Perspective Article - Imaging in Medicine (2011) Volume 3, Issue 3
Advances in contrast media for vascular imaging of atherosclerosis
Victoria EL Young†1, Andrew J Degnan1and Jonathan H Gillard1
1Addenbrooke’s Hospital, University Department of Radiology, Box 218, Hills Road, Cambridge, CB2 0QQ, UK
- *Corresponding Author:
- Victoria EL Young
Addenbrooke’s Hospital
University Department of Radiology
Box 218, Hills Road, Cambridge, CB2 0QQ, UK
Tel.: +44 1223 746 446
Fax: +44 1223 330 915
E-mail: vy207@cam.ac.uk
Abstract
Atherosclerosis is a major cause of morbidity and mortality worldwide. Imaging plays a key role in its diagnosis and management, and contrast-enhanced imaging is widely integrated into current clinical practice for the imaging of atherosclerosis. Traditional imaging methods have focused on the degree of stenosis owing to atherosclerotic plaque; however, contrast media now allow for improved detection of plaque characteristics, which may aid in identifying at-risk lesions in cardiovascular and cerebrovascular disease. Developments in contrast media may allow imaging to play a much earlier role in diagnosis and, potentially, therapy for atherosclerosis in the future. This article focuses on the developments in contrast media for MRI, ultrasound and CT in relation to atherosclerosis.
Keywords
atherosclerosis; contrast-enhanced ultrasound; contrast media; molecular imaging; MRI; vascular imaging
Cardiovascular disease, including atherosclerosis, accounts for over 850,000 deaths a year in the USA and constitutes a significant healthcare burden [201]. Stroke and myocardial infarction are the leading preventable causes of death; earlier diagnosis of atherosclerosis and greater emphasis on disease prevention, as opposed to treatment of clinical events, could reduce the impact of atherosclerosis on society [202]. Contrast-enhanced imaging plays a substantial role in the detection of disease at a subclinical or asymptomatic stage, although the clinical significance of atherosclerosis in its early stages is uncertain. This article will briefly discuss the current clinical role of contrast imaging in atherosclerosis, the wider applications of currently available contrast media for atherosclerosis imaging and new developments in imaging with contrast media for atherosclerosis. MRI, CT and ultrasound will be considered, but nuclear medicine is outside the scope of this article. Nevertheless, imaging of atherosclerosis with F18-FDG PET appears to be promising in detecting the presence of an inflamed plaque; however, limited spatial resolution prevents widespread clinical use, as there is no means of assessing the degree of luminal narrowing [1]. Ultimately, contrast agents are becoming increasingly directed at specific molecular targets; unfortunately, many of these rapid advances in contrast technology have not yet been fully vetted in the clinical arena. This article will discuss the potential for contrast media to reach clinical significance to predict the risk of vascular events in atherosclerotic disease.
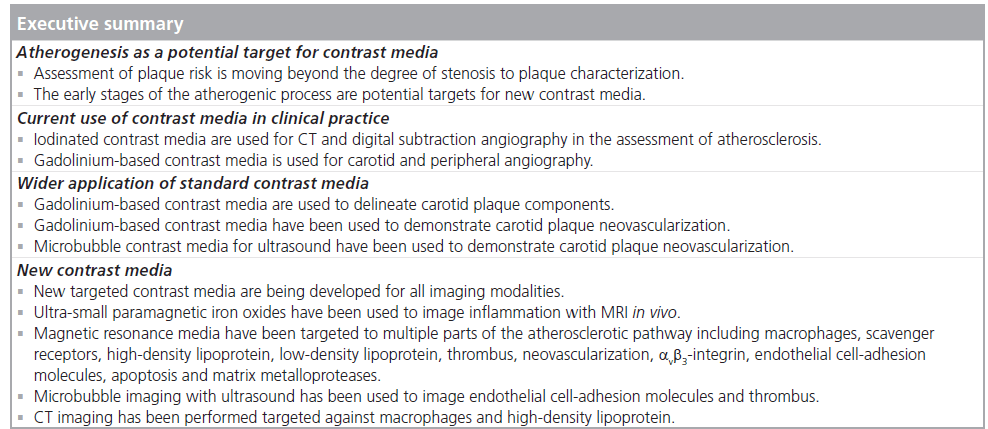
Atherogenesis as a potential target for contrast media
As the understanding of atherosclerosis has become more detailed, potential targets for imaging with contrast media have become more refined. Standard imaging for current clinical management is based on a traditional understanding of disease, which is an expanding plaque, encroaching on the lumen causing increasing stenosis, with higher degrees warranting intervention [2–7]. The next stage of understanding was the gross morphological features of the plaque itself, which differ and, depending on the configuration (i.e., the presence of lipid, fibrous cap status and the presence of hemorrhage), can put the individual at varying risks of future clinical events leading to the concept of the vulnerable plaque [8]. Therefore, the next imaging step was to look at plaque morphological characterization, although much of this work has been done without the use of contrast media, using the different tissue properties of the components on the imaging modalities [9–15]. On a microscopic level, other features were then identified, which were also linked to plaque vulnerability, in particular, plaque inflammation [15] and neovascularization [16]. These have made good potential targets for contrast media in providing the impetus for several key molecular targets, such as fibrin for intraplaque hemorrhage and low-density lipoprotein (LDL) targeted to macrophages to image inflammation. As work on the atherogenic model progressed, many mediators involved in early-stage atherogenesis were identified. Some of these mediators are endothelial cell-adhesion molecules [17], lipoproteins [18] and matrix metalloproteinases [19]. These are attractive targets for imaging as they might allow detection of plaque at an earlier stage, although the clinical significance of identifying plaque at such an early stage is uncertain considering atherosclerotic fatty streaks can be identified in the young without risk factors [20]. Thus, the challenge of novel contrast media and new applications of existing media is to specifically identify atherosclerotic processes that are likely to progress to clinical events rather than merely identifying all atherosclerotic lesions that appear to be ubiquitous in populations.
Current use of contrast media in clinical practice
Contrast media are widely used in the everyday practice of atherosclerosis diagnostic imaging, for invasive and noninvasive angiography, in all the main vascular territories. The focus of imaging for atherosclerosis is on three main vascular beds – coronary, carotid and peripheral – of which two cause life-threatening clinical events in the form of stroke and myocardial infarction.
Invasive angiography, in the form of digital subtraction angiography (DSA) with iodine contrast media, is held as the gold standard for assessment of stenotic lesions. It is currently less widely used because of the risks of serious complications, with permanent neurological sequelae reported in approximately 0.5–1% of cerebral angiograms at experienced centers [21], and the advent of noninvasive methods with good comparative sensitivities and specificities. The advantage of DSA in the coronary scenario over noninvasive methods in the acute setting is the ability to combine interventions in the same procedure.
Noninvasive methods of angiography used to assess atherosclerosis in the carotid and coronary territories are CT angiography (CTA) with iodine-based contrast media, magnetic resonance (MR) angiography with gadolinium (Gd)-based contrast media in the carotid artery and periphery and, more recently, contrast-enhanced ultrasound to better distinguish lumen from arterial wall.
Expanded applications of conventional, nontargeted contrast media
MRI
Gadolinium-based contrast media are used in imaging of carotid atherosclerosis, not only for angiography, but also for vessel wall imaging. Wall enhancement has been used to improve the delineation of fibrous cap and lipid-rich necrotic core when compared with unenhanced imaging [22,23]. The move of plaque imaging with high-resolution contrast-enhanced MRI, which is still more widely used in the research arena, to the clinical setting may be assisted by the research currently being conducted, such as the BioImage study, where the investigators are performing multimodality assessment of patients with subclinical disease, looking at long-term outcomes [24].
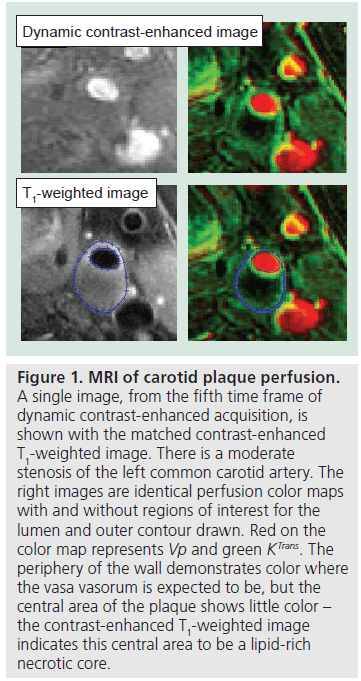
The wall enhancement produced on delayed contrast imaging of the carotid artery has also been related to plaque neovascularization, which is thought to be an early marker of atherogenesis [25]. This concept has been explored more thoroughly using dynamic contrast-enhanced (DCE) MRI of the carotid vessel wall (Figure 1). T1-weighted gradient-echo imaging was performed on 20 individuals who subsequently underwent carotid endarterectomy to calculate the fractional blood volume (fBV) of the plaque [26]. Plaques with high fBV were found to have dense microvasculature on histology. Later work has used the same imaging technique but different modeling metrics, in the form of KTrans and Vp [27]. Both parameters were correlated with the presence of both macrophages and neovascularization on histology, and other studies have suggested a link between these two pathological mechanisms indicating that these parameters may be noninvasive markers of inflammation [28,29]. KTrans was also found to be higher in smokers and those with low high-density lipoprotein (HDL) levels, suggesting a link between KTrans and pro-inflammatory atherosclerotic risk factors.
Figure 1. MRI of carotid plaque perfusion. A single image, from the fifth time frame of dynamic contrast-enhanced acquisition, is shown with the matched contrast-enhanced T1-weighted image. There is a moderate stenosis of the left common carotid artery. The right images are identical perfusion color maps with and without regions of interest for the lumen and outer contour drawn. Red on the color map represents Vp and green KTrans. The periphery of the wall demonstrates color where the vasa vasorum is expected to be, but the central area of the plaque shows little color – the contrast-enhanced T1-weighted image indicates this central area to be a lipid-rich necrotic core.
Contrast-enhanced ultrasound
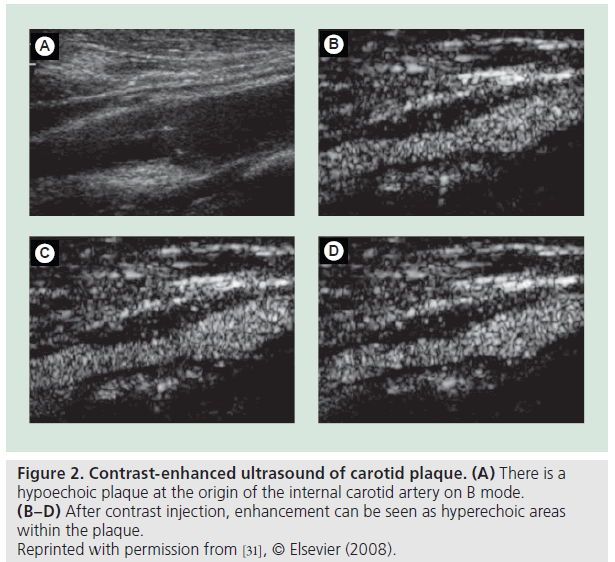
In a similar way to MRI methods, contrast media available for ultrasound – ‘microbubble’ contrast media – have been used to demonstrate plaque neovascularization. Contrastenhanced ultrasound in a femoral swine model of atherosclerosis demonstrated enhancement in the early stages of the disease process and indicated progression, with increased enhancement, as the microvascular network developed within the plaque, demonstrated with histology [30]. Two very similar studies comparing contrast-enhanced ultrasound and histology for human subjects with carotid atherosclerosis demonstrated significantly greater enhancement in the plaques with more neovascularization (Figure 2) [31,32], but neither characteristic correlated with the degree of stenosis [31]. This reinforces the ever-growing body of literature declaring degree of stenosis inadequate in identifying those individuals with high-risk disease. A larger study examining 64 patients who underwent surgery following contrast-enhanced ultrasound found that all nine acutely symptomatic patients had enhancement at the base of the plaque correlating with neovascularization, but only one of the asymptomatic cohort displayed the same enhancement [33]. This represents further evidence that neovascularization is an indicator of vulnerable disease, and is further corroborated by a different study that showed patients with symptomatic disease to have a significantly higher plaque-enhanced intensity compared with asymptomatic individuals on contrast-enhanced ultrasound [34].
It has also been suggested that late-phase microbubble contrast ultrasound can be used as a marker of inflammation, as in vitro studies have shown that the microbubbles are phagocytosed by monocytes but remain acoustically active for up to 30 min, and preclinical experiments have also shown microbubbles within monocytes [35,36]. Assessment of late-phase carotid plaque echogenicity (normalized to the lumen) following microbubble contrast injection (n = 37) demonstrated significantly greater echogenicity in symptomatic versus asymptomatic individuals, although with some overlap between groups. There was only a moderate inverse relationship between late-phase echogenicity and grayscale median score, a more traditional ultrasound assessment; a lower grayscale median score connotes greater lipid, macrophage and hemorrhage content and, therefore, greater echogenicity is associated with a low (high-risk) grayscale median score [37]. The relative weakness of the relationship reported by these authors would suggest that microbubble contrast is potentially more sensitive than grayscale median score, but there was no direct correlation with histology in this case and further studies with histological evaluation would be needed.
CT
CT provides excellent determination of lumen status and yields excellent spatial resolution. Nevertheless, contrast media has been employed infrequently for the purpose of characterizing atherosclerotic lesions. A study of contrastenhanced CTA of the carotids discovered that contrast enhancement of the arterial wall was seen more frequently in patients who were symptomatic (p = 0.041) [38]. In addition, supporting the conclusion that contrast-enhanced CT may aid in atherosclerotic detection, a study demonstrated that there was delayed enhancement in the majority of abdominal aortic aneurysms [39]. The mechanism by which CT contrast enhancement occurs within the arterial wall of plaques may be similar to those of Gd and microbubbles in the cases of MRI and ultrasound, respectively. Enhancing regions may occur because of neovascularization, a potential indicator of plaque instability. The use of contrast enhancement on CTA could be particularly useful clinically as CT is quicker, cheaper and more readily available than high-resolution MRI, and offers a more rapid means of imaging multiple vascular beds concurrently in one session. Recently, others have proposed the use of CT of the intracranial, extracranial and cardiac vessels for comprehensive ischemic stroke evaluation [40]. Thus, finding utility in traditional contrast-enhanced CTA would be a boon for the use of CT as a comprehensive single evaluation of multiple vascular beds for the assessment of the degree and risk of atherosclerotic disease. More work is needed in this field, in particular, to extend the initial findings of contrast enhancement in symptomatic carotid stenosis.
New targeted contrast media
Owing to the range of methods currently being used to image atherosclerosis, new contrast media are being developed for all three of the main modalities: MRI, CT and ultrasound.
MRI
Many of the new MR contrast media use traditional Gd within a particle as a basis for the contrast medium, which is then tagged with a marker to target a specific area of interest in the atherogenic pathway [41], while others use particles of iron oxides as the basis [42], and even fewer utilize manganese as an alternative to Gd [43,44]. There are multiple different constructs for these new media including liposomes, micelles and nanoparticles [45,46]. As much of this work is still in the preclinical setting, the new compounds are often co-tagged with an immunofluoroscent marker to allow concurrent optical imaging for histological validation of target specificity [47].
Gadolinium-based contrast media
One of the simpler compounds, gadofluorine M, has used the properties of the molecule itself, a small molecule of Gd chelate, without any additional tags [48]. The structure contains a perfluorinated side chain and sugar moiety, which makes it hydrophobic and, therefore, it forms micelles in aqueous solutions. Three rabbit models have demonstrated good delineation of aortic and femoral atherosclerotic plaque versus controls with this contrast medium [49–51]. More recently, enhancement of rabbit aortic atherosclerosis has been correlated with macrophage and microvessel density within the plaque [52].
There are newer Gd-based media that have additional tags added to their structures in order to target the macrophage and, therefore, atherosclerotic inflammation, specifically. Inflammation can be imaged using a number of different parts of the inflammatory pathway. One method that has shown good results in vitro [53], and against experimental controls in the mouse aorta model, is the use of immunomicelles targeted at the macrophage scavenger receptor, which is involved in the uptake of oxidized LDL and implicated in the induction of thrombosis and atherogenesis [47,54,55]. Nanoparticles, using Gd labeled specifically to target the macrophage scavenger receptor, have been used in combination with MRI for excised specimens of human aorta and have shown good localization to the fibrous cap, with an increased contrast-to-noise ratio compared with untargeted nanoparticles, which infiltrated the whole atheromatous plaque [56].
Another method of targeting the macrophage- laden areas of the atherosclerotic plaque is to use HDL. Contrast media combining synthetic compounds imitating HDL with Gd in nanoparticles have been successfully used in the mouse model to demonstrate significant enhancement in the macrophage-laden plaque areas [57–59]. Gd versions targeted with LDL have also been explored in tumor imaging, but not in atherosclerosis animal models [60,61].
It has long been acknowledged that lipid infiltration is a key factor in the atherosclerotic process, but current imaging needs an established lipid-rich necrotic core to be present before it can be detected, otherwise the early infiltration cannot be imaged. A polyethylene glycol (PEG) micelle combining a Gd base and functionalized with tyrosine residues (which are lipophilic) has been used in ApoE mice to demonstrate aortic signal enhancement correlated with histological presence of lipid [62].
Another component of the plaque morphology, along with a lipid-rich necrotic core, that has been linked to high-risk disease is the presence of hemorrhage, which has an association with fibrous cap rupture and also subsequent clinical events [63]. Imaging can already be used to detect the presence of large areas of plaque hemorrhage but the ability to detect much smaller areas at an earlier stage in the disease process could be beneficial. The feasibility of imaging this in the acute and subacute setting has been explored using a number of agents specifically targeting fibrin, which comprises thrombi.
Microthrombi atop the surface of ruptured plaque are thought to constitute the originating element in atheroembolic events. In a canine model of thrombus, in vivo MRI detected improved enhancement of external jugular venous thrombus with use of Gd-bound antifibrin nanoparticles (Gd–diethylene triamine pentaacetic acid [DTPA]–bis-oleate [BOA]) compared with standard Gd media [64]. This group also illustrated MRI detection of human plasma thrombi using this contrast medium. Subsequent studies have shown that the use of a fibrin-binding peptide labeled with Gd–EP-1873 (EPIX Medical Inc.) visualizes thrombi in a rabbit model of atherosclerosis and plaque rupture with positive MRI enhancement [65]. The time course of arterial wall enhancement within 30 min of contrast administration demonstrates the ability of EP-1873 to highlight atherosclerotic plaque rupture rapidly. Media targeting fibrin may offer additional insight into plaque vulnerability in the future with clinical development of agents, such as EP-1873, and improvements in the degree of enhancement with Gd–DTPA–phosphatidylethanolamine- over Gd–DTPA–BOA-complexed antifibrin [66]. EP-2104R, another similar antifibrin Gd-based contrast agent, outperformed noncontrast and nontargeted Gd–DTPA MRI in enhancing thrombus in an animal model of thrombosis from directed injury and stasis [67]. That this method detects thrombus in vivo and has a temporal signal-enhancement decrease with time with detection until 8 weeks after injury may mean that future development could produce a fibrin-targeted agent that can both detect and age thrombus. EP-2104R has been used in a small Phase II trial of patients with good results in enhancing intracardiac, aortic and carotid thrombi on MRI [68,69].
Gadolinium in its currently available commercial form has been used to explore plaque neovascularization in patients who have a reasonable degree of plaque burden. In the development of neovascularization, the endothelial cells of the microvessels express αvb3-integrin, as do the intimal and medial smooth muscle cells in the plaque. Therefore, it is suggested that αvb3-integrin can be used as an early marker of neovascularization and disease. In both rabbit and mouse aortic models, which have been used to test a Gd-based contrast media targeted to αvb3-integrin, there have been promising results, with good enhancement on MRI matched to angiogenic vessels on histology [70–72]. Improved enhancement has been gained by producing a contrast medium targeted to two ligands (instead of one) to improve uptake, namely of αvb3-integrin and galectin-1 [73].
Another potential set of targets expressed by cells involved in the atherosclerotic process (e.g., macrophages and smooth muscle cells) is the endothelial cell-adhesion molecules. Of particular interest are VCAM-1, ICAM-1, P-selectin and E-selectin. MRI of the mouse aorta using VCAM-1-targeted Gd produced signal enhancement, suggesting this as a possible methodology for atherosclerotic imaging [74].
An overlapping area of interest for many diseases, including atherosclerosis, as imaging advances, is the ability to target apoptosis. In the context of atheromatous disease, this is particularly a concern in the so-called ‘vulnerable’ plaques. Cells undergoing apoptosis may now be specifically identified through the use of annexin A5 (AA5), a 35-kDa plasma protein that binds to phosphatidylserine, which is expressed on the surface of dying macrophages and, to a lesser extent, smooth muscle cells [75]. Several animal models have looked at imaging AA5 uptake using nuclear medicine with success, and have been extended into pilot clinical studies to show SPECT detected enhancement of carotid atherosclerosis [75,76]. To extend the use of AA5 to take advantage of MRI’s higher resolution, phantom and in vitro MRI studies of AA5 have been performed using AA5 with crosslinked iron oxide [77] or Gd–DTPA-based nanoparticles to visualize apoptotic cells [78]. Early animal trials of MRI using a micellar form of AA5 with Gd–DTPA–DSA could detect atherosclerotic lesions within the abdominal aorta in a recent study of ApoE-knockout mice [79]; also in this mouse model, non-AA5 contrast agents (complexed with Gd), identified by phage display, demonstrated good colocalization between MR detection of aortic atherosclerotic lesions and histological confirmation of apoptotic cells [80,81]. The method of imaging, in the future, may also have the potential for therapeutic intervention, as suggested by a recent study showing reduced vascular inflammation in hyperlipidemic mice treated with AA5 [82]. Targeted therapies using nanoparticles for simultaneous imaging and treatment of atherosclerosis are currently in development [83]. An alternative approach being explored ties two of the previously mentioned pathways together.
Instead of using phosphatidylserine as the target of the contrast medium, it is being incorporated into the Gd liposome. The rationale behind this is that, as phosphatidylserine residues become exposed on the apoptotic cell, phagocytosis occurs through the macrophage scavenger-receptor pathways. in vitro and in vivo experiments (mouse aorta) demonstrated signal enhancement on T1-weighted MRI that corresponded to the presence of macrophages [84].
A number of matrix metalloproteinases (MMPs) are implicated in the atherogenic pathway and plaque destabilization. P947 is a Gd-based contrast medium targeted to MMPs, which has shown good discrimination between plaques containing high and low levels of MMPs, as well as excellent MR delineation of atherosclerotic plaque in the mouse model [85,86]. A similar study in rabbits also demonstrated good enhancement that correlated to MMP activity on zymography [87].
Iron oxide-based contrast media
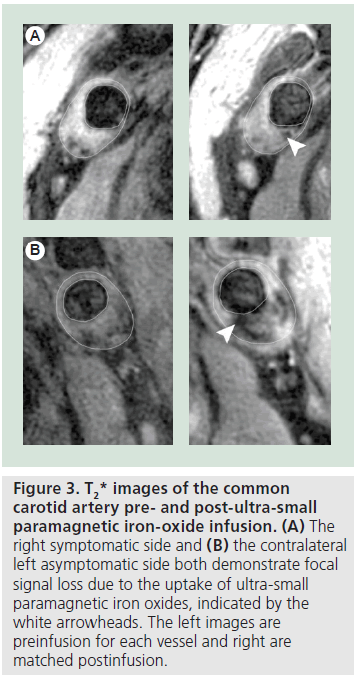
An advance in the use of contrast media for the imaging of inflammation in atherosclerosis has been the development of ultra-small paramagnetic iron oxides (USPIOs) for MRI of the carotid artery and aorta. Unlike traditional Gd, USPIOs are taken up by macrophages within the circulation, rather than being targeted to receptors on them, and then the USPIO-laden macrophages migrate into the atheromatous plaque. Owing to the long circulation time needed for USPIO to accumulate within plaque, two imaging sessions are required with a delay of 2–3 days from initial imaging and injection of contrast media; the optimal imaging window established for one of the compounds, ferumoxtran- 10, was 36–48 h postinjection [88]. When imaged, USPIOs operate as negative contrast medium, producing a signal loss rather than enhancement on T2 and T2* imaging, although there can be some enhancement on T1-weighted images at low concentrations before magnetic susceptibility effects outweigh the T1 shortening effect (Figure 3) [89]. There are serious limitations preventing widespread clinical adoption as there are concerns over the reproducibility of signal change comparison between initial and postcontrast scans, the need for two imaging sessions and the delay between imaging sessions. Nevertheless, unlike many of the other new contrast media that will be discussed, USPIOs have progressed from animal models [90–94] and have been in use in humans for some time, which has enabled contrast media to be used to demonstrate both pathogenesis and results of clinical intervention. The initial human studies of severe carotid atherosclerosis confirmed that the signal loss detected matched the presence of macrophages on histology [95,96], and that signal loss has an association with vulnerable plaque morphology [96,97]. USPIO has also been used to demonstrate inflammation in contralateral mild-to-moderate stenosis of symptomatic individuals, suggesting that although their symptomatic side has been surgically treated, their contralateral stenosis is also at high risk of causing future clinical symptoms, as atherosclerosis is a systemic, nonfocal disease [98]. One of the most powerful demonstrations of the application of contrast media in atherosclerosis has been the Atorvastatin Therapy: Effects on Reduction of Macrophage Activity (ATHEROMA) study [99]. This trial used the USPIO, ferumoxtran-10, to demonstrate that even after only 12 weeks of treatment, high-dose statin therapy had a beneficial effect on carotid plaque inflammation, whereas traditional imaging and measurements demonstrated no changes at this early stage.
Figure 3. T2* images of the common carotid artery pre- and post-ultra-small paramagnetic iron-oxide infusion. (A) The right symptomatic side and (B) the contralateral left asymptomatic side both demonstrate focal signal loss due to the uptake of ultra-small paramagnetic iron oxides, indicated by the white arrowheads. The left images are preinfusion for each vessel and right are matched postinfusion.
An alternative method of imaging inflammation with iron oxide particles, which is in the early experimental stages, has used the principle of utilizing HDL to target macrophagerich regions of plaque, but combined it with an iron oxide as opposed to a Gd base, which was described earlier [100]. in vitro experiments demonstrated uptake of the iron oxide HDL into macrophages and mouse studies histologically showed the presence of the compound with the atherosclerotic aortic plaque.
In a similar way to the Gd media, iron oxide has been used as the basis for targeted contrast media with tags being attached for specific mediators of the atherogenic pathway by conjugating with antibodies. Microparticles of iron oxide (MPIOs) targeted against endothelial cell-adhesion molecules, for both VCAM-1 and P-selectin, have been successfully used in both the mouse model and ex vivo specimens [101–103]. Early in vitro and in vivo mouse work using USPIO tagged for E-selectin showed positive results for vascular endothelium, suggesting vascular inflammation as a potential application [104]. A recent study has also used an E-selectin-targeted USPIO to demonstrate muscle inflammation, although no other studies with vascular inflammation are currently available [105].
As with Gd, platelets have also been used as a potential target for MPIOs, through their involvement in plaque hemorrhage and thrombus formation. Detection of small areas of hemorrhage was attempted by imaging activated platelets using a MPIO targeted at the IIb/IIa receptor [106]. A mouse carotid thrombus model used to test the contrast media demonstrated increased signal loss compared with control MPIOs on MRI and a reduction in the signal loss following thrombolysis.
Manganese-based contrast media
Often overlooked is the potential use of manganese- based agents in MRI. While there have been initial investigations into the use of targeting of manganese agents to atherosclerotic plaque targets, these investigations have been few in number and limited in extent. One study employed LDL modified with manganese mesoporphyrin (MnMeso) with success in vitro in shortening the T1 of foam cells, which are ubiquitous in the atheromatous plaque [44]. More recently, manganese nanobialys targeted against fibrin was demonstrated to enhance human clots in an experimental setting [43]. A carotid injury model of rabbits using ethylenediaminetetraacetic acid (EDTA)-complexed manganese showed enhancement in regions of vascular injury, but these results have not been extended further [107]. Thus, although the preponderance of contrast media research lies in Gd and iron oxide particles targeted to atherosclerotic processes, there may be some utility in examining manganese-based agents in greater detail, as manganese-based agents appear to avoid the risk of nephrogenic systemic fibrosis seen infrequently with Gd use [108].
Ultrasound
Much of the new contrast media developments have focused on MRI, but other imaging options are also being explored. Ultrasound has many benefits in terms of good resolution, wide availability and low cost, which makes it an attractive option, and a more targeted approach to using the microbubble contrast media mentioned earlier is being explored. VCAM-1 is another target in the atherogenic pathway and this has been exploited by producing lipid-shelled microbubbles with immunoglobulin against VCAM attached to the surface [109,110]. Enhancement demonstrated on ultrasound in the mouse aortic model correlated with microbubble endothelial attachment to atherosclerotic regions. Recent work that combined ultrasound imaging for both VCAM-1 and P-selectin in the mouse aortic model found that as early as at 10 weeks, wall enhancement could be detected by the microbubbles preferentially attaching to the regions of atherosclerosis [111]. This had also been previously attempted in vitro with ICAM-1 as a target [112,113], and was progressed to the stage of animal models in swine [114], but there is no recent work in the literature using this marker as a target.
Angiogenesis has been targeted with MR contrast media for early detection of atherosclerosis. This pathogenic process has so far been explored in the tumor animal model but not for atherosclerosis using markers such as av-integrin [110,115,116] and VEGF receptors [117,118].
A complication of atherosclerotic disease is intraplaque and juxtaluminal hemorrhage. Targeting the hemorrhage through the platelets has shown promising results with the use of an ‘immunomicrobubble’ containing a IIb/IIIa-receptor inhibitor, which demonstrated good detectability of carotid thrombi in a rat model [119]. Previously high mechanical index ultrasound pulses have been demonstrated to have the capacity to lyse thombi in vessels in conjunction with the administration of microbubbles [120–122]. Activated platelet-targeted microbubbles, using the glycoprotein IIb/IIIa receptor as the mediator, have been tested in swine and rats to enhance the localization of the microbubbles to the thrombus. These were associated with higher recanalization rates compared with nontargeted microbubbles when both were combined with high mechanical index impulses [123,124].
Taking it a stage further, it has been suggested that the imaging could be used to administer targeted therapy. To this end, echogenic liposomes, incorporating tissue plasminogen activator, have been developed and trialed on porcine clots [125]. ex vivo studies of the clots demonstrated release of the drug with ultrasound and clot lysis. This technique has been trialed, but is not directly applicable to atherosclerosis, which has suggested that water-soluble drugs are better options for this type of drug delivery [126].
CT
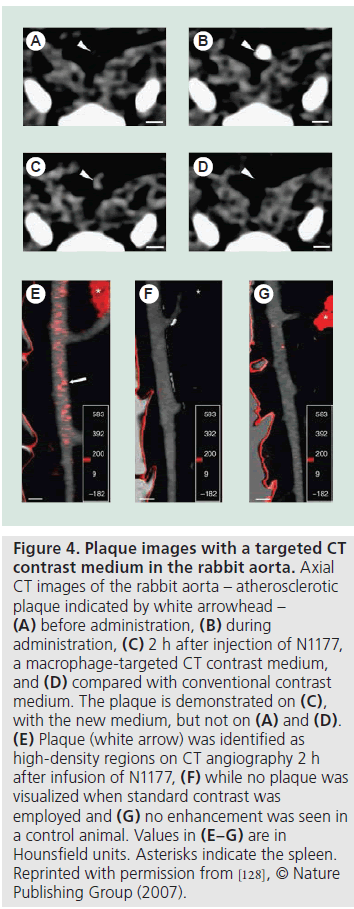
While most efforts in contrast media development have been directed towards targeted contrast media for MRI, there have been some developments in CT-compatible agents. In a manner similar to MRI, macrophages have been targeted with a CT nanoparticle as a way to detect atherosclerosis [127,128]. As with the targeted MRI nanoparticles, the contrast medium serves as the scaffold for the nanoparticle. Initial work demonstrated that this new CT medium, N1177 (Nanoscan Imaging), accumulated in the macrophages of atherosclerotic plaques in rabbits, and showed that this could be identified on CT (Figure 4) [129]. This CT enhancement has been cross-correlated with both PET and histological detection of inflammation [127].
Figure 4.Plaque images with a targeted CT contrast medium in the rabbit aorta. Axial CT images of the rabbit aorta – atherosclerotic plaque indicated by white arrowhead – (A) before administration, (B) during administration, (C) 2 h after injection of N1177, a macrophage-targeted CT contrast medium, and (D) compared with conventional contrast medium. The plaque is demonstrated on (C), with the new medium, but not on (A) and (D). (E) Plaque (white arrow) was identified as high-density regions on CT angiography 2 h after infusion of N1177, (F) while no plaque was visualized when standard contrast was employed and (G) no enhancement was seen in a control animal. Values in (E–G) are in Hounsfield units. Asterisks indicate the spleen. Reprinted with permission from [128], © Nature Publishing Group (2007).
The other goal identified, so far, for targeted contrast medium in CT is HDL, using the HDL–macrophage pathway of atherosclerosis described previously. A gold HDL-laden nanoparticle has been trialed in the mouse aorta model [129]. CT imaging in conjunction with iodinated contrast (for luminal delineation) demonstrated accumulation of the gold nanoparticles in the aorta, which was colocalized to the macrophages on histology. A potential limitation of gold nanoparticle use in humans is the limited understanding of their safety, especially as some in vivo experiments show the potential for cytotoxicity in selected cell types [130,131].
Conclusion
Contrast media is a fundamental part of current everyday practice for the assessment of atherosclerosis. New and exciting developments are taking place in the field of contrast media, which are directly linked to increasing knowledge of the pathogenesis of atherosclerosis as a disease process. The initial expansion of the use of contrast media led to the broader application of existing media for alternative imaging of the vessel wall as opposed to the lumen, aiming to delineate neovascularization. Newer contrast media have been developed for MRI, ultrasound and CT, using both cellular and molecular targets within the atherosclerotic pathway, with the aim to detect specific processes within atherogenesis and at a much earlier stage in atherosclerosis.
Future perspective
As the understanding of the disease process develops and the evidence for the features of vulnerable disease builds, the remit of contrast media will expand. We are moving from a diagnostic methodology of imaging after a clinical event to eventually, hopefully, imaging in the very early subclinical stages of disease with the aim to prevent clinical events from occurring. There is a move to advance imaging from a diagnostic aid to an interventional procedure, in which molecular targets can be employed to direct pharmacologic agents directly to the site of atherosclerosis. For contrast media, the first stage is to make this a more targeted imaging approach, which may well entail multimodality imaging to optimize spatial resolution and sensitivity to vulnerable plaque characterization. The next step in the much more distant future will be to use this targeted imaging to guide or administer therapeutic intervention, such as in the case of AA5.
The first step in this exciting field is for many of these new contrast media to progress from the preclinical arena into early-phase clinical studies to identify which of these myriad of proposed compounds are clinically safe and feasible. Ultimately, a prospective assessment of the association between imaging findings and the risk of vascular events is the true test of these nascent targeted imaging compounds. Clinically, pertinent contrast media should identify vulnerable lesions associated with a high incidence of vascular events, and have a high negative predictive value to exclude stable atherosclerotic plaque. Of the many contrast media currently in development, the most promising advances have occurred in the realm of MRI. Gd directed against fibrin for the visualization of thrombi shows great promise in terms of clinical adoption; in the case of one agent in particular, EP-2104R, initial Phase II trials have already been reported and the promise of detecting intravascular thrombus in vulnerable plaques is great. Agents employing iron oxide particles are still hampered by their delayed nature, the need for multiple imaging sessions to detect signal loss owing to paramagnetic effects and the difficulty in interpreting signal changes resultant from iron oxide uptake. Other more novel agents, such as gold nanoparticles, are presently limited by a dearth of safety documentation and scant clinical validation. These non-Gd agents should be vetted in prospective trials with longterm follow-up as they may yield great benefit in averting the uncommon, though concerning, risk of nephrogenic systemic fibrosis seen in some Gd products.
Attempts to use agents in modalities other than MRI will become of even greater importance, as costs of healthcare rise and access to care widens. A CT contrast media of recent interest is N1177, which is taken up by macrophages in inflamed atherosclerosis. The clinical potential of having a CT-based agent that is targeted to inflammatory mediators could offer the amalgamation of the speed, ease and availability of CT evaluation with detailed plaque vulnerability characterization formerly exclusive to MRI-based imaging studies. Greater research interest in the field of targeted CT-based contrast media, and particularly, media that may be jointly used in both CT and MRI modalities, would offer a broader ability to detect atherosclerosis.
Contrast-enhanced ultrasound may, in the future, compete with the high spatial resolution of MRI but, at present, its research foundation is small. The properties of microbubbles necessitate rapid imaging after infusion, and current measures of uptake are largely qualitative grading scales. An expansion of plaque imaging with contrast-enhanced ultrasound to include 3D imaging and validated quantification models is needed before this modality can attain clinical pertinence. In addition, concerns over the effect of operator skill have not yet been explored. However, the potential is great for contrast-enhanced ultrasound as ultrasound is an affordable, safe and common method of potentially ascertaining plaque vulnerability while assessing degree of stenosis in the carotid arteries.
Application of these atherosclerosis imaging methods to peripheral vessels outside of the usual cardiovascular and cerebrovascular and extracranial vessels will also be important in the future, with increasing recognition of the importance of peripheral vascular disease and its association with atherosclerosis in the other vascular beds. The challenge remains to develop contrast media that are targeted to particular aspects of the atherosclerotic process to yield clinically useful information about the risk of vascular events. Only with large, prospective trials of these novel agents will clinicians be able to infer the relative utility of these advances, which, to-date, are largely restricted to small pilot studies. Atherosclerosis is a ubiquitous problem for developed countries, and the need for risk stratification of individuals with atherosclerotic plaque will only increase with rising worldwide life expectancy. Contrast media developments, such as those discussed herein, may offer a means of addressing this growing disease burden.
Financial & competing interests disclosure
JH Gillard is a consultant to GlaxoSmithKline and currently receives study funding from Guerbet Laboratories. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
* of interest
* of considerable interest
References
- Rudd JH, Myers KS, Bansilal S et al.: Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J. Nucl. Med. 49(6), 871–878 (2008).
- Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 325(7), 445–453 (1991).
- Risk of stroke in the distribution of an asymptomatic carotid artery. The European Carotid Surgery Trialists Collaborative Group. Lancet 345(8944), 209–212 (1995).
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351(9113), 1379–1387 (1998).
- Moore WS, Young B, Baker WH et al.: Surgical results: a justification of the surgeon selection process for the ACAS trial. The ACAS investigators. J. Vasc. Surg. 23(2), 323–328 (1996).
- Rothwell PM: ACST: which subgroups will benefit most from carotid endarterectomy? Lancet 364(9440), 1122–1123; author reply 1125–1126 (2004).
- Rothwell PM, Eliasziw M, Gutnikov SA et al.: Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 361(9352), 107–116 (2003).
- Naghavi M, Libby P, Falk E et al.: From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108(14), 1664–1672 (2003).
- Cai JM, Hatsukami TS, Ferguson MS et al.: Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 106(11), 1368–1373 (2002). & Offers an excellent review of carotid plaque classification on the basis of MRI contrasts and shows that good agreement between MRI-determined plaque evaluation and American Heart Association histological classification can be achieved.
- Chu B, Kampschulte A, Ferguson MS et al.: Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35(5), 1079–1084 (2004).
- Murphy RE, Moody AR, Morgan PS et al.: Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation 107(24), 3053–3058 (2003).
- Biasi GM, Froio A, Diethrich EB et al.: Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) Study. Circulation 110(6), 756–762 (2004).
- Van Der Giessen AG, Toepker MH, Donelly PM et al.: Reproducibility, accuracy, and predictors of accuracy for the detection of coronary atherosclerotic plaque composition by computed tomography: an ex vivo comparison to intravascular ultrasound. Invest. Radiol. 45(11), 693–701 (2010).
- Maehara A, Mintz GS, Weissman NJ: Advances in intravascular imaging. Circ. Cardiovasc. Interv. 2(5), 482–490 (2009).
- Libby P: Inflammation in atherosclerosis. Nature 420(6917), 868–874 (2002).
- Libby P, Aikawa M, Jain MK: Vascular endothelium and atherosclerosis. Handb. Exp. Pharmacol. (176 Pt 2), 285–306 (2006).
- O’Brien KD, Mcdonald TO, Chait A, Allen MD, Alpers CE: Neovascular expression of e-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 93(4), 672–682 (1996).
- Libby P: Fat fuels the flame: triglyceride-rich lipoproteins and arterial inflammation. Circ. Res. 100(3), 299–301 (2007).
- Loftus IM, Thompson MM: The role of matrix metalloproteinases in vascular disease. Vasc. Med. 7(2), 117–133 (2002).
- Mcgill HC Jr, Mcmahan CA, Herderick EE et al.: Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 72(5 Suppl.), S1307–S1315 (2000).
- Willinsky RA, Taylor SM, Terbrugge K et al.: Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 227(2), 522–528 (2003).
- Zhao X, Underhill HR, Yuan C et al.: Minimization of MR contrast weightings for the comprehensive evaluation of carotid atherosclerotic disease. Invest. Radiol. 45(1), 36–41 (2010).
- Takaya N, Cai J, Ferguson MS et al.: Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J. Magn. Reson. Imaging 24(1), 203–210 (2006).
- Muntendam P, Mccall C, Sanz J, Falk E, Fuster V: The Bioimage Study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease – study design and objectives. Am. Heart J. 160(1), 49–57.E41 (2004).
- Yuan C, Kerwin WS, Ferguson MS et al.: Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J. Magn. Reson. Imaging 15(1), 62–67 (2002).
- Kerwin W, Hooker A, Spilker M et al.: Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 107(6), 851–856 (2003).
- Kerwin WS, O’Brien KD, Ferguson MS et al.: Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 241(2), 459–468 (2006). & Dynamic contrast-enhanced MRI-measured values of KTrans and Vp correlated well with the extent of macrophage content and neovasculature on histology in this well-designed study. These results suggest that dynamic contrast-enhanced MRI is able to visualize plaque inflammation and concomitant neovascularization with accuracy in patients.
- Kumamoto M, Nakashima Y, Sueishi K: Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum. Pathol. 26(4), 450–456 (1995).
- Calcagno C, Cornily JC, Hyafil F et al.: Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler. Thromb. Vasc. Biol. 28(7), 1311–1317 (2008).
- Schinkel AF, Krueger CG, Tellez A et al.: Contrast-enhanced ultrasound for imaging vasa vasorum: comparison with histopathology in a swine model of atherosclerosis. Eur. J. Echocardiogr. 11(8), 659–664 (2010).
- Coli S, Magnoni M, Sangiorgi G et al.: Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J. Am. Coll. Cardiol. 52(3), 223–230 (2008). & Contrast enhancement on ultrasound correlated well with microvessel density of carotid plaque on histology in this study of 32 patients. This study demonstrates the feasibility of microbubble ultrasound in ascertaining neovascularity in vivo with good histological validation.
- Shah F, Balan P, Weinberg M et al.: Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc. Med. 12(4), 291–297 (2007).
- Giannoni MF, Vicenzini E, Citone M et al.: Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur. J. Vasc. Endovasc. Surg. 37(6), 722–727 (2009).
- Xiong L, Deng YB, Zhu Y, Liu YN, Bi XJ: Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology 251(2), 583–589 (2009).
- Lindner JR, Dayton PA, Coggins MP et al.: Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation 102(5), 531–538 (2000).
- Lindner JR, Song J, Xu F et al.: Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 102(22), 2745–2750 (2000).
- Owen DR, Shalhoub J, Miller S et al.: Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrastenhanced US. Radiology 255(2), 638–644 (2010).
- Romero JM, Babiarz LS, Forero NP et al.: Arterial wall enhancement overlying carotid plaque on CT angiography correlates with symptoms in patients with high grade stenosis. Stroke 40(5), 1894–1896 (2009).
- Sakuta A, Kimura F, Aoka Y et al.: Delayed enhancement on computed tomography in abdominal aortic aneurysm wall. Heart Vessels 22(2), 79–87 (2007).
- Boussel L, Cakmak S, Wintermark M et al.: Ischemic stroke: etiologic work-up with multidetector ct of heart and extra- and intracranial arteries. Radiology 258(1), 206–212 (2011).
- Doiron AL, Chu K, Ali A, Brannon- Peppas L: Preparation and initial characterization of biodegradable particles containing gadolinium-DTPA contrast agent for enhanced MRI. Proc. Natl Acad. Sci. USA 105(45), 17232–17237 (2008).
- Corot C, Robert P, Idee JM, Port M: Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 58(14), 1471–1504 (2006).
- Pan D, Caruthers SD, Hu G et al.: Liganddirected nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance imaging of vascular targets. J. Am. Chem. Soc. 130(29), 9186–9187 (2008).
- Mitsumori LM, Ricks JL, Rosenfeld ME, Schmiedl UP, Yuan C: Development of a lipoprotein based molecular imaging MR contrast agent for the noninvasive detection of early atherosclerotic disease. Int. J. Cardiovasc. Imaging 20(6), 561–567 (2004).
- Cormode DP, Skajaa T, Fayad ZA, Mulder WJ: Nanotechnology in medical imaging: probe design and applications. Arterioscler. Thromb. Vasc. Biol. 29(7), 992–1000 (2009).
- Mulder WJ, Strijkers GJ, Van Tilborg GA et al.: Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res. 42(7), 904–914 (2009).
- Mulder WJ, Strijkers GJ, Briley-Saboe KC et al.: Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn. Reson. Med. 58(6), 1164–1170 (2007).
- Henning TD, Saborowski O, Golovko D et al.: Cell labeling with the positive MR contrast agent gadofluorine M. Eur. Radiol. 17(5), 1226–1234 (2007).
- Sirol M, Itskovich VV, Mani V et al.: Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation 109(23), 2890–2896 (2004).
- Barkhausen J, Ebert W, Heyer C, Debatin JF, Weinmann HJ: Detection of atherosclerotic plaque with gadofluorine-enhanced magnetic resonance imaging. Circulation 108(5), 605–609 (2003).
- Zheng J, Ochoa E, Misselwitz B et al.: Targeted contrast agent helps to monitor advanced plaque during progression: a magnetic resonance imaging study in rabbits. Invest. Radiol. 43(1), 49–55 (2008).
- Sirol M, Moreno PR, Purushothaman KR et al.: Increased neovascularization in advanced lipid-rich atherosclerotic lesions detected by gadofluorine-M-enhanced MRI: implications for plaque vulnerability. Circ. Cardiovasc. Imaging 2(5), 391–396 (2009).
- Gustafsson B, Youens S, Louie AY: Development of contrast agents targeted to macrophage scavenger receptors for MRI of vascular inflammation. Bioconjug. Chem. 17(2), 538–547 (2006).
- Amirbekian V, Lipinski MJ, Briley-Saebo KC et al.: Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl Acad. Sci. USA 104(3), 961–966 (2007). & Mouse model study that demonstrated the ability of macrophage scavenger receptortargeted, gadolinium-labeled immunomicelles to enhance atherosclerotic plaques in vivo with excellent colocalization with macrophages on microscopy.
- Lipinski MJ, Amirbekian V, Frias JC et al.: MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn. Reson. Med. 56(3), 601–610 (2006).
- Lipinski MJ, Frias JC, Amirbekian V et al.: Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc. Imaging 2(5), 637–647 (2009).
- Cormode DP, Briley-Saebo KC, Mulder WJ et al.: An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small 4(9), 1437–1444 (2008).
- Cormode DP, Chandrasekar R, Delshad A et al.: Comparison of synthetic high density lipoprotein (HDL) contrast agents for MR imaging of atherosclerosis. Bioconjug. Chem. 20(5), 937–943 (2009).
- Chen W, Vucic E, Leupold E et al.: Incorporation of an ApoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol. Imaging 3(6), 233–242 (2008).
- Corbin IR, Li H, Chen J et al.: Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia 8(6), 488–498 (2006).
- Crich SG, Lanzardo S, Alberti D et al.: Magnetic resonance imaging detection of tumor cells by targeting low-density lipoprotein receptors with Gd-loaded low-density lipoprotein particles. Neoplasia 9(12), 1046–1056 (2007).
- Beilvert A, Cormode DP, Chaubet F et al.: Tyrosine polyethylene glycol (PEG)-micelle magnetic resonance contrast agent for the detection of lipid rich areas in atherosclerotic plaque. Magn. Reson. Med. 62(5), 1195–1201 (2009).
- Altaf N, Macsweeney ST, Gladman J, Auer DP: Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 38(5), 1633–1635 (2007).
- Flacke S, Fischer S, Scott MJ et al.: Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation 104(11), 1280–1285 (2001).
- Botnar RM, Perez AS, Witte S et al.: In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation 109(16), 2023–2029 (2004). & EP-1873, a fibrin-binding peptide with gadolinium, is used in this study of simulated plaque rupture in vivo in a rabbit model to identify thrombi with great accuracy.
- Winter PM, Caruthers SD, Yu X et al.: Improved molecular imaging contrast agent for detection of human thrombus. Magn. Reson. Med. 50(2), 411–416 (2003).
- Sirol M, Aguinaldo JG, Graham PB et al.: Fibrin-targeted contrast agent for improvement of in vivo acute thrombus detection with magnetic resonance imaging. Atherosclerosis 182(1), 79–85 (2005).
- Spuentrup E, Botnar RM, Wiethoff AJ et al.: MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: initial results in patients. Eur. Radiol. 18(9), 1995–2005 (2008).
- Vymazal J, Spuentrup E, Cardenas-Molina G et al.: Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a Phase II clinical study of feasibility. Invest. Radiol. 44(11), 697–704 (2009).
- Burtea C, Laurent S, Murariu O et al.: Molecular imaging of avb3 integrin expression in atherosclerotic plaques with a mimetic of RGD peptide grafted to Gd-DTPA. Cardiovasc. Res. 78(1), 148–157 (2008).
- Winter PM, Morawski AM, Caruthers SD et al.: Molecular imaging of angiogenesis in early-stage atherosclerosis with avb3-integrintargeted nanoparticles. Circulation 108(18), 2270–2274 (2003).
- Neubauer AM, Sim H, Winter PM et al.: Nanoparticle pharmacokinetic profiling in vivo using magnetic resonance imaging. Magn. Reson. Med. 60(6), 1353–1361 (2008).
- Kluza E, Van Der Schaft DW, Hautvast PA et al.: Synergistic targeting of avb3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett. 10(1), 52–58 (2001).
- Burtea C, Laurent S, Port M et al.: Magnetic resonance molecular imaging of vascular cell adhesion molecule-1 expression in inflammatory lesions using a peptidevectorized paramagnetic imaging probe. J. Med. Chem. 52(15), 4725–4742 (2009).
- Tahara N, Imaizumi T, Virmani R, Narula J: Clinical feasibility of molecular imaging of plaque inflammation in atherosclerosis. J. Nucl. Med. 50(3), 331–334 (2009).
- Kietselaer BL, Reutelingsperger CP, Heidendal GA et al.: Noninvasive detection of plaque instability with use of radiolabeled annexin a5 in patients with carotid-artery atherosclerosis. N. Engl. J. Med. 350(14), 1472–1473 (2004).
- Schellenberger EA, Bogdanov A Jr, Hogemann D et al.: Annexin v-clio: a nanoparticle for detecting apoptosis by MRI. Mol. Imaging 1(2), 102–107 (2002).
- Prinzen L, Miserus RJ, Dirksen A et al.: Optical and magnetic resonance imaging of cell death and platelet activation using annexin a5-functionalized quantum dots. Nano Lett. 7(1), 93–100 (2007).
- Van Tilborg GA, Mulder WJ, Deckers N et al.: Annexin a5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjug. Chem. 17(3), 741–749 (2006).
- Burtea C, Laurent S, Lancelot E et al.: Peptidic targeting of phosphatidylserine for the MRI detection of apoptosis in atherosclerotic plaques. Mol. Pharm. 6(6), 1903–1919 (2009).
- Van Tilborg GA, Vucic E, Strijkers GJ et al.: Annexin a5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug. Chem. 21(10), 1794–1803 (2003).
- Ewing MM, De Vries MR, Nordzell M et al.: Annexin a5 therapy attenuates vascular inflammation and remodeling and improves endothelial function in mice. Arterioscler. Thromb. Vasc. Biol. 31(1), 95–101 (2010).
- Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM: Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J. Magn. Reson. Imaging 25(4), 667–680 (2007).
- Maiseyeu A, Mihai G, Kampfrath T et al.: Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J. Lipid Res. 50(11), 2157–2163 (2009).
- Lancelot E, Amirbekian V, Brigger I et al.: Evaluation of matrix metalloproteinases in atherosclerosis using a novel noninvasive imaging approach. Arterioscler. Thromb. Vasc. Biol. 28(3), 425–432 (2008).
- Amirbekian V, Aguinaldo JG, Amirbekian S et al.: Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo. Radiology 251(2), 429–438 (2009). & Matrix metalloproteinases were targeted successfully with use of P947 in a mouse model of atherosclerosis. This contrast media performed well with substantial in vivo MRI enhancement and colocalization with matrix metalloproteinases on microscopy.
- Hyafil F, Vucic E, Cornily JC et al.: Monitoring of arterial wall remodelling in atherosclerotic rabbits with a magnetic resonance imaging contrast agent binding to matrix metalloproteinases. Eur. Heart J. DOI: 10.1093/eurheartj/ehq413 (2010) (Epub ahead of print).
- Tang TY, Patterson AJ, Miller SR et al.: Temporal dependence of in vivo USPIOenhanced MRI signal changes in human carotid atheromatous plaques. Neuroradiology 51(7), 457–465 (2009).
- Howarth SP, Tang TY, Trivedi R et al.: Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur. J. Radiol. 70(3), 555–560 (2009).
- Schmitz SA, Coupland SE, Gust R et al.: Superparamagnetic iron oxide-enhanced MRI of atherosclerotic plaques in watanabe hereditable hyperlipidemic rabbits. Invest. Radiol. 35(8), 460–471 (2000).
- Litovsky S, Madjid M, Zarrabi A et al.: Superparamagnetic iron oxide-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: enhancement by tissue necrosis factor-a, interleukin-1b, and interferon-g. Circulation 107(11), 1545–1549 (2003).
- Herborn CU, Vogt FM, Lauenstein TC et al.: Magnetic resonance imaging of experimental atherosclerotic plaque: comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 24(2), 388–393 (2006).
- Korosoglou G, Weiss RG, Kedziorek DA et al.: Noninvasive detection of macrophagerich atherosclerotic plaque in hyperlipidemic rabbits using “positive contrast” magnetic resonance imaging. J. Am. Coll. Cardiol. 52(6), 483–491 (2008).
- Morishige K, Kacher DF, Libby P et al.: High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation 122(17), 1707–1715 (2010).
- Trivedi RA, Mallawarachi C, U-King-Im JM et al.: Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler. Thromb. Vasc. Biol. 26(7), 1601–1606 (2006).
- Kooi ME, Cappendijk VC, Cleutjens KB et al.: Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107(19), 2453–2458 (2003).
- Howarth SP, Tang TY, Trivedi R et al.: Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur. J. Radiol. 70(3), 555–560 (2008).
- Tang T, Howarth SP, Miller SR et al.: Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 37(9), 2266–2270 (2006).
- Tang TY, Howarth SP, Miller SR et al.: The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 53(22), 2039–2050 (2009). & Examined changes in ultra-small paramagnetic iron oxides (USPIOs) between patients treated over 12 weeks with either high- or low-dose atorvastatin. USPIO uptake within carotid plaque decreased in patients on the high-dose statin, suggesting the importance of USPIOs as targeted imaging agents for the detection of inflammation.
- Skajaa T, Cormode DP, Jarzyna PA et al.: The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials 32(1), 206–213 (2011).
- Nahrendorf M, Jaffer FA, Kelly KA et al.: Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114(14), 1504–1511 (2006).
- Mcateer MA, Schneider JE, Ali ZA et al.: Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler. Thromb. Vasc. Biol. 28(1), 77–83 (2008).
- Kelly KA, Allport JR, Tsourkas A et al.: Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ. Res. 96(3), 327–336 (2005).
- Boutry S, Laurent S, Elst LV, Muller RN: Specific E-selectin targeting with a superparamagnetic MRI contrast agent. Contrast Media Mol. Imaging 1(1), 15–22 (2006).
- Radermacher KA, Beghein N, Boutry S et al.: In vivo detection of inflammation using PEGylated iron oxide particles targeted at E-selectin: a multimodal approach using MR imaging and EPR spectroscopy. Invest. Radiol. 44(7), 398–404 (2009).
- Von Zur Muhlen C, Von Elverfeldt D, Moeller JA et al.: Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation 118(3), 258–267 (2008).
- Troughton JS, Greenfield MT, Greenwood JM et al.: Synthesis and evaluation of a high relaxivity manganese(II)- based MRI contrast agent. Inorg. Chem. 43(20), 6313–6323 (2004).
- Pan D, Caruthers SD, Senpan A et al.: Revisiting an old friend: manganese-based MRI contrast agents. Nanomed. Nanobiotechnol. DOI: 10.1002/wnan.116 (2010) (Epub ahead of print).
- Kaufmann BA, Sanders JM, Davis C et al.: Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 116(3), 276–284 (2007).
- Behm CZ, Kaufmann BA, Carr C et al.: Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation 117(22), 2902–2911 (2008).
- Kaufmann BA, Carr CL, Belcik JT et al.: Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler. Thromb. Vasc. Biol. 30(1), 54–59 (2010).
- Villanueva FS, Jankowski RJ, Klibanov S et al.: Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation 98(1), 1–5 (1998).
- Weller GE, Villanueva FS, Klibanov AL, Wagner WR: Modulating targeted adhesion of an ultrasound contrast agent to dysfunctional endothelium. Ann. Biomed. Eng. 30(8), 1012–1019 (2002).
- Demos SM, Alkan-Onyuksel H, Kane BJ et al.: In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J. Am. Coll. Cardiol. 33(3), 867–875 (1999).
- Ellegala DB, Leong-Poi H, Carpenter JE et al.: Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to avb3. Circulation 108(3), 336–341 (2003).
- Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR: Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to av-integrins. Circulation 107(3), 455–460 (2003).
- Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA: Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res. 13(1), 323–330 (2007).
- Rychak JJ, Graba J, Cheung AM et al.: Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol. Imaging 6(5), 289–296 (2007).
- Alonso A, Della Martina A, Stroick M et al.: Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 38(5), 1508–1514 (2007).
- Xie F, Tsutsui JM, Lof J et al.: Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med. Biol. 31(7), 979–985 (2005).
- Tachibana K, Tachibana S: Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation 92(5), 1148–1150 (1995).
- Molina CA, Ribo M, Rubiera M et al.: Microbubble administration accelerates clot lysis during continuous 2-mHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 37(2), 425–429 (2006).
- Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR: Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation 119(10), 1378–1385 (2009).
- Alonso A, Dempfle CE, Della Martina A et al.: In vivo clot lysis of human thrombus with intravenous abciximab immunobubbles and ultrasound. Thromb. Res. 124(1), 70–74 (2009).
- Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, Mcpherson DD: Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb. Res. 119(6), 777–784 (2007).
- Kopechek JA, Abruzzo TM, Wang B et al.: Ultrasound-mediated release of hydrophilic and lipophilic agents from echogenic liposomes. J. Ultrasound Med. 27(11), 1597–1606 (2008).
- Hyafil F, Cornily JC, Rudd JH et al.: Quantification of inflammation within rabbit atherosclerotic plaques using the macrophagespecific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J. Nucl. Med. 50(6), 959–965 (2009).
- Hyafil F, Cornily JC, Feig JE et al.: Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 13(5), 636–641 (2007).
- Cormode DP, Jarzyna PA, Mulder WJ, Fayad ZA: Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Deliv. Rev. 62(3), 329–338 (2011).
- Li JJ, Hartono D, Ong CN, Bay BH, Yung LY: Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 31(23), 5996–6003 (2010).
- Pernodet N, Fang X, Sun Y et al.: Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small 2(6), 766–773 (2006).
Websites
- American Heart Association: Heart Disease and Stroke Statistics – 2009 Update. American Heart Association, TX, USA (2009) www.americanheart.org/downloadable/ heart/1240250946756LS-1982%20 Heart%20and%20Stroke%20 Update.042009.pdf
- WHO: World Health Report: Facts and Figures. WHO, Geneva, Switzerland (2003) www.who.int/whr/2003/en/whr03_en.pdf