Review Article - International Journal of Clinical Rheumatology (2020)
Therapeutic management of chronic inflammatory rheumatic diseases in times of pandemic COVID 19
- Corresponding Author:
- Rim Dhahri
Department of Rheumatology
Military Hospital of Tunis, Tunisia
E-mail: rimdhahri@ymail.com
Abstract
Conoravirus 2019 or Covid-19 disease (acronym for COronaVirus Infectious Disease 2019) is an emerging highly contagious infection caused by the coronavirus strain SARS-CoV-2. In March 2020 it has been declared a pandemic by the World Health Organization (WHO). The rheumatologist is therefore faced with a challenge: an optimal management of COVID patients who are immunosuppressed not only by the disease but also by the immunosuppressive treatments they take. In fact, some of these drugs seem , oddly, to have antagonist effects on viral response and therefore are investigated in COVID treatment. Severe COVID-19 originates from a cytokine storm caused by increased levels of a number of cytokines and chemokines resulting in a multiorgan failure. The management of this cytokine storm is one of the major challenges regarding COVID-19 infection. Several pathways are investigated including drugs known to be efficient on rheumatology field (especially NSAIDs, corticosteroids, Disease Modifying Anti Rheumatic diseases (DMARDs): biological or synthetic ones such as methotrexate, salazopyrine, leflunomide and Hydroxychloroquine). Given these facts, this review aimed to summarize the existing data that may support the therapeuting decision making of rheumatologists in this pandemic context. Glucocorticoids should be used at its lowest necessary dose regardless of exposure or infection status if needed to controlee underlying disease. They should never be stopped precipitously . Stable patients with no COVID 19 infection or exposure should continue their treatment either NSAIDs or immunosuppressants (CsDMARDs, TsDMARDs or bDMARDs) with no modification or dose reduction in case of life threatening organ lesion. Anti malarial drugs is to not discontinue them, considering the antiviral efficacy and the immunomodulatory rather than immunosuppressive effect. The anti Il 6 is also not to be stopped given their action on the inflammatory reaction caused by COVID19. In case of confirmed COVID 19 infection it is recommended to temporarily stop other immunosuppressive treatments. That guidance does not replace clinical judgment of the rheumatologist based on clinical severity of the infection or the ARD. Mostly guideline panel recommends underlying general preventive measures, e.g., social distancing and hand hygiene, reducing exposure of patients with minimizing health encounters.
Keywords
COVID-19 • SARS-CoV-2 • autoinflammatory diseases • hydroxychloroquine • biologic response modifiers
Introduction
In December 2019 a new virus of the coronoviridae, called Conoravirus 2019, family has appeared in Wuhan province of China. Conoravirus 2019 is an emerging viral zoonosis-like infectious disease caused by the coronavirus strain SARS-CoV-2. In March 2020, COVID 19 has been declared a pandemic by the World Health Organization WHO [1].
Covid 19 disease can lead to death, especially in people who are frail due to age or comorbidities. Another deadly complication is an exacerbated response from the innate immune system e.g cytokine storm [2]. Despite the awareness of rheumatologists that Autoimmune Rheumatic Diseases (ARD) such as Rheumatoid Arthritis (RA) and spondyloarthritis (SpA), are associated with increased risk of serious infections, links and contraindications related to therapeutic management of those diseases still are ill defined in COVID 19 infection. This is, indeed, a subject of a challenging debate. In fact, the paucity of studies or cases reported in the Rheumatic diseases ‘population as well as the potent effects of antirheumatic therapies considered to be effective against COVID may lead to controversial conclusions
Faced with the risk of new outbreaks of this pandemic which may occur in an ARD context, urgent guidance is crucial. The availability of reliable information for both doctors and patients is also of a substantial need. Given these facts, this review aimed to summarize the existing data that may support the therapeutic decision making of rheumatologists in a previously controlled RA and SpA’s patients considering the most frequently prescribed Disease Modifying Anti Rheumatic Drugs (DMARs).
Are ARDs patients at higher risk of COVID 19 infection? Patients with rheumatic diseases are usually considered to be immunocompromised based on the underlying disease properties and targeted therapies they are usually on. In general, this population is at higher risk of infection even serious infection compared to immunocompetent population. Although comparing informations published about other coronaviruses outbreaks such as SARS and MERS, there was no evidence of an over-risk in patients with Rheumatic disease (compared to other comorbidities) [3].
NSAIDS (non steroidal anti inflammatory Drugs) and COV2 SARS infection
Various Drugs are investigated in order to seek their effectiveness on COVID19 virus in vitro and in Vivo. It is crucial to understand first the molecular pathways of this viral infection. It is suggested that this virus uses type 2 Angiotensin-Converting Enzyme (ACE2) receptor for cellular entry. ACE2 is expressed especially on the epithelial cells of oral mucosa and surfactant-producing type 2 pneumocytes which can be severely impacted in most serious COVID 19 forms [4].
Ibuprofen, a commonly used NSAIDs, is believed to increase expression of ACEs receptors thus increasing severity of viral infection. This was especially highlighted by French physicians linking some deaths among young immunocompetent patients infected with SARS COV2 to history of Ibuprofen intake. Most authors and current guidelines advise against its use until clearer proofs emerges [5].
In April 2019, the french ANSM (agence nationale de sécurité du médicament et des produits de santé), drew attention to the risks of prescribing NSAIDs (ibuprofene, ketoprofene, naproxen and others) in case of infection [6]. Recent observations suggest that NSAIDs may be more specifically associated with worsening of patients infected with COVID-19. An inhibition of the immune system and the increase in the expression of the ACE2 would be involved, which would also explain the vulnerability of diabetic or ACE-treated patients towards the coronavirus [7]. These findings are based on the similarities between the new SARS-cov-2 virus and SARS [8].
Canadian health services continue to recommend NSAIDs for fever in Covid + patients and to use them with "caution" in patients over the age of 60. They also recommend to avoid them in the event of comorbidity as well as in the elderly. However, the NHS (National Health Service), the NICE (National Institute for health and Care Excellence) and the French Directorate General for Health, recognizes the scarcity of data and agree on the fact that they no longer use NSAIDs in COVID (+) patients and preferentially use paracetamol. In this context, it seems desirable to remain cautious and to follow these latest recommendations.
Corticosteroids: should we be limiting their use?
Corticosteroids are frequently used during chronic inflammatory rheumatic diseases. They are used either by the general route (per os or bolus) or locally by intra-articular injection. Their use of is associated with an infectious over-risk beyond the daily dose of 5 mg/day [9]. A study prospectively assessed the risk of hospitalization for pneumonia in RA patients [9]. After adjustment for other potential confounders, the use of prednisone increased by 70% the risk of hospitalization for pneumonia in a dose-dependent manner [9].
Its long-term use, for the most part, limits the possibility of an abrupt stop in this pandemic context. It is therefore important to weigh the pros and cons before making a therapeutic decision. The corticosteroid therapy in COVID is controversial. It reduces excessive and harmful pulmonary inflammation but it also inhibits the beneficial immune response, which allows the patient to eliminate pathogens.
A meta-analysis published in the Lancet on February, 2020 presented the synthesis of their use of corticosteroids in SARS, MERS, syncytial virus infections in ARDS context [10]. It concluded that clinical data showed no benefit in favor of the use of corticosteroids [10]. Chen et al. reported 19 (19%) patients were treated with glucocorticoids for 3–15 days (median 5), and methylprednisolone (1–2 mg/kg per day) are recommended for patients with ARDS, for as short a duration of treatment as possible [11]. However, some evidences indicate that the benefit of the use of glucocorticoids is likely outweighed by adverse effect. Wang et al. reported 44.9% patients of COVID-19 were given glucocorticoid therapy, with no effective outcomes observed [12]. Corticosteroid therapy is therefore a double-edged sword.
It is carefully advised to manage patients on long-term corticosteroids by gradually tapering doses to 5–7.5 mg/ daily during this pandemic [13]. These concepts can evolve rapidly based on new data. In the immediate future, the French society of anesthesia and resuscitation (FSAR) proposes the following recommendations (Table 1) [14]. COVID-19 Clinical Guidance for Adult Patients with Rheumatic Diseases on April 11, 2020. Developed by the ACR COVID-19 Clinical Guidance Task Force [9]. A task force formed by rheumatologists and infectious diseases’ specialits covened on March the 26th IN ORDER to provide guidance for rheumatologists about ARD management in pandemic time [9].
Table 1. FSAR guidance for NSAIDs ans corticosteroids intake in COVI19 pandemic time.
| 1 | Patients on anti-inflammatory drugs or other immunosuppressants for chronic pathology should not interrupt their treatment, unless the doctor following them advises otherwise for this pathology |
| 2 | The treatment of ill-tolerated fever or pain in the context of COVID19 or any other respiratory virosis is based on paracetamol, without exceeding the dose of 60 mg / kg / day and 3 g / day. NSAIDs should be banned. |
| 3 | The use of NSAIDs perioperatively remains possible, but must be evaluated on a case-by-case basis and must be kept as short as possible (2 days maximum). In the case of an outpatient journey, the patient must be made aware of the need to stop them immediately at the first sign of infection. |
| 4 | Patients must be informed of the risks and benefits of these treatments and this evaluation must be traced in the patient's medical file. |
| 5 | FSAR advises that use of corticosteroids like dexamethasone, remains possible, but must be strictly limited to the intraoperative and must be evaluated on a case by case basis. |
Those guidance does not replace rheumatologist judgement of the based on clinical severity of the infection or the ARD. Mostly guideline panel recommends underlying general preventive measures, e.g., social distancing and hand hygiene, reduced exposure of patients with minimizing health encounters (Table 2).
Table 2. ACR guidance for Adult patients with Rheumatic diseases.
| 1 | Glucocorticoids should be used at it lowest necessary dose regardless of exposure or infection status if needed to contrôle underlying disease. They should never be stopped precipitously. |
| 2 | Stable patients with no COVID 19 infection or exposure shoud continue their treatment either NSAIDs or immunosuppressants (CsDMARDs, Ts DMARDs or bDMARDs) with no modification or dose reduction in case of life threatning organ lesion |
| 3 | In patients already on corticosteroids guidance does not prohibit its prescription if necessary nor its continuation in stable patients |
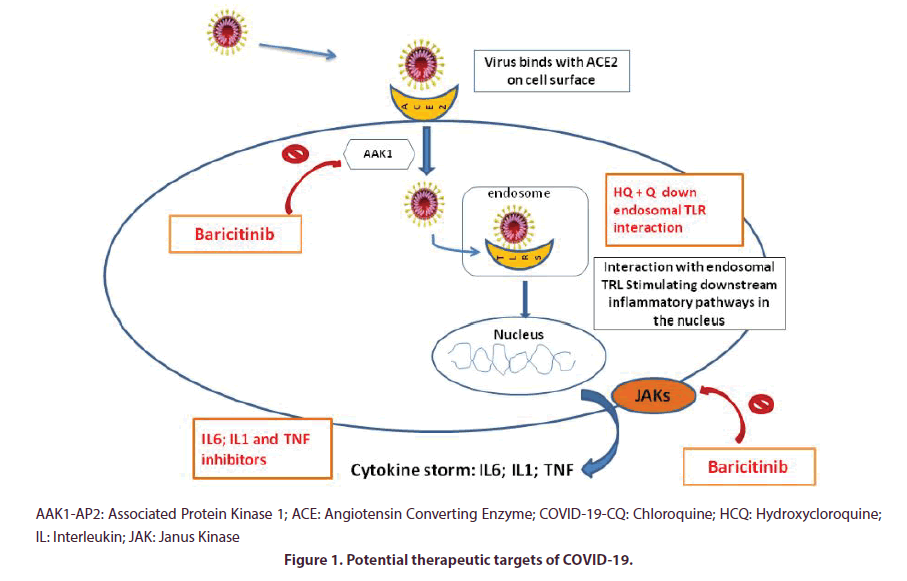
In Rheumatology,especially in chronic inflammatory rheumatic diseases, it is common to prescribe immunosuppressants drugs, such as NSAIDs, corticosteroids, DMARDs either biologic or synthetic such as methotrexate, salazopyrine, leflunomide, JAK inhibitors or hydroxychloroquine (HCQ). The latter is currently at the heart of a fervent debate on its potential therapeutic effects on COVID infection. Some of these are actually major therapeutic pathways trialled or clinically used to date (such as HCQ , tocilizumab or baricitinib) [15]. Potential therapeutic targets of DMARDs possibly effective in the fight against COVID are summarized below (Figure 1).
Hydroxychloroquine and chloroquine in COVID 19
HCQ a sulfate derivative of Chloroquine (CQ) (N4-(7-Chloro-4-quinolinyl)-N1, N1-diethyl-1,4- pentanediamine) with introduced hydroxyl group , ( both synthetic anti malarial drugs) was first commercialized in 1946 and proved to be less (~40%) toxic than CQ in animals [16]. CQ and HCQ are treatments whose rheumatological indication is broad in RA, systemic lupus. It has been widely prescribed for decades with few serious side effects and a confirmed immunomodulatory effect. Its off-label prescription in covid 19 is mainly based on its invoked intracellular action [16,17].
After receptor binding, lysosomal proteases cleave the spike protein releasing the signal peptide that mediates intracellular entry [18]. At therapeutic concentrations CQ is able to increase the endosomal pH required for virus/cell fusion in order to inhibit the toll-like receptor activity and to interfere with terminal glycosylation of the cellular receptor ACE2. Thus, CQ may negatively influence the virus-receptor binding, resulting in a potential effect of the drug on both entry and post-entry stages of the SARS CoV infection [19-21].
In fact, both CQ and HCQ can inhibit major histocompatibility complex class II expression, antigen presentation and immune activation (reducing CD154 expression by T cells) via toll-like receptor signaling and cGAS stimulation of interferon genes. As a main consequence, CQ and HCQ can reduce the production of pro-inflammatory cytokines, such as IL-1, IL-6, interferon α and Tumour Necrosis Factor (TNF), main cytokines involved in the cytokine storm. These immunomodulatory effects may synergize their antiviral effects in the treatment of COVID-19 [22].
These mechanisms may be inhibited by therapies such as CQ. Data supports its clinical benefice in the management of CoViD-19 infected patients as observed in ameliorated imaging and shortening of the diseases course, in inhibiting the worsening of pneumonia, accelerating a virus negative conversion, according to the news briefing. Severe adverse reactions to CQ phosphate were not noted in the aforementioned patients [23]. These facts still are preliminary since positive studies has been methodologically criticized. Consequently, for rheumatic patients chronically taking antimalarial drugs is not to discontinue them, considering the antiviral efficacy and the immunomodulatory rather than immunosuppressive effect [24].
Low dose Methotrexate
To date there is no evidence that low-dose of methotrexate for auto-immune disease, may be associated with more infection rates with COVID 19 virus nor with severe forms. A recent systematic review and meta-analysis confirmed the absence of an increased risk of infection in patients taking MTX (RR: 1.14;95% CI,0.98–1.34). However, there is no data on the risk of stratified infection by pathogen [25-27].
Overall, MTX was associated with increased risk of infection in RA (RR: 1.25; 95% CI, 1.01–1.56; p = 0.04; I2 = 0%) but not in other non-RA Inflammatory rheumatic disease populations. There was no increased risk of total infections (RR: 1.14; 95% CI, 0.98– 1.34; p = 0.10; I2 = 0%) or serious infections (RR: 0.76; 95% CI, 0.11–5.15; p = 0.78; I2 = 0%) in all included Inflammatory rheumatic diseases [28].
Although most patients on MTX should continue taking it, if a patient gets COVID-19 symptoms, although there isn’t any solid data at this time, rheumatologists believe that MTX should be stopped temporarily to up odds of fighting off the virus [29,30].
Leflunomide
Leflunomide is an immunosuppressive drug the mode of action of which is to block mitochondrial dihydroorotate dehydrogenase, reduce de novo pyrimidine synthesis and diminish activated lymphocytes expansion [31,32]. In addition, it inhibits protein kinase activity and the NF- κB signaling system in B and T lymphocytes [33,34]. Leflunomide has been shown to have an antiviral effect towards cytomegalovirus [35], herpes virus [36] and HIV-1[37] and has been used for treatment of polyomavirus-associated nephropathy [38].
The pathogenesis underlying the antiviral effect remains ill defined. Some of it may be explained by the partial inhibition of IL-17 production. Peripheral blood lymphocytes are activated with reduced production of IL- 17 and TNF-α, which mostly initiate proinflammatory reactions [39].
Furthermore, A771726, an active leflunomide metabolite, remarkably suppresses the production of IL- 1β and IL-6, which inhibits the differentiation of IL-17- producing cells [40]. in vivo experimentation objectified that A771726 treatment leaded to an attenuated STAT3 activity in CD4+ T cells and diminished Th17 cell numbers [41].
To date, there were no clinical trials assessing Leflunomide antiviral effect. Its suppressive effect on IL-17 production has been shown in RA patients [42]. Moreover, leflunomide–MTX combination therapy tested in these patients significantly decreased circulating Th17 cells and the plasma levels of IL-17 and was associated with ameliorated RA symptoms [42].
The most frequent infection site was respiratory tract and bacteria were responsible for three quarters of all infections [43]. Usually, infections are more serious when the patients are on biotherapy (especially etanercept and infliximub) [44]. Disease activity and disease duration were also been reported to be crucial elements in the occurrence of infections: the more active the disease, the greater the risk of infection [45,46].
In a 2-week follow-up survey contact to outpatients with chronic arthritis taking either biological diseasemodifying antirheumatic drugs (bDMARDs) or targeted synthetic disease- modifying antirheumatic drugs (tsDMARDs) at biological outpatient clinic in Pavia in? Lombardy During the first month, 320 patients were enrolled in the study (68% female, mean age 55 ± 14 years) treated with either bDMARDs or tsDMARDs (57% RA, 43% SpA , 52% treated with tumour necrosis factor inhibitors, 40% with other bDMARDs and 8% with tsDMARDs). Thirteen patients were diagnosed with COVID 19 infection. All patients with infection symptoms temporarily stopped their treatment at the time of symptom onset. During the study there have been no significant relapses of the rheumatic disease. None of them developed severe respiratory complications nor died. Nevertheless, this does not allow yet to conclude on the rate of SARS- CoV-2 infection in patients with rheumatic diseases, nor on their outcome [47].
Biological treatment during COVID-19 outbreak
Risk infection is known to be slightly higher (from 1.5 up to 2 fold) in RA patients treated with biologics DMARDs compared to those treated with conventional synthetic DMARDs [48].
To date there is no reliable data on COVID 19 infection features in patients taking biological treatment. Most of papers concerning this subject compared it with seasonal influenzae or H1N1 or try to explain the possible effects of biologics based on immune system dysregulation caused by SARS COV2 which is still ill defined. Taking into consideration all of these facts caution is still needed [49].
Pro inflammatory cytokines in COVID 19
Pro inflammatory cytokines are found to be reaching high levels (i.e. tumor necrosis factor (TNF)-α, interleukin (IL)-2R ; and IL-6, IL-8, IL10 and TNF-α and immunoglobulins (IgA, IgG and IgM) and complement proteins (C3-C4) [50].
It’s a syndrome characterized by a massive and fatal hypercytokinaemia with multiorgan failure [51,52] which are caused by an increased levels of a number of cytokines (interleukin-1β [IL-1β], IL-2, IL-6, IL-7, IL-8, tumor necrosis factor-α [TNF]), chemokines and CC-chemokine Ligand 2 [CCL2]) [50]. This syndrome could be triggered by viral infections in 50% of cases [51,52]. It could also be triggered by rheumatic diseases.
Indicators of inflammation have been sought in mild, serious and critical patients to quickly discriminate those whose pneumonia could progress to a severe or fatal form. The criteria associated with the severity of the disease were age, interleukin 2 , interleukin 6 (IL- 6), interleukin 8, interleukin 10, TNF α, C Reactive Protein (CRP), ferritin, procalcitonin, white blood count, lymphocyte count, neutrophil count and eosinophil count [53], with in conclusion a maximum risk for those over 67 years of age, with an interleukin 2 level greater than 793.5 U/mL, CRP greater than 30.7 ng / mL, ferritin greater than 2 252 μg / L, leukocytosis greater than 95 × 108 L-1 or a number neutrophils larger than 7,305 × 106 L-1 [53].
ANTI TNF α
TNF is a proinflammatory cytokine secreted in the earliest stages of inflammation. Inhibitors of TNF α may be used in autoimmune diseases, RA and diabetes treatment. Despite the fact that patients on an anti- TNF agents have a similar risk of Influenzae infection compared with the general population (seasonal and H1N1) [54] some may contraindicate it according to the general rule of stopping TNF inhibitors in active or high risk of infection. Moreover it has been objectified that in the « cytokine storm » responsible for major lung and visceral injuries, TNF α levels are relatively high [55]. That can suggest a potential « protective » effect of TNF blockers by reducing alveolar damage [56].
An author highlighted, indeed, its potential therapeutic effect in the Acute Respiratory Distress Syndrome (ARDS) caused by SARS coronavirus. He suggested the use of etanercept as first arguing that it has a long record of safety, a short half-life, and a reduced immunogenicity [57]. Another study performed on pigs observed that etanercept was not associated with decrease in disease severity in a model of acute virus-endotoxin mediated respiratory disease [58]. Moreover, anti TNF agents have a protective pulmonary effect in patients with RA. It has been reported that the frequency of Interstitial Lung Disease (ILD) in RA patients taking anti-TNF was lower than those without anti-TNF [59].
H1N1 influenza study performed on mice showed an inhibition of the overproduction of inflammatory cytokines (TNF and IL6) associated to a reduced lung injury and a low mortality of mice treated with etanercept for lethal influenza [60]. Currently, there is a randomized, open-labelled, controlled trial evaluating adalimumab in severe COVID-19 pneumonia.
Preliminary results are promising [61]. These studies need to be consolidated with more clinical trials to insure that TNF-alpha inhibition will not increase the risk of SARS-CoV-2 infection.
Interleukine 17 Inhibitors (IL17 inhibitors) IL 17 is a crucial mediator of inflammation and immunopathology as well [62] notably during psoriasis and diverse chronic inflammatory rheumatic diseases [63].
In fact, IL-17 plays a key role in protective immune response to infectious microorganisms in a controversial ways. It can enhance effective antiviral immune responses. Oddly, it may also promote and exacerbate virus-induced illnesses. Those antagonist actions on viral infection are poorly known in COVID 19 particular cases [64].
However, it is argued that IL 17 blockers may have a benefic role in regularizing COVID-19’s overwhelming host cytokine storm and thus improve the course of ARDS-related. This effect was more important when IL 17 blockers were associated with anti IL6 agents [65].
Concluding from previous data on IL 17 blockers in seasonal influenza, it is unlikely that these agents would cause higher rates of COVID-19. For instance, Secukinumab does not damage patients’ response to seasonal influenza vaccines establishing a possible proof that immune response to viruses is still active under IL 17 blockade [66].
Based on studies comparing infectious side effects occurring under IL 17 antagonists, one can presume that these agents affect poorly infection rates compared to placebo groups [67]. Real life data in the Psoriasis Longitudinal Assessment and Registry (PSOLAR), showed that serious infection rates were even lower for ustekinumab than for naïve treatment psoriasis patients [68]. Herpes virus seems to affect slightly more patients on IL 17 inhibitors with no statistically significant difference [69].
Anti CD20
Rituximab (RTX) is a monoclonal antibody against CD20 receptor, a molecule located on the B cell surfaces [70]. Leukopenia, hypogammaglobulinemia, and neutropenia are common anti CD20 side effects related to B-cell depletion [70]. Therefore, RTX has the potential to enhance infection risks [71]. In a recent systematic review and meta‐analysis, authors found that global infection frequency was to 44.9% and serious infection 4.1% in patients treated with RTX [72]. This supposes that RTX adds no infection risks for patients with RA compared to non‐RTX’s [72] Though, a granulomatosis with polyangiitis’ patient treated with RTX and corticosteroids have had severe pneumonia of COVID 19. The course of the infection was slower than habitual forms and he recovered after 29 days with a negative nasopharyngeal RT-PCR [73] Both glucocorticoids and RTX may have limited the cytokine storm and delayed the worsening of the infection.
Although we cannot draw any definitive conclusion from this observation and given this uncertainty it is wise not to start treatment with anti CD20 during this COVID 19 pandemic.
Anti Il1
IL 1 is another important cytokine of the inflammatory response during infections and autoimmune diseases especially on COVID 19 infection [41]. Currently there is no clear evidence of efficacy of IL1 inhibitors on severe forms of COVID 19 (with cytokine storm). Nevertheless, there is one study which highlighted benefits of intravenous continuous anakinra infusions (up to 2400 mg/d) in five patients with severe secondary hemophagocytic lymphohistiocytosis refractory to all other therapies including subcutaneous anakinra [74]. This method resulted in rapid reponse with clinical improvement in 4 of the 5 patients. Subsequently, 3 of them have been maintained on anakinra or canakinumab, with no recurrence of MAS. Based on physiopathologic similarities, this protocol may be considered in the treatment of cytokine storm COVID 19 presentation.
In this indication, there was only one study (abstract), in which authors proposed that inflammation by coronavirus may be inhibited by anti-inflammatory cytokines belonging to the IL-1 family [41].
IL6 AND COVID 19 : ANTI IL6 efficiency: myth or reality ?
A high level of IL-6 at admission would be associated with a severe clinical manifestation [75] The decrease in IL-6 seems to indicate the effectiveness of the treatment, while its increase indicates a progression of the disease. The evolution of the level of IL-6 is therefore a useful marker for monitoring the disease in patients with severe Covid-19 [75].
A study shows that the viral load is directly correlated with the blood level of IL 6, which makes this molecule an important prognostic marker [76]. This increase associated with a high blood iron level should lead to the search for secondary hemophagocytic lymphohistiocytosis [76].
Tocilizumab (TCZ), one of the immunosuppressants used for the treatment of RA , and a variety of clinical conditions with Cytokine Release Syndrome (CRS) such as those associated with Chimeric Antigen Receptor T-cell (CAR-T) therapy [77]. Similarities between CRS and cytokine storm may have leaded to its use in severe forms of COVID 19.
TCZ was comprised in the treatment guidelines by the National Health Commission of China after a positive study by the University Science and Technology of China [78]. A study evaluated the use of a single dose of intravenous TCZ (400 mg) in 21 patients with COVID-19 who either had respiratory distress, hypoxemia or required intensive care support [79]. Nineteen of these 21 patients recovered with discharge from hospital within 14 days and lung opacities vanished [79] suggesting efficiency of TCZ on critical viral infection with respiratory distress syndrome.
However, repeated doses were suggested in an other study including 15 critically ill patients [80]. The study showed that repeated and even lower doses may improve the clinical outcome of critically ill patients. Therefore, in addition to the safety advantage,a repeated dose of TCZ is more likely to be effective than glucocorticoid in the treatment of COVID‐19.
Based on IL6 basic levels, this study suggested the following rythm of administration:
Single dose is expected to benefit seriously ill patients with 10 times elevated IL‐6 in serum. Moderately ill patient with almost 90 times of normal, could benefit from repetitive TCZ therapy. Taking into consideration the long half-time of TCZ and the possible saturation of the dedicated receptors, dose can be reduced when repeated use in case of extremely high levels of IL6 [80].
JAK inhibitors
Janus kinases (JAK) 1 and 2 are crucial actors of inflammation and the enzyme AP-2-associated protein kinase 1 (AAK1) plays a role in viral cellular entry. Janus Kinase inhibitors type 1 and 2 are hypothesized to enhance antiviral response by reducing virus entry and regulating aberrant inflammation [81]. Based on information generated by bioinformatics analysis, baricitinib may help reduce SARS-CoV-2 infection by inhibiting AAK1 and also possibly dampening the resulting inflammation by JAK1/2 inhibition [81]. Baricitinib, a JAK inhibitor, binds the cyclin G-associated kinase (another actor of endocytosis) as well [81].
On therapeutic dosing of barcitinib (either as 2 mg or 4 mg once daily) inhibits AAK1. Some authors suggested it could be trialled using a selected patient population , in order to reduce both the viral entry and the inflammation using endpoints such as the MuLBSTA score, an early warning model for predicting mortality in viral pneumonia [81].
Inhibition of JAK 2 was proposed as a potential target in COVID 19 cytokine storm associated in TH17 response regulation under the name of fedratinib [82].
Management of rheumatic patients during the covid-19 pandemic: take home messages
Patients with chronic inflammatory rheumatism, as the rest of the general population, are called upon to follow the general hygiene measures imposed by their countries [83]. In addition to these hygiene measures. There are some precautions to take for patients with inflammatory rheumatism. Rheumatology societies elaborated recommendations during coronavirus disease 19 outbreak . They all agree on the following points:
• Not to interrupt immunosuppressive treatment
• To Follow the general guidance for infection prevention suggested by WHO
• Encouraging telecommuting
• CQ and HCQ seem to have some efficacy on SARS-CoV2 infection
As the WHO promotes that the general population take some basic protective measures, rheumatologists recommends to date the same measures to their patients [84]. The Table 3 summarizes current guidelines of the EULAR, SFR and ACR societies for ARD management in COVID19 pandemic depending on infection status of patients.
Table 3. summerizes ACR, EULAR and SFR guidelines for drug management in rheumatic diseases depending on infection status of patients.
| ACR | SFR | EULAR | |
| General statements | |||
| Glucocorticoids | If indicated should be used at the lowest dose possible (M/H) Should not be abruptly stopped, regardless of exposure or infection status (H). | To be continued | To be continued vaccination against influenza, whooping cough and pneumococcus |
| Ongoing treatment of stable patients in the absence of infection or SARS-CoV-2 exposure | |||
| HCQ/CQ, SSZ, MTX, LEF, immunosuppressants, biologics, anti-JAK and NSAIDs | To be contunued | To be continued If possible, stop NSAIDs (switch to paracetamol) | To be continued |
| Treatment of newly diagnosed or active rheumatic diseases, in the absence of infection or SARS-CoV-2 exposure | |||
| Active Inflammatory Arthritis | |||
| HCQ/CQ | Should be continued, when not available; switching to a different DMARD (M/H). | ||
| IL-6 inhibitor | Should be continued, when not available; switching to a different biologic (M). | ||
| bDMARDs | should be started if necessary | ||
| anti JAK | Uncertainty regarding the use of JAK inhibitors in this situation. (M) | ||
| csDMARDs | may be started or switched if active or newly diagnosed inflammatory arthritis (M) | ||
| Low-dose glucocorticoids or NSAIDs | If indicated may be started (M/H). | ||
| Other Rheumatic Diseases | |||
| In patients with systemic inflammatory or vital organ-threatening disease (e.g., lupus nephritis or vasculitis | High-dose glucocorticoids or immunosuppressants may be initiated (M). | ||
| In patients with newly diagnosed Sjögren’s | Given the paucity of data proving efficacy, HCQ/CQ should not be started (M). | ||
| Ongoing treatment of stable patients following SARS-CoV-2 exposure (without symptoms related to COVID-19) | |||
| HCQ, SSZ, and NSAIDs | May be continued (M/H). | ||
| Immunosuppressants, non-IL-6 biologics, and JAK inhibitors | should be stopped temporarily, pending a Negative test result for COVID-19 or after 2 weeks of symptom-free observation (M). | ||
| IL-6 inhibitors | may be continued (M) | ||
| csDMARDs | The panel noted | ||
| MTX or LEF | Uncertainty re: temporarily stopping in this situation. | ||
| Rheumatic disease treatment in the context of documented or presumptive COVID-19 infection | |||
| HCQ/CQ | may be continued | May be continued | Discuss this with your doctor or rheumatologist. |
| SSZ, MTX, LEF, immunosuppressants, non-IL-6 biologics, and JAK inhibitors | Should be stopped or held (M/H). | Should be stopped | |
| NSAIDS | Should be stopped For patients with severe respiratory symptoms, (M). | Should be stopped | |
| IL-6 inhibitors | May be continued in select circumstances, as part of a shared decision-making process (M). | ||
| Glucocorticoids | To be continued | ||
Conclusions
As the WHO promotes that the general population take some basic protective measures, rheumatologists recommends to date the same measures to their patients. Furthermore, patients should be regularly followed by their treating physicians by promoting remote consultation. In fact, guidance does not replace the rheumatologist’s judgement.
Stable patients with no COVID 19 infection or exposure should continue their treatment either NSAIDs or immunosuppressants (CsDMARDs, TsDMARDs or bDMARDs) with no modification or dose reduction in case of life threatening organ lesion. Anti malarial drugs are not to be interrupted considering their supposed antiviral efficacy and their immunomodulatory rather than immunosuppressive effect. The anti Il 6 is also not to be stopped given its action on the inflammatory reaction caused by COVID19. In case of confirmed COVID 19 infection it is advised to temporarily stop immunosuppressive treatments.
Author agreement
I, Rim DHAHRI Here by certify that all authors have seen and approved the final version of the manuscript being submitted. I on behalf of all authors warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Conflict of Interest Declaration
Authors declare having no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Mackenzie JS, Smith DW. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiol. Aust. MA20013 (2020).
- Mehta P, Daniel F, McAuley et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 395(10229) (2020).
- Figueroa-Parra G, Aguirre-Garcia GM, Gamboa-Alonso CM et al. Are my patients with rheumatic diseases at higher risk of COVID-19? Ann. Rheum. Dis. 79(6), 839-840 (2020).
- Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. 134(5), 543-545 (2020).
- Day M. Covid-19: European drugs agency to review safety of ibuprofen. BMJ. m1168 (2020).
- https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Anti-inflammatoires-non-steroidiens-AINS-et-complications-infectieuses-graves-Point-d-Information-actualise-le-20-05-2020
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet. Respir. Med. 8(4), e21 (2020).
- Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists BMJ. (2020)
- Mikuls TR, Johnson SR, Fraenkel L et al. American College of Rheumatology guidance for the management of adult patients with rheumatic disease during the COVID-19 pandemic. Arthritis. Rheumatol. 72(8), 1241-1251 (2020).
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 395(10223), 473-475 (2020).
- Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395, 507–513 (2020).
- Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020).
- Misra DP, Agarwal V, Gasparyan AY et al. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol 39(7):2055-2062 (2020).
- https://sfar.org/download/recommandations-sfar-sfetd-sur-esins/?wpdmdl=25767&refresh=5e9daf0a4441f1587392266
- Favalli EG, Ingegnoli F, De Lucia O. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun. Rev. 19(5), 102523 (2020).
- Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell. Discov. 6(1),16 (2020).
- https://www.nih.gov/news-events/news-releases/nih-clinical-trial-hydroxychloroquine-potential-therapy-covid-19-begins
- Zheng Y, Shang J, Yang Y et al. Lysosomal proteases are a determinant of coronavirus tropism. J. Virol. 92, e01504-e01518 (2018).
- Vincent MJ, Bergeron E, Benjannet S et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2(1), 69 (2005).
- Devaux CA, Rolain JM, Colson P et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. (2020).
- Savarino A, Di Trani L, Donatelli I et al. New insights into the antiviral effects of chloroquine. Lancet. Infect. Dis. 6(2), 67-69 (2006).
- Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. (2020).
- https://www.jstage.jst.go.jp/article/bst/14/1/14_2020.01047/_article
- Ceribelli A, Motta F, De Santis M et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J. Autoimm. 109, 102442 (2020).
- Yao X, Ye F, Zhang M et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71(15), 732-739 (2020).
- Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell. Discov. 6(1), 16 (2020).
- Russell B, Moss C, George G et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 14, 1022 (2020).
- Ibrahim A, Ahmed M, Conway R et al. Risk of Infection with Methotrexate Therapy in Inflammatory Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 8(1), 15 (2019).
- https://www.eular.org/eular_guidance_for_patients_covid19_outbreak.cfm
- https://creakyjoints.org/treatment/methotrexate-immunosuppressing-coronavirus
- Cheng HY, Li WJ, Li XM et al. Pathogenicity of blood orf virus isolates in the development of dairy goat contagious pustular dermatitis. Vet. Microbiol. 219, 178-182 (2018).
- Granich R, Gupta S, Hersh B et al. Trends in AIDS Deaths, New Infections and ART Coverage in the Top 30 Countries with the Highest AIDS Mortality Burden; 1990–2013. PLoS One. 10(7), e0131353 (2015).
- Schmidt ME, Varga SM. The CD8 T Cell Response to Respiratory Virus Infections. Front. Immunol. 9, 678 (2018).
- Manna SK, Mukhopadhyay A, Aggarwal BB. Leflunomide Suppresses TNF-Induced Cellular Responses: Effects on NF-κB, Activator Protein-1, c-Jun N-Terminal Protein Kinase, and Apoptosis. J. Immunol. 165(10), 5962-5969 (2000).
- Fc P. Immunity and Fibrogenesis: The Role of Th17/IL-17 Axis in HBV and HCV-induced Chronic Hepatitis and Progression to Cirrhosis. Front. Immunol. 8, 1195 (2017).
- Park H, Li Z, Yang XO et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6(11), 1133-1141 (2005).
- Huber M, Heink S, Pagenstecher A et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J. Clin. Invest. 123(1), 247-260 (2013).
- Wang S, Li J, Wu S et al. Type 3 innate lymphoid cell: a new player in liver fibrosis progression. Clin Sci 132(24), 2565-2582 (2018).
- Passos ST, Silver JS, O’Hara AC et al. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol 184(4), 1776-1783 (2010).
- Chen S, Noordenbos T, Blijdorp I et al. Histologic evidence that mast cells contribute to local tissue inflammation in peripheral spondyloarthritis by regulating interleukin-17A content. Rheumatology (Oxford). 58(4), 617-627 (2019).
- Kritas SK, Ronconi G, Caraffa AI et al. Mast Cells Contribute to Coronavirus-Induced Inflammation: New Anti-Inflammatory Strategy. J. Biol. Regul. Homeost. Agents. 34(1), 9-14 (2020).
- Zhong W, Zhao L, Liu T et al. IL-22-producing CD4+T cells in the treatment response of rheumatoid arthritis to combination therapy with methotrexate and leflunomide. Sci. Rep. 7, 41143 (2020).
- Germano V, Cattaruzza MS, Osborn J et al. Infection risk in Rheumatoid Arthritis and Spondyloarthropathy patients under treatment with DMARDs, Corticosteroids and TNF-α antagonists. J. Transl. Med. 12(1), 1-10 (2014).
- Salliot C, Gossec L, Ruyssen-Witrand A et al. Infections during tumour necrosis factor-α blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology (Oxford). 46(2), 327-334 (2007).
- Listing J, Strangfeld A, Kary S et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis & Rheumatism 52(11), 3403-3412 (2005).
- Germano V, Cattaruzza MS, Osborn J et al. Infection risk in Rheumatoid Arthritis and Spondyloarthropathy patients under treatment with DMARDs, Corticosteroids and TNF-α antagonists. J. Transl. Med. 12, 77 (2014).
- Monti S, Balduzzi S, Delvino P et al. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann. Rheum. Dis. 79(5), 667-668 (2020).
- Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford). 52(1), 53-61 (2013).
- Sriwijitalai W, Wiwanitkit V. Biological treatment during COVID-19 outbreak. J. Dermatolog. Treat. 31(4), 324 (2020).
- Wan SX, Yi QJ, Fan SB et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 Novel Coronavirus Pneumonia (NCP). medRxiv. (2020).
- Yildiz H, Van Den Neste E, Defour PJ et al. Adult haemophagocytic lymphohistiocytosis: a review. QJM. Int. J. Med. (2020).
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A et al. Adult haemophagocytic syndrome. Lancet. 383(9927), 1503–1516 (2014).
- Gong J, Dong H, Xia SQ et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. medRxiv. (2020).
- Shale M, Czub M, Kaplan GG et al. Review: Anti-tumor necrosis factor therapy and influenza: keeping it in perspective. Therap. Adv. Gastroenterol. 3(3), 173-177 (2010).
- Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223), 497-506 (2020).
- Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31(9), 2566-2573 (2001).
- Tobinick E. TNF-α inhibition for potential therapeutic modulation of SARS coronavirus infection. Curr. Med. Res. Opin. 20(1), 39-40 (2004).
- Atanasova K, Van Gucht S, Van Reeth K. Anti-TNF-α therapy does not ameliorate disease in a model of acute virus-endotoxin mediated respiratory disease in pigs. Veter. Immunol. Immunopathol. 137(1-2), 12-19 (2010).
- Herrinton LJ, Harrold LR, Liu L et al. Association between anti-TNF- α therapy and interstitial lung disease: anti-tnf- α and interstitial lung disease. Pharmacoepidemiol. Drug. Saf. 22(4), 394-402 (2013).
- Shi X, Zhou W, Huang H et al. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit. Care. 17(6), R301 (2013).
- http://www.chictr.org.cn/showprojen.aspx?proj=49889
- Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 18(6), 612-621 (2017).
- Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 9, 1682 (2018).
- Ma WT, Yao XT, Peng Q et al. The protective and pathogenic roles of IL-17 in viral infections: friend or foe? Open. Biol. 9(7), 190109 (2019).
- Casillo GM, Mansour AA, Raucci F et al. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? Pharmacol. Res. (2020).
- Noseda CE, Stevens M, Gaitatzis N et al. Treatment with the Interleukin-17A-Blocking Antibody Secukinumab Does Not Interfere with the Efficacy of Influenza and Meningococcal Vaccinations in Healthy Subjects: Results of an Open-Label, Parallel-Group, Randomized Single-Center Study. Clin. Vaccine. Immunol. 19(10), 1597–1602 (2012).
- Sbidian E, Chaimani A, Afach S et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Cochrane. Database. Syst. Rev. 12(12), CD011535 (2017).
- Kalb RE, Fiorentino DF, Lebwohl MG et al. Risk of Serious Infection With Biologic and Systemic Treatment of Psoriasis: Results From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA. Dermatol. 151(9), 961-969 (2015).
- Shalom G, Naldi L, Lebwohl M et al. Biological treatment for psoriasis and the risk of herpes zoster: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Dermatol. Treat. 30(6), 534-539 (2019).
- Boross P, Leusen JHW. Mechanisms of action of CD20 antibodies. Am. J. Cancer. Res. 2(6), 676-690 (2012).
- Barmettler S, Ong MS, Farmer JR et al. Association of Immunoglobulin Levels, Infectious Risk, and Mortality With Rituximab and Hypogammaglobulinemia. JAMA. Netw. Open. 1(7), e184169 (2019).
- Shi Y, Wu Y, Ren Y et al. Infection risks of rituximab versus non‐rituximab treatment for rheumatoid arthritis: A systematic review and meta‐analysis. Int. J. Rheum. Dis. (2019).
- Guilpain P, Bihan CL, Foulongne V et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid-19: lessons from a case with severe pneumonia. Ann. Rheum. Dis. (2020).
- Monteagudo LA, Boothby A, Gertner E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR. Open. Rheumatol. (5), 276-282 (2020).
- Liu T, Zhang J, Yang Y et al. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv. (2019).
- Chen X, Zhao B, Qu Y et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. (2020).
- Le RQ, Li L, Yuan W et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 23(8), 943-947 (2018).
- https://www.pharmaceutical-technology.com/news/roche-actemra-coronavirus-complications
- https://sfar.org/download/effective-treatment-of-severe-covid-19-patients-with-tocilizumab
- Luo P, Liu Y, Qiu L et al. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 92, 814-818 (2020).
- Richardson P, Griffin I, Tucker C et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 395(10223), e30–e31 (2020).
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Inf. (2020).
- Michaud K, Wipfler K, Shaw Y et al. Experiences of Patients with Rheumatic Diseases in the US During Early Days of the COVID-19 Pandemic. ACR Open Rheumatol. (2020).
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public