Perspective - Imaging in Medicine (2010) Volume 2, Issue 5
The role of biomarkers in thoracic imaging
William F Auffermann1, Lacey Washington1, Elizabeth B Gottlin1 & Edward F Patz Jr†1,21Department of Radiology, Duke University Medical Center, Durham, NC 27710, USA
2Department of Pharmacology & Cancer Biology, Duke University Medical Center, Durham, NC 27710, USA
- Corresponding Author:
- Edward F Patz Jr
Department of Radiology
Duke University Medical Center
Durham, NC 27710, USA
Tel: +1 919 684 7367
Fax: +1 919 684 7168
E-mail: patz0002@mc.duke.edu
Abstract
Thoracic imaging plays an essential role in understanding and diagnosing chest disease. It has traditionally relied on conventional anatomic and morphologic imaging, but as technology evolves, novel diagnostic strategies have the potential to improve accuracy. This includes incorporation of biomarkers, which are an objective measure of a biological or pathological process. Biomarkers can be used in multiple different scenarios, and may complement current imaging studies, particularly in establishing a diagnosis, determining prognosis or predicting response to therapy. However, it is important to develop and validate clinically relevant biomarkers. This is a complex, multidisciplinary subject that requires knowledge of the clinical problem, an understanding of the technology and pathology, and significant resources. Ultimately, integration of biomarkers into patient evaluation must be cost effective and improve outcomes. This article will describe the potential roles and limitations of biomarkers in thoracic diseases.
Keywords
biomarkers ▪ lung cancer ▪ molecular diagnostics ▪ molecular imaging ▪ thoracic imaging
Biomarkers are, in the broadest definition, objective indicators of a biological process. This term has become fashionable in recent years, usually pertaining to the molecular analysis of biological specimens, but the underlying concept has been used in radiology for decades. Traditional anatomical and/or morphological features can be considered as imaging biomarkers and are used daily to characterize normal and pathological processes. They are an integral part of thoracic imaging. Descriptive findings on conventional imaging studies, however, are not consistently specific, as a spectrum of diseases may have a similar radiographic appearance. In addition, morphology does not always reflect current, or predict future, biological behavior.
More recent efforts to identify novel imaging biomarkers attempt to incorporate the molecular and biochemical properties of a disease in order to improve diagnostic accuracy. As molecular mechanisms in thoracic pathology are elucidated, more specific markers should translate into advances in diagnostic capabilities. However, the possibility of developing a wide variety of novel biomarkers should not lead us to undervalue conventional anatomical and morphological markers if used in the appropriate clinical scenario. Rather, we envision that technological improvements in conventional imaging, molecular imaging and analysis of specimen biomarkers could complement each other in a focused diagnostic approach. This article describes the role of biomarkers in thoracic imaging and summarizes the spectrum of markers used in clinical medicine for diagnostic, prognostic and predictive indications.
Biomarkers in the assessment of disease, treatment & outcome
Biomarkers are employed for a variety of reasons to provide diagnostic information. In the thorax, they may be derived from noninvasive imaging and/or from biological specimens, including serum, sputum, pleural fluid, bronchoalveolar lavage or tissue. For example, if pulmonary embolism is suspected, serum d-dimer, a fibrin breakdown product, may be assessed initially but, if positive, a contrast-enhanced thoracic CT will usually be the next step. If pneumonia is suspected, a chest radiograph will be performed, and if an abnormality is seen then sputum cultures and serum white blood cell count will be examined. If there is a concern for cancer, imaging will guide tissue sampling that will be analyzed for cytology, occasionally immunohistochemistry and, more recently, genomic analysis [1,2].
While assessment of biological specimens is common, acquiring samples requires varying degrees of invasiveness. Invasive procedures are expensive, may be unpleasant for patients and are associated with some risk. Therefore noninvasive procedures are almost always preferable, providing they furnish the requisite diagnostic information. The noninvasive nature of imaging probably accounts for much of its popularity. Imaging markers may be descriptive or quantifiable. Thoracic imaging findings include anatomic or morphologic features such as size and regional distribution of an abnormality, growth characteristics, degree of intravenous contrast enhancement and extent of stenosis. PET can provide metabolic information based on the uptake of 18F-2-fluoro-2-deoxy-d-glucose (18F-FDG). These ‘radiographic biomarkers’ have different roles in addressing clinical questions and in providing specific diagnostic information.
Biomarkers are used in three fundamental areas: establishing a diagnosis, determining outcome (prognostic markers) and suggesting response to a specific therapy (predictive markers). There are numerous areas of active investigation in radiology that pursue these goals, including the development of new contrast agents for CT or MRI, new techniques such as dual-energy CT, new magnetic resonance pulse sequences and imaging probes targeted at the molecular mechanisms of a disease. The usefulness and role of these radiographic techniques need to be evaluated in the appropriate patient population, before the true clinical utility is known.
Establishing a diagnosis
One of the more common reasons that imaging is performed is to establish a diagnosis. Some patients present with symptoms, others have incidental radiographic findings that require further investigation and some patients may undergo screening procedures. In all of these scenarios, characterization of a radiographic abnormality will help refine a differential diagnosis so that the appropriate next steps can be taken. Biomarkers that can establish a diagnosis without an invasive procedure are of tremendous patient benefit.
The optimum biomarker depends on the biology and pathology of the disease. A basic understanding of the disease process should guide the diagnostic approach. A patient with a diagnosis of cancer, a fundamental disease of the genes, will require a different strategy and set of markers than a patient who presents following trauma, where there is the concern for an anatomic abnormality. Patients who present with chest pain typically require a different evaluation depending on whether there is a suspicion of myocardial ischemia, pulmonary embolism or aortic dissection. While CT has been suggested for this ‘triple rule-out’, additional biomarkers may be needed to establish a specific etiology for the patient’s symptoms.
But even within general disease categories, the selection of the appropriate biomarker may be important. For example, metastases of well differentiated thyroid cancers such as papillary and follicular thyroid cancer retain the ability to concentrate iodine. Consequently, scans with radioactive iodine may be used to diagnose metastatic disease in patients with these tumors. More poorly differentiated tumors often lose their ability to concentrate iodine, and may not be detectable with an iodine uptake scan, but may be detected with other studies such as 18F-FDG PET imaging [3]. Iodine and FDG uptake are both biomarkers; which one proves to be more informative depends on the nature of the disease.
Another example of an ongoing diagnostic dilemma is distinguishing a benign from malignant pulmonary nodule. The current standard of care for small nodules is to perform serial imaging to assess for growth and/or stability. This approach has a number of disadvantages including the cost and radiation entailed in continued surveillance for patients without disease and potential delays in diagnosis in patients with malignancy. Another method that has been used to characterize pulmonary nodules is FDG-PET imaging. However, 18F-FDG-PET imaging has lower sensitivity for small nodules. The development of new molecular imaging probes or specimen biomarkers that could complement conventional imaging studies in determining whether a pulmonary nodule is benign or malignant would have a significant impact on clinical management [4].
Prognostic & predictive biomarkers
The ultimate goal of radiology is not just to produce images, but to provide relevant information for patient management. While establishing a diagnosis is often the primary focus of radiographic studies, prognostic information and determining which patients will respond to a specific therapy are enormously important. These areas have been more difficult to address with imaging studies, and therefore have often fallen outside the purview of radiologic interpretation. Prognostic and predictive markers are difficult to develop, and depending on the disease, they are usually determined from laboratory analysis of biological specimens.
One can appreciate the need for prognostic and predictive markers in many clinical scenarios. For example, if a spiculated mass in the lung suggestive of lung cancer is detected by imaging, a patient will typically undergo a biopsy, traditional staging, surgical resection and then possibly be treated with chemotherapy and/or radiotherapy. The patient will then be followed to determine response or recurrence. While there are no clear alternatives to this established approach, imaging or specimen biomarkers that could determine cell type, outcomes and appropriate therapy at the time of diagnosis would clearly be an advantage.
In addition, biomarkers could suggest which patients would respond to specific therapies, and which patients may develop toxic side effects. Many other similar scenarios exist for a spectrum of thoracic abnormalities, including interstitial lung disease, cardiac disease, infections and trauma. Selecting the optimal therapy at the time of diagnosis would avoid subjecting patients to ineffective and potentially toxic therapy.
Assessment for treatment response
When a patient is undergoing treatment for a disease, knowing whether the currently used treatment is having an effect may help guide clinicians to continue the treatment or alter therapy. As above, it would be best to have accurate predictive markers at the time of presentation, so that multiple sequential imaging studies to determine response would not be so important. This is particularly the case in malignancy, where there is an effort to maximize treatment response while minimizing exposure to potentially toxic chemotherapeutic agents that do not affect a particular patient’s tumor growth. It is becoming increasing clear that current conventional imaging response criteria, using measurements of tumor size, are not always optimal [5], and that FDG-PET, and fusion imaging with CT-PET may be more accurate. This provides anatomic localization as well as metabolic information. Reports from early studies, however, are not consistent as tumors are heterogeneous, and FDG uptake does not necessarily correlate with the tumor cell component [6–8].
Several preliminary studies have suggested a genomic signature of the tumor as a predictive ‘biomarker’, but these are still in the early phases of development and need to be validated in larger prospective trials. In this regard, one could also envision developing imaging probes, or other imaging biomarkers that could determine if therapy was having an effect early in treatment. As long as response to therapy influences patient care, there will be a role for biomarkers to assess stability, progression or resolution of disease.
In current clinical trials overall survival is typically used as the ultimate end point, but progression-free survival is occasionally used as a surrogate marker to report trial results. Alternatively, response biomarkers have been proposed for this same purpose, as some diseases are indolent and clinical studies take years to determine whether a therapy is effective [9]. Use of biomarkers could potentially reduce the amount of time and cost required to evaluate new therapies. This could not only more efficiently suggest which therapy may be useful, but potentially reduce the necessity of clinical trials for therapies that are not effective or are associated with significant toxicity. More conventional trials assessing survival may then be used for the subset of therapies that show promising results.
Currently used imaging biomarkers
Traditional thoracic imaging studies evaluate and report a myriad of findings that represent biomarkers. They are reported because these anatomic and morphologic features provide clinically relevant diagnostic information [10]. Physiologic or serum biomarkers are useful as well. These will be reviewed as they pertain to lung or cardiac imaging.
Lung disease
In patients with interstitial lung disease, descriptive features such as reticular and ground glass opacities, interlobular septal thickening, honeycombing and architectural distortion all serve as biomarkers to assess the extent and severity of disease. These biomarkers describe a phenotype with implications concerning outcome and treatment.
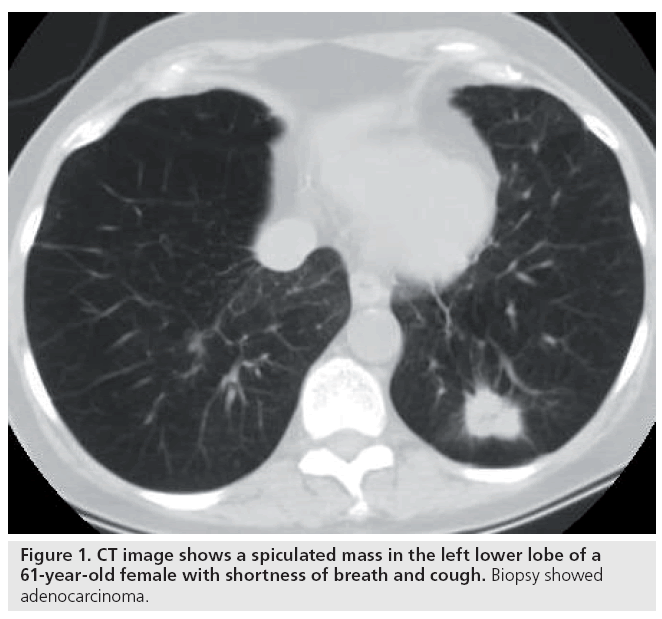
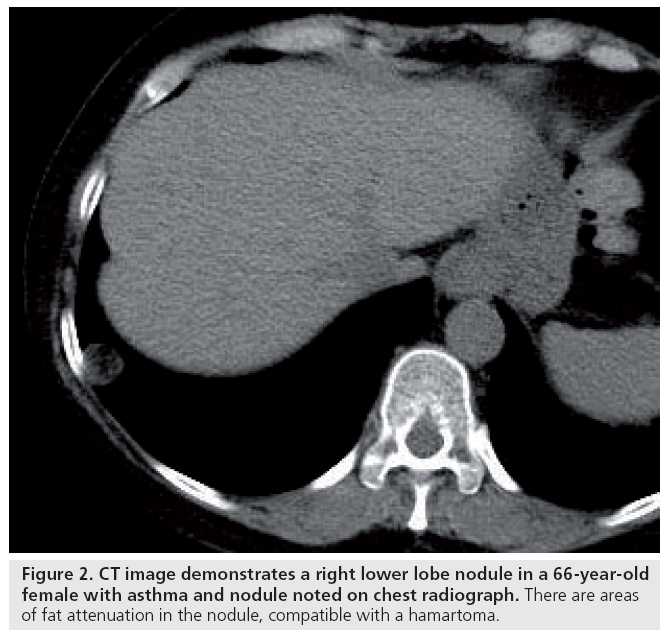
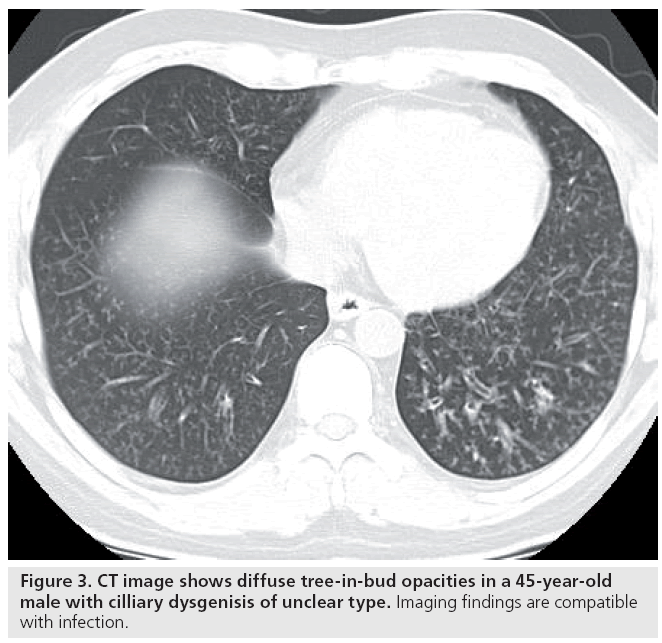
In patients with a pulmonary nodule, biomarkers include size, shape, margins, composition/ density and location. Irregular, spiculated and lobulated margins are features strongly correlated with primary lung cancer [11]. Small nodules are typically benign, and large size (>3 cm) lesions are associated with malignancy (Figure 1). Other findings may suggest a benign etiology, including central or dense calcification, typically seen with granulomas or fat, possibly indicating a hamartoma or lipoid pneumonia (Figure 2). The pattern of an abnormality in the lung is sometimes suggestive of the disorder’s etiology. For example, a tree-in-bud appearance of pulmonary nodules is a pattern often associated with infectious causes (Figure 3).
Figure 1: CT image shows a spiculated mass in the left lower lobe of a 61-year-old female with shortness of breath and cough. Biopsy showed adenocarcinoma.
Figure 2: CT image demonstrates a right lower lobe nodule in a 66-year-old female with asthma and nodule noted on chest radiograph. There are areas of fat attenuation in the nodule, compatible with a hamartoma.
For many years, change in lesion size has been used as a biomarker for suggesting a diagnosis and determining response to treatment [12]. However, there may be a time lag between the onset of a successful treatment regimen and a detectable change in lesion size [5]. In addition, since many lesions are heterogeneous and an anatomic abnormality may not completely resolve, it is currently impossible to differentiate residual disease from fibrosis based on morphologic characteristics alone.
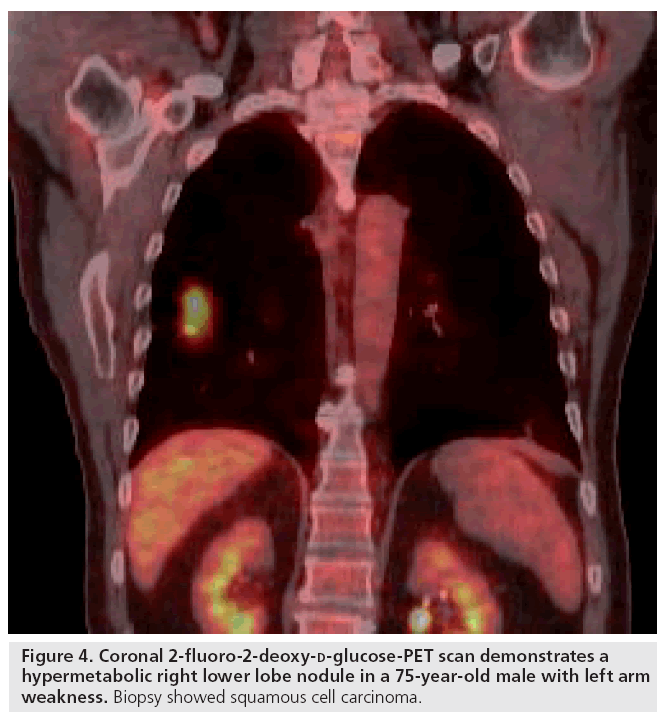
In addition to anatomical description, physiologic measurements have a role as biomarkers in thoracic imaging. For example, tumors are composed of a spectrum of cell types including malignant cells, inflammatory cells, fibroblasts and surrounding stromal cells that have increased rates of metabolism. Consequently, the tissues demand oxygen and nutrients to maintain growth and will often demonstrate increased vascularity compared with inactive lesions. One study showed intravenous contrast enhancement on CT, as an indicator of vascularity, is useful in some patients for distinguishing benign from malignant lesions [13]. The most widely used biomarker probe for tumor detection by imaging is 18F-FDG. PET imaging with 18F-FDG is a way to measure glucose metabolism, but also provides anatomic information. Recent studies have developed serum biomarkers for lung cancer and may in the future be an integral part of managing patients with pulmonary nodules (Figure 4) [14].
Figure 4: Coronal 2-fluoro-2-deoxy-d-glucose-PET scan demonstrates a hypermetabolic right lower lobe nodule in a 75-year-old male with left arm weakness. Biopsy showed squamous cell carcinoma.
2-fluoro-2-deoxy-d-glucose has also been used to monitor therapeutic responses to treatment. 18F-FDG uptake has been shown to change rapidly after initiation of chemotherapy for lung cancer, sometimes within approximately 14 days [15]. However, recent studies have shown an inconsistent relationship between changes in tumor size, tumor 18F-FDG uptake and treatment response, where survival is used as the end point [8,16]. The decrease in standardized uptake values observed after chemotherapy may therefore not be directly related to a decrease in the burden of neoplastic cells alone. Consequently, while preliminary studies have shown the potential use of 18F-FDG and other surrogate markers for prediction of tumor response to treatment, more studies are needed before such methods are used in routine clinical practice.
The exact mechanism of 18F-FDG uptake remains uncertain, and accurate differentiation between different cellular types within a tumor is not possible [6]. Therefore alternative, more specific imaging probes are being developed. The thymidine analog [18F]fluorothymidine is a marker of cell proliferation. Since tumor cells are likely to have the highest rates of proliferation of any cell type within a tumor, [18F]fluorothymidine may represent an improvement over FDG, which simply measures metabolism. [18F]fluorothymidine has recently been shown to measure early response to gefitinib therapy in lung cancer in a small clinical trial [17].
Cardiac disease
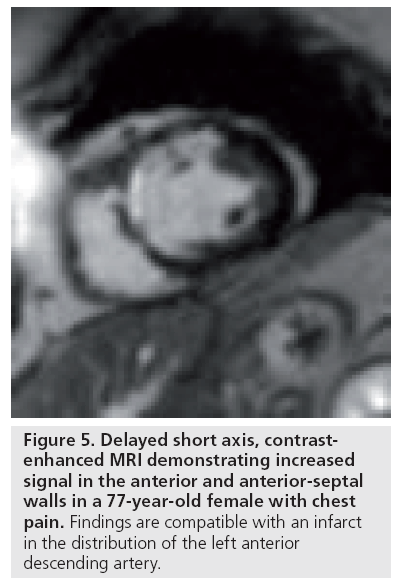
Several serum and imaging biomarkers are also used in cardiac imaging. One of the most commonly used serum biomarkers pertaining to the heart is the cardiac troponin level. Troponin is a molecule composed of three subunits, two of which are specific to cardiac muscle tissue. Following an acute myocardial infarction, an increase in serum troponin levels is observed, providing a specific marker for myocardial injury, which has found widespread clinical use [18]. While serum cardiac biomarkers are useful in disease detection, they do not allow for localization of disease. For example, when considering a patient with myocardial infarction, the vascular territory involved and presence of residual viable myocardium are factors considered when deciding on therapy. In such instances, an imaging marker such as delayed contrast enhancement may be used to supplement serum biomarkers. Delayed contrastenhancement imaging of the heart using MRI allows differentiation of viable and nonviable myocardium [19], guiding therapy towards vascular territories with viable myocardium that may benefit from revascularization (Figure 5).
Biomarker development
Developing biomarkers is not a trivial task and needs significant resources. It requires a multidisciplinary approach that involves both clinical and basic science researchers. The investigative team must clearly understand the relevant clinical questions and the technology used to address the issues. They need to develop, standardize, and validate assays or protocols, and standardize reporting. Bioinformatics is an essential part of this process to ensure the data are collected and analyzed appropriately.
The design and organization of clinical trials with suitable clinical end points, such as disease progression or overall survival, also present challenges. This involves constant monitoring, patient retention and awareness of regulatory issues. All of this requires institutional review board approval and must be Health Insurance Portability and Accountability Act compliant [20,21].
The future of thoracic imaging
As an understanding of thoracic biology increases, more specific imaging methods and new biomarkers should also be expected. These may take the form of small molecular probes with high specificity and sensitivity for a specific molecular target. An example of a biomarker in this category is octreotide, which is a somatostatin analog. This is used to image neuroendocrine tumors that express the somatostatin receptor [16].
Imaging with molecular tracers may be fused with anatomic images to form a map of the distribution of a biomarker relative to anatomic structures, as is currently widely done in PET-CT imaging. The combination of anatomic and physiologic information is often more specific for a diagnosis than either measurement alone. Other current methods of anatomic and physiologic image correlation include color Doppler imaging and SPECT-CT.
Alternatively, specimen biomarkers that examine DNA, mRNA or proteins for disease-specific properties could be used to complement imaging findings. These biomarkers must address unresolved issues in diagnostic radiology, facilitate management, reduce cost, reduce invasive procedures and improve overall outcomes. For instance, if a set of biomarkers can be identified that correlate with lung cancer, these may be used to complement the findings of conventional imaging studies.
In lung cancer, EGF receptor (EGFR) is an example of an important molecular target and biomarker. EGFR is a transmembrane tyrosine kinase receptor, which exists in a monomeric form in its nonactive state. Upon ligand binding, the receptor dimerizes and activates growth promoting signal pathways. While EGFR is a membrane constituent in many normal cells, overexpression or expression of an activating mutated form has been associated with cancer in a variety of organs. EGFR-targeted imaging agents have been demonstrated to localize to tumors with high specificity in animal models [22,23]. When compared with nonspecific biomarkers such as 18F-FDG uptake, EGFR imaging focuses on a molecular target for a subtype of malignant cells. In principle, this tracer may offer more specific diagnoses and guidance for treatment. Further studies are needed to validate its use in the diagnosis of lung cancer and for evaluating treatment response before incorporation into routine clinical medicine.
Applications of new biomarker discovery techniques can be used in patients with other thoracic abnormalities for stratification into risk categories and may guide further diagnostic evaluation. Patients at low risk for disease may be followed and patients at high risk may benefit from more definitive studies such as biopsy, to establish diagnosis and guide subsequent therapy if any is indicated.
Conclusion
Biomarkers currently play an essential role in thoracic imaging. Objective measures of disease are described on conventional imaging studies and are reported because of their clinical relevance. However, there remain a number of unresolved diagnostic issues in the chest, and new, more accurate biomarkers will undoubtedly improve diagnostic capabilities and patient care. The role of biomarkers in thoracic imaging will certainly expand as our understanding of pathophysiology improves. Novel imaging features or biomarkers derived from biological specimens should improve evaluation of patients with specific thoracic abnormalities. It is hoped that continued incorporation of novel diagnostic strategies will improve patient care and outcomes.
Future perspective
Diagnostic imaging is an essential part of patient care. While conventional imaging provides a tremendous amount of information, improvements in technology and biology will lead to novel diagnostic strategies. It will be critical for radiologists to understand and incorporate these advances for an accurate, efficient evaluation of patients with thoracic abnormalities.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C: Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax 55, 67–77 (2000).
- Knox KS, Meinke L: Role of bronchoalveolar lavage diagnostics in fungal infections. Clin. Chest Med. 30, 355–365 (2009).
- Heston TF, Wahl R: Molecular imaging in thyroid cancer. Cancer Imaging 10(1), 1–7 (2010).
- Gottlin EB, Xiangrong G, Pegram C, Cannedy A, Campa MJ, Patz EF Jr: Isolation of novel EGFR directed VHH domains with diagnostic or therapeutic potential. J. Biomol. Screen. 14, 77–85 (2009).
- Birchard KR, Hoang JK, Herndon JE, Patz EF Jr: Early changes in tumor size in patients treated for advanced stage nsclc do not correlate with survival: are current response criteria meaningful. Cancer 115(3), 581–586 (2009).
- Christensen JD, Colby TV, Patz EF Jr: Correlation of [18F]-2-fluoro-deoxy-d-glucose positron emission tomography standard uptake values with the cellular composition of stage I nonsmall cell lung cancer. Cancer 116(17), 4095–4102 (2010).
- Berghmans T, Dusart M, Paesmans M et al.: Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the european lung cancer working party for the IASLC lung cancer staging project. J. Thorac. Oncol. 3(1), 6–12 (2008).
- Tanvetyanon T, Eikman EA, Sommers E, Robinson L, Boulware D, Bepler G: Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J. Clin. Oncol. 26(28), 4610–4616 (2008).
- Wang Y: Medical imaging in pharmaceutical clinical trials: what radiologists should know. Clin. Radiol. 60, 1051–1057 (2005).
- Hansell D, Lynch D, Mcadams HP, Bankier A: Imaging of Diseases of the Chest. Elsevier Mosby, London, UK (2009).
- Truong MT, Sabloff B, Ko JP: Multidetector CT of solitary pulmonary nodules. Radiol. Clin. North Am. 48, 141–155 (2010).
- Therasse P, Arbuck SG, Eisenhauer EA et al.: New guidelines to evaluate the response to treatment in solid tumors. J. Natl Cancer Inst. 92(3), 205–216 (2000).
- Swensen SJ, Viggiano RW, Midthun DE et al.: Lung nodule enhancement at CT: multicenter study. Radiology 21, 73–80 (2000).
- Patz EF Jr, Campa MJ, Gottlin EB, Kusmartseva I, Guan XR, Herndon JE 2nd: A panel of serum biomarkers for the diagnosis of lung cancer. J. Clin. Oncol. 25, 5578–5583 (2007).
- Duong CP, Hicks R, Weih LA et al.: FDG-PET status following chemoradiotherapy provides high management impact and powerful prognostic stratification in oesophageal cancer. Eur. J. Nucl. Med. Mol. Imaging 33(7), 770–778 (2005).
- Bertino EM, Confer P, Colonna JE, Ross P, Otterson GA: Pulmonary neuroendocrine/ carcinoid tumors: a review article. Cancer 115(19), 4434–4441 (2009).
- Sohn HJ, Yang YJ, Ryu JS et al.: [18F]fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin. Cancer Res. 14(22), 7423–7429 (2008).
- Babuin L, Jaffe A: Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ 173(10), 1191–1202 (2005).
- Weinsaft JW, Klem I, Judd RM: MRI for the assessment of myocardial viability. Cardiol. Clin. 25, 35–56 (2007).
- Khleif SN, Doroshow JH, Hait WN: AACR-FDA-NCI cancer biomarkers collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin. Cancer Res. 16(13), 3299–3318 (2010).
- Pepe MS, Etzioni R, Feng Z et al.: Phases of biomarker development for early detection of cancer. J. Natl Cancer Inst. 93(14), 1054–1061 (2001).
- Huang L, Gainkam L, Caveliers V et al.: Spect imaging with 99mTc-labeled EGFRspecific nanobody for in vivo monitoring of EGFR expression. Mol. Imaging Biol. 10, 167–175 (2008).
- Pal A, Glekas A, Doubrovin M et al.: Molecular imaging of EGFR kinase activity in tumors with 124I-labeled small molecular tracer and positron emission tomography. Mol. Imaging Biol. 8, 262–277 (2006).
• Describes the development of novel tumor-specific imaging probes.
• Showed tumors to be heterogeneous and there was no correlation with cell composition.
• Did not find PET scans after neoadjuvant therapy to be useful in predicting survival. New biomarkers are needed to predict outcomes.
• Descibes the challenges of developing biomarkers for drug development.
• Illustrated the potential for imaging tumor targets.