Review Article - Imaging in Medicine (2011) Volume 3, Issue 5
Thyroid nodule sonography: assessment for risk of malignancy
Jill E Langer† and Susan J Mandel
University of Pennsylvania School of Medicine, Division of Endocrinology, Diabetes & Metabolism, 700 CRB, 415 Curie Blvd, Philadelphia, PA 19003, USA
- *Corresponding Author:
- Jill E Langer
University of Pennsylvania
Department of Radiology, 3400 Spruce Street
Philadelphia, PA 19104, USA
Tel: +1 215 662 7012
Fax:+1 215 349 5627
E-mail: jill.langer@uphs.upenn.edu
Abstract
Fine-needle aspiration (FNA) is the most reliable diagnostic tool for diagnosing thyroid cancer and should be performed on nodules considered suspicious. Traditionally, thyroid nodules were discovered by physical examination of the neck and underwent palpation-guided FNA in the physician’s office when malignancy was of concern. In recent years, the use of high-resolution neck sonography to examine the thyroid has resulted in the detection of many nonpalpable nodules. Although the majority of these nodules are benign, their discovery results in the need to determine if FNA should be performed on one or more of these nodules. The goal is to be able to diagnose all biologically significant cancers, while exposing the fewest number of patients with benign disease to unnecessary diagnostic testing. Sonography of the neck has emerged as the primary imaging means to evaluate the malignant potential of thyroid nodules. Although no single sonographic feature can be used to detect all cancerous nodules, some sonographic features such as calcifications, marked hypoechogenicity, infiltrative margins and ‘taller-than-wide’ shape are relatively specific for thyroid cancer. By using sonographic criteria to stratify risk of malignancy, invasive and costly evaluations can be avoided in many patients without missing the minority of patients who have a thyroid malignancy.
Keywords
thyroid cancer; thyroid; odule; ultrasound
Thyroid nodules are commonly noted in adults. The prevalence of palpable thyroid nodules is estimated to be 6.4% in women and 1.5% in men between 30 to 60 years of age, living in iodine-sufficient regions [1]. Imaging techniques, particularly high resolution sonography of the neck, have been shown to detect nodules with much higher frequency than physical examination of the thyroid. On ultrasound (US), nodules are detected in 19–67% of randomly selected adults, with detection rates greater in women and increasing with age for both genders [2,3]. The clinical dilemma is to determine which of these additional, nonpalpable nodules detected by imaging should undergo fine-needle aspiration (FNA) biopsy. FNA is still considered the most reliable diagnostic test to determine if a thyroid nodule is malignant. Although the incidence and prevalence of thyroid cancer is increasing, it remains a relatively uncommon cancer with approximately 46,000 new cases diagnosed annually in the US. Most clinicians have a low threshold for performing a FNA on nodules detected in patients who are at increased risk for the development of thyroid cancer. At risk patients include those who were exposed to whole body or neck irradiation as a child and those with hereditary syndromes, such as Cowden’ syndrome, MEN syndrome or a strong family history of thyroid cancer. However, because of the high prevalence of thyroid nodules, it is not cost effective to perform FNA on all detected nodules in low risk patients to exclude a malignancy. In the general population, most nonpalpable nodules are small, measuring under 1 cm and the vast majority of all incidentally detected nodules represent benign lesions. The diagnosis of thyroid malignancy is also confounded by the knowledge that many thyroids harbor malignant nodules under 1 cm, termed microcarcinomas by the WHO. The majority of these microcarcinomas lack the potential to become biologically significant and have an indolent course. However, a small percentage of these small cancers do exhibit aggressive behavior and may be invasive and metastasize. The ultimate goal is to diagnose biologically significant cancers, while exposing the fewest number of patients with benign disease or indolent malignant disease to FNA, unnecessary diagnostic testing and potentially thyroid surgery.
Prior to the advent of the widespread adoption of diagnostic thyroid US, decisions about which nodules to submit to FNA were based upon size criteria determined by physical examination. Traditionally, euthyroid patients with palpable nodules over 1 cm underwent palpation-guided biopsy as the initial diagnostic step for evaluation of their palpable lesion. As more patients with suspected nodular disease underwent thyroid sonography prior to, or contemporaneously, with FNA, it became evident that sonographic evaluation surpassed physical examination of the thyroid for nodule detection and documentation of nodule size [2–4]. In one study, 44% of patients referred for a FNA of what was thought to be a solitary nodule on physical examination, had additional nonpalpable nodules detected by sonography, including nodules larger than 1 cm in the majority of these patients [5]. In this same study, sonography also failed to identify any nodules in 16% of patients thought to have a solitary nodule on physical examination and in 30% of patients thought to have multiple nodules. Sonography is now considered the optimal method of confirming that a palpable abnormality is truly a nodule. Sonography is also recommended in patients with palpable thyroid abnormalities for the detection of additional nonpalpable nodules for which FNA may be indicated, and as a potential screening tool in patients at high risk for thyroid cancer based upon history [6,7].
Up until the mid 1990s, it was widely believed that the risk of thyroid malignancy was higher in patients with a solitary palpable nodule as compared with multinodular glands [8]. More recently, published series that define gland nodularity by imaging or by pathologic analysis note that the risk of malignancy is independent on the number of nodules as well as whether the nodule is palpable [9]. The overall incidence of thyroid cancers in patients with nodules detected by sonography that undergo FNA is approximately 9–13% regardless of the number of nodules [5,10]. In patients with multiple nodules, the cancer rate per nodule decreases, but the rate of cancer per patient remains equal to that of patients with solitary nodules [11,12]. Although cancer is most often present in the dominant or largest nodule, in over one third of patients the cancer is present in the nondominant nodule [10]. Therefore, palpability, nodule size and multiplicity do not further triage nodules into high or low risk status. Instead, analysis of the sonographic features of thyroid nodules has become the preeminent noninvasive tool for analyzing the risk of malignancy in thyroid nodules.
A large number of published reports describe the likelihood of thyroid malignancy based on nodular sonographic features. We focus on the results of fourteen large series, each comprised of over 100 nodules, that have correlated US imaging with cytology and/or histology findings to analyze sonographic features of thyroid nodules as predictors of malignancy (Table 1) [9,10,12–23]. The median sensitivity and specificity of each feature tabulated from these studies is presented in Table 2 [9,10,12–23]. There is significant variability among these studies, likely due to the differing methodologies (Table 1). One important source for the variability of certain sonographic findings relates to the proportion of malignant nodules in the study population, which varies tenfold, from 4 to over 40% [21,23]. A study that reports a relatively high rate of malignancy, as compared with the expected rate in the general population, may incorporate selection bias [7,12]. Furthermore, all 14 studies do not subclassify the different histologies of thyroid cancer (papillary and its several variants, follicular, Hürthle cell, and medullary cancers), but rather group all as ‘thyroid cancer’ for statistical analysis. Since papillary cancer accounts for 80–90% of all thyroid cancers, these criteria are inherently weighted toward detecting papillary cancers. Smaller recent studies, discussed below, provide information about different constellations of sonographic features correlated with follicular predominant histologies. In addition, the inclusion and exclusion criteria for performing a FNA and subsequently diagnosing a malignancy may alter performance of test characteristics for a given sonographic feature. For example, since some sonographic features, such as microcalcifications and taller-than-wide shape, are observed more commonly in malignant subcentimeter nodules than in larger nodules [23] inclusion of a large percentage of subcentimeter nodules with these characteristics in the study population will overestimate the sensitivity of these feature and decrease its specificity. Other differences among studies include the noncomparable definitions of specific sonographic features, such as the definition of irregular margins. Finally, results may also be affected by the quality of the sonographic equipment used, particularly the transducer frequency and whether the interpretation was based on review of static images or was based on real-time imaging, as well as the number of observers reporting the findings as interpretation of sonographic features has been shown to have relatively high interobserver variability [21,24,25].


Two additional important factors in determining the sensitivity and specificity of sonographic findings for the detection of malignancy relates to the choice of cytology results or surgical pathology as the gold standard for malignancy and the number of papillary versus follicular-predominant cancers included in the analysis. In most studies, nearly 100% of nodules with malignant cytology on FNA prove to be malignant papillary thyroid carcinoma on histological review. However, nodules that have follicular or indeterminate cytology on FNA, have a rate of malignancy between 20 and 60%.
Nodules with follicular cytology that prove to be malignant on histological analysis are either pure follicular carcinomas or are follicular variants of papillary cancer. These follicular-predominant malignancies have a very different sonographic appearance from the more common classic papillary thyroid cancer and much more closely resemble their benign counterpart, the follicular adenoma. Therefore, studies that include malignant lesions with follicular cytology often report different sensitivities and specificities for the diagnosis of malignancy as compared with studies that exclude nodules with follicular cytology.
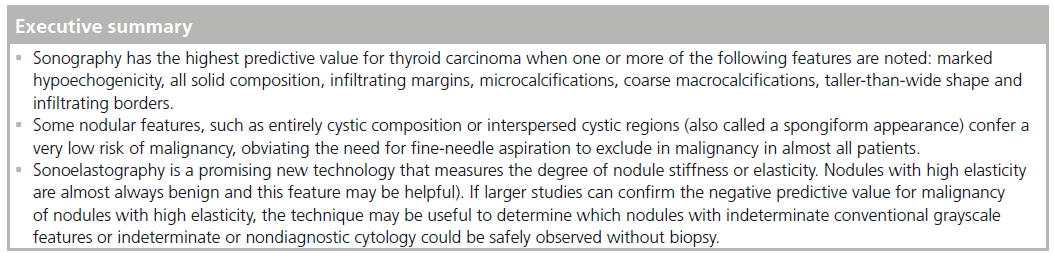
We will discuss in detail those sonographic features with either a median sensitivity and/ or a specificity of at least 80% for the detection of malignancy. A high specificity indicates that there is a high likelihood that a lesion with this feature is malignant and that very few benign lesions will possess the feature. A sonographic feature that carries a high sensitivity for malignancy indicates that very few cancers lack this feature.
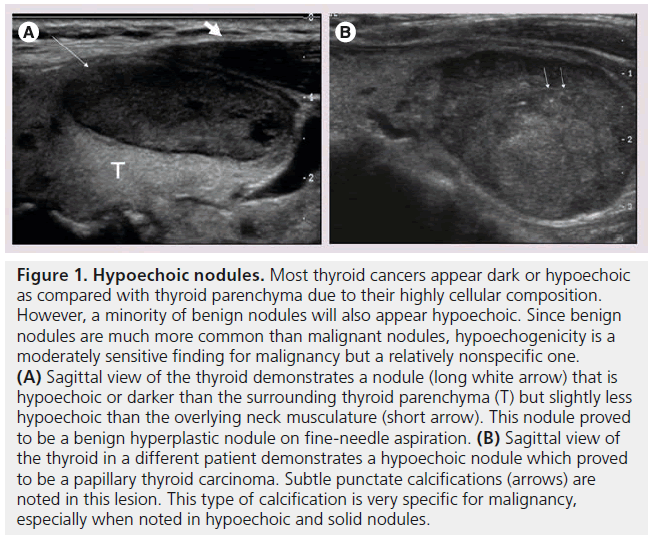
Figure 1. Hypoechoic nodules. Most thyroid cancers appear dark or hypoechoic as compared with thyroid parenchyma due to their highly cellular composition. However, a minority of benign nodules will also appear hypoechoic. Since benign nodules are much more common than malignant nodules, hypoechogenicity is a moderately sensitive finding for malignancy but a relatively nonspecific one. (A) Sagittal view of the thyroid demonstrates a nodule (long white arrow) that is hypoechoic or darker than the surrounding thyroid parenchyma (T) but slightly less hypoechoic than the overlying neck musculature (short arrow). This nodule proved to be a benign hyperplastic nodule on fine-needle aspiration. (B) Sagittal view of the thyroid in a different patient demonstrates a hypoechoic nodule which proved to be a papillary thyroid carcinoma. Subtle punctate calcifications (arrows) are noted in this lesion. This type of calcification is very specific for malignancy, especially when noted in hypoechoic and solid nodules.
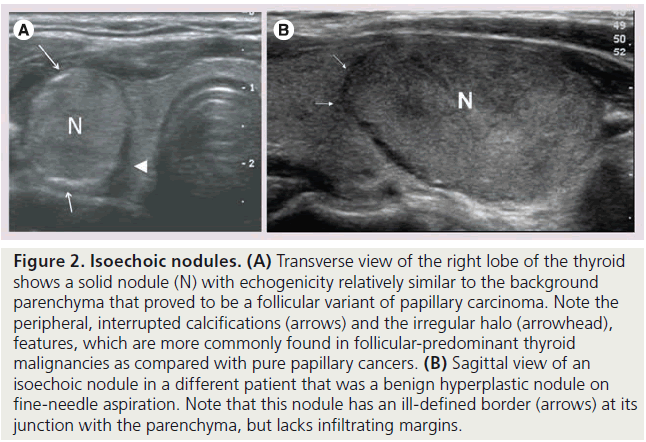
Figure 2. Isoechoic nodules. (A) Transverse view of the right lobe of the thyroid shows a solid nodule (N) with echogenicity relatively similar to the background parenchyma that proved to be a follicular variant of papillary carcinoma. Note the peripheral, interrupted calcifications (arrows) and the irregular halo (arrowhead), features, which are more commonly found in follicular-predominant thyroid malignancies as compared with pure papillary cancers. (B) Sagittal view of an isoechoic nodule in a different patient that was a benign hyperplastic nodule on fine-needle aspiration. Note that this nodule has an ill-defined border (arrows) at its junction with the parenchyma, but lacks infiltrating margins.
Echogenicity
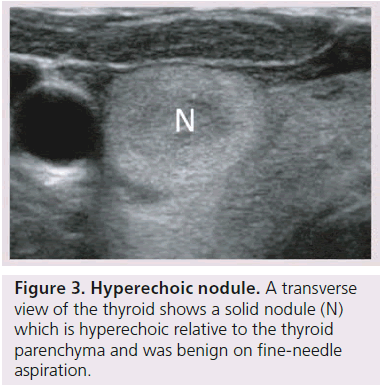
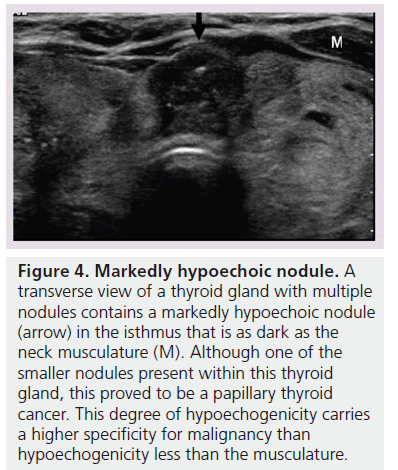
The echogenicity of a thyroid nodule refers to its brightness relative to the normal thyroid parenchyma. Normal parenchyma appears homogeneously hyperechoic or relatively bright on sonography. The bright appearance reflects the relatively high number of acoustic interfaces within the follicles that comprise normal thyroid tissue. The increased cellularity and cellular compaction and paucity of microfollicles present in classic papillary thyroid cancer and medullary thyroid cancer produces less acoustic interfaces and cause these lesions to appear hypoechoic (darker) than the surrounding normal thyroid tissue, resulting in a finding that is associated with malignancy [9,10,12–17,19,20] (Figure 1). By contrast, follicular neoplasms, either a benign follicular adenoma, a follicular carcinoma or follicular variant of papillary cancer, are composed of small microfollicles with variable amounts of colloid. Therefore, the echogenicity of these follicular predominant carcinomas and adenomas are less commonly hypoechoic and instead may be isoechoic or hyperechoic compared with the parenchyma (Figures 2 & 3) [26]. Although echogenicity may appear to be a robust observation, the interobserver reproducibility is only fair to moderate (K values: 0.3–0.5) [21,24,25]. Some authors have identified a sonographic feature termed ‘marked hypoechogenicity’ that describes a solid nodule with echogenicity as dark as the surrounding strap muscles [18,21,23]. Compared to nodules that are hypoechoic with respect to parenchyma, but not as hypoechoic as strap muscles, marked hypoechogenicity is less sensitive for identification of thyroid cancer, but much more specific (Figure 4).
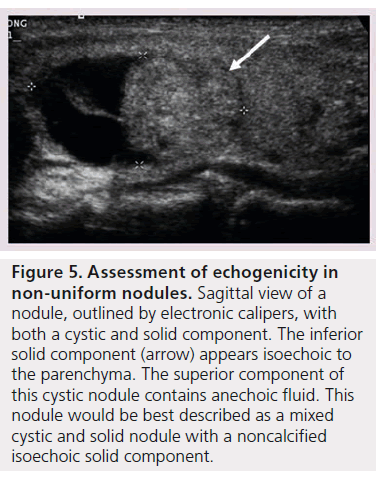
In patients with autoimmune thyroid disease, such as Hashimoto’s thyroiditis, the echogenicity of the parenchyma is much more heterogeneous in appearance making classification of the nodule’s echogenicity more subjective. Additionally nodule echogenicity may be challenging to classify when the nodule is nonuniform in its appearance. The majority of all nodules have some cystic composition, and identification of the solid component is required to determine echogenicity (Figure 5) [10].
Margins
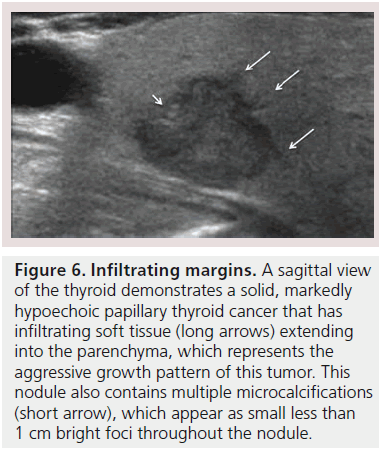
The margins of a thyroid nodule can be described as well-defined, poorly defined, irregular, microlobulated or infiltrating. A poorly defined margin that is not infiltrative, is often used to describe a nodule that appears to merge into the surrounding thyroid without discrete borders. The majority of poorly defined thyroid nodules represent focal regions of hyperplasia and are benign (Figure 2B). These lesions are unencapsulated and tend not to have a clear delineation from the adjacent parenchyma. Most thyroid cancers lack a capsule but may be differentiated from benign lesions by the presence of a microlobulated, spiculated or infiltrating margin, that histologically corresponds to invasion of a malignant nodule into the surrounding thyroid parenchyma (Figure 6). It is important to note that a well-defined margin does not exclude malignancy and is commonly observed with encapsulated follicular or Hürthle cell cancers as well as a small percentage of papillary cancers [26].
The categorization of a thyroid nodule as having irregular or infiltrating margins carries the widest range of sensitivity for malignancy and the poorest interobserver agreement [12,17,18], reflecting the relatively subjective nature of nodular margin description. Technical factors, such as the frequency of the transducer used to produce the images, may also affect the performance of margin analysis. The use of higher frequency probes and more recent improvements in postacquisition imaging software allow higher resolution imaging and will likely aid in the detection of subtle lesion margin irregularities. The size of the nodule may also affect the perception of its margins. In fact, benign nodules were more likely to have a well-defined smooth border when larger than 1 cm as compared with benign nodules under 1 cm [21].
Calcifications
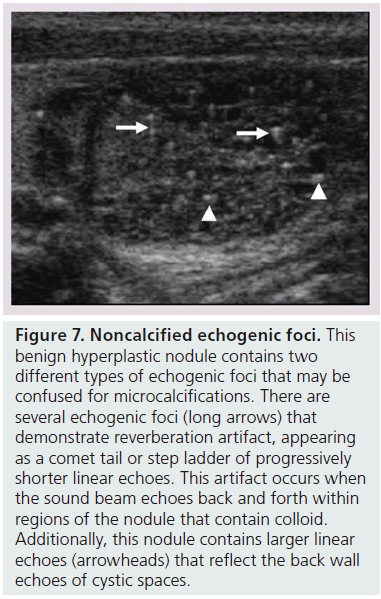
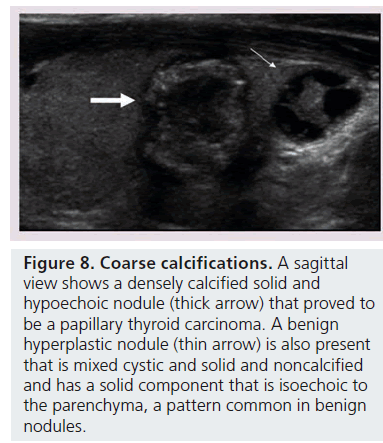
Microcalcifications are thought to represent aggregates of psammoma bodies, the laminated spherical concretions characteristic of many papillary cancers, and are rarely found in benign nodules or follicular neoplastic lesions [26,27]. (Figures 1B & Figure 6). Therefore, this finding carries a relatively high specificity for the diagnosis of thyroid cancer and interobserver variability for the identification of microcalcifications shows moderate to substantial agreement (K 0.51–0.68) [21,24,25]. Microcalcifications image as small punctuate, hyperechoic foci measuring approximately 1 mm, usually without distal acoustic shadowing, and are most apparent in solid and hypoechoic nodules. There are two types of bright foci that occur in the more common benign mixed cystic and solid or spongiform nodule (see description below) that can be mistaken for microcalcifications (Figure 7). Small hyperechoic linear or curvilinear foci located immediately posterior to small cystic regions in mixed consistency nodules are caused by a sonographic artifact. As the sound travels through the fluid filled region it causes posterior acoustic enhancement or exaggerated brightness of the back wall of the cystic space. These foci can be differentiated from microcalcifications by their size (often over 2 mm), their shape (linear or curvilinear) and their position (behind a cystic region). Small hyperechoic foci may also be seen in colloid-containing nodules. When sound strikes a colloid containing nodule it may reflect back and forth within the colloid-containing space. This produces both a bright focus within the cystic component of the nodule as well as a reverberation or comet tail artifact that appears as a series of progressively smaller linear bright echoes behind the central bright focus. The echogenic focus within the colloid-containing nodule may appear as a single bright area and if the reverberation artifact is not appreciated, may lead to the erroneous conclusion that a calcification is present [28]. Although colloid is commonly noted in benign nodules, it may also be noted in malignant nodules. Coarse, dense macrocalcifications are larger than 2 mm and cause posterior acoustic shadowing (Figure 8). These dystrophic calcifications occur in both benign and malignant lesions in areas of fibrosis, tissue degeneration and necrosis. Coarse calcifications may be associated with malignancy when they appear with microcalcifications or alone within a nodule [10,29]. Peripheral calcifications were once thought to indicate a benign process. However, this finding can be seen in malignant nodules [27]. A nodule with smooth, regular or ‘egg shell’ peripheral calcification tends to be benign, where as peripheral calcification within an identifiable capsule or in association with interruption of the calcified rim by a visible soft tissue component suggests malignant invasion of thyroid parenchyma (Figure 9). Therefore, any calcification raises the risk of malignancy: microcalcifications at least threefold and coarse calcifications and peripheral calcifications twofold, as compared with noncalcified nodules [10]. Additionally the risk for malignancy is highest when the calcification occurs in a solitary nodule and if the patient is male [10,30].
Figure 4.Markedly hypoechoic nodule. A transverse view of a thyroid gland with multiple nodules contains a markedly hypoechoic nodule (arrow) in the isthmus that is as dark as the neck musculature (M). Although one of the smaller nodules present within this thyroid gland, this proved to be a papillary thyroid cancer. This degree of hypoechogenicity carries a higher specificity for malignancy than hypoechogenicity less than the musculature.
Figure 5.Assessment of echogenicity in non-uniform nodules. Sagittal view of a nodule, outlined by electronic calipers, with both a cystic and solid component. The inferior solid component (arrow) appears isoechoic to the parenchyma. The superior component of this cystic nodule contains anechoic fluid. This nodule would be best described as a mixed cystic and solid nodule with a noncalcified isoechoic solid component.
Figure 6.Infiltrating margins. A sagittal view of the thyroid demonstrates a solid, markedly hypoechoic papillary thyroid cancer that has infiltrating soft tissue (long arrows) extending into the parenchyma, which represents the aggressive growth pattern of this tumor. This nodule also contains multiple microcalcifications (short arrow), which appear as small less than 1 cm bright foci throughout the nodule.
Solid consistency
Thyroid cancers are most commonly solid or nearly entirely solid and are more likely to be solid than benign nodules [10,12,14,17,23]. However, as benign nodules outnumber malignant ones, this feature carries a relatively low specificity for malignancy as an isolated feature. Fewer than 10% of papillary cancers are more than 50% cystic and typically also demonstrate another suspicious US feature, such as calcification. The interobserver agreement for reporting solid nodule consistency is reported to be substantial (K 0.62–0.91) [21,24,25].
Figure 7.Noncalcified echogenic foci. This benign hyperplastic nodule contains two different types of echogenic foci that may be confused for microcalcifications. There are several echogenic foci (long arrows) that demonstrate reverberation artifact, appearing as a comet tail or step ladder of progressively shorter linear echoes. This artifact occurs when the sound beam echoes back and forth within regions of the nodule that contain colloid. Additionally, this nodule contains larger linear echoes (arrowheads) that reflect the back wall echoes of cystic spaces.
Halo
A halo describes a thin hypoechoic rim that surrounds a nodule and is thought to represent compression of the extranodular blood vessels as a benign nodule slowly grows [10,13–15]. An invasive malignancy, such as unencapsulated papillary cancer or medullary cancer, lacks a halo. However, follicular and Hürthle cell adenomas and cancers are generally surrounded by a fibrous avascular capsule (Figure 3). This capsule images sonographically as a thick, irregular hypoechoic rim, which is now recognized as a more worrisome, second type of halo [16]. In a recent study, contrasting sonographic features of papillary versus follicular cancers, hypoechoic rims were demonstrated more frequently for follicular cancers (87%) rather than for papillary cancers (26%, p < 0.05) [26], and more frequently in the classic variant of papillary thyroid cancer (usually unencapsulated 25% ) than in a follicular variant of papillary cancer (3% p < 0.05) [31].
Figure 8.Coarse calcifications. A sagittal view shows a densely calcified solid and hypoechoic nodule (thick arrow) that proved to be a papillary thyroid carcinoma. A benign hyperplastic nodule (thin arrow) is also present that is mixed cystic and solid and noncalcified and has a solid component that is isoechoic to the parenchyma, a pattern common in benign nodules.
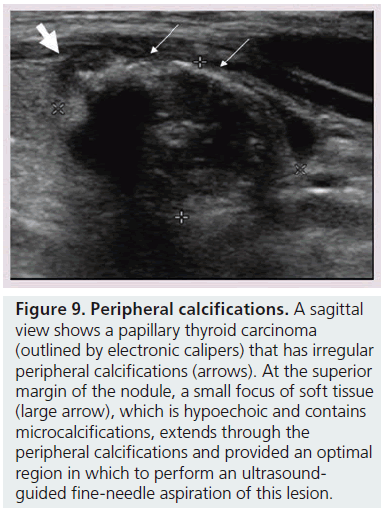
Figure 9.Peripheral calcifications.A sagittal view shows a papillary thyroid carcinoma (outlined by electronic calipers) that has irregular peripheral calcifications (arrows). At the superior margin of the nodule, a small focus of soft tissue (large arrow), which is hypoechoic and contains microcalcifications, extends through the peripheral calcifications and provided an optimal region in which to perform an ultrasoundguided fine-needle aspiration of this lesion.
Taller than wide shape
Four recent series have evaluated the shape of the nodule by looking at the ratio of the anterioposterior to transverse diameter (A/T) as measured on the transverse view, and found that a nodule with an A/T ratio greater than 1.0, (taller-thanwide) is associated with malignancy [18,19,21,23] (Figure 10). This finding replicated the results in the sonographic literature evaluating breast carcinomas [18]. Disproportionate growth in the anterioposterior dimension is considered an aggressive growth pattern across, rather than within, the normal tissue planes. This finding is not very sensitive but has specificity ranging from 82 to 93% and is most commonly noted in smaller cancers under 1 cm [23]. Based upon two studies, the interobserver variability shows moderate agreement (K 0.59–0.61) [21,24].
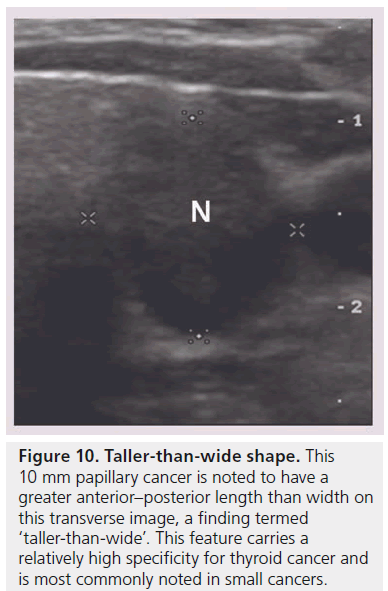
Figure 10.Taller-than-wide shape. This 10 mm papillary cancer is noted to have a greater anterior–posterior length than width on this transverse image, a finding termed ‘taller-than-wide’. This feature carries a relatively high specificity for thyroid cancer and is most commonly noted in small cancers.
There are two other grayscale imaging findings that are highly suggestive of malignancy. The first is the detection of abnormal cervical lymph nodes. Two recently published guidelines by the American Thyroid Association (ATA) [6] and American Association for Clinical Endocrinologists (AACE) [7] for evaluating patients with thyroid nodules both state that sonographic evaluation of the cervical lymph nodes should be part of a standard diagnostic thyroid US if a nodule has suspicious US features [6,7]. Cervical lymph node metastases may have a variety of abnormal findings including cystic regions, punctuate calcifications, hyperechoic foci, increased peripheral vascularity, and a rounded shape [32]. Second, extrathyroidal invasion may be occasionally seen when the tumor growth extends through either the anterior or posterior thyroid capsule, which normally appears as a bright white outline surrounding the thyroid. In such instances, the margin of the tumor has an ill-defined edge that interrupts this capsule (Figure 11) [33].
Owing to individual sonographic features having limited utility in predicting malignancy, some series have explored the association of combinations of features with cancer risk. In most series, as the specificity of a combination increases, the sensitivity decreases. For example, although less than 4% of benign nodules are hypoechoic with microcalcifications, only 26–31% of thyroid cancers will have this appearance [9,12,15]. Therefore, many thyroid cancers would be missed if only the hypoechoic nodules with microcalcifications underwent FNA. The combination of sonographic features that maximizes sensitivity and specificity is a solid, hypoechoic nodule, which identifies approximately 70% of all cancers, but still describes the appearance of 30% of benign nodules [12,14,17]. Additionally, as many as 66% of papillary thyroid cancers have at least one sonographic feature not typically associated with malignancy and 69% of benign nodules have one sonographic predictor of malignancy [25,34].
Furthermore, as discussed previously, some of the variability associated with the reported sensitivities of individual sonographic features for prediction of thyroid malignancy may depend upon the histology of the thyroid cancer. Papillary thyroid cancers are more likely to be solid, hypoechoic, and lack a halo compared with follicular thyroid cancers. Follicular cancers most commonly have a halo (90%), which is irregular (60%), and are iso- to hyperechoic [26]. In addition, the two most common histological subtypes of papillary thyroid cancer, follicular variant and classic, also have different US profiles. The classic variant is more likely to be hypoechoic with microcalcifications and spiculated margins [31]. Therefore, it is critical to recognize that ultrasound does not replace FNA cytology, rather that the two modalities are complementary.
The role of US is to confer a degree of risk to a thyroid nodule to aid in clinical decision making about whether FNA is indicated, especially for smaller nodules, where the vast majority of microcarcinomas are clinically indolent. Therefore, using constellations of US features, the high risk nodule would image on grayscale US as solid, markedly hypoechoic, with microcalcifications, and an infiltrative border with a taller-than-wide shape. An intermediate risk nodule might be a hypoechoic nodule lacking microcalcifications, with smooth borders or an iso- to hyper-echoic predominantly solid nodule [22,29].
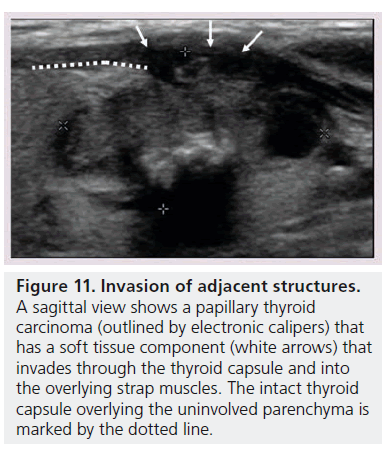
Figure 11.Invasion of adjacent structures. A sagittal view shows a papillary thyroid carcinoma (outlined by electronic calipers) that has a soft tissue component (white arrows) that invades through the thyroid capsule and into the overlying strap muscles. The intact thyroid capsule overlying the uninvolved parenchyma is marked by the dotted line
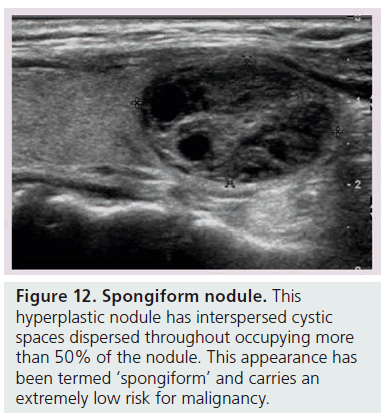
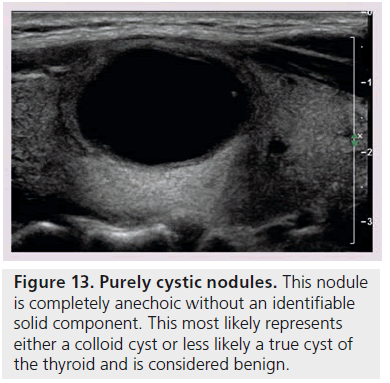
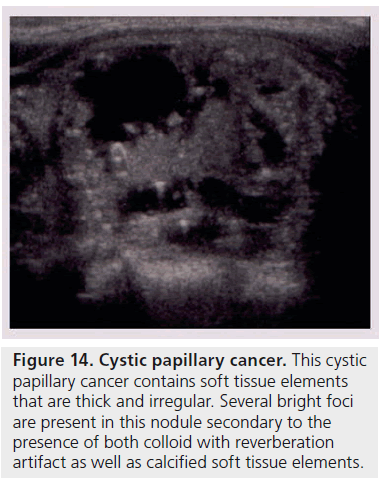
Another way to utilize sonographic features as predictors of malignancy is to identify nodules that lack all reported grayscale suspicious characteristics. Using Bayes’ theorem to determine what is termed post-test probability (i.e., the probability of disease that is recalculated after the results of a diagnostic test), the risk of malignancy can be calculated for a nodule without hypoechogenicity, taller-than-wide shape, irregular margins, and calcifications (both microcalcifications and macrocalcifications) [21]. Given a 7–9% population prevalence of thyroid cancer, for every 1000 nodules that have such a benign appearance, only nine would be malignant. Therefore, one would have to perform 1000 FNAs on nodules lacking these four US features to identify nine cancers. There are two types of thyroid nodules that not only lack these four suspicious features but have a specific appearance that carries a very high likelihood of being benign. One appearance has been termed spongiform, defined as a nodule that has multiple microcystic areas that occupy more than 50% of the nodule volume, a proverbial ‘honey comb’ of internal cystic spaces (Figure 12) [21,22,29]. In the report of Bonavita et al. [22], all 210 nonvascular nodules with this US appearance were benign, and in that of Moon et al. [21], only one of the 360 thyroid cancers appeared spongiform on US. The second sonographic appearance of a thyroid nodule that is equated with benignity is that of a pure cyst, which although rare (<2%), is virtually always benign (Figure 13). Very few thyroid cancers will be predominantly cystic and these will have another suspicious US feature (Figure 14). Often, comet tail artifact, representing reverberation of sound waves caused by inspissated colloid, is present in these pure cysts. Both ATA and AACE guidelines recognize pure cystic and spongiform nodules as low risk for malignancy and the size cut-off for FNA of such lesions is more lenient (if performed at all) than for intermediate and high-risk sonographically appearing nodules [6,7].
Figure 14.Cystic papillary cancer. This cystic papillary cancer contains soft tissue elements that are thick and irregular. Several bright foci are present in this nodule secondary to the presence of both colloid with reverberation artifact as well as calcified soft tissue elements.
There have been a limited number of reports that have evaluated the performance of color Doppler sonography in the assessment of thyroid nodules with some authors noting that internal vascularity was a useful predictor of malignancy [9,11,16] while others did not find an association [15,35]. Nodular vascularity has most commonly been classified as absent, only present in the periphery or intranodular. Several of the reports that did find intranodular vascularity to be associated with malignancy may have biased since they analyzed either only papillary carcinomas without comparing benign nodules, only hypoechoic nodules, or only nonfunctioning (cold) nodules as assessed by radioiodine scan. Some authors only investigated thyroid nodules larger than 10 mm or analyzed their results without adjusting for a mean size discrepancy between benign and malignant nodules. A recent retrospective analysis of over 1046 consecutive patients undergoing ultrasound-guided FNA of thyroid nodules, which included all thyroid nodules regardless of size, found that intranodular vascularity alone or in combination with suspicious grayscale features (defined as marked hypoechogenicity, noncircumscribed margins, microcalcifications and tallerthan- wide shape) was not useful in predicting malignancy [35]). In this study, in which papillary cancers comprised 97.4% of the malignant lesions, intranodular flow in nodules smaller than 10 mm and in hypoechoic nodules was more commonly present in benign nodules than in malignant ones. Several authors are continuing to investigate analysis of patterns of flow patterns within nodules to determine if a more complex analysis alone or in combination with other sonographic features may improve the specificity for the detection of malignancy.
Future perspective
Since approximately 20–30% of lesions with follicular cytology are malignant, surgical excision has been the recommended treatment. Similarly, nodules with persistent nondiagnostic cytology, particularly those that are not predominantly cystic, are also often managed by surgical removal due to a concern for malignancy [6]. There are two advances which appear likely to improve the negative predicative value of preoperative evaluation of these follicular lesions for the presence of malignancy: sonoelastography and molecular analysis of gene expression.
It has long been recognized that palpably hard nodules have a greater likelihood of being a cancer. Sonoelastography is a newly developed sonographic technique that can assess and quantify the stiffness or lack of elasticity of a nodule by measuring the amount of distortion that occurs when the nodule is subjected to external pressure. The sonologist applies light pressure over the nodule with a specially configured linear transducer. The degree of tissue distortion is displayed as a color map overlying the grayscale image corresponding to the degree of elasticity of the nodule. Preliminary results have shown a correlation between the stiffness of a thyroid nodule as determined by sonoelastography and malignant histopathology [36,37]. In a pilot study of selected population of 92 patients with solitary nodules undergoing surgery for a suspicion of malignancy, sonoelastography had a sensitivity of 97% and a specificity of 100% for the prediction of thyroid cancer [36]. Subsequent studies in small numbers of unselected populations have noted lower sensitivities and specificities of sonoelastography for thyroid cancer diagnosis but high negative predictive values ranging between 93 and 98.2% for nodules with high elasticity [38–40]. If larger studies of unselected patients can confirm the negative predictive value for malignancy of nodules with high elasticity, the technique may be useful to determine which nodules with indeterminate conventional grayscale features could be safely obse rved without biopsy. Sonoelastography also appears promising as an imaging tool to stratify risk of malignancy in nodules with indeterminate or nondiagnostic cytology with preliminary investigations also noting that sonoelastography had a negative predictive value of 98.6% and an accuracy of 91.3% for the prediction of malignancy among these nodules [41]. Sonoelastography may play a pivotal role differentiating those nodules with indeterminate or nondiagnostic cytology that should undergo surgical excision from those than can be safely followed.
Another important advance is the application of molecular analysis of gene expression to material obtained from thyroid nodule FNA. Somatic mutations occur in up to 50% of thyroid cancers and testing for 4 of the most common mutations, BRAF, RET/PTC rearrangements, RAS and PAX8/PPARg, is highly specific for malignancy [42]. Therefore, if a nodule with an indeterminate cytology result has one of these mutations, it is virtually always malignant, and thus, definitive surgery, near total thyroidectomy, is performed. However, the sensitivity of this panel of mutations is lower and a negative result does not preclude surgery. A new approach has been to adopt mRNA expression analysis and bioinformatics to define a pattern of gene expression associated with benign nodules, which, if present, would have a high negative predictive value for cancer, and surgery could be potentially avoided [43].
It is likely that these two techniques, elastography and molecular analysis, will be able to reduce the rate of surgical excision of benign lesions without delaying diagnosis of malignant follicular lesions.
Conclusion
In summary, sonography plays an important role in assessing the risk of malignancy based on the sonographic features and may help guide the need for FNA in incidentally detected nodules. With the exception of the identification of metastatic lymphadenopathy or direct invasion of adjacent soft tissue, no single US feature or combination is adequately sensitive to identify all malignant thyroid nodules. However, certain sonographic features and combination of features confer a higher risk of malignancy to a nodule and indicate that FNA should be performed.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending,or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
* of interest
* of considerable interest
References
- Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann. Intern. Med. 69, 537–540 (1968).
- Brander A, Viikinkoski P, Tuuhea J, Voutilainen L, Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J. Clin. Ultrasound 20, 37–42 (1992).
- Tan GH, Gharib H, Reading CC. Solitary thyroid nodule: comparison between palpation and ultrasonography. Arch. Intern. Med. 155, 2418–2423 (1995).
- Mazzaferri EL. Management of a solitary thyroid nodule. N. Engl. J. Med. 328, 553–559 (1993).
- Marqusee E, Benson CB, Frates MC et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann. Intern. Med. 133, 696–700 (2000).
- Cooper DS, Doherty GM, Haugen BR et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009). & These evidence-based guidelines outline clinical decision making for thyroid nodule fine-needle aspiration based on sonographic, size and clinical history features.
- Gharib H, Papini E, Paschke R et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr. Pract. 16, 468–475 (2010). & These evidence-based guidelines outline clinical decision making for thyroid nodule fine-needle aspiration based upon sonographic, size and clinical history features.
- Kumar H, Daykin J, Holder R, Watkinson JC, Sheppard MC, Franklyn JA. Gender, clinical findings, and serum thyrotropin measurements in the prediction of thyroid neoplasia in 1005 patients presenting with thyroid enlargement and investigated by fine-needle aspiration cytology. Thyroid 9, 1105–1109 (1999).
- Papini E, Guglielmi R, Bianchini A et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J. Clin. Endocrinol. Metab. 87, 1941–1946 (2002).
- Frates MC, Benson CB, Doubilet PM et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J. Clin. Endocrinol. Metab. 91, 3411–3417 (2006).
- Frates MC, Benson CB, Charboneau JW et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 237, 794–800 (2005).
- Nam-Goong IS, Kim HY, Gong G et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin. Endocrinol. (Oxf ) 60, 21–28 (2004).
- Brkljacic B, Cuk V, Tomic-Brzac H, Bence-Zigman Z, Delic-Brkljacic D, Drinkovic I. Ultrasonic evaluation of benign and malignant nodules in echographically multinodular thyroids. J. Clin. Ultrasound 22, 71–76 (1994).
- Takashima S, Fukuda H, Nomura N, Kishimoto H, Kim T, Kobayashi T. Thyroid nodules: re-evaluation with ultrasound. J. Clin. Ultrasound 23, 179–184 (1995).
- Rago T, Vitti P, Chiovato L et al. Role of conventional ultrasonography and color flow-Doppler sonography in predicting malignancy in ‘cold’ thyroid nodules. Eur. J. Endocrinol. 138, 41–46 (1998).
- Cerbone G, Spiezia S, Colao A et al. Power Doppler improves the diagnostic accuracy of color Doppler ultrasonography in cold thyroid nodules: follow-up results. Horm. Res. 52, 19–24 (1999).
- Leenhardt L, Hejblum G, Franc B et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J. Clin. Endocrinol. Metab. 84, 24–28 (1999).
- Kim EK, Park CS, Chung WY et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR. Am. J. Roentgenol. 178, 687–691 (2002).
- Cappelli C, Pirola I, Cumetti D et al. Is the anteroposterior and transverse diameter ratio of nonpalpable thyroid nodules a sonographic criteria for recommending fine-needle aspiration cytology? Clin. Endocrinol. (Oxf.) 63, 689–693 (2005).
- Kovacevic O, Skurla MS. Sonographic diagnosis of thyroid nodules: correlation with the results of sonographically guided fine-needle aspiration biopsy. J. Clin. Ultrasound 35, 63–67 (2007).
- Moon WJ, Jung SL, Lee JH et al. Benign and malignant thyroid nodules: US differentiation – multicenter retrospective study. Radiology 247, 762–770 (2008). & Correlates sonographic features with benign nodules and provides criteria for their identification.
- Bonavita JA, Mayo J, Babb J et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR. Am. J. Roentgenol. 193, 207–213 (2009). & Correlates sonographic features with benign nodules and also reveiws constellations of sonographic features and correlation with cancer risk.
- Ahn SS, Kim EK, Kang DR, Lim SK, Kwak JY, Kim MJ. Biopsy of thyroid nodules: comparison of three sets of guidelines. AJR. Am. J. Roentgenol. 194, 31–37 (2010).
- Choi SH, Kim EK, Kwak JY, Kim MJ, Son EJ. Interobserver and intraobserver variations in ultrasound assessment of thyroid nodules. Thyroid 20, 167–172 (2010).
- Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J. Ultrasound Med. 22, 1027–1031 (2003).
- Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J. Radiol. 8, 192–197 (2007).
- Taki S, Terahata S, Yamashita R et al. Thyroid calcifications: sonographic patterns and incidence of cancer. Clin. Imaging 28, 368–371 (2004).
- Mandel SJ. Diagnostic use of ultrasonography in patients with nodular thyroid disease. Endocr. Pract. 10, 246–252 (2004).
- Reading CC, Charboneau JW, Hay ID, Sebo TJ. Sonography of thyroid nodules: a “classic pattern” diagnostic approach. Ultrasound Q 21, 157–165 (2005). & Describes common sonographic appearances of thyroid nodules and correlates these with cancer risk.
- Khoo ML, Asa SL, Witterick IJ, Freeman JL. Thyroid calcification and its association with thyroid carcinoma. Head Neck 24, 651–655 (2002).
- Kim DS, Kim JH, Na DG et al. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J. Ultrasound Med. 28, 1685–1692 (2009).
- Langer JE, Mandel SJ. Sonographic imaging of cervical lymph nodes in patients with thyroid cancer. Neuroimaging Clin. N. Am. 18, 479–489, vii-viii (2008). & A comprehensive review of the sonographic appearance of thyroid cancer metastatic lymph nodes.
- Ito Y, Kobayashi K, Tomoda C et al. Ill-defined edge on ultrasonographic examination can be a marker of aggressive characteristic of papillary thyroid microcarcinoma. World J. Surg. 29, 1007–1011, discussion 1011–1012 (2005).
- Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J. Ultrasound Med. 22, 1083–1090 (2003).
- Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim E. Can vascularity at Power Doppler US help predict thyroid malignancy? Radiology 255, 260–269 (2010).
- Rago T, Santini F, Scutari M et al. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. Clin. Endocrinol. Metab. 92, 2917–2922 (2007).
- Lyshchick A, Higashi T, Asato R et al. Thyroid gland tumor diagnosis at US elastography. Radiology 237, 202–211 (2005).
- Asteria C, Giovanardi A, Pizzocaro A et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid 8, 523–531 (2008).
- Rubaltelli L, Corradin S, Dorigo A et al. Differential diagnosis of benign and malignant thyroid nodules at elastosonography. Ultraschall Med. 30, 175–179.
- Hong Y, Liu X, Li Z, Zhang X, Chen M, Luo Z. Real-time ultrasound elastography in the differential diagnosis of benign and malignant thyroid nodules. J. Ultrasound Med.28, 861–867.
- Rago T, Scutari M, Santini F et al. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J. Clin. Endocrinol. Metab. 95, 5274–5280 (2010).
- Ferraz C, Eszlinger M, Paschke R. Current state and future perspective of molecular diagnosis of fine-needle aspiration biopsy of thyroid nodules. J. Clin. Endocrinol. Metab. 96, 2016–2026 (2011).
- Chudova D, Wilde JI, Wang ET et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J. Clin. Endocrinol. Metab. 95, 5301–5309 (2010).