Interview - Imaging in Medicine (2013) Volume 5, Issue 2
Understanding schizophrenia and traumatic brain injury with MRI
Martha Shenton*Psychiatry Neuroimaging Laboratory, 1249 Boylston Street, Boston, MA 02215, USA
- Corresponding Author:
- Martha Shenton
Psychiatry Neuroimaging Laboratory
1249 Boylston Street, Boston, MA 02215, USA
E-mail: martha_shenton@hms.harvard.edu
Abstract
■From its early stages, your academic career has been in psychiatry, but how did you become involved in neuroimaging?
My involvement in neuroimaging was not a linear trajectory, by any means. I had no idea I would be involved in neuroimaging studies of the brain when I began my research studies as a graduate student with Professor Philip Holzman in the Department of Psychology at Harvard University (MA, USA). He was a pioneering researcher in the field of schizophrenia. I was interested in understanding the mind and how it works, what normal functioning is, and how we can understand abnormal functioning. I was completely inspired by a presentation given by Holzman as part of a graduate student seminar. He was talking about eye tracking and how patients with schizophrenia have difficulty following an object and how this abnormality was likely to be more linked to the underlying pathology of schizophrenia than the more striking symptoms including disordered thought, auditory hallucinations and delusional beliefs. The notion that something so seemingly unrelated to a disorder, such as eye tracking, may be more importantly linked to the underlying pathology was a new and intriguing idea to me. Holzman also observed that these abnormalities were present in unaffected relatives of patients with schizophrenia, suggesting that there may be a genetic component to this disorder.
Following his presentation, I sought him out and he invited me to visit his laboratory at McLean Hospital (MA, USA). I began working with him that day, in 1979, and he became my PhD dissertation advisor. I was still very interested in auditory hallucinations and the odd manner in which patients with schizophrenia spoke, called ‘formal thought disorder’. Therefore, instead of looking at eye tracking, which is what I thought I would study, I began a study to quantify disordered thinking, which is manifested in the odd speech that is such a striking symptom of this devastating disorder. Findings from my dissertation demonstrated that relatives of patients with schizophrenia also exhibited the same kind of disordered thinking, only more attenuated.
Following this work, I knew that I wanted to get closer to the brain in order to understand where in the brain disordered thought and auditory hallucinations were localized. I wondered at the time whether or not these striking symptoms would be associated with some sort of underlying substrate in the brain.
In 1984, I had the opportunity to work as a postdoctoral fellow with Professor Robert McCarley in the Department of Psychiatry at Harvard Medical School (MA, USA). Professor McCarley was interested in investigating event-related potentials, which are similar to EEG, except that the stimulus is controlled and the experimenter observes the brain wave in response to the stimulus; thus, ‘event-related potential’. As part of my postdoctoral fellowship, my job was to investigate the P300 in schizophrenia and relate it to some of the symptoms, including auditory hallucinations and formal thought disorder. The P300 is a positive waveform that occurs 300 ms after a novel event – for example, a high-pitched beep when using an auditory stimulus. It was thought that this evoked potential may be related to schizophrenia. The rationale here was that patients with schizophrenia experience difficulties in determining what is relevant from what is irrelevant in their environment. What was interesting in our findings was that patients with schizophrenia still responded to the novel stimuli by showing an increase in amplitude in the P300, but their response was more attenuated and with lower amplitude. This was also lateralized to the left and was correlated with properties of thought disorder related to language and auditory hallucinations. To my mind, this was getting closer to understanding what areas of the brain are affected in schizophrenia. Left-lateralized findings in areas of language were an important inroad. However, while event-related potential studies have temporal resolution in terms of milliseconds, the localization is not as precise and accurate. I wanted to get even closer to the brain itself.
In 1986, I met with Dr Marjorie LeMay weekly to learn about how the brain appeared on CT scans. LeMay was a wonderful neuroradiologist at Brigham and Women’s Hospital (MA, USA) who had worked with Dr Norman Geschwind and Dr Alan Galaburda. She was an expert in the area of asymmetries in the brain, which was an area we thought was also important in schizophrenia research. I sat with her for many hours each week to understand how brains were represented in CT scans and we also had a small study where we acquired CT scans from patients with schizophrenia. Then, in 1987, I applied for and received a career award from the National Institute of Mental Health (1988–1993), known as a K01 Mentored Award, to investigate brain abnormalities in schizophrenia using MRI. This collaborative effort involved Professor Ferenc Jolesz, a neuroradiologist at Brigham and Women’s Hospital, Professor Robert McCarley and Dr Ron Kikinis, who had just joined the MRI Division at Brigham and Women’s Hospital as a postdoctoral fellow. Jolesz and Kikinis welcomed me, but Jolesz cautioned me that I was not likely to find anything because if there was something there, it would have already been discovered. I believed, however, that the new tools available, both for acquiring images and for postprocessing images, were far more sophisticated than in the past, and would, therefore, make it possible for us to discover and delineate subtle brain abnormalities in schizophrenia. We were among the first to use MRI in this patient group, where we were able to image 1.5-mm slices, with no gaps between slices, which was new. We were able to cover all of the brain, and not just a part of it, or 1-cm slices. One of our first research studies was published in a psychiatry journal, Schizophrenia Research [1] but we felt that our work was not really understood at the time and so we submitted our second study to a journal outside our field (the New England Journal of Medicine [2]) and it ended up being one of the seminal papers in the field because it definitively showed that schizophrenia is a brain disorder. Although people had speculated about this for a long time, this study really demonstrated that you needed sophisticated in vivo imaging tools in order to confirm that schizophrenia was characterized by subtle brain abnormalities. So that is what we did, and we found that the brain abnormalities in schizophrenia are in areas involved with language, on the left, including the left superior temporal gyrus, and the amygdala–hippocampal complex; the latter is important for verbal memory. These two studies were my first foray into neuroimaging, and my first focus on brain abnormalities in schizophrenia.
■What has been the highlight of your career so far?
I would say there are several highlights. One was working with Philip Holzman as a graduate student. He just had this ‘can-do’ attitude that was contagious and inspiring. He was very supportive of his graduate students, helping them to flesh out their ideas, and it was also a very exciting time period to be doing research in the field of psychiatry because things had changed from looking at an interpersonal, psychosocial and psychodynamic understanding of mental illness to a more biological focus in terms of etiology. It felt like a new frontier, which was exciting. A further highlight was when we started to conduct imaging studies – early on (before the ‘Decade of the Brain’). There weren’t any guideposts – it was like being in this brand new world of discovery. We had to create guideposts as we went along, and try to standardize what we were doing. That was both exciting and anxiety provoking because we could be going down the wrong path entirely.
Another highlight for me was later, when diffusion tensor imaging became available and I started working with other investigators on this line of research in the late 1990s. Work with Dr Marek Kubicki and Dr Carl-Fredrik Westin was the start of this new effort where we developed postprocessing tools, later also with the help of Dr Sylvain Bouix and Dr Yogesh Rathi. Our first focus was to investigate white matter changes in patients with schizophrenia. Our group has now published some of the most highly cited papers applying diffusion imaging to further understand schizophrenia and we have reported findings about brain regions with reduced white matter integrity between the frontal and temporal lobe, including the uncinate fasciculus [3,4].
Then, about a decade later, it really occurred to several members of our group that diffusion tensor imaging was even more applicable to traumatic brain injury (TBI). In TBI, particularly in mild cases, you see acceleration, deceleration and rotational forces that actually stretch and sheer axons. The diffuse axonal injury in mild TBI (mTBI) is often not visible using conventional MRI or CT. Instead, more sophisticated tools such as diffusion tensor imaging are needed that will show some of the shearing and diffuse axonal injury, the latter being the most common injury in mTBI, which is not observed using conventional imaging. The big decision in an emergency room, when faced with a individual with moderate or severe TBI is: “does this individual need neurosurgery?” However, usually nothing’s going to be done for mTBI. Very often, a CT is not even performed unless there is some indication; and even if a CT is done, it does not generally show any damage. Nonetheless, the consequences for mTBI are not normally serious because, for the most part, those with mTBI recover within hours to days to weeks. However, there are a small percentage of people with mTBI (~15–30%) who do not recover. They end up experiencing symptoms long after their head injury, including dizziness, problems with concentration, memory, irritability, impulsivity and sometimes depression, without really having an explanation for it. Their doctor may tell them that there is nothing wrong with them because nothing shows up on CT or MRI; they do not have an explanation for what is going on. Imagine how disconcerting that must be for the patient.
There has been controversy in this field. Perhaps people with these symptoms may have had psychiatric symptoms before their brain injury? Perhaps there is a lot of overlap with other disorders such as post traumatic stress and/or depression? However, I really do not think that this is a fair assessment since it is not based on radiological evidence from the appropriate sophisticated imaging tools, such as diffusion tensor imaging. Fortunately, I think that is changing now and it will have a huge impact on the field of TBI. So, that to me is another highlight of my career, and it’s happening right now – I’m going in a completely different direction. I am still involved in schizophrenia research, but the work our laboratory is doing can contribute a lot towards understanding mTBI. Both schizophrenia and mTBI deal with the same kind of issue; very subtle abnormalities in the brain, which require refined tools in order to discern them. Currently, with Inga Koerte and collaborators, we have applied these tools to soccer players, where we have shown that repetitive head trauma, even in elite professional soccer players who are selected for participation with no reported history of brain concussion do show alterations in white matter compared with healthy professional swimmers [5]. We have also reported changes between pre- and post-season in hockey players, where changes in white matter have been observed [6]; see also our comprehensive review article on neuroimaging findings in mTBI [7]. We are also investigating changes in National Football Players (NFL) to determine whether or not there are early changes that might predict chronic traumatic encephalopathy (CTE), so that through detection we may be able to intercede with possible treatments to prevent the cascade of changes that lead to CTE in some players, but not in others. There is much to be done in the area of mTBI, including the area of advancing our knowledge of repetitive subconcussive blows to the head, and the work here is only just beginning, which makes it really exciting. There is also the opportunity to develop preventative neuroprotective agents prior to exposure to explosive devices in the case of soldiers, and even in the case of athletes with high exposure to head trauma.
■As a founding director of the Psychiatry Neuroimaging Laboratory, could you tell us briefly about the laboratory’s advanced imaging techniques that you have developed?
I started using these imaging tools when I was working with Kikinis and Jolesz, first, when I was a guest at Brigham and Women’s Hospital back in 1988 and then when I came to the Brigham and Women’s Hospital full time and founded the Psychiatry Neuroimaging Laboratory in 2005. The work began with using postprocessing imaging tools that were being used for presurgical planning for neurosurgery. Members of my group would use some of the algorithms that were developed by computer scientists to investigate and try to localize brain alterations in schizophrenia. We were a kind of b-testing group for tools that were being used for neurosurgery. Usually, when a computer scientist is looking at an object, it does not matter whether the object is up, down, left or right. However, if you are doing neurosurgery then it makes a big difference! Therefore, some of our early work with computer scientists involved pointing out if the corpus callosum looked as if it was going in the wrong direction, and they would fix it. Other early work we did with computer scientists was to provide feedback on segmentation algorithms to segment the brain into gray matter, white matter and cerebrospinal fluid (CSF). This sounds like something that was simple to do, but it took at least 15 years of computer vision research to develop the ability to segment all the little voxels in the brain into tissue classes. We were involved early on with computer scientists in order to provide feedback in their development of these algorithms. One example is a data set we had from Israel on post-traumatic stress disorder. The segmentation algorithm was not working well, in that the three classes of tissue were not clearly delineated. We approached the computer scientist who we had been working with, Dr William Wells III, and he noted that the homogeneity in the magnetic field was not homogeneous, which meant that the signal for white matter in one part of the brain was different than in another part. He used this data set to develop a postprocessing algorithm that would adjust for inhomogeneities in the magnetic field [8]. This algorithm is now used widely to correct for inhomogeneities in the magnetic field at the postprocessing stage.
Another area we were interested in developing was the ability to quantify the sulcal–gyral pattern of the brain in schizophrenia. A computer scientist, Dr Guido Gerig, assisted us here and we were able to differentiate between patients with schizophrenia and normal controls based on differences in the sulco–gyral pattern [9]. We were also interested in looking at brain shape, as midbrain structures in particular are affected by neurodevelopment more than other parts of the brain. Here, we worked again with Gerig on shape, as well as volume, and found that by combining both these we were able to better differentiate the amygdala–hippocampal complex between patients with schizophrenia and controls than by using either measure alone [10].
So we have been involved in developing tools to look at shape, segmentation algorithms, and we are now working on more automated measures that take the brain out of the skull. Most of the algorithms available today do not do such a good job here and quite a bit of manual editing is needed. We are also developing tools in diffusion imaging. One method developed by our group and collaborators is stochastic tractography, in which connectivity between any two brain regions of interest can be measured [11]. For example, if one is interested in language areas, one might pick an area near Wernicke’s area and Broca’s area and measure the white matter fiber tracts between these two areas.
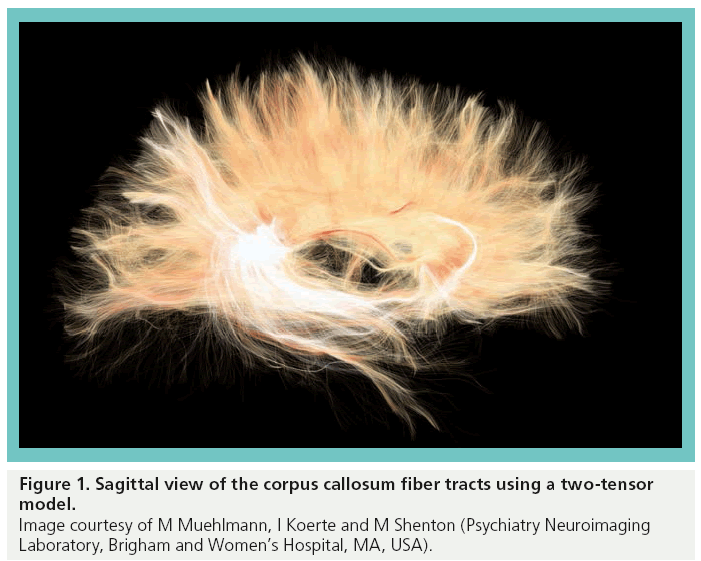
We are also working on solutions to the problem of crossing fibers in diffusion imaging. When fibers cross, where they cross is similar to what would be observed in CSF (i.e., there is movement of water in all directions instead of restricted movement of water as is the case in white matter fiber tracts). Diffusion imaging works so beautifully because in water or CSF all of the molecules move in random directions (isotropic diffusion) – there is nothing that stops the directionality of the molecules. However, if you are looking at tissue such as white matter, the water movement is restricted; white mater is perfect, as molecules are very much restricted to going in two directions (i.e., anisotropic diffusion). Thus, where you have a crossing, with one set of fibers, for example, going one way and then another set going perpendicularly, where they cross is an area that might as well look like CSF, but it is not – so how do you quantify that? We have two computer scientists who have developed a two-tensor/multitensor approach for taking into account crossing fibers [12,13]. Figure 1 shows a 3D reconstruction of the corpus callosum fiber tracts using a two-tensor model of DTI.
At the moment, we have six computer scientists who are focused on really different questions. For example, Dr Ofer Pasternak has a method to look at free water; he can separate the diffusion tensor into a component of free water that is extracellular and a component of the diffusion tensor that is more closely in line with water within/near the tissue. This is going to be important for conditions such as TBI, because you want to know if the more extracellular free water (perhaps vasogenic edema) is associated with a neuroinflammatory response of the brain to the initial head injury, which may be neuroprotective and possibly related to better outcome, whereas if more damage is present and more associated with intracellular water (perhaps cytotoxic edema), then this might indicate more neurodegenerative processes [14]. In a recent study published in the Journal of Neuroscience, it is shown that in the early stages of schizophrenia there is one component of the diffusion tensor that shows more global changes (e.g., extracellular) whereas in the frontal lobe there are already areas that may be more indicative of neurodegeneration (e.g., intracellular) [15]. Other tools being developed include building an anatomy atlas to provide individual profiles of injury for mTBI patients. Dr Sylvain Bouix is working on this project.
■One of your group’s most recent studies used 3T MRI to explore sports-induced mTBI. How have advances in imaging technologies helped to improve our understanding of TBI, & where is this field heading?
I think this is a really exciting area as it has been largely ignored because, as noted previously, neither conventional MRI nor CT have been helpful in providing knowledge about brain abnormalities in mTBI. Thus there have been, up until quite recently, very few imaging studies of mTBI. In fact, one of the first conferences I went to was on mTBI. While I was there, I noticed that not one individual showed a picture of the brain when talking about mTBI, and my colleague, Professor Ross Zafonte (Harvard Medical School), one of my new TBI collaborators, explained to me that this was because most researchers and clinicians thought that looking at the brain wasn’t helpful in mTBI. Instead, they were looking at neurocognitive function in the brain as an indicator of brain functioning being normal or abnormal, but they didn’t look at the brain itself because it was believed that there was nothing to be seen! And I say this last comment with some amazement because I had heard the same thing as a schizophrenia researcher. But the right tools were not being used to discern subtle changes in the brain.
Thankfully, I think that is changing now; people are starting to use the right tools to image the brain and see the subtle alterations. This is a tremendous step forward because you cannot properly treat something until you can properly diagnose it and definitely say which areas are really affected in mTBI.
One of the problems with mTBI, as I mentioned already, is that where you are hit in the head may determine where the head injury is, so it is often very heterogeneous. If you group people together with mTBI and then compare them with normal controls, then the differences between groups might not necessarily be a difference that categorizes any one individual.
Therefore, another question that arises, which is actually being worked on by one of our computer scientists, Bouix: is there a way to create a brain atlas based on normal controls that are matched in terms of age, gender, handedness and other variables to a group of subjects that have experienced mTBI? If so, we could compare each brain of the TBI subject with this atlas to get an individual profile of where the injury is for each patient. This would end up being very useful clinically. You could also track the injury over time and perhaps be able to predict the course of the injury. Followup studies are really needed to see what the course of illness looks like and what predicts good versus poor outcome. If you knew that you could go back and look at images of the patient’s brain 24 h or 1 week postinjury and find out what we really need to treat early before it becomes a problem and the individual experiences postconcussive symptoms.
So I think we need to know a lot more about individual injuries. Then you could actually use different statistics to do group comparisons with your brain atlas, comparing the mTBI subjects in terms of severity of the injury and number of areas affected, and use group statistics to predict if that individual’s injury has a better or worse outcome. There are different dependant variables; for example, you could look at free water measure (developed by Pasternak in our group) to see if early edema may be a good indicator in terms of outcome, whereas later edema may be a poor indicator of outcome. At the moment, we know so little about what happens at injury. Follow- up studies are clearly needed.
■Could the neuroimaging work in laboratories such as yours one day contribute to improved care for patients with psychiatric disorders? Do you see it being brought into the clinic at any point in the future?
I really hope so – I would not be doing this if I was not hoping that the advances we have in imaging technology will not only improve our understanding of TBI, but will also end up being useful for a clinician treating individual patients and help them to decide the best course of treatment based on the kind of injury. These were previously ‘invisible’ injuries, but now they are visible and we can track them and learn more about them. You diagnose and then try and find biomarkers that would allow you to be able to provide a prognosis. Then you can even look at treatment efficacy, based on the biomarkers that you have developed. My hope is that we will gain a much better understanding, particularly of conditions such as mTBI. We may even be able to add to knowledge about genetics regarding what may make some people more predisposed to developing postconcussive syndrome, and even CTE, based on their genetic profile.
For example, a study that we would like to do is a PET study looking at tau proteins in the brain. A specific tau ligand has been developed by Siemens (Munich, Germany) and has been used successfully to quantify tau proteins in one Alzheimer’s disease patient, one mild cognitively impaired patient and one normal control [16]. We would like to use this ligand to investigate a sample of NFL players selected ahead of time for having tau in their CSF, in addition to other abnormalities on neuopsychological and neurological examinations, and to select another sample of NFL players who have no such anomalies, thus having a high-risk group for CTE and a lowerrisk group. We would also like to see if the tau build-up from PET findings is associated with an aggregate risk score for tau genes, to see whether we can determine the genetic risk for developing CTE. This would possibly enable us to know ahead of time those NFL players who are most at risk for developing neurodegenerative brain diseases such as CTE prior to participating in high-impact sports. After all, not everyone who plays football ends up with such long-term injuries, and being able to determine those most at risk would go a long way in preventing CTE.
■What future developments would you like to see in advanced MRI that would allow the field to progress further?
One limitation is that among the ligands available for PET, a ligand to investigate tau protein accumulation in mTBI has not been available until quite recently. The group I am working with at Boston University (most particularly Dr Robert Stern) have found real evidence at post-mortem that shows that repetitive brain trauma probably leads to CTE in some individuals, which is likely to be more characterized by an accumulation of tau proteins in the brain than Alzheimer’s disease and other disorders. Therefore, a better understanding of this with better, more readily available, tau ligands would be helpful.
We are also beginning to combine imaging modalities, such as PET with MR spectroscopy (working with Dr Alex Lin at Brigham and Women’s Hospital), in order to look at some of the markers that may be related to neuron inflammation, and to perform multimodal imaging. This has not really been done yet in TBI.
There are also some newer diffusion imaging protocols being developed, with more information and higher resolution. That is where I think the field should be going in terms of imaging: using a multimodal approach and using more advanced techniques.
One of the downsides of center grants is that if you have some sites that are included which do not have the most advanced technology, then you just have to do the best you can, given the scanners that you have available to you. I think that some of the studies are going to have to be smaller and at sites that have the capability for advanced imaging techniques; however, there is still a need to have the larger studies – and they are only just starting in TBI.
■Finally, do you have any concluding comments, or anything else you would like to discuss?
It is hard to describe what it’s like to work in a research laboratory where people are really committed to trying to understand a disorder, solve a problem or develop a new technique because you do not yet have a way to achieve what you want to. There is a whole thrill that goes into a discovery like this – just being in an environment where people are really excited about discovery and are on the cutting edge of discovery. It is exciting to be part of, but also to know that what you are doing will hopefully be important in the end and have an impact on how a disorder such as mTBI will be looked at – a clinician will not just look at a patient and say, “This is all in your head,” all because there is not any radiological evidence that there’s anything wrong with them.
We can demonstrate radiological evidence now, and that is important and exciting. It changes the whole paradigm of how people will look at some of these ‘miserable minorities’ – people who do not recover quickly from having a concussion and who go on to have symptoms later in life. This minority of people need to be listened to, as they probably do have something wrong with them which needs to be attended to. Maybe if we know enough about the whole cascade of events that happens in the brain, we would be able to prevent people from becoming classified into the group of miserable minorities and change their lives.
I am actually going to be speaking to someone later today because they want to talk to me about the fact that they have never felt right since experiencing mTBI from falling off a bike. Everything ‘looks normal’ on conventional MRI and CT, but this individual wants to talk to someone doing research in the field. I’m not a clinician, but if I can help them or direct them to someone who is now using more sophisticated imaging methods and has more sophisticated thinking about what to do with that minority who still have symptoms despite it all ‘looking normal’, then I’ll do it.
■Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Shenton ME, Kikinis R, McCarley RW, Metcalf D, Tieman J, Jolesz FA. Application of automated MRI volumetric measurement techniques to the ventricular system in schizophrenics and normals. Schizophr. Res. 5(2), 103–113 (1991).
- Shenton ME, Kikinis R, Jolesz FA et al. Abnormalities of the left temporal-lobe and thought-disorder in schizophrenia – a quantitative magnetic-resonance-imaging study. N. Engl. J. Med. 327(9), 604–612 (1992).
- Kubicki M, Westin CF, Maier SE et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry 159(5), 813–820 (2002).
- Kubicki M, McCarley R, Westin CF et al. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatric Res. 41, 15–30 (2007).
- Koerte IK, Erl-Wagner B, Reiser M, Zafone R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 308(18), 1859–1861 (2012).
- Koerte IK, Hartl E, Kaufmann D et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 2 of 4. Neurosurg. Focus 33(6), E3: 1–7 (2012).
- Shenton ME, Hamoda HM, Schneiderman JS et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6(2), 137–192 (2012).
- Wells WM 3rd, Grimson WEL, Kikinis R, Jolesz FA. Statistical intensity correction and segmentation of MRI data. Proc. 3rd Conf. Visual. Biomed. Comput. 2359, 148–159 (1994).
- Kikinis R, Shenton ME, Gerig G et al. Temporal lobe sulco–gyral pattern anomalies in schizophrenia: an in vivo MR threedimensional surface rendering study. Neurosci. Lett. 182(1), 7–12 (1994).
- Shenton ME, Gerig G, McCarley RW, Székely G, Kikinis R. Amygdala–hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 115(1–2), 15–35 (2002).
- Friman O, Farneback G, Westin CF. A Bayesian approach for stochastic white matter tractography. IEEE Trans. Med. Imaging 25(8), 965–978 (2006).
- Malcolm JG, Shenton ME, Rathi Y. Filtered multitensor tractography. IEEE Trans. Med. Imaging 29(9), 1664–1675 (2010).
- Rathi Y, Malcolm J, Bouix S, Westin CF, Shenton ME. False positive detection using filtered tractography. Proc. Intl Soc. Mag. Reson. Med. 18, 4019 (2010).
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Mapping and eliminating free water from diffusion weighted images. Magn. Res. in Med. 62(3), 717–730 (2009).
- Pasternak O, Westin CF, Bouix S et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J. Neurosci. 32(48), 17365–17372 (2012).
- Chien DT, Bahri S, Szardenings AK et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 34(2), 457–468 (2013).