Case Report - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 2
Obesity-induced AA amyloidosis: A diagnosis of exclusion
- Corresponding Author:
- Anne F. Brunger
Department of Rheumatology & Clinical Immunology
Amyloidosis Center of Expertise, University of Groningen
University Medical Center Groningen, Groningen, The Netherlands
E-mail:a.f.brunger@umcg.nl
Abstract
A 47-year-old woman with morbid obesity (body mass index: 41 kg/m2) and a history of hypertension, pulmonary embolism and successfully treated gout (one year ago) presented with nephrotic syndrome (15 g/24 h) and loss of kidney function (endogenous creatinine clearance 27 ml/min/1.73 m2). A kidney biopsy revealed AA amyloid. An extensive investigation was performed to detect an underlying inflammatory disease process by testing blood and urine, imaging and genetic testing for autoinflammatory diseases. Serum levels of C-reactive protein (CRP) and serum amyloid A protein (SAA) were continuously elevated. Serum amyloid P component (SAP)- scintigraphy showed intense uptake in a massively enlarged liver and in the spleen. This extensive investigation did not reveal an underlying inflammatory process. Her gout became asymptomatic almost immediately after start of treatment. Therefore, we concluded that morbid obesity was the most probable cause of her AA amyloidosis. Treatment with colchicine and prednisolone did not substantially reduce the SAA and CRP levels. Also, treatment with anakinra (interleukin-1 receptor antagonist) and tocilizumab (interleukin-6 receptor antagonist) failed. The downhill course of the disease progressed to complete renal failure within three years and dialysis was started. This case indicates that the long-standing low-grade inflammation seen in morbid obesity may be a potential cause of systemic AA amyloidosis and may be difficult to treat. However, it is essentially a diagnosis of exclusion, since known underlying inflammatory conditions should be excluded first.Keywords
hobesity • AA amyloidosis • nephrotic syndrome • SAA • SAP scan
Abbreviations
AA amyloidosis: Amyloid A amyloidosis; anti-CCP: anti-Cyclic Citrulline Peptide antibody; anti-IL-1: Interleukin-1 receptor antagonist; anti-IL6: Interleukin-6 receptor antagonist; BMI: Body Mass Index; CRP: C-Reactive Protein; CT: Computer Tomography; ESR: Erythrocyte Sedimentation Rate; FLC: immunoglobulin Free Light Chain; GLP-1: Glucagon-Like Peptide-1 receptor agonists; SGLT-2 inhibitors: inhibitors of the sodium-glucose cotransporter-2; IL-1: nterleukin-1; IL-6: Interleukin-6; MCV: Mean Corpuscular Volume; MRI: Magnetic Resonance Imaging; MCP-1: Monocyte Chemotactic Protein-1; FDG-PET: 18F-Fludeoxiglucose Positron Emission Tomography; SAP: Serum Amyloid P component; SAA: Serum Amyloid A protein; SPECT: Single-Photon Emission Computed Tomography; TNF: Tumor Necrosis Factor; TRAPS: Tumor necrosis factor Receptor- Associated Periodic Syndrome
Introduction
Amyloidosis is a group of diseases characterised by extracellular deposition of insoluble amyloid fibrils leading to organ dysfunction [1]. These fibrils are derived from different soluble protein precursors, each characteristic for a specific type of amyloidosis. In AA amyloidosis the fibrils are composed of fragments of the acute phase reactant Serum Amyloid A protein (SAA) produced by the liver in response to an inflammatory process. Therefore, AA amyloidosis is considered to be a complication of long-standing inflammation, as seen in autoimmune and autoinflammatory diseases, malignancies, and chronic or recurrent infections.
Since obesity is associated with a chronic (low-grade) inflammatory state [2] it has been suggested that obesity could be a potential cause of AA amyloidosis [3,4]. In 1977 the development of amyloidosis in obese mice was observed [5]. Further experimental studies in mice confirmed that the development of renal AA amyloidosis, in combination with significantly elevated systemic SAA levels, was associated with a high fat diet [6]. In 2005 the role of obesity-induced chronic inflammation and the occurrence of AA amyloidosis in humans was described for the first time in one of two sisters with extreme obesity due to a genetic leptin receptor deficiency (Table 1) [3]. In 2009 another case of renal AA amyloidosis secondary to morbid obesity was published in Spain [7]. A recent study from France describes 13 patients with AA amyloidosis in whom no other cause of chronic inflammation and AA amyloidosis than obesity could be detected [8]. Unlike other cases published so far, the patients included in this last report had a milder degree of obesity (median Body Mass Index (BMI)=35 kg/m2) compared to the two other cases where patients were extremely obese (BMI 50 and 74 kg/m2). In addition, Blank et al. substantiated these observations in 2018 by showing that obesity is a susceptibility factor in patients with idiopathic AA amyloidosis [9].
Table 1. Literature overview: case reports describing cases with obesity-induced AA amyloidosis.
| No. patients | BMI | Serum SAA mg/l and CRP mg/l | Biopsy | Histologic conformation | Clinical picture of AA amyloidosis | Other diagnosis |
|---|---|---|---|---|---|---|
| 1 | 74 | Low grade inflammation | Kidney | CR, AG, IH | Impaired renal function and nephrotic syndrome | None reported |
| 1 | 50 | SAA 17mg/l | Kidney | CR, IH | Impaired renal function and nephrotic syndrome | Respiratory tract infection on admission. History mammillary fibroadenoma |
| 13 | Median 35 | Median SAA 19 (8-41), CRP 21 (12-67) |
Kidney (all), salivary gland (4) | CR, AG, IH | Impaired renal function (all), nephrotic syndrome (4) | Diabetes mellitus type 2 (5), osteomyelitis (1) |
| 37 | Median 31 | Median SAA 28 (1-264), CRP 15 (1-281) |
Abdominal fat and kidney (all) | CR, AG, IH | Impaired renal function or proteinuria | None reported |
| 1 | 41 | SAA 16 CRP 81 |
Kidney, liver | CR, AG, IH | Impaired renal function and nephrotic syndrome | Gout |
CR: Congo red, AG: Apple-Green birefringence, IH: Immunohistochemistry using anti-AA antibodies
This case report describes a patient with AA amyloidosis as a consequence of morbid obesity. The aim of describing this case report is firstly to demonstrate the problem of establishing the underlying cause of AA amyloidosis, namely that obesity-induced AA amyloidosis is a diagnosis of exclusion, and secondly the lack of therapy to supress low-grade inflammation and thus the failure to normalize the SAA serum levels resulting in ongoing deposition of amyloid leading to organ failure.
Case report
In 2012 a 47-year-old woman was admitted to our hospital for diagnostic evaluation of a nephrotic syndrome. Amyloidosis was diagnosed, based upon typical findings in a kidney biopsy: positive staining with Congo red and apple-green birefringence in polarized light. The amyloid was immunohistochemically characterised as AA type. Other findings during the diagnostic work-up were an increased alkaline phosphatase 376 U/l (N<120 U/l), gamma glutamyl transferase 174 U/l (N<45 U/l), and massive hepatomegaly with an inhomogeneous aspect shown by abdominal ultrasound. The patient also appeared to have hypothyroidism for which levothyroxine substitution was started. Both the electrocardiogram and echocardiography did not show any signs of cardiac involvement.
Past medical history revealed hypertension, obesity, double-sided pulmonary embolism and gout (diagnosed in 2011 based upon clinical findings), for which she had been treated successfully with allopurinol and prednisolone.
At referral the patient complained about arthralgia mainly in her left hand (dig II and III) but also in her knees and ankles, which had all started after tapering the prednisolone three days earlier. She also complained about morning stiffness which improved with motion. Furthermore, she reported to have a dry mouth, without dry eyes. History did not reveal other diagnostic clues for an underlying inflammatory autoimmune disorder, infection or malignancy.
Physical examination showed a morbidly obese woman, with a BMI of 41 kg/m2. Systolic blood pressure was 160/80 mm Hg despite using two antihypertensive drugs. Joint examination revealed an active arthritis of the left third metacarpophalangeal joint. Examination of the head and neck region, heart, lungs and abdomen did not show any abnormalities, although examination of the abdomen was very limited due to significant adiposity.
Laboratory tests showed inflammation (C-Reactive Protein (CRP) 81 mg/l (N<2.0 mg/l), SAA 16 mg/l (N<4.2 mg/l), Erythrocyte Sedimentation Rate (ESR) 115 mm/h (N<20 mm/h), haemoglobin 6.2 mmol/l (N>7.5 mmol/l), mean corpuscular volume 84 fl (N 80-96 fl), thrombocytes 519*109/l (N< 350*109/l), leucocytes 11.7*109/l (N<10*109/l) with normal differentiation), kidney failure (creatinine 177 mmol/l (N<90 mmol/l), endogenous creatinine clearance 27 ml/min/1.73 m2, urea 10.9 mmol/l (N<7.5 mmol/l), proteinuria 15 g/24 h (N< 0.5 g/24 h)), a cholestatic pattern (alkaline phosphatase 363 U/l (N<115 U/l), gamma glutamyl transferase 146 U/l (N<40 U/l), total bilirubin 5 μmol/l (N<20 μmol/l) with otherwise normal liver function (aspartate aminotransferase 25 U/l, alanine aminotransferase 16 U/l, albumin 34 g/l, prothrombin time 11 sec, activated partial thromboplastin time 26 sec) and glucose 5 mmol/l. The elevated N-terminal pro-brain natriuretic peptide 975 ng/l (N<100 ng/l), immunoglobulin Free Light Chain (FLC) kappa 30.4 mg/l (N<20 mg/l), and FLC lambda 50 mg/l (N<32 mg/l) were probably due to the decreased kidney function. Also, the uric acid level was elevated (0.47 mmol/l, N<0.35 mmol/l). There was no evidence of an underlying plasma cell dyscrasia (M-protein undetectable, kappa/lambda ratio 0.61, without Bence Jones excretion in the urine). Other laboratory tests were all normal (highly selective-troponin T 10 ng/l, morning fasting cortisol 715 nmol/l, glucose 5 mmol/l, folic acid 19 nmol/l, vitamin B12 214 pmol/l, ferritin 101 ug/l, anti-Cyclic Citrulline Peptide antibody (CCP) 1 U/ml, anti-nuclear antibodies negative, IgM rheumatoid factor 10 kIU/l, IgA 2.1 g/l, IgG 6.7 g/l, IgM 1.0 g/l, and alfa-fetoprotein 1.2 μg/l).
X-rays of the hands, feet and pelvic region did not show any abnormalities, particularly no erosive changes. Microscopic examination of the synovial fluid of the metacarpophalangeal joint did not show any crystals, therefore gout could neither be confirmed nor excluded.
To rule out infectious causes of AA amyloidosis we performed serological tests for hepatitis B and C, human immunodeficiency virus and interferon-gamma-assays for tuberculosis, which were al negative. Chest radiography did not reveal any abnormalities.A bone marrow biopsy did not reveal signs of plasma cell dyscrasia or lymphoproliferative malignancy. DNA analysis for at that moment known hereditary autoinflammatory diseases, i.e. familial Mediterranean fever (MEFV gene), mevalonate kinase deficiency (MVK gene), cryopyrin-associated periodic syndrome (NLRP3 gene), and tumor necrosis factor receptor-associated periodic syndrome (TNFRSF1A gene) and the hereditary form of transthyretin-associated amyloidosis (TTR gene) did not show a mutation.
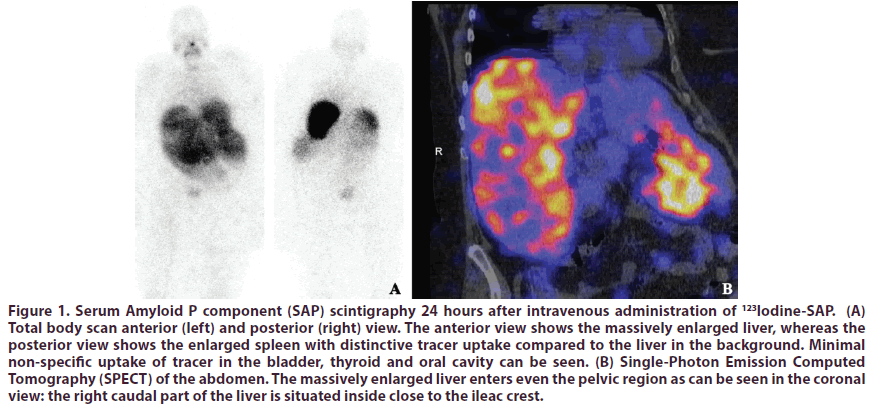
An 18F-fludeoxiglucose Positron Emission Tomography (FDG-PET)-CT scan showed slightly increased homogeneous uptake of tracer in the liver and in some joints and tendon sheets. Serum Amyloid P component (SAP) scintigraphy showed intense uptake in liver and spleen, with extravascular tracer retention of 83.5% indicating substantial amyloid deposition (Figure 1). Since abdominal ultrasound showed hepatomegaly with an inhomogeneous aspect, Computed Tomography (CT) examination of the abdomen was performed, followed by a 4-phase CT of the liver which revealed a massive hepatosplenomegaly with a craniocaudal liver diameter of 25 cm and craniocaudal spleen diameter of 14 cm and several arterial staining lesions in both the right and left liver lobes. Further evaluation of the liver with Magnetic Resonance Imaging (MRI) scan was refused because of claustrophobia. However, to rule out other liver disease such as an inflammatory hematoma or some kind of inflammation otherwise, a liver biopsy was performed showing excessive amyloid infiltration in the liver without other abnormalities. A subcutaneous abdominal fat aspirate did not show amyloid. Furthermore, there were no signs of spondyloarthropathy present on X-ray and low-dose CT of the pelvic region.
Figure 1: Serum Amyloid P component (SAP) scintigraphy 24 hours after intravenous administration of 123Iodine-SAP. (A) Total body scan anterior (left) and posterior (right) view. The anterior view shows the massively enlarged liver, whereas the posterior view shows the enlarged spleen with distinctive tracer uptake compared to the liver in the background. Minimal non-specific uptake of tracer in the bladder, thyroid and oral cavity can be seen. (B) Single-Photon Emission Computed Tomography (SPECT) of the abdomen. The massively enlarged liver enters even the pelvic region as can be seen in the coronal view: the right caudal part of the liver is situated inside close to the ileac crest.
In conclusion, this patient appeared to have AA amyloidosis characterized by a nephrotic syndrome, a massive hepatosplenomegaly and possibly related hypothyroidism with a poorly understood underlying inflammatory process. The unspecified non-erosive oligoarthritis of recent origin that was thought to be gout was not severe and long-lasting enough to be considered the cause of this far-advanced AA amyloidosis. No other infectious or inflammatory cause was found, except for the fact that the patient had been morbidly obese for many years, which can cause a chronic inflammatory state as described above.
Due to a suspected gout arthritis of the left third metacarpophalangeal joint, colchicine 0.5mg three times a day was started on admission. CRP levels improved under colchicine from 81 mg/l to 47 mg/l, but the levels never normalized (lowest CRP 35 mg/l). SAA (16 mg/l) did not change at all. Also, additional treatment with prednisolone (started by the general practitioner due to persistent arthralgia) failed to improve the inflammatory parameters (resulting CRP: 47 mg/l, SAA: 21 mg/l, and ESR 57 mm/h). Since renal failure gradually progressed and the alkaline phosphatase kept rising, suggesting progression of the amyloidosis, further treatment was required. Pending the results from the DNA analysis for hereditary autoinflammatory diseases, treatment with anakinra (interleukin-1 receptor antagonist (anti-IL-1)) was started, however this did not lead to any improvement in CRP levels (before CRP 43 mg/l; after CRP 49 mg/l). Also, second line treatment with tocilizumab (interleukin-6 receptor antagonist (anti-IL-6) and infliximab (TNF-α blocker) failed to slow down disease progression. She gradually progressed to complete renal failure within three years and hemodialysis had to be started in January 2014. Indomethacin was started to achieve medical nephrectomy, and thus completely stop the serious proteinuria. After start of the hemodialysis and medical nephrectomy her condition stabilized.
Discussion
In newly diagnosed AA amyloidosis patients without an obvious underlying cause, the underlying inflammatory process should be identified so that targeted treatment can be applied. It is generally accepted that autoimmune and autoinflammatory diseases, malignancies and underlying chronic infections have to be excluded [10]. However, in recent years more and more diseases are thought to be associated with development of AA amyloidosis [11,12]. Therefore, a diagnostic approach to detect the underlying disease in a patient with AA amyloidosis has been proposed by our group [13], and this approach is reflected in the diagnostic work-up used in this case. Once an underlying disease is found, the probability that this particular disease was the actual cause of the AA amyloidosis should be considered. For example, despite the fact that our patient was thought to suffer from gout, it was deemed unlikely that this disease had caused the far-advanced AA amyloidosis, because the arthritis was of recent onset (one year ago), was usually in remission with treatment, and had not caused any erosive changes. Rheumatoid arthritis and spondyloarthropathy were considered unlikely as other possible causes of this non-erosive oligoarthritis. The slightly increased uptake in the liver on the FDG-PET scan compelled us to perform a liver biopsy, but apart from extensive amyloid deposition no neoplastic or inflammatory process was detected. Another unrecognised underlying cause could be an unknown autoinflammatory disease. Gene panel testing for autoinflammatory diseases at that time was limited to the NLRP3, TNFRSF1A, MEFV and MVK genes. In recent years more genes have been identified to be associated with autoinflammation. The lack of suggestive clinical symptoms, suspicious family history and failure of treatment with colchicine, anakinra and tocilizumab makes it unlikely that such a disease is present in our patient. However, in future cases the use of whole exome sequencing should be considered. Taking it all together, in this case the morbid obesity was the most likely underlying condition causing longstanding chronic inflammation, which in turn caused the AA amyloidosis.
In patients with obesity, only mildly elevated CRP levels (up to 20 mg/l), have been described [14-17]. The nonproportional increased CRP (81 mg/l) on admission seems to be related to the arthritis exacerbation, as described in the case, since CRP levels decreased to 47 -35 mg/l after treatment of the arthritis with colchicine.
The basis of treatment of amyloidosis is the so-called ‘precursor-product’ concept, in which the central idea is that further growth of amyloid deposits will cease when the supply of necessary precursor proteins is put to a stop [18]. Therefore, treatment of AA amyloidosis is based on decreasing SAA serum levels to normal basal values (below 3 mg/l), since it is known that this is associated with prolonged survival and better renal outcome [19]. This can only be achieved by complete suppression or eradication of the underlying chronic inflammation. In obesity the systemic low-grade inflammation is caused by upregulation of macrophage activity resulting in secretion of the pro-inflammatory cytokines (e.g. interleukin-6 (IL-6), TNF- α) and chemokines (e.g. monocyte chemotactic protein-1 (MCP-1)) by adipocytes [20]. IL-6 subsequently stimulates the hepatic production of CRP and SAA [21]. Interestingly, this inflammatory state seems to be mainly driven by adipocytes from visceral white adipose tissue, and much less by adipocytes from subcutaneous white adipose tissue [20].
Previous studies showed that weight loss induced by either dietary changes, blood glucose lowering drugs or bariatric surgery was associated with a decreased inflammatory state [20,22-26]. For example, weight reduction of approximate 4-8 kg in 3 months by dietary intervention, led to an absolute reduction in CRP from 3.4 mg/l to 2.4 mg/l (p=0.035) and in SAA from 5.00 mg/l to 3.55 mg/l (p=0.21), in which the decrease in inflammatory parameter significantly correlated with the degree of weight reduction [22]. In patients with obesity and type 2 diabetes mellitus not only dietary changes but also various blood glucose lowering drugs have been found to have a beneficial effect on weight loss by promoting fatty acid oxidation (metformin and inhibitors of the sodium-glucose cotransporter-2 (SGLT-2 inhibitors)), or influencing brain areas that control food intake (Glucagon-like peptide-1 receptor agonists (GLP-1)) [20]. In addition, metformin has been shown to have direct anti-inflammatory properties [20].
Following bariatric surgery, systemic inflammation remains elevated in the first month post-surgery [24]. However, six months after surgery changes in both CRP and SAA were reported, in which a reduction in BMI around 10 kg/m2 led to a reduction in CRP from about 3 mg/l to 1.0 mg/l [24,26] and an absolute decrease in SAA from 11.5 mg/l to 6.7 mg/l [24]. Also, the serum levels of IL-6 and TNF-α were found to be reduced one-year post-surgery [27]. Paradoxically, the jejunoileal bypass method is brought up in a few case reports as an indirect cause of AA amyloidosis due to the development of hyperoxaluria [28,29]. We consider this association to be unlikely because there were also other more likely causes of AA amyloidosis present in these cases after jejunoileal bypass surgery [13]. To mention especially in this respect morbid obesity as the necessary condition for jejunoileal bypass as therapeutic surgical weight loss procedure.
However, the implementation of lifestyle and surgical interventions will often be difficult and time consuming. What is more, surgical interventions will not always be feasible due to comorbidities or already existing organ failure caused by amyloid deposition. To prevent further deterioration and eventually death in such cases, treatment ideally should focus on immediate decrease of inflammation and SAA production. Glucocorticoids, widely used drugs to quickly supress inflammation in many autoimmune diseases, are far from ideal in patients with obesity. Since obesity is associated with increased levels of IL-6 [15], treatment with anti-IL-6 can be considered. Also, other biological agents (e.g. anti-IL-1 or TNF-α blockers) might be a good therapeutic option, since it is known that the inflammatory cytokines IL-1, IL-6 and TNF-α amplify the production of SAA by the liver [30,31]. However, the treatment efforts in the current case proved not to be successful and we are not aware of data available on treatment with anti-IL-6 or other biological agents in obesity-induced AA amyloidosis.
Conclusion
Morbid obesity should be considered a potential cause of AA amyloidosis. However, it is a diagnosis of exclusion. Many other underlying inflammatory conditions should be ruled out first, since they require targeted therapy. Therefore, a strict and systematic diagnostic approach should be followed, such as the one proposed. Nevertheless, once an underlying disease is found, the probability of that disease to have caused the AA amyloidosis should be considered by the clinician. In patients with suspected obesity-induced AA amyloidosis, weight loss either by dietary changes or bariatric surgery seems to be a logical therapy, however this is time consuming and not always feasible. In patients with rapid deterioration of organ function treatment with biologic agents (e.g. anti-IL-6, anti-IL-1 or TNF-α blockade) might still be attempted in order to decrease SAA levels in view of the underlying pathophysiology. Further research is needed to confirm the effectiveness of different treatment options such as life style interventions, bariatric surgery, biologic agents or other drugs with anti-inflammatory properties.
Funding source
None to report.
Financial disclosure
None to report.
Conflict of interest
No potential conflict of interest was reported by the authors.
References
- Benson MD, Buxbaum JN, Eisenberg DS et al. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 25(4), 215–219 (2018).
- Scarpellini E, Tack J. Obesity and metabolic syndrome: an inflammatory condition. Dig. Dis. 30(2), 148–153 (2012).
- Poitou C, Viguerie N, Cancello R et al. Serum amyloid A: production by human white adipocyte and regulation by obesity and nutrition. Diabetologia. 48(3), 519–528 (2005).
- Gómez-Ambrosi J, Salvador J, Rotella F et al. Increased serum amyloid A concentrations in Morbid Obesity Decrease after gastric by-pass. Obes. Surg. 16(3), 262–269 (2006).
- Naeser P, Westermark P. Amyloidosis in ageing obese-hyperglycemic mice and their lean litter-mates. A morphological study. Acta. Pathol. Microbiol. Scand. 85A(6), 761–767 (1977).
- Van der Heijden RA, Bijzet J, Meijers WC et al. Obesity-induced chronic inflammation in high fat diet challenged C57BL/6J mice is associated with acceleration of age-dependent renal amyloidosis. Sci. Rep. 13(5), 16474 (2015).
- Alsina E, Martin M, Panadés MJ et al. Renal AA amyloidosis secondary to morbid obesity? Clin. Nephrol. 72(10), 312–314 (2009).
- Stankovic Stojanovic K, Georgin-Lavaille S, Poitou C et al. AA amyloidosis is an emerging cause of nephropathy in obese patients. Eur. J. Intern. Med. 39, 18–20 (2017).
- Blank N, Hegenbart U, Dietrich A et al. Obesity is a significant susceptibility factor for idiopathic AA amyloidosis. Amyloid. 25(1), 37–54 (2018).
- Hazenberg BPC, Van Rijswijk MH. Aspects cliniques de l’amylose AA. In: Grateau G, Benson MD, Delpech M, editors. Les amyloses. Paris: Flammarion. 2000, 377–427.
- Obici L, Merlini G. AA amyloidosis: basic knowledge, unmet needs and future treatments. Swiss. Med. Wkly. 142, w13580 (2012).
- Papa R, Lachmann HJ. Secondary, AA, Amyloidosis. Rheum. Dis. Clin. North. Am. 44(4), 585–603 (2018).
- Brunger AF, Nienhuis HLA, Bijzet J et al. Causes of AA amyloidosis: a systematic review. Amyloid. 27(1), 1–12 (2020).
- Visser M, Bouter LM, McQuillan GM et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 282(22), 2131 (1999).
- Khaodhiar L, Ling PR, Blackburn GL et al. Serum Levels of Interleukin-6 and C-reactive Protein Correlate with Body Mass Index Across the Broad Range of Obesity. JPEN. 28(6), 410–415 (2004).
- Sjöholm K, Palming J, Olofsson LE et al. A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J. Clin. Endocrinol. Metab. 90(4), 2233–2239 (2005).
- Paegaey AC, Genser L, Bouillot JL et al. High levels of CRP in morbid obesity: the central role of adipose tissue and lessons for clinical practice before and after bariatric surgery. Surg. Obes. Relat. Dis. 11(1), 148–154 (2015).
- Hazenberg BPC, Van Gameren II, Bijzet J et al. Diagnostic and therapeutic approach of systemic amyloidosis. Neth. J. Med. 62(4), 124–128 (2004).
- Lachmann HJ, Goodman HJB, Gilbertson JA et al. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 356(23), 2361–2371 (2007).
- Chait A, Hartigh den LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 7, 22 (2020).
- Moshage HJ, Roelofs HM, van Pelt JF et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem. Biophys. Res. Commun. 155(1), 112–117 (1988).
- O’Brien KD, Brehm BJ, Seeley RJ et al. Diet-Induced Weight Loss Is Associated with Decreases in Plasma Serum Amyloid A and C-Reactive Protein Independent of Dietary Macronutrient Composition in Obese Subjects. J. Clin. Endocrinol. Metab. 90(4), 2244–2249 (2005).
- Gomez-Ambrosi J, Salvador J, Rotellar F et al. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes. Surg. 16(3), 262–269 (2006).
- Shimizu H, Hatao F, Imamura K et al. Early Effects of Sleeve Gastrectomy on Obesity-Related Cytokines and Bile Acid Metabolism in Morbidly Obese Japanese Patients. Obes. Surg. 27, 3223–3229 (2017).
- Kjellmo CA, Karlsson H, Nestvold TK et al. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J. Clin. Lipidol. 12(1), 193–202 (2018).
- Salman MA, Salman AA, Nafea MA et al. Study of changes of obesity-related inflammatory cytokines after laparoscopic sleeve gastrectomy. ANZ. J. Surg. 89(10), 1265–1269 (2019).
- Viana EC, Araujo-Dasilio KL, Miguel GPS et al. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-α. Prospective clinical trial. Obes. Surg. 23(8), 1252–1261 (2013).
- Korzets Z, Smorijk Y, Zahavi T et al. Renal AA Amyloidosis A long-term sequela of jejuno-ileal bypass. Nephrol. Dial. Transplant. 13(7), 1843–1845 (1998).
- Cornelis T, Bammens B, Lerut E et al. AA amyloidosis due to chronic oxalate arthritis and vasculitis in a patient with secondary oxalosis after jejunoileal bypass surgery. Nephrol. Dial. Transplant. 23(10), 3362–3364 (2008).
- De Buck M, Gouwy M, Wang JM et al. The cytokine-serum amyloid A-chemokine network. Cytokine. Growth. Factor. Rev. 30, 55–69 (2016).
- Okuda Y. AA amyloidosis – Benefits and prospects of IL-6 inhibitors. Modern. Rheumatology. 29(2), 268–274 (2018).