Review Article - Imaging in Medicine (2014) Volume 6, Issue 1
Usefulness of diffusion-weighted MRI in the characterization and assessment of response to neoadjuvant therapy in rectal cancer
Luís Curvo-Semedo*Medical Imaging Department, Coimbra University Hospitals, Praceta Mota Pinto/Avenida Bissaya-Barreto, 3000-075 Coimbra, Portugal and Faculty of Medicine, University of Coimbra, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal
- Corresponding Author:
- Luís Curvo-Semedo
Medical Imaging Department
Coimbra University Hospitals, Praceta Mota Pinto/Avenida Bissaya-Barreto
3000-075 Coimbra, Portugal and Faculty of Medicine
University of Coimbra, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal
Tel: +35 123 940 0431
Fax: +35 123 948 2840
E-mail: curvosemedo@gmail.com
Abstract
The following article will focus on the role of diffusion-weighted MRI for the assessment of response to neoadjuvant combined chemoradiation therapy in rectal cancer patients. The interest of diffusion-weighted imaging for tumor characterization will be discussed, as well as the evaluation of clearance from the mesorectal fascia, nodal downstaging, assessment of complete response and the prediction of response before and during combined chemoradiation therapy.
Keywords
chemoradiation therapy • diffusion-weighted imaging • MRI • rectal cancer • therapeutic response
The prognosis of rectal cancer is dependent on a multiplicity of factors, some of which are determined by histopathological assessment of the surgical specimen. Among these are the degree of tumor invasion into and beyond the bowel wall [1,2], the presence and number of lymph nodes involved by metastases from the primary tumor [3,4], and involvement of the mesorectal fascia (MRF) [5], a factor that was shown to be also reliably assessed preoperatively by MRI [6,7]. Other factors with known prognostic importance include the level of carcinoembryonic antigen on plasma and pathological factors, such as the presence of lymphangiovascular invasion and the tumor differentiation grade [8–10].
The current trends in the management of rectal cancer patients favor a more widespread acceptance of neoadjuvant treatments. As such, there is a rising need for preoperative imaging methods to accurately select high-risk patients who could receive benefit from the more aggressive multimodality treatment approaches [11,12]. Tailored information regarding the patient’s tumor profile should permit optimization of treatment and is also prognostically relevant by yielding a way to establish the risk for local and distant recurrence [13].
Additionally, the use of preoperative combined chemoradiation therapy (CRT) induces downsizing and downstaging of the primary tumor, yielding a pathologic complete response (pCR) in up to 24% of patients. A pCR is known to be associated with a favorable oncologic outcome, with regard to both recurrence and survival [14]. Although still controversial, the trend in treatment is now toward a more conservative policy for patients identified as complete responders (CRs) after CRT [15,16]. Traditionally, a pCR is determined by histopathologic examination of the surgical specimen. However, if the determination of a CR before surgery would influence the subsequent treatment choice, an accurate preoperative assessment of response becomes essential.
Currently used methods, such as digital examination and endoscopy and/or biopsy are good but not infallible. The role of MRI in the primary staging of rectal cancer is well established, and this imaging modality is now part of the standard work-up in many countries. Nevertheless, its role in restaging after preoperative CRT is still unclear, in part because, to date, restaging by imaging has not significantly changed the treatment approach. Moreover, like other morphological imaging techniques, such as endorec-tal ultrasonography and CT, MRI is hampered by interpretation problems in evaluating the existence of residual tumor within areas of radiation-induced fibrosis [17–19].
At the moment, the inclusion of diffusion-weighted imaging (DWI) into magnetic resonance (MR) protocols is steadily rising due to its demonstrated benefit, both for tumor detection/characterization and for assessment of treatment response [20–23]. Water diffusion characteristics are dependent on several factors, such as cell density, vascularity, viscosity of extracellular fluid and cell membrane integrity [24]. By quantifying these properties and expressing them as the apparent diffusion coefficient (ADC), DWI can potentially be used as an imaging biomarker to improve selection of poor prognosis patients who will beyond doubt profit from a more aggressive neoadjuvant treatment strategy [25].
Diffusion-weighted (DW)-MRI after CRT was also shown to be more valuable than morphological MRI for the differentiation between a pCR and residual tumor, because on DWI, viable tumor remnants are more easily recognized, as they appear hyperintense compared with the low-signal intensity of the surrounding non-neoplastic tissue [26,27].
Tumor & nodal characterization
To date, the value of DWI, namely the ADC, as a quantitative biomarker in patients with rectal cancer is not yet clear. Data are scarce and most published reports on the value of DW-MRI for the prediction of response to CRT are conflicting [13,25,28–31].
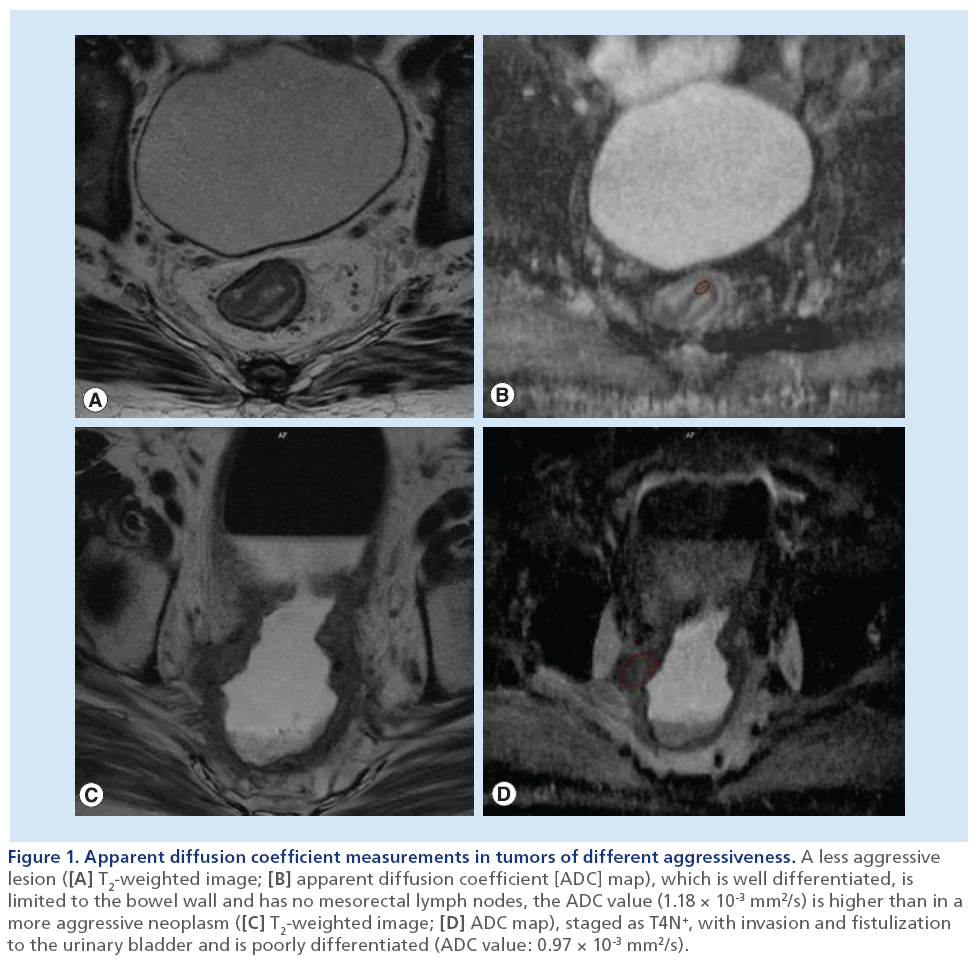
It is well-known that the aggressiveness of rectal tumors is expressed by several factors, including T stage, N stage, involvement of the MRF, carcinoembryonic antigen levels, differentiation grade of the tumor and the presence of lymphangiovascular invasion [1–7,9–10,32–34]. With this in mind, a recent report aimed to assess the value of DW-MRI, as expressed by the quantified ADC values, as a potential noninvasive imaging biomarker of tumor aggressiveness in rectal cancer, by correlating them with several prognostic factors [35]. The authors demonstrated statistically significant correlations between ADC values and the MRF and nodal status on MRI, and the tumor differentiation grade at histology [35]. Pretreatment mean ADC was significantly lower for tumors invading the MRF or tumors with nodal metastases [35]. As it is proven that both MRF involvement and metastatic lymph nodes are potent predictors of local recurrence and distant metastases, such correlation between ADC and MRF or nodal status therefore suggests that ADC correlates with prognosis. This may be related to the fact that ADC values derive from the tumor’s cellular microarchitecture and may therefore reflect the profile of aggressiveness of the tumor tissue (Figure 1). This is further suggested by the finding that less well-differentiated tumors had relatively low ADCs [35], in agreement with another recent study that showed a similar trend towards low ADC values for poorly differentiated tumors [9]. The authors also demonstrated a correlation between ADC and the distance from the tumor to the MRF, with lower ADC values associated with a shorter distance between the outermost margin of the tumor and the MRF [35].
Figure 1: Apparent diffusion coefficient measurements in tumors of different aggressiveness. A less aggressive lesion ([A] T2-weighted image; [B] apparent diffusion coefficient [ADC] map), which is well differentiated, is limited to the bowel wall and has no mesorectal lymph nodes, the ADC value (1.18 × 10-3 mm2/s) is higher than in a more aggressive neoplasm ([C] T2-weighted image; [D] ADC map), staged as T4N+, with invasion and fistulization to the urinary bladder and is poorly differentiated (ADC value: 0.97 × 10-3 mm2/s).
A more recent study by Elmi et al. concurs to these findings [36]. Furthermore, these authors have also showed that lower baseline ADC values were evident in patients who experienced tumor recurrence. In fact, in their study, a multivariate analysis identified tumor ADC as the only independently pretreatment prognostic indicator for recurrence. This may provide further evidence for the predictive value of DWI for disease recurrence in rectal cancer, suggesting that ADC by itself may correlate with disease behavior and prognosis.
Regarding lymph node characterization, a study by Cho et al. matching and analyzing 114 lymph nodes (46 metastatic and 68 nonmetastatic) demonstrated that the mean ADC of the metastatic lymph nodes was significantly lower than that of the nonmetastatic lymph nodes (0.9 ± 0.15 × 10-3 vs 1.1 ± 0.22 × 10-3 mm2/s; p < 0.0001), with an area under the receiveroperating characteristic curve of 0.734 in discriminating between these two nodal stages [37]. Yasui et al. also observed that the mean ADC value was significantly lower for metastatic nodes (1.36 × 10-3 mm2/s) than for nonmetastatic nodes (1.85 × 10-3 mm2/s) in primary rectal cancer with an accuracy of 75% [38].
Heijnen et al. showed that although the mean ADCs of the malignant and benign nodes did differ (1.15 ± 0.24 × 10-3 mm2/s vs 1.04 ± 0.22 × 10-3 mm2/s), this difference did not reach statistical significance (p = 0.10) and the area under the curve (AUC) for differentiation of malignant nodes was only moderate (0.64). Moreover, the signal intensity of benign and metastatic lymph nodes did not differ, resulting in AUCs of only 0.45−0.50. On the other hand, this study showed that the addition of DWI increased the number of detected nodes and may be beneficial in locating the nodes [39].
Similarly, Sassen et al. showed that when using visual analysis of MR images, DWI did not help in differentiating benign from malignant lymph nodes. In their study, all lymph nodes, metastatic or not, showed high-signal intensity on DWI [40].
From the analysis of these above-mentioned studies, it seems that, at present, DWI alone is not sufficiently accurate as a nodal staging tool for clinical decision-making in rectal cancer management.
MRF clearance
Recently, a Korean group evaluated the added value of DWI in combination with T2-weighted MRI compared with T2-weighted imaging alone for predicting tumor clearance of the MRF after neoadjuvant CRT in patients with locally advanced rectal cancer [41]. The study included 45 patients and key results showed that the diagnostic performance regarding prediction of tumor clearance of the MRF for two observers improved significantly after additional review of DW images: AUC improved from 0.770 to 0.918 (p = 0.017) for observer 1 and from 0.847 to 0.960 for observer 2 (p = 0.026). Diagnostic accuracy (observer 1: p < 0.001; observer 2: p = 0.022), sensitivity (observer 1: p < 0.001; observer 2: p = 0.002) and negative-predictive value (NPV; observer 1: p = 0.013; observer 2: p = 0.025) were significantly higher when both DW and T2-weighted images were evaluated than when T2-weighted images alone were reviewed for both observers. Most overstaged cases on T2-weighted images (82%) were attributed to iso- or hyper-intense masses abutting the MRF, corresponding to inflammation, fibrosis or abundant mucin components at histological examination. Understaging of tumor clearance was due to microscopic tumor cell infiltration into the MRF despite fat pads larger than 2 mm between the area of viable tumor signal intensity and the MRF at MRI [41].
Tumor invasion within the MRF appears hyperintense on DWI and hypointense on ADC maps because of the diffusion restriction of the motion of protons.
Therefore, these DWI features can help to differentiate neoplastic from non-neoplastic lesions, such as radiation-induced fibrosis and inflammation within the MRF, thus potentially improving the overall diagnostic accuracy of the prediction of tumor regression from the MRF after CRT in patients with rectal cancer. When DW images are used in combination with T2-weighted images, these serve as an anatomic reference for tumor location, which in turn leads to a more accurate assessment of the distance between viable tumor and the MRF, in spite of the comparatively low spatial resolution of the DW images alone [41].
Nodal downstaging
A Dutch group published their work on the use of DWI-MRI in restaging mesorectal lymph nodes after neoadjuvant CRT [42]. Signal intensities did not differ between benign and metastatic nodes and rendered an AUC of 0.64 (95% CI: 0.53–0.75) for reader 1 and 0.52 (95% CI: 0.40–0.64) for reader 2. The AUC for detection of metastatic nodes was 0.66 using ADC values. The optimal ADC threshold was 1.25 × 10-3 mm2/s, resulting in a sensitivity of 53%, a specificity of 82%, a positive-predictive value (PPV) of 35% and a NPV of 91% [42].
The predicted probability for the combined assessment of T2-weighted MRI plus ADC rendered an AUC of 0.91 for reader 1 and 0.96 for reader 2, which resulted in a sensitivity of 56%, a specificity of 98%, a PPV of 83% and a NPV of 92% for reader 1. These values were 56, 99, 95 and 93% for reader 2, respectively. The diagnostic performance when using ADC only was significantly lower than for T2-weighted MRI (p = 0.02 and p = 0.0003 for readers 1 and 2, respectively) and T2-weighted MRI plus ADC combined (p = 0.001 and p < 0.0001 for readers 1 and 2, respectively). There was no significant difference in diagnostic performance between T2-weighted MRI and the combination of T2-weighted MRI plus ADC (p = 0.17 and p = 0.26, respectively). ADC combined with standard T2-weighted MRI improved the diagnostic performance without, however, accomplishing a significant improvement compared with T2-weighted MRI alone [42].
Interestingly, although it did not improve the overall performance, the addition of ADC to standard T2-weighted MRI did improve the PPV from 60–61 to 83–95%, thus reducing overstaging errors. The foremost advantage from the addition of DWI in this study was the higher number of detected nodes compared with conventional T2-weighted MRI. On DWI, high-signal intensity nodes were more straightforwardly detected against the suppressed background signal of the neighboring tissues. According to the authors, DWI can thus be used to immediately focus a radiologist’s eye on the presence of nodes and their location [42]. Moreover, when radiologists evaluating post-CRT examinations will become able to provide an imaging tool for the selection of patients with truly sterilized nodes, patients with a small tumor remnant limited to the rectal wall (ypT1-2N0) may be safely stratified for local excision, while patients with a CR (ypT0N0) could be included in a ‘wait-and-see’ policy with deferral from surgery [42–45].
Assessment of CR
The introduction of preoperative, rather than postoperative, CRT has led to a decline in local recurrence rates and has become standard of care for patients with locally advanced rectal cancer [46]. If CRT is chosen for a patient with locally advanced rectal cancer, the patient is usually scheduled for surgery after completion of it, but in 10–24% of patients, no residual tumor is found at histology of the surgical specimen [14].
These CRs have been shown to have a very good prognosis, in terms of both overall and disease-free survival [14]. A CR also raises the highly debated and controversial question of whether surgery is still required for these patients, particularly because total mesorectal excision may have associated morbidity and even mortality, and also has the potential risk of a permanent colostomy. Recently, a more conservative treatment was advocated in patients who displayed a good or complete response to neoadjuvant CRT; in 2006, Habr-Gama et al. presented the long-term results of a prospective trial that investigated a ‘wait-and-see’ policy in a carefully selected group of patients with clinical and radiological evidence of a CR after neoadjuvant CRT [45]. Results at 5-year follow-up were favorable for the nonsurgical group, with an overall and diseasefree survival of 93 and 85%, respectively [45]. However, in order to securely suggest such a deferral from surgery, it is essential to accurately select the correct candidates – the true CRs.
The role of imaging for restaging rectal cancer after CRT has been the subject of a number of studies and all suggest that neither MRI nor endorectal ultrasound or PET are sufficiently accurate for identifying the true CRs, with PPVs ranging from 17 to 50% [26,47–51].
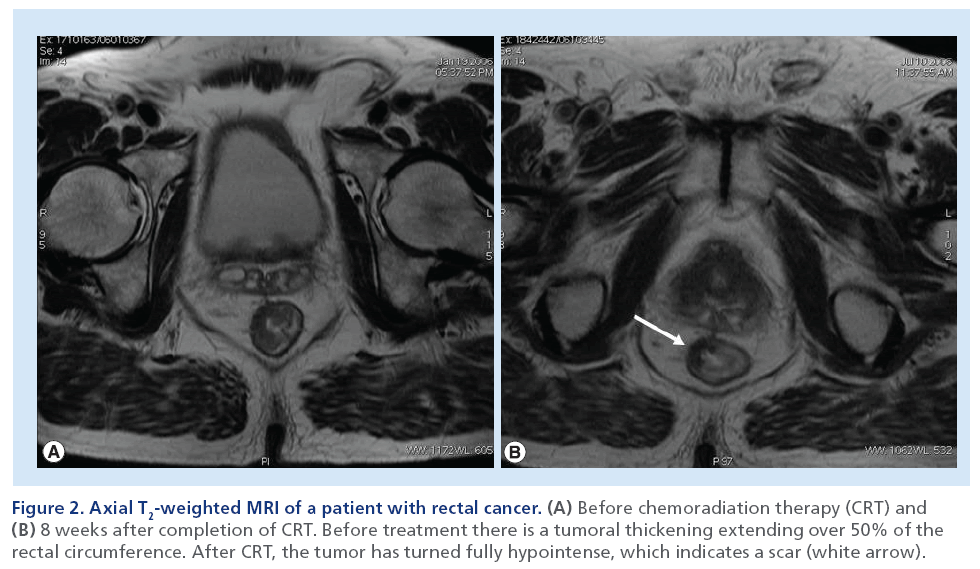
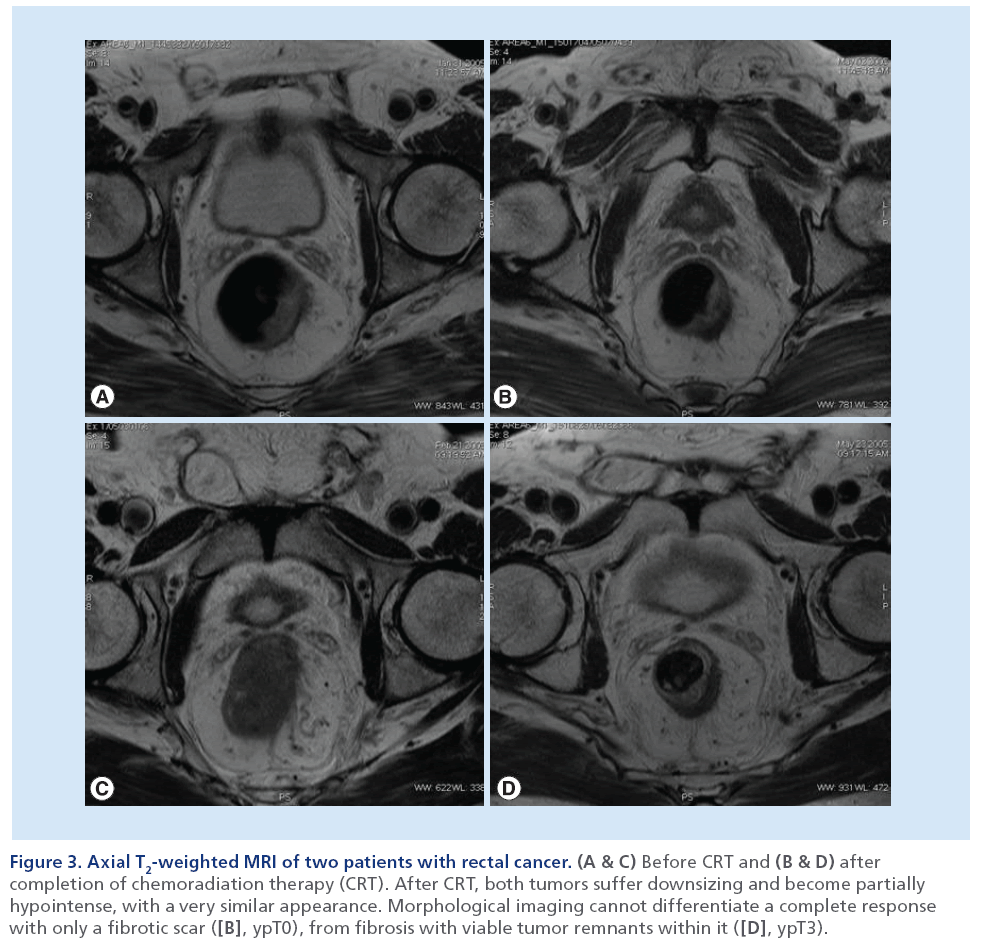
In fact, when MRI is performed 4–6 weeks after the completion of preoperative CRT for locally advanced rectal cancer, it is seldom normal, even in patients who will demonstrate a pCR at histological examination after surgery. Rather, in the majority of patients with an optimal response at MRI, a scar – represented by a focal area of low-signal intensity on T2-weighted MRI – replaces the site of disease (Figure 2). The precise cellular composition of such an area of low-signal intensity cannot be known, and a single MRI scan may not be able to diagnose CR. In fact, the major component of error on MRI is overstaging due to its limited capability to allow differentiation between viable tumor, residual fibrotic nontumor tissue and desmoplastic reaction (Figure 3) [18,52–53]. However, if surgery is deferred, then the scar can be followed with serial MRI examinations to monitor any change in size, morphology or signal intensity.
Figure 2: Axial T2-weighted MRI of a patient with rectal cancer. (A) Before chemoradiation therapy (CRT) and (B) 8 weeks after completion of CRT. Before treatment there is a tumoral thickening extending over 50% of the rectal circumference. After CRT, the tumor has turned fully hypointense, which indicates a scar (white arrow).
Figure 3: Axial T2-weighted MRI of two patients with rectal cancer. (A & C) Before CRT and (B & D) after completion of chemoradiation therapy (CRT). After CRT, both tumors suffer downsizing and become partially hypointense, with a very similar appearance. Morphological imaging cannot differentiate a complete response with only a fibrotic scar ([B], ypT0), from fibrosis with viable tumor remnants within it ([D], ypT3).
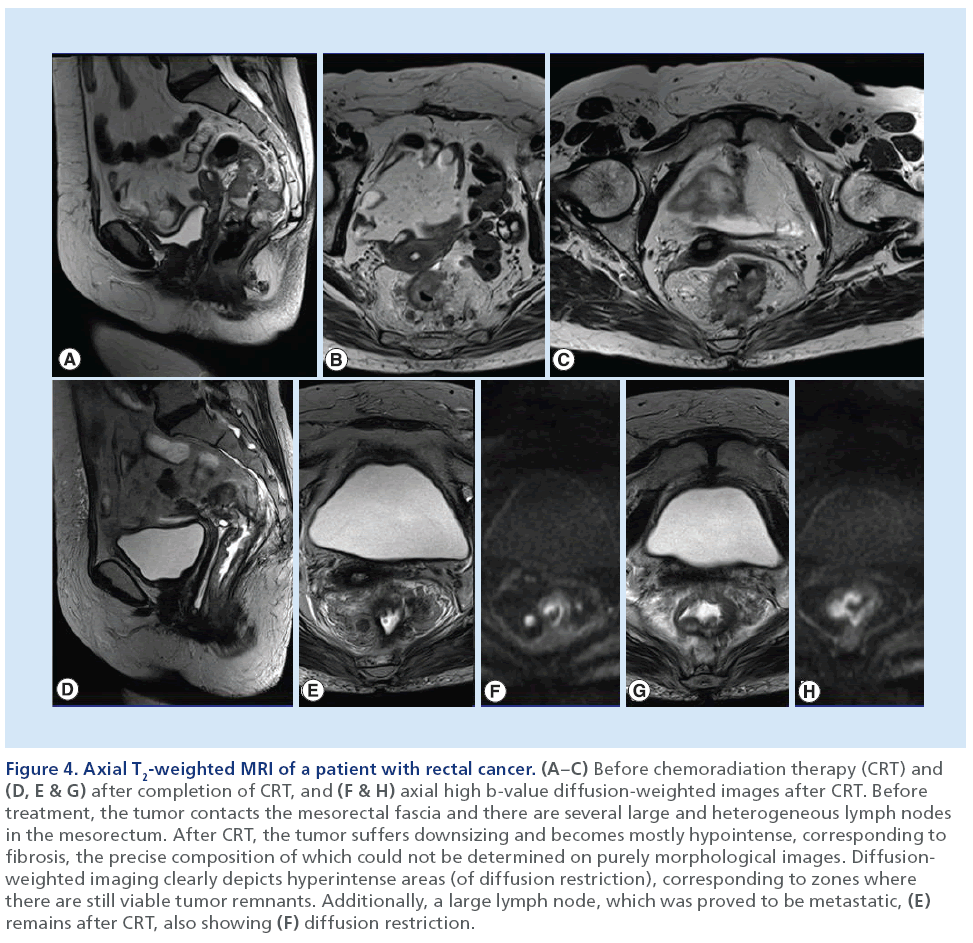
Owing to the limitations of purely morphological MRI, recent attention has been directed toward DWIMRI as a complement to standard morphological MRI for detection of CRs, because on DWI, viable tumor remnants are recognized as hyperintense foci compared with the low-signal intensity of the surrounding non-neoplastic background tissue (Figure 4) [26,27].
Figure 4: Axial T2-weighted MRI of a patient with rectal cancer. (A–C) Before chemoradiation therapy (CRT) and (D, E & G) after completion of CRT, and (F & H) axial high b-value diffusion-weighted images after CRT. Before treatment, the tumor contacts the mesorectal fascia and there are several large and heterogeneous lymph nodes in the mesorectum. After CRT, the tumor suffers downsizing and becomes mostly hypointense, corresponding to fibrosis, the precise composition of which could not be determined on purely morphological images. Diffusionweighted imaging clearly depicts hyperintense areas (of diffusion restriction), corresponding to zones where there are still viable tumor remnants. Additionally, a large lymph node, which was proved to be metastatic, (E) remains after CRT, also showing (F) diffusion restriction.
A previous study by Kim et al., including 40 patients, demonstrated that DWI, in addition to standard MRI, significantly improved the performance of radiologists to select CRs compared with standard MRI only [26].
In the same way, Lambregts et al., in a retrospective multicenter study of 120 patients, indicated that the diagnostic performance for predicting a pCR after CRT improved for the combination of standard MRI plus DWI (AUC: 0.78–0.8) compared with standard MRI only (AUC: 0.58–0.76). Moreover, it resulted in a substantial decrease in the number of equivocal scores and an improved interobserver agreement [27]. In this study, the superior sensitivity for the combination of MRI plus DWI resulted in less overestimation of residual tumor in patients with a pCR [27]. On restaging MRI without DWI, many interpretation difficulties were observed when the primary tumor bed had become ‘fibrotic’ as a result of the neoadjuvant treatment. In these cases, as previously mentioned, it becomes hard to differentiate small areas of residual tumor from simple fibrosis, and readers tend to overestimate the presence of tumor [47,49–51]. In this particular context, the functional information from DWI may be valuable; areas of fibrosis typically have low cellular density, which results in low-signal intensity on high b-value diffusion images [54]. On the other hand, areas of residual tumor have a relatively high cellular density and demonstrate high-signal intensity on DWI, easily recognizable within the low-signal intensity of the surrounding tissue/fibrosis, thus allowing a better depiction of small areas of residual tumor on DWI (Figure 5) [26,54].
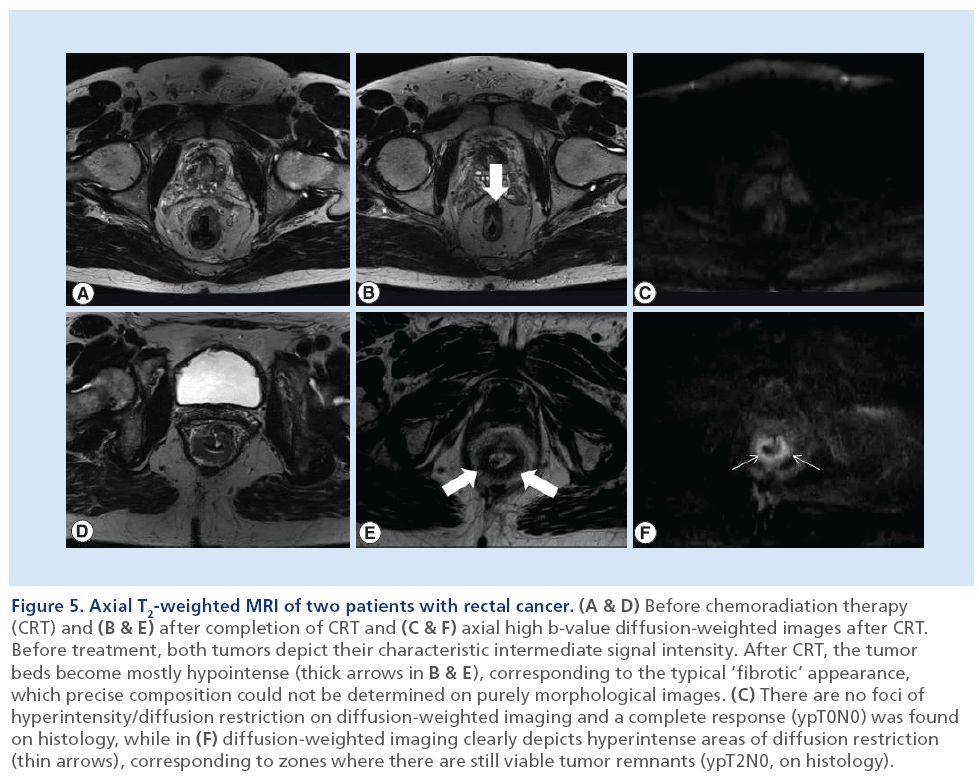
Figure 5: Axial T2-weighted MRI of two patients with rectal cancer. (A & D) Before chemoradiation therapy (CRT) and (B & E) after completion of CRT and (C & F) axial high b-value diffusion-weighted images after CRT. Before treatment, both tumors depict their characteristic intermediate signal intensity. After CRT, the tumor beds become mostly hypointense (thick arrows in B & E), corresponding to the typical ‘fibrotic’ appearance, which precise composition could not be determined on purely morphological images. (C) There are no foci of hyperintensity/diffusion restriction on diffusion-weighted imaging and a complete response (ypT0N0) was found on histology, while in (F) diffusion-weighted imaging clearly depicts hyperintense areas of diffusion restriction (thin arrows), corresponding to zones where there are still viable tumor remnants (ypT2N0, on histology).
This study reported a specificity for MRI and DWI superior to 90%, indicating that residual tumor is accurately detected and the risk for undertreatment will be less than 10% [27]. Although DWI allows detection of even small tumor volumes, the detection of microscopically small clusters of residual tumor cells, which are difficult to detect – even at histology – and are currently beyond the detection threshold of any available imaging modality (including DWI), will remain the major challenge for imaging.
In a retrospective study of 50 patients performed by Song et al., an increased diagnostic accuracy was reported for the two readers from 64–76 to 86–90% after addition of DWI-MRI to T2-weighted MRI. Sensitivity improved from 64–77 to 91–98%, with corresponding specificities of 67 to 33–50% [55]. Another Dutch group performed a retrospective analysis of 70 patients who underwent CRT followed by restaging MRI and resection [40]. Two readers with different experience levels independently scored T2 images for CR and, in a second reading, combined T2 and DWI. They demonstrated that, in agreement with the previously cited works, the interobserver agreement for the identification of CRs increased after addition of DWI from 0.35 to 0.58, and sensitivity and NPV improved from 20–30 to 40–70% and 88 to 91–95%, respectively. However, a more pronounced benefit was found for the experienced reader; the AUC (0.77–0.89; p = 0.005 vs 0.74–0.70; p > 0.05), as well as specificity and PPV improved only for him (87–93% and 27–63%, respectively).
Another approach to this subject was performed in a recent study that tried to predict CR using both conventional MRI-based and DWI-based volumetric analyses [56]. The authors showed that post-CRT DW-MR volumetry provided high diagnostic performance (AUC: 0.93) for the assessment of a CR and was significantly more accurate than post-CRT T2-weighted MR volumetry (AUC: 0.70) or post-CRT ADC (AUC: 0.54). Pre-CRT DWI-MRI and T2-weighted MR volumetry, as well as ADC, were not sufficiently reliable to identify a CR, with AUCs ranging between 0.51 and 0.63 [56].
Apparently, the tumor volumes determined on the basis of the presence (or absence) of high-signal intensity areas on DW-MRI better represented the existence of residual viable tumor. The measurement of volumes on morphological post-CRT MRI is a more complex task as it is difficult to characterize which of the fibrotic areas are still suspicious for the presence of residual tumor and should be incorporated into the volume measurements and which should not. Those problems were less obvious on DW-MRI, in which the delineation of residual tumor was more clear cut [56].
Given the high diagnostic performance of post-CRT DW-MR volumetry on the basis of signal perception on images with b = 1000 s/mm2, the authors hypothesized that a visual evaluation of the presence of a highsignal intensity area suggestive of residual tumor will be sufficient, and volumetric measurements are not even required. Such a visual approach would also be more practical and far less time consuming [56].
A high AUC (0.93) for the assessment of a CR with DW-MRI was found, and this was even higher than in the above-mentioned previous studies [26,27], which have already shown good results for a visual analysis of DW-MRI. A possible explanation for the better results for this study could be that the DW-MRI were evaluated independently from T2-weighted MRI and with objective volume measurements, whereas in the above-mentioned works, T2-weighted and DW-MRI were read side by side and by means of subjective interpretation. For example, if on the basis of the T2-weighted MRI morphlogical findings, a radiologist has already determined a strong suspicion of residual tumor, they are unlikely to be eager to alter the diagnosis even if the DW-MRI would demonstrate the contrary. This factor, together with the knowledge that, in an oncological context, when in doubt one should better err on the ‘safe’ side and should best diagnose a patient as having residual disease than to potentially incorrectly categorize that patient as having a CR, might have incorporated some bias in the evaluation of DW-MRI in the published literature. If the definition of a pCR on a DW-MRI is solely based on the absolute absence of hyperintense areas within the rectal wall and the DW-MRI are being evaluated independently from the T2-weighted images, then this bias can potentially be eliminated [56].
Ha et al. corroborated these findings and demonstrated, in a retrospective study with 100 patients, that DW-MR tumor volumetry after CRT showed significant superiority in predicting CR compared with T2-weighted MRI (AUC: 0.910 vs 0.792, respectively; p = 0.015). Using a cut-off value for the tumor volume reduction rate of more than 86.8% on DW-MRI, the sensitivity and specificity for predicting CR were 91.4 and 80%, respectively. They also showed that the mean post-CRT ADC of the CR group was significantly higher than that of the non-CR group, but there was a limitation to its use in clinical practice to assess CR because of the low diagnostic accuracy (67%) [57]. In addition, Genovesi et al. showed that the mean post- CRT ADC values in the CR group (1.79 ± 0.51 × 10-3 mm2/s) were significantly higher than that in non-CR group (1.373 ± 0.432 × 10-3 mm2/s; p = 0.003) [57]. On the contrary, Intgen et al. were not able to confirm the predictive potential of postradiochemotherapy ADC for pCR [58].
In the assessment of CR, DWI images should be interpreted with caution, as some problems remain. DWI, in the same way conventional imaging does, is unable to detect microscopic foci of residual tumor that are beyond the detection level of any imaging method, leading to false-positive results for a complete tumor response. It was also shown that CRs can show areas of high-signal intensity on DWI that may be erroneously interpreted as residual tumor. This may happen in areas of diffuse fibrosis (combined with chronic inflammation) and in mucinous lakes. Particularly important in image interpretation are artefacts that occur at interfaces of air-rectal wall and in collapsed rectal wall, which constitute a well-known pitfall that may hamper the identification of CRs. Although, it is plausible that the recognition and understanding of these problems may enhance the diagnostic performance of DWI, but this has to be proven in further studies.
Prediction of response before CRT
Previous studies have investigated the value of pretreatment tumor ADC as a prognostic factor in terms of prediction of response to CRT in the specific subgroup of patients with locally advanced rectal cancer.
Some have shown that tumors with low baseline pretreatment ADC values responded better to chemotherapy or radiation therapy than neoplasms that exhibited high pretreatment ADC values [13,20]. Sun et al. [29] observed that the mean pre-CRT ADC value (1.07 ± 0.13 × 10-3 mm2/s) in the group of tumors that showed T-downstaging (17 out of 37 patients) was lower (1.19 ± 0.15 × 10-3 mm2/s) than that in the T-nondownstaged group (p = 0.013). Similarly, a Dutch group [58] showed for low preradiochemotherapy ADC a correlation with pathological good response. In a study on a 3 T magnet, Jung et al. [59] also demonstrated that pre-CRT ADC of the histopathologic responders was significantly lower than that of the histopathologic nonresponders (p = 0.034). One possible explanation for this is that tumors with high pretreatment ADC values are likely to exhibit more necrotic areas than those with low values [21]. Necrotic tumors are frequently hypoxic, acidic and poorly perfused, which leads to reduced sensitivity to chemotherapy and radiation therapy.
However, other studies have failed to replicate those results. Kim and collaborators [28] could not reliably discriminate pCR from non-pCR based on the pre-CRT ADC, and Heo and co-authors [60] also reported that the pre-CRT ADC value was not significantly correlated with tumor regression grade after the analysis of 39 patients. Another recent study also failed to demonstrate a benefit for pre-CRT ADC, post-CRT ADC or ΔADC measurements to differentiate between patients with a CR and residual tumor [56]. According to Monguzzi et al. [61], no differences in ADC pretreatment measurements were observed between responders and nonresponders. This was also found in other studies [57,62].
There is even a recent study that showed that the mean pretreatment ADC value in responders was higher than the value in nonresponders; however, this was only marginally significant (p = 0.035) [36].
These differences may be attributable, at least partially, to the distinct definitions of response; some authors used the tumor size (50% reduction) as a criterion, while others predefined responders as the T-downstaged group and others considered pCR as the end point for response.
Another explanation could be that ADC measurements are more subject to measuring errors because of the inherently low discriminatory power and lesion conspicuity on ADC images. Even subtle variations in region of interest size and region of interest positioning between two readers may result in substantial variations in ADC. This phenomenon may have contributed to the low performance of ADC in some studies to precisely distinguish between CRs and non-CRs [56]. This factor is less an issue when the response groups are more roughly categorized in ‘responding’ and ‘nonresponding’ patient groups [13,29–31,63]. Obviously, such large subcategories will require less precise discrimination methods and is a possible reason why those published data have shown more favorable results for ADC.
The reproducibility of DWI has been insufficiently investigated, and the cut-off values used to determine treatment response vary between treatments and ADC measurement techniques. Thus, a standardized guideline to predict or assess treatment response is needed before DWI can be implemented in clinical practice.
At present, additional multicentric studies using large populations are warranted to assess the diagnostic potential of DWI to preoperatively identify those patients with rectal cancer who may benefit from a less aggressive therapeutic approach after CRT.
Assessment of response during CRT
Sun et al. [29] showed that at the end of the first week of CRT, the mean tumor ADC increased significantly from 1.07 × 10-3 to 1.32 × 10-3 mm2/s (F = 37.63; p < 0.001) in the downstaged group, but there was no significant ADC increase in the nondownstaged group (F = 1.18; p = 0.291). It is believed that increases in ADC are a consequence of cellular damage leading to necrosis [64,65]. Another reason for the increase in ADC seen within 1 week of CRT is tumor edema caused by the massive release of VEGF within hours of even the first fraction of radiation therapy. That would lead to increased vascular permeability and increased interstitial volume, which would in turn increase ADC [29]. The mean percentage of tumor ADC change in the downstaged group was significantly higher than that in the nondownstaged group at each time point (F = 18.39; p < 0.001). This phenomenon may be explained by a higher degree of cellular necrosis achieved with CRT in the downstaged group than in the nondownstaged group. Therefore, the difference of increase in the ADC after the beginning of CRT mainly reflected the different sensitivity of the tumor cells to CRT in the two groups [29].
Similarly, a study by Cai et al. [66] demonstrated a significant increase in the mean ADC at the second and fifth week during treatment, relative to the values prior to treatment, in the T-downstaged and tumor regression groups. In addition, Barbaro et al. [67] showed that at the end of the second week of CRT, the mean percentage of change in the tumor ADCs in the downstaged group was greater than that in the nondownstaged group (p < 0.0001). When the cut-off value for the percentage of ADC increase was >23%, the PPV was 85.2%, the NPV was 60% and the accuracy for response assessment was 70.9%.
In this way, these authors suggest that early temporal changes in ADC and pretherapy ADC can potentially discriminate patients with locally advanced rectal cancers that are resistant to preoperative CRT, which may allow a prompt modification of the treatment protocols [29,66].
Conclusion
In rectal cancer, tumor stage at diagnosis is a guide to treatment strategies. As such, while patients with early cancers usually may achieve cure through surgery alone, those with locally advanced cancers typically undergo preoperative therapy, which is useful for decreasing the tumor stage in order to facilitate curative resection, and to decrease the local recurrence rate. Therefore, the role of the radiologist is to identify tumors within these groups, so that a tailored treatment can be offered to each single patient in order to decrease the probability of local recurrence.
In recent years, a paradigm shift toward less invasive treatments has been witnessed, including local excision or even a – still controversial – deferral from surgery in those patients achieving a CR from the tumor following preoperative CRT.
However, no imaging techniques currently allow an accurate prediction of which tumors will respond satisfactorily to this kind of treatment, and which cases develop a CR. This is particularly true when using purely morphological imaging methods, and consequently, there has been a growing interest in more ‘functional’ imaging techniques, such as DW-MRI.
MRI is widely used for the diagnosis and staging of tumors, whereby mainly morphometric macroscopic tissue information is usually obtained. For the assessment of viability and aggressiveness of the tumor or its response to therapy, a method that gives insights at a cellular level would be desirable. DW-MRI provides images whose signal intensity is sensitized to the random motion of free water molecules. The mobility of water molecules within a given voxel is determined by the microscopic cellular structure (i.e., the presence of barriers, such as cell membranes and macromolecules). Thus, DWI offers a theoretical possibility for the assessment of viability of the tumor or its response to therapy.
DWI has the potential to become an imaging biomarker in rectal cancer. DWI-MRI (including volumetry studies) may help to assess response to neoadjuvant therapy and particularly the presence of complete tumoral response.
Future perspective
The role of DWI-MRI as a biomarker of aggressiveness and response needs still to be validated in multicentric studies. However, as an adjunct to clinical tools (digital examination, endoscopy and biopsy), the use of DWIMRI seems promising to enable a more precise selection of patients eligible to undergo minimally invasive treatments. The results published so far are obviously still premature for clinical decision-making, but its promise warrants further large and prospective patient studies.
Financial & competing interests disclosure
No writing assistance was utilized in the production of this manuscript.
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Jass JR, Love SB. Prognostic value of direct spread in Dukes’ C cases of rectal cancer. Dis. Colon Rectum 32, 477–480 (1989).
- Willett CG, Badizadegan K, Ancukiewicz M et al. Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis. Colon Rectum 42, 167–173 (1999).
- Wolmark N, Fisher B, Wieand HS. The prognostic value of the modifications of the Dukes’ C class of colorectal cancer: an analysis of the NSABP clinical trials. Ann. Surg. 203, 115–122 (1986).
- Tang R, Wang JY, Chen JS et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J. Am. Coll. Surg. 180, 705–712 (1995).
- Adam IJ, Mohamdee MO, Martin IG et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 344, 707–711 (1994).
- Beets-Tan RG, Beets GL, Vliegen RF et al. Accuracy of magnetic resonance imaging in prediction of tumor-free resection margin in rectal cancer surgery. Lancet 357, 497–504 (2001).
- Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br. J. Surg. 90, 355–364 (2003).
- Du CZ, Xue WC, Cai Y, Li M, Gu J. Lymphovascular invasion in rectal cancer following neoadjuvant radiotherapy: a retrospective cohort study. World J. Gastroenterol. 15, 3793–3798 (2009).
- Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDGPET/ CT. Mol. Imaging Biol. 13, 1020–1028 (2011).
- Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J. Surg. Oncol. 101, 396–400 (2010).
- Barrett MW. Chemoradiation for rectal cancer: current methods. Semin. Surg. Oncol. 15, 114–119 (1998).
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 358, 1291–1304 (2001).
- Kremser C, Judmaier W, Hein P, Griebel J, Lukas P, DeVries A. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther. Onkol. 179, 641–649 (2003).
- Maas M, Nelemans P, Valentini V et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 11, 835–844 (2010).
- Bujko K, Kepka L, Nowacki MP. Chemoradiotherapy alone for rectal cancer: a word of caution [letter]. Lancet Oncol. 8(10), 860–862 (2007).
- O’Neill BD, Brown G, Heald RJ, Cunningham D, Tait DM. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 8(7), 625–633 (2007).
- Barbaro B, Vitale R, Leccisotti L et al. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. RadioGraphics 30(3), 699–716 (2010).
- Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis. Colon Rectum 48(4), 722–728 (2005).
- Huh JW, Park YA, Jung EJ, Lee KY, Sohn SK. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J. Am. Coll. Surg. 207(1), 7–12 (2008).
- Koh DM, Padhani AR. Diffusion-weighted MRI: a new functional clinical technique for tumor imaging. Br. J. Radiol. 79, 633–635 (2006).
- Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am. J. Roentgenol. 188, 1622–1635 (2007). l General overview of diffusion-weighted imaging (DWI)- MRI in oncology.
- Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI – a potential new biomarker of response to cancer therapy. Nat. Clin. Pract. Oncol. 5, 220–233 (2008).
- Padhani AR, Liu G, Koh DM et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11, 102–125 (2009).
- DeSouza NM, Riches SF, Vanas NJ et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin. Radiol. 63(7), 774–782 (2008).
- Lambrecht M, Deroose C, Roels S et al. The use of FDGPET/ CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 49, 956–963 (2010).
- Kim SH, Lee JM, Hong SH et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemoand radiation therapy. Radiology 253, 116–125 (2009).
- Lambregts DM, Vandecaveye V, Barbaro B et al. Diffusionweighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann. Surg. Oncol. 18(8), 2224–2231 (2011).
- Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer Eur. Radiol. 21, 987–995 (2011).
- Sun YS, Zhang XP, Tang L et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusionweighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254, 170–178 (2010).
- Dzik-Jurasz A, Domenig C, George M et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360, 307–308 (2002).
- DeVries AF, Kremser C, Hein PA et al. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 56, 958–965 (2003).
- Ho ML, Liu J, Narra V. Magnetic resonance imaging of rectal cancer. Clin. Colon Rectal Surg. 21, 178–187 (2008).
- Lahaye MJ, Engelen SM, Nelemans PJ et al. Imaging for predicting the risk factors – the circumferential resection margin and nodal disease of local recurrence in rectal cancer: a meta-analysis. Semin. Ultrasound CT MR 26(4), 259–268 (2005).
- Wieder HA, Rosenberg R, Lordick F et al. Rectal cancer: MR imaging before neoadjuvant chemotherapy and radiation therapy for prediction of tumor-free circumferential resection margins and long-term survival. Radiology 243, 744–751 (2007).
- Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J. Magn. Reson. Imaging 35(6), 1365–1371 (2012).
- Elmi A, Hedgire SS, Covarrubias D, Abtahi SM, Hahn PF, Harisinghani M. Apparent diffusion coefficient as a non-invasive predictor of treatment response and recurrence in locally advanced rectal cancer. Clin. Radiol. 68(10), e524–e531 (2013).
- Cho EY, Kim SH, Yoon JH et al. Apparent diffusion coefficient for discriminating metastatic from nonmetastatic lymph nodes in primary rectal cancer. Eur. J. Radiol. 82(11), e662–e668 (2013).
- Yasui O, Sato M, Kamada A. Diffusion-weighted imaging in the detection of lymph node metastasis in colorectal cancer. Tohoku J. Exp. Med. 218(3), 177–183 (2009).
- Heijnen LA, Lambregts DM, Mondal D et al. Diffusionweighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur. Radiol. 23(12), 3354–3360 (2013).
- Sassen S, de Booij M, Sosef M et al. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur. Radiol. 23, 3440–3449 (2013).
- Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 260(3), 771–780 (2011).
- Lambregts DM, Maas M, Riedl RG et al. Value of ADC measurements for nodal staging after chemoradiation in locally advanced rectal cancer – a per lesion validation study. Eur. Radiol. 21(2), 265–273 (2011).
- Lezoche G, Baldarelli M, Guerrieri M et al. A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg. Endosc. 22, 352–358 (2008).
- Borschitz T, Wachtlin D, Möhler M et al. Neoadjuvant chemoradiation and local excision for T2–3 rectal cancer. Ann. Surg. Oncol. 15, 712–720 (2008).
- Habr-Gama A, Perez RO, Proscurshim I et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J. Gastrointest. Surg. 10, 1319–1328 (2006).
- Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 351, 1731–1740 (2004).
- Janssen MH, Ollers MC, Riedl RG et al. Accurate prediction of pathological rectal tumor response after two weeks of preoperative radiochemotherapy using (18) F-fluorodeoxyglucose-positron emission tomographycomputed tomography imaging. Int. J. Radiat. Oncol. Biol. Phys. 77, 392–329 (2010).
- Kristiansen C, Loft A, Berthelsen AK et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis. Colon Rectum 51, 21–25 (2008).
- Suppiah A, Hunter IA, Cowley J et al. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Colorectal Dis. 11, 249–253 (2009).
- Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am. J. Gastroenterol. 99, 109–112 (2004).
- Capirci C, Rubello D, Chierichetti F et al. Restaging after neoadjuvant chemoradiotherapy for rectal adenocarcinoma: role of F18-FDG PET. Biomed. Pharmacother. 58, 451–457 (2004).
- Kuo LJ, Chern MC, Tsou MH et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis. Colon Rectum 48(1), 23–28 (2005).
- Valentini V, Coco C, Cellini N et al. Preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation. Int. J. Radiat. Oncol. Biol. Phys. 40(5), 1067–1075 (1998).
- Vandecaveye V, De Keyzer F, Nuyts S et al. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int. J. Radiat. Oncol. Biol. Phys. 67, 960–971 (2007).
- Song I, Kim SH, Lee SJ, Choi JY, Kim MJ, Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br. J. Radiol. 85(1013), 577–586 (2013).
- Curvo-Semedo L, Lambregts DM, Maas M et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapyconventional MR volumetry versus diffusion-weighted MR imaging. Radiology 260(3), 734–743 (2011).
- Ha HI, Kim AY, Yu CS, Park SH, Ha HK. Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur. Radiol. 23(12), 3345–3353 (2013).
- Intven M, Reerink O, Philippens ME. Diffusion-weighted MRI in locally advanced rectal cancer: pathological response prediction after neo-adjuvant radiochemotherapy. Strahlenther. Onkol. 189(2), 117–122 (2013).
- Jung SH, Heo SH, Kim JW et al. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J. Magn. Reson. Imaging 35(1), 110–116 (2012).
- Heo S, Jeong S, Young J et al. A comparative study of histopathologic parameters and apparent diffusion coefficient values on 3T rectal MRI in locally advanced rectal cancer following neoadjuvant chemoradiation therapy. Presented at: European Congress of Radiology (ECR) Annual Meeting Program Vienna, Austria, 4–8 March 2010.
- Monguzzi L, Ippolito D, Bernasconi DP, Trattenero C, Galimberti S, Sironi S. Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur. J. Radiol. 82(2), 234–240 (2013).
- Genovesi D, Filippone A, Ausili Cèfaro G et al. Diffusionweighted magnetic resonance for prediction of response after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: preliminary results of a monoinstitutional prospective study. Eur. J. Surg. Oncol. 39(10), 1071–1078 (2013).
- Hein PA, Kremser C, Judmaier W et al. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur. J. Radiol. 45, 214–222 (2003).
- Thoeny HC, De Keyzer F, Chen F et al. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology 234(3), 756–764 (2005).
- Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin. Cancer Res. 3(9), 1457–1466 (1997).
- Cai G, Xu Y, Zhu J et al. Diffusion-weighted magnetic resonance imaging for predicting the response of rectal cancer to neoadjuvant concurrent chemoradiation. World J. Gastroenterol. 19(33), 5520–5527 (2013).
- Barbaro B, Vitale R, Valentini V et al. Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 83(2), 594–599 (2012).
- Jonas J, Bahr R. Neoadjuvant chemoradiation treatment impairs accuracy of MRI staging in rectal carcinoma. Gut 55, 1214–1215 (2006).
- Barbaro B, Fiorucci C, Tebala C et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 250, 730–739 (2009).
- Dresen RC, Beets GL, Rutten HJ et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 252, 71–80 (2009).
• General overview of the role of DWI as a biomarker in the oncologic setting.
• • Multicentric study on the role of DWI-MRI for assessing complete response.
• • Prospective study assessing the role of DWI and apparent diffusion coefficient (ADC) as biomarkers of response to chemoradiation therapy (CRT) before and during therapy.
• • Study on the role of DWI-MRI for assessment of tumor regression from the mesorectal fascia after CRT.
• • Study on the role of ADC for nodal restaging after neoadjuvant CRT.