Review Article - Imaging in Medicine (2011) Volume 3, Issue 4
Role and potential of modern ultrasound in pediatric abdominal imaging
A Pilhatsch†1 & M Riccabona11Department of Radiology, Division of Pediatric Radiology, University Hospital LKH Graz, Auenbruggenplatz 34, A – 8036 Graz, Austria
- Corresponding Author:
- A Pilhatsch
Department of Radiology
Division of Pediatric Radiology
University Hospital LKH Graz
Auenbruggenplatz 34, A – 8036 Graz, Austria
Tel: +43 316 3851 4202
Fax: +43 316 3851 4299
E-mail: alexander.pilhatsch@klinikum-graz.at
Abstract
Ultrasound (US) has gained its place as the preferred first-line imaging modality in the child’s abdomen. Especially in infants US has advantages – the small amount of tissue and fat creating restrictions to CT and MRI allows for application of high-resolution (especially linear) transducers, offering excellent imaging capabilities; in addition, US is applicable at the bedside and can be performed without sedation. As radiation protection has become a key issue for imaging children, exploiting US to the utmost using all its possibilities and applying modern methods that have significantly widened US potential is an indispensable and increasingly important approach. This review illustrates standard US techniques, basic and new applications throughout the entire abdomen and its different organ systems, trying to point out the enormous potential of US in different clinical scenarios, focusing on gastrointestinal and urogenital issues. Future perspectives provided by new modalities such as contrastenhanced and 3D US will further strengthen the diagnostic value of this powerful imaging tool, hopefully contributing to continuing reduction of diagnostic radiation burden to children, by diminishing the need for CT.
Keywords
abdomen ▪ children ▪ gastrointestinal ▪ infants ▪ pediatric ultrasound ▪ urinary tract
Children and especially infants are not small adults – they suffer from specific diseases, present different imaging demands and have increased radiation sensitivity. One important aspect of ongoing continuous improvement of medical imaging is the increasing potential of ultrasound (US); this may help to minimize radiation by replacing or obviating other imaging. This important task, particularly in childhood, can only be achieved by providing dedicated skilled pediatric US to children 24 h, 7 days a week.
The purpose of this review is to give some key advice on pediatric US, to list and illustrate typical pediatric conditions, to debate the relevant respective differential diagnoses and how US can reliably provide diagnostically relevant information with implications on therapy. For those who are familiar with pediatric radiology the following should be a useful review with some highlighted details. For everyone else this review should demonstrate what is achievable by modern US in children, completely different from the US potential in (obese) adult patients. We will aim to point out the significance of US in common and important pediatric abdominal queries and emphasize the difference between adults and children not only in terms of pathology, but also in the application and potential of the modality itself.
Requisites for performing pediatric US
■ US environment
Children are less cooperative and a specific handling and environment is essential to obtain optimal results. It is essential to take your time at the beginning of any examination to prevent crying or overexcitement; often it is a balancing act, particularly with toddlers who often are less compliant – here the environment is important. Soft light, nice colors, paintings on the wall, soft toys or – later on – a stereo and a TV with a popular animated movie can save you a lot of excitement. Warmed-up US gel, heating radiator and swaddling facilities are particularly important in (preterm) neonates and young infants.
■ Probes
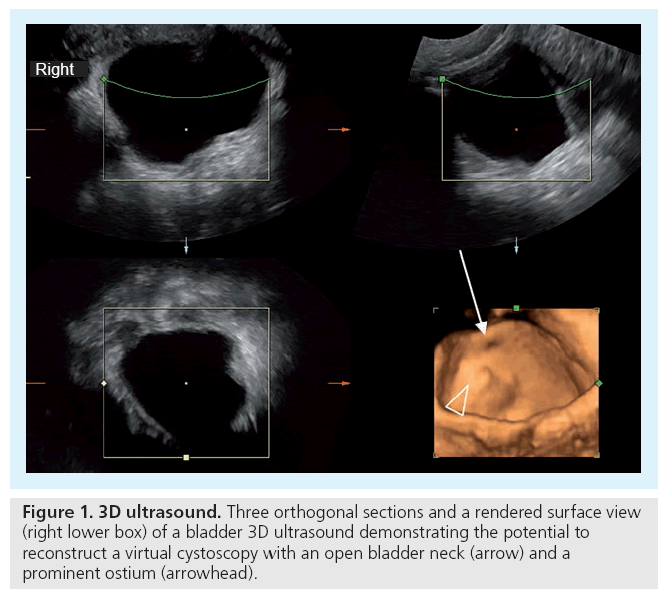
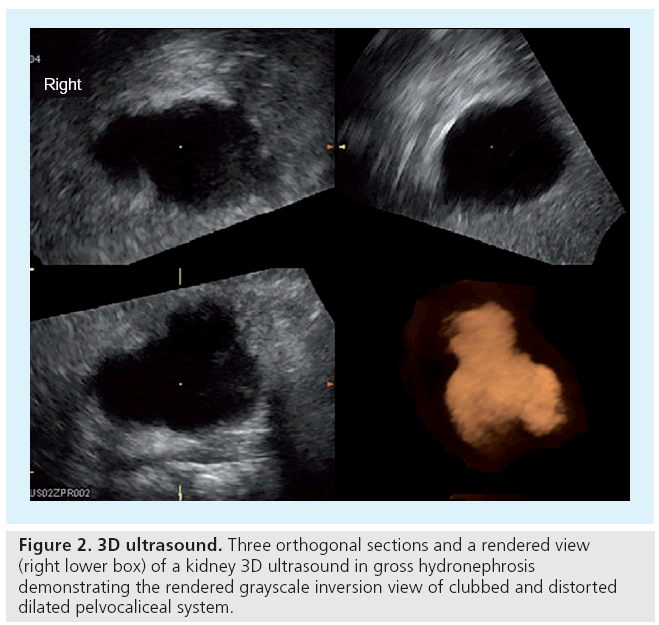
It is mandatory to have not only acceptable but appropriate equipment. A variety of (multifrequency) transducers and a state-of-the-art device with sufficient frame rates are necessary to be able to properly image all the various abdominal queries throughout childhood. For standard pediatric exams you need curved arrays from 10 to 2.5 MHz. To evaluate details or for assessing a neonatal abdomen, high-frequency, high-resolution linear probes ranging from 17 to 5 MHz are advisable. A small footprint sector probe in a similar range as the curved array can be helpful to get access to the retroperitoneum or for an intercostal approach. Color Doppler sonography (CDS) has become standard and should always be available. Extended field of view ‘panoramic’ imaging can be helpful for displaying and measuring large structures, harmonic imaging as well as image compounding is routinely applied. Special features such as dedicated contrast-enhanced (ce) US viewing software or 3D US open up new applications and give new horizons, such as US-elastography, presently under evaluation for pediatric use and not yet established (Figures 1 & 2).
■ Standard normal abdominal US
Routine standard sections for pediatric abdominal ultrasound US are defined by (national and international) documentation recommendations (e.g., issued by the Austrian and German US societies) [101,102]. Usually, an abdominal US starts with the pelvis to assess the urinary bladder, including the distal ureters, before the child voids. The bladder and the retrovesical space including the inner (female) genitalia are viewed and documented in sagittal and axial sections; potentially the major pelvic vessels are additionally assessed. Then the upper abdominal parenchymal organs (i.e., liver and gall bladder, pancreas, spleen, kidneys and adrenal glands) are imaged by longitudinal and transverse sections; the big vessels and the retroperitoneum are included and partially used to identify standard sections in a normal situs. This assessment includes all relevant aspects such as position, parenchymal echogenicity (i.e., liver echogenicity compared with right kidney) and homogeneity, contour, size, major vessels and respective hollow systems (e.g., pelvi-caliceal system, gall bladder and bile ducts). Measurements are essential: liver size is defined by the length measurements; the spleen is measured obliquely in the axis of the largest length defined by the hilar vessels and the pancreas is usually assessed in a slightly oblique axial section of the median upper abdomen through the organ’s longitudinal axis. The kidneys are always documented longitudinally and transversely, including respective diameter measurements that are used for renal volume calculation (applying the standard ellipsoid equation) [1,2].
These required images represent the minimum documentation; any pathology or specifically addressed query (e.g., ‘appendicitis?’) widens the investigation and documentation needs. All pathology must be documented in two orthogonal planes and these images suffice for documentation only; the investigation itself includes a through sweep throughout every organ entirely in two perpendicular planes with all intrinsic functional-dynamic information.
Specific queries & conditions in pediatric abdomen assessable by US
■ US in the child’s GI tract Typical conditions of the neonate & young infant
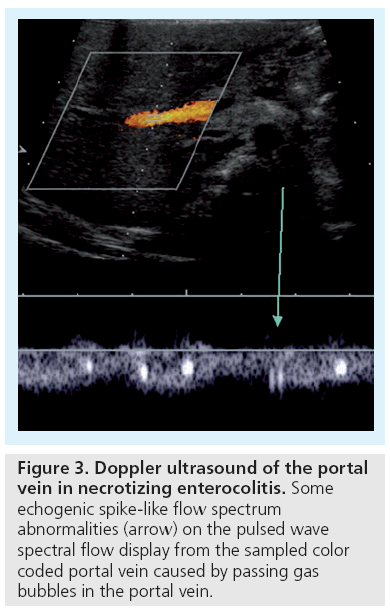
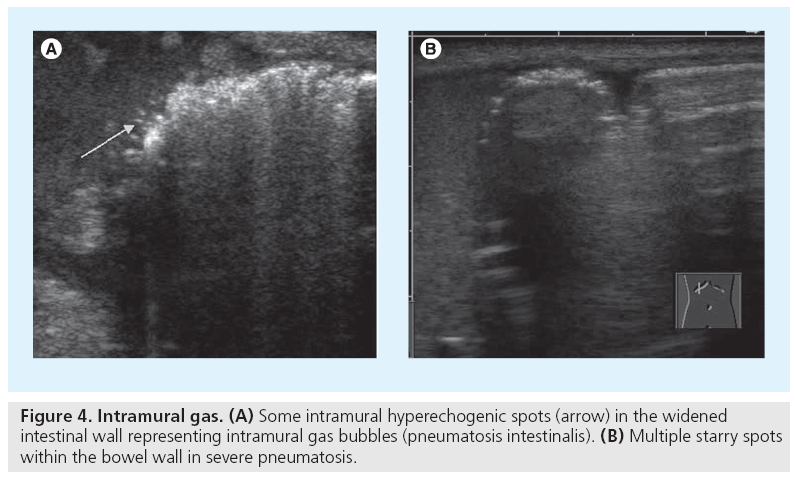
Necrotizing enterocolitis (NEC) is a life threatening condition, particularly in preterm infants, with abdominal distention, feeding intolerance and bloody stools [3]. Abdominal plain films are standard, but they become diagnostic only in a relatively late phase of the disease. Skilled dedicated US has gained importance, by visualizing bowel wall thickening and free fluid as well as hyperemia. Moving echogenic gas bubbles within the portal vein and its branches are a typical, though late US feature, convincingly confirmed and documented by the typical signal spikes in the Doppler spectrum (Figure 3) [4,5]. The neonatal bowel wall is evaluated using a highresolution linear probe; possible wall thickening or even intramural echogenic spots representing intramural gas can be detected (Figure 4). As these findings may occur segmental, a thorough but gentle examination of the entire accessible abdomen is mandatory. Whenever there is wall thickening use CDS to visualize the vasculature and to assess potential (inflammatory) hyperperfusion by consecutive spectral flow analysis; the absence of depictable wall vasculature even with proper low CDS settings may indicate necrosis and consecutive risk of perforation; usually the wall structure is altered as well in these bowel portions, with lack of peristalsis [6]. Then a thorough search for free peritoneal air becomes mandatory. The classical spot to find free air (in supine position) is the highest area between liver and abdominal wall next to the midline. Avoid transducer pressure not only to avoid pressure on the potentially painful abdomen, but also to avoid missing small amounts of trapped air that can be displaced by compressing this narrow space and thus be missed. US is used not just for an initial assessment in a clinically suspicious situation, but is useful for follow-up too, particularly whenever symptoms get worse, reducing the need for frequent abdominal plain films [7,8]. Note that intestinal pneumatosis is a typical sign for NEC, but not pathognomonic.
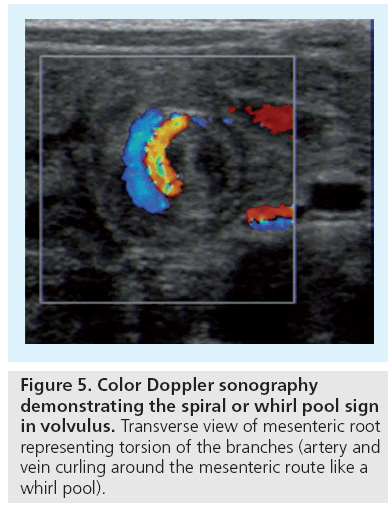
Midgut volvulus is the other life threatening emergency situation, also important as a differential diagnosis of NEC. This typical neonatal condition, however, can occur at any age and is related to predisposing bowel malfixation or malrotation. Symptoms usually have an acute onset unless there is partial, chronic or intermittent volvolus. US can reliably diagnose acute midgut volvolus by depicting a mass-like structure at the mesenteric root consisting of spiral shaped echoic and anechoic structures representing the twisted bowel lops with the respective mesentery and mesenteric veins that curl around the central mesenteric artery (‘spiral or whirlpool sign’) (Figure 5) [9,10]. In later stages one can observe US features similar to NEC – bowel wall thickening, lack of identifiable perfused bowel wall vessels, intramural and portal venous gas. Thus – in neonates and infants – assessment of the mesenteric vessels is mandatory in every case of an acute abdomen or with clinically obstructive (upper) GI symptoms. The normal topographic position of the superior mesenteric artery (SMA) and vein (SMV) is with the SMV on right side of the SMA – as with the abdominal aorta and inferior cava vein. A SMA positioned on the patients’ right side of the SMV hints at malrotation, but this malalignement is not diagnostic either for malrotation or for an acute volvulus [11]. Only the whirlpool sign with the signs of upper GI obstruction (dilated, often fluid filled upper duodenum) and the dilated curling SMV loops are indicative for a volvulus; in other cases emergent fluoroscopic upper GI studies have to be performed.
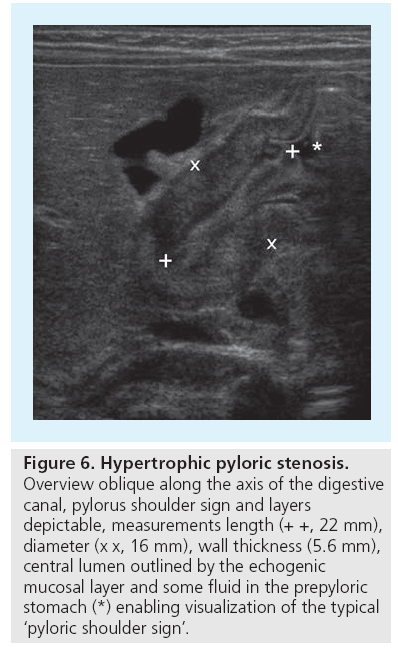
A relatively common condition in infants where US has replaced fluoroscopy is hypertrophic pylorus stenosis (HPS). Beside the typical clinical appearance, the palpable ‘olive’ at the gastric outlet and the typical laboratory constellation of alkaline vomiting, US has replaced fluoroscopy and become the gold standard for HPS assessment. The US examination using a linear probe focuses on demonstrating the pylorus and its function, but always includes US of the entire abdomen to rule out any other reason of obstruction, particularly malrotation or chronic volvulus or other conditions that may cause vomiting in infants, such as severe urinary tract infection with associated urinary tract malformations. It is best to start US with a fasted baby (one thus may also assess the amount of residual stomach filling as a result of the gastric outlet obstruction). The pylorus is usually visualized on a sagittal paramedian section through the liver, using the anterior wall of the stomach as a guide structure. If gas hinders access to the pylorus the baby can be turned into a right lateral decubitus position, sometimes a dorsal acquisition can be helpful. The pylorus can nearly always be sufficiently assessed; otherwise feeding the baby may open up an US window and additionally will allow for functional assessment. Assessment of the pylorus includes measuring the entire length, diameter and thickness of the (muscular) wall (Figure 6). Upper limits that can be used as cut-off values are 15–20 mm in length, 12–15 mm in diameter and 3–4 mm in wall width [12–14]. But these measurements are not the only criteria; some cases with pyloric enlargement may be due to intermittent spasm or thickening by mucosal swelling (e.g., after prostaglandin medication). In these situations functional observation is most helpful: observation of peristalsis and existing passage of stomach content through the intermittently open digestive canal with sufficient filling and distension of the duodenal bulb rules out HPS [15]. If US findings are evident, diagnosis is easy at first glance; in borderline cases, such as in early stages of the disease or in very small and younger neonates, a conservative observation approach with US follow-up will usually work [16,17].
Figure 6: Hypertrophic pyloric stenosis. Overview oblique along the axis of the digestive canal, pylorus shoulder sign and layers depictable, measurements length (+ +, 22 mm), diameter (x x, 16 mm), wall thickness (5.6 mm), central lumen outlined by the echogenic mucosal layer and some fluid in the prepyloric stomach (*) enabling visualization of the typical ‘pyloric shoulder sign’.
Typical conditions of later childhood
Intussusception is typically a condition of the slightly older child (aged 1–5 years), but is another pediatric specific query. The most significant clinical symptom is bloody diarrhea, with abdominal pain or cramps that may change quickly [18,19]. As long standing intussusception will endanger the bowel, this is an emergency situation, and can be sufficiently addressed (and treated) by US. As in all cases with abdominal pain a ‘sonoscopic’ overview of the entire abdomen is performed. The bowel is assessed using a linear probe and gentle graded compression, focusing on visualization of the coecum and the ileocecal junction. As in intussusception the pseudotumorous bowel is usually positioned slightly higher and more medially, bowel US starts in the upper right quadrant using a transverse section; from there one moves caudally. The most lateral bowel loop is usually the ascending colon, nicely identifiable by the haustrae, best seen after turning the transducer into a longitudinal plane. Always include a look at the small bowel and the mesenteric lymph nodes as well as a search for potential free fluid.
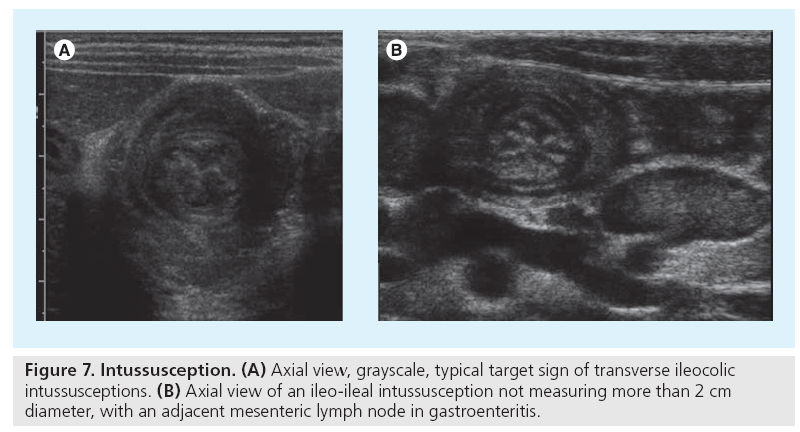
The typical appearance of ileocecal intussusception includes the (in longitudinal planes) pseudo-kidney or donut sign and the target-like appearance formed by the inner small bowel (the intussusceptum) and the outer receiving colon loop (the intussuscipiens) in cross section (Figure 7) [20]. This triple layer of bowel wall at the lead point most distally is followed by additional layers more proximally formed by some mesenteric fat and enlarged lymph nodes. Potentially entrapped fluid and bowel wall thickening can be observed, findings which in some publications are linked to a longer duration and restricted reducibility [21]. Only free peritoneal air and potentially a necrotic bowel wall (no vessels depictable by CDS in the intussuscepted loop) pose a contraindication for an attempt at image-guided reduction [22–24].
Image-guided reduction is usually performed by fluoroscopy and air or fluid enema; in some institutions and with mild or diagnostically unclear cases US hydrocolon (i.e., US with body-warmed saline enema) is performed routinely and successfully, allowing for repeated attempts without any radiation burden. Note that for any reduction procedure proper handling (analgo-sedation and fluid balance, for example) and monitoring are mandatory. As relapse can occur, particularly in the first 24 h, observation and potentially repeated reduction may become necessary, also if the initial reduction was incomplete. The smaller ileo-ileal intussusception (measuring less than 2 cm diameter) usually resolves spontaneously and does not need treatment unless fixed (e.g., in polyps or tumors). In these cases, where the image-guided procedure is less successful, surgery will often become necessary.
The most common differential diagnoses in this query are gastroenteritis and mesenteric lymphadenopathia. In these conditions one usually can find some free fluid without any echoes or sedimentations, some fluid-filled bowel loops with hyperperistalsis and some or more enlarged mesenteric lymph nodes. The task of US in these conditions is to try to rule out appendicitis, intussusception or Crohn’s disease. Different to adults, in children enlarged mesenteric lymph nodes can cause long-lasting abdominal pain, even when all other symptoms of an intestinal infection have disappeared. Persisting large nodes are typical for campylobacter infection or yersinosis, though not pathognomonic. Differentiation of mesenteric nodes to non-Hodgkin lymphoma and Burkitt lymphoma (if associated with the ileum) is improved by high-resolution linear transducers that enable demonstration of the disrupted typical nodal US-anatomy lacking the central sinus echogenicity
Meckel´s diverticulum (MD) is another entity potentially associated with intussusception. It is a rare congenital condition, a remnant of the omphaloenteric duct, commonly with gastric mucosa content, and represents a tubular diverticulum of the small bowel. Secondary inflammation, bleeding from gastric mucosa or obstruction due to bands to the umbilicus can cause problems. US appearance is similar to any other bowel loop, so one cannot differentiate MD without superposed pathology. If there is a prominent gastric mucosa, the respective layer can become wider; if MD has either a narrow or no connection with the gut’s lumen it may get a cystiform appearance [25]. The knowledge of its sonomorphologic appearance is necessary as it may represent a lead point in intussusception, as well as for differential diagnosis of appendicitis [26].
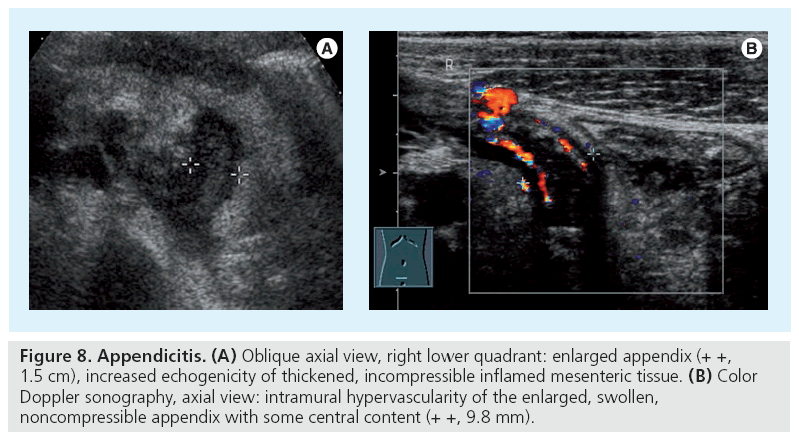
The differential diagnosis of abdominal pain, particularly in the lower right quadrant, includes appendicitis. US is often performed before surgery, particularly in girls, to rule out other conditions, for example, of the ovary, notably when the patient shows equivocal symptoms. Additionally US can often demonstrate the appendix, particularly if inflamed. The leading structures for searching the appendix are the coecum and the iliac vessels, which may be more difficult when hidden behind stool-filled meteoristic bowel with unplugged shadows. Using gently graded compression the appendix can be visualized in up to 90% [27–29]. Taking measurement of the diameter is good, but there are mimics and pitfalls; a stool-filled appendix can measure more than 6 mm in diameter but with normal wall it does not necessarily represent acute appendicitis. Typical features of acute appendicitis are an enlarged, a stiff, noncompressible appendix with a thickened wall, an appendicolith and free fluid next to coecum or in the Douglas space (Figure 8); another typical finding is the increased echogenicity of the adjacent mesenteric fat. Also look for some shinning bright spots or reverberation echoes which may indicate perforation with free peritoneal or (if covered) subperitoneal air or an abscess. One may also use CDS for evaluating the vasculature not only of the wall but also of the mesenteriolum, hypervascularity with spectral Doppler signs of (diastolic) hyperemia may be seen and thus may enhance the diagnosis. Pitfalls include a nonenlarged appendix after perforation or a thin and nonstructured wall in necrosis [30]. Note that in older obese children US may fail and – depending on symptoms and laboratory parameters – CT (or, in future, MRI) may become necessary.
Figure 8: Appendicitis. (A) Oblique axial view, right lower quadrant: enlarged appendix (+ +, 1.5 cm), increased echogenicity of thickened, incompressible inflamed mesenteric tissue. (B) Color Doppler sonography, axial view: intramural hypervascularity of the enlarged, swollen, noncompressible appendix with some central content (+ +, 9.8 mm).
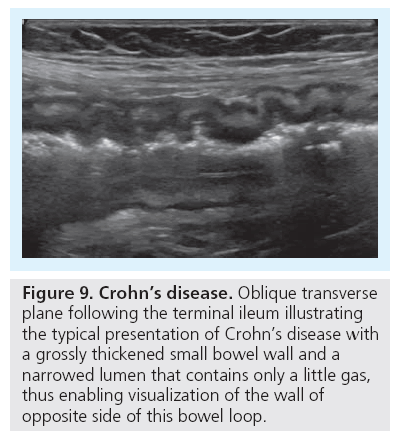
Finally, Crohn’s disease needs to be addressed as an important differential finding that can be nicely diagnosed and followed using US. It commonly affects adolescents, who tend to have a longer history. The typical location is the terminal ileum, but colon (especially the sigma) may be involved, too. The thickening of the bowel wall as well as the secondary mesenteric reaction usually allow for easy depiction of the affected bowel segment. One can discriminate the different layers of the intestinal wall: the low echogenicity mucosa, the high echogenicity of the submucosa and the low echogenicity of the muscularis propria, causing a five interphase US image [31–33]. CDS with spectral flow analysis allows for visualization and assessment of bowel wall perfusion, which correlates with inflammatory activity and thus may be used to guide therapy (Figure 9) [34,35].
Figure 9: Crohn’s disease. Oblique transverse plane following the terminal ileum illustrating the typical presentation of Crohn’s disease with a grossly thickened small bowel wall and a narrowed lumen that contains only a little gas, thus enabling visualization of the wall of opposite side of this bowel loop.
Cystic abdominal diseases
In infants cysts have a completely different differential diagnosis than in adults. In adults cysts are – increasingly with age – a normal finding in the liver and the kidneys; also cystic pancreatic tumors may occur. In infants different diseases have to be considered and a cyst is only called a ‘simple normal cyst’ after ruling out other conditions after follow-up. Kidney, liver, spleen or pancreatic cysts may be associated with autosomal recessive or dominant polycystic kidney disease. Or they may represent a duplication cyst, which can occur anywhere in the context of the digestive tract throughout the entire abdomen. These cystic tubular or even nodular looking lesions are congenital findings and may grow due to secretion [36]. They may not cause any symptoms and therefore represent an incidental finding; but special location, such as next to the pylorus, and very large cysts can cause transit problems [37]. US appearance also includes cysts with some sedimentation; additionally and pathognomonically the wall of these cysts exhibits the ‘gut signature’ (i.e., the typical layers of intestinal wall are visible when exploring with a linear probe). In large duplication cysts the wall can be thinner; then differentiating them from other cysts such as a mesenteric cyst may become more challenging. In the pelvis, differentiation from ovarian cysts can cause some problems, particularly if they have a thicker wall, sedimentations from hemorrhage and no typical daughter cysts. Cystic lymphangiomas consist of a group of cysts separated by a small septa and – though potentially with internal echoes due to bleeding – are usually easily identified.
US of the urogenital tract
■ Basic considerations & requisites for US of the child’s urogenital tract
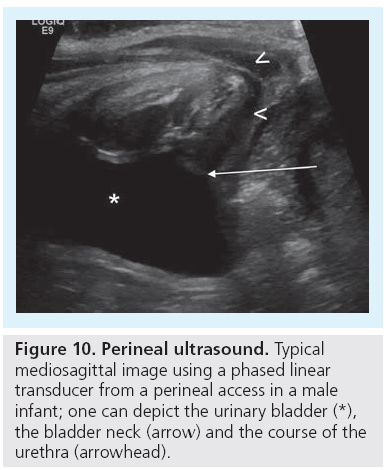
Ultrasound of the kidney is included in a standard general abdominal scan, but the detailed examination of the urinary tract has some special features that are standardized in existing procedural recommendations [38,39]. The standard urinary tract US requires well hydrated infants and children, if possible with a filled bladder, as any effort to assess the urinary tract can be useless in a collapsed collecting system. Usually, one starts with a transversal scan throughout the bladder, the bladder wall and the content. Assessment of the distal ureters in both planes with documentation of the urine inflow into the bladder by CDS is a good way to demonstrate ureteral patency, estimate hydration and identify asymmetric urine production or drainage, thus reflux can be counterproductive. Bladder volume calculation and bladder wall thickness measurements are obligatory. Both kidneys are imaged with sagittal and transversal scans, measurements are taken for mandatory volume calculations which are compared with age- or weight-related growth charts [40,41]. After detailed assessment of the renal parenchyma and its corticomedullary differentiation the renal artery and vein are identified by CDS. Any potential dilatation of the renal pelvis and/or calices has to be measured in a transverse plane. Additionally, evaluation after voiding is very important, checking for residual urine as well as to detect changes to the collecting system’s width. The advanced urinary tract US technique includes an additional amplitude-coded CDS (aCDS; Power Doppler), a perineal access and potentially a contrast-enhanced voiding urosonography as well as 3D-US (Figure 10) [42–45].
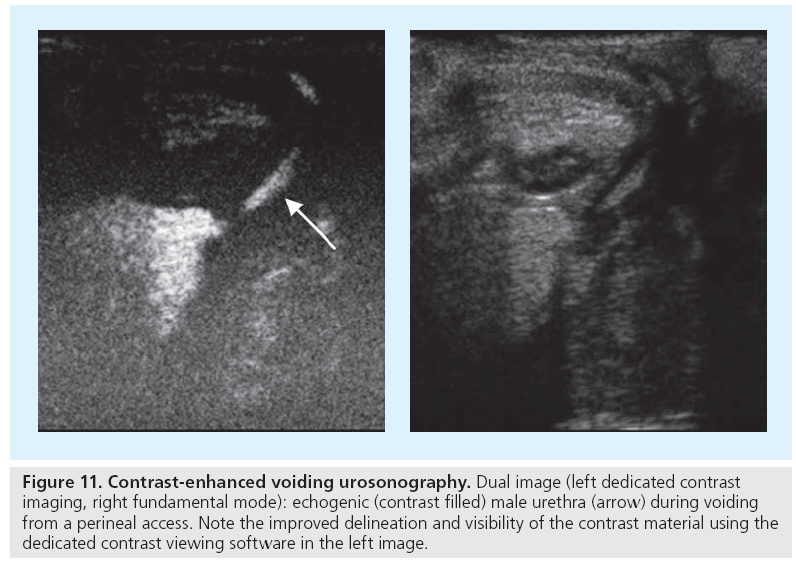
Contrast-enhanced voiding urosonography (ce-VUS) has been shown to be reliable for detection of vesicoureteral reflux (VUR) and is now considered an established imaging modality for screening purposes, for first exams in girls and for follow-up whenever available [46,47]. In newborn males conventional voiding cysto-urethrography (VCUG) is preferred to grant a reliable assessment of the urethra; furthermore, VCUG should be performed in preoperative settings to reliably assess the entire ureters’ anatomy and not to miss potential bladder diverticula that might be missed if posing only at the end of voiding and thus restricted US access [48]. At present, US contrast agents are not registered for intra-luminal or pediatric use, as often in pediatric medicine. Thus, this off-license use needs an informed consent; however, with respect to the radiation delivered by VCUG, ce-VUS appears justified for the patient groups defined above. The procedure is standardized, recommendation on its use and the respective VUR grading have been published [38,39,49]. As with VCUG, catheterization is needed; in our experience it is better to place the catheter before coming to the examination room to avoid agitated infants. Then a basic urinary tract assessment is performed: saline and then intermittent contrast infusion at physiologic filling pressure is started using a stopcock between catheter and saline infusion (always use plastic containers), alternating observing the bladder, the ureters and both kidney. During and after voiding the urethra (and vagina), the distal ureters and the renal pelvocaliceal system have to reassessed (Figure 11). Modern equipment with dedicated contrast viewing techniques or acoustic stimulated high mechanical index techniques are recommended, particularly if only few bubbles are refluxing, giving only little contrast; these methods also allow for visualization of intrarenal reflux.
Figure 11: Contrast-enhanced voiding urosonography. Dual image (left dedicated contrast imaging, right fundamental mode): echogenic (contrast filled) male urethra (arrow) during voiding from a perineal access. Note the improved delineation and visibility of the contrast material using the dedicated contrast viewing software in the left image.
■ Value of US in hydronephrosis & obstructive uropathies
Prenatal US is performed as a general screening in many countries and a growing number of often mild findings in the urinary tract are submitted for postnatal evaluation, mostly some degree of fetal hydronephrosis (HN). To standardize the degree of HN, a sonographic HN grading system has been developed and proposed that derives from the Society of Fetal Urology classification [50]. In HN 1° only the renal pelvis is seen, in HN 2° some normal calices can be depicted. In HN 3° all calices are dilated, with loss of fornix shape and papillary impression, and HN 4° defines those with significant pelvocaliceal dilatation and narrowed renal parenchyma – compared with the nonaffected side, often corresponding to a so-called ‘partial obstruction’. HN 5° is used in some places to define a gross dilatation with only a narrow parenchymal rim [38,39,51]. Considering the physiologic adaptation of renal function after birth we tend to investigate the newborns only at or after the first week of life to prevent false negative findings. Again, detailed imaging algorithms have been proposed on how and when to image children with fetal HN, based on their fetal staging and postnatal presentation [38]. Mostly low-degree HN in an otherwise normal neonatal urinary tract is unimportant, may even vanish, but rarely may also be associated with VUR, particularly if a pelvic wall thickening, a varying dilatation of the ureter and a change in pelvic diameter can be observed. These features, which may indicate follow-up or even additional imaging in a clinically symptomatic child, are summarized as ‘extended US criteria’ [52]. High-degree HN, however, is often associated with obstructive uropathy; the aim of the early imaging and assessment is not only to confirm or establish the diagnoses and differentiate high-degree dilating VUR, but also to be able to prevent avoidable deterioration of renal function and growth potential [53].
The most severe end point of fetal obstructive uropathy with dysplastic cystic damage to the renal parenchyma is multicystic dysplastic kidney disease. It is usually detected by prenatal US screening. In the area of the kidney one or a couple of large cysts are usually found, with or without recognizable connections and potentially a central remnant of a dysplastic parenchyma; sometimes remnants of the dilated pelvocaliceal system may also be seen. Although most of these spontaneously shrink and even resolve, some of them may grow and then cause mass effect to adjacent structures, requiring nephrectomy [54–57]. CDS allows depiction of some residual perfused parenchyma, which then indicates that those are at risk for complications, such as mass effect, inflammation or malignant conversion [58]. US follow-up is performed not only to monitor the development, but also to detect complications and to observe the contralateral healthy kidney.
Uretero-pelvic junction obstruction (UPJO) is the most common finding in neonates with high-grade fetal HN. The reason for the dilatation of the pelvocaliceal system is a constriction at the level of the uretero-pelvic junction, causing a typical peaking of the renal pelvis caudally towards the proximal nondilated ureter. The big challenge in UPJO is not the diagnosis but the decision, whether and when to send the child to surgery. There is no reliable imaging-based a priori pro futuro assessment in terms of which kidney will deteriorate and thus might benefit from surgery, the development during course of disease as described below is used to monitor these children. Both the grade of dilatation and the situation of the parenchyma may help to schedule the next follow-up. If an initially normal corticomedullary differentiation becomes reduced in assosiation with thinning of an increasingly echogenic parenchyma, deterioration is likely even in relatively unchanged distention of the pelvocaliceal system and additional functional imaging becomes necessary. Remember that all these parameters may vary with hydration: ‘low tide’ appears with a smaller pelvocaliceal diameter, a thickened pelvic wall and a constant resistive index from central to periphery, whereas ‘high tide’ exhibits larger pelvocaliceal diameters, a thinner pelvic wall and an elevated resistive index in the periphery – particularly compared with the opposite healthy side [56,57]. Note that decreasing renal function can mimic improvement by exhibiting less dilatation and a larger parenchymal width even on diuretic US – thus dilatation does not equal obstruction and functional imaging (usually diuretic TC99m MAG3 renal scintigraphy or functional MR-urography) is always necessary for treatment decisions. Always try to visualize the renal artery and their main branches. Often additional renal arteries can be associated with UPJO, potentially the pulsation of a vessel close to the pyeloureteric junction can induce and boost a changing obstruction. In such cases we tend to arrange shorter follow-up intervals. Also note that at any time UPJO predisposes for occurrence of urolithiasis and infection, which itself will aggravate the obstructive component.
Postoperative US follow-up after pyeloplasty is important to document good perfusion of the entire kidney in the early postoperative phase, here aCDS is particularly helpful. As at this time the pelvis may be collapsed due to pigtail drainage and some reshaping of the pelvis will have happened during surgery, dilatation is not useful as an indicator for success; sometimes air can also be seen in the pelvocaliceal system. After removal of the drain the pelvocaliceal system may physiologically become as wide as before surgery and will more or less diminish only over time.
For the future the 3D-US-based volume calculation of the renal parenchyma in hydronephrotic kidneys may improve US followup potential by providing reliable split renal size assessment not only in normal-shaped nondilated kidneys [59].
Primary obstructive megaureter (POM) is the other common congenital obstructive uropathy. VUR assessment is essential for establishing the diagnosis, only reliably achieved either by VCUG or if available ce-VUS to exclude VUR as differential. In POM with a prevesical dysplastic segment a variable part of the distal ureter may be narrowed. US can visualize the ostium and estimate its patency using CDS for demonstrating the ureteral inflow jet and the width of the ureter as well as its wall. As in UPJO, sufficient (standardized) hydration (and a full bladder) is important for any US assessment. Observation of the ostium is necessary to catch intermittent gaping as seen in VUR or combined refluxive-obstructive POM; other reasons for ureteral obstruction such as an ureterocele or an ectopic insertion should not be missed. M-Mode enables documentation of the missing peristalsis in the dysplastic part: hyperperistalis in severe obstruction or lack of peristalsis in a previously active POM as a sign of decompensation are the common findings [60]. only the effects on the kidney with growing dilatation and decreasing function or recurrent infections indicate surgery [61].
Finally, there are congenital distal or lower urinary tract obstructions. The most important entity is a posterior urethral valve. Severe forms of posterior urethral valve present in utero with huge dilatation of the entire urinary tract and parenchymal dysplasia, commonly of both kidneys. The latter is sonographically seen by fading of corticomedullary differentiation, increased parenchymal echogenicity and cysts. There may be urinary ascites or pararenal urinoma by pop-off caliceal rupture. The POM can be refluxive or obstructive or even mixed, and partially combined with intermittently posing diverticula in the vicinity of the ostium. The bladder wall is markedly thickened, with trabeculation and (pseudo-) diverticula. US can give a clue to the diagnosis by visualizing the open bladder neck and dilatation of the posterior urethra during voiding efforts, best seen from a perineal access (Figure 10). Early postnatal imaging is recommended to promote immediate diagnosis in order to prevent severe early sequelae of the condition [51]. In cases with only mild valves or semicircular rings as well as neurological lower tract obstruction these findings are variable and even distinct, thus a conventional VCUG with pressure measurements is considered mandatory.
■ Role of US in VUR & urinary tract infection
In infants VUR of varying degree is a frequent problem; however, VUR often resolves spontaneously without sequelae. Thus, imaging algorithms have changed and US preselection for more invasive tests has become more important. For US a proper bladder filling is more important than hydration; the best set-up would be a full bladder in a fasting infant. Conventional US can only detect several indirect signs, for example, an asymmetric urinary jet into the bladder, a lateralized position of the ostium, intermittent widening of the prevesical distal ureter particularly at full bladder or during voiding attempts that may exhibit the ‘urothelial sign’, an obvious change of the pelvocaliceal diameter and potential effects to the renal parenchyma. Careful renal volume calculations are essential to detect growth retardation secondary to scarring or ‘reflux nephropathy’. Look for scars by observing any region for subtle decrease of corticomedullary differentiation or parenchymal narrowing as well as caliceal clubbing, more common in the polar areas. aCDS may will sometimes improve US potential in assessing gross renal scarring, but cannot compete with the present imaging gold standard for scarring, which is Tc99m DMSA scintigraphy. aCDS is device and setting dependent. It also takes a lot of experience and finesse to achieve meaningful results that clinicians will believe. Sometimes, particularly with reflecting particles within the urine, VUR can be shown by CDS [62].
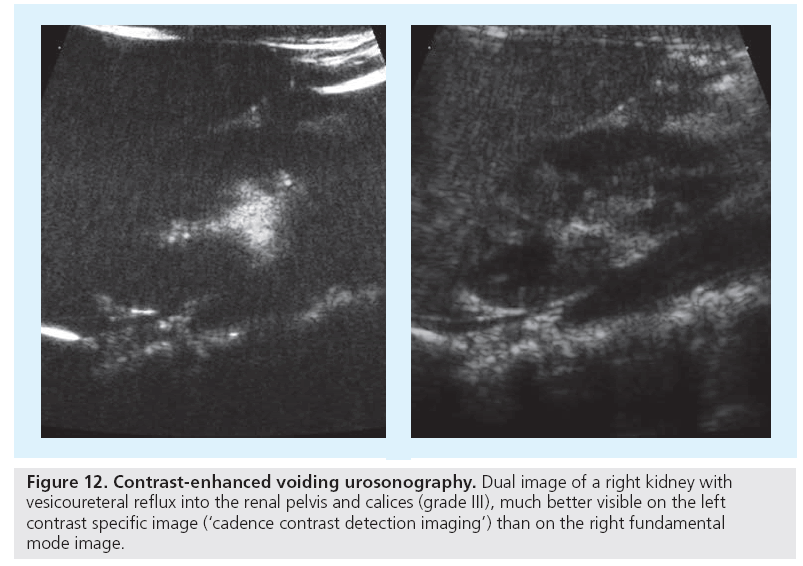
In the future, fusion of reliable aCDS or intravenous ce-US data with 3D-US acquisitions might allow an improved US assessment of split renal function and renal scars in reflux disease, but this still is an auspicious goal. Finally, virtual real-time 3D-US-based cystoscoypy may improve US potential in children with suspected VUR by enabling a functional dynamic observation of the ureter’s ostium, improving visualization of ostium lateralization and gaping, both features that often combine with VUR and thus may help to more precisely indicate the need for VCUG or ce-VUS (Figure 12) [63].
Urinary tract infection is frequent and thus important in infants and children. Urinary tract infection is diagnosed clinically and by laboratory, but urinary tract infection may be linked to congenital urinary tract malformation and renal involvement may lead to scars and long-term sequelae. While the importance of VUR in this clinical scenario has declined, other risk factors have been acknowledged and the role of imaging for pediatric urinary tract infection has consecutively changed. With this change, the importance of reliable fetal screening and high-quality US for symptomatic children has increased. The task of imaging in general and US in particular is to detect early potentially associated urinary tract malformations and dysfunctions (including VUR), avoiding harm to the kidney. Thus, differentiation of upper versus lower urinary tract infection has become the primary task [64]. More or less urinary tract infection-specific US findings include floating echoes within the bladder, bladder wall thickening, increased renal size and echogenicity, focal disruption of corticomedullary differentiation or even severe pyo-(hydro-) nephrosis. Complicated urinary tract infection may be present, with associated stones or fungus balls, xanthogranulomatous pyelonephritis, focal necroses or abscess formation, as well as pseudotumorous lobar nephronia. Modern methods improve US potential in this condition: harmonic imaging improves depiction of necrotic areas and abscess formation, and subcapsular and pararenal fluid and effusions can be seen and differentiated more easily. aCDS is a very sensitive method to look for focal inflammation in an affected kidney [65]. With this reliable performance of modern US from newborn to adolescents the need for scintigraphy has decreased and CT is practically obsolete for assessing urinary tract infection in childhood; for equivocal US findings and some rare differential diagnoses MR should be used whenever available.
As children without involvement of the upper urinary tract have a low risk of suffering renal damage and long-term sequelae, VUR assessment is not routinely indicated in this scenario, whereas all children with particularly severe or prolonged and complicated or recurrent upper urinary tract infection should still undergo VUR assessment [39].
■ US in other common & important childhood UGT conditions
The absence or agenesis of one kidney leads to some compensatory hypertrophy of the other side only after the neonatal period. If only one kidney is detected, a thorough search for an ectopic kidney or renal ectopic bud has to be performed; US can establish the diagnosis in most cases. Note that in any case of a single kidney or an ectopic renal bud a detailed and early examination of the internal, particularly female, genitalia has to be performed, as associated malformations of the uterus and ovary (on the same side) are common and can best be seen sonographically in the neonatal period due to stimulation of these structures by maternal hormones – later the diagnosis will become increasingly challenging. In male babies associated cystic abnormalities of the ipsilateral seminal vesicle may occur. 3D-US – as in adults – has been shown to be very beneficial for assessment of uterine malformations and perineal (3D/4D) US as part of a (potentially contrast-enhanced) sonographic genitography certainly is a promising future option.
Childhood stone disease is rare and has a large regional variability. But even in babies stones can arise more frequently than most adult radiologist believe. As well as infection, neonatal problems with renal failure and diuretic as well as antibiotic treatment and hereditary disease may cause uro- and nephro-lithiasis in infants. In addition, vitamin D overdose, dehydration, familiar hypercaliuria and urinary tract obstruction can facilitate precipitations that may cause even a complete obliteration of renal pelvis. The US features are similar as in adults, usually an echogenic focus of varying size with shadowing. On CDS one may observe the ‘twinkling artifact’ when using a relatively high sound energy with a focus zone at the level of the suspected stone and the maximum available scale range [66,67]. To verify stones in extended calices is simple, but it can be tricky to differentiate these from papillar calcifications in collapsed systems.
In children, different imaging algorithms and methodical application rules apply than in adults [51,68]. As in most cases US can detect the calculus in the pelvocaliceal system or the proximal and distal ureter, often even in the mid-ureter segment by following the dilated ureter towards the bladder with suitable and subtle transducer pressure. Of course, there are some sonographically dark areas between the crossing of the iliac vessels and the window behind even a sufficiently filled bladder; hence it is very important to evaluate the urine inflow into the bladder. No ureteric jet with a dilated proximal and normal size distal ureter in the respective clinical setting will give you all the information needed to initiate treatment. With time and therapy – in contrast to adults – you will be able to demonstrate the cause of colics and pain in the distal ureter or bladder on follow-up; stone CT will only exceptionally become necessary.
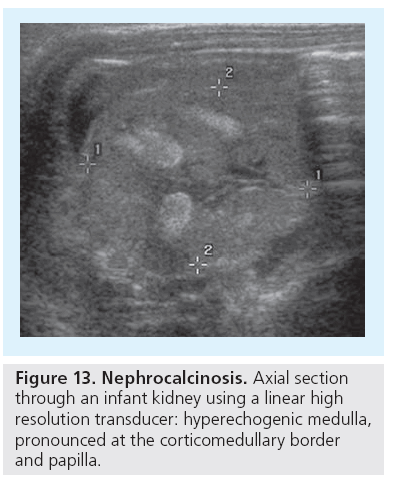
Nephrocalcinosis (NC) is another condition that is best addressed (and only) by US; both the type (medullary or cortical or mixed/global) as well as the grade (I to III) can be established by US, which is much more sensitive than plain film [69]. The typical features of medullary NC are initially some increased echogenicity at the corticomedulary bounder. Note that this finding can be mimicked by harmonic imaging, which increases the echogenicity of this junction zone and thus the clear and pronounced border may be mistaken for early NC changes (‘pseudo-NC’). The physiologically increased echogenicity of the neonatal medulla is a normal transient phenomenon and should not be mistaken for NC, or the typical pattern of ‘sickle cell nephropathy’ (Figure 13).
Potential of modern US in the pediatric liver
■ Normal findings & general remarks
As in adults one can differentiate between diffuse and focal alterations of the liver parenchyma in children, too. This assessment, however, markedly benefits from using high-resolution US techniques and linear transducers, particularly in younger children and infants and for assessing the biliary system. Size assessment is somewhat cumbersome: orienting values are obtained in the maximum sagittal length in a median section and the anterior axillar line and these are correlated with the child’s height. With enlargement the contour of the lower liver margin becomes rounded. Always assess the hepatic vessels: the portal should exhibit a laminar flow with an average velocity of 20–30 cm/s and some modest variance during breathing. The diastolic flow of the hepatic artery varies with nutritional status. Most important is the triphasic bidirectional flow undulation in the liver veins, as with increasing intrahepatic pressure and resistance the backflow of blood into the liver during atrial contraction will diminish and this pattern is lost. The biliary system is another important part; particularly congenital malformations of the bile ducts or secondary disease such as cholecystolithiasis have to be addressed accordingly.
■ Congenital liver conditions
Biliary atresia is an important entity in neonates, as early treatment is the key for success. In neonates transient jaundice is common. If jaundice persists, laboratory testing is mandatory for diagnosing endocrine, metabolic or infectious causes. US findings are usually rather unspecific, just showing an increased or altered echogenicity and size. A hint towards biliary atresia is the lack of or a very small gall bladder. Sometimes one can find the ‘triangular cord sign’ in the porta hepatis; this echogenic triangular-shaped spot or band close to the central portal vein represents a fibrotic remnant from the bile duct and is almost diagnostic for biliary atresia [70]. Rarely one can see signs of cirrhosis already in the neonate, this is more often linked to other (systemic metabolic) disease such as thyrosinosis.
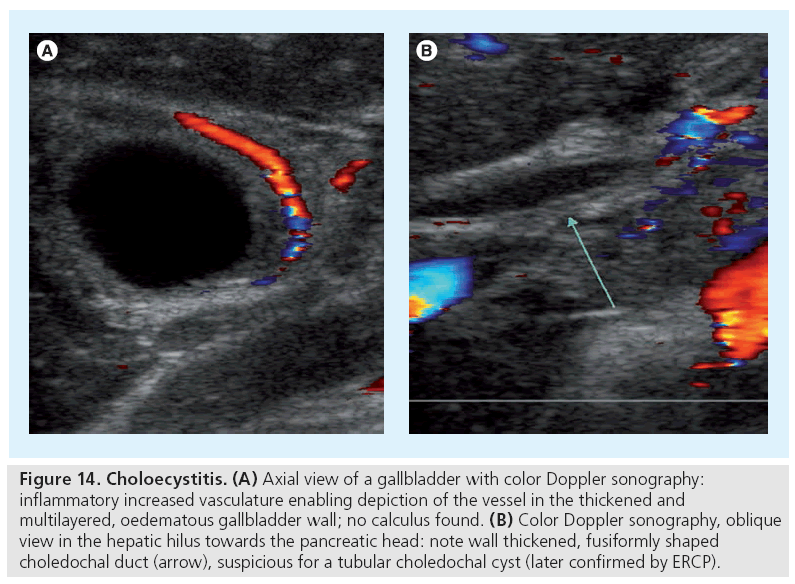
Choledochal cysts are another congenital malformation, but more commonly manifest later in infancy. US will depict the bile duct dilatation or a cystic structure arising from a bile duct; this is sometimes picked up as an incidental finding. A choledochal duct diameter of more than 2 mm should raise suspicion. Once this diagnosis is suspected, MR cholangiopancreatographie or conventional endoscopic retrograde cholangiopancreatographie as the gold standard is performed for confirmation. Note that an acute noncalculous childhood cholecystitis during childhood may be secondary to a choledochal cyst, as well as (recurrent) cholangitis (Figure 14).
Figure 14: Choloecystitis. (A) Axial view of a gallbladder with color Doppler sonography: inflammatory increased vasculature enabling depiction of the vessel in the thickened and multilayered, oedematous gallbladder wall; no calculus found. (B) Color Doppler sonography, oblique view in the hepatic hilus towards the pancreatic head: note wall thickened, fusiformly shaped choledochal duct (arrow), suspicious for a tubular choledochal cyst (later confirmed by ERCP).
The most severe cystic malformation of the biliary tract is Carolis’ disease. US will reveal paraportal cystic structures or irregularly dilated intrahepatic bile ducts. These have to be differentiated from cystic liver changes and liver fibrosis as seen in ARPKD and ADPKD. Besides initial diagnosis, all these conditions will undergo repeated US follow-up for monitoring liver growth or early depiction of portal hypertension as well as fibrotic or cirrhotic transformation.
Fatty liver
The most common diffuse parenchymal abnormality is the fatty liver. There are several causes, from obesity and metabolic changes to toxic, particularly after chemotherapy. Morphologically the fatty liver shows an increased echogenicity and a decreased sonolucency. In case of a minor uneven fatty liver you find geographically distributed hyperechoic areas typically next to the gallbladder, the ligamentum falciforme or in segment 4, usually with sharp margins. Conversely, if the fatty change does not concern the entire parenchyma, one can delineate hypoechoic areas as isles of normal tissue. In storage disease with diffuse liver involvement the entire organ is enlarged and shows an increased echogenicity.
■ Liver masses
Focal liver lesions represent a small group of entities in childhood. Malignancy is very rare. Benign infantile hepatic hemangiomas (IHH) are seen most frequently [71]. These IHH differ from those in adults. Some of them involute quickly, some persist or even grow. Usually IHH are hyperechogenic, but this depends on fat content and histological composition (more vessels or more sinusoids). Small IHH usually cause no problem and are just observed; however, particularly in a subcapsular location they may rupture, for example, during birth, and cause severe acute bleeding. Always apply CDS to evaluate for an increased blood flow, though in capillary or low-flow hemangioma not much flow will be depictable. In the case of hemangiomatosis there are multiple lesions of different size, potentially causing early heart failure if severe shunting is present.
Liver hemangioendothelioma are commonly hypoechoic and well demarked, with an increased flow and secondary changes to the hepatic artery – thus they are more easily distinguishable by US and CDS [72,73]. They represent a histologically separate, biologically benign entity, but may – due to their origin ‘in the vessels’ – lead to metastasis.
The most important differential diagnoses in multiple hepatic nodules are metastases of neuroblastoma, leukemia or lymphoma or infection (e.g., abscess and fungus, for example). Many of these conditions are better distinguished by laboratory parameters and other sectional dynamic imaging such as MRI.
Primary liver tumors are rare in childhood. The typical hepatoblastoma occurs after 6 months of age and is mostly detected as a large liver mass at the time of diagnosis, with elevated a-fetoprotein levels. On US the mass is inhomogeneous and may contain calcifications or cysts and necrosis. Besides detecting the mass, US must focus on assessment of occlusion or invasion of the different vessels, mainly the inferior cava, portal and liver vein or the bile ducts. Other childhood liver tumors are rhabdomyosarcoma (of the bile ducts) or hepatic embryonic cell sarcoma, whereas the typical hepatocellular carcinoma of adults is rare and only occurs in children with predisposing conditions. Later in childhood, adenomas and focal nodular hyperplasia may occur, too. These lesions are often hard to detect and even more difficult to characterize by US. In future, intravenous. ce-US may become – as already established in adults – a helpful nonionizing US approach to improve lesion detection and characterization in children with these queries. Adenomas or focal nodular hyperplasia may occur particularly after chemotherapy or toxic damage.
US of other abdominal parenchymal organs in children
■ Pancreas
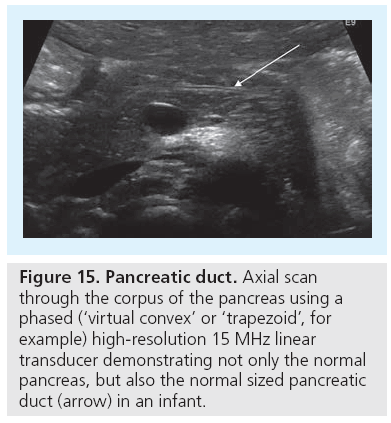
US of the pancreas is completely different in adults than in infants. In infants one can commonly visualize the entire organ, particularly if the stomach is filled with fluids, to create a superb US window to access the pancreatic tail. Even the pancreatic duct can often be visualized as a thin central band-like double contour when using high-resolution (linear) transducers, measuring not more than 1 mm in neonates and up to 2 mm in older infants (Figure 15). The depth of the corpus at its isthmus (ventral to the abdominal aorta) varies with age, starting from 10 mm and reaching 22 mm [74]. The parenchymal echogenicity changes with age, too.
Focal echogenicity alterations exist as a normal finding, but tumors or inflammatory infiltrations as well as hemorrhage are rare in children, as is malformation. Only traumatic, posttraumatic and suspected (post-)inflammatory changes usually indicate US.
■ Spleen
Similar to liver, the common nontraumatic pathology of spleen includes mostly cysts and hemangiomas. The most common reason for spleen US is, however, size assessment. But – similarly to the liver, due to the difficulty in taking reproducible standardized 2D measurements – these size measurements are very variable, inaccurate and unreliable, the size is often overestimated and reliable follow-up measurements are problematic, particularly if only subtle size changes have occurred (e.g., in short-term follow-up). Size and its changes often correlate poorly with any disease type, presence or activity.
More than any other organ the child’s spleen is prone to injury after blunt trauma. Note that in the early post-traumatic period even severe lesions may be missed – thus a follow-up US is mandatory. Post-traumatic arteriovenous fistula may occur, thus CDS is an essential part in these investigations. Intravenous ce-US will in future become more important for traumatic assessment of the spleen, promising to give similar reliability and accuracy as CT for early and reliable detection of spleen injuries. And – if inclusion of the entire organ into a 3D-US data set can be achieved – 3D-US may help to overcome US restrictions in size determination [75,76].
■ Adrenal gland
A notable obvious difference of US potential in children compared with adults is the superb visualization of the adrenal gland in neonates and infants, with their characteristic V-shaped appearance and well-defined differentiation of medulla and cortex [77]. A typical pediatric condition is the sometimes clinically silent perinatal hemorrhage, which may lead to mass effect, circulation instability or hormonal insufficiency [78].
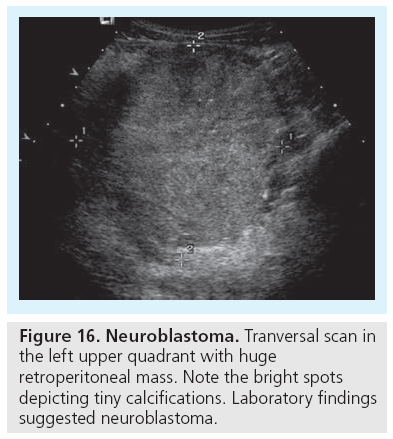
The other important condition is neuroblastoma, one of the common tumors in early infancy. Neuroblastoma can be very distinct but also huge. They usually exhibit a mixed low-density echogenicity with possible stippled echogenic calcifications with or without shadowing, which are nearly pathognomonic (Figure 16). Laboratory and genetic investigations help differentiation from Wilms’ tumor, if large and not easily characterized or differentiated by US (and other imaging) [79].
■ Retroperitoneum
Another area where US is more helpful in children than in adults – due to the better access and higher resolution, allowed by less fat and more water content of the tissue – is the retroperitoneum, with the large vessels and the lymphatic structures. Anomalies, some even without clinical sequelae, are depictable, such as a doubled cava vein, a retroaortal course of the left renal vein or aneurysms of the aorta or the renal, hepatic and mesenteric artery (e.g., in fibromuscular dysplasia); the depiction of the latter condition is of significant clinical impact due to the danger of rupture [80,81]. Also renal vein thrombosis in sepsis or dehydration or Wilms’ tumor is a typical US diagnosis in childhood, with US being superior to other sectional imaging [82]. And the depiction and follow-up of cystic lymphangioma or mesenteric nodes as described above is a domain of US throughout childhood. However, as in adults, particularly with increasing age and body weight, intestinal gas as well as poor cooperation, particularly in toddlers, may restrict US in the pediatric abdomen.
Conclusion & future perspective
The numerous options and enormous potential of modern dedicated abdominal pediatric US, listed and discussed above, indicates that US is the ideal first-line imaging modality for most abdominal queries throughout childhood; often US is sufficient or even the best method to provide and establish a certain and distinct diagnosis for a reliable final statement, offering all clinically relevant information, and thus no additional imaging is needed. To depict all the conclusive findings, all options and possibilities of modern US have to be applied and exploited. To really impact imaging algorithms and use this powerful imaging tool, however, dedicated skilled pediatric US with adequate equipment still has to be made available for children everywhere 24 h, 7 days a week.
In future, we hope ce-US will improve lesion detection (e.g., in trauma) or lesion differentiation (as already now known from adults, e.g., in the liver). 3D-US will provide not only more accurate volume calculation, but will also improve standardization and may become a superior and comprehensive visualization method using similar approaches and rendering tools as other sectional imaging techniques, enabling conspicuous demonstration of yet sonographically inaccessible structures, such as by virtual endoscopy.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Riccabona M, Fritz GA, Schöllnast H, Schwarz T, Deutschmann MJ, Mache CJ. Hydronephrotic kidney: pediatric threedimensional US for relative renal size assessment-initial experience. Radiology 236, 276–283 (2005).
- Fritz GA, Riccabona M, Bohdal G, Quehenberger F. Accuracy of renal volume assessment in children by three-dimensional sonography. Rofo 175, 540–546 (2003).
- Epelman M, Daneman A, Navarro OM et al. Necrotizing enterocolitis: review of state-ofthe- art imaging findings with pathologic correlation Radiographics 27, 285–302 (2007).
- Riccabona M, Joannou A, Maurer U, Müller W, Schwinger W. Doppler ultrasound in necrotizing enterocolitis. Z Geburtshilfe Perinatol. 197, 87–89 (1993).
- Dördelmann M, Rau GA, Bartels D et al. Evaluation of portal venous gas detected by ultrasound examination for diagnosis of necrotising enterocolitis. Arch. Dis. Child Fetal Neonatal Ed. 94, 183–187 (2009).
- Kurscheid T, Holschneider AM. Necrotizing enterocolitis (NEC) – mortality and long-term results. Eur. J. Pediatr. Surg. 3, 139–143 (1993).
- Buonomo C. The radiology of necrotizing enterocolitis. Radiol. Clin. North Am. 37, 1187–1198 (1999).
- Kosloske AM, Musemeche CA, Ball WS Jr, Albin DS, Bhattacharyya N. Necrotizing enterocolitis: value of radiographic findings to predict outcome. AJR Am. J. Roenthenol. 151, 771–774 (1988).
- Patino MO, Munden MM. Utility of the sonographic whirlpool sign in diagnosing midgut volvulus in patients with typical clinical presentations. J. Ultrasound Med. 23, 397–401 (2004).
- Shimanuki Y, Aihara T, Takano H et al. Clockwise whirlpool sign at color Doppler US: an objective and definite sign of midgut volvulus. Radiology 199, 261–264 (1996).
- Lampl B, Levin TL, Berdon WE, Cowles RA. Malrotation and midgut volvulus: a historical review and current controversies in diagnosis and management. Pediatr. Radiol. 39, 359–366 (2009).
- Riccabona M. Sonography of hypertrophic pyloric stenosis – attempt at prognostic evaluation based on sonographic criteria. Wien. Klin. Wochenschr. 102, 13–15 (1990).
- Yip WC, Wong ML, Tay JS, Prabhakaran K, Wong HB. Infantile hypertrophic pyloric stenosis: evaluation of sonographic criteria. J. Singapore Paediatr. Soc. 31, 111–115 (1989).
- Mollitt DL, Golladay ES, Williamson S, Seibert JJ, Sutterfield SL. Ultrasonography in the diagnosis of pyloric stenosis. South Med. J. 80, 47–50 (1987).
- Keckler SJ, Ostlie DJ, Holcomb Iii GW, St Peter SD. The progressive development of pyloric stenosis: a role for repeat ultrasound. Eur. J. Pediatr. Surg. 18, 168–170 (2008).
- Riccabona M, Weitzer C, Lindbichler F, Mayr J. Sonography and color Doppler sonography for monitoring conservatively treated infantile hypertrophic pyloric stenosis. J. Ultrasound Med. 20, 997–1002 (2001).
- Forster N, Haddad RL, Choroomi S, Dilley AV, Pereira J. Use of ultrasound in 187 infants with suspected infantile hypertrophic pyloric stenosis. Australas. Radiol. 51, 560–563 (2007).
- Justice FA, Auldist AW, Bines JE. Intussusception: trends in clinical presentation and management. J. Gastroenterol. Hepatol. 21, 842–846 (2006).
- Yalcin S, Ciftci AO, Karaagaoglu E, Tanyel FC, Senocak ME. Presenting clinical features and outcome in intussusception. Indian J. Pediatr. 76, 401–405 (2009).
- Hryhorczuk AL, Strouse PJ. Validation of US as a first-line diagnostic test for assessment of pediatric ileocolic intussusception. Pediatr. Radiol. 39, 1075–1079 (2009).
- Tareen F, Ryan S, Avanzini S, Pena V, Mc Laughlin D, Puri P. Does the length of the history influence the outcome of pneumatic reduction of intussusception in children? Pediatr. Surg. Int. 49(1), 30–34 (2011).
- Lagalla R, Caruso G, Novara V, Derchi LE, Cardinale AE. Color Doppler ultrasonography in pediatric intussusception. J. Ultrasound Med. 13, 171–174 (1994).
- Patsikas MN, Papazoglou LG, Jakovljevic S, Dessiris AK. Color Doppler ultrasonography in prediction of the reducibility of intussuscepted bowel in 15 young dogs. Vet. Radiol. Ultrasound 46, 313–316 (2005).
- Kong MS, Wong HF, Lin SL, Chung JL, Lin JN. Factors related to detection of blood flow by color Doppler ultrasonography in intussusception. J. Ultrasound Med. 16, 141–144 (1997).
- Baldisserotto M. Color Doppler sonographic findings of inflamed and perforated Meckel diverticulum. Ultrasound Med. 23, 843–848 (2004).
- Elsayes KM, Menias CO, Harvin HJ, Francis IR. Imaging manifestations of Meckel’s diverticulum. AJR Am. J. Roentgenol. 189, 81–88 (2007).
- Hennelly KE, Bachur R. Appendicitis update. Curr. Opin. Pediatr. 23(3), 281–285 (2011).
- Hahn H, Höpner F, von Kalle T et al. Appendicitis in childhood. Radiologe 37, 454–458 (1997).
- Schulte B, Beyer D, Kaiser C, Horsch S, Wiater A. Ultrasonography in suspected acute appendicitis in childhood-report of 1285 cases. Eur. J. Ultrasound 8, 177–182 (1998).
- Jangjoo A, Amouzeshi A, Jalali AN. Gangrenous appendicitis in a child with Henoch-Schonlein purpura. J. Pediatr. Surg. 43, e33–35 (2008).
- Alison M, Kheniche A, Azoulay R, Roche S, Sebag G, Belarbi N. Ultrasonography of Crohn disease in children. Pediatr. Radiol. 37, 1071–1082 (2007).
- Cammarota T, Sarno A, Robotti D et al. US evaluation of patients affected by IBD: how to do it, methods and findings. Eur. J. Radiol. 69, 429–437 (2009).
- Darge K, Anupindi S, Keener H, Rompel O. Ultrasound of the bowel in children: how we do it. Pediatr. Radiol. 40, 528–536 (2010).
- Ripollés T, Martínez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 253, 241–248 (2009).
- Robotti D, Cammarota T, Debani P, Sarno A, Astegiano M. Activity of Crohn disease: value of Color-Power-Doppler and contrastenhanced ultrasonography. Abdom. Imaging 29, 648–652 (2004).
- Sutcliffe J, Munden M. Sonographic diagnosis of multiple gastric duplication cysts causing gastric outlet obstruction in a pediatric patient. J. Ultrasound Med. 25, 1223–1226 (2006).
- Master V, Woods RH, Morris LL, Freeman J. Gastric duplication cyst causing gastric outlet obstruction. Pediatr. Radiol. 34, 574–576 (2004).
- Riccabona M, Avni FE, Dacher JN et al. ESPR uroradiology task force and ESUR paediatric working group: imaging and procedural recommendations in paediatric uroradiology, part III. Minutes of the ESPR uroradiology taskforce minisymposium on intravenous urography, uro-CT and MR-urography in childhood. ESPR uroradiology task force and ESUR paediatric working group. Pediatr. Radiol. 40, 1315–1320 (2010).
- Riccabona M, Avni FE, Blickman JG et al. Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr. Radiol. 38, 138–45 (2008).
- Christophe C, Cantraine F, Bogaert C et al. Ultrasound: a method for kidney size monitoring in children. Eur. J. Pediatr. 145, 532–538 (1986).
- Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr. Radiol. 15, 38–43 (1985).
- Riccabona M. Modern pediatric ultrasound: potential applications and clinical significance. A review. Clin. Imaging 30, 77–86 (2006).
- Schoellnast H, Lindbichler F, Riccabona M. Sonographic diagnosis of urethral anomalies in infants: value of perineal sonography. J. Ultrasound Med. 23, 769–776 (2004).
- Riccabona M, Fritz G, Ring E. Potential application of three-dimensional ultrasound in pediatric urinary tract: pictorial demonstration based on preliminary results. Eur. Radiol. 13, 2680–2687 (2003).
- Riccabona M. Imaging of the neonatal genito-urinary tract. Eur. J. Radiol. 60, 187–198 (2006).
- Darge K, Grattan-Smith JD, Riccabona M. Pediatric uroradiology: state of the art. Pediatr. Radiol. 41, 82–91 (2011).
- Kopac M, Riccabona M, Haim M. Contrast-enhanced voiding urosonography and genitography in a baby with ambiguous genitalia and urogenital sinus. Ultraschall. Med. 30, 299–300 (2009).
- Riccabona M. Cystography in infants and children: a critical appraisal of the many forms with special regard to voiding cystourethrography. Eur. Radiol. 12, 2910–2918 (2002).
- Darge K, Troeger J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. Eur. J. Radiol. 43, 122–128 (2002).
- Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr. Radiol. 23, 478–480 (1993).
- Riccabona M, Avni FE, Blickman JG et al. Imaging recommendations in paediatric uroradiology. Minutes of the ESPR uroradiology task force session on childhood obstructive uropathy, high-grade fetal hydronephrosis, childhood haematuria, and urolithiasis in childhood. ESPR Annual Congress, Edinburgh, UK, June 2008. Pediatr. Radiol. 39, 891–898 (2009).
- Avni FE, Hall M, Collier F, Schulman C. Abnormalities of renal pelvis and ureter. In: Pediatric Uroradiology, Second Edition. Fotter R (Ed.). Springer, Berlin, Germany, 61–90 (2008).
- Riccabona M. Obstructive diseases of the urinary tract in children: lessons from the last 15 years. Pediatr. Radiol. 40, 947–955 (2010).
- Lin CC, Tsai JD, Sheu JC, Lu HJ, Chang BP. Segmental multicystic dysplastic kidney in children: clinical presentation, imaging finding, management, and outcome. J. Pediatr. Surg. 45, 1856–1862 (2010).
- Mattioli G, Pini-Prato A, Costanzo S et al. Nephrectomy for multicystic dysplastic kidney and renal hypodysplasia in children: where do we stand? Pediatr. Surg. Int. 26, 523–528 (2010).
- Riccabona M, Ring E, Fueger G, Petritsch P, Villits P. Doppler sonography in congenital ureteropelvic junction obstruction and multicystic dysplastic kidneys. Pediatr. Radiol. 23, 502–505 (1993).
- Brkljacić B, Kuzmić AC, Dmitrović R, Rados M, Vidjak V. Doppler sonographic renal resistance index and resistance index ratio in children and adolescents with unilateral hydronephrosis. Eur. Radiol. 12, 2747–2751 (2002).
- Kullendorff CM. Surgery in unilateral multicystic kidney. Z Kinderchir. 45, 235–237 (1990).
- Riccabona M, Fritz GA, Schöllnast H, Schwarz T, Deutschmann MJ, Mache CJ. Hydronephrotic kidney: pediatric threedimensional US for relative renal size assessment – initial experience. Radiology 236 (1), 276–83 (2005).
- Riccabona M, Sorantin E, Ring E. Application of M-mode sonography to functional evaluation in pediatric patients. Eur. Radiol. 8, 1457–1461 (1998).
- Riccabona M. Lower urinary tract – the ureter and vesicoureteral reflux. In: Caffey’s Pediatric Diagnostic Imaging, 11th Edition, Volume 2, Part 13. Slovis L (Ed.). Mosby, Elsevier, PA, USA, 2315–2355 (2007).
- Haberlik A. Detection of low-grade vesicoureteral reflux in children by color Doppler imaging mode. Pediatr. Surg. Int. 12, 38–43 (1997).
- Riccabona M, Pilhatsch A, Haberlik A, Ring E. Three-dimensional ultrasonographybased virtual cystoscopy of the pediatric urinary bladder: a preliminary report on feasibility and potential value. J. Ultrasound Med. 27, 1453–1459 (2008).
- Riccabona M. Urinary tract imaging in infancy. Pediatr. Radiol. 39, 436–445 (2009).
- Brader P, Riccabona M, Schwarz T, Seebacher U, Ring E. Value of comprehensive renal ultrasound in children with acute urinary tract infection for assessment of renal involvement: comparison with DMSA scintigraphy and final diagnosis. Eur. Radiol. 18, 2981–2989 (2008).
- Darge K, Heidemeier A. Modern ultrasound technologies and their application in pediatric urinary tract imaging. Radiologe 45, 1101–1111 (2005).
- Darge K. Be aware and beware of the ‘twinkling sign’. Pediatr. Radiol. 35, 351–352 (2005).
- Riccabona M, Avni FE, Dacher JN et al.; ESPR uroradiology task force and ESUR paediatric working group. Imaging and procedural recommendations in paediatric uroradiology, part III. Minutes of the ESPR uroradiology task force minisymposium on intravenous urography, uro-CT and MR-urography in childhood. Pediatr. Radiol. 40 (7), 1315–1320 (2010).
- Dick PT, Shuckett BM, Tang B, Daneman A, Kooh SW. Observer reliability in grading nephrocalcinosis on ultrasound examinations in children. Pediatr. Radiol. 29, 68–72 (1999).
- Lee MS, Kim MJ, Lee MJ et al. Biliary atresia: color doppler US findings in neonates and infants. Radiology 252, 282–289 (2009).
- Christison-Lagay ER, Burrows PE, Alomari A et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J. Ped. Surg. 42, 62–68 (2007).
- Hoti E, Adam R. Liver transplantation for primary and metastatic liver cancers. Transpl. Int. 21, 1107–1117 (2008).
- Da Ines D, Petitcolin V, Joubert-Zakeyh J, Demeocq F, Garcier JM. Epitheloid Hemangioendothelioma of the liver with metastatic celiac lymph nodes in an 11-year-old boy. Pediatr. Radiol. 40, 1293–1296 (2010).
- Ueda D. Sonographic measurement of the pancreas in children. J. Clin. Ultrasound 17, 417–423 (1989).
- Wilson SR, Gupta C, Eliasziw M, Andrew A. Volume imaging in the abdomen with ultrasound: how we do it. AJR Am. J. Roentgenol. 193, 79–85 (2009).
- Lamb PM, Lund A, Kanagasabay RR, Martin A, Webb JA, Reznek RH. Spleen size: how well do linear ultrasound measurements correlate with three-dimensional CT volume assessments? Br. J. Radiol. 75, 573–577 (2002).
- Kangarloo H, Diament MJ, Gold RH et al. Sonography of adrenal glands in neonates and children: changes in appearance with age. J. Clin. Ultrasound 14, 43–47 (1986).
- Velaphi SC, Perlman JM. Neonatal adrenal hemorrhage: clinical and abdominal sonographic findings. Clin. Pediatr. 40, 545–548 (2001).
- Hörmann M. Neuroblastoma in children. Radiologe 48, 940–945 (2008).
- Hobbs DJ, Barletta GM, Mowry JA, Bunchman TE. Renovascular hypertension and intrarenal artery aneurysms in a preschool child. Pediatr. Radiol. 39, 988–990 (2009).
- Shimada O, Fukui K, Yanishi M et al. Case with rupture of renal arterial aneurysm caused by fibromuscular dysplasia. Hinyokika Kiyo. 55 (1)19–22 (2009).
- Laplante S, Patriquin HB, Robitaille P, Filiatrault D, Grignon A, Décarie JC. Renal vein thrombosis in children: evidence of early flow recovery with Doppler US. Radiology 189, 37–42 (1993).
- Standarddokumentation der Sonografie des kindlichen Abdomens www.oegum.at/component/option,com_ docman/task,doc_download/gid,10/
- ACR–AIUM practice guideline for the performance of an ultrasound examination of the abdomen and/or retroperitoneum www.acr.org/ SecondaryMainMenuCategories/quality_ safety/guidelines/us/us_abdomen_retro.aspx
■ Websites