Review Article - Interventional Cardiology (2013) Volume 5, Issue 6
Stem cells in vascular graft tissue engineering for congenital heart surgery
- Corresponding Author:
- Massimo Caputo
Congenital Heart Surgery, Bristol Royal Hospital for Children, Level 7
Bristol Royal Infirmary, Marlborough Street
Bristol, BS2 8HW, UK
Tel: +44 117 3428854
Fax: +44 117 3429737
E-mail: m.caputo@bristol.ac.uk
Abstract
Congenital heart disease (CHD) affects approximately 1% of all live births, making it the most common birth defect worldwide. CHD remains the primary cause of death among infants in North America and Europe. Moreover, improvements of cardiac surgery techniques have led to a remarkable improvement in early survival rate, with a parallel increase in the number of patients with adult CHD (5% increase annually in UK). The 2009 WHO annual report puts the disability-adjusted life year for CHD higher than diseases such as diabetes or hypertension
Keywords
congenital heart surgery, stem cell, tissue engineering
Congenital heart disease (CHD) affects approximately 1% of all live births, making it the most common birth defect worldwide. CHD remains the primary cause of death among infants in North America and Europe. Moreover, improvements of cardiac surgery techniques have led to a remarkable improvement in early survival rate, with a parallel increase in the number of patients with adult CHD (5% increase annually in UK). The 2009 WHO annual report puts the disability-adjusted life year for CHD higher than diseases such as diabetes or hypertension [1,2].
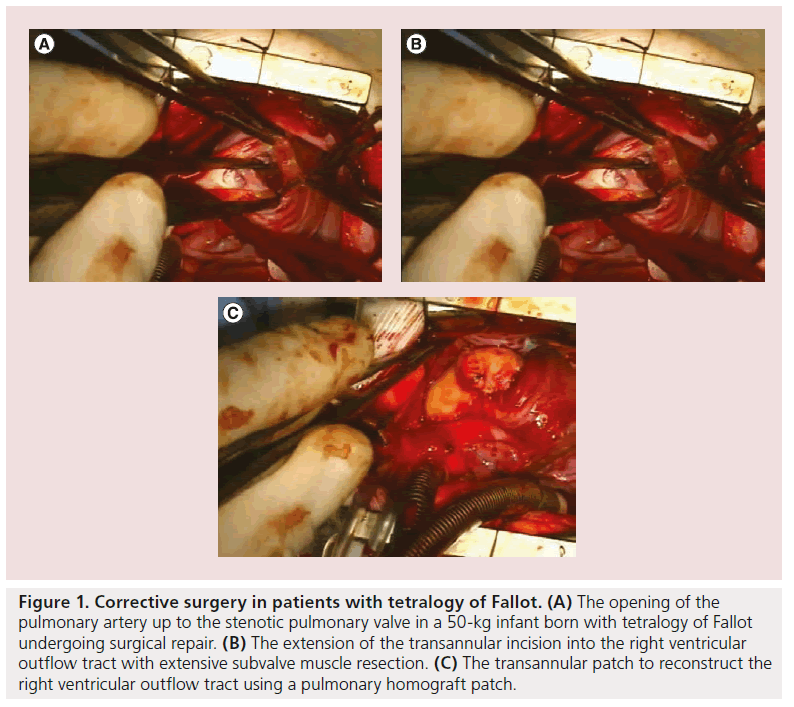
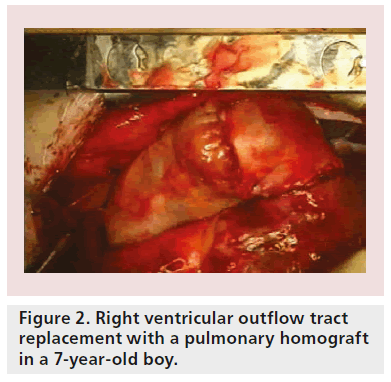
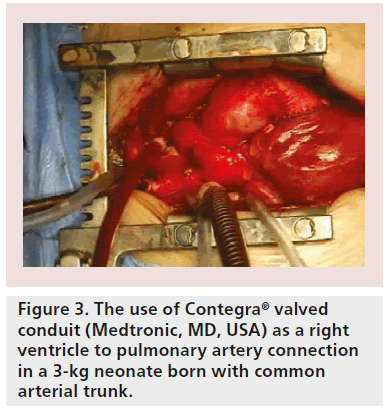
Although primary repair is sometimes possible, prosthetic replacement grafts are more often required. Patients with tetralogy of Fallot, for example, require extensive opening and resection of the pulmonary artery valve (Figure 1A) and right ventricular outflow tract (Figure 1B), implantation of patches to enlarge the whole area (Figure 1C) and, subsequently (after several years), pulmonary valve replacement with either homografts (Figure 2) or other types of biological valves (Figure 3).
Figure 1: Corrective surgery in patients with tetralogy of Fallot. (A) The opening of the pulmonary artery up to the stenotic pulmonary valve in a 50-kg infant born with tetralogy of Fallot undergoing surgical repair. (B) The extension of the transannular incision into the right ventricular outflow tract with extensive subvalve muscle resection. (C) The transannular patch to reconstruct the right ventricular outflow tract using a pulmonary homograft patch.
Even though these grafts may be life saving, they have limited durability and are prone to infection, immunological reactivity and thrombosis. As a result, repeat operations are often required. Of particular concern is that these currently available grafts do not grow as the patient ages. This may predicate additional procedures during childhood or adolescence. Moreover, it has been variably estimated that between 800,000 and 1 million patients with CHD are now adults, and many will require some form of valve or valve conduit replacement due to failure of their previous grafts [3,4].
Currently available valve replacements include mechanical prostheses, several types of gluteraldehyde-fixed xenografts, homografts or autograft with the Ross procedure. The major limitation of xenografts is their relatively short lifetime, while mechanical valves require anticoagulation with an increased risk of thrombosis and hemolysis especially in the pediatric population. Using a pulmonary autograft in the aortic position (the Ross procedure) provides favorable hemodynamics, and freedom from anticoagulation and immunological reactivity. Moreover, it provides a graft that grows as the patient grows, potentially reducing the need for further surgery. However, the pulmonary autograft in the aortic position is prone to dilatation with subsequent aortic valve regurgitation. Pulmonary homografts are the most commonly used conduits for right ventricular outflow tract reconstruction in children due to their excellent characteristics; they integrate into surrounding tissue, are easily manipulated by surgeons and are stable enough to hold suture material. However, like other types of biological valve, their durability is limited by the calcification process and intimal proliferation [3,4].
Therefore, it is clear that the ‘ideal’ replacement heart valve, which should be durable, biocompatible, nonimmunogenic, nonthrombogenic, readily available, easy to handle, and have the potential to grow and remodel in vivo, does not yet exist.
Tissue engineering is a very promising approach to overcome the limitations of existing surgical repair methods [5–8]. This approach involves seeding cells in 3D matrices to form living tissue products with structural and functional properties that can be used to restore, maintain or improve tissue function. The repopulation of a decellularized matrix with a patient’s own cells before surgery can potentially create a living structure with subsequent longterm preservation of mechanical and biological properties.
Scaffolds used in vascular tissue engineering
▪ Prosthetic materials
Clinically available vascular prosthetic replacements have many disadvantages. Indeed, these prosthetic replacements are foreign materials that predispose the patients to life-long risks of infection and lack the ability to grow, repair and remodel, which are vital for pediatric populations. Engineering a complete ‘living’ autologous vascular material may overcome many of these limitations associated with the current clinically used prosthetic substitutes. These synthetic valvular and vascular prostheses have been made from a range of materials (Table 1). Dacron, GORE-TEX® (WL Gore & Associates, Inc., DE, USA) and polytetrafluoroethylene (PTFE) made in various forms are commercially available and clinically used in patients with no alternative vascular structures. The use of these vascular conduits is satisfactory in large-diameter applications. However, in smaller vessel replacement they perform poorly. The blood-contacting surface and the biomechanical properties of the graft are believed to be important variables in causing patency in small vessels. Many attempts have been made to improve these prosthetic devices by lining them with autologous endothelium [9–11]. Early studies have procured endothelial cells from dogs’ external jugular vein segments and seeded these autologous cells in Dacron vascular grafts immediately prior to implantation [12]. The experimental grafts studied had better clot-free surfaces than the control grafts at 14- and 28-day periods [12]. In addition, endothelial cell (EC) coverage of experimental graft surfaces after 4 weeks was up to eight-times higher than in control grafts [9]. Later investigations have shown that PTFE grafts can be lined with ECs harvested from dog jugular veins and implanted in dogs with similar results to the Darcon grafts [13]. Other studies demonstrated that ECs cultured from human veins could be used to line PTFE grafts [14]. Furthermore, PTFE grafts lined with autologous ECs isolated from veins have been implanted in human [10,11]. However, clinical trials failed to confirm the successful results observed in animals [10,11].
| Type of scaffold | Materials | Advantages | Disadvantages |
|---|---|---|---|
| Prosthetic materials | Dacron, Gortex, PTFE | Satisfactory in large-diameter applications | Infection, lack the ability to grow, repair and remodel |
| Natural protein | Collagen, elastin, fibronectin | Produce a completely biological vascular conduit | Lack sufficient physical stability and mechanical properties |
| Synthetic biodegradable polymers | PGA, PGA-PHO copolymer, PCL-PLA copolymer reinforced with woven PGA |
Better controlled physical and mechanical properties | Challenge the host’s ability to remodel the implant Side effects caused by the degradation by-products |
| Decellularized vessels | Small intestinal
submucosa Porcine aorta carotid artery, Iliac arteries |
‘Off-the-shelf’ graft, short
maturation time Abundant supply of construct materials Natural composition and mechanical properties |
Inhomogeneous distribution of cells Difficulty in retaining all extracellular matrix Immunogenicity upon incomplete decellularization |
Table 1. Scaffolds in vascular tissue engineering.
▪ Vascular conduits made of layers of vascular cells
In 1986, Weinburg and Bell were the first group to produce a completely biological vascular conduit made of layers of bovine vascular cells, ECs, smooth muscle cells (SMCs) and fibroblasts, and animal collagen; however, this lacked sufficient mechanical properties [15]. More recently, such a living conduit was created by wrapping a cultured sheet of human vascular SMCs cultured into a sheet around a tubular support followed by a sheet of human fibroblasts [16]. After these layers had matured, the tube was removed and the lumen was seeded with ECs, thus creating the three layers of the blood vessel (Table 1). The vessel showed promise in a dog model, achieving good patency, and had potential as the method used no synthetic or exogenous biological materials [16]. However, there was an increased risk of contamination as there was a delay in culturing the vessel in vitro.
▪ Decellularized natural scaffolds
Prosthetic substitutes commonly used for vascular reconstruction are mainly synthetics and fixed tissue grafts. Within these replacements, graft infections are common, compliance mismatch is significant and handling qualities are poor [17]. Natural biological tissues that are unfixed offer a better alternative, particularly since they have been shown to resist infections and be durable and compliant. However, one disadvantage of these grafts is their potential antigenicity to the recipient.
Acellular or decellularized scaffolds (Table 1) have been shown to be useful as they provide the natural cues needed for recipient’s cells to grow on the scaffold in terms of mechanics, composition and ultrastructure [18], and it has been shown that the extracellular matrix can still support cell growth, even though many of its components have been disrupted [19,20]. As it is hard to remove 100% of the cytoplasmic debris and nuclear materials, the consequence of remaining material in terms of host response remains unclear [21].
Early data indicated that decellularization of animal tissues is capable of producing stable vascular conduits that exhibit long-term functionality in other species [22]. In this study, the SynerGraft ® tissue engineering strategy was used to minimize antigenicity and produce stable unfixed vascular grafts from nonvascular bovine tissues. These grafts were used to replace the abdominal aortas of dogs that were followed for up to 10 months [22]. Early evaluation indicated rapid recellularization by recipient actin-positive SMCs, which become arranged circumferentially into the media. Arterioles were present in the adventitial areas and ECs covered lumenal surfaces. After 10 months, grafts were patent and not aneurysmal.
Other forms of vascular grafts include decellularized tubular organs (Table 1). Examples include porcine aorta, and common carotid and iliac arteries [23–25]. A study in a dog model showed that the jejunal submucosa became endothelialized and developed a histological appearance, not dissimilar to the native vessel, when used as a vascular graft showing no evidence of long-term failure [26]. Other studies have shown similar results with bovine tissue [27].
This encouraging work has led to implanting SynerGraft decellularized porcine valves into pediatric patients [28]. Unfortunately, it has been reported that three out of four children implanted with SynerGraft valves died, two of which were sudden with severely degenerated SynerGraft valves 1 year after implantation [28]. The third child died on day 7 due to SynerGraft rupture. Subsequently, the fourth graft was explanted prophylactically 2 days after implantation [28]. Macroscopically, all four grafts showed severe inflammation and degeneration of the leaflets and wall. Histology demonstrated severe foreign body-type reaction. In addition significant calcific deposits were demonstrated [28]. Surprisingly, preimplant samples of the Syner- Graft revealed incomplete decellularization and calcific deposits. No cell repopulation of the porcine matrix occurred [28]. It is thought that the strong inflammatory response in humans may be due to matrix immunogenicity being conserved or may indicate insufficient decellularization to remove all xenoantigens [28]. This report resulted in the implanting the porcine SynerGraft treated heart valves being stopped.
Synthetic scaffolds seeded with cells
Many attempts have been made to create hybrid vascular grafts constructed from synthetic materials (polymers; Table 1) and cells, thus providing a natural interface with circulating blood. Initial results with regard to graft patency have been encouraging. However, the composite conduits are only a partial imitation of a living blood vessel. The use of biodegradable polymers as scaffolds for growing cells offered great potential to engineer a living blood conduit. Autologous cells are expanded in vitro and then seeded onto the polymer. Once cells are attached to the 3D polymer structure, the resulting cell–polymer construct can be reimplanted into the experimental subject. As cells grow and the extracellular matrix develops, the polymer degrades, leaving only the ‘engineered’ tissue without any foreign material. The feasibility of this technique to tissue engineer a heart valve leaflet was shown in a large animal model in 1995 [29]. This study showed that a tissue-engineered valve leaflet constructed from its cellular components can function in the pulmonary valve position [29]. However, this work necessitated the sacrifice of intact vascular donor structures to harvest the autologous cells used to repopulate the polyglycolic acid fibers, a synthetic biodegradable scaffold [29]. Another study also succeeded in constructing lamb heart valve leaflets by seeding a concentrated suspension of autologous myofibroblasts onto a biodegradable synthetic polymeric scaffold composed of fibers made from polyglycolic acid and polylactic acid [30]. In both studies, the evaluation of pulmonary valve function by Doppler echocardiography demonstrated valve function without evidence of stenosis and with only trivial regurgitation under normal physiologic conditions [29,30]. Histologically, the tissue-engineered heart valve leaflets resembled native valve leaflet tissue [29,30]. A later study used autologous cells seeded onto synthetic biodegradable tubular scaffolds (polyglactin/ polyglycolic acid) to replace a small segment of the pulmonary artery in lambs. Tissueengineered grafts were patent and demonstrated an increase in diameter. The biodegradable polymer scaffold disappeared from the tissue-engineered graft by 11 weeks [31]. Collagen content in tissue-engineered grafts was 25% lower than in the adjacent native pulmonary artery. The elastic fibers were present in the media layer of the tissue-engineered vessel wall and endothelialspecific factor VIII was identified on the luminal surface. Calcium content of tissue-engineered grafts was elevated but no macroscopic calcification was found. The acellular control graft (polymer tube) developed progressive obstruction and thrombosis [31]. Unfortunately, the use of a polyglactin–polyglycolic acid copolymer to create vascular substitutes in the systemic circulation resulted in aneurysm formation [32]. However, the polyglycolic acid–polyhydroxyalkanoate copolymer, which has a much longer degradation time, seemed to better withstand systemic pressure and offered a better option to create a vascular autograft for use in the aortic position [32]. Indeed, ovine pulmonary arteries and valve leaflets have been created from autologous cells from carotid arteries and a scaffold made from polyglycolic acid and polyglactin copolymers [32]. The scaffold was seeded with a mixture of ECs, SMCs and fibroblasts. Results showed that by 5 months, the polyglycolic acid was replaced by tissue that resembled native tissue in morphology and matrix orientation, as well as similar amounts of elastin and collagen. The cells aligned with the blood flow and von Willebrand (vWF) factor were shown to be present on the inner surface, showing endothelium formation [32]. Again, however, in all of these studies, as the polymer degraded slowly it was released in the circulation and caused damage to intact vascular structures.
Another study demonstrated the feasibility of seeding bone marrow cells onto a biodegradable scaffold (copolymer of lactic acid and caprolactone) to construct tissue engineered vascular autografts [17]. It showed that the bone marrow cells seeded onto the scaffold adhered, proliferated and differentiated into components of a new vessel wall [17]. Bone marrow cells appeared to be a promising cell source for tissue engineering. However, the use of nondifferentiated cells presents the risk of neoplasm formation and calcification [33]. In a clinical study, Shin’oka et al. showed that when autologous bone marrow cells, extracted on the day of surgery, were seeded onto a polymer tube scaffold composed of a copolymer of l-lactide and e-caprolactone (50:50) and implanted into patients, there were no complications and the tube remained patent at 32 months [33]. In addition, it was shown that the diameter of the tube graft increased with time, indicating that it may have properties allowing it to grow, repair and remodel. In addition, the copolymer scaffold was degraded by hydrolysis after just a few months, only leaving the living tissue by 2 years. This study highlighted the possibility of direct use of cells in tissue engineering without the need to culture them first, a useful feature in emergency cases. However, the durability of these grafts is still unknown and complications related to the long degradation time may occur.
The above studies had led to the successful reconstruction of a pulmonary artery in a 4-year-old child using their own venous cells in 2000 [34]. This patient, with a single right ventricle and pulmonary atresia had undergone pulmonary artery angioplasty and the Fontan procedure at the age of 3 years and 3 months. Angiography 7 months later revealed total occlusion of the right intermediate pulmonary artery [34]. A small segment of peripheral vein was explanted and cells from its walls were isolated. The cells were cultured and expanded, and then seeded onto a biodegradable polycaprolactone–polylactic acid copolymer tube reinforced with woven polyglycolic acid. The graft was transplanted 10 days after seeding. The occluded pulmonary artery was reconstructed with the tissue-engineered vessel graft [34]. On follow-up angiography, the transplanted vessel was completely patent. At 7 months after implantation, the patient was doing well, with no evidence of graft occlusion or aneurysmal changes on chest radiography.
Later investigations used a pulse duplicator culture environment to speed up the process of tissue formation [35,36]. The first study sequentially seeded bioabsorbable polymers (polyglycolic acid/poly-4-hydroxybutyrate) with ovine vascular myofibroblasts and ECs. After 14 days of exposure to pulsatile flow, conduits appeared to be grossly intact but without properties of a native artery. However, grafts exhibited suture retention strength appropriate for surgical implantation [35]. The second study used umbilical cord cells as a source for tissue engineering [36]. Human umbilical cord cells were harvested and expanded in culture, and then seeded onto pulmonary conduits fabricated from rapidly bioabsorbable polymers. The seeded conduits were grown in vitro in a pulse duplicator bioreactor [36]. Histology of the conduits revealed viable, layered tissue and extracellular matrix formation. However, extracellular matrix protein levels were significantly lower compared with native tissue. In addition, cells were positive for vimentin and a-smooth muscle actin. The mechanical strength of the pulsed constructs was comparable with native tissue [36].
▪ Decellularized & cell-seeded natural scaffolds
For scaffold materials, it has been shown that natural materials have a better patency compared with artificial grafts [37]. Although biodegradable scaffold studies showed promising results, the durability of these grafts remains unknown. Decellularized tissues and organs (Table 1) are currently used in tissue engineering and have a favorable outcome [21]. They are thought to be promising as removal of cellular material decreases immunogenicity of the tissue caused by foreign antigens. Therefore, preventing the deterioration of the implanted grafts by the host’s immune response [38,39]. It has been shown that decellularized grafts elicite significantly lower levels of class I and II HLA antibody formation at 1, 3 and 12 months after implantation than standard cryopreserved allografts [38]. There are many different methods used to decellularize scaffolds, dependent on the type of tissue. The idea of this technique is that decellularization leaves the extracellular matrix intact while removing all of the ‘allogeneic and xenogeneic’ cell antigens that are not tolerated and lead to an immune response [34]. A successful cell removal from the scaffold is important as this determines the body’s response to the tissue after insertion. Despite the wide range of techniques used to remove cells, only a few are successful with minimum extracellular matrix disruption. Some disruption is necessary to expose cells; however, alterations such as glycosaminoglycan, removal may decrease cell migration onto the scaffold or increase the chance of in vivo enzymatic degradation [21].
Decellularized grafts seeded with vascular ECs
The pioneering work by Eberl and colleagues demonstrated the feasibility of endothelializing biological heart valve leaflets in vitro with cultured autologous ECs [40]. A decade later, Dohmen et al. described the first human implantation of a tissue-engineered heart valve made from a decellularized pulmonary allograft seeded with autologous vascular ECs obtained from the forearm vein 4 weeks before being implanted in patient’s pulmonary position during the Ross procedure [41]. At 1-year follow-up, the valve had a normal function with no signs of calcification.
Decellularized grafts seeded with stem cells
A study by Shin’oka et al. confirmed that animals receiving tissue-engineered pulmonary valve leaflets seeded with autologous bone marrow cells developed fewer infections and had less deterioration when compared with valves seeded with allogenic cells [33]. The success achieved in animals has led to clinical testing. An early investigation reported the first clinical implantation of pulmonary heart valves engineered with autologous endothelial progenitor cells (EPCs) and the results of 3.5 years of follow- up [42]. In this study, human pulmonary heart valve allografts were decellularized and resulting scaffolds were seeded with peripheral mononuclear cells isolated from human blood. Cultivation and differentiation of the seeded scaffold in a dynamic bioreactor system for up to 3 weeks resulted in the expression of endothelial markers in the seeded cells. The pulmonary valves seeded with autologous cells were then implanted into two pediatric patients (aged 11 and 13 years) with congenital pulmonary valve failure. Results showed reasonable mechanical properties and resistance. Over 3.5 years of follow-up, the valve annuluses increased in both patients and there were no signs of valve degeneration observed after 3.5-year follow-up [42]. This showed that seeding decellularized tissue is a feasible and safe method for pulmonary valve replacement.
These initial data for the development of tissue-engineered valves are encouraging. However, in these cases, the tissue engineering focused on reconstitution of the endothelial lining without any attempt to repopulate the extracellular matrix of the connective tissue with cells of the appropriate phenotype. A more recent study developed a tissue-engineered artery using autologous bone marrow-derived mesenchymal stem cells (MSCs) and a decellularized arterial scaffold. MSC-derived vascular smooth muscle-like and endothelial-like cells were seeded onto decellularized ovine carotid arteries, which were inserted into the carotid arteries in an ovine host model. The scaffold retained the main structural components of the blood vessel, such as collagen and elastin. The engineered vessels were patent, antithrombogenic and mechanically stable for 5 months in vivo, whereas nonseeded grafts occluded within 2 weeks. Histological, immunohistochemical, and electron microscopic analyses of the engineered vessels demonstrated the existence of endothelium and smooth muscle, and the presence of collagen and elastin at 2 and 5 months, respectively [43]. In 2012, Olausson et al. used stem cells to grow a replacement blood vessel for a 10-year-old girl who developed portal vein obstruction and liver failure [44]. A deceased donor vein was decellularized and subsequently recellularized with ECs and SMCs differentiated from stem cells obtained from the bone marrow of the recipient. The patient did well following the transplant and received no immunosuppressive drugs. This case report showed the great potential of tissue engineering and stem cell technology for vascular reconstruction, even though it was limited to the vein rather than the arterial system of the body.
Perinatal stem cells in vascular tissue engineering
Autologous stem cells provide a powerful source for all types of cell regeneration and would be of great advantage in cardiovascular tissue engineering. These cells are easy to obtain and can be manipulated for multiple passages. Repopulating scaffolds with patients’ own stem cells before surgery can potentially create a vital conduit with a high physiological replacement of all components and subsequent long-term preservation of mechanical and biological properties.
▪ Major functional cell types in vascular tissue engineering
ECs, SMCs and fibroblasts are the three major types of cells composing the vascular wall. ECs regulate the permeability, migration, remodeling, proliferation and metabolism of the blood vessel, as well as modulate the contractility of vascular SMCs by producing biochemical substances [45]. Vascular SMCs can perform both contractile and synthetic functions, which maintain the mechanical properties and radial compliance of the blood vessels. SMCs produce most of the extracellular matrix proteins. They play crucial roles for long-term adaptation, via structural remodeling by changing cell number and connective tissue composition [46]. It has been widely accepted that functional tissue-engineered blood vessels (TEBVs) cannot be achieved without ECs, SMCs, scaffolds, proper molecular signals and the unique vessel-engineering techniques [47].
It is known that the majority of the cells in the adult blood vessel are terminally differentiated, even cells isolated from umbilical cord veins still have limited proliferation potential and will lose their biological function during in vitro proliferation [48]. Although some TEBVs have been reported to be successfully constructed by seeding cells isolated from autologous vessels [49,50], the limited proliferation potential of harvested cells makes it impossible to obtain a large amount of cells from a small-vessel biopsy.
Many attempts have been made to improve the proliferation potential of ECs and SMCs; for example, the genetic manipulation by introducing human telomerase reverse transcriptase subunit into human SMCs [51]. However, the safety of the cells after genetic manipulation is still a great concern. Long-term follow-up of modified cells in vivo is necessary before clinical application of these cells. To date, there is no promising way to solve the proliferation problem of ECs and SMCs. It is of great interest to find alternative cell sources for vessel engineering.
▪ Stem cells in regenerative medicine
Stem cells are able to self-renew and differentiate into varying specialized mature cells under the correct conditions. The discovery and application of stem cells make it possible to produce a sufficient amount of targeted functional cells for tissue regeneration [52].
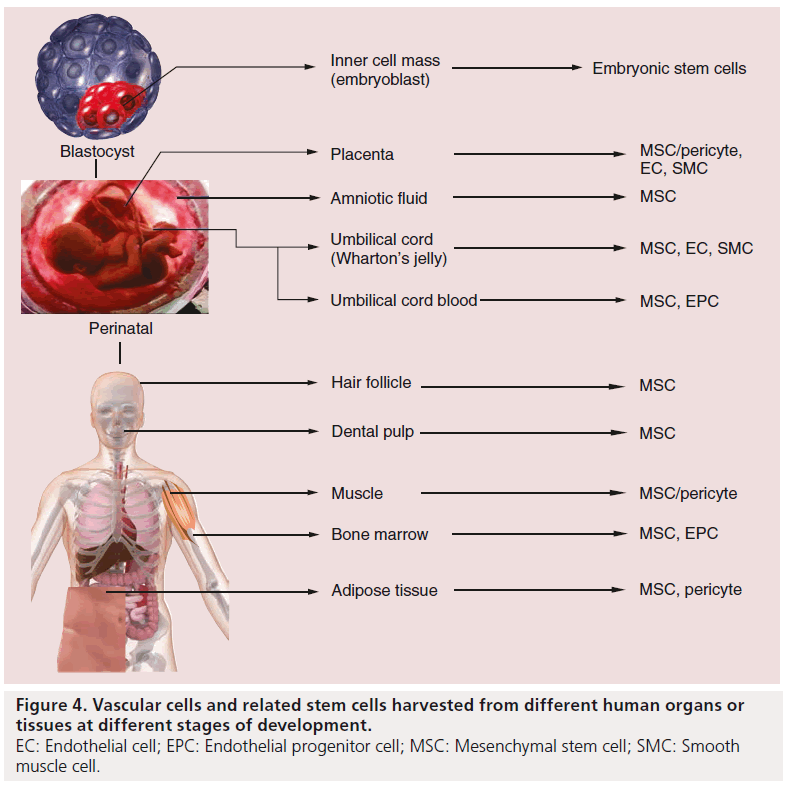
Besides the induced pluripotent stem cells (iPSCs), stem cells could be roughly categorized into three groups based on their origin in human: embryonic stem cells (ESCs), perinatal stem cells (PSCs) and adult stem cells (Figure 4).
iPSCs are reprogrammed adult somatic cells, with regained pluripotency by introducing four defined transcription factors, Oct3/4, Sox2, c-Myc and Klf4. Human fibroblast is the first and most commonly used somatic cell type in generation of human iPSCs [53,54]. The use of iPSCs could avoid the ethical concern of using ESCs and give researchers more choices in autologous transplantation.
Deriving human vascular ECs and SMCs from iPSCs has been demonstrated by different research groups [55,56]. In 2012, Cheung et al. reported the direct differentiation of iPSCs into SMCs by a chemically defined method via three intermediate lineages, which could benefit disease modeling [57]. Kim et al. demonstrated that coimplanting iPSC-derived ECs and SMCs significantly improved wound healing in a murine model 1 year later [58].
Although many studies have produced vascular-like EC and SMC from iPSCs, some researchers still question the variation in gene expression, DNA methylation and the pluripotent potential of iPSCs. Furthermore, Gore and colleagues raised questions about the somatic mutations, for example, ‘protein-coding point’ mutations, occurring after reprogramming [59]. Hussein’s research team found a remarkable increase in copy number variations in iPSCs compared with their origin fibroblasts and ESCs [60].
ESCs are pluripotent stem cells derived from the inner cell mass of a blastocyst. Theoretically, they are able to differentiate into all cell lines derived from the three primary germ layers. Researchers have described various methodologies of differentiating human ESCs into ECs and SMCs, with or without embryoid-body intermediates [61–65]. Furthermore, other studies have indicated that ESC-engineered vessels could integrate into the recipient’s circulatory system [66,67]. However, the mechanisms of controling ESCs to differentiate into vascular cells are poorly defined. Meanwhile, the utilization of ESCs is still under ethical controversy, as the isolating process destroys the fertilized human embryo.
Adult stem cells, also known as somatic stem cells, have been identified throughout organs and tissues from the whole body. Most of the adult stem cells are multipotent, that is, they can differentiate into all cell types existing in their original organ. Under the correct conditions, cross-embryonic-linage differentiation potential has also been found in some adult stem cell types, for instance, MSCs derived from adipose tissue are able to differentiate into osteocytes, skeletal muscle cells and SMCs [68]. Unfortunately, harvesting adult stem cells generally requires invasive collection; more tissue is required to obtain sufficient cells due to their limited proliferation potential compared with ESCs. These operations increase the risk to newborns and are not acceptable according to the criteria of regenerative medicine.
PSCs: the term perinatal refers to the period around birth, from the 20th week of pregnancy to the fourth week of life. The perinatal tissues, which include umbilical cord blood (UCB), the umbilical cord (including blood vessels and Wharton’s jelly matrix) and the placenta, are mostly treated as medical waste and are discarded at the time of birth. Therefore, the isolation of stem cells from these sources is noninvasive and safe to both the mothers and newborns. The stem cells obtained from those tissues are collectively called ‘PSCs’.
In the field of CHD, this perinatal tissue is of great practical and strategic importance. A detailed fetal echocardiography can detect CHD as early as 24 weeks of gestation. It is therefore possible to harvest perinatal stem cells at the time of delivery and use them for tissue engineering grafts to be used in congenital heart surgery. We will therefore concentrate in this review on the optimal stem cell populations, which can be harvested from perinatal tissues and are able to generate sufficient ECs and SMCs for the creation of tissue-engineered autologous grafts.
▪ Deriving ECs from PSCs
The endothelial lining of any graft is crucial to its success, and it appears likely that only living, autologous ECs are able to meet the joint challenges of being nonthrombogenic and immune tolerated [25].
EPCs are a population of rare mononuclear cells circulating in the peripheral blood as well as residing in the bone marrow. EPCs can be isolated from UCB by gradient centrifugation followed by direct plating or further sorting by cell surface markers, such as CD34 or CD133 [69,70]. They express both hematopoietic and EC markers at an early stage.
Previous studies have demonstrated that angiogenic stimuli, such as VEGF and b-FGF, could trigger the proliferation and differentiation of EPCs into mature ECs in vitro [71,72]. These cells not only display a matured EC phenotype, including a ‘cobblestone’ morphology, expression of EC surface markers, such as CD31, Flk-1, VE-cadherin and vWF, and the uptake of LDL particles from medium; however, they also are able to form capillary-like structures when plated at subconf luent densities with extracellular matrix support in vitro, or migrate to incorporate into neovessels in vivo. This tube-formation ability indicates that these matured ECs could be functionally involved in angiogenesis [73].
Kaushal et al. reported that TEBVs seeded with EPCs, isolated from peripheral blood, revealed better antithrombogenic properties compared with grafts not covered with EPCs [25]. Schmidt and colleagues started to test UCBderived EPCs (UCB-EPCs) in vascular tissue engineering 3 years later. They found a monolayer of matured ECs attached on the lumen of the polyglycolic acid scaffold after 12-days incubation. The tissue was characterized by endothelium functions [74].
UCB has been revealed to contain tenfold more EPCs compared with adult peripheral blood, which may be sufficient for vascular tissue engineering purpose [75]. UCB EPCs have shown stable phenotype, and proliferative and differentiation potential when cultured in defined expansion and differentiation medium, which indicates that this cell type could be a promising EC candidate for the surgical treatment of pediatric CHD.
▪ Deriving SMCs from PSCs
Deriving SMCs from adult MSCs
MSCs are a group of stem cells isolated from multiorigin tissues in the human body, including the bone marrow, fat, blood, liver, spleen and perinatal tissues [76–80]. The SMC differentiation potential of adult BM MSCs has been intensively studied [76,81], mainly for the purpose of understanding the mechanism and evaluating the treatment of arteriosclerosis.
These cells have been shown to be involved in angiogenesis, through the interaction with vascular cells, by releasing or responding to particular vascular growth factors [82]. Studies have shown that BM MSCs could be differentiated into endothelial phenotypic cells in the presence of VEGF and b-FGF [83], or into SMC lineage under the treatment with PDGF and/or TGF-b1 [84–87].
In 2008, Gong and Niklason reported the first small-diameter vessel tissue engineered using human BM MSC-derived SMCs and a biodegradable scaffold in a biomimetic condition in vitro. The BM MSC-derived SMCs only expressed α-smooth muscle actin (α-SMA) and calponin, the early differentiation stage markers. This may be the major cause that resulted in the weak mechanical strength and improper ECM protein component ratio, although engineered vessels displayed similar morphology and histology compared with natural blood vessels [88].
Perinatal sources for MSCs & their SMC differentiation potential
UCB-derived MSCs
UCB is a rich source of stem cells including hematopoietic stem cells and MSCs, without any ethical concern owing to its autologous origin and as there is no risk from harvesting UCB. Besides using UCB as an alternative hematopoietic stem cell source [89], several research groups have reported successful isolation of MSCs from cord blood with full adult MSC mutipluripontency [81,90,91].
The isolation of MSCs from UCB is mostly based on MSCs adherence-to-plastic property when cultured in selective media. Mononuclear cells from fresh cord blood are cultured in medium containing fetal bovine serum, with or without growth factors, such as PDGF-BB, EGF and b-FGF, among others, and are plated on extracellular matrix protein-coated plates. Visible colonies can be observed from days 5–14. Cells grow with unified fibroblast-like morphology and display a typical ‘hill and valley’ phenomenon when reaching confluence [81,90,91].
These cells are negative for hematopoietic lineage markers CD34 and CD45, but positive for human MSC markers CD73, CD90 and CD105, indicating the MSC nature of these UCB-derived cells. These cells have a high proliferative capacity and a remarkable capacity to differentiate into multiple mesoderm lineages, for example, adipocytes, chondrocytes, osteoblasts and hepatic cells similar to BM MSCs [92–94].
The major bottleneck in the clinical application of UCB MSCs is the low collection success rate [95].
Umbilical cord stem cell (Wharton’s jelly stem cell)
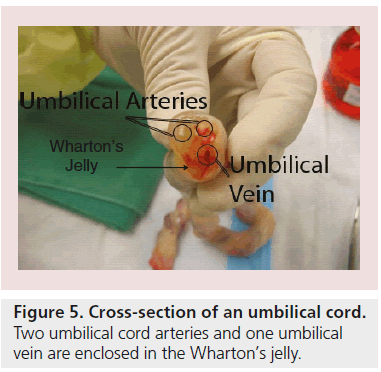
Wharton’s jelly is a gelatinous substance within the umbilical cord, largely made up of hyaluronic acid and chondroitin sulfate (Figure 5) [96]. Since 1991, fibroblast-like cells were isolated from Wharton’s jelly by McElreavey and colleagues [97]. Weiss and other scientists’ reports indicate that those cells among the umbilical cord are multipotent stem cells displaying a bone marrow MSC-like phenotype; for example, adherence to plastic surfaces and expressing cell surface markers, including CD73, CD90 and CD105, but simultaneously show no expression for CD14, CD19, CD34, CD45 and HLA-DR [97–99]. It has been reported that Wharton’s jelly cells (WJCs) are capable of differentiating into all mesodermal lineage and nervous cells [100–102].
Figure 5: Cross-section of an umbilical cord. Two umbilical cord arteries and one umbilical vein are enclosed in the Wharton’s jelly.
In 2002, Hoerstrup et al. applied WJCs in TEBVs by seeding these cells on to a biodegradable scaffold in a biomimetic growing system using a pulse duplicator bioreactor. Histological analysis showed that the seeded cells displayed some SMC markers and produced ECM proteins, for example, glycosaminoglycans and collagens [36]. Other researchers also found that cryopreserved WJCs maintain their myofibroblast- like morphology and are able to generate functional heart valves when seeded on a biodegradable trileaflet scaffold [103].
The unique advantages of WJCs are their pure fetal origin, large primary harvesting amount and nearly 100% isolation success rate by both enzymatical digestion and/or outgrowth methods. In addition, WJCs have been found to have a better proliferation capability compared with MSCs derived from bone marrow [104]. Although our unpublished data suggest that WJCs may have limited potential to give rise to contractile SMCs compared with BM MSCs and UCB MSCs, Wharton’s jelly is still a potential MSC source for vascular tissue engineering and is worth exploring further.
Placenta-derived stem cells
The placenta is an organ providing a link between the fetus and the mother to allow nutrient uptake, waste elimination and gas exchange via the maternal blood supply, while keeping the maternal and fetal circulations separated, although both exist witihin the placenta.
Pericyte
Perivascular cells, principally pericytesor mural cells, which exist in capillaries and microvessels, have been isolated from different human organs including, placenta, pancreas, skeletal muscle and adipose tissue. These cells express phenotypic markers, including CD146, NG2, PDGF-Rb and most of the major MSC markers, but lack endothelial, hematopoietic and myogenic cell markers. Pericytes derived from tissues of different origins could retain their myogenicity in long-term in vitro culture [105]. Many researchers use ‘MSCs’ to name placentaderived multipotent stem cells according to their BM MSC-like phenotype. However, considering their microvessel origin and expression of additional vascular cell-related markers, we prefer to define those cells as placenta-derived ‘pericytes’.
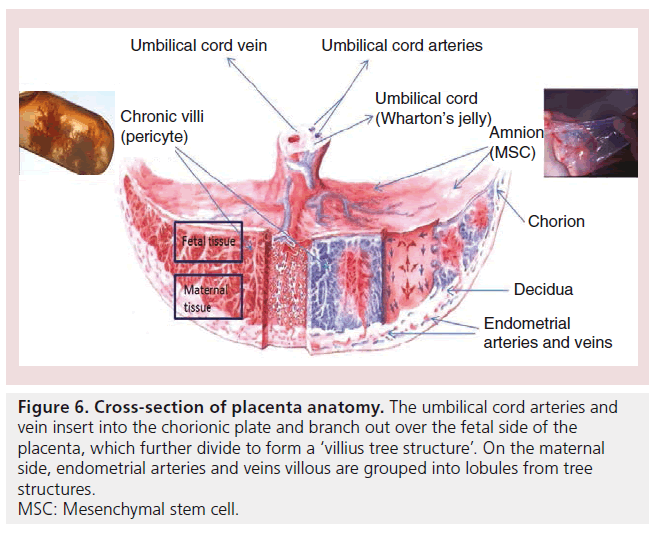
To isolate the pericytes from the placenta, Crisan and colleagues first obtained single cells by digesting placenta chronic villi (Figure 6). Pericytes were then sorted out as CD146+, CD34-, CD45- and CD56- cells from the single-cell suspension [105].
Figure 6: Cross-section of placenta anatomy. The umbilical cord arteries and vein insert into the chorionic plate and branch out over the fetal side of the placenta, which further divide to form a ‘villius tree structure’. On the maternal side, endometrial arteries and veins villous are grouped into lobules from tree structures. MSC: Mesenchymal stem cell.
Pericytes are multipotent cells retaining the capacity to differentiate into vascular cells and a variety of other MSC-derived cell types. Pericytes have been reported to have the capacity to differentiate into either vascular SMCs or ECs under the inf luence of PDGF-BB, TGF-b1 and/or VEGF, respectively [105–107].
He and colleagues developed a small-diameter TEBV by only seeding human skeletal musclederived pericytes, which were isolated by the same sorting criteria, into a biodegradable poly(esterurethane) urea scaffold. After implanting the graft into Lewis rats for 8 weeks, they found, in the remodeled tissue multilayer, the expression of some SMC markers, such as a-SMA and calponin. The lumen monolayer was positive in endothelial marker (vWF) staining. Their results proved the feasibility of pericyte-based TEBV and indicated the pericytes functional involvement in maintaining patency of the graft and repopulating vascular cells into the TEBV [107].
Although some reports suggest that placentaderived pericytes may have immunomodulatory effects [108], the clinical utilization of placentaderived stem cells is still controversial because both fetal and maternal tissues are closely integrated within the placenta. There is no readyto- use purification technique to avoid fetal or maternal cell contamination.
MSCs derived from amnion
Amnion, also named as amniotic membrane, is the extra embryonic fetal membrane derived from the ectoderm ECs and mesoderm MSCs that forms the amniotic cavity containing amniotic fluid that protects the fetus during the gestation shortly before birth [109]. This membrane can be simply peeled away from the placenta after the placenta is removed after birth (Figure 6).
Human amniotic membrane transplantation has been used in the treatment of skin burn wounds [110] and ocular surface reconstruction [111], before becoming an attractive stem cell source for other applications. Previous studies discovered that the amnion stem cells (ASCs) isolated from the amniotic membrane contain both amniotic ECs (AECs) and amniotic MSCs (AMSCs), which could be observed in the primary culture dishes under an optical microscope [109,112].
Amnion-derived stem cells did not express the hematopoietic (e.g., CD34 and CD45) and endothelial markers (CD31); however, they showed a varying and significantly lower positive rate of MSC surface markers, such as CD29, CD44, CD73, CD90 and CD105 [113,114]. This could be evidence to confirm that there are multiple populations with different phenotypes existing among ASCs. However, both AECs and AMSCs have been found to express some pluripotent cell markers, including OCT4, NANOG and SOX2 [115], which indicates that either AMSCs or AECs may have potential to differentiate into all three germ layer cells [115,116]. However, this hypothesis, generated based on their immune phenotype, has not been fully approved by the researchers. Compared with BM MSCs in similar differentiation conditions (beside the mesoderm lineage), the differentiated ASCs do not consistently express the characteristics of endodermal cells (e.g., hepatocytes and pancreatic cells) and/or ectodermal cells, such as neural cells [109,115,117].
Some researchers reported that AMSCs have osteocytic, chondrocytic and adipocytic differentiation potential similar to that of BM MSCs [113,114]. However, Soncini et al. and other researchers’ data challenged the persistence of the multipotent capacity of ASCs. They found that only the freshly isolated ASCs could differentiate towards the trilineage of mesoderm with satisfying ratio. Additionally, this differentiation ability decreased sharply and the proliferation speed slowed down during in vitro culture. Their result confirmed Portmann-Lanz and colleagues finding that most ASCs stop growing at cell passage four or five [109,118].
There has not been any significant achievement in deriving vascular SMCs from AMSCs. Considering the technical difficulty to isolate AMSCs from whole ASCs and their limited proliferation ability, ASCs can not be considered proper candidates in vascular tissue engineering based on the current research techniques.
Conclusion
Repeated operations are distressing for both young children and their families, and are increasingly risky. Since 1% of babies born have congenital defects, multiple operations are also costly. Tissue-engineered valves, conduits and vascular patches made of living tissue could: function like a native structure with the potential to grow, repair and remodel; have a longer lasting therapeutic effect than acellular grafts; reduce the number of reoperations and the cost of to the National Health Service; and improve survival and quality of life.
The improved knowledge of stem cell biology and the development of the human embryo has greatly advanced our understanding of PSC’s potential in the last decade. Perinatal tissues show great promise as an alternative stem cell source, and PSCs give clinical scientists more choices and the possibility of planning the building of the tissue-engineered grafts ready for when the surgical correction is required. However, there are still many limitations to be overcome to produce a well-defined, easy-to-harvest PSC population and for their seeding into appropriate matrices that are going to be used for the treatment of newborns or infants with CHD.
Future perspective
Synthetic biodegradable and decellularized natural materials seeded with PSC-derived vessel cells may have the greatest potential and will continue to be used in tissue engineering of vascular grafts for corrective congenital heart surgery. Although it is hard to predict which approach of the two will see wider use, decellularized and cell-seeded scaffolds promise better-engineered materials for corrective congenital heart surgery. More trials will be needed to confirm the superiority of this approach. Techniques and protocols for decellularization of natural scaffolds and differentiation of PSCs into ECs and SMCs will see further optimization. The synthetic biodegradable scaffolds seeded with autologous cells approach may see a breakthrough if new polymers are developed with the advantage of not causing any damage to intact vascular structures when degraded and released into the circulation.
Financial & competing interests disclosure
This work was supported by the Garfield Weston Trust and the Bristol NIHR BRU in Cardiovascular Medicine. MT Ghorbel was a recipient of an Intermediate Research Fellowship from the British Heart Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Many children with congenital heart disease need prosthetic and other replacement grafts in the form of new valves, conduits and patches.
▪ Although prosthetic and other replacement grafts may be life saving, they have limited durability and are prone to infection, immunological reactivity and thrombosis.
▪ The repopulation of a decellularized matrix with a patient’s own stem cells before surgery can potentially create a living structure with subsequent long-term preservation of mechanical and biological properties.
▪ The key vessel cells, endothelial and smooth muscle cells, can be derived from perinatal stem cells (umbilical cord blood endothelial progenitor cells and mesenchymal stromal cells, respectively).
▪ Prosthetic materials like Dacron or Gortex predispose the patients to life-long risks of infection and lack the ability to grow, repair and remodel, which are vital for pediatric populations.
▪ Acellular scaffolds, made by decellularization of natural biological tissues, have been shown to resist infections and be durable and compliant in animal studies. However, human study showed that these grafts triggered a strong inflammatory response due to matrix immunogenicity being conserved.
▪ Synthetic biodegradable scaffolds seeded with autologous cells offer a better alternative to acellular scaffolds. However, as the polymer degrades slowly it is released in the circulation and can cause damage to intact vascular structures.
▪ Decellularized and cell-seeded scaffolds promise even better engineered materials for corrective congenital surgery; although more trials are needed to confirm the superiority of this approach.
References
Papers of special note have been highlighted as:
▪ of interest
- Green A. Outcomes of congenital heart disease: a review. Pediatr. Nurs. 30(4), 280–284 (2004).
- Ohye RG, Bove EL. Advances in congenital heart surgery. Curr. Opin. Pediatr. 13(5), 473–481 (2001).
- Tweddell JS, Pelech AN, Frommelt PC et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation 102(19 Suppl. 3), III130–III135(2000).
- Selamet Tierney ES, Gersony WM, Altmann K et al. Pulmonary position cryopreserved homografts: durability in pediatric Ross and non-Ross patients. J. Thorac. Cardiovasc. Surg. 130(2), 282–286 (2005).
- Zimmermann WH, Cesnjevar R. Cardiac tissue engineering: implications for pediatric heart surgery. Pediatr. Cardiol. 30(5), 716–723 (2009).
- Hoerstrup SP, Sodian R, Daebritz S et al. Functional living trileaflet heart valves grown in vitro. Circulation 102(19 Suppl. 3),III44–III49 (2000).
- Mayer JE Jr, Shin’oka T, Shum-Tim D. Tissue engineering of cardiovascular structures. Curr. Opin. Cardiol. 12(6), 528–532 (1997).
- Stock UA, Mayer JE Jr. Valves in development for autogenous tissue valve replacement. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2, 51–64 (1999).
- Graham LM, Vinter DW, Ford JW, Kahn RH, Burkel WE, Stanley JC. Endothelial cell seeding of prosthetic vascular grafts: early experimental studies with cultured autologous canine endothelium. Arch. Surg. 115(8), 929–933 (1980).
- Deutsch M, Meinhart J, Vesely M et al. In vitro endothelialization of expanded polytetrafluoroethylene grafts: a clinical case report after 41 months of implantation.J. Vasc. Surg. 25(4), 757–763 (1997).
- Leseche G, Ohan J, Bouttier S, Palombi T, Bertrand P, Andreassian B. Above-knee femoropopliteal bypass grafting using endothelial cell seeded PTFE grafts: five-year clinical experience. Ann. Vasc. Surg. 9(Suppl.), S15–S23 (1995).
- Graham LM, Burkel WE, Ford JW, Vinter DW, Kahn RH, Stanley JC. Immediate seeding of enzymatically derived endothelium in Dacron vascular grafts. Early experimental studies with autologous canine cells. Arch. Surg. 115(11), 1289–1294 (1980).
- Graham LM, Burkel WE, Ford JW, Vinter DW, Kahn RH, Stanley JC. Expanded polytetrafluoroethylene vascular prostheses seeded with enzymatically derived and cultured canine endothelial cells. Surgery 91(5), 550–559 (1982).
- Leseche G, Bikfalvi A, Dupuy E, Tobelem G, Andreassian B, Caen J. Prelining of polytetrafluoroethylene grafts with cultured human endothelial cells isolated from varicose veins. Surgery 105(1), 36–45 (1989).
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science 231(4736), 397–400 (1986).
- L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 12(1), 47–56 (1998).
- Jones L, Braithwaite BD, Davies B, Heather BP, Earnshaw JJ. Mechanism of late prosthetic vascular graft infection. Cardiovasc. Surg. 5(5), 486–489 (1997).
- Wang B, Borazjani A, Tahai M et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J. Biomed. Mater. Res. A 94(4), 1100–1110 (2010).
- Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, Deruiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur. J. Cardiothorac. Surg. 27(4), 566–571 (2005).
- Schenke-Layland K, Vasilevski O, Opitz F et al. Impact of decellularization ofxenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J. Struct. Biol. 143(3), 201–208 (2003).
- Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 27(19), 3675–3683 (2006).
- Clarke DR, Lust RM, Sun YS, Black KS, Ollerenshaw JD. Transformation of nonvascular acellular tissue matrices into durable vascular conduits. Ann. Thorac. Surg. 71(5 Suppl.), S433–S436 (2001).
- Bader A, Steinhoff G, Strobl K et al. Engineering of human vascular aortic tissue based on a xenogeneic starter matrix. Transplantation 70(1), 7–14 (2000).
- Tamura N, Nakamura T, Terai H et al. A new acellular vascular prosthesis as a scaffold for host tissue regeneration. Int. J. Artif. Organs 26(9), 783–792 (2003).
- Kaushal S, Amiel GE, Guleserian KJ et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 7(9), 1035–1040 (2001).
- Lantz GC, Badylak SF, Hiles MC et al. Small intestinal submucosa as a vascular graft: a review. J. Invest. Surg. 6(3), 297–310 (1993).
- Huynh T, Abraham G, Murray J, Brockbank K, Hagen PO, Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat. Biotechnol. 17(11), 1083–1086 (1999).
- Simon P, Kasimir MT, Seebacher G et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur. J. Cardiothorac. Surg. 23(6), 1002–1006; discussion 1006 (2003).
- Shinoka T, Breuer CK, Tanel RE et al. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann. Thorac. Surg. 60(6 Suppl.), S513–S516 (1995).
- Breuer CK, Shin’oka T, Tanel RE et al. Tissue engineering lamb heart valve leaflets. Biotechnol. Bioeng. 50(5), 562–567 (1996).
- Shinoka T, Shum-Tim D, Ma PX et al. Creation of viable pulmonary artery autografts through tissue engineering.J. Thorac. Cardiovasc. Surg. 115(3), 536–545;discussion 545–536 (1998).
- Shum-Tim D, Stock U, Hrkach J et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann. Thorac. Surg. 68(6), 2298–2304; discussion 2305 (1999).
- Shin’oka T, Matsumura G, Hibino N et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc.Surg. 129(6), 1330–1338 (2005).
- Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery.N. Engl. J. Med. 344(7), 532–533 (2001).
- Hoerstrup SP, Zund G, Sodian R, Schnell AM, Grunenfelder J, Turina MI. Tissue engineering of small caliber vascular grafts. Eur. J. Cardiothorac. Surg. 20(1), 164–169(2001).
- Hoerstrup SP, Kadner A, Breymann C et al. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann. Thorac. Surg. 74(1), 46–52; discussion 52 (2002).
- Sales KM, Salacinski HJ, Alobaid N, Mikhail M, Balakrishnan V, Seifalian AM. Advancing vascular tissue engineering: the role of stem cell technology. Trends Biotechnol. 23(9), 461–467 (2005).
- Hawkins JA, Hillman ND, Lambert LM et al. Immunogenicity of decellularizedcryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J. Thorac. Cardiovasc. Surg. 126(1), 247–252; discussion252–243 (2003).
- Allaire E, Bruneval P, Mandet C, Becquemin JP, Michel JB. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery 122(1), 73–81 (1997).
- Eberl T, Siedler S, Schumacher B, Zilla P, Schlaudraff K, Fasol R. Experimental in vitro endothelialization of cardiac valve leaflets. Ann. Thorac. Surg. 53(3), 487–492 (1992).
- Dohmen PM, Lembcke A, Hotz H, Kivelitz D, Konertz WF. Ross operation with a tissue-engineered heart valve. Ann. Thorac. Surg. 74(5), 1438–1442 (2002).
- Cebotari S, Lichtenberg A, Tudorache I et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 114(1 Suppl.), I132–I137 (2006).
- Zhao Y, Zhang S, Zhou J et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials 31(2), 296–307 (2010).
- Olausson M, Patil PB, Kuna VK et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet 380(9838), 230–237 (2012).
- Chien S. Effects of disturbed flow on endothelial cells. Ann. Biomed. Eng. 36(4), 554–562 (2008).
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84(3), 767–801 (2004).
- Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354(Suppl. 1), S132–S134 (1999).
- Liu Y, Zhang YZ, Chen JJ et al. [Experimental study on constructing small-caliber artery by tissue engineering approach. ] Zhonghua Wai Ke Za Zhi 41(9), 679–683 (2003).
- Weinberg CB, Bell E. A blood-vessel model constructed from collagen and cultured vascular cells. Science 231(4736), 397–400 (1986).
- L’Heureux N, Dusserre N, Konig G et al. First use of a completely biological human tissue engineered blood vessel in a primate model. Circulation 110(17), 508–508 (2004).
- McKee JA, Banik SSR, Boyer MJ et al. Human arteries engineered in vitro. EMBO Rep. 4(6), 633–638 (2003).
- Cohen S, Leshanski L, Itskovitz-Eldor J. Tissue engineering using human embryonic stem cells. Methods Enzymol. 420, 303–315 (2006).
- Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5), 861–872 (2007).
- Yu J, Vodyanik MA, Smuga-Otto K et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858), 1917–1920 (2007).
- Drukker M, Tang C, Ardehali R et al. Isolation of primitive endoderm, mesoderm, vascular endothelial and trophoblast progenitors from human pluripotent stem cells. Nat. Biotechnol. 30(6), 531–542 (2012).
- Majesky MW, Mummery CL. Smooth muscle diversity from human pluripotent cells. Nat. Biotechnol. 30(2), 152–154 (2012).
- Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 30(2), 165–173 (2012).
- Kim KL, Song SH, Choi KS, Suh W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng. Part A doi:10.1089/ten.TEA.2012.0768(2013) (Epub ahead of print).
- Gore A, Li Z, Fung HL et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 471(7336), 63–67 (2011).
- Hussein SM, Batada NN, Vuoristo S et al. Copy number variation and selection during reprogramming to pluripotency. Nature 471(7336), 58–62 (2011).
- Woll PS, Morris JK, Painschab MS et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood 111(1), 122–131 (2008).
- Cheung C, Sinha S. Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation. J. Mol. Cell. Cardiol. 51(5), 651–664 (2011).
- Ferreira LS, Gerecht S, Shieh HF et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ. Res. 101(3), 286–294 (2007).
- Hill KL, Obrtlikova P, Alvarez DF et al. Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. Exp. Hematol. 38(3), 246–257.e1 (2010).
- Vazao H, Das Neves RP, Graos M, Ferreira L. Towards the maturation and characterization of smooth muscle cells derived from human embryonic stem cells. PLoS ONE 6(3), e17771 (2011).
- Vo E, Hanjaya-Putra D, Zha Y, Kusuma S, Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev. 6(2), 237–247 (2010).
- Wang ZZ, Au P, Chen T et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo.Nat. Biotechnol. 25(3), 317–318 (2007).
- Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, Dimuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J. Surg. Res. 168(2), 306–314 (2011).
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 287(3),C572–C579 (2004).
- Siddique A, Shantsila E, Lip GY, Varma C. Endothelial progenitor cells: what use for the cardiologist? J. Angiogenes Res. 2, 6 (2010).
- Asahara T, Takahashi T, Masuda H et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18(14), 3964–3972 (1999).
- Guo S, Yu L, Cheng Y et al. PDGFRbeta triggered by bFGF promotes the proliferation and migration of endothelial progenitor cells via p-ERK signalling. Cell Biol. Int. 36(10), 945–950 (2012).
- Griese DP, Ehsan A, Melo LG et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts – implications for cell-based vascular therapy. Circulation 108(21), 2710–2715 (2003).
- Schmidt D, Breymann C, Weber A et al. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann. Thorac. Surg. 78(6), 2094–2098 (2004).
- Murohara T, Ikeda H, Duan J et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 105(11), 1527–1536 (2000).
- Gong Z, Calkins G, Cheng EC, Krause D, Niklason LE. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng. Part A 15(2), 319–330 (2009).
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol. Histopathol. 25(6), 807–815 (2010).
- Fouraschen SM, Pan Q, De Ruiter PE et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 21(13), 2410–2419 (2012).
- Hoogduijn MJ, Crop MJ, Peeters AM et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 16(4), 597–604 (2007).
- Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J. Orthop. Res. 30(4), 634–642 (2012).
- Kang XQ, Zang WJ, Bao LJ, Li DL, Xu XL, Yu XJ. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol. Int. 30(7), 569–575 (2006).
- Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J. Cell Biol. 177(3), 489–500 (2007).
- Lin H, Shabbir A, Molnar M et al. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 216(2), 458–468 (2008).
- Wang D, Park JS, Chu JS et al. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J. Biol. Chem. 279(42), 43725–43734 (2004).
- Cho SW, Lim SH, Kim IK et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann. Surg. 241(3), 506–515 (2005).
- Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature 428(6979), 138–139 (2004).
- Hibino N, Shin’oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J. Thorac. Cardiovasc. Surg. 129(5), 1064–1070 (2005).
- Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 22(6), 1635–1648 (2008).
- Gluckman E, Broxmeyer HA, Auerbach AD et al. Hematopoietic reconstitution in apatient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N. Engl. J. Med. 321(17), 1174–1178 (1989).
- Gang EJ, Hong SH, Jeong JA et al. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 321(1),102–108 (2004).
- Nishiyama N, Miyoshi S, Hida N et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells 25(8), 2017–2024 (2007).
- Chang YJ, Tseng CP, Hsu LF, Hsieh TB, Hwang SM. Characterization of two populations of mesenchymal progenitor cells in umbilical cord blood. Cell Biol. Int. 30(6), 495–499 (2006).
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5), 1294–1301 (2006).
- Lu LL, Liu YJ, Yang SG et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91(8), 1017–1026 (2006).
- Divya MS, Roshin GE, Divya TS et al. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res. Ther. 3(6), 57 (2012).
- Meyer T, Pfeifroth A, Höcht B. Isolation and characterisation of mesenchymal stem cells in Wharton’s jelly of the human umbilical cord: potent cells for cell-based therapies in paediatric surgery? Eur. Surg. 40, 239–244 (2008).
- McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 19(1), 29S (1991).
- Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3), 591–599 (2008).
- Weiss ML, Anderson C, Medicetty S et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 26(11), 2865–2874 (2008).
- La Rocca G, Anzalone R, Corrao S et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem. Cell Biol. 131(2), 267–282 (2009).
- Majore I, Moretti P, Stahl F, Hass R, Kasper Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 7(1), 17–31 (2010).
- Kedong S, Xiubo F, Tianqing L et al. Simultaneous expansion and harvest of hematopoietic stem cells and mesenchymal stem cells derived from umbilical cord blood. Mater. Sci. Mater. Med. 21(12), 3183–3193(2010).
- Sodian R, Lueders C, Kraemer L et al. Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. Ann. Thorac. Surg. 81(6), 2207–2216 (2006).
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25(6), 1384–1392 (2007).
- Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3), 301–313 (2008).
- Crisan M, Huard J, Zheng B et al. Purification and culture of human blood vessel-associated progenitor cells. Curr. Protoc. Stem Cell Biol. Chapter 2,2B.2.1–2B.2.13 (2008).
- He W, Nieponice A, Soletti L et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials 31(32), 8235–8244 (2010).
- Jang MJ, Kim HS, Lee HG et al. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta Haematol. 129(4), 197–206 (2013).
- Soncini M, Vertua E, Gibelli L et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 1(4), 296–305 (2007).
- John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol. Clin. North Am. 16(1), 43–65, vi(2003).
- Maharajan VS, Shanmuganathan V, Currie A, Hopkinson A, Powell-Richards A, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction: indications and outcomes. Clin. Exp. Ophthalmol. 35(2), 140–147 (2007).
- Alviano F, Fossati V, Marchionni C et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 7, 11 (2007).
- Nogami M, Tsuno H, Koike C et al. Isolation and characterization of human amniotic mesenchymal stem cells and their chondrogenic differentiation. Transplantation 93(12), 1221–1228 (2012).
- Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T et al. Isolation and characterization of mesenchymal stem cells from human amniotic membrane. Tissue Eng. Part C Methods 17(1), 49–59 (2011).
- Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 77(3), 577–588 (2007).
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells 23(10), 1549–1559 (2005).
- Takashima S, Ise H, Zhao P, Akaike T, Nikaido T. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct. Funct. 29(3), 73–84 (2004).
- Portmann-Lanz CB, Schoeberlein A, Huber A et al. Placental mesenchymal stem cells aspotential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 194(3), 664–673 (2006).
▪ Early clinical attempt of transplanting a pulmonary artery engineered using autologous vein cells seeded onto a polycaprolactone–polylactic acid copolymer tube.
▪ Pioneering work demonstrating the feasibility of endothelializing biological heart valve leaflets in vitro with cultured autologous endothelial cells.
▪ Tissue-engineered vessels developed in this study were patent, antithrombogenic and mechanically stable for 5 months in vivo.
▪ Present proof-of-concept results that provide promise for the clinical transplantation of bioengineered blood vessels.
▪ First group to produce a completely biological vascular conduit made of layers of bovine vascular cells, endothelial cells, smooth muscle cells and fibroblasts, and animal collagen.
▪ Wharton’s jelly stem cell isolation, purification and characterization methods are illustrated in this paper.