Review Article - Interventional Cardiology (2015) Volume 7, Issue 6
Bioengineering stents with proactive biocompatibility
- Corresponding Author:
- Martin K C Ng
Department of Cardiology, Royal Prince Alfred Hospital, Sydney, Australia
Tel: +612 9515 6111
Fax: +612 9550 6262
E-mail: mkcng@med.usyd.edu.au
Percutaneous coronary intervention has revolutionized the treatment of coronary artery disease. Successive improvements in implantation techniques, stent materials and design, combined with dual antiplatelet therapy have improved stent safety. However, optimal biocompatibility and long-term effectiveness in the absence of pharmaceutical intervention remains elusive. Drug-eluting stents, introduced to combat in-stent restenosis was found to impair endothelial regeneration, increasing thrombotic risk. Innovations in polymer technology and new stent designs have improved, but not solved, these issues. Despite the drawbacks of drug elution it remains a key component of stent platforms, leaving the need for a truly biocompatible platform with lasting clinical efficacy and safety unmet. This review will examine current stent designs and explore proactive approaches to enhance stent biocompatibility.
Submitted: 20 August 2015; Accepted: 1 October 2015; Published online: 12 November 2015
Abstract
Percutaneous coronary intervention has revolutionized the treatment of coronary artery disease. Successive improvements in implantation techniques, stent materials and design, combined with dual antiplatelet therapy have improved stent safety. However, optimal biocompatibility and long-term effectiveness in the absence of pharmaceutical intervention remains elusive. Drug-eluting stents, introduced to combat in-stent restenosis was found to impair endothelial regeneration, increasing thrombotic risk. Innovations in polymer technology and new stent designs have improved, but not solved, these issues. Despite the drawbacks of drug elution it remains a key component of stent platforms, leaving the need for a truly biocompatible platform with lasting clinical efficacy and safety unmet. This review will examine current stent designs and explore proactive approaches to enhance stent biocompatibility.
Keywords
biocompatibility, coronary stents, drug-eluting stents, endothelialization, thrombogenicity
Cardiovascular disease (CVD) has had a tremendous health impact across the globe. A rise in obesity levels, sedentary lifestyle and diabetes (in particular in developing countries) has led to CVD becoming a leading cause of mortality. Coronary artery disease (CAD) is by far the single largest contributor to the burden of CVD [1]. Percutaneous treatment of CAD has been one of the most significant advancements in clinical cardiovascular medicine, offering the advantage of a minimally invasive procedure with rapid recovery time and short hospital stay. While the prognostic benefit of percutaneous coronary intervention (PCI) has been questioned in patients with stable angina, robust evidence for PCI in acute coronary syndrome exists with reductions in mortality and myocardial reinfarction [2]. After nearly four decades of use, PCI has evolved from balloon angioplasty to contemporary drugeluting stents (DES). Driven by a desire to improve clinical efficacy and to reduce adverse outcomes related to PCI, refinements have been made in stent technology with promising novel strategies. This update will briefly review the biocompatibility shortcomings of bare metal stents (BMS) and DESs. We will explore promising new stent platforms and shine a light on nondrug-eluting strategies with proactive biocompatibility.
Concept of biocompatibility
Biocompatibility is a broad term used to describe the interaction of an implanted prosthesis with the human body. A key requirement of a biomaterial is to cause the least amount of harm to the host environment. The choice of biomaterial reflects this goal, 316L stainless steel, titanium and cobalt chromium alloys used extensively in clinical and vascular applications are highly corrosion resistant [3], thereby reducing tissue toxicity from in vivo deterioration following implantation.
Our understanding of biocompatibility has evolved with more in depth knowledge of tissue and cellular responses to biomaterials. A prosthesis is not simply a passive nonreactive entity but can be an active participant in the host response to its presence. Biocompatibility has been redefined to reflect this realization, incorporating three important tenets: a biomaterial has an active functional role in local tissue, should elicit an appropriate biological response and the response to the biomaterial needs to be appropriate and will depend on the intended role [4]. This represented a shift from the simple goal of ‘do no harm’ to one of active modulation of the biological response, specific to the clinical application and local tissue environment. In the context of coronary stents, biocompatibility encompasses hemocompatibility (freedom from thrombosis) and modulation of intimal hyperplasia that must be reconciled with the mechanical needs of scaffolding an artery to maintain vessel patency.
Clinically available stents
Bare metal stents
The predominant material used for construction of coronary stents are metallic alloys (316L stainless steel and more recently cobalt chromium and platinum chromium), borne out of the need for mechanical strength, deformability and radio-opacity [5]. Two early landmark trials, the Benestent and STRESS studies ushered in the stent era by demonstrating high rates of procedural success, improved immediate and long-term vessel luminal diameter over balloon angioplasty [6–8]. However, the hemocompatibility of metallic stents were brought into question. Inherent thrombogenicity of metallic alloys coupled with disruption of the endothelium following stent expansion led to a high incidence of subacute (1–30 days) stent thrombosis [9]. In contemporary practice, higher pressure stent deployment combined with dual antiplatelet therapy (DAPT) have reduced early thrombosis rates to <1% [10]. Nonetheless, the price of combination antiplatelet therapy is the increase in major and gastrointestinal bleeding [11].
Inability of metal only platforms to modulate the local host response led to additional biocompatibility issues. High incidences of in stent restenosis (ISR) presenting clinically as recurrent angina to acute coronary syndrome have been reported, necessitating repeat revascularization [12]. ISR is an inflammatory fibrocellular healing reaction to arterial injury and damage, an inevitable consequence of stent implantation [13]. Vascular smooth muscle cells (VSMCs) and extracellular matrix (ECM) rich in proteoglycans contributes to the neointima [14]. An increase in neointimal thickness and degree of restenosis is seen when stent struts penetrate the lipid plaque core causing fracture of the tunica media [15], indicating the healing response is directly proportional to the extent of vascular trauma.
Drug-eluting stents
The sirolimus (rapamycin) eluting Cypher stent and the paclitaxel eluting Taxus stent were shown in pivotal trials to be markedly superior to BMS at preventing ISR [16,17]. Both drugs act nonspecifically, inhibiting the proliferation of VSMCs and endothelial cells. Use of DAPT reduces early stent thrombosis rate to less than 1% for both BMS and DES [18]. Analysis of randomized DES trials revealed an increase in very late (>1 year) stent thrombosis in both paclitaxel- and sirolimus-eluting stents, a risk which appeared to persist at a rate of 0.35–0.6% annually [19–21]. DES thrombosis is associated with a mortality of 20–40% and myocardial infarction of 50–70% [22].
One of the main differences in the biological response to BMS and DES is the rate of stent strut endothelialization, occurring significantly faster in BMS [13]. In contrast high-resolution optical coherence tomography (OCT) showed incompletely endothelialized DES struts, 2 years after implantation [23,24]. Impairment of poststenting endothelial healing leads to continued exposure of metallic struts to blood, negatively impacting hemocompatibility.
Second-generation everolimus (EES) and zotarolimus (ZES) DES have largely replaced Cypher and Taxus stents. Using cobalt chromium (CoCr) for construction strut thickness was significantly lowered, reducing stent footprint, potentially limiting vessel injury, intimal hyperplasia [25] and thrombogenicity [26]. More recently platinum–chromium has been adopted in the Element platform (Boston Scientific) with excellent radial strength and corrosion resistant [27].
Preclinical assessment of second-generation DES showed improved biocompatibility with reduced inflammation and thrombogenicity, improved endothelial adhesion and more complete strut endothelialization [28]. In a 13-month OCT study of EES and ZES, 92.6–94.2% of struts were endothelialized [29]. In recent randomized trials, the XIENCE V stent (CoCr, fluropolymer, everolimus elution) exhibited a reduction in stent thrombosis and nonfatal myocardial infarction compared with first-generation paclitaxeleluting stents [30,31]. Despite the improvements in biocompatibility, continued requirement for prolonged DAPT highlights a need for further improvements in hemocompatibility without a decline in the modulation of intimal hyperplasia.
New stent platforms to improve biocompatibility
Drug eluting platforms
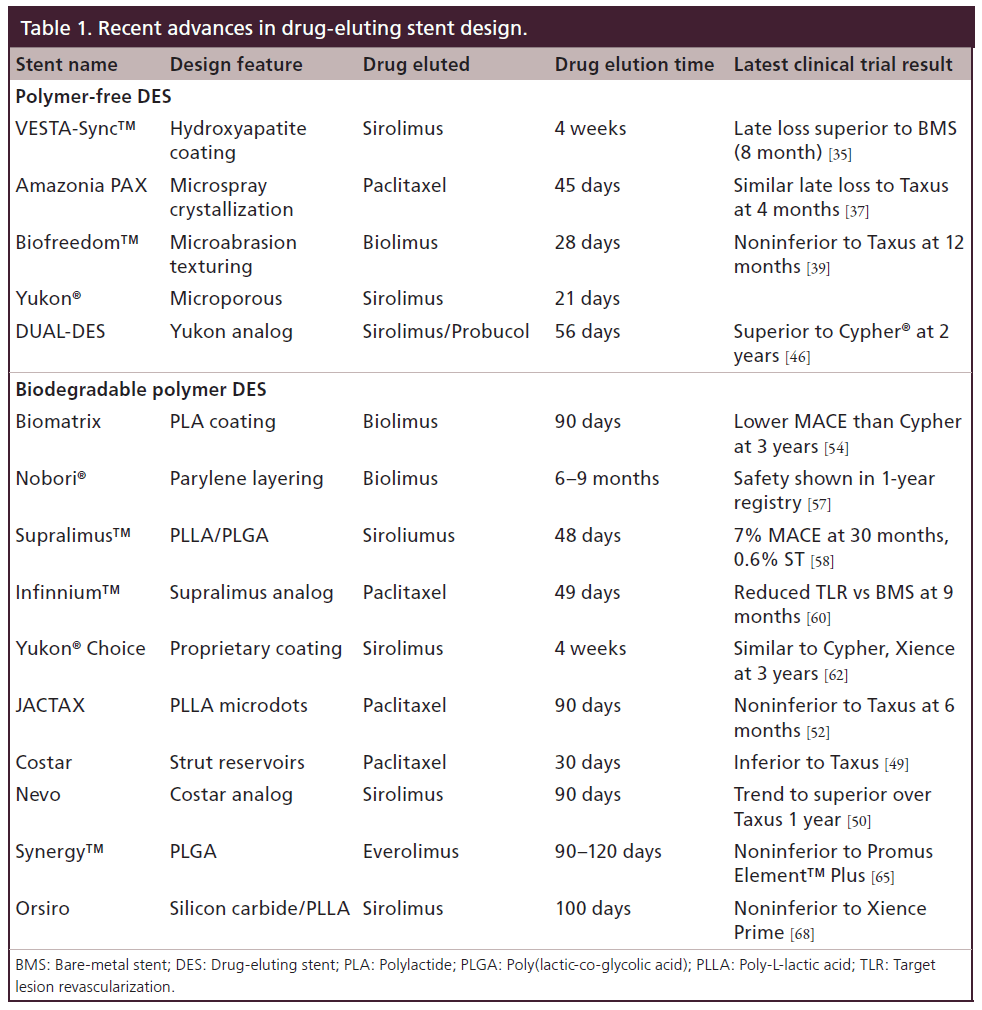
Polymer-free DES
Inflammation and hypersensitivity concerns over permanent polymer-coated DES have led to innovative stent designs aiming to develop alternate means of drug delivery. A stent only delivery system allows drug elution without concerns of polymer peeling and polymer-driven inflammatory reactions. Prominent examples of this approach are discussed below and summarized in Table 1.
The VESTA-sync™ stent is a stainless steel platform employing a microporous hydroxyapatite coating carrying oil-based sirolimus. Hydroxyapatite remains stable for 4 months with complete dissolution by 9–12 months, while elution of sirolimus is complete within 4 weeks [32]. The FIM trial, consisting of 15 patients with simple de novo coronary lesions, showed in-stent late loss of 0.30 mm and percent stent obstruction of 2.8% at 4 months and 0.36 mm and 4% at 9 months, respectively [32,33]. No MACE was reported at 2 years [34], one target lesion revascularization (TLR) was noted at 3 years [35]. Preliminary results from the Vestasync II trial comparing Vestasync to BMS revealed 8-month in-stent late loss of 0.39 mm for VESTA-sync stent compared with 0.74 mm with BMS [35].
The Amazonia PAX stent uses a microspray crystallization process to deposit paclitaxel onto the abluminal surface of a CoCr stent. A relatively rapid drug elution occurs within 45 days [36]. The PAX A trial randomizing 30 patients to the Amazonia stent or Taxus stent reported early 4-month results showing similar in-stent late loss between the two stent groups [37]. A larger 100 patient nonrandomized PAX B trial is ongoing. Similarly, the abluminal surface of the Biofreedom stent is treated with a microabrasion method to create a textured surface [36]. Biolimus A9, a highly lipophilic rapamycin analog is then coated onto the surface [38]. 12-month angiographic follow-up from the BIOFREEDOM FIM trial found noninferiority in in-stent late loss with the standard dose (15.6 μg/mm of stent) Biofreedom stent when compared with the Taxus stent [39]. Larger trials are needed to evaluate the Biofreedom stent to establish superiority and safety.
The Yukon® DES also has a microporous surface created by mechanical treatment of a stainless steel stent [40]. Sirolimus is sprayed on in the catheterization suite, using a proprietary coating methodology. The majority of the drug is eluted in the first 6 days followed by prolonged release over 21 days [41]. The Yukon stent was found in the ISAR-TEST trial to be noninferior in late loss and TLR when compared with the Taxus stent. However, results from the ISAR-TEST 3 comparing the Yukon and Cypher® stent found inferior late loss results [42]. The short drug elution time does not provide adequate control of intimal hyperplasia. Efforts to counter the suboptimal results from the rapamycin only elution gave rise to the Dual-DES, in which a combination of 1.0% rapamycin, 1.0% probucol and 0.4% shellac resin was coated onto the stent [43]. Probucol, an antihyperlipdemic agent has potent antioxidant effects and can inhibit VSMC proliferation [44]. In the ISAR TEST-2 trial, the DUAL-DES was found to be comparable in binary restenosis and TLR to Cypher and significantly better than the Endeavor stent [45]. Results were maintained out to 2 years, importantly the ‘late catch up’ phenomenon noted in the Cypher stent group was absent in the DUAL DES [46]. The recently released large ISAR-TEST 5 trial demonstrated very promising results with the DUAL-DES stent shown to be noninferior to the next-generation Resolute stent in the composite primary endpoint of cardiac death, target vesselrelated myocardial infarction or TLR [47].
Biodegradable polymer DES
An alternative approach to avoiding adverse polymer reactions is to use a biodegradable polymer based drug carrier which erodes over a defined time period. This potentially delivers the benefit of superior drug release kinetics but leaves no permanent source of inflammation. The compositions of these polymers are predominantly based on lactic acid/lactide analogs. Variations between the stents include the polymer coating, the drugs eluted, kinetics of elution and the degradation time for the polymers.
The Costar stent consists of struts with reservoirs filled with a biodegradable polymer polylactide-co-glycolide impregnated with paclitaxel [48]. Results from the Costar II trial [49] comparing against the Taxus stent were disappointing due to reduced efficacy from the lower dose of paclitaxel used and the short release kinetics. The Nevo stent is based on the same reservoir system but uses sirolimus with more prolonged elution. Despite promising results from the NEVO RES-ELUTION I study [50] problems identified in the balloon delivery system resulted in suspension of the reservoir platform.
The JACTAX stent utilizes a biodegradable polylactide (PLA) polymer placed in a focal microdot fashion onto the abluminal surface of the stent. Noninferiority in MACE, TLR compared with historical data from the Taxus Atlas trial was reported in the FIM JACTAX HD trial (HD = higher dose, 9.2 μg each of drug and polymer per 16 mm stent) [51]. The OCTDESI OCT based trial examined the degree of stent strut coverage at 6 months, no benefit in endothelialization was reported for the JACTAX stent over Taxus [52].
The Biomatrix Stent consists of a stainless steel platform coated with a polylactic acid and Biolimus coating. The coating is directed toward the abluminal surface [53], full degradation of the polymer occurs over 9 months. The LEADERS trial randomized patients to Biomatrix stent or Cypher stent. At 12-month followup the composite primary endpoint (cardiac death, myocardial infarction and target vessel revascularization) was noninferior in the Biomatrix group versus the cypher group [54]. Clinical follow-up now to 3 years show a trend toward a lower MACE rate in Biomatrix stent group (Biomatrix 15.7 vs 19% Cypher; P for superiority = 0.09) with cumulative very late ST rates remaining low at 0.2% [54].
The Nobori stent employs the same stent design and biodegradable polymer as Biomatrix, but with the addition of an ultrathin nondegradable parylene coating between the stent and the polymer to improve polymer attachment. Trials with the Nobori® stent have further reinforced the positive results of this approach. The Nobori I trial confirmed noninferiority at 9 months in in-stent late loss against the Taxus stent [55], with recently reported clinical follow-up out to 5 years showing no ST [56]. 1-year clinical data from the 3068 patient all comers registry (Nobori 2 study) reported excellent efficacy and safety result with target lesion failure of 3.6% and MACE of 4.8% [57]. The Nobori stent will be further evaluated in the SORTOUT V trial (vs Cypher), COMPARE II (vs Xience), SECURITY (vs Resolute with 6-month DAPT arm and 2-month DAPT arm) and BASKET PROVE 2 (vs ProKinectic and Xience).
The Supralimus™ sirolimus-eluting stent utilizes two biodegradable coatings on a stainless steel stent platform. The undercoat consists of a matrix of poly L-Lactic acid, poly DL-lactide-co-glycolide and polyvinyl pyrrolidone intermixed with 1.4 mcg/mm2 of sirolimus. A top coat of polyvinyl pyrrolidone prevents early release of the drug. The dissolution of the top coat within 2 h after implantation leads to a rapid burst release over 7 days of half of the drug. At 48 days the drug is completely eluted. Complete polymer degradation occurs over 7 months. The FIM Series I study (nonrandomized 100 patients) showed at 6 months a low in-stent late loss of 0.09 mm and binary restenosis of 0%. At 30 months MACE was 7%. 2-year data from the acute coronary syndrome all comers E-Series registry had reassuringly low ST rates of 0.6% [58]. The Series III trial currently in progress will evaluate the Supralimus against the Xience stent. The Supralimus and Supralimus Core using a thinner CoCr platform have both recently received European CE Mark. The Infinnium stent utilizes the same biodegradable polymer components and has the same release kinetics as the Supralimus. The polymers are mixed with paclitaxel in various ratios to form three layers with distinct fast, medium and slow release kinetics. A good safety and efficacy profile was shown in the nonrandomized SIMPLE II registry [59]. The PAINT study randomized patients to the Infinnium stent, Supralimus stent or a BMS. In brief, both the Infinnium and Supralimus stents showed significant reductions in angiographic in-sent late loss at 9 months, with reduced TLR at 12 months compared with the BMS. The Supralimus stent had superior angiographic but equivalent clinical results to the Infinnium stent [60].
The Yukon Choice PC stent platform is based on the microporous Yukon stent. A proprietary biodegradable matrix (degraded in 6–9 weeks) is mixed with sirolimus and a natural resin and then applied in the catheterization laboratory. In the ISAR-3 trial the efficacy of the Yukon Choice stent was noninferior to the Cypher stent [42]. ISAR-4 was a clinically driven large randomized study evaluating the Yukon Choice against Cyper and Xience stents. MACE at 1 year was comparable between the three stents. Cardiac death, myocardial infarction, TLR and ST were not significantly different [61]. Efficacy and safety remained similar between the stents in a recent report of 3-year results [62].
The Synergy™ platform elutes everolimus from a poly-lactide-co-glycolide polymer (degraded over 4 months) coated on to the abluminal surface of a platinum chromium constructed stent (strut thickness 74 μm). Evaluated in porcine models the Synergy stent was found to have equivalent vascular compatibility to Promus element and bare metal platinum chromium Element stent [63]. Moving forward, the randomized first in man Evolve trial assessed the safety and efficacy of low and standard dose everolimus eluting Synergy stent against the Promus element stent. At 6 month, both formulations of the Synergy stent were found to be noninferior in the primary angiographic endpoint of in-sent late loss (0.15 ± 0.34 mm PROMUS Element, 0.10 ± 0.25 mm Synergy and 0.13 ± 0.26 mm Synergy half-dose). Encouragingly no stent thrombosis occurred in any group at 6 months [64]. The Evolve 2 USA pivotal trial randomized over 1600 patients with stable coronary or non-ST-segment-elevation acute coronary syndrome to Synergy or Promus element plus stent. Noninferiority of the 12-month primary end point of target lesion failure was demonstrated (6.7% Synergy and 6.5% Promus Element plus, p = 0.0003 for noninferiority). Definite/probable stent thrombosis was noted in 0.4 versus 0.6% (p = 0.50), for Synergy and Promus Element plus, respectively [65]. Moving toward US FDA approval, the already CE marked Synergy stent may become the first biodegradable polymer stent permitted in USA.
Similarly, the Orsiro stent is undergoing evaluation in the currently enrolling Bioflow-V study with the goal of obtaining FDA approval. Fashioned from CoCr, the 60 μm thick stent is coated with a sealant layer of silicon carbide preventing release of ions from the metal alloy. A biodegradable poly-L-lactic acid (PLLA) polymer is used as carrier for sirolimus with complete drug elution in 100 days. Encouraging instent late lumen loss of 0.12 ± 0.19 mm and 0.05 ± 0.22 mm at 4 and 9 months, respectively and a low MACE rate of 10% was reported in the BIOFLOW-I first in man study [66]. BIOFLOW-II, a noninferiority study against Xience Prime stent, demonstrated comparable levels of in-stent late lumen loss at 9 months 0.1 ± 0.32 mm and 0.11 ± 0.29 mm, and target lesion failure of 6.5 and 8.0% for Orsiro and Xience Prime, respectively. No stent thrombosis was reported in both groups [67]. Building on these results the BIOSCIENCE study compared the Orsiro stent against the Xience Prime stent in a larger all comers cohort powered for clinical endpoints. Target lesion failure (cardiac death, target vessel myocardial infarction and target lesion revascularization) at 12 months was noninferior to Xience Prime [68].
Conclusive long-term safety data for biodegradable polymer stents remain incomplete. Studies have been relatively small, often based on surrogate angiographic endpoints and powered to establish noninferiority to contemporary stents. While the ISAR-TEST 3 and ISAR-TEST 4 demonstrated similar efficacy and safety to the leading first- and second-generation DES, superiority in safety have yet to be demonstrated to validate the theoretical benefit of biodegradable polymers. In this regard a recent meta-analysis by Bryne et al. [69] of pooled 3-year data from the LEADERS, ISAR-TEST 3 and ISAR-TEST 4 trials encompassing 4000 patients found a significant reduction in ST in the biodegradable polymer group at 1.2% than the nonerodible polymer group at 2.1% (p = 0.013). A reduction in the composite clinical end points (cardiac death, MI, TLR) was also noted in the biodegradable polymer group (18.2 vs 20.1%, p = 0.04). This was largely due to a reduction in TLR.
Biodegradable stents
Biodegradable polymeric and metallic stent structures have been developed as an alternative to persistent metallic substrates. The aim is to deliver a temporary scaffold, which can mechanically support the vessel against recoil while negating the issue of unfavorable host response to a permanent prosthesis. Late stent thrombosis although rare can occur with BMS particularly with discontinuation of aspirin [70] highlighting the ongoing thrombotic risk of metallic alloys.
A biodegradable stent (BDS) has a number of theoretical benefits, reducing the dependence on prolonged antiplatelet therapy, removing the chronic negative effect of a rigid metallic stent on vasomotor tone and positive vessel remodeling, providing greater options for future coronary intervention, bypass surgery and safety for MRI imaging [71]. However, ongoing challenges include preventing acute vessel recoil soon after deployment and maintaining stable medium-term support as the stent starts to degrade [72], developing an appropriate time frame of degradation, ensuring adequate radio-opacity and preventing vessel inflammation to the polymer.
PLA the main polymer used in BDS undergoes hydrolysis, breaking down to carbon dioxide, lactic acid and water. [71] Animal models demonstrated robust acute radial strength was maintained out to 1 month. Degradation occurred over a 9-month period with good vascular biocompatibility and a low toxicity profile [73]. The Igaki-Tamai PLA stent evaluated in 15 patients with 6-month follow-up validated the mechanical integrity of the stent in the prevention of acute recoil, comparable restenosis rates to BMS and an adequate safety profile with no MACE or stent thrombosis [74]. A larger 50 patient trial demonstrated complete resorption of the stents, a low subacute and late stent thrombosis rate and TLR of 12.7% at 1 year. The main impediment to the wide spread adoption of the Igaki-Tamai stent has been over its method of deployment. The stent requires heating (to between 65 and 75°C) prior to implantation for expansion, leading to concerns of arterial wall necrosis and accentuating intimal hyperplasia [75].
Biodegradability may enhance hemocompatibility it does not however address the issue of restenosis caused by vessel injury. Full absorption of BDS takes more than 6 months [71] and both vessel recoil and negative remodeling occur within this time. [76] Persistence of struts beyond this time may lead to greater intimal hyperplasia without providing any additional support benefit [77]. The Abbot Vascular BDS (BVS) seeks to address the shortcomings of BDS by combining biodegradability with an antirestenotic strategy. Based on a PLLA backbone, the BVS additionally releases the antiproliferative drug everolimus. Thicker 150 μm stent struts than is commonly used for metal alloy platforms were used to maintain radial strength. At 1 year, good clinical safety and efficacy with no TLR and MACE of 3.3% (1 non-Q wave MI) was noted [78]. Safety was maintained with a steady MACE rate (3.4%) reported at 3 years [79]. The 6-month late loss (0.44 mm) [78] was higher than the metallic equivalent (Xience stent) (0.11 mm) [80] due to a reduction in the stent area from both acute recoil [81] and progressive loss of radial strength with absorption of PLLA. [78] Encouragingly at 2 years there was no further late catch up in late loss and stent strut resorption was noted [82].
A redesigned stent geometry (in-phase zig zag hoops linked by bridges) was used to provide more uniform and improved radial support. Angiographic followup at 6 months of 45 patients were more encouraging demonstrating a lower late loss of 0.19 mm than revision 1.0, suggesting improved radial strength. A larger 1000 patient Absorb Extended trial is currently in progress to further evaluate the BVS in patients with more complex coronary lesions.
Alternatives to drug eluting platforms
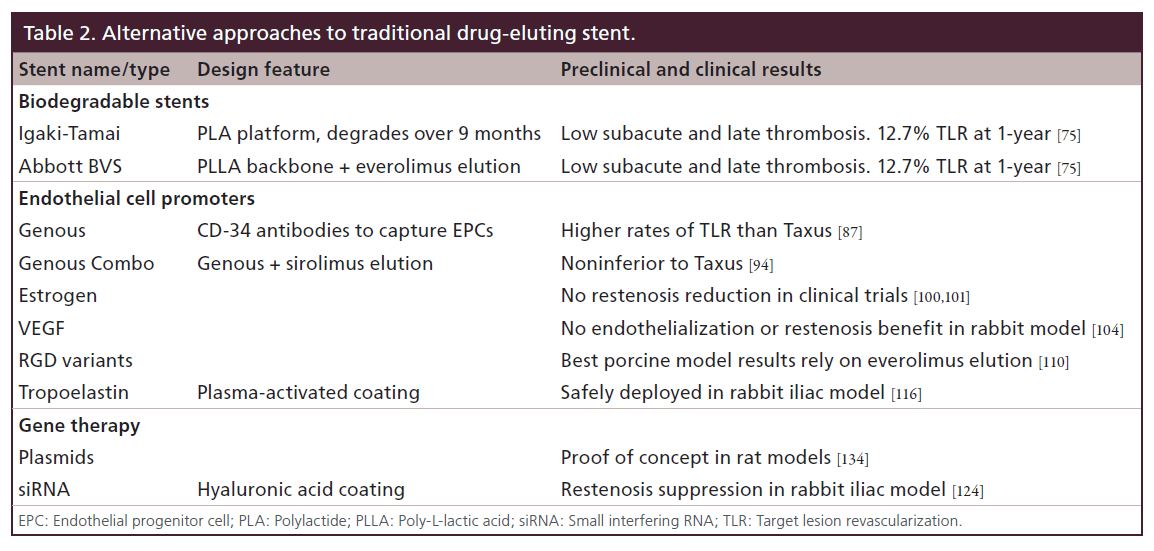
The majority of new stent platforms under clinical investigation are dependent on drug elution to control intimal hyperplasia. Eluted cytotoxic agents are indiscriminate in the inhibition of cell activity. The impact on endothelial cells can delay endothelialization of the stent struts. Below we highlight some alternative strategies either with clinical data or in preclinical stages designed to modulate smooth muscle cell activity. Ranging from coatings which accelerate endothelialization to functionalizing stent surfaces with smooth muscle cell-regulating biomolecules (Table 2).
Proendothelial surface coatings
EPC capture
Coatings designed to enhance healing have attempted to leverage the antithrombotic and antirestenosis functions of the endothelium for improved stent biocompatibility. A functional and intact endothelium has important ramifications in restenosis and neoatherosclerosis. The degree of intima formation correlates with the area of endothelial denudation, with increasing thickness with more endothelium injured [83]. Moreover, endothelial regeneration over the injured vasculature appear to retard smooth muscle growth and extracellular deposition [84]. The endothelial secretion of NO maintains VSMC in a quiescent phenotype [85]. Poorly regenerated and dys-functional endothelium has reduced NO secretion.
The Genous endothelial progenitor cell (EPC) capture stent (GS) (OrbusNeich Medical) employs murine monoclonal antihuman CD-34 antibodies coupled to a polysaccharide coating to capture EPCs. Preclinical studies showed promising results with reduced thrombogenicity, more EPC coverage and increased endothelialization on the GS when compared with BMS [86]. The HEALING-FIM trial examined this stent in 16 patients, reported no ST despite only 1 month of DAPT and a MACE rate of 6.3% with one TLR at 9 months [89]. The HEALING-II registry, with 63 patients again showed no ST with 1 month of DAPT. At 6 months in-stent late loss was 0.78 mm, however at 18 months regression and remodeling was seen with reduction of 16.9% in late loss [90]. The much larger e-HEALING registry with 4939 patients, reported at 1 year an overall and late ST rate of 1.1 and 0.2% respectively, with only 34% of patients remaining on DAPT and a low rate of TLR of 5.7%. [91]
Randomized data have raised concerns over the efficacy of this platform. Beijk et al. randomized 193 patients with high risk for restenosis to either the Genous stent or Taxus Liberte stent. 1-year target vessel failure driven largely by increase TLR was significantly higher at 17.3% in the Genous group versus 10.5% in the Taxus group [87]. The TRIAS-HR trial was stopped early after disappointing results from 622 randomized patients. Patients randomized to the Genous stents had at 1 year significantly higher TLR 17.4 versus 7% in DES groups [88]. In order to counter these results the prohealing qualities of the Genous R stent has been combined with sirolimus drug elution. A permanent polymer [92] and a bioabsorbable polymer variant (Combo stent) are being investigated at present [93]. Recent results from a randomized trial against the Taxus stent demonstrated noninferior angiographic restenosis, low rates of complications and no ST [94]. These findings will need to be validated in larger randomized trials.
The inability of the Genous stent to make a meaningful contribution to restenosis does not necessarily invalidate the prohealing strategy. Coated antibody to CD34+ is used to capture EPCs. However, EPCs are a heterogeneous group of cells with overlapping surface markers. To date, no unique set of surface markers can definitive identify an EPC [95]. The CD34+ marker is nonspecific and can be found on a variety of cells including primitive hematopoietic cells [96] and mature endothelial cells [97]. Furthermore, EPCs only represent a small fraction of the cells bearing CD34+ surface markers. Taken together, the effectiveness and specificity of CD34+ based EPC capture is uncertain. It is likely a rather heterogonous group of cells is captured on to the stent surface, with varying contributions to endothelialization and modulation of vascular smooth muscle cell activity.
Endothelial cell stimulation & promotion
Estrogen enhances the gene expression of endothelial growth factor and increases NO production, with estrogen releasing stents showing promise in animal studies with improved endothelialization [98,99]. Disappointingly, clinical studies failed to show any advantage in restenosis reduction [100,101]. In a similar attempt to accelerate endothelialization VEGF releasing stents were conceived. VEGF is a specific endogenous signaling protein that simulates angiogenesis [102]. It has a broad range of effects on endothelial cells including proliferation, migration and actin reorganization. Small animal models demonstrated locally delivered VEGF-enhanced endothelial recovery and control of intimal hyperplasia after vessel injury [103]. On the back of these promising results, VEGF-eluting stents were designed to prolong the exposure to local tissue. However in stented rabbit iliac arteries no benefit on endothelialization or restenosis was evident [104].
Cells interact with ECM proteins via transmembrane surface integrin. These interactions give vital signaling cues to direct cellular activity including migration, differentiation, adhesion and proliferation [105]. Ligands for integrin reside within short amino acid sequences (motifs) on the extracellular protein. The RGD (Arg– Glu–Asp) tripeptide is one of the most prevalent integrin binding motifs [106] and supports adhesion to extracellular proteins for a wide range of cells [107]. Structurally altered cyclic RGD (cRGD) can preferentially promote cell adhesion of one cell type over another. cRGD functionalized stents were found in preclinical models to substantially reduce neointimal area by accelerating endothelial coverage presumably through augmenting EPC activity without stimulating VSMCs [108,109]. Dual cRGD and everolimus-coated stents have recently been investigated in porcine models finding a good balance between retardation of hyperplasia while supporting endothelialization [110]. Certainly promising, in man studies are needed to see if preclinical findings are maintained. Potentially a more selective novel biofunctional peptide, RRETAWA has been designed with in vitro data pointing to a more endothelial specific interaction without platelet stimulation [111].
Extending further the benefit of ECM-based surface functionalization, our laboratory has developed a proprietary coating technology that facilitates the covalent attachment of biomolecules to almost any substrate, while retaining bioactivity [112]. The plasma-activated coating has been used successfully to functionalize stainless steel surfaces with recombinant human tropoelastin, the soluble precursor of elastin [113]. In in vitro assays tropoelastin modified stainless steel surfaces showed enhanced endothelial cell attachment and proliferation in combination with very low thrombogenicity [114,115]. In vivo implantation in a rabbit iliac stenting model demonstrated stability of the plasma polymer coating and lack of immunogenic response to the tropoelastin functionalized coronary stents [116]. Efficacy in the control of intimal hyperplasia is undergoing further preclinical testing.
Gene therapy has also been investigated as a strategy to specifically modulate cellular protein production for biointegration of coronary stents. Plasmids, double-stranded DNA found in bacteria, have been exploited as a vehicle for intranuclear delivery of DNA. Relative ease of construction, along with lack of pro-oncogenic potential are the main advantages of plasmids [117]. Proof-of-concept animal studies have shown successful transfection of rat aortic smooth muscle cells using green fluorescent protein plasmid DNA eluted from a polymer-coated stent [118]. Phosphorylcholine polymer covered VEGF plasmidinfused stents implanted in rabbit iliac arteries augmented endothelialization, reduced restenosis and enhanced endothelial function by increasing NO production [119]. Endothelial NO synthase DNA plasmid can successfully transfect rabbit endothelial cells raising NO production and suppressing smooth muscle cell proliferation [120]. Inhibitory proteins can also be induced with plasmids. Anti-MCP-1 gene, which inhibits mononuclear and VSMCs can be eluted from a stent. A reported reduction in neointimal formation after 6 months was found in monkeys [121]. Alternative delivery methods include viral vectors such as retrovirus and adenovirus. Highly efficient as a gene delivery vehicle [122] concerns over mutagenesis from tumor suppressor gene disruption or oncogene activation has limited its broad investigation for stent biocompatibility. Animal models show that an adenovirus- coated stents can successfully transduce TIMP-3 into smooth muscle cells resulting in decreased neointimal formation [123].
Direct elution of small interfering RNA (siRNA) has also been used. Hyaluronic-coated stent surfaces can deliver siRNA nanoparticles against the Akt1 gene/protein responsible for stimulating cell growth. Following balloon injury, siRNA-eluting stents deployed in the rabbit iliac segments suppressed smooth muscle growth and restenosis [124]. To date, human studies have yet to be conducted with gene therapy stents. This is an exciting area of development still some time away from actual clinical utilization.
Conclusion
The issue of stent biocompatibility remains unsolved. At present, there is no stent that can successfully bio-integrate into the vasculature with low enough throm-bogenicity as to not require prolonged DAPT. There is also no platform that can simultaneously promote a rapid reinstitution of a functional vascular endothelium while controlling the reactive smooth muscle response. The constant juggling act between the three cornerstones of stent biocompatibility healing, restenosis and thrombogenicity is complex and inter connected.
In the past two decades the field of interventional cardiology shifted from the mechanical support of vessel with the introduction of the bare metal stent to the goals of controlling of intimal hyperplasia and improving thrombogenicity. While a host of different passive and bioactive coatings improved thrombogenicity they alone failed to control restenosis. DES quite successfully emerged as the dominant strategy to address intimal hyperplasia.
The cytoinhibitory approach to restenosis comes at the cost of endothelial recovery and along with permanent polymers led to issues of late stent thrombosis and a necessity for prolonged DAPT. Despite the introduction of next generation DES and new stents under investigation, a definite and significant advancement in clinical safety and efficacy over previous platforms remains to be seen. In the short-term drug elution strategies remain the mainstay of clinical treatment but developments in alternative strategies provide hope for a more comprehensive solution in the future.
Future perspective
Improving the biocompatibility of coronary stents needs a multifaceted approach addressing three key aspects: thrombogenicity, intimal hyperplasia and endothelialization. Currently, there are promising evolutions in stent technology to improve biocompatibility but no solution encompasses all three components. A dominant platform has yet to emerge to truly replace DES in the coming few years. BDS are certainly a promising next step however lingering concerns and the need for more real world clinical data means we are still some time away from embracing this technology. Further into the future, over the next decade we predict a shift away from antiproliferative agents as focus shifts to more holistic, proactive biocompatibility. The application of biomolecules with differential and selective biological activities to stent surfaces may accelerate endothelial healing, attenuate intimal hyperplasia and cloak against clotting elements.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• Coronary artery stenting has become the main procedural modality for treatment of coronary artery disease.
Clinically available stents
• Today a truly histocompatible coronary artery remains elusive.
• Stent thrombosis and restenosis while improved with contemporary drug-eluting stents remain as clinical manifestations of suboptimal biocompatibility.
New drug-eluting stent platforms
• Polymer free stents, degradable polymer stents and completely biodegradable stents are been investigated as potential replacements for current generation stents. Robust long term clinical data are lacking and as yet these new platforms have not replaced traditional drug-eluting stents in clinical practice.
Alternative strategies to drug elution
• Proactive stents functionalized with bioactive molecules offer an attractive strategy with the potential to be prohealing and/or control vascular smooth muscle cell activity.
References
- Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. World Health Organization, (2011).
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J. Am. Coll. Cardiol. 48(7), 1319–1325 (2006).
- Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials 28(9), 1689– 1710 (2007).
- Williams DF. On the mechanisms of biocompatibility. Biomaterials 29(20), 2941–2953 (2008).
- Schatz RA. A view of vascular stents. Circulation 79(2), 445–457 (1989).
- Serruys PW, De Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331(8), 489–495 (1994).
- Kiemeneij F, Serruys PW, Macaya C et al. Continued benefit of coronary stenting versus balloon angioplasty: five-year clinical follow-up of Benestent-I trial. J. Am. Coll. Cardiol. 37(6), 1598–1603 (2001).
- Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 331(8), 496–501 (1994).
- Baim DS, Carrozza JP Jr. Stent thrombosis. Closing in on the best preventive treatment. Circulation 95(5), 1098–1100 (1997).
- Schomig A, Neumann FJ, Kastrati A et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N. Engl. J. Med. 334(17), 1084–1089 (1996).
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345(7), 494–502 (2001).
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 151(6), 1260–1264 (2006).
- Farb A, Sangiorgi G, Carter AJ et al. Pathology of acute and chronic coronary stenting in humans. Circulation 99(1), 44–52 (1999).
- Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Del. Rev. 58(3), 358–376 (2006).
- Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation 105(25), 2974–2980 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Ong ATL, Mcfadden EP, Regar E, De Jaegere PPT, Van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J. Am. Coll. Cardiol. 45(12), 2088–2092 (2005).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J. Am. Coll. Cardiol. 48(12), 2584–2591 (2006).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4 year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Holmes DR Jr, Kereiakes DJ, Garg S et al. Stent thrombosis. J. Am. Coll. Cardiol. 56(17), 1357–1365 (2010).
- Jimenez-Valero S, Moreno R, Sanchez-Recalde A. Very late drug-eluting stent thrombosis related to incomplete stent endothelialization: in-vivo demonstration by optical coherence tomography. J. Invasive Cardiol. 21(9), 488–490 (2009).
- Chen BX, Ma FY, Luo W et al. Neointimal coverage of bare-metal and sirolimus-eluting stents evaluated with optical coherence tomography. Heart 94(5), 566–570 (2008).
- Zahedmanesh H, Lally C. Determination of the influence of stent strut thickness using the finite element method: implications for vascular injury and in-stent restenosis. Med. Biol. Eng. Comput. 47(4), 385–393 (2009).
- Kolandaivelu K, Swaminathan R, Gibson WJ et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 123(13), 1400–1409 (2011).
- O’brien BJ, Stinson JS, Larsen SR, Eppihimer MJ, Carroll WM. A platinum–chromium steel for cardiovascular stents. Biomaterials 31(14), 3755–3761 (2010).
- Chin-Quee SL, Hsu SH, Nguyen-Ehrenreich KL et al. Endothelial cell recovery, acute thrombogenicity, and monocyte adhesion and activation on fluorinated copolymer and phosphorylcholine polymer stent coatings. Biomaterials 31(4), 648–657 (2010).
- Gutierrez-Chico JL, Van Geuns RJ, Regar E et al. Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13 month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur. Heart J. 32(19), 2454–2463 (2011).
- Kedhi E, Joesoef KS, Mcfadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Stone GW, Midei M, Newman W et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents. Circulation 119(5), 680–686 (2009).
- Costa JR Jr, Abizaid A, Costa R et al. Preliminary results of the hydroxyapatite nonpolymer-based sirolimus-eluting stent for the treatment of single De novo Coronary lesions: a first-in-human analysis of a third-generation drug-eluting stent system. J. Am. Coll. Cardiol. Interv. 1(5), 545–551 (2008).
- Costa JR Jr., Abizaid A, Costa R et al. 1 year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc. Interv. 2(5), 422–427 (2009).
- Costa JR Jr. Very long term results (>2 years) of the VestaSync I trial with a novel, third-generation, hydroxyapatite polymer-free sirolimus eluting stent. J. Am. Coll. Cardiol. 55(10), S0735-1097, 61680-4 (2010).
- Abizaid A. The MIV Vestasync polymer-free sirolimus-eluting stent program (microporous hydroxyapatite surface). Presented at: Transcatheter Cardiovascular Therapeutics 21st Annual Scientific Symposium. San Francisco, CA, USA, 21–26September 2009.
- Abizaid A, Costa JR. New drug-eluting stents. Circ. Cardiovasc. Interv. 3(4), 384–393 (2010).
- Abizaid A. PAX A trial (Amazonia Pax versus Taxus Liberté): 4 month follow up: IVUS and optical coherence tomography evaluation. Presented at: EuroPCR meeting. Paris, France, 25–28 May 2010.
- Tada N, Virmani R, Grant G et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model/clinical perspective. Circ. Cardiovasc. Interv. 3(2), 174–183 (2010).
- Grube E. BioFreedom: a prospective randomized trial of polymer-free Biolimus A9-eluting stents and paclitaxel-eluting stents in patients with coronary artery disease. Presented at: TCT 2010: Transcatheter Cardiovascular Therapeutics 22nd Annual Scientific Symposium. Washington,DC, USA, 21–25 September 2010.
- Wessely R, Hausleiter J, Michaelis C et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coating. Arterioscler. Thromb. Vasc. Biol. 25(4), 748–753 (2005).
- Tsujino I, Ako J, Honda Y, Fitzgerald PJ. Drug delivery via nano-, micro and macroporous coronary stent surfaces. Expert Opin. Drug Deliv. 4(3), 287–295 (2007).
- Mehilli J, Byrne RA, Wieczorek A et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur. Heart J. 29(16), 1975–1982 (2008).
- Steigerwald K, Merl S, Kastrati A et al. The pre-clinical assessment of rapamycin-eluting, durable polymer-free stent coating concepts. Biomaterials 30(4), 632–637 (2009).
- Deng YM, Wu BJ, Witting PK, Stocker R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation 110(13), 1855–1860 (2004).
- Byrne RA, Mehilli J, Iijima R et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur. Heart J. 30(8), 923–931 (2009).
- Byrne RA, Kastrati A, Tiroch K et al. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based cypher and endeavor, drug-eluting stents. J. Am. Coll. Cardiol. 55(23), 2536–2543 (2010).
- Massberg SMD, Byrne RaMBB, Kastrati AMD et al. Polymer-free sirolimus- and probucol-eluting versus new generation Zotarolimus-Eluting stents in coronary artery disease: the intracoronary stenting and angiographic results: test efficacy of tirolimus- and probucol-eluting versus zotarolimus-eluting stents (ISAR-TEST 5) trial. Circulation 124(5), 624–632 (2011).
- Finkelstein A, Mcclean D, Kar S et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation 107(5), 777–784 (2003).
- Wang TY, Hasselblad V, Peterson JL et al. The Cobalt chromium STent with Antiproliferative for Restenosis II (COSTAR II) trial study design: advancing the active-control evaluation of second-generation drug-eluting stents. Am. Heart J. 153(5), 743–748 (2007).
- Mauri L. Twelve-month follow-up of NEVO RES-Elution I: a randomized multi-center comparison of the NEVO TM sirolimus-eluting coronary stent system using RES technology with the TAXUS liberté paclitaxel-eluting stent. Presented at: TCT 2010: Transcatheter Cardiovascular Therapeutics 22nd Annual Scientific Symposium. Washington,DC, USA, 21–25 September 2010.
- Grube E, Schofer J, Hauptmann KE et al. A novel paclitaxel-eluting stent with an ultrathin abluminal biodegradable polymer 9 month outcomes with the JACTAX HD stent. JACC Cardiovasc. Interv. 3(4), 431–438 (2010).
- Guagliumi G, Sirbu V, Musumeci G et al. Strut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: Optical Coherence Tomography Drug-Eluting Stent Investigation (OCTDESI). Circ. Cardiovasc. Interv. 3(4), 367–375 (2010).
- Grube E, Hauptmann KE, Buellesfeld L, Lim V, Abizaid A. Six-month results of a randomized study to evaluate safety and efficacy of a Biolimus A9 eluting stent with a biodegradable polymer coating. EuroIntervention 1(1), 53–57 (2005).
- Garg S, Sarno G, Serruys PW et al. The twelve-month outcomes of a biolimus eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymer. EuroIntervention 6(2), 233–239 (2010).
- Chevalier B, Serruys PW, Silber S et al. Randomised comparison of Nobori, biolimus A9-eluting coronary stent with a Taxus(R), paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the Nobori 1 trial. EuroIntervention 2(4), 426–434 (2007).
- Chevalier B. NOBORI 1 pivotal trial clinical outcomes at 5 years. Presented at: EuroPCR 2011: Congress of the European Association of Percutaneous Cardiovascular Interventions. Paris,France, 17–20 May 2011.
- Gian D. Safety and efficacy of Biolimus A9 eluting stent with biodegradable polymer in real life Norbori 2 trial. Presented at: EuroPCR meeting. Paris, France, 25–28 May 2010.
- Costa JR Jr. Supralimus bioabsorbable polymer sirolimus eluting stent in patients with acute coronary syndrome: two year results of the e-series registry. Presented at: The American College of Cardiology 59th Annual Scientific Session/ i2 Summit. Atlanta, GA, USA, 14–16, March 2010.
- Vranckx P, Serruys PW, Gambhir S et al. Biodegradable-polymer-based, paclitaxel-eluting Infinnium stent: 9-Month clinical and angiographic follow-up results from the SIMPLE II prospective multi-centre registry study. EuroIntervention 2(3), 310–317 (2006).
- Lemos PA, Moulin B, Perin MA et al. Randomized evaluation of two drug-eluting stents with identical metallic platform and biodegradable polymer but different agents (paclitaxel or sirolimus) compared against bare stents: 1-Year results of the PAINT trial. Catheter. Cardiovasc. Interv. 74(5), 665–673 (2009).
- Byrne RA, Kastrati A, Kufner S et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) trial. Eur. Heart J. 30(20), 2441– 2449 (2009).
- Byrne RA, Kastrati A, Massberg S et al. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J. Am. Coll. Cardiol. 58(13), 1325–1331 (2011).
- Wilson GJ, Huibregtse BA, Pennington DE, Dawkins KD. Comparison of the SYNERGY with the PROMUS (XIENCE V) and bare metal and polymer-only Element control stents in porcine coronary arteries. EuroIntervention 8(2), 250–257 (2012).
- Meredith IT, Verheye S, Dubois CL et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J. Am. Coll. Cardiol. 59(15), 1362–1370 (2012).
- Kereiakes DJ, Meredith IT, Windecker S et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial. Circ. Cardiovasc. Interv. 8(4), pii: e002372 (2015).
- Hamon M, Niculescu R, Deleanu D, Dorobantu M, Weissman NJ, Waksman R. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in-man study. EuroIntervention 8(9), 1006–1011 (2013).
- Windecker S, Haude M, Neumann F-J et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ. Cardiovasc. Interv. 8(2), e001441 (2015).
- Pilgrim T, Heg D, Roffi M et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet 384(9960), 2111–2122 (2014).
- Byrne RA. Biodegradable polymer versus durable polymer drug-eluting stents for patients with coronary artery disease: three year pooled analysis of individual patient data from the ISAR-TEST 4, LEADERS, and ISAR-TEST 3 randomised trials. Presented at: EuroPCR 2011: Congress of the European Association of Percutaneous Cardiovascular Interventions. Paris,France, 17–20 May 2011.
- Ferrari E, Benhamou M, Cerboni P, Marcel B. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J. Am. Coll. Cardiol. 45(3), 456–459 (2005).
- Garg S, Serruys PW. Coronary stents: looking forward. J. Am. Coll. Cardiol. 56(Suppl. S 10), S43–S78 (2010).
- Onuma Y, Serruys PW, Gomez J et al. Comparison of in vivo acute stent recoil between the bioresorbable everolimus-eluting coronary scaffolds (revision 1.0 and 1.1) and the metallic everolimus-eluting stent. Catheter. Cardiovasc. Interv. 78(1), 3–12 (2011).
- Stack RS, Califf RM, Phillips HR et al. Interventional cardiac catheterization at Duke Medical Center. Am. J. Cardiol. 62(10 Pt 2), F3–F24 (1988).
- Tamai H, Igaki K, Kyo E et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 102(4), 399–404 (2000).
- Ramcharitar S, Serruys PW. Fully biodegradable coronary stents: progress to date. Am. J. Cardiovasc. Drugs 8(5), 305–314 (2008).
- Di Mario C, Gil R, Camenzind E et al. Quantitative assessment with intracoronary ultrasound of the mechanisms of restenosis after percutaneous transluminal coronary angioplasty and directional coronary atherectomy. Am. J. Cardiol. 75(12), 772–777 (1995).
- Colombo A, Karvouni E. Biodegradable stents: “fulfilling the mission and stepping away”. Circulation 102(4), 371–373 (2000).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371(9616), 899–907 (2008).
- Onuma Y, Serruys PW, Ormiston JA et al. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention 6(4), 447–453 (2010).
- Serruys PW, Ruygrok P, Neuzner J et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent:the SPIRIT II trial. EuroIntervention 2(3), 286–294 (2006).
- Tanimoto S, Serruys PW, Thuesen L et al. Comparison of in vivo acute stent recoil between the bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting cobalt chromium coronary stent: insights from the ABSORB and SPIRIT trials. Catheter. Cardiovasc. Interv. 70(4), 515–523 (2007).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2 year outcomes and results from multiple imaging methods. Lancet 373(9667), 897–910 (2009).
- Azuma H, Niimi Y, Terada T, Hamasaki H. Accelerated endothelial regeneration and intimal hyperplasia following a repeated denudation of rabbit carotid arteries: morphological and immunohistochemical studies. Clin. Exp. Pharmacol. Physiol. 22(10), 748–754 (1995).
- Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab. Invest. 41(5), 407–418 (1979).
- Tahir H, Bona-Casas C, Hoekstra AG. Modelling the effect of a functional endothelium on the development of in-stent restenosis. PLoS One 8(6), e66138 (2013).
- Larsen K, Cheng C, Tempel D et al. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur. Heart J. 33(1),120–128 (2012).
- Beijk MA, Klomp M, Verouden NJ et al. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur. Heart J. 31(9), 1055–1064 (2010).
- Klomp M, Beijk MA, Varma C et al. 1-year outcome of TRIAS HR (TRI-Stent Adjudication Study-High Risk of Restenosis): a multicenter, randomized trial comparing genous endothelial progenitor cell ca444444pturing stents with drug-eluting stents. J. Am. Coll. Cardiol. Interv. 4(8), 896–904 (2011).
- Aoki J, Serruys PW, Van Beusekom H et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) registry. J. Am. Coll. Cardiol. 45(10), 1574–1579 (2005).
- Duckers HJ, Soullie T, Den Heijer P et al. Accelerated vascular repair following percutaneous coronary intervention by capture of endothelial progenitor cells promotes regression of neointimal growth at long term follow-up: final results of the Healing II trial using an endothelial progenitor cell capturing stent (Genous R stent). EuroIntervention 3(3), 350–358 (2007).
- Silber S, Damman P, Klomp M et al. Clinical results after coronary stenting with the Genous bio-engineered R stent: 12 month outcomes of the e-HEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) worldwide registry. EuroIntervention 6(7), 819–825 (2011).
- Nakazawa G, Granada JF, Alviar CL et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. J. Am. Coll. Cardiol. Interv. 3(1), 68–75 (2010).
- Granada JF, Inami S, Aboodi MS et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ. Cardiovasc. Interv. 3(3), 257–266 (2010).
- Haude M, Lee SW, Worthley SG et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc. Interv. 6(4), 334–343 (2013).
- Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J. Cell. Mol. Med. 13(1), 87–102 (2009).
- Stella C, Cazzola M, De Fabritiis P et al. CD34-positive cells: biology and clinical relevance. Haematologica 80(4), 367–387 (1995).
- Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34−/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circul. Res. 98(3), e20–e25 (2006).
- Chandrasekar B, Tanguay J-F. Local delivery of 17-beta-estradiol decreases neointimal hyperplasia after coronary angioplasty in a porcine model. J. Am. Coll. Cardiol. 36(6), 1972–1978 (2000).
- Chandrasekar B, Sirois MG, Geoffroy P, Lauzier D, Nattel S, Tanguay J. Local delivery of 17beta-estradiol improves reendothelialization and decreases inflammation after coronary stenting in a porcine model. Thromb. Haemost.-Stuttgart- 94(5), 1042 (2005).
- Adriaenssens T, Mehilli J, Wessely R et al. Does addition of estradiol improve the efficacy of a rapamycin-eluting stent?: results of the ISAR-PEACE randomized trial. J. Am. Coll. Cardiol. 49(12), 1265–1271 (2007).
- Airoldi F, Di Mario C, Ribichini F et al. 17-beta-estradiol eluting stent versus phosphorylcholine-coated stent for the treatment of native coronary artery disease. Am. J. Cardiol. 96(5), 664–667 (2005).
- Ferrara N, Gerber H-P, Lecouter J. The biology of VEGF and its receptors. Nat. Med. 9(6), 669–676 (2003).
- Asahara T, Bauters C, Pastore C et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation 91(11), 2793–2801 (1995).
- Swanson N, Hogrefe K, Javed Q, Malik N, Gershlick AH. Vascular endothelial growth factor (VEGF)-eluting stents: in vivo effects on thrombosis, endothelialization and intimalhyperplasia. J. Invasive Cardiol. 15(12), 688–692 (2003).
- Van Der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 305(3), 285–298 (2001).
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J. Cell Sci. 119(Pt 19), 3901–3903 (2006).
- D’souza SE, Ginsberg MH, Plow EF. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem. Sci. 16(7), 246–250 (1991).
- Blindt R, Vogt F, Astafieva I et al. A novel drug-eluting stent coated with an integrin-binding cyclic arg-gly-asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J. Am. Coll. Cardiol. 47(9), 1786–1795 (2006).
- Joner M, Cheng Q, Schonhofer-Merl S et al. Polymer-free immobilization of a cyclic RGD peptide on a nitinol stent promotes integrin-dependent endothelial coverage of strut surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 100(3), 637–645 (2012).
- Vogt F, Mause S, Schröder J et al. Improved safety and efficacy of a novel dual cRGD- and everolimus-coated coronary stent compared to everolimus-eluting stents in the Porcine Coronary Model. Circulation 130(Suppl. 2), Abstract 16008 (2014).
- Meyers SR, Kenan DJ, Khoo X, Grinstaff MW. A bioactive stent surface coating that promotes endothelialization while preventing platelet adhesion. Biomacromolecules 12(3), 533–539 (2011).
- Bilek MMM, Bax DV, Kondyurin A et al. Free radical functionalization of surfaces to prevent adverse responses to biomedical devices. Proc. Natl Acad. Sci. USA 108(35), 14405–14410 (2011).
- Waterhouse A, Bax DV, Wise SG et al. Stability of a therapeutic layer of immobilized recombinant human tropoelastin on a plasma-activated coated surface. Pharm. Res. 28(6), 1415–1421 (2011).
- Yin Y, Wise SG, Nosworthy NJ et al. Covalent immobilisation of tropoelastin on a plasma deposited interface for enhancement of endothelialisation on metal surfaces. Biomaterials 30(9), 1675–1681 (2009).
- Waterhouse A, Yin Y, Wise SG et al. The immobilization of recombinant human tropoelastin on metals using a plasma-activated coating to improve the biocompatibility of coronary stents. Biomaterials 31(32), 8332–8340 (2010).
- Waterhouse A, Wise SG, Yin Y et al. In vivo biocompatibility of a plasma-activated, coronary stent coating. Biomaterials 33(32), 7984–7992 (2012).
- Williams PD, Kingston PA. Plasmid-mediated gene therapy for cardiovascular disease. Cardiovasc. Res. 91(4), 565–576 (2011).
- Klugherz BD, Jones PL, Cui X et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat. Biotechnol. 18(11), 1181–1184 (2000).
- Walter DH, Cejna M, Diaz-Sandoval L et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation 110(1), 36–45 (2004).
- Brito LA, Chandrasekhar S, Little SR, Amiji MM. Non-viral eNOS gene delivery and transfection with stents for the treatment of restenosis. Biomed. Eng. Online 9, 56 (2010).
- Egashira K, Nakano K, Ohtani K et al. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Atertio. Thromb. Vasc. Biol. 27(12), 2563–2568 (2007).
- Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J. Pathol. 208(2), 299–318 (2006).
- Johnson TW, Wu YX, Herdeg C et al. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Atertio. Thromb. Vasc. Biol. 25(4), 754–759 (2005).
- Che HL, Bae IH, Lim KS et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials 33(33), 8548–8556 (2012).