Research Article - Clinical Practice (2020) Volume 17, Issue 1
Vascular endothelial growth factor (VEGF) as a biochemical marker for the diagnosis of hepatocellular carcinoma (HCC)
- Corresponding Author:
- Neelima Gupta
Centre of Excellence Laboratory
Department of Animal Science
MJP Rohilkhand University, India
E-mail: guptagrawal@rediffmail.com
Abstract
Hepatocellular Carcinoma (HCC), a malignant tumor is common in Egypt. Early diagnosis of HCC is important; therefore, this study evaluated the diagnostic potential of VEGF for diagnosis of HCC and its capability to differentiate HCC from benign tumor and CLD in early stages. Three groups of patients: Group I with HCC (n=116), Group II with Chronic Liver Diseases (CLD) (n=30) and Group III control (n=30) were subjected to full history, clinical examination, liver function tests and radiological investigations. Peripheral blood samples were assayed for VEGF by sandwich ELISA technique, quantitation was achieved by the construction of a standard curve. Serum VEGF and AFP were significantly higher in HCC. A significant positive correlation between VEGF and AFP, Child score and class, Okuda class and total bilirubin and a significant negative correlation with albumin was observed. The cut-off to discriminate HCC and CLD from the healthy group was ≥ 280 pg/mL. VEGF is a highly useful marker for the diagnosis of HCC in patients with normal serum AFP concentrations. Its serum levels beyond threshold show its discriminatory potential for HCC and CLD suggesting that suspected patients with high ALT, AST and albumin levels may be screened for AFP and VEGF.
Keywords
alpha fetoprotein, chronic liver disease, hepatocellular carcinoma, vascular endothelial growth factor, Hepatitis C-virus
Abbreviations
AFP: Alpha Feto Protein; ALT: Alanine Transaminase; AST: Aspartate Transaminase; CLD: Chronic Liver Diseases; CT: Computed Tomography; ELISA: Enzyme Linked Immune Sorbent Assay; GGT: Gamma-Glutamyl Transferase; HBV: Hepatitis B-Virus; HCC: Hepatocellular Carcinoma; HCV: Hepatitis C-Virus; PT: Prothrombin Time; VEGF: Vascular Endothelial Growth Factor; VEGFR: Vascular Endothelial Growth Factor Receptor
Introduction
Hepatocellular Carcinoma (HCC) is the second common cause of cancer-related death mortalities [1]. Its incidences are increasing worldwide ranging between 3% and 9% annually [2]. The rates of HCV in Egypt are among the highest in the world [3,4]. In Egypt, the annual proportion of HCC showed a significant rising trend from 4.7% in 1993 to 7.2% in 2002 [5]. Studies show that there are 6 million cases of HCC per year with annual 600,000 deaths [6,7]. Hypoxia is the major trigger for HCC; thus, it is hypervascular in nature, with an important role of angiogenesis in its pathophysiology [8,9]. Deregulation of several angiogenic pathways are been reported in HCC [10]. There are diverse causes of HCC as chronic Hepatitis B and Hepatitis C infections, alcohol use or nonalcoholic fatty liver disease in association with cirrhosis, hereditary hemochromatosis, obesity or primary biliary cirrhosis, with cirrhosis as the most important risk factor [11-13].
If HCC is left untreated, liver cancer has a poor prognosis [14]. Most of HCC patients visit clinics at advance disease with the survival of approximately 5 years [15,16]. Complete surgical resection and liver transplant are at present the only curative treatment options [17]. Unfortunately, the majority of patients with liver cancer present advanced unresectable disease, therefore, they are not surgical candidates [17,18]. Hence, screening programs of patients at risks, such as chronic infections of hepatitis B and C represent attractive strategies for potential improvement of the outcome of HCC patients. Therefore, it is very important to detect this disease and the recurrence during its early phase.
Serum Alpha Fetoprotein (AFP) is the most widely used tumor marker for diagnosis as well as surveillance of HCC [19-21]. However, AFP levels may be normal in up to 40% of patients with HCC, particularly during the early stages (low sensitivity) resulting in 40% cases of missed diagnosis [22]. Furthermore, elevated AFP levels may be seen in patients with cirrhosis or exacerbations of chronic hepatitis (low specificity), normal levels of serum AFP are shown in 15%-20% while advanced stages may have AFP concentration as low as <20 ng/ml which account for 30%-40% of HCC [22,23]. Thus, the identification of novel biochemical markers for HCC remains an important goal for many laboratories around the world [24]. It has been suggested that elevated ALT levels are strongly associated with the incidence of HCC regardless of hepatitis virus positivity. Therefore, the ALT level may be considered as a good independent determinant for intervention [25]. Serum ALP level is increased up to 4 times in HCC patients. Total GGT activity increases in both hepatic and extra-hepatic tumors. This mostly decreases both the sensitivity and specificity of this enzyme in detecting HCC [20].
Hepatocellular carcinoma is a highly vascular tumor characterized by neovascularization and a high propensity for venous invasion [26]. Vascular Endothelial Growth Factor (VEGF) is the most potent directly acting angiogenic factor known so far. It is a soluble, homo-dimer of 34-42 kDa heparin-binding glycoproteins that specifically stimulates endothelial cell proliferation and enhances vascular permeability [26]. VEGF promotes extravasation of plasma fibrinogen, leading to the formation of fibrin scaffolding that facilitates cell migration during invasion [19,27]. As an endothelial growth factor, VEGF stimulates endothelial cell proliferation, thus inducing the budding of new blood vessels around the growing tumor masses [19]. Serum VEGF levels were significantly elevated in HCC patients as compared to patients with benign liver lesions and normal controls, however, VEGF levels of serum in the previous reports were not significantly different between the patients with benign liver lesions and normal controls [19,20]. However, many recent studies have reported significantly higher levels of VEGF in HCC as compared to others [8,9,28]. These show that it has the potential to be used as a diagnostic and prognostic marker as well as VEGF/VEGFR pathway as a potential therapeutic target for HCC [29,30].
The aim of the present study was to investigate the clinical utility of VEGF in HCC patients and to compare the results with those having CLD.
Patients and Methods
Patients
The present study included 116 patients from the Department of Tropical Medicine, Ain Shams University Hospital, Cairo, Egypt with HCC, 30 with CLD as pathological control and 30 apparently healthy individuals as the healthy control group. The HCC group was included prior to starting any kind of treatment. The diagnosis was confirmed by a triphasic spiral abdominal CT. They were 95 males and 21 females ranging in age from 40 to 77 years (mean 56.1 ± 7.7 years). The CLD group was matched in age and sex with HCC patients. The diagnosis was based on clinical, biochemical, and sonographic criteria of chronic liver disease. They were 24 males and 6 females ranging in age from 40 to 72 years (mean 55.0 ± 8.4 years). The healthy individuals were matched in age and sex with HCC patients. They were 24 males and 6 females whose age ranged from 43 to 72 years (mean 56.8 ± 7.9 years).
Clinical examinations
All subjects of the study were subjected to full history, detailed clinical examination, routine liver function tests including AST, ALT, albumin, total bilirubin, Prothrombin Time (P.T.), and serum AFP as follows.
AST and ALT
The analysis was done on Synchron CX-5 autoanalyzer by a kinetic method using reagents provided by humans [31].
Albumin
Albumin was analysed on the Synchron CX-5 autoanalyzer by albumin-bromocresol green (BCG) binding method using reagents provided by Spectrum [32].
Total bilirubin
Total bilirubin was estimated on Synchron CX-5 autoanalyzer by a timed endpoint Diazo method using reagents supplied by Synchron System Beckman Coulter [33].
Prothrombin time (PT)
The analysis was done on STA- Stago Compact C.T. autoanalyzer using reagents supplied by Dade- Behring [34].
Alfa feto protein
Assay was carried out by a sandwich ELISA technique using reagents provided by BioCheck, Inc. [35].
Radiological investigations
Radiological investigations included CT scan and abdominal ultrasound of the HCC patients using abdominal Ultrasound apparatus and abdominal Triphasic Spiral Computed Tomography (CT).
Histopathology
Liver biopsy and histopathological examinations were done whenever needed following routine techniques.
VEGF quantitation
Peripheral blood samples were assayed for VEGF by sandwich ELISA technique employing two VEGF specific antibodies. VEGF quantitation was performed by Sandwich Enzyme-Linked Immunosorbent Assay using reagents provided by Bender MedSystems. Quantitation was achieved by the construction of the standard curve using known concentrations of VEGF.
Results
The study was initiated with age and sexmatched control, CLD and HCC subjects. TABLE 1 shows the patients of HCC and CLD groups classified according to Child-Pugh criteria. A maximum number of patients in both groups HCC and CLD were in B class followed by A and C.
| Group | HCC | CLD |
|---|---|---|
| Child classification | ||
| A | 44 (37.9%) | 11 (36.7%) |
| B | 48 (41.4%) | 13 (43.3%) |
| C | 24 (20.7%) | 6 (20.0%) |
| Child score | 7.5 ± 2.0 | 7.5 ± 1.9 |
TABLE 1. Comparison between HCC and CLD groups with respect to child classification.
The HCC and CLD groups have been compared according to Okuda TABLE 2 classification. Group II Okuda showed the highest values for both HCC and CLD groups, followed by I and III.
| N | % | |
|---|---|---|
| Okuda I | 49 | 42.2 |
| Okuda II | 54 | 46.6 |
| Okuda III | 13 | 11.2 |
| Total | 116 | 100 |
TABLE 2. Classification of HCC group according to Okuda classification.
TABLE 3 shows that there is no significant difference between HCC and CLD groups regarding a number of cases and percentage of HBV and HCV, however, HCV is more common in HCC and CLD patients as compared to HBV.
| HCC | CLD | χ2 | p | |
|---|---|---|---|---|
| HBV | 11 (9.5%) | 2 (6.7%) | 0.233 | 0.629 |
| HCV | 107 (92.2%) | 27 (90.0%) | 0.159 | 0.69 |
χ2: Chi square test; p>0.05: Non-significant difference; p<0.05: Significant difference; p<0.01, <0.001: Highly significant difference
TABLE 3. Comparison between HCC and CLD with respect to the number of cases and percentage of HBV and HCV infection.
TABLE 4 with respect to the clinical picture, the only difference between the CLD and HCC group was that tremors were significantly more frequent in the former group as compared to the latter.
| HCC | CLD | χ2 | p | |
|---|---|---|---|---|
| Bleeding Tendency | 68 (58.6%) | 12 (40.0%) | 3.336 | 0.068 |
| Hematemsis | 19 (16.4%) | 4 (13.3%) | 0.167 | 0.683 |
| Jaundice | 34 (29.3%) | 11 (36.7%) | 0.605 | 0.437 |
| Ascites | 51 (44.0%) | 11 (36.7%) | 0.52 | 0.471 |
| Edema | 62 (53.4%) | 17 (56.7%) | 0.099 | 0.753 |
| Fever | 11 (9.5%) | 5 (16.7%) | 1.261 | 0.262 |
| Encephalopathy | 20 (17.2%) | 5 (16.7%) | 0.006 | 0.941 |
| Tremors | 1 (0.9%) | 2 (6.7%) | 3.99 | 0.046* |
| DM | 33 (28.4%) | 8 (26.7%) | 0.037 | 0.847 |
χ2: Chi square test; p>0.05: Non-significant difference; p<0.05: Significant difference; p<0.01: Highly significant difference
TABLE 4. Clinical comparison between HCC and CLD groups.
Different radiological aspects showed nonsignificant differences between the two groups in spleen size, ascites, portal vein, its diameter, common bile duct, IHBRD, and collaterals. However, hepatomegaly was significantly more frequent in CLD group than HCC group TABLE 5.
| HCC | CLD | χ2 | p | |
|---|---|---|---|---|
| Liver size | ||||
| Average | 71 (61.2%) | 5 (16.7%) | 31.55 | <0.001* |
| Shrunken | 19 (16.4%) | 2 (6.7%) | ||
| Enlarged | 26 (22.4%) | 23 (76.7%) | ||
| Spleen size | ||||
| Average | 78 (67.2%) | 21 (70.0%) | 2.249 | 0.325 |
| Shrunken | 30 (25.9%) | 9 (30.0%) | ||
| Enlarged | 8 (6.9%) | 0 (0.0%) | ||
| Ascites | ||||
| Present | 46 (39.7%) | 11 (36.7%) | 0.089 | 0.765 |
| Absent | 70 (60.3%) | 19 (63.3%) | ||
| Portal Vein | ||||
| Thrombosed | 10 (8.6%) | 1 (3.3%) | 0.956 | 0.328 |
| Not | 106 (91.4%) | 29 (96.7%) | ||
| Portal Vein Diameter | ||||
| Dilated | 19 (16.4%) | 3 (10.0%) | 0.758 | 0.384 |
| Not | 97 (83.6%) | 27 (90.0%) | ||
| Common Bile Duct | ||||
| Dilated | 0 (0.0%) | 0 (0.0%) | 0 | 1 |
| Not | 116 (100.0%) | 30 (100.0%) | ||
| IHBRD | ||||
| Present | 2 (1.7%) | 1 (3.3%) | 0.307 | 0.58 |
| Absent | 114 (98.3%) | 29 (96.7%) | ||
| Collaterals | ||||
| Present | 11 (9.5%) | 0 (0.0%) | 3.077 | 0.079 |
| Absent | 105 (90.5%) | 30 (100.0%) | ||
χ2: Chi square test; p>0.05: Non-significant difference; p<0.05: Significant difference; p<0.01, <0.001: Highly significant difference
TABLE 5. Radiological comparison between HCC and CLD groups.
ALT, albumin, hemoglobin was significantly higher in HCC group than CLD group, though AST and INR were also higher in HCC than CLD group, but the difference was statistically non-significant, while there was no significant difference between HCC and CLD groups with regards to platelets TABLE 6. On the other hand, total bilirubin was significantly higher in CLD group as compared to HCC group.
| HCC | CLD | Z | p | |||
|---|---|---|---|---|---|---|
| (N=116) | (N=30) | |||||
| Median (IQR) | Range | Median (IQR) | Range | |||
| ALT | 47.5 | 8.0-200.0 | 35 | 120.0-135.0 | -2.715 | 0.007* |
| (37.3-67.0) | (19.5-53.8) | |||||
| AST | 65 | 10.0-215.0 | 52 | 15.0-285.0 | -1.444 | 0.149 |
| (46.5-84.5) | (33.5-84.3) | |||||
| Albumin | 3.2 | 1.8-4.9 | 2.9 | 1.7-4.6 | 2.268 | 0.023* |
| (2.7-3.5) | (2.3-3.2) | |||||
| Bilirubin | 1.5 | 0.3–5.7 | 1.8 | 0.7–19.4 | -1.953 | 0.050* |
| (1.0–2.5) | (1.5–3.6) | |||||
| Hb | 12 | 8.1-20.7 | 10.6 | 7.1-16.0 | 3.294 | 0.001* |
| (10.8-13.5) | (8.8-12.0) | |||||
| Platelets | 110.5 | 15.0–661.0 | 112 | 1.0–376.0 | -0.242 | 0.809 |
| (81.3–151.5) | (56.3–192.8) | |||||
| INR | 1.4 | 0.9-2.7 | 1.3 | 0.9-2.6 | 1.49 | 0.136 |
| (1.2-1.7) | (1.1-1.5) | |||||
*Z: Mann Whitney test;
**IQR: Inter-quartile range; p>0.05: Non-significant difference; p<0.05: Significant difference; p<0.01, <0.001: Highly significant difference
TABLE 6. Comparison between HCC and CLD groups based on laboratory results.
AFP was significantly different between HCC, CLD and healthy groups being highest in HCC group, followed by CLD group and lowest in healthy group TABLE 7.
| Group | N | Median (IQR) | Range | HCC | HCC | CLD |
|---|---|---|---|---|---|---|
| I (HCC) | 116 | 25.9 (10-281.3) | 0.9-3308 | CLD | Healthy | Healthy |
| II (CLD) | 30 | 6.66 (4.1-15) | 2-107.5 | Z**=4.318 | Z=5.789 | Z=1.079 |
| III (Healthy) | 30 | 6.1 (5.3-7.4) | 2.9-10.2 | p<0.0001* | p<0.0001* | p=0.0280* |
IQR*: Inter-Quartile Range;
Z**: Mann Whitney test; p>0.05: Non-significant difference, p<0.05: Significant difference; p<0.01, <0.001: Highly significant difference
TABLE 7. Comparison between HCC, CLD and healthy control groups with respect to AFP.
VEGF was significantly different amongst HCC, CLD and healthy groups were highest in HCC group followed by C
LD group and lowest in healthy group TABLE 8.
| Group | N | Median (IQR) | Range | HCC | HCC | CLD |
|---|---|---|---|---|---|---|
| I (HCC) | 116 | 530 (248.5-915) | 40.0-3028.0 | CLD | Healthy | Healthy |
| II (CLD) | 30 | 166 (113-340) | 13.0-482.0 | Z=5.168 | Z=6.606 | Z= 2.285 |
| III (Healthy) | 30 | 129 (37-212) | 12.0-280.0 | p<0.000* | p<0.0001* | p=0.0223* |
IQR*: Inter-Quartile Range;
Z**: Mann Whitney test; p>0.05: Non-significant difference; p<0.05: Significant difference; p<0.01, <0.001: Highly significant difference
TABLE 8. Comparison between HCC, CLD and healthy groups with respect to VEGF.
There was a significant positive correlation between AFP and VEGF, ALT and AST as well as AFP and VEGF TABLE 9.
| rs | p | |
|---|---|---|
| VEGF | 0.347 | <0.001* |
| Age | -0.01 | 0.91 |
| Child Score | 0.021 | 0.807 |
| Child Class | 0.016 | 0.851 |
| ALT | 0.268 | <0.001* |
| AST | 0.212 | 0.011* |
| Albumin | 0.135 | 0.109 |
| Total Bilirubin | 0.002 | 0.983 |
| Hemoglobin | 0.157 | 0.062 |
| Platelets | -0.056 | 0.56 |
| INR | -0.106 | 0.209 |
rs: Spearman correlation test;
p>0.05: Non-significant difference;
p<0.05: Significant difference;
p<0.01, <0.001: Highly significant difference
TABLE 9. Correlation between AFP and other parameters.
There was a significant positive correlation between VEGF and AFP, Child Score, Child class and total bilirubin and a significant negative correlation with albumin TABLE 10.
| rs | p | |
|---|---|---|
| AFP | 0.347 | <0.001* |
| Age | -0.025 | 0.768 |
| Child Score | 0.388 | <0.001* |
| Child Class | 0.372 | <0.001* |
| Okuda Class | 0.309 | <0.001* |
| ALT | 0.114 | 0.172 |
| AST | 0.13 | 0.118 |
| Albumin | -0.186 | 0.024* |
| Total Bilirubin | 0.204 | 0.013* |
| Hemoglobin | -0.06 | 0.472 |
| Platelets | -0.059 | 0.531 |
| INR | 0.102 | 0.221 |
rs: Spearman correlation test;
p> 0.05: Non-significant difference;
p<0.05: Significant difference;
p<0.01, <0.001: Highly significant difference
TABLE 10. Correlation between VEGF and other parameters.
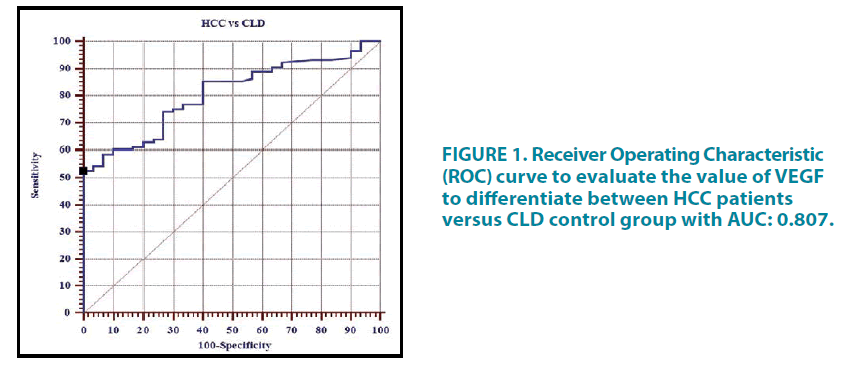
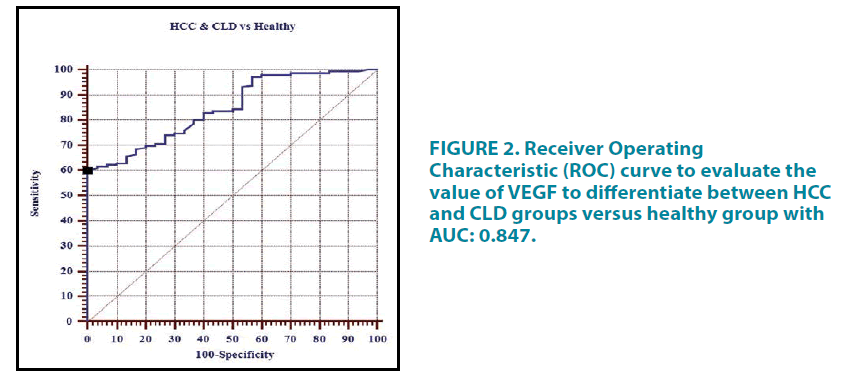
The Receiver Operating Characteristic (ROC) curve analysis was done to assess the diagnostic performance of serum VEGF (pg/mL) in HCC patients versus CLD control group TABLE 11. The curve is represented in FIGURE 1 whereas the ROC curve to evaluate the value of VEGF to differentiate between HCC and CLD groups versus healthy group is shown in TABLE 12 and FIGURE 2.
| Cut-off | SN% | SP% | PPV% | NPV% | EFF% | |
|---|---|---|---|---|---|---|
| HCC vs CLD | >482 | 52.59 | 100 | 100 | 35.3 | 61.6 |
EFF: Efficacy (Diagnostic Accuracy); PPV: Positive Predictive Value; SN: Sensitivity; SP: Specificity; NPV: Negative Predictive Value.
TABLE 11. Receiver operating characteristic (ROC) curve analysis to assess the diagnostic performance of serum VEGF (pg/mL) in HCC patients versus CLD control group.
| Cut-off | SN% | SP% | PPV% | NPV% | EFF% | |
|---|---|---|---|---|---|---|
| HCC and CLD vs Healthy | > 280 | 60.27 | 100 | 100 | 34.1 | 67 |
EFF: Efficacy (Diagnostic Accuracy); PPV: Positive Predictive Value; SN: Sensitivity; SP: Specificity; NPV: Negative Predictive Value.
TABLE 12. Receiver operating characteristic (ROC) curve analysis to assess the diagnostic performance of serum VEGF (pg/mL) in HCC and CLD groups versus Healthy group.
TABLE 13 shows that VEGF was significantly elevated (>482 pg/ml) in both groups of AFP especially in a group with normal levels of AFP (<200 ng/ml), representing its useful role as a serological marker for diagnosis of HCC patients with a normal level of AFP.
| AFP>200 | AFP<200 | χ2 | p | |
|---|---|---|---|---|
| VEGF>482 | 29 (25%) | 32 (27.6%) | 11.387 | 0.0007 |
| VEGF<482 | 9 (7.8%) | 46 (39.6%) |
χ2: Chi square test; p>0.05: Non-significant difference; p<0.05: Significant difference; p<0.01, <0.001: Highly significant difference
TABLE 13. Classification of HCC patients according to their AFP and VEGF levels.
TABLE 14 The levels of AFP in different classes of OKUDA showed significant differences in class wise. Our study shows high levels of AFP in OKUDA I with reduced levels in II but >200 in OKUDA II and very high levels in OKUDA III, while VEGF was very high in all three classes of OKUDA.
| AFP | VEGF | |||||
|---|---|---|---|---|---|---|
| Okuda classes | I | II | III | I | II | III |
| N | 49 | 54 | 13 | 49 | 54 | 13 |
| Mean | 452.67 | 272.2 | 2931.9 | 525.55 | 626.44 | 1324.54 |
| Std. Error | 156.03 | 81.05 | 1907.62 | 63.78 | 71.63 | 181.87 |
| F-value | 6.99** | 12.85*** | ||||
p<0.001 is represented as**; p<0.0001 is represented as***
TABLE 14. Comparative analysis of AFP and VEGF in different classes of Okuda using one-way analysis of variance. a and b represent significant differences in the means of I versus II and II versus III.
Discussion
Hepatocellular carcinoma is the fifth most common malignancy in the world and the third most common cause of cancer-related mortality [19,36,37]. In Egypt, it has been reported that the incidence rate of HCC increases annually and the burden of HCC has been increasing with doubling of the incidence rate in the past 10 years [38].
Hepatocellular carcinoma is often asymptomatic at the early and most curable stages. Unfortunately, patients are usually diagnosed at very advanced stages when effective treatments are not possible. Therefore, early detection of HCC is a critical goal to improve patient health [39].
Our study revealed that 44, 48 and 24 patients with HCC were included in Child’s classification, while the majority of HCC patients were in OKUDA II. The results suggest that initial changes based on Child’s classification should be taken up seriously for the diagnosis of HCC.
With respect to the number of cases and percentage of HBV and HCV, the present study revealed that there is no significant difference between HCC and CLD regarding HBV, HCV, also in patients with HCC, HCV was positive in 92.2% and HBV in 9.5% cases, therefore HCV is more common in HCC patients than HBV. Hepatitis C virus (HCV) infection was commonly observed in both HCC and CLD groups. HCV infection was also found to be associated with the onset of CLD. In patients with HCC, HCV was positive in 73.3% and HBV in 16.3% cases and our results are in agreement [5].
However, the majority of our HCC patients showed average liver size suggesting ultrasonography may not be a useful modality for diagnosis of HCC (22.4% patients) while CLD was significantly associated with hepatomegaly (76.7% patients). Thus, radiological findings indicated that there is no significant difference between HCC and CLD except for hepatomegaly which is significantly more frequent in CLD group than HCC group; this was similar to earlier results [40,41].
The laboratory results revealed that ALT, AST, Albumin, and hemoglobin are significantly higher in HCC group than CLD group. The results suggest their upregulation in HCC. Results also showed that AST and INR are higher in HCC group than CLD group, but the difference was statistically non-significant. This was similar to the results of Zekri et al. who found that aminotransferases were significantly elevated in HCC when compared to CLD patients [42]. On the other hand, no significant difference between HCC patients and CLD patients regarding aminotransferases were reported, none of the liver function tests were specific enough to be diagnostic of HCC and were not distinct from those found in cirrhosis [43]. The present study indicated that there is no significant difference between HCC and CLD with regard to platelets, while total bilirubin was significantly higher in CLD group than HCC group.
Taking the clinical picture into consideration, there is no significant difference between HCC and CLD except for tremors which were more frequent in CLD group than HCC group, this finding was in agreement where many patients of HCC could be asymptomatic and diagnosed during screening of high-risk patients [44].
AFP was significantly high in HCC group as compared to CLD group. High AFP is suggestive of its diagnostic potential in HCC. Currently, AFP represents the most commonly used serological marker for the diagnosis of HCC, despite its low sensitivity which reaches only 45%. Moreover, alterations of AFP serum levels are commonly observed in cirrhotic patients [45]. The development of false-negative or falsepositive rates with AFP has been reported to be as high as 30%-40% respectively for patients with small hepatocellular carcinomas [23]. This prompted the search for more reliable, noninvasive biochemical markers with better sensitivity and specificity for the early diagnosis of HCC. VEGF is one of the platelet-derived growth factor family members who have been found to be overexpressed in HCC [46]. The increase in the VEGF level in CLD patients and the marked increase in HCC patients may be attributed to the increased hepatocyte and hepatoma cell production of VEGF in response to hypoxia resulting from regeneration or proliferation [47].
Our results showed significantly higher VEGF levels in HCC as compared to CLD and healthy controls. Therefore, we evaluated the clinical utility of serum levels of VEGF in HCC patients and compared the results with those having CLD. Yvamoto et al. demonstrated VEGF to be a useful biomarker for the detection of HCC [48]. However, AFP was important to discriminate patients with HCC and cirrhosis or HCV.
Our study was in accordance with other studies who reported that AFP levels were higher in HCC as compared to CLD patients with statistically significant differences between the two [45,49,50]. Di Bisceglie et al. observed that AFP production is enhanced in the presence of inflammation, necrosis and hepatocellular injury, possibly resulting from increased hepatocyte turnover [51]. However, Abdelgawad et al. opined that elevated serum AFP levels may be the result of altered hepatocyte-hepatocyte interaction associated with a loss of normal architectural arrangements rather than necrosis or active regeneration [52].
In the present study, serum VEGF level in HCC patients was significantly higher than the healthy controls and CLD groups. This finding coincides with other reports and they demonstrated that VEGF levels in HCC patients has shown promise for HCC screening, whether used alone or combined with serum AFP [19,49,53].
Angiogenesis is an important requirement of almost every solid tumor. HCC being highly invasive and vascular tumor has angiogenesis for tumor progression and metastasis, which is also a leading cause of mortality of advance HCC after curative resection. High expression of VEGF and its receptors (VEGFR-1, -2 and -3) are observed to be high in HCC cell lines and serum of HCC patients [8,9,54,55].
VEGF overexpression was reported in precancerous stages as dysplastic and cirrhotic liver tissues, suggesting its important role in angiogenesis in liver cancer [56]. High expression of VEGF was associated with the development of HCC, but was also correlated with the tumor grading of HCC [57]. VEGF and its receptor VEGFR-2 are important angiogenic pathways, and its blockade is the first strategy for cancer therapy [58].
In a case control immune histochemical study performed on 36 patients with HCC and 6 healthy controls, VEGF was detected in 32 out of 36 patients (89%) tumor specimens, but none in the 6 healthy controls [59]. In the present study, there was a significant difference between CLD patients and healthy control groups in the level of VEGF which agreed with El-Houseini et al. [49].
In contrast to our finding, Zhao et al. had detected that serum levels of VEGF were insignificantly higher in CLD patients than in healthy controls [60].
The results of the current study revealed a positive correlation between AFP levels and VEGF and serum aminotransferases. There is a significant positive correlation between VEGF and AFP, Child score class and Okuda class and total bilirubin and a significant negative correlation with albumin. In addition, the present study showed no correlation between VEGF and aminotransferases, hemoglobin, platelets, and INR which is in agreement with Assy et al. who stated that no correlation was found between VEGF serum levels and aminotransferases [61]. On the contrary, Makhlouf et al. found that there was a significant positive correlation between circulating VEGF and aminotransferases which indicates a degree of hepatic dysfunction and this may be attributed to the release of VEGF from the damaged hepatocytes [47]. However, studies reported that VEGF showed no significant correlation to any of the clinicopathological variables in HCC patients [19,53,60].
Also, this study shows no significant correlation between serum VEGF and platelet count, thereby resembling the data given by Assy et al. who stated that no correlation was found between VEGF serum levels and platelets count [61]. In addition, Li et al. reported that there was a weak correlation of platelet number and VEGF level [62]. On the other hand, few studies found that there was a significant positive correlation between the circulating VEGF and the platelet count [47,63,64].
Obtaining plasma VEGF levels is an easy and simple procedure, which makes long-term monitoring of VEGF levels feasible, even after local intervention and therapies for HCC. Moreover, the absence of correlation between plasma VEGF levels with aminotransferases, hemoglobin, platelets and INR suggests that VEGF is not affected by minor changes in blood metabolism or inflammation.
Our findings are in accordance with Elmezayen and Darwish [65]. These also support the work of Mukozu et al., who reported significantly high serum VEGF levels in HCC patients than in non-HCC patients [66].
Likewise, Guo et al. also reported that the median serum VEGF level in the HCC patients (285 pg/mL) was significantly higher than that of healthy controls (p=0.021) [29,67-69]. Elmezayen generated a score incorporating both plasma VEGF and serum AFP beside other parameters for early detection of HCC [65].
Assessment of the diagnostic performance of serum VEGF in our study revealed that the best cut-off for serum VEGF to discriminate HCC from CLD patients was ≥ 482 pg/mL. This cut-off provides sensitivity, specificity, PPV, NPV and efficacy of 52.59%, 100.0%, 100.0%, 35.3% and 61.6% respectively. These results were consistent with the results of El-Houseini et al. who stated that in order to discriminate between HCC patients and CLD patients, the cut-off point for VEGF was established at 355.2 pg/mL with a sensitivity, specificity, PPV, NPV and efficacy of 86.4%, 60.0%, 78.1%, 82.6%, and 33.3% respectively [49]. The results on the diagnostic performance of VEGF in HCC and CLD patients versus the healthy control group revealed that the best cut-off was ≥ 280 pg/mL that yielded sensitivity, specificity, PPV, NPV and efficacy of 60.27%, 100%, 100%, 34.1% and 67% respectively.
According to the European Association for the study of the liver, the AFP level is diagnostic for HCC above 200 ng/mL [50,70]. In the present study, there were 78 HCC patients with AFP <200 ng/mL and 38 patients with AFP level >200 ng/mL. From the 78 HCC patients with AFP <200 ng/mL, there were 32 (27.6%) patients with VEGF >482 pg/mL and 46 (39.7%) patients with VEGF <482 pg/mL and from the 38 patients with AFP level >200 ng/ mL there were 29 (25%) patients with VEGF >482 pg/mL and 9 (7.8%) patients with VEGF <482 pg/mL. Therefore, VEGF was significantly elevated (>482 pg/ml) in both groups of AFP especially in the group with a normal level of AFP (<200 ng/ml), representing its useful role as a serological marker for diagnosis of HCC patients with the normal level of AFP.
In a study by Atta et al. for diagnosis of HCC the cutoff value of VEGF in plasma was 271.85 pg/mL that had a sensitivity of 90%, the specificity of 90% with 87.3% accuracy and 92.3% positive predictive value [28]. Using AFP and VEGF both for diagnosis of HCC, 100% and 98.7% increase was observed in the sensitivity and specificity respectively and accuracy of 98.9% [28]. Thus using both the markers simultaneously has increased the HCC detection sensitivity [28]. In our study, AFP and VEGF both were significantly different in different classes of Okuda. The study shows AFP levels were reduced in Okuda II while very high values were observed for Okuda I and III. However, the increase in VEGF levels was found with increasing classes, suggesting its reliability for use as a diagnostic marker and therapeutic target. It suggests that VEGF can serve as a diagnostic marker for detection of HCC in Okuda I, II and III with or without AFP but also that these patients are suitable for anti-VEGF/VEGFR therapy as well. A study by Shigeta et al., suggest that anti VEGFR-2 therapy combined with other therapies as PD1 can overcome resistance to treatment and increase survival in HCC patients [71]. It can thus be used with other therapies for increasing the survival of HCC patients.
A study by Zhuang et al. showed that HCC tissues have a high expression of VEGFR-1, and VEGFR-3 and VEGF-C [72]. It may have an important role in progression of HCC.
Expression of these three factors in the peritumoral tissues may contribute to the assessment of the risk of tumor recurrence in HCC patients and to optimize postoperative treatment to prevent tumor recurrence [72].
For targeting the cancerous cells usage of Sorafenib (multiple kinase inhibitors) prolongs the survival time of advanced HCC patients [73]. Sorafenib specifically acts on VEGFR1, VEGFR2, VEGFR3, PDGFRβ, c-KI, and Raf kinases (Raf1, B-Raf, MEK, ERK). Major targets of sorafenib are VEGF and its receptor VEGFR. Peng et al, have shown that autocrine VEGF is capable of promoting the proliferation of HCC cells. Sorafenib treatment is believed to target the autocrine VEGF signaling pathway in HCC patients [74].
Thus, our study is able to establish that patients with HCV infection with a liver abnormality, high ALT, AST and Albumin should be evaluated for the levels of AFP and VEGF in their serum. If the values of these markers, particularly VEGF are above a definite level, they should be seriously monitored for HCC diagnosis and treatment.
Conclusion
It can be concluded that the results of the current study are encouraging, as serum VEGF could be a promising tumor marker that could be added to the current standard tests for diagnosis of HCC in order to detect the disease at an early stage and hence improve the prognosis and survival rate of the patient. Thus, measurement of VEGF especially in patients with normal AFP is highly recommended, especially in known cirrhotic patients who deteriorate rapidly without any apparent etiology.
Inclusion of serum VEGF evaluation to the current standard tests for HCC would provide diagnostic, prognostic as well as a therapeutic target for better management. This, in turn, could greatly improve the ability to identify such patients and thus could allow them to benefit from earlier treatment. The validation and cost-effectiveness of VEGF as a diagnostic and prognostic tool along with therapeutic target requires further confirmation for largescale studies in clinical practice.
Declaration of Interests
None
Acknowledgment
National Research Centre, Cairo, Egypt for providing funds for the execution of the work and Ain Shams Faculty of Medicine Hospital, for providing necessary facilities.
Authorship
Guarantor of the article: Neelima Gupta
Author Contributions
1. Concept and design of the study: KYS, EMB
2. Acquisition of data: MNH, MAMA, EMB
3. Analysis and interpretation of data: MNH, KYS, MAMA
4. Drafting and revision of the article: MNH, KYS, SIS, NG, VG
5. Final approval of the version to be published: SIS, NG
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136, 59-86 (2015).
- Velázquez E, Tournier HA, Mordujovich de Buschiazzo P, et al. Antioxidant activity of Paraguayan plant extracts. Fitoterapia. 74, 91-97 (2003).
- Arafa N, El Hoseiny M, Rekacewicz C, et al. Changing pattern of Hepatitis C virus spread in rural areas of Egypt. J Hepatol. 43, 418-424 (2003).
- El Gaafary MM, Rekacewicz C, Abdel-Rahman AG, et al. Surveillance of acute Hepatitis C in Cairo, Egypt. J Med Virol. 76, 520-525 (2005).
- El-Zayadi AR, Badran HM, Barakat EM, et al. Hepatocellular carcinoma in Egypt: A single-center study over a decade. World J Gastroenterol. 11, 5193-5198 (2005).
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 127, 2893-2917 (2010).
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 61, 69-90 (2011).
- McKeown SR. Defining normoxia, physoxia and hypoxia in tumors implications for treatment response. Br J Radiol. 87, 20130676 (2014).
- Xiong XX, Qiu XY, Hu DX, et al. Advances in hypoxia-mediated mechanisms in hepatocellular carcinoma. Mol Pharmacol. 92, 246-255 (2017).
- Morse MA, Sun W, Kim R, et al. The Role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 25, 912-920 (2019).
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 69, 182-236 (2018).
- Bruix J, Sherman M. AASLD practice guideline: Management of hepatocellular carcinoma. Hepatology. 42, 1208-1236 (2005).
- Nadinskaia MY, Kodzoeva KB, Ulyanova KA, et al. Risk factors associated with portal vein thrombosis in liver cirrhosis: A case-control study. Ter Arkh. 91, 73-81 (2019).
- Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 56, 6-30 (2006).
- Arciero CA, Sigurdson ER. Liver-directed therapies for hepatocellular carcinoma. J Natl Compr Canc Netw. 4, 768-774 (2006).
- Connell LC, Harding JJ, Abou-Alfa GK. Advanced hepatocellular cancer: The current state of future research. Curr Treat Options Oncol. 17, 43 (2016).
- Schwartz JM, Ham JM. Treatment of hepatocellular carcinoma. Curr Treat Options Gastroenterol. 6, 465-472 (2003).
- Lopez LJ, Marrero JA. Hepatocellular carcinoma. Curr Opin. Gastroenterol. 20, 248-253 (2004).
- Kaseb AO, Hanbali A, Cotant M, et al. Vascular endothelial growth factor in the management of Hepatocellular Carcinoma. Cancer. 115, 4895-4906 (2009).
- Zhou L, Liu J, Luo F. Serum tumor markers for the detection of hepatocellular carcinoma. World J Gastroenterol. 12, 1175-1181 (2006).
- Hsia CY, Huo TI, Chiang SY, et al. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol. 33, 208-212 (2006).
- Wang M, Devarajan K, Singal AG, et al. The Doylestown algorithm: A test to improve the performance of AFP in the detection of hepatocellular carcinoma. Cancer Prev Res (Phila). 9, 172-179 (2016).
- Wu W, Yao DF, Yuan YM, et al. Combined serum hepatoma-specific alpha-fetoprotein and circulating alphafetoprotein-mRNA in diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 5, 538-544 (2006).
- Nakatsura T, Yoshitake Y, Senju S, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 306, 16-25 (2003).
- Ishiguro S, Inoue M, Tanaka Y, et al. Serum aminotransferase level and the risk of hepatocellular carcinoma: a population-based cohort study in Japan. Eur J Cancer Prev. 18, 26-32 (2009).
- Poon RT, Ng IO, Lau C, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: A prospective study. Ann Surg. 233, 227-235 (2001).
- Dvorak HF, Brown IF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hypermeability, and angiogenesis. Am J Pathol. 146, 1029-1039 (1995).
- Atta MM, Atta HM, Gad MA, et al. Clinical significance of vascular endothelial growth factor in hepatitis C related hepatocellular carcinoma in Egyptian patients. J Hepatocellular Carcinoma. 3, 19-24 (2016).
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 359, 378-390 (2008).
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16, 859-870 (2015).
- Bergmeyer HU, Rej R, Horder M. Methods of enzymatic analysis Eds. Verlag Chemie, Weinheim, F.R.G. 3rd Ed, 416-424 (1983).
- Doumas BAT, Watson WA, Biggs HG. Method of albumin analysis. Clin Chim Acta. 31, 87-96 (1971).
- Malloy HT, Evelyn KA. The determination of Bilirubin with the photoelectrons colorimeter. J Biol Chem. 119, 481-490 (1973).
- Quick AJ. Quick on “Quick agglutination venostasis” bleeding time technique. J Lab Clin Med. 26, 1812-1822 (1973).
- Engall E. Methods in Enzymology 70, Van Vunakis, H. and Langone, JJ. (eds.), Academic Press. 419-492 (1980).
- El-Serag HB. Hepatocellular carcinoma an epidemiologic view. J Clin Gastroenterol. 35, 72-78 (2002).
- Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden. Int J Cancer. 94, 153-156 (2001).
- Sayed HA, El Ayyat A, El Dusoki H, et al. A cross sectional study of Hepatitis B, C, some trace elements, heavy metals, aflatoxin B1 and schistosomiasis in a rural population. J Egypt Public Health Assoc. 80, 355-388 (2005).
- Wong KF, Xu Z, Chen J, et al. Circulating markers for prognosis of hepatocellular carcinoma. Expert Opin Med Diagn. 7, 319-329 (2013).
- Abdel-Hamid H (ed.), Basic Gastroenterology and Hepatology, Second edn., Medicine Series. (1998).
- Haseeb AF. Study on the relation between hepatocellular carcinoma, Hepatitis B virus, hepatitis C virus and Aflatoxin among Egyptians. MSc Thesis, Tropical Medicine, Cairo University, Egypt (2000).
- Zekri AN, Alam El-Din HM, Bahnassy AA, et al. Serum levels of soluble fats, soluble tumor necrosis factor-receptor II, interleukin-2 receptor and interleukin-8 as early predictors of hepatocellular carcinoma in Egyptian patients with hepatitis C virus genotype-4 Comp. Hepatol. 9, 1-12 (2010).
- Gameel M, El Assaly N, Madani H, et al. Evaluation of tumor markers panel in the detection of HCC in HCV Egyptian patients and its correlation with AFP. Res J Med. 4(2), 402-410 (2009).
- Di Biscelgie AM, CarithesRL Jr, Gores GJ. Hepatocellular carcinoma. Hepatology. 28, 1161-1165 (1998).
- Giannelli G, Marinosci F, Sgarra C, et al. Clinical role of tissue and serum levels of SCCA antigen in hepatocellular carcinoma. Int J Cancer. 116, 579-583 (2005).
- Grizzi F, Franceschini B, Hamrick C, et al. Usefulness of cancer-testis antigens as biomarkers for the diagnosis and treatment of hepatocellular carcinoma. J Transl Med. 5, 3-45 (2007).
- Makhlouf MM, Awad A, Zakhari MM, et al. Vascular endothelial growth factor level in chronic liver diseases. J Egypt Soc Parasitol. 32, 907-921 (2002).
- Yvamoto EY, Ferreira RF, Nogueira V, et al. Influence of vascular endothelial growth factor and alpha-fetoprotein on hepatocellular carcinoma. Genet Mol Res. 14, 17453-17462 (2015).
- el-Houseini ME, Mohammed MS, Elshemey WM, et al. Enhanced detection of Hepatocellular Carcinoma. Cancer Control. 12, 248-253 (2005).
- Hussein MM, Ibrahim AA, Abdella HM, et al. Evaluation of serum squamous cell carcinoma antigen as a novel biomarker for diagnosis of hepatocellular carcinoma in Egyptian patients. Indian J Cancer. 45, 167-172 (2008).
- Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein in patients with advanced hepatitis C: Results from the HALT-C Trial. J Hepatol. 43, 434-441 (2005).
- Abdelgawad MR, Abdel-Wahab M, Mostafa M. Evaluation of serum alpha-fetoprotein levels in chronic Hepatitis C patients with or without Hepatocellular Carcinoma. Arab J Nucl Sci Applic. 43, 271-280 (2010).
- Poon RT, Lau C, Pang R, et al. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: Importance of tumor biomarker in ablative therapies. Ann Surg Oncol. 14, 1835-1845 (2007).
- Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 29, 4989-5005 (2010).
- Poon RT, Ho JW, Tong CS, et al. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg. 91, 1354-1360 (2004).
- El-Assal ON, Yamanoi A, Soda Y, et al. Clinical significance of micro vessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: Possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 27, 1554-1562 (1998).
- Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 28, 68-77 (1998).
- Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res. 312, 527-537 (2006).
- Huang Y, Fan XG, Wang ZM, et al. Identification of Helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J Clin Pathol. 57, 1273-1277 (2004).
- Zhao J, Hu J, Cai J, et al. Vascular endothelial growth factor expression in serum of patients with hepatocellular carcinoma. Chin Med J (Engl). 116, 772-776 (2003).
- Assy N, Paizi M, Gaitini D, et al. Clinical implication of VEGF serum levels in cirrhotic patients with or without portal hypertension. World J Gastroenterol. 5, 296-300 (1999).
- Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and the effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 10, 2878-2882 (2004).
- Jinno K, Tanimizu M, Hyodo I, et al. Circulating VEGF is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 33, 376-382 (1998).
- Genesca J, González A, Mujal A, et al. Vascular endothelial growth factor levels in liver cirrhosis. Dig Dis Sci. 44, 1261-1262 (1999).
- El-mezayen HA, Darwish H. Development of a novel scores for early detection of hepatocellular carcinoma among high-risk Hepatitis C virus patients. Tumour Biol. 35, 6501-6509 (2014).
- Mukozu T, Nagai H, Matsui D, et al. Serum VEGF as a tumor marker in patients with HCV related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 33, 1013-1021 (2013).
- Guo RP, Zhong C, Shi M, et al. Clinical value of apoptosis and angiogenesis factors in estimating the prognosis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 132, 547-555 (2006).
- Kamiyama T, Takahashi M, Nakanishi K, et al. α-fetoprotein, vascular endothelial growth factor receptor-1 and early recurrence of hepatoma. World J Gastroenterol. 18, 340-348 (2012).
- Niizeki T, Sumie S, Torimura T, et al. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J Gastroenterol. 47, 686-695 (2012).
- European Association for the study of the liver (EASL) 36th Annual Meeting (2000).
- Shigeta K, Datta M, Hato T, et al. Dual PD-1 and VEGFR-2 blockade promotes vascular normalization and enhances anti-tumor immune responses in HCC. Hepatology. (2019).
- Zhuang PY, Shen J, Zhu XD, et al. Prognostic roles of cross-talk between peritumoral hepatocytes and stromal cells in hepatocellular carcinoma involving peritumoral VEGF-C, VEGFR-1 and VEGFR-3. PLoS One. 8, 64598 (2013).
- Barontoaldo M, Salvatore V, Marinelli S, et al. Use of VEGFR-2 targeted ultrasound contrast agent for the early evaluation of response to sorafenib in a mouse model of hepatocellular carcinoma. Mol Imaging Biol. 17, 29-37 (2015).
- Peng S, Wang Y, Peng H, et al. Autocrine vascular endothelial growth factor signaling promotes cell proliferation and modulates sorafenib treatment efficacy in hepatocellular carcinoma. Hepatology. 60, 1264-1277 (2014).