Contrast Agent Evaluation - Imaging in Medicine (2012) Volume 4, Issue 2
Perflutren lipid microsphere injectable suspension for cardiac ultrasound
Sahar S Abdelmoneim1,2 & Sharon L Mulvagh*11Mayo Clinic Cardiovascular Ultrasound Imaging & Hemodynamic Laboratory, Division of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA
2Division of Cardiovascular Medicine, Assiut University, Assiut, Egypt
- Corresponding Author:
- Sharon L Mulvagh
Mayo Clinic Cardiovascular Ultrasound Imaging & Hemodynamic Laboratory
Division of Cardiovascular Medicine
Mayo Clinic, Rochester, MN, USA
Tel: +1 507 284 9601
Fax: +1 507 266 0228
E-mail: smulvagh@mayo.edu
Abstract
Echocardiography is a widely available noninvasive procedure for diagnosing cardiovascular disease. With the development of clinically useful microbubble contrast agents to enhance echocardiographic images and advantages of portability without radiation, microbubble contrast agents have become essential established adjunctive tools to perform state-of-the-art echocardiography. Several manufacturers produce commercially available stabilized microbubbles for intravenous use. Perflutren lipid microsphere (DEFINITY®) is one of the second generation microbubble contrast agents approved for left ventricular opacification and left ventricular endocardial border detection, and is the subject of this review.
Keywords
contrast echocardiography ▪ endocardial border definition ▪ left ventricular opacification ▪ perflutren lipid microsphere ▪ stress echocardiography ▪ ultrasound microbubble contrast agents
Contrast imaging was introduced to diagnostic ultrasound more than 40 years ago [1]. The first ultrasound contrast agent described in the late 1960s was agitated saline. More than two decades were required to develop a microbubble contrast agent (MCA) that consisted of a high molecular weight gas encapsulated by an insoluble shell. These MCAs can be injected intravenously and circulate throughout the systemic circulation to produce an excellent arterial blood pool effect, termed left ventricular opacification (LVO) and left ventricular endocardial border detection (EBD) [2,3]. Successful evaluations of MCAs were undertaken by a number of researchers and pharmaceutical companies in both animals and human clinical trials. Incremental benefits of improved accuracy and reproducibility for assessment of left ventricular (LV) function have been demonstrated with the use of MCAs in both stress and rest transthoracic echocardiography (TTE) [4]. It was also shown that the use of MCA for LVO may contribute to a more cost-effective pattern of patient care [5].
In addition to LVO, microbubbles can be deliberately disrupted by the ultrasound beam and the rate at which fresh microbubbles replenish the scan plane can be observed in subsequent images, thus enabling the quantification of microvascular flow rate and relative vascular volume. Although the use of MCAs for myocardial perfusion has been extensively researched, it has not yet been routinely implemented in clinical practice and is limited to off-label use and in research studies. In addition to MCA use in echocardiography, noncardiac application of contrast ultrasound imaging has expanded the range of spatial and temporal resolution in radiological ultrasound diagnostics [6].
In 2008, the American Society of Echocardiography (ASE) guidelines provided the rationale of MCA use for the purposes of improving endocardial visualization in all subjects with two or more suboptimal contiguous LV wall segments. In addition, the guidelines highlighted specific clinical scenarios requiring the use of MCA to obtain the best diagnostic information [2]. Subsequently, in 2009, the European Association of Echocardiography [7], similarly documented the clinical value of MCAs. In 2010, the Intersocietal Commission for the Accreditation of Echocardiography Laboratories (ICAEL) affirmed the necessity of MCA availability and skilled use for laboratory accreditation and further emphasized the need for a written policy per individual laboratories for the use of MCAs or for alternative imaging if MCAs were not used [101]. In addition, the use of MCAs has been acknowledged in the 2011 American College of Cardiology/ASE Appropriateness Criteria for TTE [8]. Subsequently, the International Ultrasound Contrast Society [102] was organized to provide cohesive information on MCAs in different ultrasound fields.
To date, the US FDA has approved two perfluoropropane MCAs for the indications of EBD and LVO in patients with suboptimal baseline images. These commercially available MCAs include: FSO 69 (OPTISON™; GE Healthcare Inc., Princeton, NJ, USA), which was approved in 1998 and DMP 115 (DEFINITY®; Lantheus Medical Imaging, North Billerica, MA, USA), which was approved in 2001. These and several other MCAs are approved for use in other parts of the world [2].
This article will focus on commercially available perflutren lipid microsphere injectable suspension pharmacologic properties and evidence from the literature on efficacy and safety. Additional highlights from major studies of the off-label use of these agents will be also discussed.
Overview of ultrasound contrast agents
The commercial development of MCAs began in the 1980s with the first agent Echovist® (Schering, Berlin, Germany), which enabled right ventricle enhancement but it did not survive passage through the pulmonary circulation [9]. In 1985, Levovist® (Schering, Berlin, Germany), a galactose palmitic acid encapsulated air microbubbles was developed and approved in Europe, Japan and Canada. Both Albunex® (Molecular Biosystems Inc., San Diego, CA, USA) and Levovist are transpulmonary MCAs but with a reduced longevity in vivo (half-life <5 min) resulting in less than optimal LVO [10]. In North America, the production of Albunex enabled LVO after an intravenous injection [11]. Albunex was the first agent approved by the FDA in 1994.
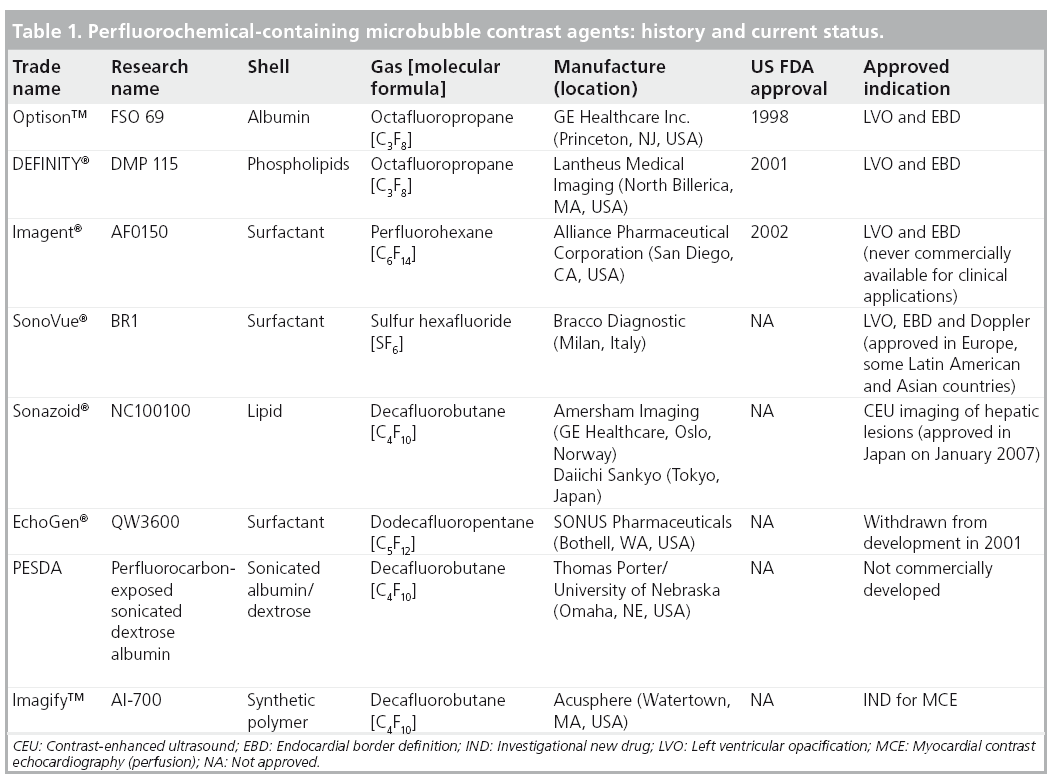
With increasing recognition of the potential usefulness of MCAs, the focus shifted to the production of stabilized shelf-ready microbubbles. An increasing number of manufacturers produced forms of stabilized MCAs with smaller diameter (≤10 μm) able to cross the pulmonary circulation, having a longer effect. To achieve a persistent clinically useful effect, small amounts of high molecular weight gases known as perfluorocarbons (PFCs) were utilized. PFCs (i.e., perfluorocarbon, perfluorobutane, perfluoropropane, and perfluorohexane) are inert compounds with a low surface tension, and can be intravenously injected if emulsified. These ‘second generation’ MCAs containing PFCs demonstrated clear superiority to earlier agents with respect to the degree and duration of LVO, EBD and Doppler enhancement, and further as off-label use for myocardial perfusion [12]. Table 1 illustrates the current market overview of PFCs containing MCAs. Latest research efforts are ongoing to develop microbubbles for molecular imaging (third generation agents) and for targeted drug/gene delivery (fourth generation agents) as a site-specific, ultrasound directed, drug or gene therapeutic tool [13].
Concomitantly, unique contrast-specific imaging techniques that are now routinely available on ultrasound machine platforms were being developed. Indeed, the ultrasound market has experienced a dynamic transition over the last decade driven by the need for enhanced contrast detection. The primary mechanisms for MCA signal enhancement are microbubble backscattering, oscillation and rupture, all of which are primarily dependent on the acoustic power (mechanical index) of the transmitted ultrasound beam. These improvements have enabled not only excellent visualization of MCAs within cardiac chambers but also within the myocardial microvasculature [2].
The only commercially available perflutren lipid microsphere injectable suspension in the USA is DEFINITY, approved by FDA in July 2001 and was also approved by the European Medicine Agency in September 2006 under the name LUMINITY® (Lantheus Medical Imaging, North Billerica, MA, USA). Up to this date, 3.3 million patients have received DEFINITY, for contrast-enhanced echocardiographic procedures since the first product approval [103].
Introduction to the compound
■ Chemistry
The main two components of DEFINITY, include a core of perflutren gas (octafluoropropane and perfluoropropane) and an outer lipid shell. The chemical name (molecular formula) for perflutren is 1,1,1,2,2,3,3,3-octafluoropropane [C3F8; CF3-CF2-CF3]. Its molecular weight is 188.02 g/mol. It is a colorless and odorless gas that is chemically inert and thermally stable because of the strength of the carbon–fluorine bond. Figure 1 shows the structural formula of the active substance in DEFINITY.
The lipid shell is composed of three phospholipids (0.75 mg/ml) including DPPA (1,2-dipalmitoyl-sn-glycero-3-phosphatidic acid, monosodium salt); DPPC (1,2-dipalmitoyl- sn-glycero-3-phosphatidylcholine); and MPEG5000 DPPE: N-(methoxypolyethylene glycol 5000 carbamoyl)-1,2-dipalmitoyl-snglycero- 3-phosphatidylethanolamine, monosodium salt. DPPA and DPPC are major constituents of human cell membranes. DPPA negatively charges the microspheres reducing coalescence. Other ingredients included in the formulation matrix are phosphate buffer (for phospholipid stability purposes); sodium chloride (used as a tonicity agent); propylene glycol (improves the hydrophilic properties of the phospholipids during compounding); and glycerol (increases viscosity and stability) [14,103].
■ Pharmacodynamics Mechanism of DEFINITY actions
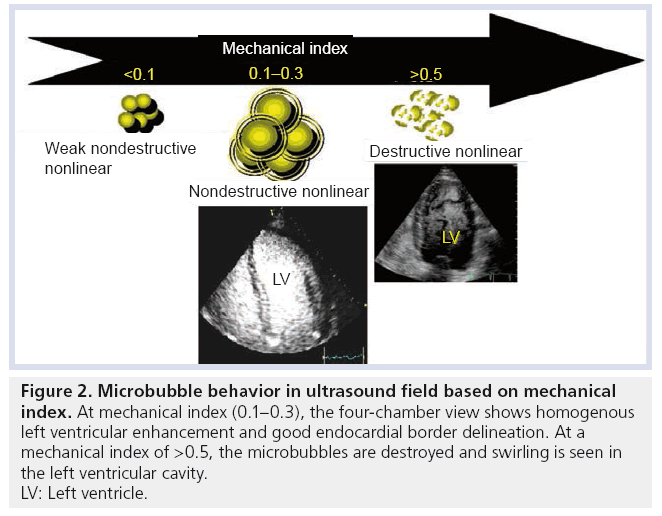
Activated DEFINITY is small enough to pass through the lungs and circulate in the vascular system. The difference in density and compressibility between the MCA and the surroundings creates an efficient reflector of the ultrasound beam, thus enhancing blood echogenicity and EBD [15]. The mechanical index is a major parameter affecting microbubble oscillation [2]. These oscillations can result in weak nonlinear backscatter at a very low mechanical index (<0.1); nonlinear signals (ultraharmonics) at low–medium mechanical index (0.1–0.5); and microbubble destruction at high mechanical index (>0.5) where the shell is broken and the gas rapidly diffuses into the surroundings [16]. Figure 2 illustrates microbubble behavior in an ultrasonic field as a function of mechanical index. In general, the acoustic properties of activated DEFINITY are characterized by improved stability and favorable resonance behavior at or below a mechanical index of 0.8 [103]. While conventional 2D ultrasound can detect high concentrations of microbubbles, in practice optimal assessment requires contrast-specific imaging modes. These specific ultrasound modes are based on the cancellation and separation of the linear signals arising from tissue and utilization of the nonlinear response from MCA [17]. Detection of the nonlinear response from DEFINITY was observed with variable underlying imaging approaches including conventional harmonic imaging, subtraction techniques performed using single pulse (coherent imaging mode), or multiple pulses (pulse or phase inversion mode) and combined for multiframe subtraction techniques. The characteristics of activated DEFINITY response to ultrasound waves and its effectiveness in enhancing ultrasound imaging for LVO and myocardial perfusion were established in animal models and several human clinical trials [2]. A correlation between increased mechanical index and decreased persistence of DEFINITY microbubbles in circulation was demonstrated both in in vitro and in vivo models [15]. DEFINITY was shown to exhibit a peak mean backscatter power at concentrations between 1 and 10 × 106 microbubbles/ml with a linear reduction of mean backscatter power in concentrations lower than this [18]. The persistence of clinically useful LVO was demonstrated for approximately 3.4 min for activated DEFINITY bolus dose (3–10 μl/kg) and 7 min for continuous diluted infusion in 50 ml normal saline (10 μl/kg, at a rate of 4 ml/min). Such effects permit the interrogation of multiple views during an echocardiographic study. Although higher DEFINITY doses have been shown to produce a longer LVO effect, definite dose–response relationship could not be a established [19,20].
Figure 2: Microbubble behavior in ultrasound field based on mechanical index. At mechanical index (0.1–0.3), the four-chamber view shows homogenous left ventricular enhancement and good endocardial border delineation. At a mechanical index of >0.5, the microbubbles are destroyed and swirling is seen in the left ventricular cavity. LV: Left ventricle.
Hemodynamic & tissue effects of DEFINITY in preclinical & clinical studies
It was observed that the activated DEFINITY microbubbles had identical velocity to red blood cells within the arterioles, capillaries and venules, and no obstruction of the vessels occurred [21,22]. The effects of activated DEFINITY on cardiovascular hemodynamics was evaluated in preclinical studies in pigs at dose levels ranging from 0.5 to 10 μl/kg showing no changes in heart rate, systemic pressure or arterial oxygen saturations. However, a mild reversible increase in pulmonary arterial pressure was observed [23,24]. Such a dose (10 μl/kg) is currently the highest recommended dose for clinical use [103]. In a multicenter randomized, placebo-controlled, double-blind trial study by Kitzman and colleagues in patients with suboptimal baseline echocardiographic images, two doses of activated DEFINITY (5 vs 10 μl/kg) were given. The study showed no clinically significant change in hemodynamic, electrocardiography or hematological values between the two dosage groups [3]. The differences in hemodynamic responses between humans and pigs are attributed to unique species-specific thromboxane release from porcine pulmonary intravascular macrophages, which induces pulmonary hypertension. Indeed, Szebeni proposed that the pig represents a highly sensitive testing model for assessing hypersensitivity reactions [25], although the extrapolation to humans who do not have pulmonary intravascular macrophages is not clinically relevant. In a prospective controlled human clinical study by Wei et al., no changes in peak or mean pulmonary arterial pressures were observed using invasive monitoring after a full dose of DEFINITY in patients with normal or elevated predose pulmonary arterial pressures [26].
Studies examining the effect of activated DEFINITY on cerebral vasculature utilizing histopathological sections of the brain indicated no adverse effect seen at doses that are five-times that of the human dose within 5 min of administration. Although another study in rats looking at a different PFC-based MCAs revealed disruption of the blood–brain barrier that lasted 24 h [27]. A study investigating the influence of perflutren on microvessels in the rat mesentery and myocardium reported no evidence of microvessel bleeding and/or endothelial cell injury unless high mechanical index ultrasound exposure occurred [21]. While mechanistic reports studying the potential for hypersensitivity response of the immune system to DEFINITY, suggested possible complement factor (C3a) activation as an immediate response, declining gradually over 30 min, the actual mechanism has not been clearly established [104].
■ Pharmacokinetics
DEFINITY is presented as a vial containing a translucent liquid phospholipid mixture with octafluoropropane gas in the head space. After activation (emulsification) using a mechanical shaking device (Vialmix®, Lantheus Medical Imaging, North Billerica, MA, USA) for 45 s, it becomes an opaque suspension (1 ml of the milky white dispersion contains a maximum of 1.2 × 1010 perflutren lipid microspheres with a diameter ranging from 1.1 to 3.3 μm, and approximately 1.1-mg/ml octafluoropropane) [104].
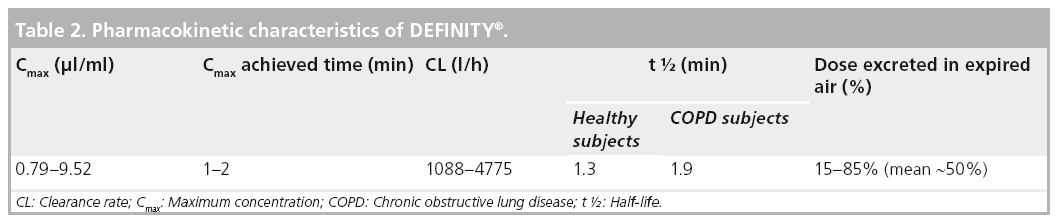
The pharmacokinetics of an intravenous dose of activated DEFINITY was evaluated in healthy humans and those with chronic obstructive pulmonary disease. Using validated gas chromatographic methods, perflutren gas was found to mainly be cleared by the lungs in the expired air in an unchanged state. The rapid elimination of perflutren gas in the expired air was also consistent with the rapid disappearance of ultrasound contrast enhancement after activated DEFINITY administration. Furthermore, there were no statistically significant differences observed in the concentration-time curves of blood perflutren in normal versus chronic obstructive pulmonary disease subjects [104,105]. The lipid shell contains both DPPC and DPPA, which are endogenous lipids that are metabolized to free fatty acids. MPEG5000 DPPE is not an endogenous phospholipid and is cleared from the circulation via urine as MPEG5000 [19]. Table 2 shows the pharmacokinetic characteristics of DEFINITY.
Clinical efficacy
We review below the pivotal Phase I, II and III studies that were conducted in support of the use of DEFINITY for contrast-enhanced echocardiography.
■ Phase I studies
Two Phase I clinical trials were performed to determine the safety and tolerance of a single ascending dose or multiple doses of DEFINITY in 30 and 18 healthy adult male subjects, respectively [28]. These Phase I trials further assessed the contrast enhancement of the cardiac chambers visually and by quantitative videodensitometric measurements. The efficacy results of these Phase I studies indicated that both median visual scores for contrast enhancement and mean changes from baseline in quantitative videodensitometric measurements were higher in the DEFINITY-treated group compared with the placebo group. Both studies reported on the hemodynamic safety with no treatment-related changes in vital signs, ECG or arterial oxygen saturation (15 min–24 h after DEFINITY administration) and that the doses were well tolerated in humans with no significant side effects [28].
■ Phase II studies
Pantely and colleagues evaluated 19 patients referred for diagnostic echocardiography in a Phase II study [29]. DEFINITY was administered as a single intravenous bolus injection at doses of 5, 10 or 15 μl/kg. Optimal enhancement was observed for approximately 1.5–2 min in the 10-μl/kg dose group, while excessive attenuation occurred in the 15-μl/kg dose group. Based on these findings, the dose of 5–10 μl/kg was recommended for use in the Phase III efficacy cardiology trials.
■ Phase III studies
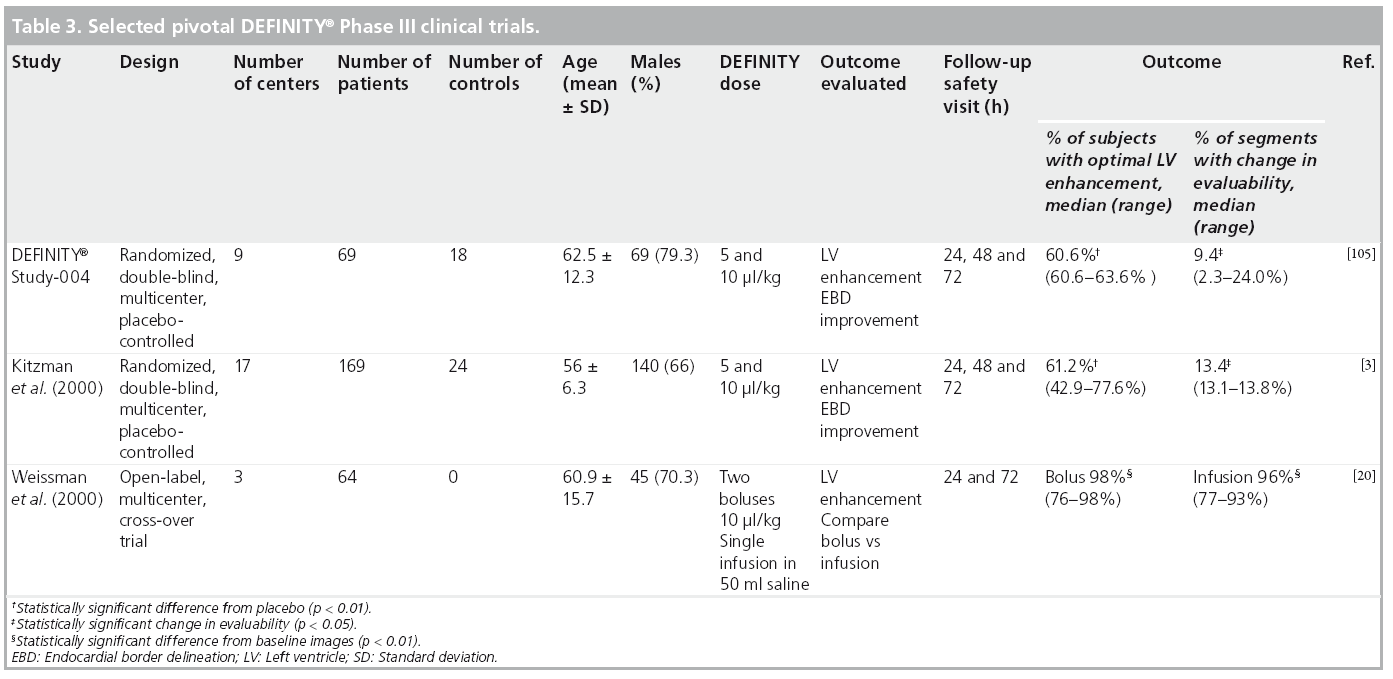
Three pivotal Phase III multicenter clinical trials on DEFINITY were evaluated by the FDA [3,20,105]. These were performed in patients with known or suspected cardiac disease and suboptimal (≥2 nonevaluable segments) echocardiographic images with doses ranging from 5 to 10 μl/kg. Outcomes evaluated were the percentage of subjects who demonstrated optimal LVO and the percentage of segments with change in evaluability from nonevaluable at baseline to evaluable post-DEFINITY. In all trials, subjects with optimal LVO intensity were higher in the contrast than in noncontrast (placebo) groups. No serious adverse events were reported, however, the most frequent new-onset adverse events (≥1%) were fatigue, dyspnea, headache, chest pain, flushing, nausea and dizziness. Furthermore, no dose relationship could be detected for the overall frequency of treatment-related, new-onset adverse events in subjects receiving bolus doses of DEFINITY. Table 3 illustrates the results of pivotal DEFINITY Phase III trials that were submitted to the FDA for approval.
DEFINITY was further evaluated in two abdominal ultrasound Phase III multicenter clinical trials enrolling 209 patients (130 with suspected liver pathology and 79 with suspected kidney pathology). The authors concluded that the addition of the DEFINITY contrast agent to standard grayscale abdominal ultrasound provided both additional diagnostic information and substantive changes in patient management, with 23% of patients judged to not require additional diagnostic examinations [14].
Clinical applications of contrast echocardiography
DEFINITY is approved in the USA for use in suboptimal echocardiograms [103] and use of MCAs is required for echocardiography laboratory accreditation [101].
■ Enhancement of endocardial border during rest TTE
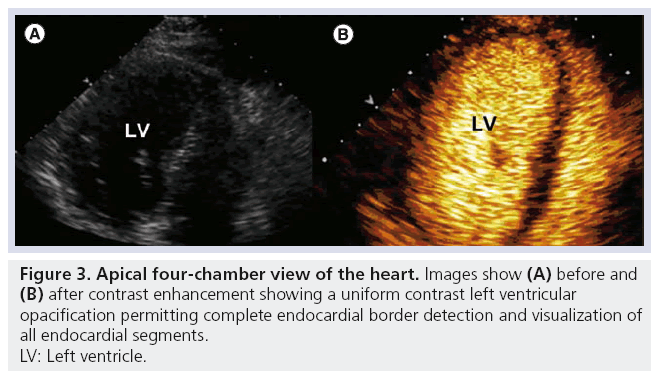
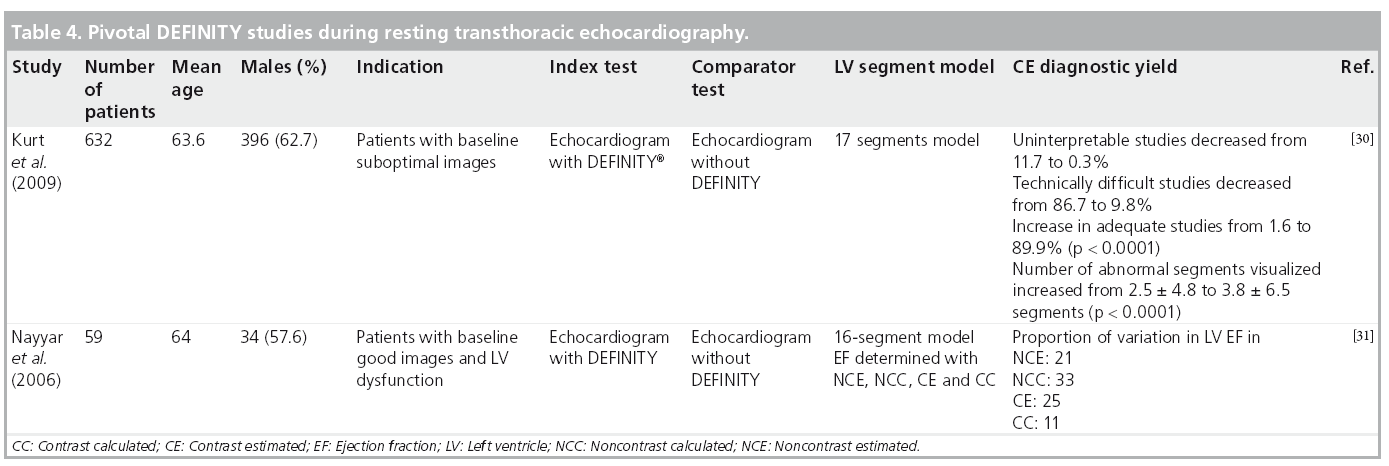
Beyond enhanced endocardial visualization, the use of MCA for quantification of LV volumes and ejection fraction (EF) has been shown to have excellent correlation with comparative imaging modalities, demonstrating improved intra- and inter-observer agreement and physician interpretation confidence [2]. In addition to the two pivotal Phase III trials identified above [3,20], two additional studies in which DEFINITY was used to enhance resting TTE confirmed these findings [30,31]. Table 4 summarizes DEFINITY studies demonstrating the benefit of contrast utilization for LVO during resting TTE. These studies [3,30] further assessed the direct impact of contrast-enhanced echocardiography on health outcomes in patients requiring assessment of ventricular function. Kitzman and colleagues concluded that the use of DEFINITY provided information that eliminated the need for additional testing in 60% of patients and aided EF determinations in 81% of all patients when compared with those who received placebo [3]. These rates of salvaged echocardiographic studies were in a similar range to other MCAs (51% for perflenapent emulsion [32] and 74% for albumin perfluoropropane microbubbles [12]). It is notable that these findings were obtained even in the era of fundamental imaging, prior to optimization of ultrasound imaging technology as is routinely used today for enhanced MCA detection. In the landmark paper by Kurt and colleagues, the use of DEFINITY for LVO significantly impacted diagnostic accuracy, resource utilization and directly benefited patient management [30]. Such benefit was reflected in change of therapy, procedure or both in 35.6% of suboptimal quality studies. Figure 3 illustrates on apical four-chamber view pre- and post-contrast enhancement showing uniform LVO and EBD with improved visualization of all endocardial segments.
■ Enhancement of endocardial border during stress TTE
The use of MCAs with stress echocardiography (SE) improves the diagnostic accuracy of stress echo in the diagnosis of coronary artery disease (CAD) [33]. Several nonrandomized studies compared noncontrast with contrast SE utilizing DEFINITY [34–37] or albumin perfluoropropane microbubbles [38–44] primarily to identify wall motion abnormalities in clinically indicated SE studies. Table 5 summarizes pivotal studies utilizing specifically DEFINITY for LVO in SE.
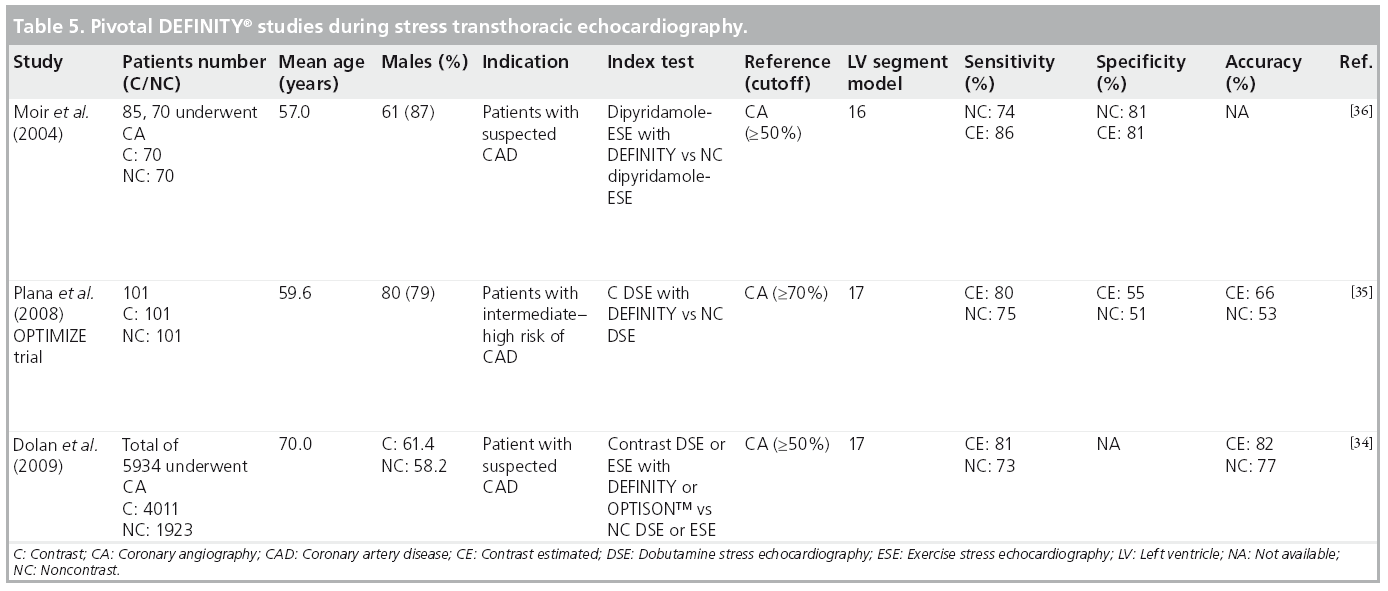
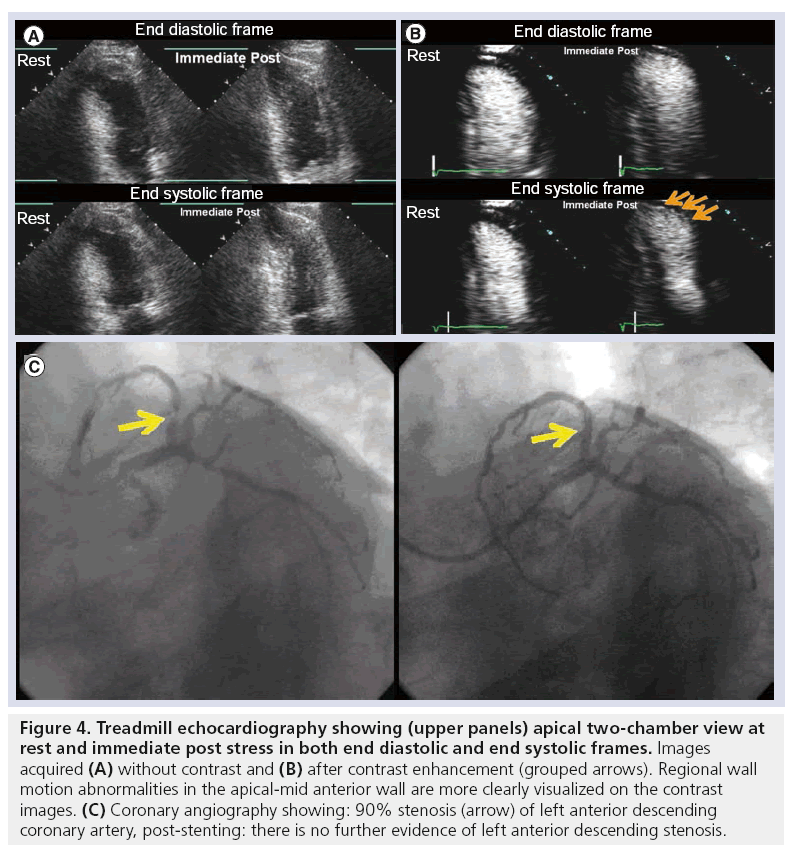
Other studies utilizing different PFC-based MCAs have shown similar results. Using an albumin perfluoropropane MCA, Thanigaraj and colleagues, reported that 53% of patients with poor resting images who underwent SE were referred to subsequent nuclear stress testing versus 3% of those who received MCA [41]. Furthermore, two studies [45,46] utilizing an airbased MCA (Levovist) demonstrated the value of contrast-enhanced SE over noncontrast SE for the prediction of total cardiac events [47,48]. Figure 4 depicts an apical two-chamber view preand post-contrast enhancement, demonstrating the effect of uniform LVO with MCA to allow complete visualization of endocardial segments and detection of regional wall motion abnormalities in the apical and mid-anterior wall, not otherwise seen on noncontrast images, and correlating with significant coronary artery stenosis confirmed at coronary angiography.
Figure 4: Treadmill echocardiography showing (upper panels) apical two-chamber view at rest and immediate post stress in both end diastolic and end systolic frames. Images acquired (A) without contrast and (B) after contrast enhancement (grouped arrows). Regional wall motion abnormalities in the apical-mid anterior wall are more clearly visualized on the contrast images. (C) Coronary angiography showing: 90% stenosis (arrow) of left anterior descending coronary artery, post-stenting: there is no further evidence of left anterior descending stenosis.
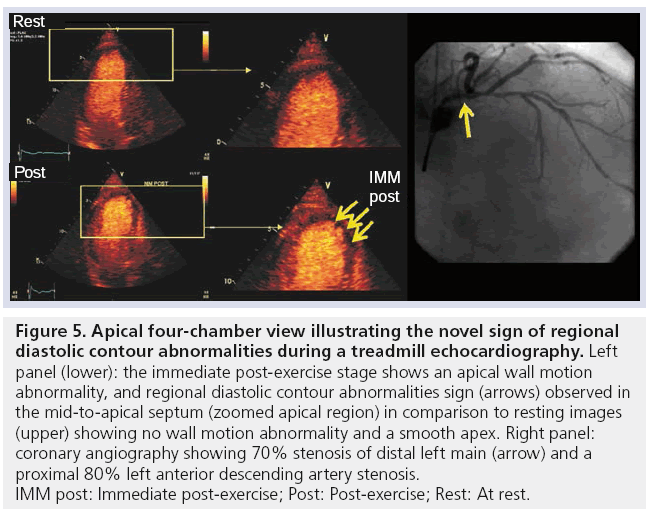
The very clear endocardial border definition that occurs with MCA has allowed enhanced observation not only of systolic abnormalities of wall thickening, but also diastolic abnormalities. These regional diastolic contour alterations of the endocardium provide incremental information to detect the presence of angiographically significant CAD compared with LV systolic wall thickening abnormalities alone [49]. Figure 5 demonstrates Regional diastolic contour alterations that were observed in the apical four-chamber view (apical region) during immediate post-stress imaging; a significant coronary stenosis was confirmed at angiography.
Figure 5: Apical four-chamber view illustrating the novel sign of regional
diastolic contour abnormalities during a treadmill echocardiography. Left
panel (lower): the immediate post-exercise stage shows an apical wall motion
abnormality, and regional diastolic contour abnormalities sign (arrows) observed in
the mid-to-apical septum (zoomed apical region) in comparison to resting images
(upper) showing no wall motion abnormality and a smooth apex. Right panel:
coronary angiography showing 70% stenosis of distal left main (arrow) and a
proximal 80% left anterior descending artery stenosis.
IMM post: Immediate post-exercise; Post: Post-exercise; Rest: At rest.
■ Assessment of abnormal cardiac structural anatomy & hemodynamic flow
MCAs are of value in the detailed structural assessment of the left and right ventricles, the atria and the great vessels [2]. In particular, the diagnoses of apical LV pathology (e.g., apical variant of hypertrophic cardiomyopathy and thrombus), postmyocardial infarction complications (e.g., LV rupture or pseudoaneurysm, or ventricular septal defect) and intracardiac masses [50] are significantly enhanced. Suboptimal Doppler signals and their spectral envelopes can be clearly demarcated to permit determination of peak velocities and time velocity integrals, in order to improve assessment of right ventricular systolic pressures, valvular stenosis, intracardiac shunts and diastolic function [2].
■ Off-label use of DEFINITY for myocardial perfusion
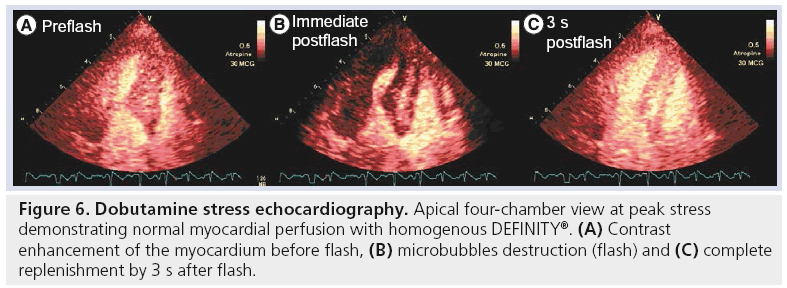
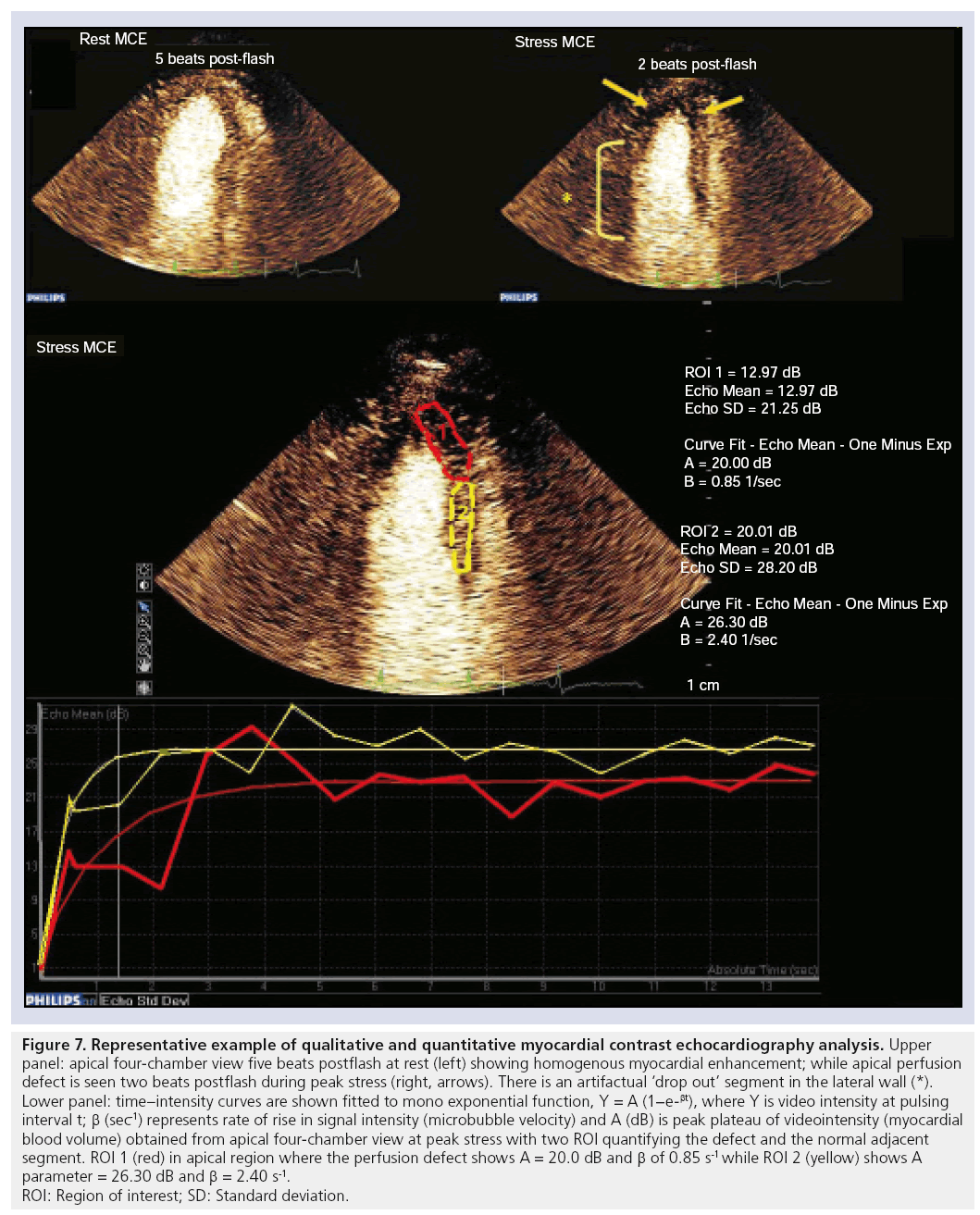
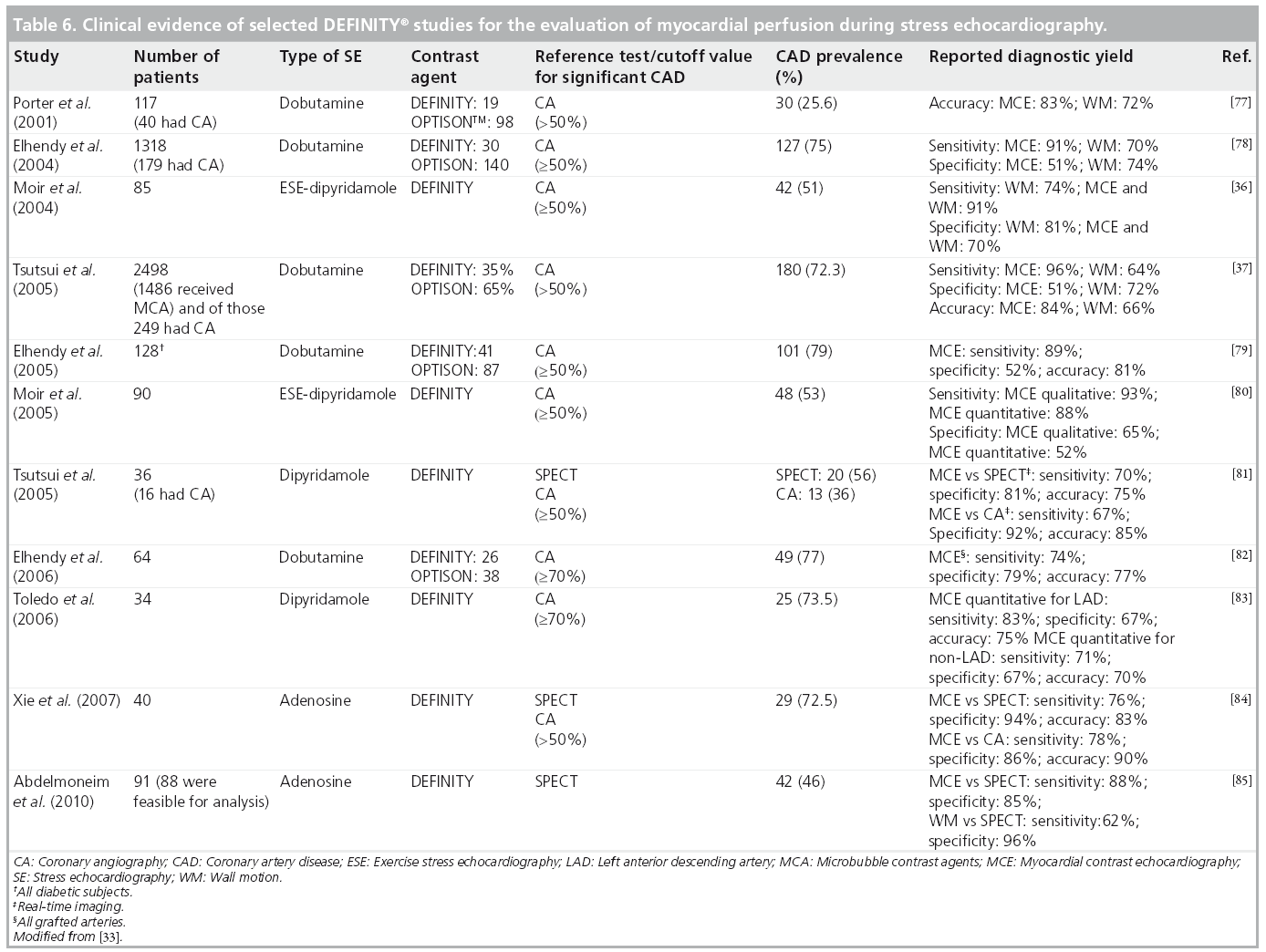
MCAs enhance the backscattered ultrasound signals and can be seen not only in the LV cavity, but also in the myocardium. Thus, myocardial perfusion can be detected, and quantified using MCA. A growing number of published articles have documented the use of DEFINITY and other MCAs for use as myocardial perfusion agents to detect perfusion abnormalities both at rest and in conjunction with exercise and pharmacological SE [51–59]. The fundamental concept for evaluation of perfusion using real-time contrast echocardiography is dependent on the instantaneous depletion (destruction) of microbubbles and observation of their gradual refill (replenishment) into the myocardial microvasculature. We and others have performed metaanalyses to evaluate the diagnostic accuracy of contrast echocardiography for detection of perfusion abnormalities quantitatively [60] and qualitatively [61]. Dijkmans and colleagues reported [61] that perfusion abnormalities detected by myocardial perfusion echocardiography showed equivalent noninferior results to those of SPECT dobutamine SE for the detection of CAD. An example of normal myocardial perfusion as evaluated by myocardial contrast echocardiography is illustrated in Figure 6. We have also reported on the pooled sensitivity and specificity for the quantitative perfusion parameters of myocardial blood flow velocity reserve (b) and coronary flow reserve (Ab reserve) as: 81% (95% CI: 76–85) and 77% (95% CI: 73–80), and 80% (95% CI: 75–84) and 81% (95% CI: 77–84), respectively [60]. An example of a myocardial perfusion defect observed using myocardial perfusion echocardiography is shown in Figure 7. Selected SE studies utilizing DEFINITY for evaluation of myocardial perfusion with coronary angiography and/or SPECT as a reference standard test for CAD diagnosis is shown in Table 6 with reported range for sensitivities and specificities of 67–91% and 51–96%, respectively.
Figure 6: Dobutamine stress echocardiography. Apical four-chamber view at peak stress demonstrating normal myocardial perfusion with homogenous DEFINITY®. (A) Contrast enhancement of the myocardium before flash, (B) microbubbles destruction (flash) and (C) complete replenishment by 3 s after flash.
Figure 7: Representative example of qualitative and quantitative myocardial contrast echocardiography analysis.
Upper
panel: apical four-chamber view five beats postflash at rest (left) showing homogenous myocardial enhancement; while apical perfusion
defect is seen two beats postflash during peak stress (right, arrows). There is an artifactual ‘drop out’ segment in the lateral wall (*).
Lower panel: time–intensity curves are shown fitted to mono exponential function, Y = A (1−e-βt), where Y is video intensity at pulsing
interval t; β (sec-1) represents rate of rise in signal intensity (microbubble velocity) and A (dB) is peak plateau of videointensity (myocardial
blood volume) obtained from apical four-chamber view at peak stress with two ROI quantifying the defect and the normal adjacent
segment. ROI 1 (red) in apical region where the perfusion defect shows A = 20.0 dB and b of 0.85 s-1 while ROI 2 (yellow) shows A
parameter = 26.30 dB and b = 2.40 s-1.
ROI: Region of interest; SD: Standard deviation.
A prognostic role of myocardial perfusion during SE has been demonstrated in multiple studies. Tsutsui and colleagues studied 788 patients with real-time myocardial perfusion during dobutamine SE with a median follow-up of 20 months [62]. The authors reported that abnormal myocardial perfusion had significant incremental value over clinical factors, resting EF and wall motion in the prediction of cardiac events. In addition, Dawson and colleagues, in a study enrolling patients with known or suspected CAD and comparing dipyridamole perfusion echocardiography with simultaneous SPECT, noted that during a mean follow-up period of 14 months, abnormal perfusion echocardiography was found to be an independent predictor of adverse cardiac outcome (odds ratio: 23, 95% CI: 6–201; p < 0.001) and further provided an incremental prognostic value over clinical variables, LV systolic function, inducible wall-thickening abnormalities and SPECT results [63].
■ Cost–effectiveness of DEFINITY
Several studies have evaluated the cost–effectiveness of MCAs by comparing outcomes within patients undergoing echocardiography with and without MCA [5,40,64]. Moir and colleagues utilized DEFINITY for LVO during pharmacologic and/or exercise SE in 135 patients undergoing coronary angiography. The authors noted that LVO was of benefit to 14% of patients, unrelated to resting image quality. They also reported on improvement of sensitivity from 80 to 91%, p = 0.03, while nonsignificant improvement in specificity was observed (72–77%; p = 0.25) [64]. Despite that, the authors concluded that the use of DEFINITY was not cost effective in all patients; however, their methods were limited only to assessment of improvement in diagnostic accuracy in order to neutralize the cost of the intervention (contrast agent) and did not take into account the clinical impact and cost-savings in avoidance of additional downstream testing. In another study of 315 patients (277 received contrast vs 38 patients with no contrast) undergoing SE and SPECT studies, a net US$238 saving in the group receiving MCA was reported [41]. Other cost–effectiveness analyses by Shaw and colleagues [5], revealed 2.7-fold improvement in the diagnostic accuracy for patients with a nondiagnostic echocardiogram receiving contrast, with a cost saving of $319 per patient. Furthermore, Kurt and colleagues illustrated the impact of utilizing contrast echocardiography on the clinical management of 632 consecutive patients with technically difficult echocardiographic studies. The authors reported 32.8% of patients in whom additional diagnostic procedures were avoided, and alteration of drug management in 10% and concluded with a savings using contrast agent of $122 per patient on a cost–benefit analysis [30].
■ New technology with contrast echocardiography
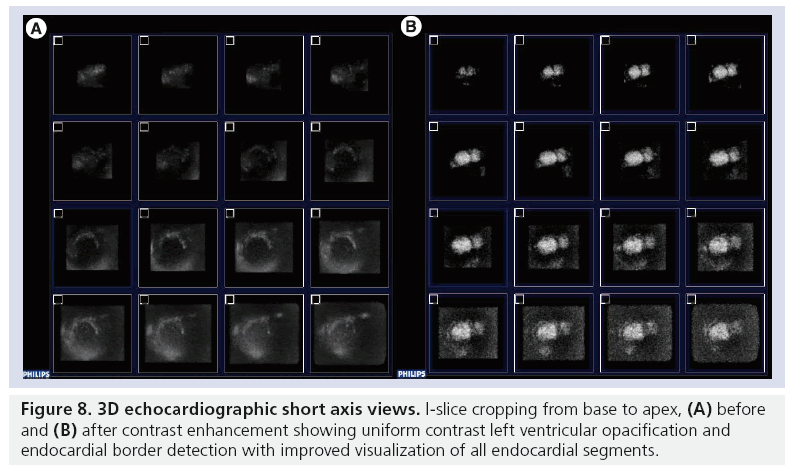
The diagnostic accuracy of echocardiography has been enhanced by the combined advances in 3D contrast echocardiography [65]. Recent improvements in 3D contrast technology include the incorporation of power modulation imaging, which provides incremental benefit for visualization of microbubbles in the myocardial microcirculation and significant advantages in image acquisition time [66]. We and others have reported on the preliminary observations of feasibility and potential benefit of 3D over 2D echocardiography in the assessment of myocardial perfusion utilizing DEFINITY [66–68]. 3D transducer technology improvements including increased operating frequency and bandwidth, transducer miniaturization, and flash technology have been developed, and continue to be refined. Figure 8 demonstrates 3D echocardiographic short axis views in 16 segments I-slice cropping format, base to apex, pre- and post-contrast enhancement. Complete LVO with improved visualization of all endocardial segments can be seen.
Postmarketing surveillance
Postmarketing surveillance is mandated by the FDA for routine postapproval monitoring of drug safety. As part of this process, a sequence of events occurred that have affected labeling of the MCA class. A ‘box warning’ was placed in October 2007 after rare fatalities (n = 4) and uncommon serious cardiopulmonary adverse events (n = 197) thought to be temporally related to the administration of MCA were reported [104]. This action followed that in Europe, as the European Medicine Agency halted use of a sulfurhexafluoride-based MCA for similar concerns [106]. In May 2008, after review of the accumulated safety data, including additional safety reports on larger cohorts of patients, validations of the usefulness of MCA in patient management and cost–effectiveness, the FDA revised the labeling changes to reflect the established clinical safety record of MCAs [104]. More recently, in May 2011, the FDA has again revised DEFINITY label (see safety data) [104]. At present, the only contraindications to DEFINITY are those originally placed in the label, including patients with known hypersensitivity or those with known or suspected right-to-left or bidirectional shunts.
Safety
Several reviews have indicated that all the ultrasound MCAs have microscale bioeffects in vivo when given at sufficient concentrations and exposed to higher ultrasound energy for longer times [69]. Two main bioeffect concerns included the increased frequency of premature ventricular contractions and the possibility of vascular injury and cardiac injury [70,71]. These were associated mostly with imaging at a high mechanical index [72,73]. However, these bioeffects were not reported with low mechanical index imaging [37]. Given the physical and chemical structure similarities of the MCA, the safety risks for one agent of class microbubbles may represent risks for all members of the drug class. The body of evidence drawn from large databases followed the FDA box warning, aiming to evaluate the safety of PFC-based MCA. Table 7 reports on the safety published studies on MCA focusing on DEFINITY.
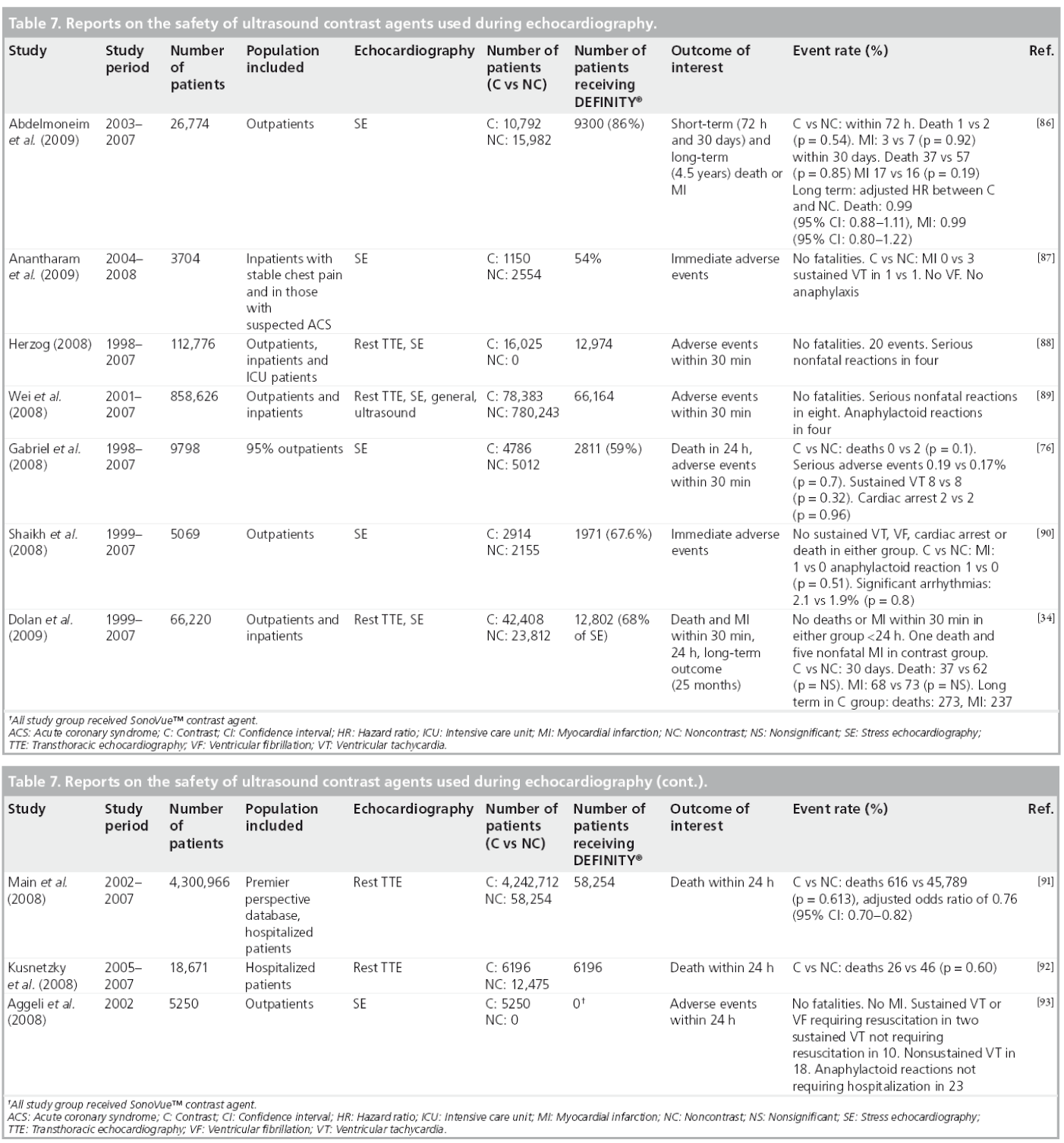
In a recent meta-analysis of published studies (up to October 2009) on the adverse cardiovascular events (myocardial infarction and all-cause mortality) with MCAs [74], the authors noted that the cumulative evidence was not suggestive for any MCA-related increase in the incidence of mortality or myocardial infarction with a pooled odds ratio of 0.57 (95% CI: 0.32–1.01; p = 0.05); and 0.85 (95% CI: 0.35–2.05; p = 0.72), respectively.
In response to the FDA call for postmarketing registries to evaluate clinical outcomes and potential MCA serious adverse events, two recent Phase IV studies were conducted and presented at the ASE 21st Annual Scientific Sessions. Additionally the data were presented in the FDA joint meeting of the Cardiovascular and Renal Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee discussing perflutren lipid microsphere in May 2011 [107]. The CARES multicenter safety registry was one of the Phase IV studies implemented in the setting of routine clinical practice per MCA guideline indications. The CARES study evaluated all adverse events within 30 min and 24 h of DEFINITY administration including life-threatening cardiopulmonary events in 1053 dosed patients (mean age: 61.3 ± 12.9 years) at 15 USA sites. This study reported no deaths or life-threatening events at 24 h post-DEFINITY [107].
Reported findings on pulmonary hemodynamics in preclinical studies [23,24] on pigs raised additional concerns regarding the possibility of increased risk for serious cardiopulmonary reactions in patients with significant pulmonary hypertension and receiving MCA. In response to the FDA call for postmarketing registries to evaluate safety in patients with pulmonary hypertension, Wei and colleagues presented a prospective multicenter open-label nonrandomized, placebo-controlled Phase IV safety study in 32 patients (mean age: 57.2 ± 12.9) [26]. The authors evaluated pulmonary and systemic hemodynamics invasively in patients with (pulmonary artery systolic pressure, [PASP] >35 mmHg) and without pulmonary hypertension (PASP ≤35 mmHg) and receiving DEFINITY. No deaths or serious adverse events were reported. In addition, no change in PASP hemodynamics was associated with DEFINITY [107]. However, this study excluded patients with baseline PASP of >75 mmHg.
We [75] and others [76] have also demonstrated the safety of MCAs in pulmonary hypertension patients. Gabriel and colleagues reported no evidence of serious adverse events in 57 patients with moderate-to-severe pulmonary hypertension (right ventricular systolic pressure >50 mmHg) who underwent SE [76]. Our group has also reported no significant difference in short- or long-term events (4.6 years) between the contrast and noncontrast cohorts, with an adjusted hazard ratio (95% CI) for death and myocardial infarction of 1.10 (0.80–1.50; p = 0.56) and 0.34 (0.11–1.03; p = 0.06), respectively [75].
Following the DEFINITY safety data presented for FDA review in September 2010 and in May 2011, the boxed warning contained within the DEFINITY label was re-revised in respect to patients with pulmonary hypertension or unstable cardiopulmonary conditions, in whom the 30 min post-DEFINITY monitoring was no longer required. In addition, the committee concurred with the safety and efficacy data presented for DEFINITY during exercise and pharmacologic stress testing and hence the statement regarding lack of such evidence was removed. Furthermore, a new label change included a statement about serious reactions uncommonly occurring but if they do, it would usually be within 30 min of administration, which negates the need for additional monitoring beyond the timeframe of performing the echocardiographic study [104].
Regulatory affairs
DEFINITY was approved in Canada for use with echocardiography and radiology in December 2000 and in the USA in 2001 for use with echocardiography only. It is also available for echocardiographic and or radiologic use in Latin America, Europe, Australia and some Asian countries [104].
Conclusion
Based on data available from original publications, reviews and guidelines, the use of MCA with echocardiography in patients with suboptimal images is considered part of standard clinical echocardiographic practice and is supported by ultrasound professional societies (ASE, International Contrast Ultrasound Society, ICAEL). Test feasibility and performance, confidence of interpretation, and cost–effectiveness is significantly greater with contrast enhancement when indicated. After rigorous evaluation, DEFINITY MCA has demonstrated a good safety profile with documented benefit, and low risk, which is comparable to other noninvasive imaging modalities, with the distinct advantages of portability, lack of nephrotoxicity or radiation risk.
Future perspective
MCAs provide an established tool in contemporary echocardiography practice. Clinical applications for the use of MCAs for LVO and EBD are well established and regulated. The off-label applications of MCA for evaluation of myocardial perfusion, as well as perfusion of noncardiac vascular beds as assessed in radiologic imaging, are being evaluated in numerous clinical trials, while also being utilized daily
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Gramiak R, Shah PM, Kramer DH. Ultrasound cardiography: contrast studies in anatomy and function. Radiology 92(5), 939–948 (1969).
- Mulvagh SL, Rakowski H, Vannan MA et al. American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J. Am. Soc. Echocardiogr. 21(11), 1179–1201; quiz 1281 (2008).
- Kitzman DW, Goldman ME, Gillam LD, Cohen JL, Aurigemma GP, Gottdiener JS. Efficacy and safety of the novel ultrasound contrast agent perflutren (definity) in patients with suboptimal baseline left ventricular echocardiographic images. Am. J. Cardiol. 86(6), 669–674 (2000).
- Hoffmann R, von Bardeleben S, Ten Cate F et al. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur. Heart J. 26(6), 607–616 (2005).
- Shaw LJ, Monaghan MJ, Nihoyannopolous P. Clinical and economic outcomes assessment with myocardial contrast echocardiography. Heart 82(Suppl. 3), III16–III21 (1999).
- Wilson SR, Burns PN. Microbubbleenhanced US in body imaging: what role? Radiology 257(1), 24–39 (2010).
- Senior R, Becher H, Monaghan M et al. Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. Eur. J. Echocardiogr. 10(2), 194–212 (2009).
- Douglas PS, Garcia MJ, Haines DE et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/ SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J. Am. Soc. Echocardiogr. 24(3), 229–267 (2011).
- Ayida G, Harris P, Kennedy S, Seif M, Barlow D, Chamberlain P. Hysterosalpingo-contrast sonography (HyCoSy) using Echovist-200 in the outpatient investigation of infertility patients. Br. J. Radiol. 69(826), 910–913 (1996).
- Bouakaz A, de Jong N, Cachard C, Jouini K. On the effect of lung filtering and cardiac pressure on the standard properties of ultrasound contrast agent. Ultrasonics 36(1–5), 703–708 (1998).
- Feinstein SB, Cheirif J, Ten Cate FJ et al. Safety and efficacy of a new transpulmonary ultrasound contrast agent: initial multicenter clinical results. J. Am. Coll. Cardiol. 16(2), 316–324 (1990).
- Cohen JL, Cheirif J, Segar DS et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a Phase III multicenter trial. J. Am. Coll. Cardiol. 32(3), 746–752 (1998).
- Unger EC, Hersh E, Vannan M, Matsunaga TO, Mccreery T. Local drug and gene delivery through microbubbles. Prog. Cardiovasc. Dis. 44(1), 45–54 (2001).
- Rosenberg ML, Carpenter AP. Contrast material-enhanced abdominal US examinations with DMP 115 (DEFINITY) provides additional diagnostic information with potential for changes in patient management. Acad. Radiol. 9(Suppl. 1), S243–S245 (2002).
- Schutt EG, Klein DH, Mattrey RM, Riess JG. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: the key role of perfluorochemicals. Angew. Chem. Int. Ed. Engl. 42(28), 3218–3235 (2003).
- Porter TR, Xie F, Li S, D’Sa A, Rafter P. Increased ultrasound contrast and decreased microbubble destruction rates with triggered ultrasound imaging. J. Am. Soc. Echocardiogr. 9(5), 599–605 (1996).
- Averkiou M, Powers J, Skyba D, Bruce M, Jensen S. Ultrasound contrast imaging research. Ultrasound Q. 19(1), 27–37 (2003).
- Moran CM, Watson RJ, Fox KA, McDicken WN. In vitro acoustic characterisation of four intravenous ultrasonic contrast agents at 30 MHz. Ultrasound Med. Biol. 28(6), 785–791 (2002).
- Hutter JC, Luu HM, Mehlhaff PM, Killam AL, Dittrich HC. Physiologically based pharmacokinetic model for fluorocarbon elimination after the administration of an octafluoropropane-albumin microsphere sonographic contrast agent. J. Ultrasound Med. 18(1), 1–11 (1999).
- Weissman NJ, Cohen MC, Hack TC, Gillam LD, Cohen JL, Kitzman DW. Infusion versus bolus contrast echocardiography: a multicenter, open-label, crossover trial. Am. Heart J. 139(3), 399–404 (2000).
- Kobayashi N, Yasu T, Yamada S et al. Influence of contrast ultrasonography with perflutren lipid microspheres on microvessel injury. Circ. J. 67(7), 630–636 (2003).
- Li P, Armstrong WF, Miller DL. Impact of myocardial contrast echocardiography on vascular permeability: comparison of three different contrast agents. Ultrasound Med. Biol. 30(1), 83–91 (2004).
- De Chantal M, Amyot R, Diodati JG, Leblanc AR, Pharand C. Absence of clinically significant increase in pulmonary artery pressure after intravenous perflutren injection for myocardial perfusion imaging in pigs. J. Am. Soc. Echocardiogr. 18(12), 1299–1303 (2005).
- Grauer SE, Pantely GA, Xu J et al. Myocardial imaging with a new transpulmonary lipid-fluorocarbon echo contrast agent: experimental studies in pigs. Am. Heart J. 132(5), 938–945 (1996).
- Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology 216(2–3), 106–121 (2005).
- Wei K, Main ML, Lang RM et al. The effect of Definity on systemic and pulmonary hemodynamics in patients. J. Am. Soc. Echocardiogr. doi:10.1016/j.echo.2012.01.019 (2012) (Epub ahead of print).
- Mychaskiw G 2nd, Badr AE, Tibbs R, Clower BR, Zhang JH. Optison (FS069) disrupts the blood–brain barrier in rats. Anesth. Analg. 91(4), 798–803 (2000).
- Fritz TA, Unger EC, Sutherland G, Sahn D. Phase I clinical trials of MRX-115. A new ultrasound contrast agent. Invest. Radiol. 32(12), 735–740 (1997).
- Pantely GA, Joyce M, Grauer SE, Giraud GD, Shiota T, Sahn D. Phase II, placebo-controlled trial of MRX115 (Aerosomes) for left heart and myocardial perfusion imaging. Acad. Radiol. 5(Suppl. 1), S66–S68; discussion S72–S74 (1998).
- Kurt M, Shaikh KA, Peterson L et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J. Am. Coll. Cardiol. 53(9), 802–810 (2009).
- Nayyar S, Magalski A, Khumri TM et al. Contrast administration reduces interobserver variability in determination of left ventricular ejection fraction in patients with left ventricular dysfunction and good baseline endocardial border delineation. Am. J. Cardiol. 98(8), 1110–1114 (2006).
- Grayburn PA, Weiss JL, Hack TC et al. Phase III multicenter trial comparing the efficacy of 2% dodecafluoropentane emulsion (EchoGen) and sonicated 5% human albumin (Albunex) as ultrasound contrast agents in patients with suboptimal echocardiograms. J. Am. Coll. Cardiol. 32(1), 230–236 (1998).
- Abdelmoneim SS, Mulvagh SL. Stress echocardiography-state of the art. US Cardiology 6(2), 16–26 (2009).
- Dolan MS, Gala SS, Dodla S et al. Safety and efficacy of commercially available ultrasound contrast agents for rest and stress echocardiography a multicenter experience. J. Am. Coll. Cardiol. 53(1), 32–38 (2009).
- Plana JC, Mikati IA, Dokainish H et al. A randomized cross-over study for evaluation of the effect of image optimization with contrast on the diagnostic accuracy of dobutamine echocardiography in coronary artery disease: the OPTIMIZE trial. JACC Cardiovasc. imaging 1(2), 145–152 (2008).
- Moir S, Haluska BA, Jenkins C, Fathi R, Marwick TH. Incremental benefit of myocardial contrast to combined dipyridamole-exercise stress echocardiography for the assessment of coronary artery disease. Circulation 110(9), 1108–1113 (2004).
- Tsutsui JM, Elhendy A, Xie F, O’Leary EL, McGrain AC, Porter TR. Safety of dobutamine stress real-time myocardial contrast echocardiography. J. Am. Coll. Cardiol. 45(8), 1235–1242 (2005).
- Dolan MS, Riad K, El-Shafei A et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am. Heart J. 142(5), 908–915 (2001).
- Rainbird AJ, Mulvagh SL, Oh JK et al. Contrast dobutamine stress echocardiography: clinical practice assessment in 300 consecutive patients. J. Am. Soc. Echocardiogr. 14(5), 378–385 (2001).
- Xie F, Tsutsui JM, Mcgrain AC et al. Comparison of dobutamine stress echocardiography with and without real-time perfusion imaging for detection of coronary artery disease. Am. J. Cardiol. 96(4), 506–511 (2005).
- Thanigaraj S, Nease RF Jr, Schechtman KB, Wade RL, Loslo S, Perez JE. Use of contrast for image enhancement during stress echocardiography is cost-effective and reduces additional diagnostic testing. Am. J. Cardiol. 87(12), 1430–1432 (2001).
- Almeida AG, David CN, Gabriel HM et al. Role of real-time myocardial contrast echocardiography in the assessment of viability after acute myocardial infarction and angioplasty. Rev. Port. Cardiol. 23(10), 1277–1287 (2004).
- Vlassak I, Rubin DN, Odabashian JA et al. Contrast and harmonic imaging improves accuracy and efficiency of novice readers for dobutamine stress echocardiography. Echocardiography 19(6), 483–488 (2002).
- Yokoyama N, Schwarz KQ, Steinmetz SD, Li X, Chen X. Prognostic value of contrast stress echocardiography in patients with image quality too limited for traditional noncontrast harmonic echocardiography. J. Am. Soc. Echocardiogr. 17(1), 15–20 (2004).
- Wake R, Takeuchi M, Yoshitani H et al. Role of contrast-enhanced dobutamine stress echocardiography in predicting outcome in patients with known or suspected coronary artery disease. Echocardiography 23(8), 642–649 (2006).
- Wake R, Takeuchi M, Yoshikawa J, Yoshiyama M. Effects of gender on prognosis of patients with known or suspected coronary artery disease undergoing contrast-enhanced dobutamine stress echocardiography. Circ. J. 71(7), 1060–1066 (2007).
- Biagini E, Elhendy A, Bax JJ et al. Seven-year follow-up after dobutamine stress echocardiography: impact of gender on prognosis. J. Am. Coll. Cardiol. 45(1), 93–97 (2005).
- Cortigiani L, Dodi C, Paolini EA, Bernardi D, Bruno G, Nannini E. Prognostic value of pharmacological stress echocardiography in women with chest pain and unknown coronary artery disease. J. Am. Coll. Cardiol. 32(7), 1975–1981 (1998).
- Cukon-Buttignoni S, Abdelmoneim SS, Ehrsam JE et al. Regional diastolic contour abnormalities during contrast stress echocardiography: improved detection of coronary artery disease. J. Am. Soc. Echocardiogr. 21(10), 1109–1115 (2008).
- Ward RP, Weinert L, Spencer KT et al. Quantitative diagnosis of apical cardiomyopathy using contrast echocardiography. J. Am. Soc. Echocardiogr. 15(4), 316–322 (2002).
- Abdelmoneim SS, Mankad SV, Bernier M et al. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J. Am. Soc. Echocardiogr. 22(11), 1249–1255 (2009).
- Abdelmoneim SS, Wijdicks EF, Lee VH et al. Real-time myocardial perfusion contrast echocardiography and regional wall motion abnormalities after aneurysmal subarachnoid hemorrhage. Clinical article. J. Neurosurg. 111(5), 1023–1028 (2009).
- Shimoni S, Zoghbi WA, Xie F et al. Real-time assessment of myocardial perfusion and wall motion during bicycle and treadmill exercise echocardiography: comparison with single photon emission computed tomography. J. Am. Coll. Cardiol. 37(3), 741–747 (2001).
- Korosoglou G, Dubart AE, Dasilva KG Jr et al. Real-time myocardial perfusion imaging for pharmacologic stress testing: added value to single photon emission computed tomography. Am. Heart J. 151(1), 131–138 (2006).
- Chiou KR, Huang WC, Lin SL et al. Real-time dobutamine stress myocardial contrast echocardiography for detecting coronary artery disease: correlating abnormal wall motion and disturbed perfusion. Can. J. Cardiol. 20(12), 1237–1243 (2004).
- Malm S, Frigstad S, Torp H, Wiseth R, Skjarpe T. Quantitative adenosine real-time myocardial contrast echocardiography for detection of angiographically significant coronary artery disease. J. Am. Soc. Echocardiogr. 19(4), 365–372 (2006).
- Olszowska M, Kostkiewicz M, Tracz W, Przewlocki T. Assessment of myocardial perfusion in patients with coronary artery disease. Comparison of myocardial contrast echocardiography and 99mTc MIBI single photon emission computed tomography. Int. J. Cardiol. 90(1), 49–55 (2003).
- Peltier M, Vancraeynest D, Pasquet A et al. Assessment of the physiologic significance of coronary disease with dipyridamole real-time myocardial contrast echocardiography. Comparison with technetium-99m sestamibi single-photon emission computed tomography and quantitative coronary angiography. J. Am. Coll. Cardiol. 43(2), 257–264 (2004).
- Winter R, Gudmundsson P, Willenheimer R. Real-time perfusion adenosine stress echocardiography in the coronary care unit: a feasible bedside tool for predicting coronary artery stenosis in patients with acute coronary syndrome. Eur. J. Echocardiogr. 6(1), 31–40 (2005).
- Abdelmoneim SS, Dhoble A, Bernier M et al. Quantitative myocardial contrast echocardiography during pharmacological stress for diagnosis of coronary artery disease: a systematic review and meta-analysis of diagnostic accuracy studies. Eur. J. Echocardiogr. 10(7), 813–825 (2009).
- Dijkmans PA, Senior R, Becher H et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J. Am. Coll. Cardiol. 48(11), 2168–2177 (2006).
- Tsutsui JM, Elhendy A, Anderson JR, Xie F, Mcgrain AC, Porter TR. Prognostic value of dobutamine stress myocardial contrast perfusion echocardiography. Circulation 112(10), 1444–1450 (2005).
- Dawson D, Kaul S, Peters D et al. Prognostic value of dipyridamole stress myocardial contrast echocardiography: comparison with single photon emission computed tomography. J. Am. Soc. Echocardiogr. 22(8), 954–960 (2009).
- Moir S, Shaw L, Haluska B, Jenkins C, Marwick TH. Left ventricular opacification for the diagnosis of coronary artery disease with stress echocardiography: an angiographic study of incremental benefit and cost–effectiveness. Am. Heart J.154(3), 510–518 (2007).
- Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur. Heart J. 30(1), 98–106 (2009).
- Lang RM, Mor-Avi V, Sugeng L, Nieman PS, Sahn DJ. Three-dimensional echocardiography: the benefits of the additional dimension. J. Am. Coll. Cardiol. 48(10), 2053–2069 (2006).
- Abdelmoneim SS, Bernier M, Dhoble A et al. Assessment of myocardial perfusion during adenosine stress using real time threedimensional and two-dimensional myocardial contrast echocardiography: comparison with single-photon emission computed tomography. Echocardiography 27(4), 421–429 (2010).
- Aggeli C, Felekos I, Roussakis G et al. Value of real-time three-dimensional adenosine stress contrast echocardiography in patients with known or suspected coronary artery disease. Eur J Echocardiogr. 12(9), 648–55 (2011).
- Miller DL, Averkiou MA, Brayman AA et al. Bioeffects considerations for diagnostic ultrasound contrast agents. J. Ultrasound Med. 27(4), 611–632; quiz 633–636 (2008).
- Vancraeynest D, Kefer J, Hanet C et al. Release of cardiac bio-markers during high mechanical index contrast-enhanced echocardiography in humans. Eur. Heart J. 28(10), 1236–1241 (2007).
- Borges AC, Walde T, Reibis RK et al. Does contrast echocardiography with Optison induce myocardial necrosis in humans? J. Am. Soc. Echocardiogr. 15(10 Pt 1), 1080–1086 (2002).
- van der Wouw PA, Brauns AC, Bailey SE, Powers JE, Wilde AA. Premature ventricular contractions during triggered imaging with ultrasound contrast. J. Am. Soc. Echocardiogr. 13(4), 288–294 (2000).
- Raisinghani A, Wei KS, Crouse L et al. Myocardial contrast echocardiography (MCE) with triggered ultrasound does not cause premature ventricular complexes: evidence from PB127 MCE studies. J. Am. Soc. Echocardiogr. 16(10), 1037–1042 (2003).
- Khawaja OA, Shaikh KA, Al-Mallah MH. Meta-analysis of adverse cardiovascular events associated with echocardiographic contrast agents. Am. J. Cardiol. 106(5), 742–747 (2010).
- Abdelmoneim SS, Bernier M, Scott CG et al. Safety of contrast agent use during stress echocardiography in patients with elevated right ventricular systolic pressure: a cohort study. Circ. Cardiovasc. Imaging 3(3), 240–248 (2010).
- Gabriel RS, Smyth YM, Menon V et al. Safety of ultrasound contrast agents in stress echocardiography. Am. J. Cardiol. 102(9), 1269–1272 (2008).
- Porter TR, Xie F, Silver M, Kricsfeld D, Oleary E. Real-time perfusion imaging with low mechanical index pulse inversion Doppler imaging. J. Am. Coll. Cardiol. 37(3), 748–753 (2001).
- Elhendy A, O’Leary EL, Xie F, Mcgrain AC, Anderson JR, Porter TR. Comparative accuracy of real-time myocardial contrast perfusion imaging and wall motion analysis during dobutamine stress echocardiography for the diagnosis of coronary artery disease. J. Am. Coll. Cardiol. 44(11), 2185–2191 (2004).
- Elhendy A, Tsutsui JM, O’Leary EL, Xie F, Mcgrain AC, Porter TR. Noninvasive diagnosis of coronary artery disease in patients with diabetes by dobutamine stress real-time myocardial contrast perfusion imaging. Diabetes Care 28(7), 1662–1667 (2005).
- Moir S, Haluska BA, Jenkins C, McNab D, Marwick TH. Myocardial blood volume and perfusion reserve responses to combined dipyridamole and exercise stress: a quantitative approach to contrast stress echocardiography. J. Am. Soc. Echocardiogr. 18(11), 1187–1193 (2005).
- Tsutsui JM, Xie F, Mcgrain AC et al. Comparison of low-mechanical index pulse sequence schemes for detecting myocardial perfusion abnormalities during vasodilator stress echocardiography. Am. J. Cardiol. 95(5), 565–570 (2005).
- Elhendy A, Tsutsui JM, O’Leary EL, Xie F, Porter TR. Noninvasive diagnosis of coronary artery bypass graft disease by dobutamine stress real-time myocardial contrast perfusion imaging. J. Am. Soc. Echocardiogr. 19(12), 1482–1487 (2006).
- Toledo E, Jacobs LD, Lodato JA et al. Quantitative diagnosis of stress-induced myocardial ischemia using analysis of contrast echocardiographic parametric perfusion images. Eur. J. Echocardiogr. 7(3), 217–225 (2006).
- Xie F, Hankins J, Mahrous HA, Porter TR. Detection of coronary artery disease with a continuous infusion of definity ultrasound contrast during adenosine stress real time perfusion echocardiography. Echocardiography 24(10), 1044–1050 (2007).
- Abdelmoneim SS, Bernier M, Dhoble A et al. Diagnostic accuracy of contrast echocardiography during adenosine stress for detection of abnormal myocardial perfusion: a prospective comparison with technetium-99 m sestamibi single-photon emission computed tomography. Heart Vessels 25(2), 121–130 (2010).
- Abdelmoneim SS, Bernier M, Scott CG et al. Safety of contrast agent use during stress echocardiography: a 4‑year experience from a single-center cohort study of 26,774 patients. JACC Cardiovasc. Imaging 2(9), 1048–1056 (2009).
- Anantharam B, Chahal N, Chelliah R, Ramzy I, Gani F, Senior R. Safety of contrast in stress echocardiography in stable patients and in patients with suspected acute coronary syndrome but negative 12-hour troponin. Am. J. Cardiol. 104(1), 14–18 (2009).
- Herzog CA. Incidence of adverse events associated with use of perflutren contrast agents for echocardiography. JAMA 299(17), 2023–2025 (2008).
- Wei K, Mulvagh SL, Carson L et al. The safety of Definity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J. Am. Soc. Echocardiogr. 21(11), 1202–1206 (2008).
- Shaikh K, Chang SM, Peterson L et al. Safety of contrast administration for endocardial enhancement during stress echocardiography compared with noncontrast stress. Am. J. Cardiol. 102(11), 1444–1450 (2008).
- Main ML, Ryan AC, Davis TE, Albano MP, Kusnetzky LL, Hibberd M. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients). Am. J. Cardiol. 102(12), 1742–1746 (2008).
- Kusnetzky LL, Khalid A, Khumri TM, Moe TG, Jones PG, Main ML. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent: results in 18,671 consecutive studies. J. Am. Coll. Cardiol. 51(17), 1704–1706 (2008).
- Aggeli C, Giannopoulos G, Roussakis G et al. Safety of myocardial flash-contrast echocardiography in combination with dobutamine stress testing for detection of ischemia in 5250 studies. Heart 94(12), 1571–1577 (2008).
- Intersocietal Commission for the Accreditation of Echocardiography Laboratories (ICAEL). ICAEL Standards for Accreditation in Adult Echocardiography 2010: Parts I through IV. www.icael.org/intersocietal.htm (Accessed 1 September 2011)
- International Ultrasound Contrast Society. www.icus-society.org (Accessed 1 September 2011)
- Definity Imaging. Product prescribing information. www.definityimaging.com (Accessed 1 November 2011)
- US FDA. Information for healthcare professionals. www.fda.gov/drugs/drugsaety/postmarketd rugsafetyinformationforpatientsandproviders (Accessed 29 September 2011)
- US FDA. Food and Drug Administeration Center for Drug Evaluation and Research. www.accessdata.fda.gov/scripts/cder/ drugsatfda/index.cfm?fuseaction=search. drugdetails (Accessed 10 July 2011)
- European Medicines Agency. Information on SonoVue. www.ema.europa.eu/ema (Accessed 10 July 2011)
- US FDA. Joint Meeting of the Cardiovascular and Renal Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee-DEFINITY®. www.fda.gov/downloads/ advisorycommittees/committeesmeeting materials/drugs/cardiovascularand renaldrugsadvisorycommittee/ ucm254502.pdf (Accessed 1 November 2011)
• Mandatory for all performers of contrast echocardiography.
• • Provides the guidelines for the appropriate use of echocardiography.
• Provides an overview on stress echocardiography, including contrast echocardiography.
• • Important study discussing the cost–effectiveness of contrast echocardiography.
• Comprehensive review on studies addressing the safety of contrast agents.
■ Websites