Research Article - Diabetes Management (2021)
Evaluation of antidiabetic activity using methanolic extract of Eichhornia crassipes against streptozotocin induced diabetes in rats
- Corresponding Author:
- Vivek Kumar Tiwari
Department of Pharmacology,
Gokaraju Rangaraju College of Pharmacy,
Bachupally,
Kukatpally, Hyderabad,
India
E-mail: vivek.tiwari087@gmail.com
Abstract
Objective: Plants not only provide food for man but also provide number of active compounds with potent and varied therapeutical value. The antioxidant activity and quantitative estimation of Eichhornia crassipes from methanolic extract of whole plant are widely used traditional medicine in many parts of the world for the management, control, and/or treatment of a plethora of human ailments, including diabetes mellitus. Methods: The animals used were divided into five groups. Normal control, Disease Control animals induced with streptozotocin 55 mg/kg bw administered i.p, while treated groups received 200 and 400 mg/kg between of methanolic extract of Eichhornia crassipes (MEEC) administered orally and treatment with standard drug glibenclamide 10 mg/kg between administered i.p daily for 21 days. The antioxidant activities of MEEC are on reducing power assay and hydrogen peroxide scavenging assay using spectrophotometric methods. Statistical analysis was performed as Mean ± Standard Error Mean (SEM). The IC50 values were also calculated by linear regression analysis. Results: The yield of MEEC was found to be 11.2% w/w. The preliminary phytochemical investigation of MEEC revealed the presence of alkaloids, flavonoids, phenolics, sterols, terpenoids, and tannins. Acute toxicity studies revealed the non-toxic nature of MEEC and LD50 was found to be more than 2000 mg/kg bw. The MEEC inhibited the free radicals in dose dependent manner in reducing power assay and hydrogen peroxide scavenging assay. It revealed its antioxidant activity. Blood glucose levels and body weights are checked in streptozotocin induced diabetic rats. Conclusion: It is concluded that the phytochemicals involved in MEEC represent good sources of natural antioxidants. The above results depicted that this plant exhibits significant antidiabetic activity.
Keywords
■ Eichhornia crassipes ■ diabetes mellitus ■ antioxidant activity ■ therapeutical value
Introduction
Diabetes Mellitus (DM) is not a single disease entity, but rather a group of metabolic disorders sharing the common underlying feature of hyperglycemia. Hyperglycemia in diabetes results from defects in insulin secretion, insulin action, or most commonly, both. The chronic hyperglycemia and attendant metabolic dysregulation may be associated with secondary damage in multiple organ system, especially the kidneys, eyes, nerves, and blood vessels [1]. According to Global Health and Aging (2010), 8% of the world’s population is over 65 years of age in 2010, and the number of people over 65 years of age is expected to rise to 16% by the year 2050 [2]. The American Diabetes Association reports that the Fasting Plasma Glucose (FPG) for normal individuals should be <100 mg/dL. Here fasting is defined as, no caloric intake for at least 8 hours (usually implying overnight fasting). Approximately 2 hours after a meal, plasma glucose levels should be less than 140 mg/dL. The diagnosis of diabetes requires fasting glucose levels greater than 126 mg/dL on two occasions [3,4]. Test administration: Blood is drawn from the vein following overnight fasting (for fasti.ng blood glucose levels) or at any random moment during the day (to measure random glucose levels). Risks: There may be some bruising, infection, and soreness at the site of puncture for drawing blood. The subject may also feel some dizziness [5].
The cornerstone of diabetes management is tight glycemic control. The first line of action upon noticing any symptoms of type 2 diabetes should be lifestyle changes, i.e., managing blood glucose levels by regulating diet (both quality and quantity of nutritional intake) and exercise [6,7]. Through these changes, individuals diagnosed as prediabetic can often check their progression to full-blown disease. Individuals diagnosed with diabetes, who are taking medications can also benefit from these lifestyle changes [8,9].
High blood sugar levels can be managed by reducing caloric intake, either by careful portion control or by choosing foods that have lower glycemic loads. Foods made from refined sugars and easily digestible starches, such as white bread, sugary drinks, etc., lead to sudden increases (spikes) in blood glucose levels and are likely to promote metabolic imbalances [10]. On the other hand, foods rich in fibre, such as whole grains, fruits, and vegetables take time to be digested, do not prompt rapid rise in blood glucose levels, and favour metabolic balance. Exercise has many health benefits, the most obvious of which may be improved glucose homeostasis and insulin sensitivity [11,12]. Exercise stimulates skeletal muscles to take up more glucose from the blood. In addition, it reduces the amount of lipids stored in fat cells, while also making them more sensitive to insulin action.
The main strategies for managing blood glucose levels are increasing insulin secretion may be accomplished by various classes of drugs. Some drugs increase release of insulin stored within the pancreatic β-cells, while other drugs promote insulin production and release. Treatment is with a drug, Glibenclamide, an oral antidiabetic drug [13]. Sulfonylureas are a class of organic compounds used in medicine and agriculture. These are antidiabetic drugs widely used in the management of T2D [14]. They act by increasing insulin release from the beta cells in the pancreas. Sulfonylureas bind to close ATP-sensitive K+ (KATP) channels on the cell membrane of pancreatic beta cells, which depolarizes the cell by preventing potassium from exiting. This depolarization opens voltage-gated Ca2+ channels [15]. The rise in intracellular calcium leads to increased fusion of insulin granule with the cell membrane, and therefore increased secretion of mature insulin [16,17].
Eichhornia crassipes commonly known as water hyacinth (family: Pontederiaceae), a native of South America, is one of the free floating macrophytes found in the aquatic environment such a ditches, ponds and lakes. It is rich in phytochemicals [18]. Water hyacinth is a source of many compounds with radical-scavenging activity, such as vitamins, terpenoids, phenolic acids, lignin, stilbenes, alkaloids, sterols and other metabolites with high antioxidant activity [19]. Phenolic compounds have therapeutic potential against different diseases because of their antioxidant property. They are known to possess antiviral, anti-inflammatory, antiulcer, antidiarrheal and antitumor activities [20]. Flavonoids are a group of polyphenolic substances present inmost plants and are responsible for various biochemical and antimicrobial activities. They exert their antioxidant activity via radical scavenging, metal ion chelation and membrane protective efficacy [21,22].
Free radicals or Reactive Oxygen Species (ROS) are produced as by product of normal metabolism also by xenobiotic compounds, drugs or ionizing radiations [23]. Plants also generate ROS as signalling molecules to control various processes such as programmed cell death, pathogen defence and stomatal behavior [24]. The ROS or free radicals are highly toxic which cause damage to genetic material and lipid peroxidation also inactivates membrane bound enzymes. It also causes chronic and degenerative disease like alzheimer, aging, pulmonary disease, cardiovascular disease, cancer and rheumatoid arthritis. Many medicinal plants have great antioxidant potential, antioxidants reduce oxidative stress in cells and therefore are useful in treatment of disease like cancer, cardiovascular and inflammatory diseases [25,26].
Antioxidants are the compounds which possess the ability to protect the cell organelles from damage caused by free radicals induced oxidative stress either by inhibiting the initiation or propagation of oxidative chain reactions [27]. Antioxidants exhibit their antioxidant activity either by inhibiting lipid peroxidation, by scavenging free radicals and active oxygen species, preventing the decomposition of hydrogen peroxides into free radicals or by chelating heavy metal ions [28,29].
The study revealed the antioxidant activity of MEEC of different plant parts that showed therapeutically and pharmacological importance of ethnomedicinal properties [30,31].
Materials and Methods
■ Plant materials
The whole plant of Eichhornia crassipes was collected from a river named Sundalavani Kunta, Balanagar during the month of December 2018. This plant material was identified and authenticated by botanist. The freshly collected plant was cleared from dirt and then the plant were cut into pieces and dried under shade for about 7 days and coarsely powered in a mixer grinder.
■ Preparation of Methanolic Extract of Eichhornia crassipes (MEEC)
The MEEC was prepared as described by Almeida. with some modifications. Briefly, 500 g of powdered material was taken up for Soxhlet extraction process using 1000 ml methanol as a solvent. The solvent was completely removed under reduced pressure using rotary evaporator. The amount of extract obtained was 56 g. The present yield of methanolic extract was found to be 11.2% w/w respectively.
■ Animals
Adult male wistar rats weighing 200-250 g were obtained from the Animal House of the Gokaraju Rangaraju College of Pharmacy, Bachupally, Hyderabad, India (Reg.No.1175/ PO/Re/S/08/CPCSEA) from a stock originally purchased from albino research, Hyderabad, India. The rats were group housed (three per cage) and maintained at 23 ± 2ºC under 12:12 h light/dark cycle with free access to rodent’s chow and tap water. The animal studies were approved by the Institutional Animal Ethics Committee (IAEC), constituted for the purpose of control and supervision of experimental animals by Ministry of Environment and Forests, Government of India, New Delhi, India. The rats were acclimatized for 2 weeks. They were assigned into five groups. Disease Control rats did not receive any extract. Animals in the test groups were orally fed using oral cannula once daily with 200 mg/kg, 400 mg/kg, body weight of extract respectively and standard drug Glibenclamide 10 mg/kg bw was administered intraperitonially (i.p). This should be carried out for 21 days.
■ Drugs and chemicals
Streptozotocin (Albino Research laboratories), Glibenclamide (Apollo pharmacy), Methanol (Crescent trading company), Potassium ferric cyanide, Hydrogen peroxide (SD Fine Chem. Limited), Trichloro acetic acid (SD Fine Chem. Limited), Ferric chloride, Potassium dihydrogen phosphate, Sodium hydroxide, Disodium hydrogen phosphate, Sodium dihydrogen phosphate (Himedia laboratories), Glucose estimation kit (K.K. Diagnostics).
■ Experimental induction of diabetes
Diabetes was induced in rats by using earlier reported method (Umathe, kochar, jain, and Dixit, 2009). In brief, STZ was dissolved in 0.1M sodium citrate buffer, (pH 4.4) and administered at the dose of 55 mg/kg i.p. route. STZ treated rats received 5% glucose solution instead of water for 24 h after injection of STZ in order to reduce death due to hypoglycaemic shock. Blood sample is collected through retro- orbital puncture and fasting blood glucose level will be estimated by glucose oxidase-peroxidase method using semi autoanalyzer. Only animals with fasting blood glucose levels over 250 mg/dl were considered diabetic and used for the further study [32].
■ Treatment schedule
After diabetes induction, a group of rats (n=6) were orally administered MEEC at different doses (200 and 400 mg/kg, bw) and standard drug glibenclamide at a dose of (10 mg/kg, bw) was administered for 21 days. Evaluation of blood glucose levels and body weight: Blood glucose levels were measured with a semi autoanalyzer (Tulip, India). In brief, blood sample was withdrawn from the retro orbital puncture and fasting blood glucose level will be estimated by glucose oxidase-peroxidase method using semi autoanalyzer. During the experiment, blood glucose levels and body weights were verified in the interim (0, 7th, 14th and 21st days) of the treatment.
■ In vitro antioxidant assays
Reducing power assay: Principle: This method is based on the principle of increase in the absorbance of the reaction mixture. Increase in the absorbance indicates an increase in the antioxidant activity. In this method, substances, which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferricyanide (Fe2+), which then reacts with ferric chloride to form ferric ferrous complex that has an absorption maximum at 700 nm.
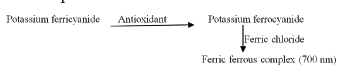
Phosphate buffer pH 6.6: Potassium dihydrogen phosphate (62.5 ml of 0.2 M) was added to 250 ml volumetric flask and also 20.5 ml of 0.2 M NaOH and made up to volume 250 ml with distilled water. Potassium dihydrogen phosphate (0.2 M) solution: Potassium dihydrogen phosphate (2.72 g) was dissolved in distilled water and volume made up to 100 ml. Sodium hydroxide solution (0.2 M) solution: 0.8 g of sodium hydroxide was dissolved in distilled water and volume was made up to 100 ml. Potassium ferric cyanide (1% w/v) solution: Potassium ferricyanide (l g) was dissolved in water and volume was made up to 100 ml in volumetric flash. Ferric chloride solution (0.1% w/v): Ferric chloride (25 mg) was dissolved in distilled water and volume was made up to 25 ml in volumetric flask.
Method: To 1 ml of test and standard compounds, 2.5 ml of potassium ferricyanide (1% WM, 2.5 ml of phosphate buffer pH 6.6 were added and incubated at 50°C for 30. To 2.5 ml of above supernatant liquid 2.5 ml of distilled water and 0.5 ml, 0 Kcl; solution (0.1% w/v) were added. The absorbance of ferric complex was measured using phosphate buffer pH 6.6 as control at 700 nm using UV-Visible spectrophotometer and estimated the increase in absorbance [33]. The percent increase in reducing power was calculated using the following equations,
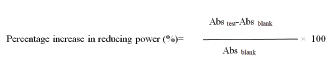
Where ‘Abs test’ is absorbance of test solution, ‘Abs blank’ is absorbance of blank.
Hydrogen peroxide radical scavenging assay: Principle: Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. Hydrogen peroxide can cross cell membranes rapidly, once inside the cell, H2O2 can probably react with Fe2+, and possibly Cu2+ ions to form hydroxyl radical and this may be the origin of many of its toxic effects. It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate.
Preparation of reagents: Phosphate buffer solution pH-7.4: Add 250.0 ml of 0.2 M potassium dihydrogen phosphate to 393.4 ml of 0.1 M sodium hydroxide and make up the volume to 1000 ml with the distilled water. Potassium dihydrogen phosphate (0.2 M) solution: Potassium dihydrogen phosphate (2.72 g) was dissolved in distilled water and volume made up to 100 ml. Sodium hydroxide solution (0.1 M) solution: 0.4 g of sodium hydroxide was dissolved in distilled water and volume made up to 100 ml.
Method: A solution of hydrogen peroxide (2 mmol/l) was prepared in phosphate buffer (pH-7.4). Test compounds (10-50 ug/M) were added to hydrogen peroxide solution (0.6 ml). Absorbance of hydrogen peroxide at 230 nm was determined after 10 min against a blank solution containing phosphate buffer without hydrogen peroxide and compared with ascorbic acid, the reference compound [34].

■ Statistical analysis
Results were expressed as mean ± SEM. The data were analysed by one- way Analysis of Variance (ANOVA) followed by Dunnett’s test respectively. Statistical significance was considered at *p<0,005, **p<0.01.
Results
■ Preliminary phytochemical analysis
The Preliminary phytochemical investigation of methanolic extract of whole plant of Eichhornia crassipes showed the presence of phenolic compounds, alkaloids, flavonoids, phenols, sterols, terpenoids, and tannins.
■ Acute toxicity studies of MEEC
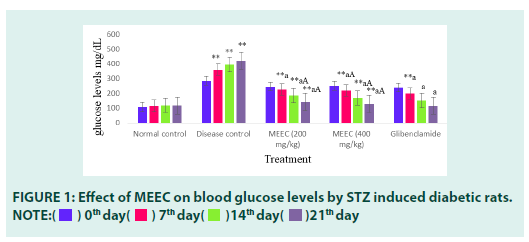
Methanolic Extract of Eichhornia crassipes (MEEC) was treated on albino swiss mice up to a dose of 2000 mg/kg bw. The animal did not exhibit any signs of toxicity or mortality up to 2000 mg/kg bw. Various morphological and behavioural characters were observed during the study. The other parameters like food and water consumption were also observed. All the animals were found to be safe even after 14 days of observation. Hence the extract was found to be safe up to 2000 mg/kg (FIGURE 1).
■ Effect of MEEC on blood glucose levels (mg/dL)
Values are expressed as mean ± SEM, (n=6). Statistical analysis was performed by using ANOVA followed by Dunnett’s test. Results were expressed as (**=p<0.01) when compared to normal control, (a=p<0.01, b=p<0.05) when compared to disease control, (A=p< 0.01, B=p<0.05) when compared to standard. There was a significant increase (P<0.01) in BGL’s in disease control group when compared to Normal control on 0th (285.1 ± 8.43) 7th (359.5 ± 8.61) 14th (395 ± 1.93) and 21st (420 ± 0.96) days indicating the induction of diabetes. Treatment with MEEC at 200 and 400 mg/kg doses significantly reduced the BGL’s on 7th,14th and 21st days. The effect of 400 mg/kg (128 ± 2.36) of MEEC was found to be better than that of 200 mg/kg (141 ± 0.96). The potency of 400 mg/ kg dose of MEEC was found to be comparable to that of standard glibenclamide (115.1 ± 0.6) (FIGURE 2).
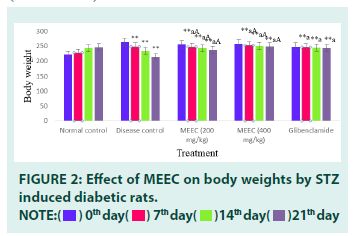
■ Effect of MEEC on body weight
Values are expressed as mean ± SEM, (n=6). Statistical analysis was performed by using ANOVA followed by Dunnett’s test. Results were expressed as (**=p<0.01) when compared to normal control, (a=p<0.01) when compared to disease control, (A=p<0.01) when compared to standard. Administration of streptozotocin (55 mg/kg) resulted in significant attenuation of body weights in disease control group indicting the induction of diabetes (213.3 ± 0.08).
Treatment of diabetic animals with MEEC at 200 and 400 mg/kg doses significantly antagonised the streptozotocin induced reduction of body weights. The effect of 400 mg/kg of MEEC (248.6 ± 0.02) was found to be better than that of 200 mg/kg (237.5 ± 0.06). The efficacy of 400 mg/kg dose of MEEC was found to be similar to that of standard glibenclamide (243.3 ± 0.06).
■ In vitro antioxidant assays
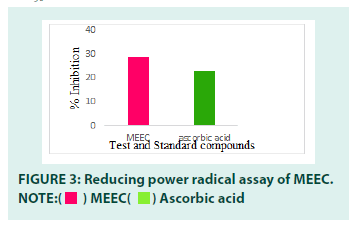
Reducing power assay: The MEEC has shown antioxidant activity in reducing power assay. The reducing ability of a compound generally depends on the presence of a compound generally depends on the presence of reductants which have been exhibiting antioxidative potential by breaking the free radical chain and donating a hydrogen atom. The reducing capacity of a compound can be known by measuring Fe3+–Fe2+ complex. Fe3+ reduction is due to electron donating activity of MEEC, which is an important mechanism of antioxidant action. Increase in absorbance indicates an increase in reductive ability of MEEC. The reducing power activity of MEEC might be due to presence of hydroxyl groups. It might be due to the presence of phenols and flavonoids in the extract with adequate number of hydroxyl group. The results were expressed in FIGURE 3. MEEC had shown dose dependent inhibition of free radicals and its IC50 value was found to be 29 μg/ml. The potential of the extract was comparable to that of standard ascorbic acid (IC50=23 μg/ml).
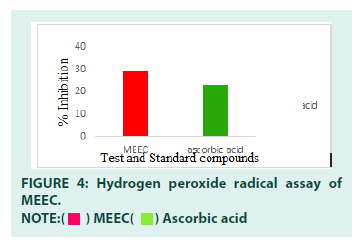
Hydrogen peroxide scavenging assay: The in vitro antioxidant activity was performed using hydrogen peroxide radical scavenging assay. The IC50 value of the MEEC was 20 μg/ml and ascorbic acid was 12 μg/ml respectively. From the results, it is clear that the MEEC showed antioxidant activity. The results were expressed in FIGURE 4. With increase in concentration the % inhibition of MEEC was found to increase and its IC50 value was found to be 20 mg/ml. The antioxidant potential of MEEC was found to be similar to that of standard ascorbic acid (IC50=12mg/dl). The antioxidant activity of the extract was further confirmed by hydrogen peroxide radical scavenging assay. The standard ascorbic acid showed prominent dose dependent inhibition of hydrogen peroxide radicals and its IC50 value was found to be 12 μg/ml. similarly, the test extract exhibited the dose dependent inhibition of free radicals and its IC50 value (20 μg/mL) was comparable to that of standard. The MEEC has shown antioxidant activity in hydrogen peroxide radical scavenging assay. Hydrogen peroxide is a weak oxidizing agent that inactivates a few enzymes directly, usually by oxidation of essential thiol (-SH) group. It can cross cell membrane rapidly: once inside the cell, it can probably react with Fe2+ and possibly Cu2+ ions to form hydroxyl radicals and this may be the origin of many of its toxic effects. The scavenging capacity of a compound may serve as a significant indicator of its potential antioxidant activity. However, the activities of antioxidants have been attributed to various mechanisms such as prevention of chain initiation, decomposition of peroxides, reducing capacity and radical scavenging. The MEEC have shown dose dependent inhibition of hydrogen peroxide and good scavenging ability which was comparable to ascorbic acid. It might be due to the presence of phenols and flavonoids in the extract with adequate number of hydroxyl groups.
Discussion
Diabetes mellitus is a heterogeneous group of diseases characterized by chronic elevation of glucose in the blood. It arises because the body is unable to produce enough insulin for its own needs, either because of impaired insulin secretion, impaired insulin action, or both [2]. Chronic exposure to high blood glucose is a leading cause of renal failure, visual loss and a range of other types of tissue damage. Diabetes also predisposes to arterial disease, not least because it is often accompanied by hypertension, lipid disorders and obesity [3-6]. Many cases of diabetes and almost all of its unwanted longterm consequences are potentially avoidable, but this will require intervention at a societal as well as at a medical level.
Antidiabetic, psychotropic drugs, antioxidants like vitamin A, C and E and SOD, CAT enzymes have been found to prevent the progression of diabetes and occurrence of complications resulted from DM [35]. However, these drugs are associated with several adverse effects which have provoked research in field of traditional system of medicine to deduce the drugs with less toxicity and better tolerability. From the vast array of materiamedica of the indigenous system, many plants have been reported to have activity against DM that act as very useful remedies for the alleviation of human suffering. Eichhornia crassipes is one of them and it is reported to contain phytoconstituents like alkaloids, flavonoids which have been reported to decrease the levels of blood glucose and glycosylated haemoglobin significantly and increase the serum insulin to normal level [36].
The MEEC has been studied for its effect on diabetes by streptozotocin model. Streptozotocin is capable of inducing diabetes upon intraperitoneal injection in experimental animals been taken up cellularly by the Glucose Transporter 2 (GLUT2) which is a low affinity protein situated in cell membrane of insulin producing β-cells of the pancreas as well as in cell of other organs like kidney and liver, leading to selective toxicity due to DNA alkylation resulting into cellular necrosis [37]. The resulting hyperglycaemia due to reduced insulin secretion results in enhanced formulation of reactive oxygen species both centrally and peripherally. These free radicals contribute to the increased neuronal death by oxidizing protein, damaging DNA, and augmenting lipid peroxidation [38].
In this study, the effect of MEEC towards hyperglycaemia was significantly ameliorated the fasting blood glucose levels at both 200 and 400 mg/kg doses. This might be given by their antioxidant properties in reducing oxidative stress in the pancreas caused by streptozotocin induced effect, hence reduces the glucose metabolism in diabetic rats. The streptozotocin induction exhibits a significant lower bodyweight in diabetic control group as compared to the normal control. Treatment with MEEC caused a significant improvement in body weight as compared to the diabetes control rats. This proved the beneficial effect of the selected plant in DM.
The effect of MEEC on streptozotocin induced diabetes in rodent models was assessed by in vitro model such as hydrogen peroxide radical scavenging assay and reducing power assay. The role of oxidative stress in complications of diabetes has been studied extensively in experimental diabetes models and diabetic patients. Due to the hyperglycaemia associated with diabetes enhanced formation of reactive oxygen occurs, which contributes to the increased normal death by oxidising proteins, damaging DNA and augmenting lipid peroxidation. Oxidative damage to the rat synapse in the cerebral cortex and hippocampus has been previously reported to contribute to the deficit of cognitive functions.
Therefore, antioxidants might be of general use in the prevention of complications associated with diabetes. In the present study, MEEC significantly scavenged hydrogen peroxide radicles and their subsequent reducing power. The antioxidant property of Eichhornia crassipes may reduce oxidative damage. In general, it was found that Eichhornia crassipes significantly ameliorated the blood glucose levels in streptozotocin induced diabetic rats. The presence of phytoconstituents like phenols, alkaloids, flavonoids might be responsible for the beneficial role of Eichhornia crassipes in diabetes and its related complications.
Conclusion
Diabetes is a metabolic disorder which results from pancreatic beta cells dysfunction and insulin resistance and is characterized by hyperglycemia. The MEEC inhibited the free radicals in dose dependent manner in reducing power assay and hydrogen peroxide scavenging assay. It revealed its antioxidant activity. The plant Eichhornia crassipes chosen to find its antidiabetic efficacy against streptozotocin induced diabetic rats. The present study reveals the MEEC possess significant antidiabetic activity.
Acknowledgement
The authors are grateful to thank the management of Gokaraju Rangaraju College of Pharmacy for providing laboratory facilities and assistance for antidiabetic and antioxidant activity.
References
- Iyer SR, Iyer RR, Upasani SV, et al. Diabetes Mellitus in Dombivli: An Urban Population Study. J Assoc Phys. 49(7):713-716 (2001).
- Misra A, Pandey RM, Devi JR, et al. High Prevalence of Diabetes, Obesity and Dyslipidaemia in Urban Slum Population in Northern India. Int J Obes. 25(2):1722–9 (2001).
- Gupta A, Gupta R, Sarna M, et al. Prevalence of Diabetes, Impaired Fasting Glucose and Insulin Resistance Syndrome in an Urban Indian Population. Diab Res Clin Pract. 61(1):69–76 (2003).
- Prabhakaran D, Shah P, Chaturvedi V, et al. Cardiovascular Risk Factor Prevalence among Men in a Large Industry of Northern India. Natl Med J India. 18(2):59–65 (2005).
- Ramachandran A, Snehalatha C, Kapur A, et al. High Prevalence of Diabetes and Impaired Glucose Tolerance in India: National Urban Diabetes Survey. Diabetologia. 44(2):1094-101 (2001).
- DeFronzo RA. Pathogenesis of Type 2 Diabetes Mellitus. (Eds: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM), Internationl Textbook of Diabetes Mellitus. John Wiley Sons, USA. 371-400 (2015).
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of Diabetes, Impaired Fasting Glucose, and Impaired Glucose Tolerance in US. Adults. Diabetes Care. 5(1):1-3(1998).
- Misra A, Pandey RM, Devi JR, et al. High Prevalence of Diabetes, Obesity and Dyslipidaemia in Urban Slum Population in Northern India. Int J Obes. 25(4): 1722–1729 (2001).
- Wild S, Roglic G, Green A, et al. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care. 27(5):1047-53 (2004).
- Huizinga MM, Rothman RL. Addressing the Diabetes Pandemic: A Comprehensive Approach. Indian J Med Res. 124(5):481-484 (2006).
- Adisakwattana S, Sompong W, Meeprom A, et al. Blood glucose levels to promote metabolic imbalances. Int J Mol Sci. 13(7):1778-1789 (2012).
- Boynes JW. Role of oxidative stress in development of complication in diabetes. Diabetes. 40(4):405-411(1991).
- Gale EAM. Lessons from the Glitazones: A Story of Drug Development. Lancet. 357(9271):1870-1875(2001).
- Guan Y, Hao C, Cha DR, et al. Thiazolidinediones Expand Body Fluid Volume Through Ppar Gama Stimulation of Enac-mediated Renal Salt Absorption. Nat Med. 11(1):861-865(2005).
- Burns JM, Donnelly JE. Peripheral Insulin and Brain Structure in Early Alzheimer Disease. Neurology. 69 (11):1094-104 (2007).
- Craft S, Asthana S. Insulin Metabolism in Alzheimer’s Disease Differs According to Apolipoprotein E Genotype and Gender. Neuroendocrinology 70(7):146-152 (1999).
- Sridhar GR, Thota H. Alzheimer’s Disease and Type 2 Diabetes Mellitus: The Cholinesterase Connection? Lipids Health Dis. 5(2):28-30 (2006).
- Anjali P, Manoj KM. Same Comments on Diabetes and Herbal Therapy. Ancient Sci Life. 15(1):27-29(1995).
- Jayanthi P, P Lalitha. Antimicrobial Activity of Solvent Extracts of Eichhornia Crassipes (Mart) Solms. Der Pharma Chemica. 5(2):135-140(2013).
- Kumar M, P Mondal, S Borah, et al. Physico-chemical Evaluation, Preliminary Phytochemical Investigation, Fluorescence and Tlc Analysis of Leaves of the Plant Lasia Spinosa (Lour) Thwaites. Int J Pharm Pharm Sci. 5(2):306-310 (2013).
- Quayum SL. Effect of Water Hyacinth Root Extract on Arsenic Level in Different Organs of Arsenic-treated Rat. Bangladesh J Pharmacol. 2(2):73-80 (2007).
- Malik A. Environmental Challenge Vis a Vis Opportunity: The Case of Water Hyacinth. Environ Int. 33(1):122-138 (2007).
- Collier A, Wilson R, Bradley H, et al. Free Radical Activity Is Type 2 Diabetes. Diabetic Med. 7(1): 27-30 (1990).
- Jain V, SK Verma, SS Katewa, et al. Free Radical Scavenging Property of Bombax Ceiba Linn Root. Res J Med Plant. 5(4):462-470 (2011).
- De Martino L, V de Feo, F Fratiamni, et al. Chemistry, Antioxidant, Antibacterial and Antifungal Activities of Volatile Oils and Their Components. Nat Prod Commun. 4(12):1741-1750 (2009).
- Kumar S, AK Pandey. Chemistry and Biological Activities of Flavonoids: An Overview. Scient World J. 9(1):7-11(2013).
- Sabu MC, Kuttan R. Anti-diabetic Activity of Medicinal Plants and Its Relationship with Their Antioxidant Property. J Ethanopharmacol. 81(2): 155-160 (2002).
- Pham-Huy LA, H He, C Pham-Huy. Free Radicals, Antioxidants in Disease and Health. Int J Biomed Sci. 4(2): 89-96 (2008).
- Krishnaiah D, R Sarbatly, R Nithyanandam. A Review of the Antioxidant Potential of Medicinal Plant Species. Food Bioprod Process. 89(3):217-233 (2011).
- Ghimeray AK, C Jin, BK Ghimine, et al. Antioxidant Activity and Quantitative Estimation of Azadirachtin and Nimbin in Azadirachta Indica A. Juss Grown in Foothills of Nepal. Afr J Biotechnol. 8(13):3084-3091(2009).
- Ghaffar FRA, IA El-Elaimy. In Vitro, Antioxidant and Scavenging Activities of Hibiscus Rosa Sinensis Crude Extract. J Applied Pharm Sci. 2(2):51-58 (2012).
- Umathe kochar, Jain Dixit. Intracerebroventricular Streptozotocin Induced Diabetes. J Sci Food Agric. 94(9):2266-2273(2009).
- Kanatt SR, Chander R, Sharma A. Antioxidant Potential of Mint (Mentha Spicata L.) In Radiation-processed Lamb Meat. Food Chem. 100(2):451-458 (2007).
- Jiang ZY, Hunt JV. Ferrous Ion Oxidation in the Presence of Xylenol Orange for Detection of Lipid Hydroperoxide in Low Density Lipoprotein. Anal Biochem. 202(2):384-389 (1992).
- Madhu CG, Devi DB. Protective Antioxidant Effect of Vitamins C and E in Streptozotocin Induced Diabetic Rats. Ind J Exp Biol. 38(5):101-104(2000).
- Cooperstein SJ, Watkins D. Action of Toxic Drugs an Islet Cells. In: The Islets of Langerhans. Academic Press, New York, USA. 387-425(1981).
- Davie SJ, Gould BJ, Yudkin JS. Effect of Vitamin C as Glycosylation of Proteins. Diabetes. 41(2):167-169 (1992).
- Elizabeth K, Rao MNA. Oxygen Radical Scavenging Activity of Curcumin. Int J Pharm. 58(3):237-240(1990).