Review Article - Imaging in Medicine (2010) Volume 2, Issue 6
Endovascular treatment of intracranial aneurysms
Timothy J Phillips1 & Peter J Mitchell†11Neurointervention Service, Department of Radiology, The Royal Melbourne Hospital, Parkville, VIC 3051, Australia
- Corresponding Author:
- Peter J Mitchell
Neurointervention Service
Department of Radiology, The Royal Melbourne Hospital
Parkville, VIC 3051, Australia
E-mail: peter.mitchell@.mh.org.au
Abstract
Subarachnoid hemorrhage (SAH) is a potentially lethal disease with high morbidity and mortality. The goal of endovascular intracranial aneurysm treatment of intracranial aneurysms is the prevention of rebleeding after primary SAH, the prevention of SAH in unruptured aneurysms or the alleviation of other symptoms attributable to the aneurysm. Securing ruptured aneurysms improves outcome after SAH, and there is high level evidence that endovascular coiling of ruptured aneurysms offers lower morbidity and mortality than neurosurgical clipping. However, the natural history and treatment of unruptured aneurysms is controversial. Endovascular techniques can be classified into deconstructive arterial sacrifice, and endosaccular and endoluminal reconstruction. Detachable microcoils, arterial stents, detachable balloons and liquid embolic agents are devices used in aneurysm treatment. The most serious neurological risks of endovascular techniques are thromboembolic infarction, aneurysm rupture and arterial dissection. Immediate angiographic outcome is measured by the degree of persistent aneurysm or neck filling and is often classified by the ‘modified Montreal’ or ‘Raymond’ system. A better immediate angiographic result is shown to reduce aneurysm recurrence. Immediate residual filling of the aneurysm sac and delayed aneurysm recurrence are both risk factors for repeat SAH. The aim of long-term follow-up is to monitor for aneurysm recurrence, and magnetic resonance angiography is now replacing catheter angiography as the first line follow-up imaging modality.
Keywords
endovascular coiling ▪ flow-diverting stent ▪ interventional neuroradiology ▪ intracranial aneurysm ▪ neurointervention ▪ pipeline embolization device ▪ subarachnoid hemorrhage
Intracranial aneurysms
Types
‘Intracranial aneurysm’ is a collective term encompassing saccular/berry aneurysms, pseudoaneurysms, dissection-related aneurysms, mycotic (infective) aneurysms, fusiform intradural aneurysms and oncotic aneurysms. A detailed discussion on the pathology of these lesions is beyond the scope of this review, but a short overview is provided.
Saccular/ berry aneurysms
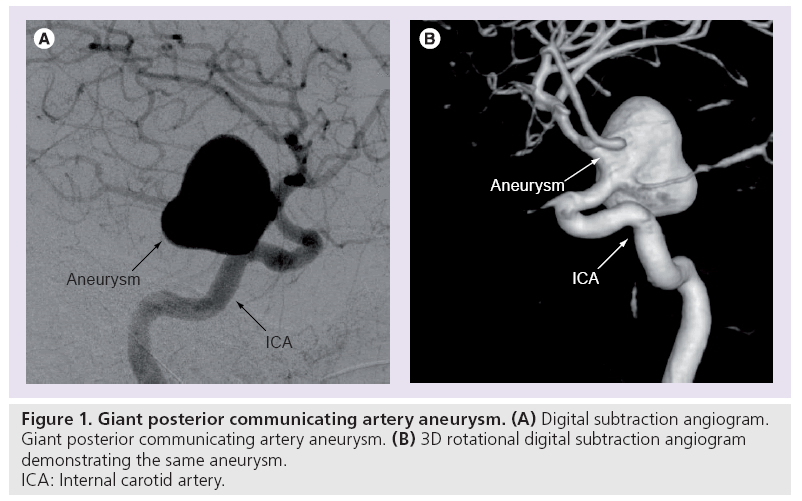
The most common form of aneurysm results from a combination of arterial wall weakness and flow factors; the end result being a ‘ballooning’ of the vessel wall (Figure 1). Small unruptured saccular aneurysms have a thin (30–150 μm) wall of endothelium and adventitia, with larger aneurysms often becoming thickened with collagen and elastic fibers. The incidence of intracranial saccular aneurysms varies between autopsy and angiographic series between 0.2 and 8.9%. A total of 15–30% of patients with at least one aneurysm will have multiple aneurysms [1–3]. Risk factors for aneurysm development include autosomal dominant polycystic kidney disease, Ehlers–Danlos type IV, neurofibromatosis type 1, Marfan disease, fibromuscular dysplasia, aortic coarctation, smoking, hypertension and a family history of saccular aneurysms [4].
Blister-like internal carotid artery (ICA) aneurysms are a subset that behave differently to branch-point saccular ICA aneurysms. They arise from nonbranching points, can grow very quickly and have been shown to carry higher procedural risk [5–9].
Pseudoaneurysms
Pseudoaneurysms are an organized hematoma with a cavity that remains in communication with the circulation through a perforation in the artery wall. The arterial wall injury may be caused by trauma, dissection, infection, ruptured berry aneurysm or iatrogenic injury.
Mycotic aneurysms
Impaction of a septic embolus in a peripheral cerebral artery may result in focal inflammatory arteritis and aneurysm formation. The term mycotic aneurysm is generally applied to these lesions regardless of whether the infective agent is fungal or bacterial.
Dissection-related aneurysms
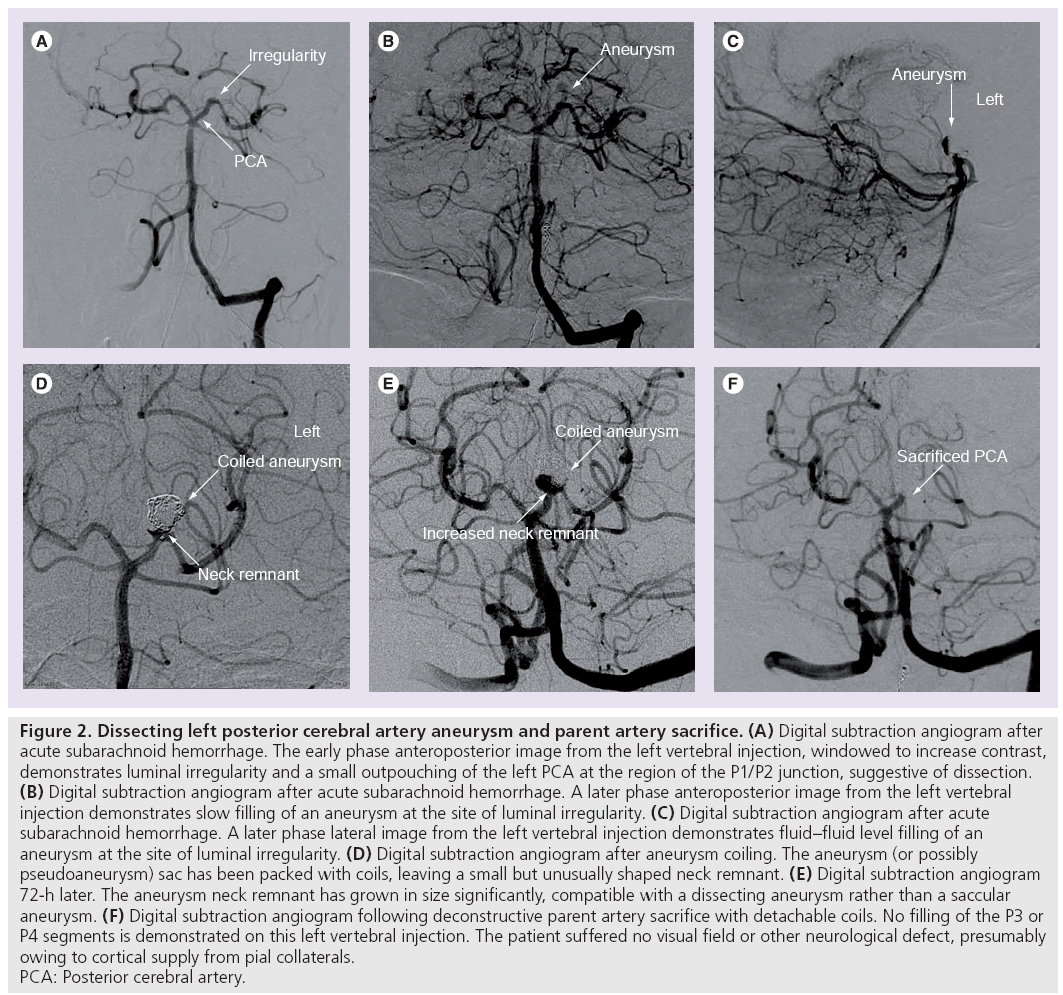
When an arterial dissection creates a communication between the lumen and a cavity between the separated layers of the arterial wall it results in a ‘dissecting aneurysm’ (Figure 2). These may present with aneurysmal outpouching or stenosis.
Figure 2: Dissecting left posterior cerebral artery aneurysm and parent artery sacrifice. (A) Digital subtraction angiogram after acute subarachnoid hemorrhage. The early phase anteroposterior image from the left vertebral injection, windowed to increase contrast, demonstrates luminal irregularity and a small outpouching of the left PCA at the region of the P1/P2 junction, suggestive of dissection. (B) Digital subtraction angiogram after acute subarachnoid hemorrhage. A later phase anteroposterior image from the left vertebral injection demonstrates slow filling of an aneurysm at the site of luminal irregularity. (C) Digital subtraction angiogram after acute subarachnoid hemorrhage. A later phase lateral image from the left vertebral injection demonstrates fluid–fluid level filling of an aneurysm at the site of luminal irregularity. (D) Digital subtraction angiogram after aneurysm coiling. The aneurysm (or possibly pseudoaneurysm) sac has been packed with coils, leaving a small but unusually shaped neck remnant. (E) Digital subtraction angiogram 72-h later. The aneurysm neck remnant has grown in size significantly, compatible with a dissecting aneurysm rather than a saccular aneurysm. (F) Digital subtraction angiogram following deconstructive parent artery sacrifice with detachable coils. No filling of the P3 or P4 segments is demonstrated on this left vertebral injection. The patient suffered no visual field or other neurological defect, presumably owing to cortical supply from pial collaterals. PCA: Posterior cerebral artery.
Fusiform intradural aneurysms
Hypertension and atherosclerosis are associated with formation of fusiform aneurysms, most commonly in the internal carotid and basilar arteries.
Oncotic aneurysms
Direct malignant invasion of the arterial wall with aneurysm formation is a rare entity described in atrial myxoma, renal cell carcinoma, choriocarcinoma, bronchogenic carcinoma and glioblastoma [10–16].
Subarachnoid hemorrhage
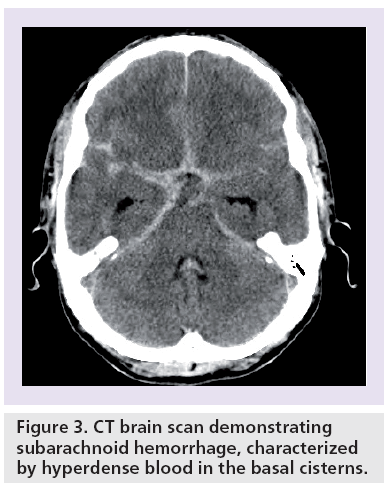
Subarachnoid hemorrhage (SAH) secondary to rupture is the most common and severe complication of intracranial aneurysms (Box 1 & Figure 3) . A total of 80% of nontraumatic SAH is caused by rupture of an intracranial aneurysm [17].
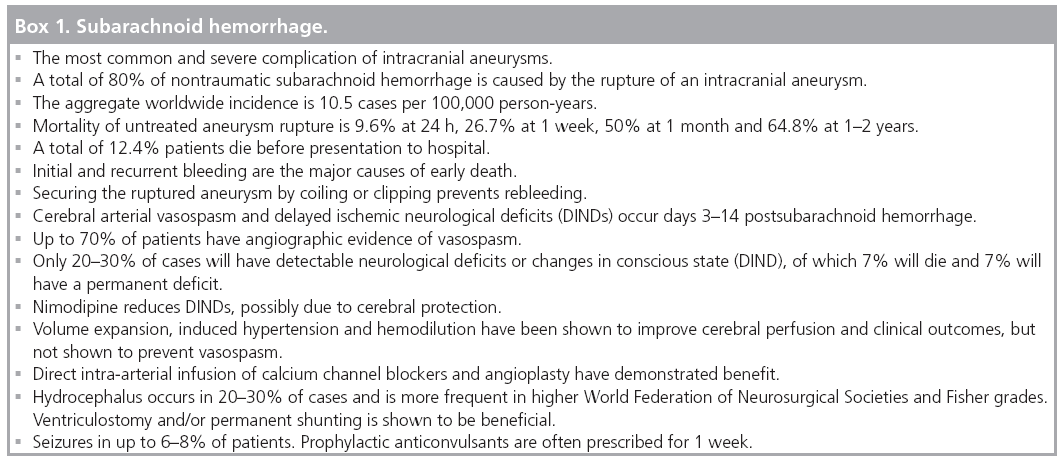
Figure 3: CT brain scan demonstrating subarachnoid hemorrhage, characterized by hyperdense blood in the basal cisterns.
Subarachnoid hemorrhage represents 2–5% of all new strokes, but overall loss of productive life years is similar to that of ischemic stroke or intraparenchymal bleeds [18]. The aggregate worldwide incidence is approximately 10.5 cases per 100,000 person-years [19], with Australian studies quoting 8.5–9.4 per 100,000 person-years [20,21]. The mean age at presentation is 55 years, and SAH is more common in females, Finnish, Japanese, African–Americans and New Zealand Maori individuals [20,22,23].
The natural history of SAH is poor. The 1966 cooperative aneurysm study reported the mortality of untreated single aneurysm ruptures as 9.6% at 24 h, 26.7% at 1 week, 50% at 1 month and 64.8% at 1–2 years [24]. A metaanalysis reported death before presentation to hospital as 12.4% [25].
Initial and recurrent bleeding are the major causes of early death [26]. The acute brain injury responsible for early death is secondary to the subarachnoid bleed leading directly to elevated intracranial pressure, reduced cerebral perfusion and blood flow, reduced brain oxygenation, blood–brain barrier breakdown, brain edema and neuronal apoptosis. The mechanism of injury is complex and involves direct microvascular injury, vascular occlusion and disruption of metabolic pathways [27,28].
For patients admitted to hospital there is a 3–4% or higher risk of repeat hemorrhage in the first 24 h, and 1–2% per day for the first month [29]. Some series quote ultra-early rebleeding at 15%, and up to 20% in World Federation of Neurological Surgeons (WFNS) grade 4 and 5 patients [30,31]. Repeat hemorrhage is associated with over 50% mortality [31–34].
Cerebral arterial vasospasm and delayed ischemic neurological deficits (DINDs) are complications of SAH with poorly understood pathophysiological mechanisms, controversial treatment and preventative strategies, and potentially devastating outcomes. Characterized by a reversible narrowing of proximal cerebral arteries in days 3–14 post-SAH, in some series up to 70% of patients have angiographic evidence of vasospasm. Only 20–30% will have detectable neurological deficits or changes in conscious state (DIND). Of patients suffering DIND in SAH, 7% will die and 7% will have a permanent deficit [35]. The recent American Heart Association/American Stroke Association guidelines recommend prophylactic oral nimodipine, early management of the ruptured aneurysm and avoiding hypovolemia. There is evidence that nimodipine reduces DINDs, but this may be due to cerebral protection as no reduction in angiographic vasospasm has been demonstrated in controlled trials [29,36]. Volume expansion, induced hypertension and hemodilution have been shown to improve cerebral perfusion and clinical outcomes, but have not been definitively shown to prevent vasospasm [29]. Endovascular techniques including direct intra-arterial infusion of calcium channel blockers and angioplasty have demonstrated low level evidence of improved outcome in cases refractory to medical therapy [35].
Hydrocephalus has an overall incidence of 20–30% and is more frequent in higher WFNS and Fisher grades. Ventriculostomy and/or permanent shunting are shown to be beneficial without conclusive evidence of any increased incidence of rebleeding [29].
There are varying published incidences of seizures in SAH. One recent retrospective review quoted this incidence to be 6–18%. Prophylactic anticonvulsants are often prescribed for 1 week after initial bleed, although there is a lack of high level evidence that this improves outcome [29].
Hyponatremia is more common in higher WFNS grades, anterior communicating artery aneurysms and hydrocephalus. It is associated with volume contraction and may be an independent risk factor for poor outcome. Fludrocortisone and hypertonic 3% NaCl have been shown to correct Na levels but not volume contraction [29]. Other medical complications include pulmonary edema (23%) and cardiac arrhythmias (35%) [34].
Carotid-cavernous fistula
Intracavernous carotid aneurysms account for 1.9–9.0% of intracranial aneurysms and their rupture more often leads to a direct carotidcavernous fistula (CCF) than to SAH. A direct CCF complicates approximately 6–9% of intracavernous carotid aneurysms. Aneurysmal CCFs account for approximately 20% of direct CCFs [37].
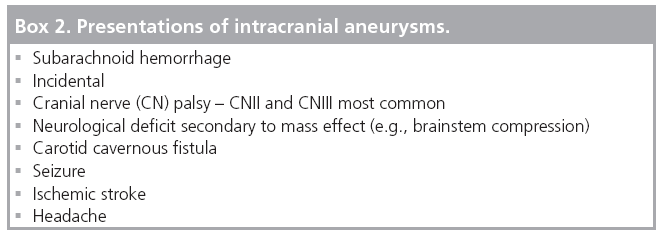
Clinical presentations of unruptured aneurysms
The majority of detected unruptured aneurysms are identified incidentally on CT or MRI performed for another reason. Unruptured aneurysms can, however, present with headaches, embolic strokes and mass effect.
Headaches were the most common symptom (36%) in the 1449 patients enrolled in the retrospective arm of the International Study of Unruptured Intracranial Aneurysms (ISUIA) trial [38]. A Korean study reported a statistically significant rate of headache improvement (90%) following aneurysm treatment in a cohort of patients that had chronic headaches prior to diagnosis of an unruptured aneurysm [39].
In a retrospective review, a total of nine out of 269 patients (3.3%) with unruptured aneurysms had embolic strokes in distributions attributable to the aneurysm locations [40].
A total of 5.7% of the patients enrolled in the retrospective arm of the ISUIA trial had symptoms of mass effect [38]. In a single-center 11-year retrospective review of electively clipped aneurysms less than 1 cm in size, 10.3% of the aneurysms presented with third nerve palsy or visual acuity/field loss attributable to mass effect from the aneurysm [41]. The presentations of intracranial aneurysms are outlined in Box 2.
Decision to treat
Aneurysmal SAH
A sound argument can be made for always treating aneurysmal SAH by securing the aneurysm when possible. As noted above, the natural history of aneurysmal SAH is very poor, and it is generally regarded that the risks of conservative management are greater than the procedural risks of securing the ruptured aneurysm [24,42]. Randomized trials in the 1960s and 1970s demonstrated that the benefits exceeded the risks of surgery in some anatomical locations [43–46], and it is generally accepted that advances in microsurgery and endovascular intervention have further reduced the procedural risk profile.
With treatment, the 60-day case fatality was 7.2% and the 1-year mortality was 9.0% overall in the largest controlled trial of aneurysmal SAH treatment [47,48]. It should be noted that this group excludes patients who die before presenting to a treatment facility, estimated to be 12.4% [25], and that the patients were predominantly low WFNS clinical grade.
In the ISUIA cohort the 5-year mortality of patients with unruptured aneurysms that subsequently ruptured was 65%, but little information regarding these patients, the treatment (if any) they received and the small number (n = 33) makes extrapolation of this data difficult.
Unruptured aneurysms
The elective treatment of unruptured asymptomatic aneurysms is a far more controversial topic. Like the management of any asymptomatic disease, the risk without treatment must exceed the risk of intervention if the treatment is to be justified.
Natural history
The natural history of unruptured aneurysms has been the topic of considerable research and debate.
The results of earlier small observational studies and neurosurgical opinions were challenged by the publication of two reports from the ISUIA trial. In the second prospective arm of the study, the ISUIA authors reported an 0.1% annual risk of rupture (RR) for asymptomatic unruptured aneurysms less than 7 mm in size in patients with no history of SAH. Cumulative 5-year rupture rates for ICA excluding posterior communicating artery origin, middle cerebral artery (MCA), and anterior cerebral artery/anterior communicating artery aneurysms were 0, 2.6, 14.5 and 40% for less than 7, 7–12, 13–24 and 25 mm or more, respectively. This compared with rates of 2.5, 14.5, 18.4 and 50%, respectively, for the same size categories involving posterior circulation and posterior communicating artery aneurysms. Increased relative RR was reported in 7–12 mm aneurysms (3.3), greater than 12 mm aneurysms (17), posterior communicating artery origin (2.1) and basilar tip (2.3) aneurysms [38,49].
Considerable literature was subsequently devoted to critiquing the methodology, anatomical stratification and selection bias of these studies; and whether the results were applicable to decisions on patient management [50–55]. A vital criticism is that the conservative management arm of an observational study is heavily biased towards lesions that are considered low risk.
A large Finnish single-center retrospective study reported significantly higher rupture rates than ISUIA, which were more congruent with the previously accepted neurosurgical views. They found the overall annual RR was 1.1% for aneurysms that were 2–6 mm in size, 2.3% for 7–9 mm and 2.8% for 10–26 mm. In this large cohort 70% of ruptures were in patients with aneurysms that were less than 6 mm in size. The authors demonstrated increasing rupture risk with aneurysm size (RR 1.11/mm >6 mm in diameter) [56].
Meta-analyses performed with and without the inclusion of the ISUIA data reported annual rupture risks of 0.5–2%, and significantly increased risk with advancing age, female gender, Japanese or Finnish descent, aneurysm size larger than 5 mm, posterior circulation aneurysms and symptomatic aneurysms [57,58].
The lack of available solid evidence prompted one group to begin a multinational, multicenter, prospectively randomized trial comparing conservative management to endovascular coiling for unruptured intracranial aneurysms [53,55,59–61]. Unfortunately the cancellation of this trial owing to low enrollment was announced in 2009 [62].
Risk of treatment
The procedural risk of aneurysm treatment was the subject of a systematic review that found a 0.6% case-fatality rate and 7% permanent morbidity associated with endovascular coiling of unruptured aneurysms in patients treated from 1990–2002. In this analysis, morbidity was reduced (to 4.5%) in studies performed after 1994 [63].
Subsequent single-center large cohort longterm follow-up papers have reported lower mortalities (0%, 0.5%) and morbidities (3.4%, 3.5%) [64,65]. A large prospective multicenter Canadian and French study of endovascular treatment in unruptured aneurysms is following up 649 patients. The investigators have published immediate clinical outcomes, reporting 1-month mortality and morbidity of 1.4 and 1.7%, respectively [66–68].
The ISUIA study reported 1-year mortality and morbidity rates of 2.7 and 12.6% for surgical treatment of unruptured aneurysms in patients who had never had documented SAH. The corresponding values were 3.4 and 9.8% for endovascular treatment. Included in the morbidity group was a modified Rankin score of 3–5, death and impaired cognitive status. Statistical tests of significance between the surgical and endovascular groups were not applied, as the groups were vastly different, the endovascular group had older patients with larger unruptured aneurysms, and a higher proportion of aneurysms in the posterior circulation [38,49].
This trend towards better outcomes in the literature is likely to reflect a worldwide learning curve in endovascular management, as well as ongoing improvements in techniques, equipment and devices.
The efficacy and durability of aneurysm treatment is also a consideration in elective treatment, and is discussed further later.
Neurosurgical versus endovascular treatment
Ruptured aneurysms The International Subarachnoid Aneurysm Trial
The International Subarachnoid Aneurysm Trial (ISAT) is a multinational, multicenter, randomized trial of endovascular coiling versus neurosurgical clipping in aneurysmal SAH (Box 3). Equipoise was defined as an aneurysm deemed appropriate for either treatment method by both a neurosurgeon and interventional neuroradiologist, with uncertainty as to the better option. A total of 2143 patients with aneurysmal SAH were randomly assigned to neurosurgical clipping or endovascular coiling [42].
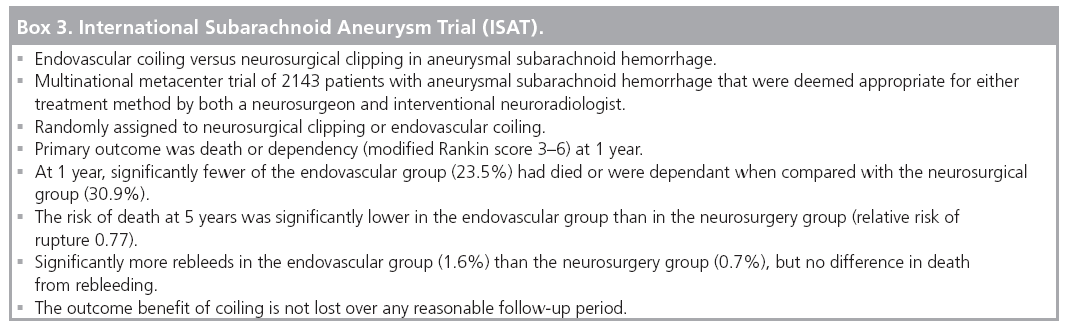
The primary outcome was death or dependency (modified Rankin score of 3–6) at 1 year. At 1 year, significantly fewer of the endovascular group (23.5%) were dead or dependant than the neurosurgical group (30.9%). The risk of death at 5 years was significantly lower in the endovascular group than the neurosurgery group (RR 0.77) [42,48,69].
Long-term follow-up has demonstrated significantly more repeat SAH in the endovascular group (1.6%) than the neurosurgery group (0.7%), but no difference in death from rebleeding [69].
The proportion of SAH patients at participating centers that were entered into the trial varied from 1–44%. Overall, 6745 out of 9559 (70.6%) patients were judged unsuitable for the trial by the neurosurgeons/neuroradiologists assessing them, presumably because equipoise did not exist and one treatment was felt to be the better option.
A high proportion (83%) of the aneurysms were arising from the ICA or anterior cerebral artery–anterior communicating artery complex. A lower proportion of MCA aneurysms (14.1%) and posterior circulation aneurysms (2.7%) were enrolled than the expected proportion in SAH [42]. This spread of aneurysm location in the ISAT patients is an important point, and emphasizes that the results and conclusions can be applied to aneurysms deemed suitable for either treatment modality.
In many centers, clipping is the preferred treatment for MCA aneurysms owing to both the relatively atraumatic surgical access and the difficulty faced to achieve complete endovascular occlusion with preservation of the MCA branches [70,71]. Several groups continue to report good results with endovascular coiling, but there remains a lack of high level evidence comparing the two modalities in this location [72,73].
Posterior circulation aneurysms and in particular basilar tip aneurysms require higher risk surgical access but are readily accessible to endovascular treatment in the majority of cases [74,75].
Across the ISAT groups the mean time to treatment was 1.1 days for endovascular coiling and 1.8 days for neurosurgical clipping [42]. The authors’ adherence to intention-to-treat analysis means that the patients who died before receiving their randomized neurosurgical clipping are included in the results. This is a point that many neurosurgical groups criticize [76].
The results and ramifications of the ISAT are debated in neurosurgical literature with each new publication of ISAT data [76–78]. It is, however, the only large prospective randomized trial comparing the two treatment methods. Systematic reviews, meta-analyses and published guidelines have subsequently reinforced its conclusions, and recommended that in cases of ruptured aneurysms judged by interventional neuroradiologists and cerebrovascular surgeons to be amenable to either technique, endovascular therapy offers lower morbidity and mortality [29,79,80]. Another common conclusion in published guidelines is that aneurysms are best managed in centers where both endovascular and neurosurgical treatment is available, facilitating case-by-case discussion as to which treatment is preferable [29,34,79,80].
Unruptured aneurysms
The ISAT data cannot be directly applied to unruptured aneurysms. There have been no randomized trials comparing endovascular and neurosurgical treatment of unruptured aneurysms.
The Trial on Endovascular Aneurysm Management (TEAM) aimed to compare conservative management with endovascular treatment of unruptured aneurysms, but did not include a neurosurgical clipping cohort to allow comparison of the two modalities [55,59,60]. Unfortunately the cancellation of this trial owing to low enrollment was announced in 2009 [62].
Procedural complications
The major neurological risks associated with endovascular aneurysm treatment are thromboembolic ischemia, aneurysm perforation, parent vessel occlusion and arterial dissection.
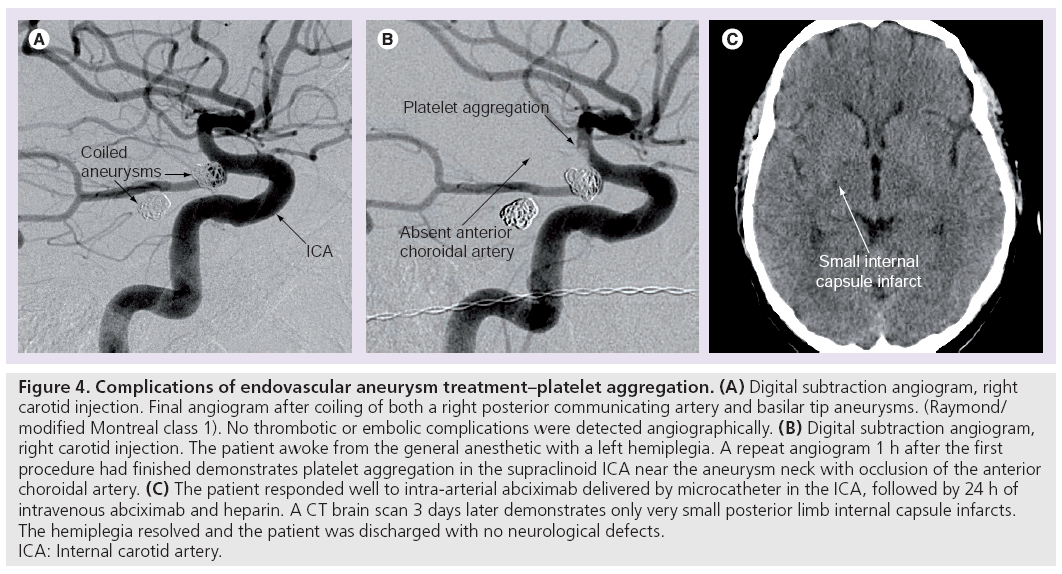
Thromboembolic complications can result from thrombus formation on catheters, wires, coils, within aneurysmal sacs and secondary to iatrogenic arterial dissection (Figure 4). Reported rates of symptomatic thromboembolic complications range from 2.4 to 5.2% [81–85], however, studies specifically investigating the rate of asymptomatic emboli using diffusion-weighted MR have reported incidences of 28–69%. Some authors criticize the methodology of such data, pointing out that no preoperative diffusionweighted imaging was obtained in these patients, the majority of whom presented with SAH [86–92].
Figure 4: Complications of endovascular aneurysm treatment–platelet aggregation. (A) Digital subtraction angiogram, right
carotid injection. Final angiogram after coiling of both a right posterior communicating artery and basilar tip aneurysms. (Raymond/
modified Montreal class 1). No thrombotic or embolic complications were detected angiographically. (B) Digital subtraction angiogram,
right carotid injection. The patient awoke from the general anesthetic with a left hemiplegia. A repeat angiogram 1 h after the first
procedure had finished demonstrates platelet aggregation in the supraclinoid ICA near the aneurysm neck with occlusion of the anterior
choroidal artery. (C) The patient responded well to intra-arterial abciximab delivered by microcatheter in the ICA, followed by 24 h of
intravenous abciximab and heparin. A CT brain scan 3 days later demonstrates only very small posterior limb internal capsule infarcts.
The hemiplegia resolved and the patient was discharged with no neurological defects.
ICA: Internal carotid artery.
Proven risk factors for procedural thromboembolic complications include ruptured aneurysms and aneurysms greater than 10 mm in size. Embolism is less likely in the posterior circulation [86–93].
Use of periprocedural intravenous acetylsalicylic acid was reported to reduce thromboembolic complications without an increase in hemorrhage-related complications in a retrospective noncontrolled review of cases before and after this became standard treatment in a single center, and in smaller retrospective reviews [94,95]. More rigorous research is required to validate this potential benefit and assess any hemorrhagic side effects.
Intraprocedural anticoagulation is standard practice to minimize the risk of thromboembolic complications. Full heparinization for 24 h after treatment of ruptured aneurysms in patients without recent craniotomy is reported to be safe in one case series [96]. Concern exists regarding the risk of hemorrhage with recently inserted external ventricular drains, however, one large retrospective series reported that with tight control of the activated prothrombin time, heparinization is safe in this setting [96–98].
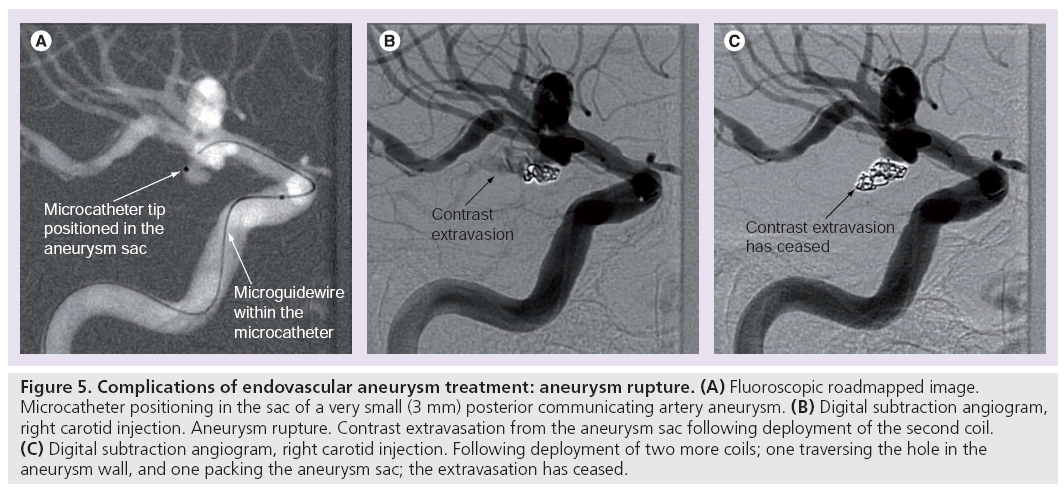
Procedural rupture of the aneurysm can be ascribed to instrument perforation or spontaneous rerupture (Figure 5). Reported rates in published case series are 2.3–6.5% for ruptured aneurysms and 0.5–2.4% for unruptured aneurysms [81–85,93,99]. A 2002 meta-analysis found that the risk of death or disability after procedural perforation was 38% in previously ruptured aneurysms and 28% in previously unruptured aneurysms. Heparinization at the time of rupture did not influence morbidity and mortality [99].
Figure 5: Complications of endovascular aneurysm treatment: aneurysm rupture. (A) Fluoroscopic roadmapped image. Microcatheter positioning in the sac of a very small (3 mm) posterior communicating artery aneurysm. (B) Digital subtraction angiogram, right carotid injection. Aneurysm rupture. Contrast extravasation from the aneurysm sac following deployment of the second coil. (C) Digital subtraction angiogram, right carotid injection. Following deployment of two more coils; one traversing the hole in the aneurysm wall, and one packing the aneurysm sac; the extravasation has ceased.
The Cerebral Aneurysm Rerupture After Treatment (CARAT) trial found that nonwhite race, chronic obstructive pulmonary disease, and lower initial Hunt and Hess grade were predictors of procedural rupture in coiling cases [100]. There are case series reporting higher rates of procedural rupture during endovascular treatment of ICA blister-like aneurysms [8]. Balloon-assisted coiling/balloon remodeling is shown, in some case series, to increase rupture rates in ruptured aneurysms [101].
Several authors have demonstrated increased procedural rupture rates associated with coiling very small (≤3 mm) aneurysms [102–105].
Long-term outcomes
The degree of immediate angiographic aneurysm occlusion and the incidence of delayed aneurysm recurrence are separate outcome factors that are associated both with each other and with delayed SAH. They are considered separately first.
Immediate angiographic outcome
Although all published results are generally based around angiographic opacification of the neck and/or aneurysm sac, the classification systems for initial angiographic results vary across different groups [85,106–109]. Many now adopt the so-called ‘modified Montreal’ or ‘Raymond’ classification, which stratifies results into complete occlusion of aneurysm and neck (class 1), persistence of the neck or any portion of the original defect of the arterial wall as seen on any single projection but without opacification of the aneurysmal sac (class 2), and any opacification of the aneurysmal sac (class 3) [110]. A major source of disagreement in the application of this system relates to the coiled aneurysm with no neck remnant but contrast filling the interstices of the coil mesh [66]. More recently some authors have proposed a more comprehensive 6-point grading system to expand the Montreal classification [91].
In large case series from high-volume centers and one prospective multicenter study, the reported incidence of total angiographic occlusion (class 1) varies from 36 to 76%, neck remnant (class 2) 22–46% and aneurysm remnant (class 3) 1.7–20%. The wide ranges likely reflect the varied classifications and definitions applied to the immediate postcoiling appearance [81–83,106,108,109,111]. In a systematic review covering 37 studies with 6991 aneurysms, total angiographic occlusion (class 1) was reported in 62.3%, neck remnant (class 2) in 29.5% and aneurysm remnant (class 3) in 8.2% [111].
In the large prospective multicenter Clinical and Anatomical Results in the Treatment of Ruptured Intracranial Aneurysms (CLARITY) and Analysis of Treatment by Endovascular Approach of Nonruptured Aneurysms (ATENA) series; initial angiographic results as judged by the performing physician are compared with the results judged by consensus between two independent reviewers. In both studies the interobserver agreement between the treating physician and the reviewers was low (k-values = 0.395 and 0.33), highlighting the heterogeneity of the assessment and classification of immediate angiographic results [66,112].
Aneurysm recurrence, regrowth or recanalization
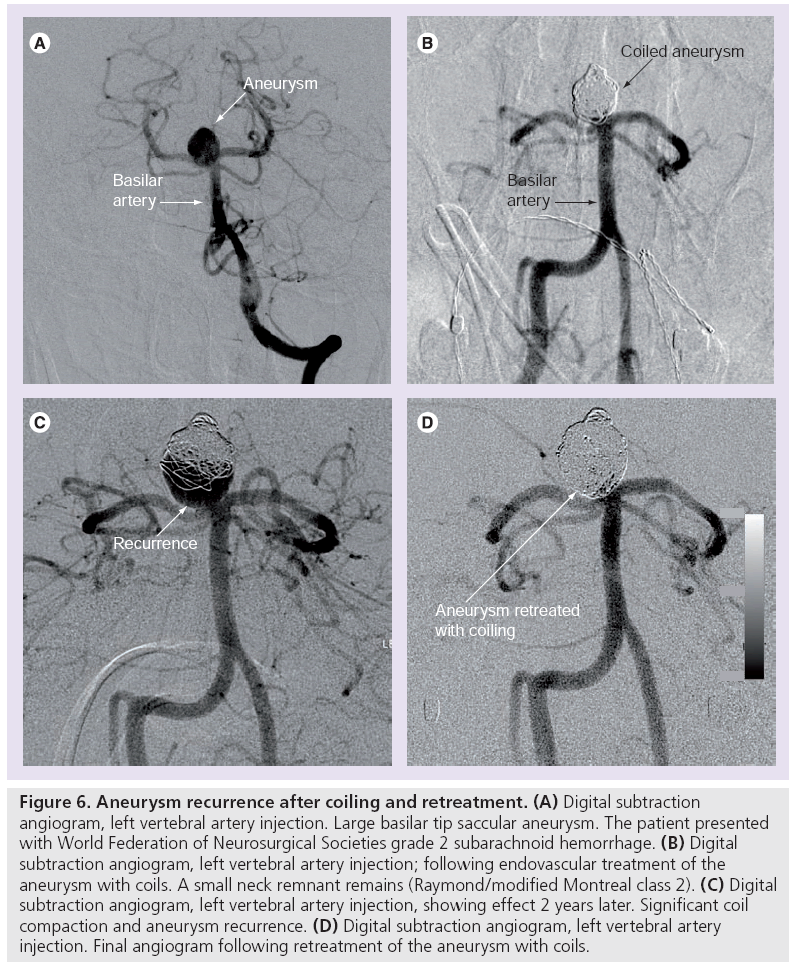
Over time more follow-up data on coiled aneurysms has become available from many high volume centers, most reporting aneurysm recurrence of between 20 and 34% [81–83,85,109]. A 2009 systematic review encompassing 46 studies totaling 8161 coiled aneurysms found that 20.8% of all coiled intracranial aneurysms showed recurrence, half of which were retreated (Figure 6) [111].
Figure 6: Aneurysm recurrence after coiling and retreatment. (A) Digital subtraction angiogram, left vertebral artery injection. Large basilar tip saccular aneurysm. The patient presented with World Federation of Neurosurgical Societies grade 2 subarachnoid hemorrhage. (B) Digital subtraction angiogram, left vertebral artery injection; following endovascular treatment of the aneurysm with coils. A small neck remnant remains (Raymond/modified Montreal class 2). (C) Digital subtraction angiogram, left vertebral artery injection, showing effect 2 years later. Significant coil compaction and aneurysm recurrence. (D) Digital subtraction angiogram, left vertebral artery injection. Final angiogram following retreatment of the aneurysm with coils.
Non-modifiable patient factors, including aneurysm size of greater than 10 mm, neck diameter greater than 4 mm and high dome:neck ratio, are statistically significant predictors of recurrence following aneurysm coiling, both in ruptured and unruptured aneurysms [83,85,106,108,109,113].
Association between immediate angiographic result & aneurysm recurrence
Recurrence is usually attributable to regrowth from an unsecured part (remnant) of the aneurysm, or recanalization secondary to instability of the coil-thrombus mass (coil compaction). If no remnant was reported at the original treatment, some groups refer to any recurrence as ‘de novo’ aneurysm formation [107].
Two large case series have reported significant associations between the initial angiographic result and the incidence of aneurysm recurrence or recanalization [85,109].
One large consecutive series found no difference in the rate of recurrence between complete occlusion (Raymond class 1) and neck remnants (Raymond class 2). They conclude from this that the increased risk of procedural complications associated with pursuing a complete occlusion is not justified by the outcome [85].
The multicenter retrospective CARAT study reported a rerupture risk of 25% in coiled aneurysms with residual sac filling (Raymond class 3), 4.9% with large neck remnants, 2.3% with small neck remnants (both class 2) and 1.8% with complete occlusion (Raymond class 1). They concluded that these results justify attempts to completely occlude aneurysms [108]. The authors note the limitation of their study owing to very small numbers. It is important to note that the very high (25%) rebleed rate occurred in a subset of Raymond 3 (<70% occlusion) and cannot be extrapolated to relate to all Raymond 3 residual aneurysms. The methodology of the statistical analysis applied to the CARAT data is also the subject of negative criticism from other authors [114].
The heterogeneity of physician assessments and classification of immediate angiographic results discussed earlier is likely to be a factor in the differing hemorrhage risk and recommendations associated with neck remnants.
There is evidence that more objective measures of angiographic results may predict stability. Two studies which compared the volume ratio of embolic coils to aneurysm volume (packing ratio) demonstrated that a high packing ratio is a statistically significant predictor of stability. One author reported that no aneurysm with packing greater than 24% demonstrated recurrence at 6-month follow-up [115,116].
Long-term prevention of SAH
The greatest long-term limitation of coil embolization is that several reports have shown that rebleeding or delayed hemorrhage is more frequent after coiling than clipping [106–108].
The reported risk of repeat hemorrhage from the target aneurysm following coiling of ruptured aneurysms varies from 0.6 to 2.1% across the largest published series, which includes the ISAT and CARAT trials [81–83,85,106–109].
Recanalization or recurrence after aneurysm treatment is a known risk factor for delayed hemorrhage, but in all published series the rate of recurrence is much higher than the rate of hemorrhage [85,106,108,109]. Several large case series have also demonstrated an association between incomplete aneurysm occlusion and delayed hemorrhage [106,108,109]. The absolute numbers of rebleeds or delayed hemorrhage from the target aneurysm in most series and both the ISAT and CARAT trials is very low, which limits the power of statistical associations [106–108].
The most recent ISAT follow-up data demonstrates that the slightly higher repeat hemorrhage and retreatment rates in the coiling arm are not associated with any increase in death. The clinical outcome benefit of coiling over clipping would not be lost over any reasonable follow-up. This confirms that coiling is the appropriate treatment for ruptured aneurysms treatable by either technique regardless of the recanalization/recurrence rate [69,107].
At present no clear conclusions can be drawn on factors predicting which recurrences will rebleed. The decision to treat an aneurysm recurrence amenable to endovascular therapy is therefore an individual case-by-case assessment [113]. In the ISAT, 17% of coiled patients were retreated. Younger age, larger aneurysm size and incomplete occlusion were predictors of retreatment [107].
Follow-up
The aim of imaging follow-up of aneurysms treated with endovascular techniques is to monitor for recurrence, as detailed previously. Traditionally this was done with intra-arterial catheter angiography. In the last 5 years there has been a shift to the use of magnetic resonance angiography (MRA), both time-of-flight and gadolinium-enhanced techniques, using 1.5 and 3 Tesla magnets. Several publications have reported MRA having similar accuracy to catheter angiography [85,117–121]. The lack of procedural risk and radiation make MRA a superior choice if the detected of recurrence is shown to be equivalent. There is no consensus on whether sensitivity and specificity in detection of aneurysm remnants is improved by using contrastenhanced techniques or 3 Tesla field strength over noncontrast time-of-flight and 1.5 Tesla field strength, respectively.
Endovascular techniques
Approaches
The overall goal of endovascular aneurysm treatment is to stop flowing blood from entering the aneurysm sac, excluding it from the circulation. In broad terms, the endovascular treatment options can be divided into ‘deconstructive’, which prevents aneurysm filling by deliberately occluding the parent vessel; and ‘reconstructive’, which aims to occlude the aneurysm sac, preserve the parent vessel lumen, and reconstruct a nonaneurysmal parent artery wall. Many authors further divide the reconstructive goals into endosaccular, referring to occlusion of the aneurysmal sac, and ‘endoluminal’ referring to the reconstruction of a nonaneurysmal parent artery wall.
Armamentarium
Procedural instructions are beyond the scope of this article, but a brief explanation of the tools and basic methods applicable to most techniques is provided. The term ‘guide catheter’ refers to a stiff 5F or greater caliber wide lumen catheter which is positioned below the skull base, usually in the cervical internal carotid or vertebral artery. ‘Microcatheters’ are much narrower (usually less than 2 French caliber) catheters which are advanced over ‘microwires’ (usually 0.010–0.018 inch in caliber). Using digital subtraction roadmap images for guidance a microcatheter is introduced into the guide catheter, and advanced into the intracranial system.
Today, most endovascular aneurysm treatment procedures are performed under general anesthesia, allowing complete paralysis to eliminate motion artifact, and better control of hemodynamic parameters. Therapeutic heparinization is standard procedure. Dual antiplatelet therapy – commonly with acetylsalicylic acid and clopidogrel – is usually required for elective aneurysm treatment requiring stent-assisted techniques. This usually restricts these techniques to unruptured aneurysms. This is discussed further later.
Reconstructive approach
The first endosaccular occlusion of an ICA aneurysm was performed in 1941 via craniotomy, direct puncture of the aneurysm and the insertion of 9 m of wire into the aneurysm sac [122].
Detachable coil embolization: endosaccular reconstructive approach
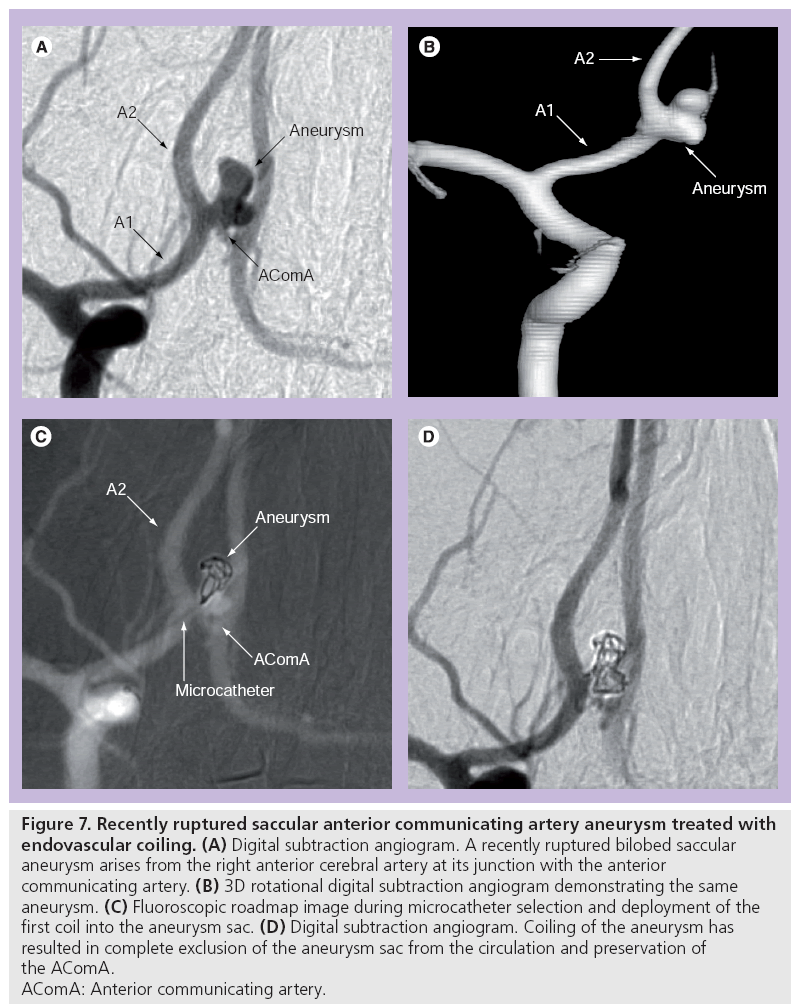
Detachable coils can be used for ‘endosaccular reconstruction’ by occluding the aneurysm sac, inducing thrombosis and excluding the sac from the circulation (Figure 7). They are also used in deconstructive occlusion of the parent artery (vessel sacrifice).
Figure 7: Recently ruptured saccular anterior communicating artery aneurysm treated with
endovascular coiling. (A) Digital subtraction angiogram. A recently ruptured bilobed saccular
aneurysm arises from the right anterior cerebral artery at its junction with the anterior
communicating artery. (B) 3D rotational digital subtraction angiogram demonstrating the same
aneurysm. (C) Fluoroscopic roadmap image during microcatheter selection and deployment of the
first coil into the aneurysm sac. (D) Digital subtraction angiogram. Coiling of the aneurysm has
resulted in complete exclusion of the aneurysm sac from the circulation and preservation of
the AComA.
AComA: Anterior communicating artery.
A ‘detachable coil’ is introduced through a microcatheter either into the aneurysm sac or an artery lumen depending on the deconstructive or reconstructive goal. The coil can be withdrawn and repositioned until the operator is satisfied with its position. It is detached by using electrolytic, hydraulic or thermomechanical methods depending on the manufacturer and product. All microcatheters designed for detachable coil deployment have radio-opaque markers at their distal tip and 30 mm proximal to this. Compatible detachable coils have a radio-opaque marker 30 mm proximal to the detachment zone.
When these two proximal markers are aligned fluoroscopically the operator can be certain the detachment zone is at the distal end of the catheter when it is often obscured by previously deployed coils.
The first human use of the Guglielmi detachable coil (GDC) in 1990 led to wider clinical use in 1991, approval by the US FDA for surgically unmanageable aneurysms in 1995, and emergence as the preferable modality for the management of ruptured aneurysms following the first results of the ISAT in 2002 [42,122].
The success of the archetypal GDC has led to a wide array of bare metal coils from the manufacturers of GDC and other vendors. Bare metal coils vary in the caliber of their constrained delivery form or ‘wire thickness’, the morphology and diameter of the shape they form when unconstrained, their length and their relative ‘softness’. For example, a Boston GDC-10 360° 4 mm/15 cm soft refers to a 0.010˝ wire thickness, a 3D shape consisting of alternating small and large loops (360™), a 4 mm unconstrained diameter and a 15 cm length.
Bioactive coils
The incidence of aneurysm recurrence with bare metal coiling has prompted the introduction of coils with ‘bioactive’ resorbable polymer either coated on or loaded within the coil. Experimental animal studies suggested that these materials induce an increased tissue response, increased fibrous tissue and intimal layer covering the aneurysm neck, conditions thought more likely to result in complete aneurysm occlusion and less recurrence [123–126].
Several bioactive coils have been in use since 2002. The products include the Cerecyte® microcoil (Micrus Endovascular, CA, USA), containing polyglycolic acid suture material; the Matrix® detachable coil (Boston Scientific, MA, USA) and Nexus microcoils (ev3, CA, USA) coated with a combination of polyglycolic and polylactic acid; and the HydroCoil® (Microvention, CA, USA) coated in hydrogel.
A total of 32 published studies evaluating coated or bioactive coils in aneurysm treatment were analyzed in a systematic review. All studies were clinical series rather than randomized trials and provided very little evidence in favor of bioactive or coated coils over bare platinum coils [127].
Randomized controlled trials comparing bioactive coils with bare platinum coils are in progress for the Matrix, HydroCoil and Cerecyte products. All have finished recruiting but have not released outcome data at the time of writing [128,201–204]. When complete, these trials will provide scientific evidence as to the benefit, if any, of bioactive coil technology.
Several reports of aseptic meningitis and hydrocephalus associated with the use of hydrogel-coated coils have been published [129– 132]. The first publication from the randomized HydroCoil Endovascular Aneurysm Occlusion and Packing Study (HELPS) trial found no difference in meningitic symptoms or hydrocephalus between the bare platinum and HydroCoil groups [128].
Balloon remodeling/ balloon-assisted coiling
The difficulty in packing wide necked aneurysms with standard coiling technique led to the development and publication of the ‘balloon remodeling’ or ‘balloon-assisted coiling’ technique. This involves positioning of a compliant microcatheter-delivered balloon at the aneurysm neck. The balloon is intermittently inflated to support the primary microcatheter in the aneurysm sac and to prevent the coils from prolapsing into the parent artery during deployment [133–137].
The initial publications are single-center case series which report high success rates, and the ability to treat wide necked aneurysms not previously treatable with standard coiling technique [133–137]. Improved technology and increased compliance of the balloons has been reported to improve the efficacy of bifurcation aneurysm treatment [134].
The additional instruments theoretically increase the risk of complication. The presence of an additional microcatheter, microwire and depending on the technique an additional guide catheter increase the risk of thromboembolism and arterial dissection. There is low-grade evidence in published case series that cases with more catheter repositioning are associated with higher rates of thromboembolic complications [88,92].
Balloon inflation adjacent to the aneurysm sac may increase the RR, particularly in recently ruptured aneurysms. Balloon-assisted coiling is shown in case series to increase rupture rates in ruptured aneurysms [101,138]. Reassuringly, a comparison of cases using balloon-assisted coiling with standard coiling of unruptured aneurysms in the ATENA study found no increase in morbidity and mortality associated with the technique [139].
Balloon overinflation can cause arterial rupture. If two groin punctures are performed the risk of femoral artery complications is doubled.
Liquid embolic agents: balloon-assisted endosaccular approach with alternative embolic material
The use of liquid embolic agents such as Onyx® (ev3, CA, USA) to treat wide necked, large and giant unruptured aneurysms has been the subject of several case series and one multicenter prospective observational study [140–143]. The endovascular technique involves microcatheter selection of the aneurysm and inflation of a compliant microcatheter delivered balloon across the aneurysm neck. Injection of contrast into the aneurysm with the balloon inflated is performed to confirm that none leaks into the parent artery. If this test is successful, Onyx is injected into the aneurysm sac. Stent assistance to improve the stability of the Onyx cast has also been reported [140–144]. The Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial reported subtotal or incomplete occlusion in 21%, neurological morbidity in 8.3%, procedural or disease related death in 3.1%, and delayed occlusion of the parent artery in 13% [143]. Many authors described procedural mortality of 2–3% and morbidity of 7–8% as being similar to endovascular or surgical treatment of similar aneurysm subgroups. The fact that the aneurysm occlusion rates and adverse events are possibly similar but certainly no better than coil, balloon and stent technology has seen embolic liquids sidelined in favor of other device technologies.
Stent-assisted coiling: endoluminal & endosaccular reconstructive approach
The use of a stent in the parent artery across the aneurysm neck in addition to coil packing of the aneurysm sac has proven mechanical benefits, a well as hemodynamic and biological benefits demonstrated in flow models and animal studies, respectively.
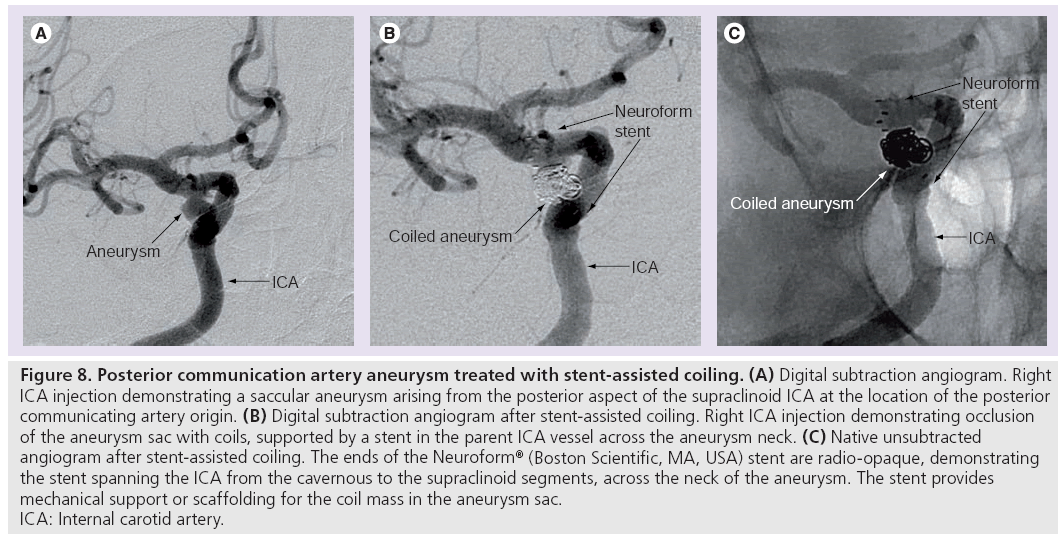
The wall of the stent provides a mechanical scaffolding to prevent coils prolapsing out of the aneurysm sac into the parent artery during deployment. This enables more complete packing of the aneurysm sac (Figure 8).
Figure 8: Posterior communication artery aneurysm treated with stent-assisted coiling. (A) Digital subtraction angiogram. Right
ICA injection demonstrating a saccular aneurysm arising from the posterior aspect of the supraclinoid ICA at the location of the posterior
communicating artery origin. (B) Digital subtraction angiogram after stent-assisted coiling. Right ICA injection demonstrating occlusion
of the aneurysm sac with coils, supported by a stent in the parent ICA vessel across the aneurysm neck. (C) Native unsubtracted
angiogram after stent-assisted coiling. The ends of the Neuroform® (Boston Scientific, MA, USA) stent are radio-opaque, demonstrating
the stent spanning the ICA from the cavernous to the supraclinoid segments, across the neck of the aneurysm. The stent provides
mechanical support or scaffolding for the coil mass in the aneurysm sac.
ICA: Internal carotid artery.
The stent mesh across the aneurysm neck has been shown in in vitro models to alter the flow dynamics. Increasing the circulation time through the aneurysm sac theoretically enhances the disturbance of flow induced by the coils and assists occlusion. Reducing the flow-related stress on the aneurysm wall theoretically reduces the RR [145,146].
Canine models have demonstrated endothelialization of the stent struts [147,148]. A human postmortem histological study demonstrated endothelialization of a Neuroform stent placed across an ICA aneurysm neck [149]. Endothelialization is desirable to secure the aneurysm neck, and to reduce the thromboembolic complications by removing the thrombogenic interface between flowing blood and the metallic foreign body.
Self-expanding microstents for aneurysm treatment have been in use since 2002 and several large case series have been recently published, although not all outcome parameters are reported in every series. Immediate postprocedural total aneurysm occlusion (or >90% occlusion) is reported as 57–79%. Recanalization is reported as 9–28%. The largest series reports a 15.1% retreatment rate [150–155].
Arterial stenting carries a proven risk of platelet aggregation and thromboembolic complications. For this reason, most authors currently recommend acetylsalicylic acid and clopidogrel therapy for 3–4 days preoperatively and for a variable duration postoperatively, often 6 weeks. Acetylsalicylic acid is often continued for life [150–154,156,157]. Analysis of individual testing of patient response to antiplatelet agents is ongoing. As well as full procedural heparinization, most operators continue full heparinization for 24–48 h postoperatively.
In a series of elective stent-assisted aneurysm treatment, reported thromboembolic complications range from 2.4 to 8.8%, permanent morbidity 2.4 to 7.4% and mortality 2.0 to 4.6% [150–155].
The need for aggressive antiplatelet and anticoagulant therapy makes the use of stents in treatment of acutely ruptured aneurysms difficult. One multicenter series reported the use of stents to treat wide necked aneurysms in acute SAH. They found a 21% rate of procedural complications, most of them thromboembolic and attributable to the use of stent assistance. The overall 30-day mortality was 20% [156].
In-stent stenosis is a known major complication of intracranial arterial stenting. The largest series of stent-assisted coiling with Neuroform stents reports a 5.6% (10 of 177) rate of moderate or severe in-stent stenosis. Three of these presented with focal neurological deficits, two required angioplasty and one eventually a surgical bypass. One of the seven asymptomatic patients progressed to total occlusion [150].
Comparison of stent-assisted immediate angiographic occlusion rates in nonrandomized series with standard coiling results is illogical because stent assistance is selected for the most anatomically challenging cases. In the published series, thromboembolic complications are slightly higher with stent assistance than coiling alone, which reinforces the importance of antiplatelet therapy. The 21% complication rate and 20% morbidity reported in acute SAH cases suggests that further research is warranted into the use of stent assistance in this setting. Several authors advocate delayed definitive stent treatment under full antiplatelet and anticoagulation therapy once the aneurysm is partially treated in the acute setting with coils only.
Flow-diverting stents: endoluminal reconstructive approach
New stents have recently been developed to treat aneurysms by endoluminal reconstruction, without the need for endosaccular coil embolization (Figures 9 & 10). Ideally, the stent will divert flow away from the aneurysm sac to promote thrombosis and involution of the aneurysm. The porosity of the stent is such that the high flow into the ostia of perforating branches is not occluded. A second aim is neo-intimal formation and endothelialization of the stent struts that lie across the aneurysm neck and against the parent artery wall, but not the struts crossing the ostia of perforating branches.
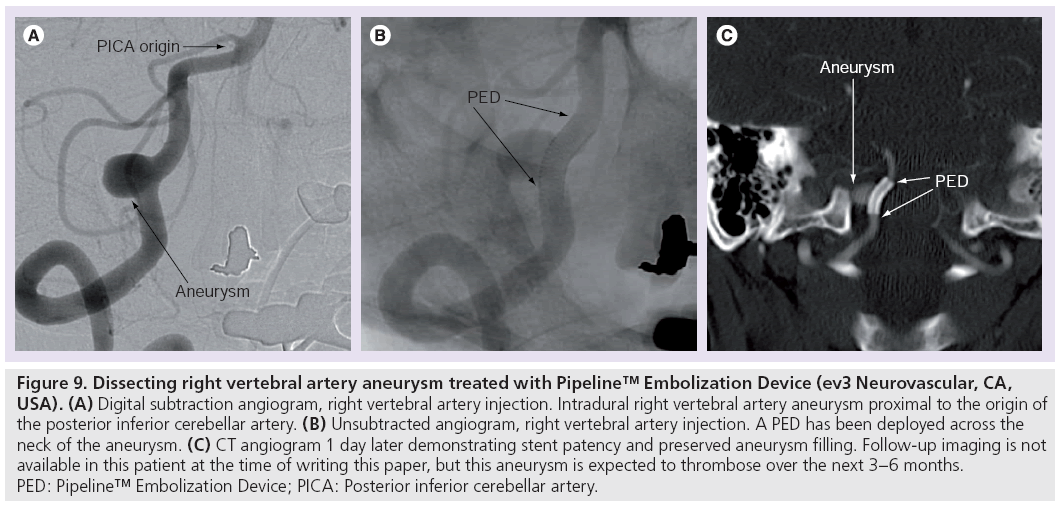
Figure 9: Dissecting right vertebral artery aneurysm treated with Pipeline™ Embolization Device (ev3 Neurovascular, CA, USA). (A) Digital subtraction angiogram, right vertebral artery injection. Intradural right vertebral artery aneurysm proximal to the origin of the posterior inferior cerebellar artery. (B) Unsubtracted angiogram, right vertebral artery injection. A PED has been deployed across the neck of the aneurysm. (C) CT angiogram 1 day later demonstrating stent patency and preserved aneurysm filling. Follow-up imaging is not available in this patient at the time of writing this paper, but this aneurysm is expected to thrombose over the next 3–6 months. PED: Pipeline™ Embolization Device; PICA: Posterior inferior cerebellar artery.
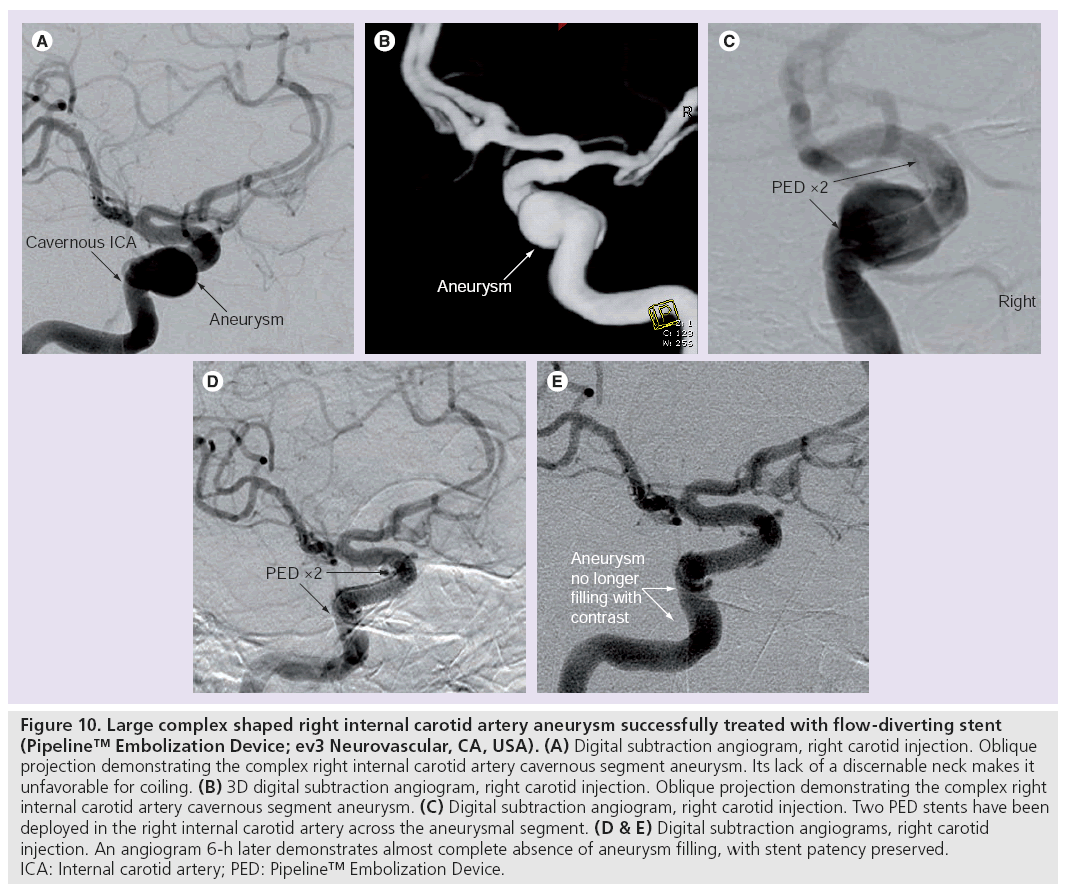
Figure 10: Large complex shaped right internal carotid artery aneurysm successfully treated with flow-diverting stent (Pipeline™ Embolization Device; ev3 Neurovascular, CA, USA). (A) Digital subtraction angiogram, right carotid injection. Oblique projection demonstrating the complex right internal carotid artery cavernous segment aneurysm. Its lack of a discernable neck makes it unfavorable for coiling. (B) 3D digital subtraction angiogram, right carotid injection. Oblique projection demonstrating the complex right internal carotid artery cavernous segment aneurysm. (C) Digital subtraction angiogram, right carotid injection. Two PED stents have been deployed in the right internal carotid artery across the aneurysmal segment. (D & E) Digital subtraction angiograms, right carotid injection. An angiogram 6-h later demonstrates almost complete absence of aneurysm filling, with stent patency preserved. ICA: Internal carotid artery; PED: Pipeline™ Embolization Device.
Mid-1990s animal studies on the use of various stent types to treat aneurysms predicted that porous self-expanding stents may become a useful aneurysm treatment in humans [147,148]. The encouraging animal and human experimental data on altered aneurysm flow dynamics and stent endothelialization was reinforced by reports describing the use of self-expanding remodeling stents (designed for stent assistant coiling) without coils to treat selected complex aneurysms [147,148,158–163]. Chinese groups reported positive results with the use of the covered Willis stent to treat cisternal segment ICA aneurysms, but cautioned that tortuous anatomy and perforator coverage were contraindications [164].
Animal studies of prototype flow-diverting stents produced angiographic, histological and electron microscopic results, which showed flow diversion, aneurysm occlusion and appropriate neo-intimal formation and endothelialization, and preservation of perforating branches [165,166].
The new generation of flow-diverting stents, the Pipeline™ Embolization Device (PED; ev3 Neurovascular, CA, USA), and the SILK stent (Balt Extrusion, Montmorency, France) are the two intracranial flow-diverting stents currently available.
Pipeline embolization device
The PED is a self-expanding cylindrical device composed of 48 braided strands of cobalt chromium and platinum. When fully deployed within an appropriately size-matched vessel, a single PED has 30–35% metal surface area.
The largest reported PED series describes treatment of 63 aneurysms in 53 patients and reports complete angiographic occlusion in 93%, and 95% of aneurysms at 6 and 12 months, respectively. At the time of writing this paper, 6 months or greater follow-up imaging was only available in 28 of the 53 patients. They report no strokes, deaths or decrease in modified Rankin scores. A giant circumferential basilar aneurysm was the only lesion that was not successfully treated. The authors hypothesize that a pre-existing self-expanding stent used for stentassisted coiling in this case may have impaired the wall apposition of the PED construct. A total of 19% of patients available for follow-up at 3 months had asymptomatic in-stent stenosis. Three of these seven patients demonstrated angiographic improvement at 12 months [167].
The Pipeline for the Intracranial Treatment of Aneurysms (PITA) trial enrolled 31 patients, most with large and wide necked ICA sidewall aneurysms. A total of 93% were angiographically cured at 6 months and 6% suffered complicating strokes. One case (3%) of asymptomatic in-stent stenosis was observed [168].
Other single-center smaller series have reported similar 6 months occlusion and in-stent stenosis rates [168,169].
Two international trials (Complete Occlusion of Coilable Aneurysms using Pipeline Embolization Device [COCOA] and Pipeline Embolization Device for Uncoilable or Failed Aneurysms [PUFS]) are running at present. The COCOA trial randomizes ‘coilable’ extra- and intra-dural ICA aneurysms to coiling or PED therapy. The PUFS trial examines PED treatment of large and wide necked ICA aneurysms without a control arm [168].
Although the 35% porosity of the PED theoretically does not occlude the perforating branches, a case of anterior cerebral artery A1 lenticulostriate occlusion and basal ganglia infarction associated with overlapping PED stents has been reported [170].
SILK stent
The SILK stent is a self-expanding stent composed of 48 braided nitinol strands. It is delivered via microcatheter and can be resheathed and repositioned before final deployment [171]. Case reports of its use in successful treatment of successful treatment of aneurysms which were not amenable to coiling have been published [171].
The Marco Polo trial is a multicenter randomized trial of SILK stents versus standard coiling techniques (including balloon and stent assistance) for ICA and vertebrobasilar aneurysms [205].
A case report was published earlier this year of fatal SAH secondary to rupture of an ophthalmic segment ICA aneurysm, which had been apparently successfully treated 20 days earlier with a SILK stent [172]. Recently, a number of deaths shortly after elective treatment of large and giant aneurysms with SILK stents led the manufacturer to issue a notice advising that it does not currently have the clinical data to support the use of the SILK device without the use of embolization coils [206].
Flow-directing stents are an exciting new development in the endovascular treatment of aneurysms. More will be understood regarding their precise role when further data are available on long-term safety and efficacy.
Deconstructive approach Vessel sacrifice
Although the endosaccular and endoluminal reconstructive approaches to aneurysm treatment are preferable, there remains a subset of aneurysms that are not amenable to these techniques. Giant size, a very wide neck, complex anatomy, a surgically inaccessible location and previous failed reconstructive approaches are factors that can make some aneurysms impossible or too high risk to treat with endosaccular coiling and or endoluminal stenting. Infective mycotic, vasculitic and dissecting aneurysms are sometimes best treated with vessel sacrifice (Figure 2) [173].
Sacrifice of the parent artery may have a higher success rate and potentially be safer, particularly if adequate collateral vascular support can be demonstrated with balloon test occlusion (BTO) before definitive artery occlusion [173,174].
Balloon test occlusion
Balloon test occlusion involves the inflation of an endovascular balloon in the target parent artery (usually the ICA or vertebral artery) under full heparinization for up to 20–30 min depending on the results obtained. A microcatheter- delivered device or one that is part of a larger double lumen balloon catheter can be used. Mild hypotension is often pharmacologically initiated to increase the sensitivity of the test. Ipsilateral conventional coronary angiography is performed during the occlusion to assess external carotid artery–ICA collateral supply. Awake neurological examination is performed and compared with the baseline status before the procedure.
Balloon test occlusion has a positive predictive value and specificity approaching 100%; that is to say that all patients who demonstrate neurological decline during test occlusion will suffer permanent deficit if the vessel is sacrificed. Its negative predictive value and sensitivity is more limited; investigators have reported rates of 4.7–25% of stroke in vessel sacrifice following a normal BTO. In order to increase the sensitivity of the test, several modifications have been reported [173].
During BTO, EEG, transcranial Doppler, ICA pressure measurements, Xenon CT, SPECT, PET and perfusion MRI are all adjunctive tests that have been reported to increase sensitivity in small nonrandomized series [173].
An alternative method, the ‘venous phase BTO,’ is reported to increase positive predictive value to 98%, and can also be performed under general anesthetic. It involves catheterization of the contralateral ICA and angiography during ipsilateral BTO. The synchronicity of cerebral cortical vein opacification between the index and contralateral sides is the outcome measure. A delay of between 2 and 4 s on the index side is reported to be indicative of poor collateral reserve, and predictive of deficit following sacrifice. A delay of greater than 4 s is regarded as a contraindication to sacrifice [175,176].
The BTO itself is a relatively safe procedure, with a reported risk profile marginally greater than diagnostic catheter angiography. In large series permanent neurological complication rates of 0.33–0.40% are reported [173,177].
Parent artery sacrifice
Proximal ICA sacrifice for aneurysms of the petrous and cavernous segment can be performed with detachable balloons, detachable devices such as the Amplatzer® Vascular Plug (AGA Medical Corporation, MN, USA) or coils. The use of pushable 0.35˝ coils has largely been replaced by the detachable microcoils. A trapping technique is usually performed, with sacrifice of the vessel proximal and distal to the aneurysm neck, preventing retrograde filling of the aneurysm sac.
Sacrifice of the supraclinoid ICA or circle of Willis vessels can be performed with detachable microcoils. Careful planning of the extent of sacrifice is needed with regard to potential collateral supply to the aneurysm. Distal vessels sacrifice is performed with microcoils or liquid embolic agents.
Vessel sacrifice is highly effective at removing aneurysms from the circulation. A meta-analysis found that endovascular sacrifice thrombosed 97.5% of the cavernous ICA aneurysms being treated [174].
As noted previously, proximal ICA sacrifice carries a risk of cerebral infarction variously quoted as 4.7–25% after successful BTO. The ischemia may be delayed and caused by thrombus forming in the vessel immediately distal to the sacrificed segment as the dynamics change over time. Sacrifice of distal vessels carries a higher and less predictable risk depending on pial collaterals [173].
Conclusion
Aneurysmal SAH is a potentially lethal condition that leaves many survivors disabled. Endovascular treatment of intracranial aneurysms is shown to improve clinical outcome after SAH and the treatment of high-risk unruptured aneurysms is likely to improve life expectancy by preventing SAH. There is high level evidence that endovascular coiling offers better outcomes than neurosurgical clipping in the treatment of aneurysmal SAH. Endovascular treatment, including advanced coil techniques and newer generation devices, is an area of continued technological development. Ongoing improvement of devices and techniques aims to reduce complications and improve angiographic and clinical outcomes.
Future perspective
The past two decades have witnessed major changes in management of intracranial aneurysms, with the broad introduction of endovascular coil-based techniques. ISAT was crucial in providing robust evidence on which to base this expansion of endovascular aneurysm repair in ruptured aneurysms, and there has been similar application to unruptured aneurysms, albeit without the same level of evidence.
Key issues which will inform a discussion on the future of aneurysm management over the next 5–10 years are:
▪ Incomplete aneurysm exclusion – both remnants and late recurrences
▪ Significance of aneurysm remnants
▪ Antiplatelet therapy and stent use
▪ Treatment of unruptured aneurysms
▪ Management of delayed neurological deficit post-SAH
Complete aneurysm exclusion remains the goal, but further evidence will confirm that not all remnants are the same. We will look back at the period between 1995 and 2010, and the vigor with which a perfect ‘imaging outcome’ was pursued, and question the acceptance of associated re-treatment risk. Further information will allow identification of low-risk remnants that can either be followed up using MRA, or safely ignored. Those remnants that are growing, or have other features associated with higher rupture risk will be treated with coils that will promote healing of the aneurysm neck, or with stents that can lead to endothelialization and healing of the wall defect at the neck.
Endovascular therapy will be more specific for sites and types of aneurysms. Flow-diverting stent constructs will be the primary treatment option, with high obliteration rates – either alone or with concomitant coil placement – for paraclinoid ICA aneurysms, and many intradural vertebral and basilar artery aneurysms. Both untreated aneurysms of complex geometry and post-treatment remnants will benefit from stent usage. Issues related to required antiplatelet therapy to allow use of stents – either as scaffolding or as flow diverters – will remain, but coated stents will become available, which will decrease the likelihood of thromboembolic complications, and delayed stenosis. Stents that do not promote platelet aggregation, neointimal hyperplasia or dissolve with time will further widen the options available.
Liquid embolic agents and endosaccular devices will progress, and may find a niche role for aneurysms unsuited to the coils and/or stent approaches, but are unlikely to be applicable for the majority of intracranial aneurysms.
Magnetic resonance angiography-based imaging workup will replace digital subtraction angiography and CT angiography – both of which use ionizing radiation and therefore have risks associated with this, particularly for ongoing follow-up. Model-based computed flow dynamics, serial imaging and aneurysm wall assessment will progress and allow more accurate risk stratification – such that we will be able to identify aneurysms at greater rupture risk, quantify that risk and provide understandable risk–benefit data on which patients and their physicians can make decisions on best management. The modeling will also allow virtual treatment to be performed – with benefits to training and procedure preparation.
Will there be a role for open surgery, or will the decreasing proportion of surgical clipping cases continue to effectively 0%? The role for open microneurosurgical aneurysm treatment will be in selected cases – likely to be more complex and of higher risk. The majority of cases will be treated using endovascular techniques – but with major referral centers for complex cerebrovascular management where a significant (20–25%) proportion of cases will be treated surgically. Concentrating such cases in major centers will be necessary to maintain surgical expertise in the face of increasingly complex cases, and decreasing numbers of more ‘simple’ aneurysms for surgical clipping.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Menghini VV, Brown RD Jr, Sicks JD, O’Fallon WM, Wiebers DO: Incidence and prevalence of intracranial aneurysms and hemorrhage in Olmsted county, Minnesota, 1965 to 1995. Neurology 51(2), 405–411 (1998).
- McCormick WF, Nofzinger JD: Saccular intracranial aneurysms: an autopsy study. J. Neurosurg. 22, 155–159 (1965).
- Atkinson JL, Sundt TM Jr, Houser OW, Whisnant JP: Angiographic frequency of anterior circulation intracranial aneurysms. J. Neurosurg. 70(4), 551–555 (1989).
- Schievink WI: Intracranial aneurysms. N. Engl. J. Med. 336(1), 28–40 (1997).
- Kim BM, Chung EC, Park SI, Choi CS, Won YS: Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent-within-a-stent technique. Case report. J. Neurosurg. 107(6), 1211–1213 (2007).
- Lee BH, Kim BM, Park MS et al.: Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J. Neurosurg. 110(3), 431–436 (2009).
- Meling TR, Sorteberg A, Bakke SJ, Slettebo H, Hernesniemi J, Sorteberg W: Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J. Neurosurg. 108(4), 662–671 (2008).
- Park JH, Park IS, Han DH et al.: Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J. Neurosurg. 106(5), 812–819 (2007).
- Shimizu H, Matsumoto Y, Tominaga T: Non-saccular aneurysms of the supraclinoid internal carotid artery trunk causing subarachnoid hemorrhage: acute surgical treatments and review of literatures. Neurosurg. Rev. 33(2), 205–216 (2010).
- Seigle JM, Caputy AJ, Manz HJ, Wheeler C, Fox JL: Multiple oncotic intracranial aneurysms and cardiac metastasis from choriocarcinoma: case report and review of the literature. Neurosurgery 20(1), 39–42 (1987).
- Kalafut M, Vinuela F, Saver JL, Martin N, Vespa P, Verity MA: Multiple cerebral pseudoaneurysms and hemorrhages: the expanding spectrum of metastatic cerebral choriocarcinoma. J. Neuroimaging 8(1), 44–47 (1998).
- Iihara K, Kikuchi H, Nagata I: [Left atrial myxoma with cerebral oncotic aneurysms with special reference to the importance of serial angiography]. No Shinkei Geka 19(9), 857–860 (1991).
- Hove B, Andersen BB, Christiansen TM: Intracranial oncotic aneurysms from choriocarcinoma. Case report and review of the literature. Neuroradiology 32(6), 526–528 (1990).
- Helmer FA: Oncotic aneurysm. Case report. J. Neurosurg. 45(1), 98–100 (1976).
- Gallo P, Fabiao Neto OM, Raupp SF, Ordovas CA, Oppitz PP: Cerebral metastasis from choriocarcinoma and oncotic aneurysms. Case report. Arq. Neuropsiquiatr. 51(2), 275–280 (1993).
- Friedman DP, Rapoport RJ: Giant fusiform oncotic aneurysm: MR and angiographic findings. AJR Am. J. Roentgenol. 167(2), 538–539 (1996).
- van Gijn J, Rinkel GJE: Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124(2), 249–278 (2001).
- Johnston SC, Selvin S, Gress DR: The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 50(5), 1413–1418 (1998).
- Linn FHH, Rinkel GJE, Algra A, van Gijn J: Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke 27(4), 625–629 (1996).
- Epidemiology of aneurysmal subarachnoid hemorrhage in australia and new zealand: incidence and case fatality from the australasian cooperative research on subarachnoid hemorrhage study (across). Stroke 31(8), 1843–1850 (2000).
- Wood MJ, Nowitzke AM: Epidemiological aspects of spontaneous subarachnoid haemorrhage in Queensland, Australia. J. Clin. Neurosci. 12(7), 770–774 (2005).
- Rinkel G, Djibuti M, Algra A, Van Gijn J: Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29(1), 251 (1998).
- van Gijn J, Kerr R, Rinkel G: Subarachnoid haemorrhage. The Lancet 369(9558), 306–318 (2007).
- Locksley H: Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. J. Neurosurg. 25(3), 321–368 (1966).
- Huang J, van Gelder JM: The probability of sudden death from rupture of intracranial aneurysms: a meta-analysis. Neurosurgery 51(5), 1101–1107 (2002).
- Broderick J, Brott T, Duldner J, Tomsick T, Leach A: Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25(7), 1342–1347 (1994).
- Sehba FA, Bederson JB: Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol. Res. 28(4), 381–398 (2006).
- Cahill J, Zhang JH: Subarachnoid hemorrhage: is it time for a new direction? Stroke 40(Suppl. 3), S86–S87 (2009).
- Bederson JB, Connolly ES Jr, Batjer HH et al.: Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke 40(3), 994–1025 (2009).
- Laidlaw JD, Siu KH: Poor-grade aneurysmal subarachnoid hemorrhage: outcome after treatment with urgent surgery. Neurosurgery 53(6), 1275–1280; discussion 1280–1272 (2003).
- Ohkuma H, Tsurutani H, Suzuki S: Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke 32(5), 1176 (2001).
- Kassell N, Torner J: Aneurysmal rebleeding: a preliminary report from the cooperative aneurysm study. Neurosurgery 13(5), 479 (1983).
- Laidlaw J, Siu K: Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J. Neurosurg. 97(2), 250–258; discussion 247–249 (2002).
- Suarez JI, Tarr RW, Selman WR: Aneurysmal subarachnoid hemorrhage. N. Engl. J. Med. 354(4), 387–396 (2006).
- Eddleman CS, Hurley MC, Naidech AM, Batjer HH, Bendok BR: Endovascular options in the treatment of delayed ischemic neurological deficits due to cerebral vasospasm. Neurosurgical Focus 26(3), E6 (2009).
- Weyer GW, Nolan CP, Macdonald RL: Evidence-based cerebral vasospasm management. Neurosurg. Focus 21(3), E8 (2006).
- Kobayashi N, Miyachi S, Negoro M et al.: Endovascular treatment strategy for direct carotid-cavernous fistulas resulting from rupture of intracavernous carotid aneurysms. AJNR Am. J. Neuroradiol. 24(9), 1789–1796 (2003).
- The International Study of Unruptured Intracranial Aneurysms Investigators: Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. N. Engl. J. Med. 339(24), 1725–1733 (1998).
- Kong D-S, Hong S-C, Jung Y-J, Kim JS: Improvement of chronic headache after treatment of unruptured intracranial aneurysms. Headache 47(5), 693–697 (2007).
- Qureshi AI, Mohammad Y, Yahia AM et al.: Ischemic events associated with unruptured intracranial aneurysms: multicenter clinical study and review of the literature. Neurosurgery 46(2), 282 (2000).
- Friedman JA, Piepgras DG, Pichelmann MA, Hansen KK, Brown RD Jr, Wiebers DO: Small cerebral aneurysms presenting with symptoms other than rupture. Neurology 57(7), 1212–1216 (2001).
- Molyneux A: International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360(9342), 1267–1274 (2002).
- Graf CJ, Nibbelink DW: Cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Report on a randomized treatment study: III. intracranial surgery. Stroke 5(4), 557–601 (1974).
- Henderson W, Torner J, Nibbelink D: Intracranial aneurysms and subarachnoid hemorrhage – report on a randomized treatment study. IV-B. Regulated bed rest – statistical evaluation. Stroke 8(5), 579 (1977).
- Nibbelink D, Torner J, Henderson W: Intracranial aneurysms and subarachnoid hemorrhage – report on a randomized treatment study. IV-A. Regulated bed rest. Stroke 8(2), 202–218 (1977).
- Sahs AL: Cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Report on a randomized treatment study: I. Introduction. Stroke 5(4), 550–551 (1974).
- Risselada R, Lingsma H, Bauer-Mehren A et al.: Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: results from the international subarachnoid aneurysm trial (ISAT). Eur. J. Epidemiol. 25(4), 261–266 (2010).
- Molyneux AJ, Kerr RSC, Yu L-M et al.: International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366(9488), 809–817 (2005).
- Wiebers DO, Whisnant JP, Huston J 3rd et al.: Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362(9378), 103–110 (2003).
- Ausman J: Why the international study of unruptured intracranial aneurysms has lost credibility with neuroscientists. Surgical Neurology 58(3–4), 287–290 (2002).
- Ausman J: The unruptured intracranial aneurysm study-II: a critique of the second study. Surg. Neurol. 62(2), 91–94 (2004).
- Ausman J, Roitberg B: A response from the ISUIA. International study on unruptured intracranial aneurysms. Surg. Neurol. 52(4), 428 (1999).
- Raymond J: Incidental intracranial aneurysms: rationale for treatment. Curr. Opin. Neurol. 22(1), 96 (2009).
- Raymond J, Guillemin F, Proust F et al.: Unruptured intracranial aneurysms a critical review of the international study of unruptured intracranial aneurysms (ISUIA) and of appropriate methods to address the clinical problem. Interv. Neuroradiol. 14(1), 85 (2008).
- Raymond J, Molyneux A: Unruptured intracranial aneurysms: evidence and speculations. Radiology 247(1), 294 (2008).
- Juvela S, Porras M, Poussa K: Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J. Neurosurg. 93(3), 379 (2000).
- Clarke G, Mendelow AD, Mitchell P: Predicting the risk of rupture of intracranial aneurysms based on anatomical location. Acta Neurochir. (Wien) 147(3), 259–263; discussion 263 (2005).
- Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ: Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 38(4), 1404–1410 (2007).
- Raymond J, Roy D, Weill A et al.: Unruptured intracranial aneurysms and the trial on endovascular aneurysm management (team): the principles behind the protocol. J. Vasc. Interv. Neurol. 1(1), 22–26 (2008).
- Raymond J, Molyneux A, Fox A, Johnston S, Collet J, Rouleau I: The team trial: safety and efficacy of endovascular treatment of unruptured intracranial aneurysms in the prevention of aneurysmal hemorrhages: a randomized comparison with indefinite deferral of treatment in 2002 patients followed for 10 years. Trials 9(1), 43 (2008).
- Raymond J, Meder JF, Molyneux AJ et al.: Unruptured intracranial aneurysms: the unreliability of clinical judgment, the necessity for evidence, and reasons to participate in a randomized trial. J. Neuroradiol. 33(4), 211–219 (2006).
- Gounis M, De Leo III M, Wakhloo A: Advances in interventional neuroradiology. Stroke 41(2), e81 (2010).
- Lanterna LA, Tredici G, Dimitrov BD, Biroli F: Treatment of unruptured cerebral aneurysms by embolization with guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding – a systematic review of the literature. Neurosurgery 55(4), 767–775; discussion 775–768 (2004).
- Holmin S, Krings T, Ozanne A et al.: Intradural saccular aneurysms treated by guglielmi detachable bare coils at a single institution between 1993 and 2005: clinical long-term follow-up for a total of 1810 patient-years in relation to morphological treatment results. Stroke 39(8), 2288–2297 (2008).
- Standhardt H, Boecher-Schwarz H, Gruber A, Benesch T, Knosp E, Bavinzski G: Endovascular treatment of unruptured intracranial aneurysms with guglielmi detachable coils: short- and long-term results of a single-centre series. Stroke 39(3), 899–904 (2008).
- Pierot L, Spelle L, Vitry F: Immediate anatomic results after the endovascular treatment of unruptured intracranial aneurysms: analysis of the ATENA series. AJNR Am. J. Neuroradiol. 31(1), 140 (2010).
- Pierot L, Spelle L, Vitry F: Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 39(9), 2497 (2008).
- Pierot L, Spelle L, Vitry F; ATENA investigators: Similar safety in centers with low and high volumes of endovascular treatments for unruptured intracranial aneurysms: evaluation of the analysis of treatment by endovascular approach of nonruptured aneurysms study. AJNR Am. J. Neuroradiol. 31(6), 1010–1014 (2010).
- Molyneux AJ, Kerr RSC, Birks J et al.: Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol. 8(5), 427–433 (2009).
- Regli L, Uske A, de Tribolet N: Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J. Neurosurg. 90(6), 1025–1030 (1999).
- Regli L, Dehdashti AR, Uske A, de Tribolet N: Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir. (Suppl. 82), 41–46 (2002).
- Suzuki SMDPD, Tateshima SMD, Jahan RMD et al.: Endovascular treatment of middle cerebral artery aneurysms with detachable coils: angiographic and clinical outcomes in 115 consecutive patients. Neurosurgery 64(5), 876–889 (2009).
- Iijima A, Piotin M, Mounayer C, Spelle L, Weill A, Moret J: Endovascular treatment with coils of 149 middle cerebral artery berry aneurysms. Radiology 237(2), 611 (2005).
- Lozier AP, Connolly ES Jr, Lavine SD, Solomon RA: Guglielmi detachable coil embolization of posterior circulation aneurysms: a systematic review of the literature. Stroke 33(10), 2509–2518 (2002).
- Gruber D, Zimmerman G, Tomsick T, van Loveren H, Link M, Tew Jr J: A comparison between endovascular and surgical management of basilar artery apex aneurysms. J. Neurosurg. 90(5), 868 (1999).
- Bakker NA, Metzemaekers JD, Groen RJ, Mooij JJ, Van Dijk JM: International subarachnoid aneurysm trial 2009: endovascular coiling of ruptured intracranial aneurysms has no significant advantage over neurosurgical clipping. Neurosurgery 66(5), 961–962 (2010).
- Raper DM, Allan R: International subarachnoid trial in the long run: critical evaluation of the long-term follow-up data from the isat trial of clipping vs coiling for ruptured intracranial aneurysms. Neurosurgery 66(6), 1166–1169; discussion 1169 (2010).
- Harbaugh RE, Heros RC, Hadley MN: More on ISAT. The Lancet 361(9359), 783–784 (2003).
- Qureshi A, Janardhan V, Hanel R, Lanzino G: Comparison of endovascular and surgical treatments for intracranial aneurysms: an evidence-based review. Lancet Neurol. 6(9), 816–825 (2007).
- van der Schaaf I, Algra A, Wermer M et al.: Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. (4), CD003085 (2005).
- Gallas S, Januel AC, Pasco A et al.: Long-term follow-up of 1036 cerebral aneurysms treated by bare coils: a multicentric cohort treated between 1998 and 2003. AJNR Am. J. Neuroradiol. 30(10), 1986–1992 (2009).
- Gallas S, Pasco A, Cottier J-P et al.: A multicenter study of 705 ruptured intracranial aneurysms treated with guglielmi detachable coils. AJNR Am. J. Neuroradiol. 26(7), 1723–1731 (2005).
- Murayama Y, Nien YL, Duckwiler G et al.: Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J. Neurosurg. 98(5), 959–966 (2003).
- Pelz D, Lownie S, Fox A: Thromboembolic events associated with the treatment of cerebral aneurysms with guglielmi detachable coils. AJNR Am. J. Neuroradiol. 19(8), 1541 (1998).
- Willinsky RA, Peltz J, da Costa L, Agid R, Farb RI, terBrugge KG: Clinical and angiographic follow-up of ruptured intracranial aneurysms treated with endovascular embolization. AJNR Am. J. Neuroradiol. 30(5), 1035–1040 (2009).
- Rordorf G, Bellon RJ, Budzik RF Jr et al.: Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am. J. Neuroradiol. 22(1), 5–10 (2001).
- Soeda A, Sakai N, Sakai H et al.: Thromboembolic events associated with guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusionweighted MR imaging. AJNR Am.
- J. Neuroradiol. 24(1), 127–132 (2003). 88 Albayram S, Selcuk H, Kara B et al.: Thromboembolic events associated with balloon-assisted coil embolization: evaluation with diffusion-weighted MR imaging. AJNR Am. J. Neuroradiol. 25(10), 1768–1777 (2004).
- Biondi A, Oppenheim C, Vivas E et al.: Cerebral aneurysms treated by guglielmi detachable coils: evaluation with diffusionweighted MR imaging. AJNR Am.
- J. Neuroradiol. 21(5), 957–963 (2000). 90 Biondi A, Soeda A, Sakai N, Iihara K, Nagata I: Diffusion-weighted imaging of thromboembolic events associated with coil embolization of intracranial aneurysms. AJNR Am. J. Neuroradiol. 25(10), 1861–1862 (2004).
- Meyers PM, Schumacher HC, Higashida RT et al.: Reporting standards for endovascular repair of saccular intracranial cerebral aneurysms. AJNR Am. J. Neuroradiol. 31(1), E12–E24 (2010).
- Soeda A, Sakai N, Murao K et al.: Thromboembolic events associated with guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am. J. Neuroradiol. 24(10), 2035–2038 (2003).
- Fiehler JA, Byrne JVB: Factors affecting outcome after endovascular treatment of intracranial aneurysms. Curr. Opin. Neurol. 22(1), 103–108 (2009).
- Ries T, Buhk J-H, Kucinski T et al.: Intravenous administration of acetylsalicylic acid during endovascular treatment of cerebral aneurysms reduces the rate of thromboembolic events. Stroke 37(7), 1816–1821 (2006).
- Yamada NK, Cross DT III, Pilgram TK, Moran CJ, Derdeyn CP, Dacey RG Jr: Effect of antiplatelet therapy on thromboembolic complications of elective coil embolization of cerebral aneurysms. AJNR Am. J. Neuroradiol. 28(9), 1778–1782 (2007).
- Vance AZ, Jayaraman MV, Dubel GJ, Doberstein CE, Haas RA: Safety of intravenous heparin administration after endovascular treatment for ruptured intracranial aneurysms. J. Neurointerv. Surg. 1(2), 136–141 (2009).
- Ross IB, Dhillon GS: Ventriculostomy-related cerebral hemorrhages after endovascular aneurysm treatment. AJNR Am. J. Neuroradiol. 24(8), 1528–1531 (2003).
- Hoh BL, Nogueira RG, Ledezma CJ, Pryor JC, Ogilvy CS: Safety of heparinization for cerebral aneurysm coiling soon after external ventriculostomy drain placement. Neurosurgery 57(5), 845–849 (2005).
- Cloft HJ, Kallmes DF: Cerebral aneurysm perforations complicating therapy with guglielmi detachable coils: a meta-analysis. AJNR Am. J. Neuroradiol. 23(10), 1706–1709 (2002).
- Elijovich L, Higashida RT, Lawton MT et al.: Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: the CARAT study. Stroke 39(5), 1501–1506 (2008).
- Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC: Balloon-assisted coil embolization of intracranial aneurysms: incidence, complications, and angiography results. J. Neurosurg. 105(3), 396–399 (2006).
- Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF: Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: report of a consecutive series and a meta-analysis. Stroke 41(1), 116–121 (2010).
- van Rooij WJ, Keeren GJ, Peluso JPP, Sluzewski M: Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am. J. Neuroradiol. 30(4), 835–839 (2009).
- Ioannidis I, Lalloo S, Corkill R, Kuker W, Byrne JV: Endovascular treatment of very small intracranial aneurysms. J. Neurosurg. 112(3), 551–556 (2010).
- Suzuki S, Kurata A, Ohmomo T et al.: Endovascular surgery for very small ruptured intracranial aneurysms. Technical note. J. Neurosurg. 105(5), 777–780 (2006).
- Johnston SC, Dowd CF, Higashida RT et al.: Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the cerebral aneurysm rerupture after treatment (CARAT) study. Stroke 39(1), 120–125 (2008).
- Campi A, Ramzi N, Molyneux AJ et al.: Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (ISAT). Stroke 38(5), 1538–1544 (2007).
- CARAT Investigators: Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke 37, 1437–1442 (2006).
- Raymond J, Guilbert F, Weill A et al.: Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 34, 1398–1403 (2003).
- Roy D, Milot G, Raymond J: Endovascular treatment of unruptured aneurysms. Stroke 32(9), 1998–2004 (2001).
- Ferns SP, Sprengers MES, van Rooij WJ et al.: Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 40(8), e523–e529 (2009).
- Pierot L, Cognard C, Ricolfi F, Anxionnat R; CLARITY Investigators: Immediate anatomic results after the endovascular treatment of ruptured intracranial aneurysms: analysis in the clarity series. AJNR Am. J. Neuroradiol. 31(5), 907–911 (2010).
- Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J: Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am. J. Neuroradiol. 28(9), 1755–1761 (2007).
- van Rooij WJ, Sluzewski M: Reply. AJNR Am. J. Neuroradiol. 30(9), E132 (2009).
- Sluzewski M, van Rooij WJ, Slob MJ, Bescós JO, Slump CH, Wijnalda D: Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 231(3), 653–658 (2004).
- Tamatani S, Ito Y, Abe H, Koike T, Takeuchi S, Tanaka R: Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am. J. Neuroradiol. 23(5), 762–767 (2002).
- Agid R, Willinsky RA, Lee S-K, Terbrugge KG, Farb RI: Characterization of aneurysm remnants after endovascular treatment: contrast-enhanced mr angiography versus catheter digital subtraction angiography. AJNR Am. J. Neuroradiol. 29(8), 1570–1574 (2008).
- Anzalone N, Scomazzoni F, Cirillo M et al.: Follow-up of coiled cerebral aneurysms at 3 T: comparison of 3D time-of-flight MR angiography and contrast-enhanced MR angiography. AJNR Am. J. Neuroradiol. 29(8), 1530–1536 (2008).
- Leclerc X, Navez J-F, Gauvrit J-Y, Lejeune J-P, Pruvo J-P: Aneurysms of the anterior communicating artery treated with guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. AJNR Am. J. Neuroradiol. 23(7), 1121–1127 (2002).
- Majoie CBLM, Sprengers ME, van Rooij WJJ et al.: MR angiography at 3 T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. AJNR Am. J. Neuroradiol. 26(6), 1349–1356 (2005).
- Shankar JJS, Lum C, Parikh N, dos Santos M: Long-term prospective follow-up of intracranial aneurysms treated with endovascular coiling using contrast-enhanced mr angiography. AJNR Am. J. Neuroradiol. 31(7), 1211–1215 (2010).
- Guglielmi G: History of the genesis of detachable coils. A review. J. Neurosurg. 111(1), 1–8 (2009).
- Matsumoto H, Terada T, Tsuura M, Itakura T, Ogawa A: Basic fibroblast growth factor released from a platinum coil with a polyvinyl alcohol core enhances cellular proliferation and vascular wall thickness: an in vitro and in vivo study. Neurosurgery 53(2), 402–408 (2003).
- Murayama Y, Tateshima S, Gonzalez NR, Vinuela F: Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 34(8), 2031–2037 (2003).
- Szikora I, Seifert P, Hanzely Z et al.: Histopathologic evaluation of aneurysms treated with guglielmi detachable coils or matrix detachable microcoils. AJNR Am. J. Neuroradiol. 27(2), 283–288 (2006).
- Yoshino Y, Niimi Y, Song J, Silane M, Berenstein A: Endovascular treatment of intracranial aneurysms: comparative evaluation in a terminal bifurcation aneurysm model in dogs. J. Neurosurg. 101(6), 996–1003 (2004).
- White PM, Raymond J: Endovascular coiling of cerebral aneurysms using ‘bioactive’ or coated-coil technologies: a systematic review of the literature. AJNR Am. J. Neuroradiol. 30(2), 219–226 (2009).
- White PM, Lewis SC, Nahser H et al.: Hydrocoil endovascular aneurysm occlusion and packing study (helps trial): procedural safety and operator-assessed efficacy results. AJNR Am. J. Neuroradiol. 29(2), 217–223 (2008).
- Fanning NF, Willinsky RA: Wall enhancement, edema, and hydrocephalus after endovascular coil occlusion of intradural cerebral aneurysms. J. Neurosurg. 108(6), 1074–1086 (2008).
- Im S-H, Han MH, Kwon BJ, Jung C, Kim JE, Han DH: Aseptic meningitis after embolization of cerebral aneurysms using hydrogel-coated coils: report of three cases. AJNR Am. J. Neuroradiol. 28(3), 511–512 (2007).
- Kang HS, Han MH, Lee TH et al.: Embolization of intracranial aneurysms with hydrogel-coated coils: result of a korean multicenter trial. Neurosurgery 61(1), 51–59 (2007).
- Mitha AP, Wong JH, Lu JQ, Morrish WF, Hudon ME, Hu WY: Communicating hydrocephalus after endovascular coiling of unruptured aneurysms. J. Neurosurg. 108(6), 1241–1244 (2008).
- Aletich VA, Debrun GM, Misra M, Charbel F, Ausman JI: The remodeling technique of balloon-assisted guglielmi detachable coil placement in wide-necked aneurysms: experience at the university of Illinois at Chicago. J. Neurosurg. 93(3), 388–396 (2000).
- Baldi S, Mounayer C, Piotin M, Spelle L, Moret J: Balloon-assisted coil placement in wide-neck bifurcation aneurysms by use of a new, compliant balloon microcatheter. AJNR Am. J. Neuroradiol. 24(6), 1222–1225 (2003).
- Moret J, Cognard C, Weill A, Castaings L, Rey A: [Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases]. J. Neuroradiol. 24(1), 30–44 (1997).
- Lefkowitz MA, Gobin YP, Akiba Y et al.: Balloon-assisted guglielmi detachable coiling of wide-necked aneurysma: part II – clinical results. Neurosurgery 45(3), 531–537; discussion 537–538 (1999).
- Nelson PK, Levy DI: Balloon-assisted coil embolization of wide-necked aneurysms of the internal carotid artery: medium-term angiographic and clinical follow-up in 22 patients. AJNR Am. J. Neuroradiol. 22(1), 19–26 (2001).
- Sluzewski M, Bosch J, van Rooij W, Nijssen P, Wijnalda D: Rupture of intracranial aneurysms during treatment with guglielmi detachable coils: incidence, outcome, and risk factors. J. Neurosurg. 94(2), 238–240 (2001).
- Pierot L, Spelle L, Leclerc X, Cognard C, Bonafe A, Moret J: Endovascular treatment of unruptured intracranial aneurysms: comparison of safety of remodeling technique and standard treatment with coils. Radiology 251(3), 846 (2009).
- Piske R, Kanashiro L, Paschoal E, Agner C, Lima S, Aguiar P: Evaluation of Onyx hd-500 embolic system in the treatment of 84 wide-neck intracranial aneurysms. Neurosurgery 64(5), E865 (2009).
- Cekirge H, Saatci I, Ozturk M et al.: Late angiographic and clinical follow-up results of 100 consecutive aneurysms treated with Onyx reconstruction: largest single-center experience. Neuroradiology 48(2), 113–126 (2006).
- Weber W, Siekmann R, Kis B, Kuehne D: Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx hd 500. AJNR Am. J. Neuroradiol. 26(8), 1909 (2005).
- Molyneux AJ, Cekirge S, Saatci I, Gal G: Cerebral aneurysm multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 european centers. AJNR Am. J. Neuroradiol. 25(1), 39–51 (2004).
- Simon S, Lopes D, Mericle R: Use of intracranial stenting to secure unstable liquid embolic casts in wide-neck sidewall intracranial aneurysms. Neurosurgery 66(3), 92–97 (2010).
- Barath K, Cassot F, Rufenacht DA, Fasel JHD: Anatomically shaped internal carotid artery aneurysm in vitro model for flow analysis to evaluate stent effect. AJNR Am. J. Neuroradiol. 25(10), 1750–1759 (2004).
- Canton G, Levy DI, Lasheras JC, Nelson PK: Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J. Neurosurg. 103(5), 891–902 (2005).
- Wakhloo A, Tio F, Lieber B, Schellhammer F, Graf M, Hopkins L: Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am. J. Neuroradiol. 16(5), 1043–1051 (1995).
- Wakhloo AK, Schellhammer F, de Vries J, Haberstroh J, Schumacher M: Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. AJNR Am. J. Neuroradiol. 15(3), 493–502 (1994).
- Lopes DMD, Sani SMD: Histological postmortem study of an internal carotid artery aneurysm treated with the neuroform stent. Neurosurgery 56(2), E416 (2005).
- Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG: Neuroform stent assisted aneurysm treatment: evolving treatment strategies, complications and results of long term follow-up. J. Neurointerv. Surg. 2(1), 16–22 (2010).
- Sedat J, Chau Y, Mondot L, Vargas J, Szapiro J, Lonjon M: Endovascular occlusion of intracranial wide-necked aneurysms with stenting (neuroform) and coiling: mid-term and long-term results. Neuroradiology 51(6), 401–409 (2009).
- Mocco J, Snyder KV, Albuquerque FC et al.: Treatment of intracranial aneurysms with the enterprise stent: a multicenter registry. J. Neurosurg. 110(1), 35–39 (2009).
- Lubicz B, Bandeira A, Bruneau M, Dewindt A, Balériaux D, De Witte O: Stenting is improving and stabilizing anatomical results of coiled intracranial aneurysms. Neuroradiology 51(6), 419–425 (2009).
- Liang G, Gao X, Li Z, Wei X, Xue H: Neuroform stent-assisted coiling of intracranial aneurysms: a 5 year single-center experience and follow-up. Neurol. Res. 32(7), 721–727 (2009).
- Piotin M, Blanc R, Spelle L et al.: Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke 41(1), 110–115 (2010).
- Tähtinen OI, Vanninen RL, Manninen HI et al.: Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (<72 hours) subarachnoid hemorrhage – experience in 61 consecutive patients. Radiology 253(1), 199–208 (2009).
- Lylyk P, Ferrario A, Pasbón B, Miranda C, Doroszuk G: Buenos aires experience with the neuroform self-expanding stent for the treatment of intracranial aneurysms. J. Neurosurg. 102(2), 235–241 (2005).
- Zenteno M, Gomez C, Santos-Franco J, Vinuela F, Aburto-Murrieta Y, Lee A: Ten-year follow-up of giant basilar aneurysm treated by sole stenting technique: a case report. J. Med. Case Reports 4(1), 64 (2010).
- Pavlisa G, Ozretic D, Murselovic T, Rados M: Sole stenting of large and giant intracranial aneurysms with self-expanding intracranial stents-limits and complications. Acta Neurochir. (Wien) 152(5), 763–769 (2010).
- Zenteno M, Santos-Franco J, Freitas-Modenesi J et al.: Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J. Neurosurg. 108, 1104–1118 (2008).
- Van Rooij W, Peluso J, Sluzewski M, Beute G: Dangerous sophistication? J. Neurosurg. 108(3), 633–634 (2008).
- Aziz-Sultan A, Heros R: Sole stenting. J. Neurosurg. 108, 1101–1103 (2008).
- Zenteno M, Santos-Franco J, Aburto-Murrieta Y et al.: Superior cerebellar artery aneurysms treated using the sole stenting approach. Technical note. J. Neurosurg. 107, 860–864 (2007).
- Zhu YQ, Li MH, Fang C et al.: Application of the willis covered stent in the treatment of aneurysm in the cisternal segment of the internal carotid artery: a pilot comparative study with midterm follow-up. J. Endovasc. Ther. 17(1), 55–65 (2010).
- Sadasivan C, Cesar L, Seong J et al.: An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke 40(3), 952 (2009).
- Sadasivan C, Lieber BB, Cesar L, Miskolczi L, Jaehoon S, Wakhloo AK: Angiographic assessment of the performance of flow divertors to treat cerebral aneurysms. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 3210–3213 (2006).
- Lylyk P, Miranda C, Ceratto R et al.: Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the buenos aires experience. Neurosurgery 64(4), 632 (2009).
- Fiorella D, Lylyk P, Szikora I et al.: Curative cerebrovascular reconstruction with the pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J. Neurointerv. Surg. 1(1), 56–65 (2009).
- Szikora I, Berentei Z, Kulcsar Z et al.: Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am. J. Neuroradiol. 31(6), 1139–1147 (2010).
- van Rooij WJ, Sluzewski M: Perforator infarction after placement of a pipeline flow-diverting stent for an unruptured a1 aneurysm. AJNR Am. J. Neuroradiol. 31(4), E43–E44 (2010).
- Appelboom G, Kadri K, Hassan F, Leclerc X: Infectious aneurysm of the cavernous carotid artery in a child treated with a new-generation of flow-diverting stent graft: case report. Neurosurgery 66(3), E623–E624 (2010).
- Turowski B, Macht S, Kulcsár Z, Hänggi D, Stummer W: Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-stent).
- Neuroradiology (2010) (Epub ahead of print). 173 Lesley WS, Rangaswamy R: Balloon test occlusion and endosurgical parent artery sacrifice for the evaluation and management of complex intracranial aneurysmal disease. J. Neurointerv. Surg. 1(2), 112–120 (2009).
- van der Schaaf IC, Brilstra EH, Buskens E, Rinkel GJE: Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 33(1), 313–318 (2002).
- Abud DG, Spelle L, Piotin M, Mounayer C, Vanzin JR, Moret J: Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am. J. Neuroradiol. 26(10), 2602–2609 (2005).
- van Rooij WJ, Sluzewski M, Slob MJ, Rinkel GJ: Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am. J. Neuroradiol. 26(1), 175–178 (2005).
- Mathis J, Barr J, Jungreis C et al.: Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am. J. Neuroradiol. 16(4), 749–754 (1995).
- Maps trial: Matrix and platinum science http://clinicaltrials.gov/ct2/show/ NCT00396981
- Hcatrial
- www.hcatrial.org CERECYTE® trial www.cerecytecoiltrial.com/tp. asp?Section=Protocol
- PRET study
- www.pretstudy.org/doc/index.html MARCO POLO – SILK trial http://clinicaltrials.gov/ct2/show/ NCT01084681
- Medical device alert: Intracranial stent manufactured by balt extrusion (mda/2010/023) www.mhra.gov.uk/Publications/ Safetywarnings/MedicalDeviceAlerts/ CON076289
Websites