Review Article - Interventional Cardiology (2023) Volume 15, Issue 2
Imbalance of mitochondrial fusion and fission in ischemic heart disease
- Corresponding Author:
- Alexander V. Blagov Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia, E-mail: al.blagov2014@gmail.com
- Alexander N. Orekhov Petrovsky Russian Scientific Center of Surgery, 2, Abrikosovsky lane, 119991 Moscow, Russia, E-mail: a.h.opexob@gmail.com
Received date: 14-Feb-2023, Manuscript No. FMIC-23-89338; Editor assigned: 16-Feb-2023, PreQC No. FMIC-23-89338 (PQ); Reviewed date: 02- Mar-2023, QC No. FMIC-23-89338; Revised date: 09-Mar-2023, Manuscript No. FMIC-23-89338 (R); Published date: 16-Mar-2023, DOI: 10.37532/1755-5310.2023.15(2).655
Abstract
Cardiovascular diseases are one of the leading causes of death in the world. One of the main diseases of this group is coronary heart disease, the treatment of which does not always have a positive effect. The development of new drugs requires finding new pathological targets that affect the development of the disease. Proteins that regulate mitochondrial dynamics, namely, mitochondrial division and mitochondrial fusion, can act as such targets, since a change in these processes has been noted for a number of cardiovascular diseases. In this review, we will consider the mechanism of changes in these processes in the context of the pathogenesis of ischemia, as well as note the current state of therapeutic developments in this area.
Keywords
Mitochondria • Ischemic heart disease • Ischemia • Coronary artery
Introduction
Ischemic Heart Disease (IHD) is a condition in which there is insufficient supply of blood and oxygen to the myocardium. This occurs as a result of occlusion of the coronary arteries and leads to a mismatch in oxygen demand. This is usually associated with the formation of plaques in the lumen of the coronary arteries, which impede blood flow. It is the leading cause of death worldwide. Mortality from coronary artery disease peaked in the mid-1960s and then declined, but it still remains the leading cause of death worldwide [1].
Arrhythmia, acute coronary syndrome, congestive heart failure, mitral regurgitation, ventricular free wall rupture, pericarditis, aneurysm formation, and mural thrombi are the main complications associated with coronary heart disease [2]. Ischemic heart disease is a multifactorial phenomenon. Etiological factors can be divided into non- modifiable and modifiable factors. Non-modifiable factors include gender, age, family history, and genetics. Modifiable risk factors include smoking, obesity, lipid levels, and psychosocial variables. In the world, the fast-paced lifestyle has led people to eat more fast food and junk food, which has led to an increase in the prevalence of coronary heart disease. Comorbidities, including diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease, also play a role in overall outcome [3]. The male sex is more predisposed than the female sex. Hypercholesterolemia remains an important modifiable risk factor for IHD. Elevated Low-Density Lipoprotein (LDL) levels increase the risk of IHD, and elevated High-Density Lipoprotein (HDL) levels reduce the incidence of IHD [4].
Ischemic heart disease is very common in both developed and developing countries. In one study, it was estimated that coronary artery disease accounts for 2.2% of the total global burden of disease and 32.7% of cardiovascular disease. The incidence of IHD increases with age regardless of gender. The incidence of coronary artery disease was about 1% in the age group from 45 to 65 years, and as the age group reached 75 to 84 years, it increased to about 4% [5].
The main goals of the pharmacological treatment of coronary heart disease are to reduce the number of pulling symptoms and prevent cardiovascular complications. Ischemic heart disease can present with either Stable Ischemic Heart Disease (SIHD) or Acute Coronary Syndrome (ACS). The former is manifested in a chronic form, while the latter is more often manifested in an acute form [6]. Treatment of SIHD includes both non-drug and pharmacological interventions. Lifestyle modifications include quitting smoking, regular exercise, weight loss, good control of diabetes and hypertension, and a healthy diet. The main groups of drugs used: Short- and long-acting nitrates, β-blockers, calcium channel antagonists, lipid-lowering drugs, anticoagulants, Angiotensin-Converting Enzyme inhibitors (ACE inhibitors). Both medical and surgical treatment of coronary heart disease is associated with their side effects and complications. For example, beta-blockers can cause bradycardia and hypotension. ACE inhibitors can cause hypotension, dizziness, increased creatinine levels, cough, and allergic reactions, including angioedema [7]. Statin therapy can cause myalgia, diarrhea, and arthralgia among side effects [8].
Despite great advances made in recent years, modern IHD medications are associated with numerous side effects and complications. Thus, the development and implementation of highly effective drugs or combinations of drugs with favorable side effect profiles for patients is an important goal today.
Literature Review
Ischemic heart disease pathogenesis
A distinctive feature of the pathogenesis of IHD is the development of atherosclerotic plaques. A plaque is an accumulation of fatty material that narrows the lumen of the vessel and impedes blood flow. The first step in this process is the formation of a “fat strip” [9]. This stage begins with the outflow of Low-Density Lipoprotein (LDL) cholesterol into the sub endothelial space, which can then be modified and oxidized by various agents. Oxidized/modified LDL particles are potent chemotactic molecules that induce the expression of vascular cell adhesion molecules and intercellular adhesion molecules on the endothelial surface and promote adhesion and migration of monocytes into the subendothelial space. Monocytes differentiate into macrophages in the intima- media. Recently, different subsets of monocytes have been identified and their roles appear to differ depending on the phase of atherosclerosis in which they are involved [10]. Macrophages bind oxidized LDL via scavenger receptors to become foam cells and also have pro-inflammatory functions, including the release of cytokines such as interleukins and tumor necrosis factor. The end result of this process is the formation of the first typical atherosclerotic focus, the fatty streak, in which foam cells are present in the subendothelial space [11].
Other types of leukocytes, such as lymphocytes and mast cells, also accumulate in the subendothelial space [12]. The interaction between monocytes, macrophages, foam cells and T cells leads to cellular and humoral immune responses and ultimately to a chronic inflammatory state with the production of several pro- inflammatory molecules [13]. This process continues with the migration of smooth muscle cells from the medial layer of the artery to the intima, which leads to a transition from the fatty streak to a more complex lesion [12]. Once the smooth muscle cells enter the intima media, they produce extracellular matrix molecules, creating a fibrous cap that covers the original fat streak. Foam cells within the fibrous cap die and release lipids, which accumulate in the extracellular space, forming a lipid-rich pool known as the necrotic nucleus. The result of this process is the formation of a second atherosclerotic focus-a fibrous plaque [9].
Over time, this plaque may increase in size or become stable if no further damage to the endothelium occurs. If it becomes stable, the lesion will gradually calcify. Over time, the lesion may become hemodynamically significant enough that at the time of increased demands on the myocardial tissue, sufficient blood does not flow, and symptoms of angina pectoris occur. However, symptoms decrease at rest as oxygen demand decreases. For a lesion to cause angina at rest, it must be at least 90% stenotic. Some plaques can rupture and become exposed to tissue factor, leading to thrombosis. This thrombosis can cause subtotal or complete lumen occlusion and can lead to the development of Acute Coronary Syndrome (ACS) in the form of unstable angina [14].
In acute ischemia, oxygen deficiency disrupts the oxidation of glucose and Free Fatty Acids (FFA), so enzymatic cytoplasmic glycolysis becomes the main source of energy. Secreted catecholamines (epinephrine and norepinephrine) increase the hydrolysis of fats reaching the heart. As a result, a reduced glucose supply promotes free fatty acid oxidation, thus becoming the only source of energy in which oxygen consumption increases and the supply decreases rapidly, thereby forcing the cell to switch to anaerobic glycolysis. This causes the accumulation of lactates and hydrogen ions. A few seconds of ischemia impair myocardial contractility and relaxation. Lack of return of myocardial reperfusion within 45-60 minutes leads to necrosis of cardiac cells, i.e., to infarction [15].
Mechanism of mitochondrial fission
The result of the mitochondrial fission process is the division of the mitochondria into two smaller organelles. The main role in this reaction is played by the GTP-hydrolyzing enzyme, Dynamin- related protein 1 (Drp1). Drp1 is a member of the GTPase dynamin superfamily and, like other dynamins, acts primarily in membrane remodeling through oligomerization and conformational changes. The primary sequence of this protein consists of four conserved regions: The N-terminal domain of GTPase, the middle, variable, and effector domain of GTPase (GED) at the C-terminus [16]. Drp1 is primarily localized in the cytosol as a mixture of dimers and tetramers, and in response to specific cellular signals, it is recruited via mitochondrial Outer Membrane (OMM) receptors, where it oligomerizes into filaments that wrap and constrict membranes. In mammals, four proteins have been described that are involved in Drp1 recruitment to OMM: Mitochondrial fission factor (Mff), mitochondrial dynamics protein 49 kDa (MID49), 51 kDa (MID51), and mitochondrial fission protein 1 (Fis1) [17].
Mff is considered the primary receptor for Drp1 in both mitochondria and peroxisomes. Mff is distributed in discrete foci along the OMM, where it specifically recruits highly oligomeric forms of Drp1. Moreover, it has been suggested that this protein increases the activity of Drp1 GTPase, thereby promoting pinching of Drp1 helices around mitochondrial membranes and, consequently, mitochondrial division [18].
MIDs proteins attached to the OMM via an N-terminal transmembrane domain are Drp1 receptors present on mitochondria and not on peroxisomes. Crystal structure studies have shown that both MID49 and MID51 possess a non-canonical nucleotidyl transferase (NTase) domain, but lack critical residues required for transferase activity; however, it is possible that the NTase fold acts as a platform for protein recruitment and assembly of Drp1 oligomers on the OMM [19,20]. In any case, structural differences between these two Drp1 receptors have been reported, for example, unlike MID49, MID51 does bind Adenosine Diphosphate (ADP) as a cofactor; moreover, MID49 has a monomeric structure, while MID51 is dimeric. These differences may reflect their differential regulation of mitochondrial division. To this end, it was suggested that the dimeric structure of MID51 promotes the recruitment of Drp1 in the basal state, which is considered to be dimeric. In addition, it is assumed that the ability of MID51 to bind ADP allows it to sense metabolic changes in the cell [19].
Fis1 was the first identified receptor for Drp1 in yeast, where it recruits Dnm1p (a yeast homologue of Drp1) through interaction with adapter proteins Mdv1p and Caf4p [21]. However, the absence of homologues of Mdv1 and Caf4 in mammals makes the role of human Fis1 in the process of mitochondrial division unclear. In fact, Fis1 has been reported to bind Drp1 and take part in Drp1-dependent mitochondrial division. Later, after the identification of MIDs and Mff receptors, the role of Fis1 in mitochondrial division was reconsidered, and it was demonstrated that Drp1 activity is not affected or little affected by silencing or overexpression of human Fis1 [22]. However, Fis1 has been reported to play a specific role in the mitophagy process by complexing with several Endoplasmic Reticulum (ER) proteins and Mff-recruited Drp1, thereby promoting mitochondrial division required for mitochondrial autophagy [22].
Although Drp1 and its receptors/adapters are at the core of the division mechanism, both the initial and final stages of this process depend on the action of other cellular structures such as the Endoplasmic Reticulum (ER), actin cytoskeleton, and Dynamin-2 (Dnm2). It has been observed that mitochondrial division occurs exclusively at the ER-mitochondrial-cytoskeleton interface, where ER membranous tubules span elongated mitochondria to mark the sites of upcoming mitochondrial division [23]. Moreover, it has been suggested that both myosin II and the formin-2 protein localized in the ER control actin polymerization at the ER-mitochondrial interface, ensuring efficient division [24]. Considering that mitochondrial tubules have a diameter approximately five times greater than that of Drp1 helices, the interaction of the ER-cytoskeletal apparatus is necessary for preliminary constriction of mitochondria to a diameter that allows assembly of the DRP1 oligomer [21].
Mechanism of mitochondrial fusion
Mitochondrial fusion is the process that results in the physical union of two adjacent organelles. At the site of collision, fusion occurs in two stages: Fusion of the outer membrane followed by fusion of the inner membrane. The result of this fusion process is the transfer of information through the exchange of mtDNA, proteins, lipids, and metabolites. The fusion mechanism consists of three dynamin-associated GTPases: Mitofusins 1 and 2 (Mfn1, Mfn2) and optic nerve atrophy protein 1 (OPA1) [25].
The mitofusins responsible for OMM fusion contain conserved catalytic GTP-binding domains at the N-terminus and two C-terminal helical domains (also called heptad repeat domains, HR1 and HR2) that surround two transmembrane I domains [26]. Mitofusins are directly involved in OMM docking and fusion. Docking activity depends on their ability to self-assemble into oligomers within the same membrane or across opposite membranes. Two possible models for the OMM merger have been proposed. The first suggests that the HR2 domain promotes membrane docking during the formation of dimers of antiparallel helical mitofusins that bring opposite mitochondrial membranes closer together at a distance of ~10 nm from each other; then, the HR1 domain, which has a conserved amphipathic helix, interacts with the lipid membrane, bringing OMM closer and disrupting the structure of their lipid bilayer [27]. In the second case, it is assumed that binding of GTP triggers the oligomerization of Mfns dimers and the subsequent hydrolysis of GTP, leading to a conformational change that brings the membranes closer together [28]. Similar to mitochondrial fission, mitochondrial fusion also occurs at ER-mitochondrial contact sites, and it has been suggested that Mfn2 directly mediates ER-mitochondrial binding in mammals [29].
Despite the central role of Mfn, Misato (MSTO1) has been reported to be involved in the OMM fusion process as its depletion has been found to cause mitochondrial fragmentation. MSTO1 is a soluble cytoplasmic protein that translocates to the outer surface of the OMM where it interacts with mitochondrial fusion proteins. It is believed that MSTO1 can support mitochondrial fusion by enhancing or initiating OMM fusion [30]. In addition, mitoPLD, a member of the phospholipase D family, is thought to be involved in the fusion process. MitoPLD binds to OMM, where it converts cardiolipin to phosphatidic acid, whose negatively charged lipid group induces negative curvature of lipid bilayers, allowing recruitment of adapter proteins required for mitochondrial fusion [31].
One way or another, the dynamin-like GTPase OPA1 plays a key role in the fusion of the inner mitochondrial membrane in mammals. Like other dynamins, it contains three highly conserved regions open to the Intermembrane Space (IMS); A GTP binding domain, a GTP effector domain, and a middle domain. In addition, the N-terminal region includes a mitochondrial- targeting sequence followed by a transmembrane helix required for anchoring the mitochondrial Inner Membrane (IMM) [32]. In humans, OPA1 is present in eight different isoforms. OPA1 mRNA variants result from alternative splicing, and the resulting progenitors are targeted to the mitochondria via their leader sequence. Upon import into mitochondria, mitochondrial processing peptidase cleaves a mitochondrial-targeted sequence, producing long GTPase isoforms (L-OPA1) that attach to the IMM [33]. L-OPA1 isoforms can undergo further proteolytic processing at the N-terminus to form short forms (S-OPA1). Previous work describes that only L-OPA1 is capable of fusion [34]. In vitro studies have shown that membrane-anchored L-OPA1 binds to opposing membrane Cardiolipin (CL), allowing binding and subsequent fusion by GTP hydrolysis, even in the absence of opposing membrane OPA1. These results revealed two important features: The presence of OPA1 even in only one of the two opposite mitochondria can drive the fusion process, while cardiolipin is directly involved in membrane remodeling and dynamics [35].
Another protein involved in mitochondrial fusion is F-box and leucine-rich repeat 4 (FBXL4). This protein, localized in the mitochondrial intermembrane space, contains a leucine-rich repeating domain necessary for protein-protein interactions and through which it forms quaternary protein complexes [36]. The role of FBXL4 in the process of mitochondrial fusion is suggested, since defects in this protein lead to mitochondrial fragmentation, while overexpression of FBXL4 promotes mitochondrial hyperfusion [37]. Comparative scheme of mitochondrial fission and fusion is presented in Table 1.
| Parameters | Fission | Fusion |
|---|---|---|
| Role in mitochondrial homeostasis | Separation of mitochondria into dysfunctional and functional in order to improve quality control and increase the number of new mitochondria | Improving the functionality of mitochondria through uniform distribution of mitochondrial components |
| Influence on energetic cell state | Providing increased energy requirements in specific cellular compartments | Uniform stabilization of energy needs in the cell |
| Main regulatory proteins | Drp1, Mff, Fis1 | OPA1, Mfn1, Mfn2 |
| Imbalance in IHD | Increased | Decreased |
| Therapeutic targets | Drp1 | OPA1 and Mfn2 |
Table 1: Comparative scheme of mitochondrial fission and fusion.`
Disruption of mitochondrial division during ischemia
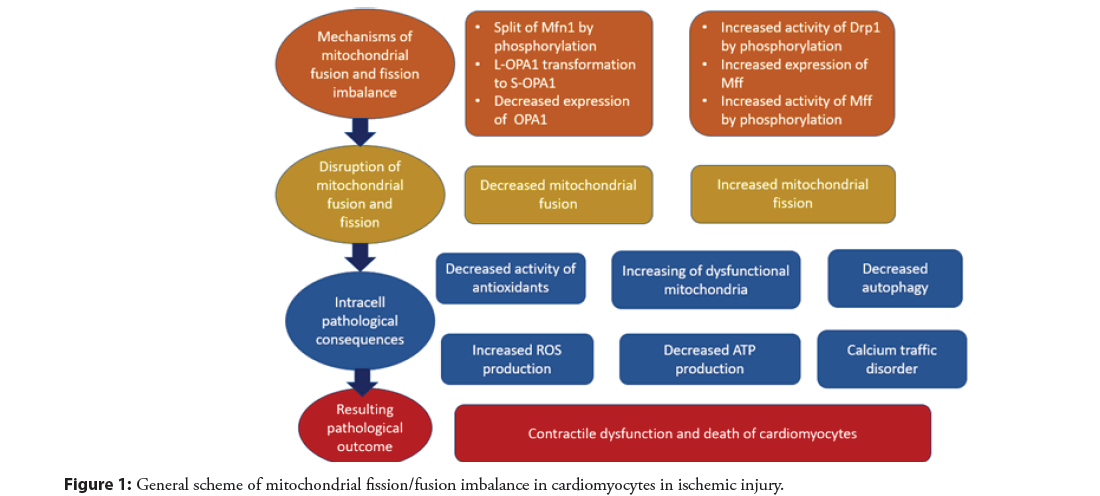
Normally, the main regulatory protein of mitochondrial division DRP1 is present in the cytoplasm in an inactive form, unable to interact with receptors located on the outer membrane of mitochondria. Therefore, under normal physiological conditions, mitochondrial division proceeds at a low level. However, when stress conditions occur, an increase in mitochondrial division was noted, which occurs as a result of DRP1 activation through post-transcriptional modifications, such as acetylation, phosphorylation, and ubiquitination [38,39]. As a result of molecular conformational changes, the DRP1 binding site becomes accessible to receptors on the outer mitochondrial membrane, which promotes DRP1 translocation to the outer mitochondrial membrane [40]. At the same time, it should be taken into account that different transcriptional modifications of DRP1 can regulate mitochondrial division in different ways. Thus, DRP1 can be phosphorylated at Ser616 and Ser637, which leads to opposite effects. Thus, phosphorylation at the Ser616 site leads to the oligomerization of DRP1 molecules around the outer mitochondrial membrane, which is the initiating step for mitochondrial division [41]. Conversely, when Ser637 is phosphorylated, DRP1 oligomerization is inhibited, which leads to the arrest of mitochondrial division [42].
With ischemic heart damage, there is a decrease in DRP1 phosphorylation at the Ser637 site, and phosphorylation at the Ser616 site increases, which causes an increase in the mitochondrial localization of DRP1, its subsequent oligomerization, and, accordingly, an increase in mitochondrial division [42,43]. In addition to changes in DRP1 phosphorylation during ischemia, phosphorylation of Mff, the mitochondrial DRP1 receptor, at the Ser146 site increases, and its expression in cardiomyocytes also increases, which is another important factor in enhancing mitochondrial division [44]. Increased division of mitochondria has a number of negative consequences, which may eventually contribute to apoptosis of cardiomyocytes. First of all, excessive division, which leads to an increase in the number of mitochondria, including dysfunctional ones, leads to an increase in ROS production and, accordingly, to the development of oxidative stress [45]. ROS can damage mitochondria, which leads to an even greater increase in the proportion of dysfunctional mitochondria, and under conditions of a decrease in the expression of a number of antioxidant enzymes and a decrease in the level of autophagy [46], this becomes widespread. As a result of an increase in the number of dysfunctional and damaged mitochondria, ATP production decreases, which negatively affects the energy state of cardiomyocytes, and the internal pathway of apoptosis is initiated, which leads to cell death [47]. The initiation of apoptosis of cardiomyocytes and the preceding contractile dysfunction of the myocardium are caused by impaired calcium traffic in the cell, another consequence of the disruption of the mitochondrial network [48].
Disruption of mitochondrial fusion in ischemia
It is known that the fusion of mitochondria is a process that protects cells under stress conditions. First of all, mitochondrial fusion is the reverse process of mitochondrial division and therefore inhibits the initiation of apoptosis, which, as noted above, is characteristic of mitochondrial division. In addition, as a result of the fusion of mitochondria within the network of elongated mitochondria, a common electrochemical potential is generated, which facilitates the detection of damaged mitochondrial regions [49]. Finally, mitochondrial fusion results in a uniform distribution of mitochondrial components, including mitochondrial proteins, cDNA, and metabolites, which improves the functional capacity of mitochondria [50]. Despite the marked decrease in the level of mitochondrial fusion in ischemic heart injury, changes in the activity of mitochondrial fusion regulatory proteins are not so obvious at first glance. In cell culture studies and animal models of ischemia, it was mainly shown that deficiency of the regulatory protein Mfn2 leads to the development of oxidative stress and death of cardiomyocytes, while Mfn1 deficiency had no significant effect on the change in the pathological state [51]. A possible mechanism of Mfn2 inhibition during cardiac ischemia may be phosphorylation, which changes its activity. Thus, phosphorylation of the Mfn2 c-Jun N-terminal kinase is known to lead to its cleavage, decrease in mitochondrial fusion, and death of cardiomyocytes [52]. It is fairly well characterized that inhibition of the function of the mitochondrial division regulatory protein OPA1 leads to cardiac dysfunction and death of cardiomyocytes. The molecular mechanism underlying this inhibition is associated with the activation of the OMA1 enzyme, which, in turn, promotes the transformation of the L-form of OPA1 into the S-form, which leads to increased mitochondrial division and initiation of apoptosis of cardiomyocytes [53]. In addition, OPA1 expression in cardiomyocytes depends on STAT3 activity, which is significantly reduced in ischemic injury [54]. Thus, an imbalance in the division and fusion of mitochondria plays an important role in the dysfunction and death of cardiomyocytes during ischemia. The general scheme of the indicated pathological changes is indicated in Figure 1.
Potential therapeutic modulation of mitochondrial fission and fusion
Restoring the balance of mitochondrial dynamics processes in coronary heart disease requires the use of compounds that either enhance mitochondrial fusion or weaken mitochondrial fission. To weaken mitochondrial division, it is logical to use compounds that have a high inhibitory activity against the main regulatory protein of mitochondrial division DRP1. One of the most frequently considered options for attenuating mitochondrial fission is the use of the small molecule Mdivi-1, which is a direct inhibitor of the DRP1 protein. The introduction of Mdivi-1 showed a positive effect both in cell culture and in an animal model of coronary heart disease. Thus, Mdivi-1 treatment of HL-1 cells led to an increase in the proportion of elongated mitochondria, decreased mitochondrial membrane permeability, and reduced cell death in modeling ischemic damage [55]. In a diabetic mouse model with induced ischemic injury, it was demonstrated that the administration of Mdivi-1 led to a decrease in the localization of DRP1 in mitochondria and, accordingly, a decrease in mitochondrial division, and also led to an increase in the activity of the natural cellular antioxidant enzyme, superoxide dismutase, and a decrease in cardiomyocyte apoptosis. In addition, an improvement in cardiac function and a decrease in myocardial infarction were noted [56]. However, it should be said that the use of Mdivi-1 may also be associated with negative effects on cardiomyocytes. Thus, the administration of Mdivi-1 in the early stages of ischemia, on the contrary, leads to a deterioration in cardiac function, which may be explained by the fact that an increase in mitochondrial division occurs during the progression of the disease, while at an early stage it proceeds at a normal level [57]. In addition, a possible side effect against the background of the weakening of apoptosis is an increase in cardiomyocyte necroptosis, which was shown in one of the studies on mice treated with Mdivi-1 [58]. As enhancers of mitochondrial fusion, promising compounds are those that restore the activity and level of regulatory proteins of mitochondrial fusion: Mfn2 and OPA1. The anesthetic sevoflurane, which has shown the ability to reduce ischemic damage to the heart, can be considered as such an enhancer. Using a model of rat cardiomyocytes, it was shown that the mechanism of its action is based on an increase in the activity of OPA1 and MFN2 [59]. The hormone melatonin was shown to be able to enhance the expression of OPA1, which contributed to the inhibition of apoptosis during ischemic injury [60].
Discussion
In the context of the analysis of the therapeutic effect of promising candidates that can potentially be used in the treatment of coronary heart disease and whose mechanism is based on the modulation of mitochondrial fission or fusion, the issue of a possible off-target effect of these compounds remains important. High specificity of drug action is essential, especially for cardiomyocytes, which are highly dependent on mitochondrial function, in order to avoid severe side effects. An equally important factor is the degree of therapeutic effect, since excessive inhibition of division and increased mitochondrial fusion will also disrupt proper mitochondrial homeostasis and can lead to fatal consequences for cardiomyocytes. In addition, an important step in this direction is the search for new therapeutic targets, whose mechanism of influence on mitochondrial fission and fusion is not as obvious as for DRP1 and Mfn1/2, but nevertheless plays an important role in mitochondrial dynamics and disruption of which can lead to progression of IHD. One of such candidates for new therapeutic targets is the so-called anti-aging gene Sirtuin 1. Its product is responsible for the regulation of a number of important processes directly related to the aging of the organism: Carbohydrate and lipid metabolism, DNA repair and cell proliferation [61,62]. A decrease in Sirtuin 1 activity has been noted for some chronic diseases, including cardiovascular diseases [61]. In a study in a mouse model, it was found that deficiency of Sirtuin 1 leads to aggravation of cardiac dysfunction during ischemia/reperfusion, which, in particular, was associated with impaired mitochondrial respiration and mitochondrial dynamics [63]. Thus, studies on the use of Sirtuin 1 activators as potential therapeutic agents in the treatment of coronary artery disease may have great promise. Catching the right balance between mitochondrial fusion and fission is a necessary task for successful restoration of mitochondrial dynamics. Due to the close relationship between the processes of mitochondrial dynamics, it is important to understand what changes occur with other processes of mitochondrial dynamics, including mitochondrial transport and mitophagy, in order to see the whole picture of the modulation of mitochondrial homeostasis in coronary heart disease. It was indicated above that increased mitochondrial division leads to a weakening of autophagy, a special case of which is mitophagy. At the same time, it is known that the division of mitochondria is usually preceded by mitophagy, which indicates the need for further study of this issue. Also, an important step in understanding the pathogenesis of coronary heart disease seems to be the answer to the question: What kind of damage to mitochondrial structures during oxidative stress leads to mitochondrial dysfunction. It also makes sense to study the effect of a healthy diet on the development of coronary artery disease, namely the mechanism of antioxidant compounds action on the pathogenesis of coronary artery disease, in particular, associated with disruption of mitochondrial dynamics [64]. The review considers the effect of carotenoids on reducing the risk of developing cardiovascular diseases. Thus, in a prospective study [65], a decrease in the risk of developing coronary artery disease was found with the consumption of diet rich in α-carotene or β-carotene. However, in addition to the direct antioxidant effect, the mechanism of the carotenoids effect on the pathogenesis of IHD is not completely clear. It is also not clear whether antioxidants influence mitochondrial dynamics, including fission and fusion. The discovery of new mechanisms of antioxidants action will make them more popular as therapeutic compounds.
Conclusion
An imbalance between mitochondrial fission and fusion is a pathological event seen in ischemic heart disease. Disturbance of this balance is caused by modulation of expression and activity of regulatory proteins of mitochondrial fission and fusion. As a result, a situation arises in which mitochondrial division sharply prevails over their fusion, which leads to detrimental consequences for cardiomyocytes, which include the development of oxidative stress, mitochondrial and subsequently contractile dysfunction, energy deficiency, impaired calcium flow, which ultimately leads to death cardiomyocytes. Currently, there are several promising drug developments that are able to modulate the balance of mitochondrial fission and fusion. However, their clinical use requires more in-depth research on side effects and off-target effects.
Author Contributions
Writing-original draft preparation, AVB, MAP; writing-review and editing, VNS, MBE, IIN, ANO
Funding
This work was supported by the Russian Science Foundation (Grant#23-25-00063).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalen JE, Alpert JS, Goldberg, RJ, et al. The epidemic of the 20th century: Coronary heart disease. Am J Med. 127(9): 807-812 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Michniewicz E, Mlodawska E, Lopatowska P, et al. Patients with atrial fibrillation and coronary artery disease–double trouble. Adv Med Sci. 63(1): 30-35. (2018).
[CrossRef] [Google Scholar] [PubMed]
- Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults-United States, 2016. MMWR Morb Mortal Wkly Rep. 67(2): 53-59 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int J Cardiol. 168(6): 5126-5134 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Bauersachs R, Zeymer U, Brière J, et al. Burden of coronary artery disease and peripheral artery disease: A literature review. Cardiovasc Ther. (2019).
[CrossRef] [Google Scholar] [PubMed]
- Bahit MC, Kochar A, Granger CB, et al. Post-myocardial infarction heart failure. JACC: Heart Fail. 6(3): 179-186 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Straka BT, Ramirez CE, Byrd JB, et al. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol. 140(1): 242-248.e2 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Elam MB, Majumdar G, Mozhui K, et al. Patients experiencing statin-induced myalgia exhibit a unique program of skeletal muscle gene expression following statin re-challenge. PLOS ONE. 12(8): e0181308 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Nakahara T, Dweck MR, Narula N, et al. Coronary artery calcification. JACC: Cardiovasc Imaging. 10(5): 582-593 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis. J Am Coll Cardiol. 62(17): 1541-1551 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Sayols-Baixeras S, Lluís-Ganella C, Lucas G, et al. Pathogenesis of coronary artery disease: Focus on genetic risk factors and identification of genetic variants. Appl Clin Genet. 7: 15-32 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Witztum, JL, Lichtman, AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 9(1): 73-102 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 32(9): 2045-2051 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Conna UV, Cvejic E, Granville SI, et al. Characterizing acute coronary syndrome-associated depression: Let the data speak. Brain Behav Immun. 48: 19-28 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Jankowski P, Czarnecka D, Badacz L, et al. Practice setting and secondary prevention of coronary artery disease. Arch Med Sci. 14(5): 979-987 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Francy CA, Alvarez FJD, Zhou L, et al. The mechanoenzymatic core of dynamin-related protein 1 comprises the minimal machinery required for membrane constriction. J Biol Chem. 290(18): 11692-11703 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Losón OC, Song Z, Chen H, et al. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 24(5): 659-667 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Liu R, Chan DC. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol Biol Cell. 26(24): 4466-4477 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Losón OC, Meng S, Ngo H, et al. Crystal structure and functional analysis of MiD49, a receptor for the mitochondrial fission protein Drp1. Protein Sci. 24(3): 386-394 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Richter V, Palmer CS, Osellame LD, et al. Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. J Cell Biol. 204(4): 477-486 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Di Nottia M, Verrigni D, Torraco A, et al. Mitochondrial dynamics: Molecular mechanisms, related primary mitochondrial disorders and therapeutic approaches. Genes. 12(2): 247 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Osellame LD, Singh AP, Stroud DA, et al. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J Cell Sci. 129(11): 2170-2181 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat Rev Mol Cell Biol. 13(10): 607-615 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Korobova F, Gauvin TJ, Higgs HN, et al. A role for myosin ii in mammalian mitochondrial fission. Curr Biol. 24(4): 409-414 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Yang L, Long Q, Liu J, et al. Mitochondrial fusion provides an “initial metabolic complementation” controlled by mtDNA. Cell Mol Life Sci. 72(13): 2585-2598 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Cohen MM, Tareste D. Recent insights into the structure and function of Mitofusins in mitochondrial fusion. F1000Res. 7: 1983 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Daste F, Sauvanet C, Bavdek A, et al. The heptad repeat domain 1 of Mitofusin has membrane destabilization function in mitochondrial fusion. EMBO Reports. 19(6): e43637 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Qi Y, Yan L, Yu C, et al. Structures of human mitofusin 1 provide insight into mitochondrial tethering. J Cell Biol. 215(5): 621-629 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Filadi R, Greotti E, Pizzo P. Highlighting the endoplasmic reticulum-mitochondria connection: Focus on mitofusin 2. Pharmacological Research. 128: 42-51 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Gal A, Balicza P, Weaver D, et al. MSTO1 is a cytoplasmic pro-mitochondrial fusion protein, whose mutation induces myopathy and ataxia in humans. EMBO Mol Med. 9(7): 967-984 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Baba T, Kashiwagi Y, Arimitsu N, et al. Phosphatidic Acid (PA)-preferring phospholipase a1 regulates mitochondrial dynamics. J Biol Chem. 289(16): 11497-11511 (2014).
[CrossRef] [Google Scholar] [PubMed]
- MacVicar T, Langer T. OPA1 processing in cell death and disease–the long and short of it. J Cell Sci. 129(12): 2297-2306 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Belenguer P, Pellegrini L. The dynamin GTPase OPA1: More than mitochondria? Biochim Biophys Acta. 1833(1): 176-183
[CrossRef] [Google Scholar] [PubMed]
- Zhang K, Li H, Song Z, et al. Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage. EMBO Rep. 15(5): 576-585 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Ban T, Ishihara T, Kohno H, et al. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat Cell Biol. 19(7): 856-863 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Gai X, Ghezzi D, Johnson MA, et al. Mutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathy. Am J Hum Genet. 93(3): 482-495 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Sabouny R, Wong R, Lee-Glover L, et al. Characterization of the C584R variant in the mtDNA depletion syndrome gene FBXL4, reveals a novel role for FBXL4 as a regulator of mitochondrial fusion. Biochim Biophys Acta Mol Basis Dis. 1865(11): 165536 (2019).
- Rosdah AA, Holien JK, Delbridge LM, et al. Mitochondrial fission—a drug target for cytoprotection or cytodestruction? Pharmacol Res Perspect. 4(3): e00235 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Schulz R, Agg B, Ferdinandy P. Survival pathways in cardiac conditioning: Individual data vs meta-analyses. What do we learn? Basic Res Cardiol. 113(1): 4 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Dorn GW. Mitochondrial fission/fusion and cardiomyopathy. Curr Opin Genet Dev. 38: 38-44 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Xu S, Wang P, Zhang H, et al. CaMKII induces permeability transition through Drp1 phosphorylation during chronic beta-AR stimulation. Nat Commun. 7: 13189 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Sharp WW, Fang YH, Han M, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia–reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. Faseb J. 28(1): 316-326 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Zaja I, Bai X, Liu Y, et al. Cdk1, PKCdelta and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 453(4): 710-721 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Zhou H, Hu S, Jin Q, et al. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. 6(3): e005328 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Chen Q, Moghaddas S, Hoppel CL, et al. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 294(2): C460-C466 (2008).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Yu P, Zhang J, Yu S, et al. Protective effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One. 10: e0134666 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Maneechote C, Palee S, Chattipakorn SC, et al. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med. 21(11): 2643-2653 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Hom J, Yu T, Yoon Y, et al. Regulation of mitochondrial fission by intracellular Ca2+ in rat ventricular myocytes. Biochim Biophys Acta. 1797(6-7): 913-921 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Pirzeh L, Babapour V, Badalzadeh R, et al. Pretreatment with vildagliptin boosts ischemic–postconditioning effects on cardioprotection and expression profile of genes regulating autophagy and mitochondrial fission/fusion in diabetic heart with reperfusion injury. Naunyn Schmiedebergs Arch Pharmacol. 392(11): 1371-1382 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Wang Q, Xu J, Li X, et al. Sirt3 modulate renal ischemia–reperfusion injury through enhancing mitochondrial fusion and activating the ERK–OPA1 signaling pathway. J Cell Physiol. 234(12): 23495-23506 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Chen H, Ren S, Clish C, et al. Titration of mitochondrial rescues Mff-deficient cardiomyopathy. J Cell Biol. 211: 795-805 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Leboucher GP, Y Tsai C, Yang M, et al. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 47: 547-557 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Nan J, Nan C, Ye J, et al. EGCG protects cardiomyocytes against hypoxia–reper injury through inhibition of OMA1 activation. J Cell Sci. 132(2): e220871 (2019).
- Nan J, Hu H, Sun Y, et al. TNFR2 stimulation promotes mitochondrial fusion via Stat3- and NF-κB-dependent activation of OPA1 expression. Circ Res. 121(4): 392-410 (2017).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Ong SB, Subrayan S, Lim SY, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 121(18): 2012-2022 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Ding M, Dong Q, Liu Z, et al. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovasc Diabetol. 16(1): 19 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Maneechote C, Palee S, Kerdphoo S, et al. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci (Lond). 132: 1669-1683 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Dong Y, Undyala VVR, Przyklenk K. Inhibition of mitochondrial fission as a molecular target for cardioprotection: Critical importance of the timing of treatment. Basic Res Cardiol. 111(5): 59 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Yu J, Wu J, Xie P, et al. Sevoflurane postconditioning attenuates cardiomyocyte hypoxia/reoxygenation injury via restoring mitochondrial morphology. PeerJ. 4: e2659 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Zhang Y, Wang Y, Xu J, et al. Melatonin attenuates myocardial ischemia–reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK–OPA1 signaling pathways. J Pineal Res. 66(2): e12542 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Martins IJ. Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epigenet. 3(3): 24 (2017).
- Martins IJ. Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research. 5: 9-26 (2016).
- Zhang J, He Z, Fedorova J, et al. Alterations in mitochondrial dynamics with age-related Sirtuin1/Sirtuin3 deficiency impair cardiomyocyte contractility. Aging Cell. 20(7): e13419 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Ciccone MM, Cortese F, Gesualdo M, et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 2013: 782137 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Shaish A, Harari A, Hananshvili L, et al. 9-cis β-carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis. 189(1): 215-221 (2006).
[CrossRef] [Google Scholar] [PubMed]