up these particles by fluorescence and MRI constitutes a crucial feature (but not sufficient) for the successful development of original therapeutic agents.
To improve the therapeutic efficiency of these gadolinium oxide nanoparticles, current studies are focused on the conjugation of biotargeting groups in order to selectively target the tumor.
Future perspective
Hybrid gadolinium oxide nanoparticles appear as an excellent substitute to the molecular gadolinium chelates that are commonly used as contrast agents for clinical MRI and have been proposed to replace boron compounds in the case of the neutron capture therapy. Besides the drawbacks inherent to the molecular state, gadolinium chelates were incriminated in the nephrogenic systemic fibrosis cases. Indeed, the presence of endogenous calcium, zinc and iron ions generates the leakage of toxic gadolinium. Such a competition can not occur in the case of gadolinium oxide nanoparticles. This safer behavior that was strengthened by the encapsulation in the polysiloxane shell and the multifunctional character that gives the resulting nanoparticles the ability to combine detection, imaging and therapy, constitute solid arguments for replacing gadolinium chelates by gadolinium oxide nanoparticles.
Financial & competing interests disclosure
This work was supported by a grant (JL Bridot) from the regional council of Rhône-Alpes (France), by a grant (AC Faure) from the French Ministry of Research (Ministère de l’Enseignement Supérieur et de la Recherche, France) and by the national agency of research Agence Nationale de la Recherche ANR-05-NANO-037–02 (C Mandon, C Billotey, M Janier, P Perriat, S Roux and O Tillement). COST D38 is acknowledged for its financial support and for fruitful discussions with the members of this network. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Ferrari M: Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer 5, 161–171 (2005).
- Piao Y, Burns A, Kim J, Wiesner U, Hyeon T: Designed fabrication of silica-based nanostructured particles systems for nanomedicine applications. Adv. Funct. Mater. 18, 1–14 (2008).
- Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H: Nanomedicine – challenge and perspectives. Angew. Chem. Int. Ed. 48, 872–897 (2009).
- Sarin H: Recent progress towards the development of effective systemic chemotherapy for the treatment of malignant brain tumors. J. Translational Med. 7, 77 (2009).
- Yong KT, Roy I, Swihart MT, Prasad PN: Multifunctional nanoparticles as biocompatible targeted probes for human cancer diagnosis and therapy. J. Mater. Chem. 19, 4655–4672 (2009).
- Kim J, Piao Y, Hyeon T: Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 38, 372–390 (2009).
- Gao JH, Gu HW, Xu B: Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc. Chem. Res. 42, 1097–1107 (2009).
- Boisselier E, Astruc D: Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 38, 1759–1782 (2009).
- Sharma R, Chen CJ: Newer nanoparticles in hyperthermia treatment and thermometry. J. Nanopart. Res. 11, 671–689 (2009).
- Dennis CL, Jackson AJ, Borchers JA et al.: Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology 20, 395103 (2009).
- Thomas LA, Dekker L, Kallumadil M et al.: Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J. Mater. Chem. 19, 6529–6535 (2009).
- Laurent S, Forge D, Port M et al.: Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008).
- Hainfeld JF, Slatkin DN, Focella M, Smilowitz HM: Gold nanoparticles: a new x-ray contrast agents. Brit. J. Radiol. 79, 248–253 (2006).
- Popovtzer R, Agrawal A, Kotov NA et al.: Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 8, 4593–4596 (2008).
- Hainfeld JF, Slatkin DN, Smilowitz HM: The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 49, N309–N315 (2004).
- Alric C, Serduc R, Mandon C et al.: Gold nanoparticles designed for combining dual modality imaging and radiotherapy. Gold Bull. 41, 90–97 (2008).
- Loo C, Lowery A, Halas N, West J, Drezek R: Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 5, 709–711 (2005).
- Huang X, El-Sayed IH, Qian W, El-Sayed M: Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128, 2115–2120 (2006).
- Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL: Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 7, 1929–1934 (2007).
- Chen J, Wang D, Xi J et al.: Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 7, 1318–1322 (2007).
- Debouttière PJ, Roux S, Vocanson F et al.: Design of gold nanoparticles for magnetic resonance imaging. Adv. Funct. Mater. 16, 2330–2339 (2006).
- Alric C, Taleb J, Le Duc G et al.: Gadolinium chelate coated gold nanoparticles as contrast agents for both x-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 130, 5908–5915 (2008).
- Caravan P, Ellison JJ, McMury TJ, Lauffer RB: Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 99, 2293–2352 (1999).
- Bottrill M, Kwok L, Long NJ: Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 35, 557–571 (2006).
- Soloway AH, Tjarks W, Barnum BA et al.: The chemistry of neutron capture therapy. Chem. Rev. 98, 1515–1562 (1998).
- Garber K: Neutron Cross Sections Report A, BNI-325 (3rd Edition). Brookhaven National Laboratory, NY, USA (1976).
- Shi JL, Brugger RM: Gadolinium as a neutron capture therapy agent. Med. Phys. 19, 733–744 (1992).
- Hofmann B, Fischer CO, Lawaczeck R et al.: Gadolinium neutron capture therapy (GdNCT) of melanoma cells and solid tumors with the magnetic resonance imaging contrast agent gadobutrol. Invest. Radiol. 34, 126–133 (1999).
- De Stasio C, Casalbore P, Pallini R et al.: Gadolinium in human glioblastoma cells for gadolinium neutron capture therapy. Cancer Res. 61, 4272–4277 (2001).
- Li SD, Huang L: Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharmaceutics 5, 496–504 (2008).
- Wiener EC, Auteri FP, Chen JW et al.: Molecular dynamics of ion-chelate complexes attached to dendrimers. J. Am. Chem. Soc. 118, 7774–7782 (1996).
- Daldrup-Link HE, Brasch RC: Macromolecular contrast agents for MR mammography: current status. Eur. Radiol. 13, 354–365 (2003).
- Farokhzad OC, Langer R: Impact of nanotechnology in drug delivery. ACS Nano 3, 16–20 (2009).
- Huber MM, Staubli AB, Kustedjo K et al.: Fluorescently detectable magnetic resonance imaging agents. Bioconjug. Chem. 9, 242–249 (1998).
- Yan GP, Liu ML, Li LY: Polyaspartamide gadolinium complexes containing sulfadiazine groups as potential macromolecular MRI contrast agents. Bioconjug. Chem. 16, 967–971 (2005).
- Bull SR, Guler MO, Bras RE, Venkatasubramanian PN, Stupp SI, Meade TJ: Magnetic resonance imaging of self-assembled biomaterial scaffolds. Bioconjug. Chem. 16, 1343–1348 (2005).
- Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI: Self-assembled peptide amphiphile nanofibers conjugated to MRI contrast agents. Nano Lett. 5, 1–4 (2005).
- Anderson EA, Isaacman S, Peabody DS, Wang EY, Canary JW, Kirshenbaum K: Viral nanoparticles donning a paramagnetic coat: conjugation of MRI contrast agents to the MS2 capsid. Nano Lett. 6, 1160–1164 (2006).
- Langereis S, de Lussanet QG, van Genderen MHP, Backes WH, Meijer EW: Multivalent contrast agents based on gadolinium– diethylenetriaminepentaacetic acid-terminated poly(propylene imine) dendrimers for magnetic resonance imaging. Macromolecules 37, 3084–3091 (2004).
- Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW: Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 6, 1459–1463 (2006).
- Mulder WJM, Strijkers GJ, Griffioen AW et al.: A liposomal system for contrastenhanced magnetic resonance imaging of molecular targets. Bioconjug. Chem. 15, 799–806 (2004).
- Accardo A, Tesauro D, Roscigno P et al.: Physicochemical properties of mixed micellar aggregates containing CCK peptides and Gd complexes designed as tumor specific contrast agents in MRI. J. Am. Chem. Soc. 126, 3097–3107 (2004).
- Parac-Vogt TN, Kimpe K, Laurent S et al.: Gadolinium DTPA-monoamide complexes incorporated into mixed micelles as possible MRI contrast agents. Eur. J. Inorg. Chem. 17, 3538–3543 (2004).
- Reynolds CH, Annan N, Beshah K et al.: Gadolinium-loaded nanoparticles: new contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 122, 8940–8945 (2000).
- Turner JL, Pan D, Plummer R, Chen Z, Whittaker AK, Wooley KL: Synthesis of gadolinium-labeled shell-crosslinked nanoparticles for magnetic resonance imaging applications. Adv. Funct. Mater. 15, 1248–1254 (2005).
- Toth E, Bolskar RD, Borel A et al.: Water-soluble gadofullerenes: toward high-relaxivity, pH-responsive MRI contrast agents. J. Am. Chem. Soc. 127, 799–805 (2005).
- Sitharaman B, Kissell KR, Hartman KB et al.: Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem. Commun. 3915–3917 (2005).
- Balkus KJ, Shi J: A study of suspending agents for gadolinium(III)-exchanged hectorite – an oral magnetic resonance imaging contrast agent. Langmuir 12, 6277–6281 (1996).
- Lin YS, Hung Y, Su JK et al.: Gadolinium(III)-incorporated nanosized mesoporous silica as potential magnetic resonance imaging contrast agents. J. Phys. Chem. B 108, 15608–15611 (2004).
- Frias JC, Williams KJ, Fisher EA, Fayad ZA: Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 126, 16316–16317 (2004).
- Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA: Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 6, 2220–2224 (2006).
- Mulder WJ, Koole R, Brandwijk RJ et al.: Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 6, 1–6 (2006).
- van Tilborg GA, Mulder WJ, Chin PT et al.: Annexin A5-conjugated quantum dots with a paramagnetic lipidic coating for the multimodal detection of apoptotic cells. Bioconjug. Chem. 17, 865–868 (2006).
- Vuu K, Xie J, McDonald MA et al.: Gadolinium-rhodamine nanoparticles for cell labeling and tracking via magnetic resonance and optical imaging. Bioconjug. Chem. 16, 995–999 (2005).
- Ghaghada K, Hawley C, Kawaji K, Annapragada A, Mukundan S: T1 relaxivity of core-encapsulated gadolinium liposomal contrast agents – effect of liposome size and internal gadolinium concentration. Acad. Radiol. 15, 1259–1263 (2008).
- Roberts D, Zhu WL, Frommen CM, Rosenzweig Z: Synthesis of gadolinium oxide magnetoliposomes for magnetic resonance imaging. J. Appl. Phys. 87, 6208–6210 (2000).
- Engström M, Klasson A, Pedersen H, Vahlberg C, Käll PO, Uvdal K: High proton relaxivity for gadolinium oxide nanoparticles. Magn. Reson. Mater. Phy. 19, 180–186 (2006).
- McDonald MA, Watkin KL: Small particulate gadolinium oxide and gadolinium oxide albumin microspheres as multimodal contrast and therapeutic agents. Invest. Radiol. 38, 305–310 (2003).
- Evanics F, Diamente PR, van Veggel F, Stanisz GJ, Prosser RS: Water-soluble GdF3 and GdF3/LaF3 nanoparticles: physical characterization and NMR relaxation properties. Chem. Mater. 18, 2499–2505 (2006).
- Kumar R, Nyk M, Ohulchanskyy TY, Flask CA, Prasad PN: Combined optical and MR bioimaging using rare earth ion doped NaYF4 nanocrystals. Adv. Funct. Mater. 19, 853–859 (2009).
- Li IF, Su CH, Sheu HS et al.: Synthesis and applications of magnetic resonance contrast agents. Adv. Funct. Mater. 18, 766–776 (2008).
- Hu KW, Jhang F, Su CH, Yeh CS: Fabrication of Gd2O(CO3)2.H2O/silica/gold hybrid particles as a bifunctional agent for MR imaging and photothermal destruction of cancer cells. J. Mater. Chem. 19, 2147–2153 (2009).
- Prezado Y, Fois G, Le Duc G, Bravin A: Gadolinium dose enhancement studies in microbeam radiation therapy. Med. Phys. 36, 3568–3574 (2009).
- Bazzi R, Flores MA, Louis C et al.: Synthesis and properties of europium-based phosphors on the nanometer scale: Eu2O3, Gd2O3:Eu, and Y2O3. Eur. J. Colloid Interface Sci. 273, 191–197 (2004).
- Bazzi R, Flores-Gonzalez MA, Louis C et al.: Synthesis and luminescent properties of sub-5-nm lanthanide oxides nanoparticles. J. Lumin. 102–103, 445–450 (2003).
- Bridot JL, Faure AC, Laurent S et al.: Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J. Am. Chem. Soc. 129, 5076–5084 (2007).
- Louis C, Lebbou K, Flores-Gonzalez MA et al.: Correlation of the structure and the luminescence properties of Eu3+-doped Gd2O3 oxide between fiber single crystal and the nano-size powders. J. Crystal Growth 265, 459–465 (2004).
- Flores-Gonzalez M, Ledoux G, Roux S, Lebbou K, Perriat P, Tillement O: Preparing nanometer scaled Tb-doped Y2O3 luminescent powders by the polyol method. J. Solid State Chem. 178, 989–997 (2005).
- Flores-Gonzalez MA, Louis C, Bazzi R et al.: Elaboration of nanostructured Eu3+-doped Gd2O3 phosphor fine spherical powders using polyol-mediated synthesis. Appl. Phys. A: Mater. Sci. Process. A81, 1385–1391 (2005).
- Louis C, Bazzi R, Marquette CA et al.: Nanosized hybrid particles with double luminescence for biological labelling. Chem. Mater. 17, 1673–1682 (2005).
- Faure AC, Hoffmann C, Bazzi R et al.: Functionalization of luminescent aminated particles for facile bioconjugation. ACS Nano 2, 2273–2282 (2008).
- Faure AC, Dufort S, Josserand V et al.: Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethylene glycol) coatings. Small 5, 2565–2575 (2009).
- Louis C, Roux S, Ledoux G et al.: Luminescence enhancement by energy transfer in core-shell structures. Chem. Phys. Lett. 429, 157–160 (2006).
- Louis C, Roux S, Ledoux G et al.: Gold nano-antennas for increasing luminescence. Adv. Mater. 16, 2163–2166 (2004).
- Martini M, Perriat P, Montagna M et al.: How gold particles suppress concentration quenching of fluorophores encapsulated in silica beads. J. Phys. Chem. C 113, 17669–17677 (2009).
- Barbillon G, Faure AC, El Kork N et al.: How nanoparticles encapsulating fluorophores allow a double detection of biomolecules by localized surface plasmon resonance and luminescence. Nanotechnology 19, 035705 (2008).
- Coderre JA, Button TM, Micca PL, Fisher CD, Nawrocky MM, Liu HB: Neutron capture therapy of the 9L rat gliosarcoma using the p-boronphenylalanine-fructose complex. Int. J. Radiat. Oncol. Biol. Phys. 30, 643–652 (1994).
- Sarin H, Kanevsky AS, Wu HT et al.: Physiologic upper limit of pore size in the blood tumor barrier of malignant solid tumors. J. Translational Med. 7, 51 (2009).
- Fizet J, Rivière C, Bridot JL et al.: Multi-luminescent hybrid gadolinium oxide nanoparticles as potential cell labeling. J. Nanosci. Nanotech. 9, 1–9 (2009).
- Bridot JL, Dayde D, Rivière C et al.: Hybrid gadolinium oxide nanoparticles combining imaging and therapy. J. Mater. Chem. 19, 2328–2335 (2009).
- Sarin H, Kanevsky AS, Wu HT et al.: Effective transvascular delivery of nanoparticles across the blood–brain tumor barrier into malignant glioma cells. J. Translational Med. 6, 80 (2008).
- Le Duc G, Roux S, Tillement O et al.: Utilisation de nanoparticules à base de lanthanides comme agents de contraste. WO2009053644 (2009).
• Clear introduction to nanomedicine that emphasizes the potential and limitations of nanotechnology in the fight against cancer.
• • Depicts the multiple facets of nanomedicine.
• • Inventory of various nanomaterials combining different imaging techniques or imaging and therapy.
• • Highlights the numerous opportunities that the gold nanostructures provide for imaging and therapy.
• • An exhaustive state-of-the-art review of magnetic iron oxide nanoparticles for biological applications.
• • Review on gadolinium chelates as MRI contrast agents.
• • After a clear introduction on neutron capture therapy, this article describes the numerous strategies for the development of neutron capture therapy agents.
Patent