Review Article - Imaging in Medicine (2013) Volume 5, Issue 3
Neuromuscular imaging in muscular dystrophies and other muscle diseases
Arne Fischmann*1 & Dirk Fischer2,3
1University of Basel Hospital, Department of Radiology & Nuclear Medicine, Division of Diagnostic & Interventional Neuroradiology, Petersgraben 4, CH-4031 Basel, Switzerland
2University of Basel Childrens Hospital, Division of Neuropediatrics, Spitalstrasse 33, CH-4056 Basel, Switzerland
3University of Basel Hospital, Department of Neurology, Petersgraben 4, CH-4031 Basel, Switzerland
- Corresponding Author:
- Arne Fischmann
University of Basel Hospital
Department of Radiology & Nuclear Medicine
Division of Diagnostic & Interventional Neuroradiology
Petersgraben 4, CH-4031 Basel, Switzerland
Tel: +41 61 328 6556
Fax: +41 61 265 4660
E-mail: arne.fischmann@unibas.ch
Abstract
Keywords
CT ▪ fat fraction ▪ MRI ▪ muscular dystrophy ▪ neuromuscular disorder ▪ quantification
Muscular dystrophies (MDs) are a group of more than 30 genetic inherited diseases that cause increasing degeneration of skeletal muscles and progressive muscle weakness. The word ‘dystrophy’ is derived from the Greek: ‘dys’, translated as ‘faulty’, and ‘troph’ as ‘nourish’. The term dystrophy is, therefore, indicative of the pathologic features of fibrosis and fatty replacement, particularly late in the disease course. While many dystrophies present in childhood, some forms can remain silent until adolescence or even late adulthood. Histopathologic changes may remain mild despite the clinical classification. However, the common features of all MDs are increasing symptoms and progressive muscle weakness. Over time many patients lose the ability to walk and will often die prematurely.
The US Office of Rare Diseases Research characterizes all MDs as rare diseases – as neither the group of MDs nor each single dystrophy will reach the level of 200,000 patients in the USA [101].
MDs are clinically divided by age of onset, rate of progression, severity of symptoms, distribution of muscle weakness and pattern of inheritance. Modern genetic techniques enabled the detection of an increasing number of causative genes, and resulted in further division into multiple subgroups.
Imaging as a diagnostic tool in MDs & inflammatory myopathies
One of the major uses of muscle imaging, especially in pediatric neuromuscular disorders, is to identify the underlying disease in a child with increasing weakness. While the clinical pattern of the disease is often suggestive of the larger group (e.g., limb-girdle MD [LGMD] or distal myopathy), there are multiple subgroups and often several underlying genetic defects. While sequencing the whole genome has become increasingly simple and feasible, the search for a causative genetic mutation is still expensive.
Dystrophic changes in muscles, on the other hand, do not occur in a random fashion but rather develop in a specific pattern, which is often characteristic for the underlying protein change and sometimes even for the causative gene. Detecting the specific pattern of muscular involvement is, therefore, often used to narrow down the genes to be sequenced and evaluated. Several groups have examined patients with neuromuscular disorders and established protocols either using a limited approach including only the lower limbs [1] or in some cases including the whole body [2].
In addition to pattern description in specific diseases and targeting the potential underlying genetic cause of a disease, muscle imaging can also be used to guide muscle biopsy. Especially in diseases with highly heterogeneous distribution, it is preferable to target an involved area, which is not yet completely replaced with fatty tissue. Therefore, imaging is also very useful to indicate muscles to be preferably biopsied.
The detection of numerous new genetic entities of MDs and other primary myopathies, many of which have a characteristic involvement, confines an exhaustive description of all possible patterns to a larger volume [3]. The authors, therefore, limited their discussion to the most common neuromuscular disorders and mainly focused on adult diseases.
■Duchenne MD & Becker MD
The most common MDs are Duchenne MD (DMD; the severe form) and Becker MD (BMD; the less severe form), which affect approximately one in every 3500–5000 boys or between 400 and 600 live male births each year in the USA [4]. Both disorders are the result of a defect in the dystrophin gene. As an X-linked recessive disease, DMD mainly affects boys, although women carrying the defective gene sometimes also show milder symptoms. Approximately one-third of patients carry de novo mutations, the remaining are familiar [4]. First symptoms in DMD usually appear when the children begin to walk. After a short period of physical improvement, boys develop increasing weakness and muscle wasting, which results in progressive difficulties and loss of independent ambulation in the early teenage years. Replacement of muscle with fatty tissue results in increased limb circumference, often referred to as pseudohypertrophy. Impaired breathing, lung weakness, recurrent infections and cardiomyopathy finally lead to an early death in the late teens or early 20s [4]. A less severe but closely related disease is BMD. While boys with DMD have a complete loss of dystrophin function, residual but impaired function of dystrophin can be found in patients with BMD. Disease onset is later, usually in the early teens, but may be beyond the age of 25 years. In addition, muscle atrophy and weakness progresses slower and many patients are independently ambulatory into their 30s.
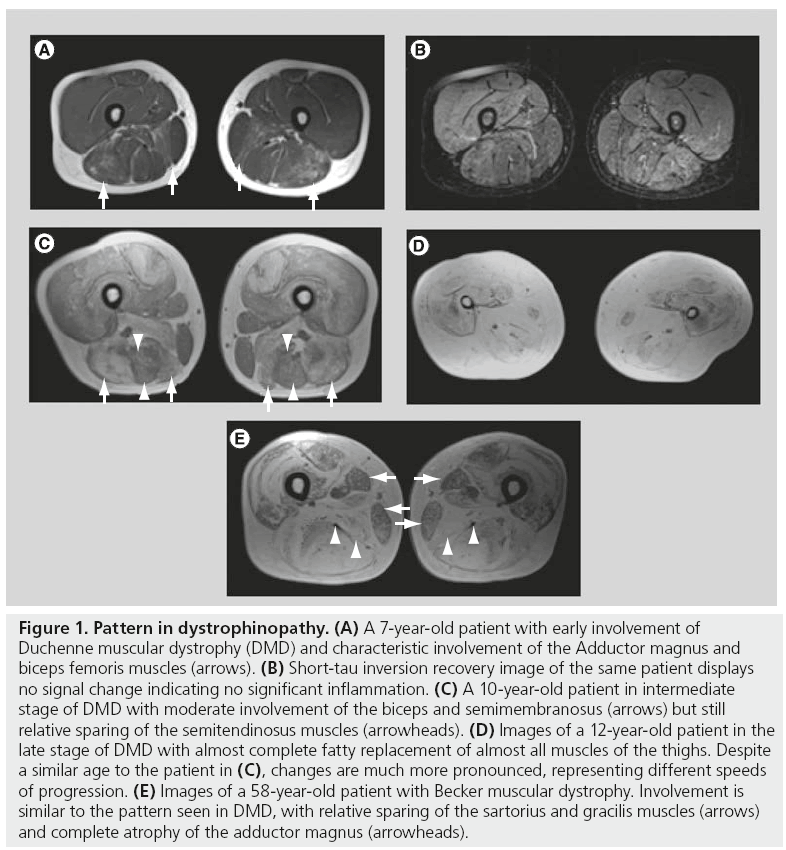
The pattern of muscle involvement on muscle imaging in DMD is characteristic, with fatty infiltration and muscular atrophy with no or only minimal inflammatory change, initially and most severe in the adductor magnus, the biceps femoris, and a successive involvement of the quadriceps (Figure 1A–D). A characteristic relative sparing of the long adductor can also often be detected [5]. Sparing of the gracilis and sartorius muscles is also common in patients with other MDs and was described as a noncharacteristic sign in neuromuscular disorders in the 1990s [6]. Although female carriers usually show no or only mild clinical symptoms, progressive muscle involvement in a typical pattern similar to DMD is clearly detectable [7]. The involvement seen on MRI can be quite extensive in women with more severe clinical findings presenting with weakness and increased creatine kinase levels [8].
Figure 1: Pattern in dystrophinopathy. (A) A 7-year-old patient with early involvement of Duchenne muscular dystrophy (DMD) and characteristic involvement of the Adductor magnus and biceps femoris muscles (arrows). (B) Short-tau inversion recovery image of the same patient displays no signal change indicating no significant inflammation. (C) A 10-year-old patient in intermediate stage of DMD with moderate involvement of the biceps and semimembranosus (arrows) but still relative sparing of the semitendinosus muscles (arrowheads). (D) Images of a 12-year-old patient in the late stage of DMD with almost complete fatty replacement of almost all muscles of the thighs. Despite a similar age to the patient in (C), changes are much more pronounced, representing different speeds of progression. (E) Images of a 58-year-old patient with Becker muscular dystrophy. Involvement is similar to the pattern seen in DMD, with relative sparing of the sartorius and gracilis muscles (arrows) and complete atrophy of the adductor magnus (arrowheads).
In patients with BMD, disease onset is at a later age; however, disease distribution at onset is similar to DMD. The disease often starts in the adductor magnus and long head of the biceps femoris. Different to DMD, involvement of the semimembranosus usually precedes the quadriceps (Figure 1E). Sparing of the sartorius, gracilis and semitendinosus is comparable with DMD [9]. There is also bilateral involvement of both heads of the gastrocnemius muscle and – in later stages – of the soleus and peroneus muscles. Patternbased differential diagnosis of BMD includes LGMD2I, which has a similar distribution but less involvement of the rectus femoris muscle [10].
■Limb-girdle MD
Autosomal dominant and recessive LGMDs are a heterogeneous group of genetic diseases with a wide spectrum of clinical involvement and severity, grouped by the common finding of predominant proximal muscle involvement [11]. As indicated by the name, weakness is typically noticed first around the hips and proximal leg, resulting in a waddling gait and difficulties rising from a chair or climbing up stairs. This clinical distribution of muscle weakness is similar to dystrophinopathies in DMD and BMD, but most LGMDs start later during adolescence. There are currently at least eight autosomal dominant forms designated as LGMD1A–H and at least 16 autosomal recessive forms designated LGMD2A–Q. Often these forms are also referred to according to the involved protein, for example, calpainopathy (LGMD2A), dysferlinopathy (LGMD2B) or sarcoglycanopathies (LGMD2C–2F) [11]. Onset, progression and distribution of the weakness and wasting can vary greatly between individual patients and genetic subtypes. Most LGMD patients have typical histopathological dystrophic changes consisting of degeneration and regeneration of muscle fibers, which usually leads to elevated serum creatine kinase levels. Diagnosis can often be achieved when performing protein immunostaining or blotting. In addition, a specific pattern of muscular involvement on muscle MRI can be observed in many different LGMD subtypes. Apart from some small populations with founder mutations, recessive forms of LGMDs are more prevalent. They usually show symptoms in late childhood or early adolescence, while dominant forms usually present later in life. Disease severity is generally linked to age of onset and markedly elevated levels of serum creatine kinase are often found. Most patients become severely disabled within 20 years of disease onset.
Muscle imaging using MRI and CT has been described in detail for LGMD2A [12], LGMD2B [13], LGMD2I [13] and LGMD2L [9,14].
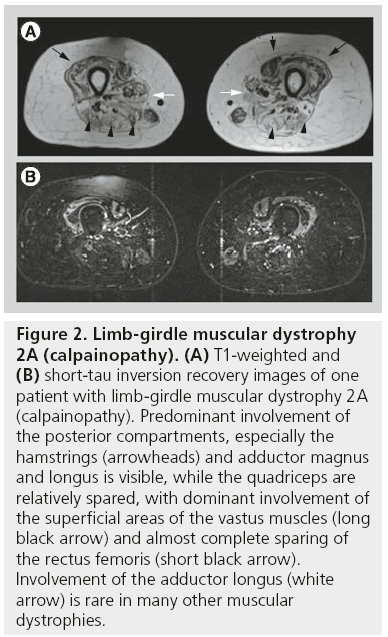
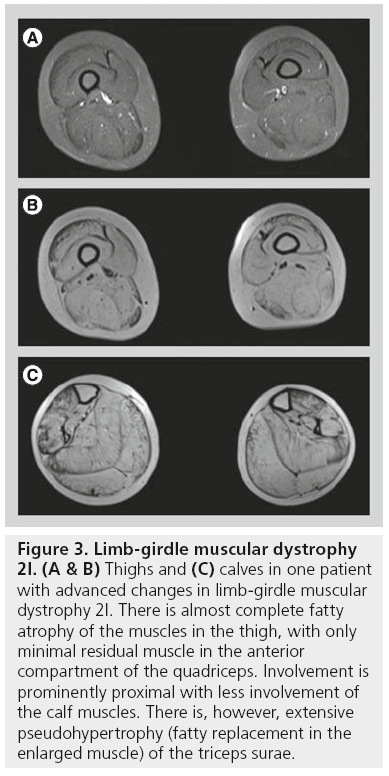
Patients with LGMD2A (calpainopathy) show a relatively typical pattern of muscle involvement on MRI studies. Often there is moderate-to-severe atrophy of the gluteus maximus muscle. In the thigh (Figure 2), the medial (adductor) and posterior compartment (hamstring) muscles are usually the most severely affected muscles, while the quadriceps muscle is much less affected. In particular, involvement of the adductor magnus and semimembranosus muscles is the most common finding. Sartorius and gracilis are usually spared. The adductor longus, biceps femoris, semitendinosus and vastus intermedius will be affected only later in the course of the disease. In the lower legs, the first muscles to be involved are the medial head of the gastrocnemius muscle and the soleus muscle with relative sparing of the lateral head of the gastrocnemius muscle [12,13]. Similar to LGMD2A, in LGMD2I muscle MRI has shown that changes occur predominantly in the medial and posterior compartments of both the thigh and the lower leg (Figure 3). The biceps femoris long head muscle, adductor magnus muscle, semitendinosus muscle, and semimembranosus muscle are affected early, while the gracilis muscle, sartorius muscle, vastus lateralis muscle, and the rectus femoris muscle are relatively spared until later. In the lower leg, predominant involvement is diffusely seen in the gastrocnemii and soleus muscles, often accompanied by pseudohypertrophy [13].
Figure 2: Limb-girdle muscular dystrophy 2A (calpainopathy). (A) T1-weighted and (B) short-tau inversion recovery images of one patient with limb-girdle muscular dystrophy 2A (calpainopathy). Predominant involvement of the posterior compartments, especially the hamstrings (arrowheads) and adductor magnus and longus is visible, while the quadriceps are relatively spared, with dominant involvement of the superficial areas of the vastus muscles (long black arrow) and almost complete sparing of the rectus femoris (short black arrow). Involvement of the adductor longus (white arrow) is rare in many other muscular dystrophies.
Figure 3: Limb-girdle muscular dystrophy 2I. (A & B) Thighs and (C) calves in one patient with advanced changes in limb-girdle muscular dystrophy 2I. There is almost complete fatty atrophy of the muscles in the thigh, with only minimal residual muscle in the anterior compartment of the quadriceps. Involvement is prominently proximal with less involvement of the calf muscles. There is, however, extensive pseudohypertrophy (fatty replacement in the enlarged muscle) of the triceps surae.
LGMD2L is a less common type with adult onset and is characterized by a posterior involvement of both the thighs (especially hamstrings and adductors) and the calves (triceps surae) with relative sparing of the quadriceps and the foot extensors. Despite the name, involvement in MRI appears to be more distal with milder changes in the pelvic muscles [9,14]. Different to most other MDs, LGMD2L presents marked fatty changes in the gracilis and sartorius muscles [15]. Interestingly, the pattern of involvement in this cohort was constant and similar in all patients with LGMD2L, despite remarkable clinical variability, underlining the value of MRI as a diagnostic tool [14].
While most studies describe patterns in LGMDs, data on diagnostic accuracy are limited. ten Dam et al. evaluated selective patterns with CT in relation to the genetic diagnosis and found a sensitivity of 40% with a specificity of 58%; data for BMD and Bethlem myopathy were higher with sensitivities above 90%; however, their data only examined a limited extent of possible diagnostic parameters as CT is less sensitive to minor fatty changes and is unable to examine edematous changes on T2 images [9].
■Myotonic dystrophies
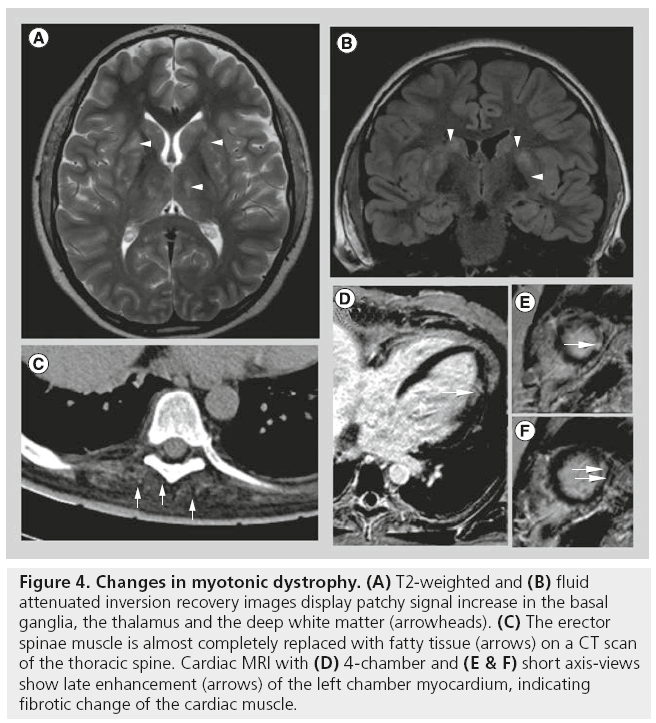
Myotonic dystrophy type I is also known as Steinert’s disease or dystophia myotonica (DM) and is the second most common MD after DMD, and probably the most common adult form of MD, affecting approximately one in 8000 individuals worldwide. DM type 2 (DM2; also referred to as proximal myotonic dystrophy) is much more rare than DM1 [16]. Both are autosomal dominant disorders caused by a lengthened triplet repeat in the DMPK gene (DM1) or ZNF9 gene (DM2). DM1 and DM2 are complex multisystem disorders. Both are characterized by progressive skeletal muscle weakness, wasting and myotonia. DM1 and DM2 involve a wide range of nonmuscular tissues. In addition to peripheral muscle symptoms, there is involvement of the CNS (Figure 4A & B) presenting with mental impairment and often resulting in poor compliance, as well as of the cardiac muscles (Figure 4D–F). Ages of onset correlate with CTGrepeat size lengths. The classical adult-onset DM1 phenotype is characterized by facial and distal muscle weakness and wasting of forearm and lower leg ankle dorsiflexor muscles. By contrast, muscle weakness in proximal myotonic dystrophy is predominantly found in proximal lower limb muscles.
Figure 4: Changes in myotonic dystrophy. (A) T2-weighted and (B) fluid attenuated inversion recovery images display patchy signal increase in the basal ganglia, the thalamus and the deep white matter (arrowheads). (C) The erector spinae muscle is almost completely replaced with fatty tissue (arrows) on a CT scan of the thoracic spine. Cardiac MRI with (D) 4-chamber and (E & F) short axis-views show late enhancement (arrows) of the left chamber myocardium, indicating fibrotic change of the cardiac muscle.
Imaging in DM has mainly been performed on brain scans, especially using functional imaging techniques as well as structural MRI. Using structural imaging in patients with DM, gray matter volume was found to be decreased in all cerebral areas examined, accompanied by reduced fractional anisotropy, indicating disrupted white matter integrity [17]. Patchy areas with hyperintense signal on T2-weighted images can be found predominantly in the frontal areas, but also in the deep white matter and basal ganglia (Figure 4A & B) [18]. Functional imaging showed increased activation of secondary motor areas [19]. On muscle imaging, all patients examined demonstrated some pathologic changes (Figure 4C), usually beginning in the anterior tibialis and medial gastrocnemius muscles [20] and sparing of the posterior tibialis muscles, as well as relative sparing of the lateral gastrocnemius muscle [20]. However, edema was most prominent in the posterior compartment of the calves [16] and may represent an early stage of the disease.
The thighs mainly show involvement of the vasti muscles, while the rectus femoris is relatively spared, resulting in a characteristic semilunar pattern of involvement [16]. While most patients present with early symptoms in the forearms, only one study reported on findings using a dedicated low-field extremity MRI. They found predominant involvement of the extensors compared with the flexor muscles and relative sparing of the pronators and supinators [20].
Compared with DM1, systematic muscular imaging data on DM2 are still lacking. In general, DM2 patients are less affected based on skeletal muscle MRI findings. Muscles of the anterior and posterior thigh, such as the quadriceps muscles, the semimembranosus and semitendinosus muscles, may be affected in DM2.
■Facioscapulohumeral MD
Facioscapulohumeral MD (FSHD) is the third most common MD worldwide, affecting approximately one in 20,000 individuals. FSHD is also known as Landouzy–Dejerine disease. It is usually passed on in an autosomal dominant form. As the name suggests, patients develop (asymmetric) weakness of the face, the shoulder and the proximal arm, usually beginning in their teenage years [21]. Although patients usually have a normal life expectancy, the disease progression can be quite debilitating. Wasting of shoulder muscles results in winged scapula and slanting shoulders. While symptoms are most prominent in the face and forelimbs, the legs are also affected in a characteristic pattern. MRI findings in FSHD patients are often asymmetric and show distal effects more than in the proximal leg muscle [21]. The tibialis anterior muscle is often the most severely involved muscle, followed by the gastrocnemius medialis muscle. The peroneal muscles are usually spared. In the thighs, the hamstring and adductor muscles are most affected. Most severe fatty replacement is seen in the semimembranosus muscle, followed by the biceps femoris, the semitendinosus muscles and the adductor group muscles, while the vasti muscles are least affected [21,22]. While fatty changes will be found later in the disease progression, muscles without relevant increases in fat content will already display increases in T2 signals, indicating inflammatory/edematous changes [23]. Genetic analysis of biopsies revealed distinct transcription profiles in muscles that are short-tau inversion recovery (STIR) positive compared with muscles that are negative on STIR imaging [24].
■Distal myopathies
Distal myopathies comprise a group of multiple hereditary diseases that are clinically defined by predominantly distal weakness of the limbs. However, imaging can detect characteristic patterns of involvement depending on the underlying genetic defect. In patients with distal myopathy and rimmed vacuoles (also called hereditary inclusion body myopathy) caused by a defect in the GNE gene, involvement of the thigh is detectable from disease onset; however, involvement was more prominent in the distal leg and the quadriceps muscles were spared to a high degree [25]. By contrast, patients with distal myopathy caused by a mutation in the gene for the human MYH7 showed involvement of both the anterior and posterior compartments of the thighs, often more prominent than in the distal leg [26]. In patients with myofibrillar myopathies, muscle imaging shows distinguished patterns, that can separate desminopathies from myofibrillar myopathies based on other genetic defects [22].
■Oculopharyngeal MD
Oculopharyngeal MD is one of the least common MDs with adult onset. It is, in most cases, an autosomal dominant disease, which is mainly found in a French–Canadian community, Bukhara Jews and Hispanic residents of northern New Mexico (USA). Often cases can be traced back to a founder mutation in one ancestor; however, novel mutations have also been identified. The underlying defect is a short GCG repeat on chromosome 14 and disease severity depends on the repeat length [27].
Symptoms usually start in patients aged 40–50 years. These include drooping eyelids and swallowing symptoms; unlike chronic progressive external ophthalmoplegia, the external eye muscles are not involved and ptosis can be compensated with a characteristic downward gaze also known as the ‘astrologers pose’. The slow progression to other muscle groups often causes delays in diagnosis of up to 20 years. Peripheral muscle involvement is predominantly in the proximal lower limb and lifespan is usually not diminished, with most patients remaining independently ambulatory. Swallowing problems, however, may cause increasing problems to involved patients [22].
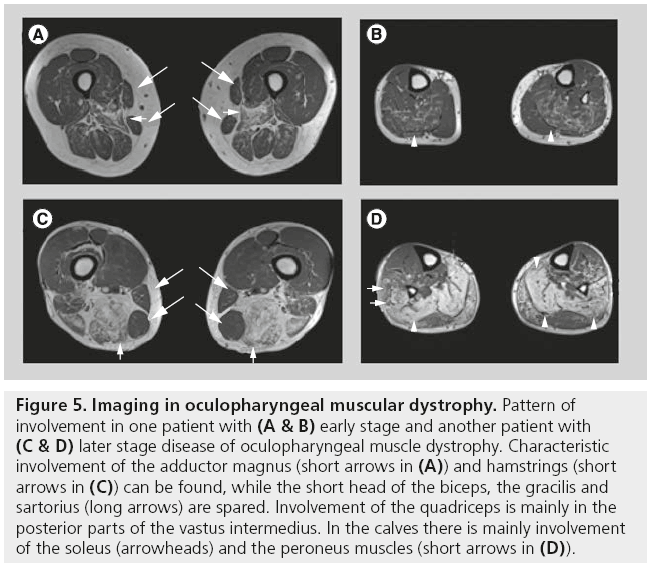
There are only a few reports on muscle imaging in oculopharyngeal MD. Abnormal fatty infiltration of the tongue, masseter, neck, shoulder girdle, lumbar paraspinous and gluteus muscles have been described. On MRI and CT scans, predominant involvement of the thighs is visible, especially in the adductor magnus and hamstrings, while the quadriceps are mainly involved in the deeper layers of the vastus intermedius muscle (Figure 5) [28].
Figure 5: Imaging in oculopharyngeal muscular dystrophy. Pattern of involvement in one patient with (A & B) early stage and another patient with (C & D) later stage disease of oculopharyngeal muscle dystrophy. Characteristic involvement of the adductor magnus (short arrows in (A)) and hamstrings (short arrows in (C)) can be found, while the short head of the biceps, the gracilis and sartorius (long arrows) are spared. Involvement of the quadriceps is mainly in the posterior parts of the vastus intermedius. In the calves there is mainly involvement of the soleus (arrowheads) and the peroneus muscles (short arrows in (D)).
■Inflammatory myopathies
Idiopathic inflammatory myopathies represent a heterogeneous group of acquired myopathies characterized by inflammatory infiltrates in skeletal muscle tissue. Among them, the most common disorders are sporadic inclusion body myositis (IBM), polymyositis (PM), dermatomyositis and autoimmune necrotizing myopathy [29]. These rare sporadic disorders are sometimes associated with connective tissue diseases such as scleroderma. Unlike the hereditary dystrophic myopathies, these diseases show an activation of the immune system, which seems to trigger the disease initiation. These diseases differ from each other in clinical and pathological terms. PM, dermatomyositis and IBM can be discriminated particularly on histopathological criteria. In PM and IBM, cytotoxic CD8+ T cells are the main feature, whereas a B-cell-mediated microangiopathy is observed in dermatomyositis.
■Inclusion body myositis
Despite similarities to PM and dermatomyositis, IBM has a distinctive clinical and imaging presentation; clinically relevant is the older age, usually beginning after 50 years. In addition, in contrast to PM and dermatomyositis, IBM usually does not respond to immunosuppressive or immunomodulatory treatment. The most frequently involved muscles are the quadriceps muscles and finger flexors. Involvement of pharyngeal muscles in a later stage may lead to dysphagia. On imaging, inflammatory changes with an increased STIR signal are less pronounced than in PM and dermatomyositis, and patients in advanced stages develop extensive muscle atrophy and fatty replacement of large muscles. These are most prominent in the quadriceps femoris muscles, as well as in the flexors of the distal arm and hand. All lower leg muscles can be involved, but mainly the medial gastrocnemius and the anterior compartment muscles. The lateral gastrocnemius is often spared.
A rare hereditary form of IBM is based on a mutation in the valosin-containing protein. These patients present with a different pattern to those in sporadic IBM, with variations even within a single family [30].
■Polymyositis
PM begins with a subacute onset of symmetrical weakness of proximal more than distal muscles. Muscle pain, tenderness, fever and nondestructive arthritis may also be complaints. PM usually presents after the second decade of life. Earlier onset is suggestive of an alternative diagnosis, such as MDs with secondary inflammatory infiltrates (LGMD2B and FSHD). PM does not display the subsequent muscle degeneration and protein abnormalities seen in IBM, and PM tends to respond well to treatments, while IBM does not [31].
On muscle MRI, inflammation is far more common than fatty infiltration. Therefore, fat suppressed T2-weighted images are the most useful sequences to detect abnormalities in PM. Inflammation and fatty infiltration are usually found symmetrically in proximal muscles. Inflammation is frequently present in the vasti muscles, medial gastrocnemius and the tibialis anterior muscle. In contrast to IBM, isolated inflammation in the absence of fatty infiltration can be observed.
■Dermatomyositis
Dermatomyositis shares a number of similar physical symptoms with PM, such as subacute onset of proximal weakness, but it often exhibits a skin rash not seen in PM or IBM. It may have different root causes unrelated to either PM or IBM. In contrast to PM and IBM, dermatomyositis is considered to be a B-cell-dependent complementmediated microangiopathy. Muscle biopsy usually demonstrates a perifascicular distribution of atrophic and degenerating muscle fibers. The perifascicular location is probably due to the destruction of capillaries in this region caused by a pathological deposition of the membrane attack complex of complement complexes [32]. This is also visible in the MRI pattern, which presents predominant inflammatory changes as indicated by a STIR signal increase, mainly in the intermuscular septi, a finding rarely detectable in either PM or any of the MDs described above [33]. Although inflammation can only be visualized directly using dedicated tracers and PET imaging, indirect changes of inflammation-like tissue edema can be detected on MRI and correspond to an inflammatory area in biopsies of patients with dermatomyositis [32].
Monitoring neuromuscular disorders with quantitative MRI
Until recently, disease progression in MD and other neuromuscular disorders was evaluated using clinical examinations and tests of varied physical abilities, such as timed walking distances or time to stand up from a lying position, as well as more elaborate scoring systems, such as the motor function measurement scale [34]. All these measurements, however, depend on the cooperation of the patient and are also dependent on variation in performance as well as on inter-rater variability. Imaging methods are superior in that the raw data can be stored and direct comparisons from data at several time points are possible. The Translational Research in Europe for the Assessment and Treatment of Neuromuscular Disease (Treat-NMD) campaign, therefore, suggested to use standardized imaging methods as surrogate outcome parameters in the evaluation of several neuromuscular disorders [35].
Probably the easiest and most widely available imaging method to evaluate muscular disorders is ultrasound and quantitative ultrasound has been shown to correlate to disease progression in boys with DMD [36]. A meta-analysis on the reliability of ultrasound to measure muscle size found good inter- and intra-reader reliability, limited by the moderate quality of the studies included [37]. However, ultrasound measurements are even more susceptible to changes in equipment and examiner bias compared with CT and MRI scans. While many trials used ultrasound in the follow-up of neuromuscular disorders, only a few trials compared imaging methods and found less sensitivity to changes in ultrasound compared Cross-sectional areas can be evaluated with either of these imaging methods and they have a high inter- and intra-reader reliability, as well as a high with CT and even less compared with MRI [38]. repeatability [39].
For standard evaluation of muscular disorders with MRI, standard T1- and T2-weighted sequences, as well as STIR sequences are in use in almost all MRI departments. However, these sequences only permit visual and semiquantitative analysis of muscle properties, with all the limitations described below. Quantitative measurements would therefore be highly advantageous to monitor neuromuscular disorders, especially in longitudinal studies. The suggested evaluation methods are fat quantifications and T2 relaxation times [35].
Several techniques to evaluate the fat content can be performed using MRI. One easily applicable method uses a standard T1 technique. Within a given region of interest (ROI), the T1 values are evaluated using simple histogram analysis and pixels above a predefined threshold are designated as fat tissue [40]. However, this method cannot detect minimal fatty changes within each voxel and may, therefore, miss early or minimal fatty replacement. This problem is overcome using 2- or 3-point Dixon techniques, whereby two or three echos in in- and opposed-phase are acquired and relative fat fractions for each voxel are calculated using simple or multipeak spectral models [41,42]. Another variant of the Dixon techniques is the ‘iterative decomposition of water and fat with echo asymmetry and least-squares estimation’ (IDEAL) [43]. Both IDEAL and 3-point Dixon techniques have the advantage of partially compensating for B0 and B1 field inhomogeneities. In addition, adiabatic pulses can be used to selectively excite fat or water [44], but are sensitive to field inhomogeneities and may therefore result in false-positive apparent changes.
T2 calculation is usually performed using a multiecho multicontrast sequence and leastsquare fitting of the signal [40]; however, this method can not separate increases in T2 caused by increased fat content from those caused by increased edema. A new sequence combining IDEAL fat-water separation and T2 calculation solved this problem by calculating separate T2 values for fat and water images [45]. In combining IDEAL fat-water separation and T2 calculation with corrections for B1 inhomogeneities, calculated T2 values and fat fractions will represent underlying pathology more reliably.
All of these methods have been used in patients with neuromuscular disorders; for example, in boys with DMD, relative muscle fat content correlated to clinical parameters, such as time to run 10 m [46] and manual strength measurements [47], and provided a possible surrogate parameter to predict time until loss of ambulation [5]. In addition, boys with DMD had increased T2 values with and without fatsaturation compared with healthy controls, with heterogeneous distribution of signal throughout dystrophic muscles [48].
Even at a single time point, involvement of muscles and degree of fatty atrophy correlates to clinical parameters as well as to disease progression and may even provide a possible predictor [5].
Muscle imaging can also be used to determine the remaining muscle area combining both muscle atrophy and fat replacement [49]. While these data correlated highly to muscle strength, intraobserver variability in MRI was significantly lower compared with clinical testing [50], making quantitative MRI less prone to observer bias.
The main advantage of quantitative methods to determine fat content is their independence from subjective reader bias and the higher repeatability. In patients with slowly progressive muscle disease, such as oculopharyngeal MD, T2 measurements can detect subclinical disease progression, while visual semiquantitative scores and even direct comparison of images could not yet detect any change [5]. In patients with genetically proven DM, changes in muscle imaging could even be detected before clinical symptoms became apparent [51]. Quantitative MRI has also been used to monitor treatment effects in children with DMD [52] and is a promising surrogate parameter in future therapeutic trials; however, use in clinical trials requires further validation of the imaging methods, especially the quantification of possible treatment effects that can be detected and the calculation of age-adjusted normal values in healthy volunteers.
While visual scores of fat fraction correlated highly with quantitative fat fractions [53], they were in general higher compared with quantitative fat measurements of the same muscles [42]. Quantitative measurements on the other hand showed excellent correlation to spectroscopic data [54], as well as to histologic and chemical quantification of relative fat fractions [41,55].
Currently, widespread use of quantitative MRI in clinical practice is limited by time consuming evaluation of the images, usually requiring manual segmentation of muscles or manual delineated ROIs. Methods to segment subcutaneous fat from intermuscular fat and muscle tissue are promising in healthy volunteers [56]. In patients with MD, however, increases in intramuscular fat are of special interest and it remains difficult to separate these from changes in intermuscular fat tissue.
Several factors may influence quantitative measurements and should be considered if patients are monitored over time periods. Exercise prior to an examination will induce a shift from extra- to intra-cellular water and may increase T2 measurements and a decrease in intracellular lipids [57], but will also change T1 measurements [58]. In addition, even a single exercise session may reduce calculated fat fractions in healthy volunteers by amounts similar to expected changes over a trial period [59]. However, these effects were short lasting and no change in T2 parameters could be detected 4 days after an exercise session in children with DMD [60]. Physical examinations and performance tests should therefore not be undertaken before the MRI session if both are scheduled on a single patient visit.
■Advanced techniques in MRI for inherited muscle diseases
These methods to evaluate fatty atrophy and inflammatory changes with T2-weighted images will gain more widespread distribution when evaluation protocols are standardized and commercially available on MRI scanners, similar to the developments in brain imaging for Alzheimer’s disease and multiple sclerosis. Nevertheless, they only regard a few aspects of imaging and modern imaging technologies could be used to broaden the analysis.
Diffusion-weighted imaging is based on the Brownian motion of water molecules. Increased diffusion is often seen in inflamed tissues with enlarged perivascular spaces, as can be found in inflamed but not in normal-appearing muscles of patients with PM [61]. Diffusion tensor imaging will also measure directed diffusion and may serve as a measure for structural integrity. Using fiber tracking algorithms, it is possible to show the anatomic structure within the muscle [62,63]. In animal models of MD, fractional anisotropy was increased after tractions injury compared with healthy controls [64].
Blood oxygenation level-dependent (BOLD) imaging is currently used in functional MRI to measure brain perfusion as an indirect parameter of neuronal activity. In skeletal muscles, BOLD-MRI has been proposed as a noninvasive tool to objectively measure peripheral limb perfusion [65]. Whether similar changes can be found in patients with neuromuscular disorders either with or without limited symptoms has to be evaluated.
With increasing field strength, it is getting easier to implement advanced spectroscopic techniques, such as 23Na-spectroscopy or 31P-spectroscopy to evaluate tissue metabolism and physiology [66]. 23Na-spectroscopy could detect permanently increased muscle sodium levels in children with DMD, which may contribute to muscle degeneration [67]. Nonproton imaging, however, requires scanners with broadband radio frequency capabilities, using dedicated radio frequency amplifiers and send/receive coil combinations. In addition, nonproton spectroscopy sequences are currently not commercially available, necessitating cooperation with a dedicated MRI physics and engineering team.
However, changes in tissue composition influences the binding of water to protein, which can be measured using magnetization transfer imaging, which is commercially available on several systems [68].
Analysis of standard parameters with new evaluation tools is also increasingly used, such as the distribution of T2 measurements in muscle. While most studies report on average T2 for each muscle ROI, distribution of T2 values within the ROI using histogram analysis will add another level of information and may be useful in future trials [48]. In addition, it is possible to separate inflammatory changes from fatty atrophy when evaluating T2 maps. This could either be achieved by correcting the T2 maps with separately acquired fat/ water-maps [69] or by the separate calculation of T2 values for fat and water images in one acquisition [45]. These methods to gain additional information from the T2 signal may prove especially useful in light of the new finding that the genetic transcription profile appears to be correlated to STIR signal in patients with FSHD [24].
Physical properties, such as tissue elasticity or stiffness of skeletal muscle, can also be evaluated using MR elastography. During the scanning session, a pneumatic driver will set the patient’s muscles into repeated motion and the propagating shear wave results in a signal change that can later be evaluated. Studies in patients with PM and dermatomyositis found increased tissue elasticity compared with healthy volunteers, probably due to disrupted tissue integrity [70].
While dedicated systems for nonproton spectroscopy or MRI-elastography require expensive additional equipment, BOLD-imaging, T2-mapping or diffusion tensor imaging can be applied to standard clinic scanners at reasonable additional costs and may gain widespread use in the future; however, evaluation of clinical validity, sensitivity, specificity and prognostic values of these parameters first need to be validated in clinical trials, many of which are already underway or in a planning phase. The next few years may, therefore, prove to be a golden age for neuromuscular imaging research.
Future perspective
The increasing number of multicenter trials in rare types of MDs will result in more and better defined patterns of disease. Dedicated software algorithms can peruse large databases of muscle patterns to help in the diagnosis of individual patients with newly diagnosed muscle disease but without genetic confirmation. On the other hand, we will see more and more treatments of individual patients with new genetic therapies in ‘one-patient trials’. Quantitative MRI, together with imaging modalities such as magnetizationtransfer imaging or spectroscopy, will provide monitoring for these patients. Children with neuromuscular disorders will live longer and remain ambulatory, resulting in the involvement of more organs outside the musculoskeletal system. Whole body imaging will be necessary to detect these changes and monitor disease progression.
Financial & competing interests disclosure
D Fischer is supported by a grant from the Lorenzo-Piaggio Foundation (Zürich, Switzerland). D Fischer has received, in the past year, consulting fees for participating on advisory boards from Teva Pharmaceutical Industries Ltd (Petah Tikva, Israel) and Genzyme Corporation (MA, USA). The Department of Radiology is supported by a nonspecific grant from Bracco (Switzerland). The sponsor played no role in manuscript preparation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
•of interest
• •of considerable interest
- Straub V, Carlier P, Mercuri E. TREAT-NMD workshop: pattern recognition in genetic muscle diseases using muscle MRI: 25–26 February 2011, Rome, Italy. Neuromuscul. Disord. 22 (Suppl. 2), S42–S53 (2012).
- Quijano-Roy S, Avila-Smirnow D, Carlier RY; WB-MRI muscle study group. Whole body muscle MRI protocol: pattern recognition in early onset NM disorders. Neuromuscul. Disord. 22 (Suppl. 2), S68–S84 (2012).
- 3 Neuromuscular Imaging. Wattjes MP, Fischer DE (Eds). Springer, NY, USA (2013).
- Bushby K, Finkel R, Birnkrant DJ et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 9(1), 77–93 (2010).
- Fischmann A, Hafner P, Gloor M et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J. Neurol. 260(4), 969–974 (2012).
- Schwartz MS, Swash M, Ryan J. Why is the gracilis muscle relatively uninvolved in neuromuscular disorders? Neuromuscul. Disord. 1(5), 365–369 (1991).
- Forbes SC, Lott DJ, Finkel RS et al. MRI/MRS evaluation of a female carrier of Duchenne muscular dystrophy. Neuromuscul. Disord. 22(Suppl. 2), S111–S121 (2012).
- Tasca G, Monforte M, Iannaccone E et al. Muscle MRI in female carriers of dystrophinopathy. Eur. J. Neurol. 19(9), 1256–1260 (2012).
- ten Dam L, van der Kooi AJ, van Wattingen M, de Haan RJ, de Visser M. Reliability and accuracy of skeletal muscle imaging in limbgirdle muscular dystrophies. Neurology 79(16), 1716–1723 (2012).
- Tasca G, Iannaccone E, Monforte M et al. Muscle MRI in Becker muscular dystrophy. Neuromuscul. Disord. 22(Suppl. 2), S100–S106 (2012).
- Flanigan KM. The muscular dystrophies. Semin. Neurol. 32(3), 255–263 (2012).
- Mercuri E, Bushby K, Ricci E et al. Muscle MRI findings in patients with limb girdle muscular dystrophy with calpain 3 deficiency (LGMD2A) and early contractures.
- Neuromuscul. Disord. 15(2), 164–171 (2005). 13 Fischer D, Walter MC, Kesper K et al. Diagnostic value of muscle MRI in differentiating LGMD2I from other LGMDs. J. Neurol. 252(5), 538–547 (2005).
- Sarkozy A, Deschauer M, Carlier RY et al. Muscle MRI findings in limb girdle muscular dystrophy type 2L. Neuromuscul. Disord. 22(Suppl. 2), S122–S129 (2012).
- Mahjneh I, Bashir R, Kiuru-Enari S et al. Selective pattern of muscle involvement seen in distal muscular dystrophy associated with anoctamin 5 mutations: a follow-up muscle MRI study. Neuromuscul. Disord. 22(Suppl. 2), S130–S136 (2012).
- Kornblum C, Lutterbey G, Bogdanow M et al. Distinct neuromuscular phenotypes in myotonic dystrophy types 1 and 2: a whole body highfield MRI study. J. Neurol. 253(6), 753–761 (2006).
- Franc DT, Muetzel RL, Robinson PR et al. Cerebral and muscle MRI abnormalities in myotonic dystrophy. Neuromuscul. Disord. 22(6), 483–491 (2012).
- Minnerop M, Weber B, Schoene-Bake JC et al. The brain in myotonic dystrophy 1 and 2: evidence for a predominant white matter disease. Brain 134(12), 3530–3546 (2011).
- Caramia F, Mainero C, Gragnani F et al. Functional MRI changes in the central motor system in myotonic dystrophy type 1. Magn. Reson. Imaging 28(2), 226–234 (2010).
- Messineo D, Cremona A, Trinci M, Francia A, Marini A. MRI in the study of distal primary myopathopies and of muscular alterations due to peripheral neuropathies: possible diagnostic capacities of MR equipment with low intensity field (0.2 T) dedicated to peripheral limbs. Magn. Reson. Imaging 16(7), 731–741 (1998).
- Olsen D, Gideon P, Jeppesen TD, Vissing J. Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI. J. Neurol. 253(11), 1437–1441 (2006).
- Wattjes MP, Kley RA, Fischer D. Neuromuscular imaging in inherited muscle diseases. Eur. Radiol. 20(10), 2447–2460 (2010).
- Kan HE, Scheenen TW, Wohlgemuth M et al. Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul. Disord. 19(5), 357–362 (2009).
- Tasca G, Pescatori M, Monforte M et al. Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS ONE 7(6), e38779 (2012).
- Tasca G, Ricci E, Monforte M et al. Muscle imaging findings in GNE myopathy. J. Neurol. 259(7), 1358–1365 (2012).
- Tasca G, Ricci E, Penttilä S et al. New phenotype and pathology features in MYH7- related distal myopathy. Neuromuscul. Disord. 22(7), 640–647 (2012).
- Brais B. Oculopharyngeal muscular dystrophy: a polyalanine myopathy. Curr. Neurol. Neurosci. Rep. 9(1), 76–82 (2009).
- Fischmann A, Gloor M, Fasler S et al. Muscular involvement assessed by MRI correlates to motor function measurement values in oculopharyngeal muscular dystrophy. J. Neurol. 258(7), 1333–1340 (2011).
- Dimachkie MM, Barohn RJ. Idiopathic inflammatory myopathies. Semin. Neurol. 32(3), 227–236 (2012).
- Peyer A, Kinter J, Hench J et al. Novel valosin containing protein mutation in a Swiss family with hereditary inclusion body myopathy and dementia. Neuromuscul. Disord. 23(2), 149–154 (2013).
- Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat. Rev. Neurol. 7(6), 343–354 (2011).
- Yoshida K, Kurosaka D, Joh K et al. Fasciitis as a common lesion of dermatomyositis, demonstrated early after disease onset by en bloc biopsy combined with magnetic resonance imaging. Arthritis Rheum. 62(12), 3751–3759 (2010).
- Del Grande F, Carrino JA, Del Grande M, Mammen AL, Christopher Stine L. Magnetic resonance imaging of inflammatory myopathies. Top. Magn. Reson. Imaging 22(2), 39–43 (2011).
- Vuillerot C, Girardot F, Payan C et al. Monitoring changes and predicting loss of ambulation in Duchenne muscular dystrophy with the Motor Function Measure. Dev. Med. Child Neurol. 52(1), 60–65 (2010).
- Hollingsworth K, de Sousa PL, Straub V, Carlier P. Towards harmonization of protocols for MRI outcome measures in skeletal muscle studies: consensus recommendations from two TREAT-NMD NMR workshops, 2 May 2010, Stockholm, Sweden, 1–2 October 2009, Paris, France. Neuromuscul. Disord. 22(Suppl. 2), S54–S67 (2012).
- Jansen M, van Alfen N, Nijhuis van der Sanden MW et al. Quantitative muscle ultrasound is a promising longitudinal followup tool in Duchenne muscular dystrophy. Neuromuscul. Disord. 22(4), 306–317 (2012).
- English C, Fisher L, Thoirs K. Reliability of real-time ultrasound for measuring skeletal muscle size in human limbs in vivo: a systematic review. Clin. Rehabil. 26(10), 934–944 (2012).
- Schedel H, Reimers CD, Nägele M et al. Imaging techniques in myotonic dystrophy. A comparative study of ultrasound, computed tomography and magnetic resonance imaging of skeletal muscles. Eur. J. Radiol. 15(3), 230–238 (1992).
- Gille O, de Sèze MP, Guérin P et al. Reliability of magnetic resonance imaging measurements of the cross-sectional area of the muscle contractile and non-contractile components. Surg. Radiol. Anat. 33(8), 735–741 (2011).
- Gloor M, Fasler S, Fischmann A et al. Quantification of fat infiltration in oculopharyngeal muscular dystrophy: comparison of three MR imaging methods. J. Magn. Reson. Imaging 33(1), 203–210 (2011).
- Gloor M, Hampe EI, Fischer D, Bieri O, Fischmann A. Comparison of 2- and 3-point dixon muscle fat content with chemical analysis. Presented at: International Society for Magnetic Resonance in Medicine 20th Annual Meeting. Melbourne, Australia, 5–11 May 2012 (Poster 1429).
- Wokke BH, Bos C, Reijnierse M et al. Comparison of dixon and T1-weighted MR methods to assess the degree of fat infiltration in duchenne muscular dystrophy patients. J. Magn. Reson. Imaging doi:10.1002/ jmri.23998 (2013) (Epub ahead of print).
- Adachi Y, Sato N, Okamoto T et al. Brachial and lumbar plexuses in chronic inflammatory demyelinating polyradiculoneuropathy: MRI assessment including apparent diffusion coefficient. Neuroradiology 53(1), 3–11 (2011).
- Ma J. Dixon techniques for water and fat imaging. J. Magn. Reson. Imaging 28(3), 543–558 (2008).
- Janiczek RL, Gambarota G, Sinclair CD et al. Simultaneous T2 and lipid quantitation using IDEAL-CPMG. Magn. Reson. Med. 66(5), 1293–1302 (2011).
- Gaeta M, Messina S, Mileto A et al. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments. Preliminary experience. Skeletal Radiol. 41(8), 955–961 (2011).
- del Porto L, Nicholson G, Ketheswaren P. Correlation between muscle atrophy on MRI and manual strength testing in hereditary neuropathies J. Clin. Neurosci. 17(7), 874–878 (2010).
- Arpan I, Forbes SC, Lott DJ et al. T₂ mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 26(3), 320–328 (2013).
- Morrow J, Sinclair CDJ, Fischmann A et al. 1700 MRI quantification of lower limb muscle fatty atrophy: a potential outcome measure in chronic neuromuscular diseases. J. Neurol. Neurosurg. Psychiatry 83(3), 9–10 (2012).
- Hiba B, Richard N, Hébert LJ et al. Quantitative assessment of skeletal muscle degeneration in patients with myotonic dystrophy type 1 using MRI. J. Magn. Reson. Imaging 35(3), 678–685 (2012).
- Damian MS, Bachmann G, Herrmann D, Dorndorf W. Magnetic resonance imaging of muscle and brain in myotonic dystrophy. J. Neurol. 240(1), 8–12 (1993).
- Kim HK, Laor T, Horn PS, Wong B. Quantitative assessment of the T2 relaxation time of the gluteus muscles in children with Duchenne muscular dystrophy: a comparative study before and after steroid treatment. Korean J. Radiol. 11(3), 304–311 (2010).
- Hollingsworth K, Willis T, Coombs A. Muscle fat infiltration in limb girdle muscular dystrophy 2I: a comparison of qualitative T1w and quantitative Dixon imaging. Proc. Intl Soc. Mag. Reson. Med. 18, 853 (2010).
- Fischer MA, Nanz D, Shimakawa A et al. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR Imaging with single-voxel MR spectroscopy. Radiology 266(2), 555–563 (2013).
- Gaeta M, Scribano E, Mileto A et al. Muscle fat fraction in neuromuscular disorders: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification – a feasibility study. Radiology 259(2), 487–494 (2011).
- Makrogiannis S, Serai S, Fishbein KW et al. Automated quantification of muscle and fat in the thigh from water-, fat-, and nonsuppressed MR images. J. Magn. Reson. Imaging 35(5), 1152–1161 (2012).
- Vermathen P, Saillen P, Boss A, Zehnder M, Boesch C. Skeletal muscle (1) H MRSI before and after prolonged exercise. I. muscle specific depletion of intramyocellular lipids. Magn. Reson. Med. 68(5), 1357–1367 (2012).
- Takahashi K, Tohdoh Y, Matsubayashi T, Jellúš V, Maruyama K. Fatigue-induced changes in longitudinal relaxation time determined by magnetic resonance imaging. Clin. Imaging 36(6), 816–820 (2012).
- Fischmann A, Kaspar S, Reinhardt J et al. Exercise might bias skeletal-muscle fat fraction calculation from Dixon images. Neuromuscul. Disord. 22(Suppl. 2), S107–S110 (2012).
- Garrood P, Hollingsworth K, Eagle M et al. MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J. Magn. Reson. Imaging 30(5), 1130–1138 (2009).
- Qi J, Olsen NJ, Price RR, Winston JA, Park JH. Diffusion-weighted imaging of inflammatory myopathies: polymyositis and dermatomyositis. J. Magn. Reson. Imaging 27(1), 212–217 (2008).
- Heemskerk AM, Sinha TK, Wilson KJ, Ding Z, Damon BM. Repeatability of DTI-based skeletal muscle fiber tracking. NMR Biomed. 23(3), 294–303 (2010).
- Noseworthy MD, Davis AD, Elzibak AH. Advanced MR imaging techniques for skeletal muscle evaluation. Semin. Musculoskelet. Radiol. 14(2), 257–268 (2010). n Good overview of advanced imaging techniques.
- McMillan AB, Shi D, Pratt SJ, Lovering RM. Diffusion tensor MRI to assess damage in healthy and dystrophic skeletal muscle after lengthening contractions. J. Biomed. Biotechnol. 2011, 970726 (2011).
- Jacobi B, Bongartz G, Partovi S et al. Skeletal muscle BOLD MRI: From underlying physiological concepts to its usefulness in clinical conditions. J. Magn. Reson. Imaging 35(6), 1253–1265 (2012).
- Chang G, Wang L, Cárdenas-Blanco A et al. Biochemical and physiological MR imaging of skeletal muscle at 7 tesla and above. Semin. Musculoskelet. Radiol. 14(2), 269–278 (2010).
- Weber MA, Nagel AM, Wolf MB et al. Permanent muscular sodium overload and persistent muscle edema in Duchenne muscular dystrophy: a possible contributor of progressive muscle degeneration. J. Neurol. 259(11), 2385–2392 (2012).
- Sinclair CD, Samson RS, Thomas DL et al. Quantitative magnetization transfer in in vivo healthy human skeletal muscle at 3 T. Magn. Reson. Med. 64(6), 1739–1748 (2010).
- Yao L, Gai N. Fat-Corrected T2 Measurement as a marker of active muscle disease in inflammatory myopathy. Am. J. Roentgenol. 198(5), W475–W481 (2012).
- McCullough M, Domire Z, Reed A et al. Evaluation of muscles affected by myositis using magnetic resonance elastography. Muscle Nerve 43(4), 585–590 (2011).
- US Office of Rare Diseases Research, Genetic and Rare Diseases Information Center. Muscular dystrophy. http://rarediseases.info.nih.gov/gard/7922/ muscular-dystrophy/resources/1 (Accessed 11 April 2013)
• General overview of pattern recognition and MRI techniques.
• • Excellent overview of pattern recognition with useful pathways for differential diagnosis.
• Standard protocols for MRI in neuromuscular disorders.
■Website