Research Article - Journal of Experimental Stroke & Translational Medicine (2009) Volume 2, Issue 2
Rodent Stroke Model Guidelines for Pre-clinical Stroke Trials (1st edition).
- *Corresponding Author:
- Shimin Liu, M.D., Ph.D.
Department of Neurology, Mount Sinai School of Medicine NYU. 1468 Madison Avenue, New York, NY 10029
E-mail: shimin.liu@mssm.edu
Phone: (212) 241-2252
Fax: (212) 241-6971
Abstract
Translational stroke research is a challenging task that needs long term team work of the stroke research community. Highly reproducible stroke models with excellent outcome consistence are essential for obtaining useful data from preclinical stroke trials as well as for improving inter-lab comparability. However, our review of literature shows that the infarct variation coeffi-cient of commonly performed stroke models ranges from 5% to 200%. An overall improvement of the commonly used stroke models will further improve the quality for experimental stroke research as well as inter-lab comparability. Many factors play a significant role in causing outcome variation; however, they have not yet been adequately addressed in the Stroke Therapy Academic Industry Roundtable (STAIR) recommendations and the Good Laboratory Practice (GLP). These critical factors include selection of anesthetics, maintenance of animal physiological environment, stroke outcome observation, and model specific factors that affect success rate and variation. The authors have reviewed these major factors that have been re-ported to influence stroke model outcome, herewith, provide the first edition of stroke model guidelines so to initiate active discussion on this topic. We hope to reach a general agreement among stroke researchers in the near future with its succes-sive updated versions.
Keywords
Acute stroke; animal model; neuroprotection; middle cerebral artery occlusion; guidelines; consistency
Abbreviation
AC: Alternating Current
ACA: Anterior Cerebral Artery
AComA: Anterior Communicating Arteries
AMPA: Amino-3-Hydroxy-5-M Ethyl-4-Isoxazol-Propionic Acid
ATP: Adenosine Triphosphate
BBB: Blood-Brain-Barrier
CBF: Cerebral Blood Flow
CCA: Common Carotid Artery
CNS: Central Nervous System
DC: Direct Current
dMCAO: Distal MCA Occlusion
ECA: External Carotid Artery
GLP Good Laboratory Practice
GABA: γ-Aminobutyric-Acid
H&E: Hematoxylin And Eosin
ICA: Internal Carotid Artery
ISCBFM: International Society For Cerebral Blood Flow And Metabolism
LDF: Laser Doppler Flowmetry
MAP2: Microtubule-Associated Protein 2
MCA: Middle Cerebral Artery
MCAO: Middle Cerebral Artery Occlusion
NMDA: N-Methyl-D-Aspartate
PaCO2: Partial arterial Carbon Dioxide Pressure
PaO2: Partial arterial Oxygen Pressure.
PComA: Posterior Communicating Arteries
PID: Proportional–Integral–Derivative
PLL: Poly-L-Lysine
pMCAO: Proximal MCA Occlusion
pSDM: Percentage Of Standard Deviation To Mean
PTA: Pterygopalatine Artery
rCBF: Regional Cerebral Blood Flow
SAH: Subarachnoid Hemorrhage
SD: Standard Deviation
SEM: Standard Error Of Mean
SFES: Society For Experimental Stroke
SOP: Standard Operational Procedures
STAIR: Stroke Therapy Academic Industry Roundtable
tCCAO: Temporary Common Carotid Artery Acclusion
TTC: 2,3,5-Triphenyltetrazolium Chloride
Definition
pSDM: percent of SD to mean
Introduction
The Stroke Therapy Academic Industry Roundtable (STAIR) was established in order to address the challenges encountered in finding an effective neuroprotective therapy for acute stroke (Fisher et al. 2007; Stroke Therapy Aca-demic Industry Roundtable 1999). Besides the STAIR’s concern for study design, other issues relating to experi-mental stroke research, especially animal stroke modeling have been raised (Dirnagl 2006; Savitz 2007; van der Worp et al. 2005). This is an important issue, and it is now rec-ommended that any stroke model that aims to achieve scientific and therapeutic value must meet certain require-ments. The model must be both highly consistent in induc-ing injury, performed under conditions to avoid co-founding factors influencing outcomes and widely available to most investigators. With the STAIR guidelines providing an ex-cellent framework for the design of preclinical stroke trials, a detailed guidance for conducting individual experiments using stroke models will further improve model consistency, reliability and inter-lab comparability. A review of literature shows that the infarct variation coefficient of commonly performed stroke models ranges from 5% to 200% (see following paragraphs). Many factors play a significant role in causing outcome variation; however, they are not fully defined in the STAIR guidelines. These critical factors in-clude selection of anesthetics, maintenance of animal phy-siological environment, stroke outcome observation, and animal species used.
Here we provide the first edition of stroke model guidelines (SMG) so as to initiate an active discussion on this topic, with the ultimate aim of reaching a general agreement among stroke researchers in future up-dated versions. Be-cause rodents are the mostly commonly used animals for preclinical stroke trials, the first version of SMG starts with rodent stroke models. In its successive versions, the con-tents may expand into stroke models of other species. The SMG ,starts with a general overview of the design and set-up of a typical preclinical stroke trial, followed by more de-tailed guidelines on the implementation of each step.
General Guidance for a Prec-Linical Stroke Trial
Before starting an experimental preclinical stroke trial the following steps are necessary for ensuring a good quality study. Specific guidance for most steps is provided in sub-sequent paragraphs.
1. Revisit the latest STAIR criteria for achieving an optimized study design
2. Select the most appropriate stroke model for your study
3. Determine stroke model parameters, such as an-ticipated infarct size and surgical procedure
4. Determine the use of anesthetics
5. Determine the components of the inhaled gas
6. Determine the necessity and settings of intubation and ventilation
7. Set up monitoring for arterial blood pressure, blood gases, blood glucose
8. Set up temperature monitoring and maintenance
9. Set up regional cerebral blood flow monitoring
10. Determine a protocol for post-operational care
11. Determine the method and timing for infarct vo-lume measurement
12. Determine the appropriate tests and timing for the assessment of functional deficits
13. Do a pilot study and adjust the experimental set-tings for further optimization
14. Assess the compliance of the trial with “Good La-boratory Practice” standards.
Revisiting the Stair Criteria
Guidance
The design of a preclinical stroke trial should start with a revisiting of the latest version of stroke therapy academic industry roundtable (STAIR) criteria. Receiving some useful suggestions with caution from the STAIR criteria may help with improving the study design to some extent although there are debates on some issues that STAIR addressed.
Supporting Discussion
The STAIR criteria provides some useful recommendations for improving the design of preclinical stroke trials.
To date, the STAIR group has met six times discussing and revising their recommendations for preclinical and clinical stroke trials (Fisher 2003; Fisher 2005; Fisher et al. 2009; Fisher et al. 2007; Saver et al. 2009; STAIR Group 1999; STAIR Group 2001). Recommendations provided by the STAIR consortia emphasize the design quality of both ex-perimental and clinical stroke trials. With respect to experi-mental animal stroke trials, STAIR recommendations have highlighted the need for investigators to consider factors such as species and gender differences, clinical relevance of animal models, dose-response determinations, thera-peutic time windows, blood-brain-barrier (BBB) permeability and tissue drug levels, treatment randomization,, physio-logical monitoring, and at least 2 outcome measures cover-ing both acute and long-term endpoints.
The STAIR criteria should be used with caution because there may be conflicts between its suggested late treatment and therapeutic windows.
In the STAIR I, the ideal neuroprotective drug trial was de-scribed thus: “should demonstrate efficacy in at least 2 species, in at least 2 laboratories that use different models, is effective in both permanent and transient focal ischemia, and improves short-term and long-term histological and functional outcomes, even when administered several hours after the onset of ischemia” (STAIR Group 1999). Keeping the therapeutic window in mind, it may be simply impossible to demonstrate robust neuroprotection when the treatment is delivered too late. The therapeutic window is roughly a few hours in rodent MCA occlusion (MCAO) models. For example, in a 300 g rat, a 2-hour duration of transient MCAO produces a large infarct volume of 400-450 mm3, which is similar in size to the infarct caused by permanent MCAO after 24 hours (Greco et al. 2007; Masa-da et al. 2001). Hence, it is likely that a preclinical stroke trial using a 2 hour transient MCAO model and a late treatment time point (e.g., 6h post-MCAO) (Simard et al. 2009; Yin and Zhang 2005) would have missed the thera-peutic window and the opportunity to observe a treatment effect. In this instance, a baseline injury quantification study, performed at different treatment time points (e.g.,, 2, 4, 6 hours post-MCAO) would improve study design and increase the chance of obtaining a positive neuroprotective effect.. Some histopathological methods and diffusion-weighted imaging techniques described in the infarct vo-lume measurement section of this guideline can be used for the detection and quantification of baseline injury start-ing several hours after ischemia.
The STAIR criteria should be used with caution because there may be conflicts between its suggested observational time and the natural history of stroke evolution.
As discussed in more detail below, both the infarction evo-lution and changes in functional deficits have their own natural histories. Measuring infarct volume evolution is time sensitive and methodology dependent (see section on In-farction volume measurement). Assessment of functional recovery is even more complicated, is model depen-dent,and is time sensitive in relation to the recovery pattern and functional test being used (see section of Functional evaluation). Therefore, it may sound arbitrary to recom-mend all outcome measures be performed in 1-3 days and in 7-30 days.
Would the STAIR criteria help with increasing the chance of a true discovery (no, but this was not really its aim)?
As stated in the STAIR I, the purpose of STAIR is “to pro-pose recommendations for ways to optimally preclinically assess neuroprotective and restorative drugs for acute ischemic stroke”. However, these recommendations were condensed into a few designing principles even with the latest update (Fisher et al. 2009), which has been function-ing like an elevated threshold limiting experimental discov-ery entering into clinical trials. These STAIR criteria may help with improving the design quality of preclinical stroke trials, reducing bias and false positive conclusions (Fisher et al. 2009), but has little to do with increasing the chance of scientific discoveries. It is the research direction that holds the chance of scientific breaking through while the optimized methodologies increase the sensitivity for posi-tive findings. The research directions that hold the contin-ued promise for neuroprotection for ischemic stroke has been discussed in our previous review paper (Liu and Le-vine 2008). Optimization of preclinical stroke trials is more complicated than that the STAIR has recommended, which is what we need to address in this stroke model guidelines
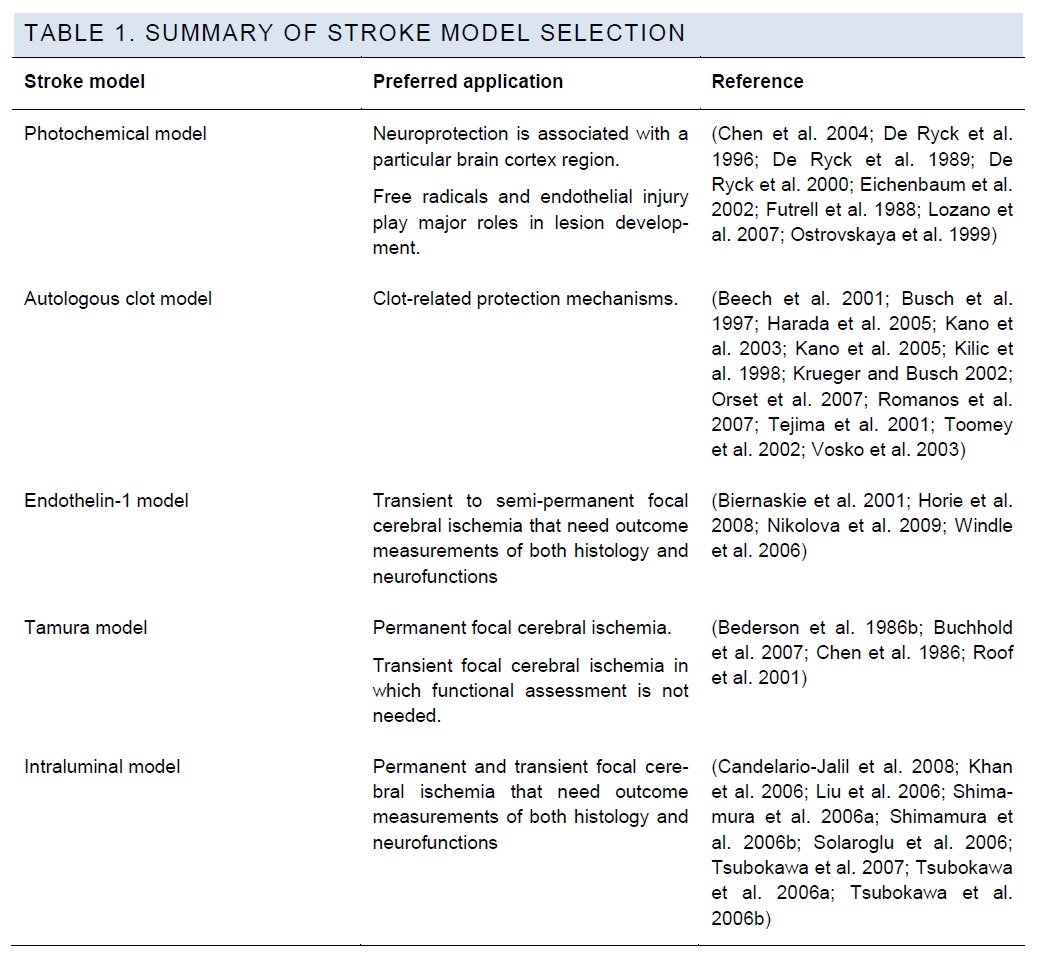
Stroke Model Selection
Various stroke models have been developed to mimic dif-ferent stroke subtypes or pathological mechanisms and can be generally classified into two categories: focal cerebral ischemia models and global cerebral ischemia models. Global ischemia models mimic the clinical conditions of brain ischemia following cardiac arrest or profound system-ic hypotension, focal models represent ischemic stroke, the most common clinical stroke subtype. The most commonly used focal ischemia models are the intraluminal filament model (Koizumi et al. 1986) and the Tamura model (Tamu-ra et al. 1981a). Some additional stroke models involve special mechanisms to induce artery occlusion/ischaemia, such as the thromboembolic, endothelin and photochemical models.
Guidance
There are mainly two factors that influence the selection of in vivo stroke models for preclinical trials. These are the potential protection mechanism of the neuroprotective can-didate and the highest achievable model quality with a par-ticular lab setting.
For examples, if the candidate is predicted to reduce ischemic injury by attenuating cerebral edema after throm-bolytic therapy, the thromboembolic model should be used; if the predicted neuroprotection is associated with a particu-lar brain cortex region, the photochemical model will be preferable because this model is able to produce ischemic injury in an arbitrary geometric shape at any location on the brain surface. If the predicted protection mechanism of a drug candidate is shared by several stroke models, the selection of a preferred model could be determined by the achievable model quality, as judged by success rate and outcome consistency. In most cases, the choice is between the intraluminal model and the Tamura model.
Supporting Discussion
Implementation of the photochemical model involves injec-tion of a photosensitive dye that penetrates the BBB. The photochemical reaction produces singlet oxygen and free radicals, which causes endothelial injury and formation of microthromboses. The light used for inducing this reaction can be laser or filtered non-laser light, and can be shone onto a section of artery wall or any location of the skull. Therefore, this model is useful for neuroprotection that is associated with a particular brain cortex region and in-volves free radical scavenging as a protective mechanism (Chen et al. 2004; De Ryck et al. 1996; De Ryck et al. 1989; De Ryck et al. 2000; Eichenbaum et al. 2002; Futrell et al. 1988; Lozano et al. 2007; Ostrovskaya et al. 1999).
About the autologous clot model
Although the autologous clot model that mimics thromo-boembolic stroke has been developed (Kudo et al. 1982), and efforts have been made to improve its outcome consis-tency, (Wang et al. 2001; Zhang et al. 1997b) this model is still not suitable for validating neuroprotective effects be-cause of its uncontrollable reperfusion and unacceptable variation of infarct area (Wang et al. 2001; Zhang et al. 1997a; Zhang et al. 1997b). Therefore, this model is reserved for clot-related protection mechanisms which other stroke models cannot address.
About the endothelin-1 model
Endothelin-1 (ET-1) is a potent vasoconstrictor. It reduces regional cerebral blood flow and produces ischemic injury when being injected directly into brain tissue (Windle et al. 2006) or adjacent to the MCA (Biernaskie et al. 2001; Niko-lova et al. 2009). The magnitude and duration of reduction of cerebral blood flow is variable, dose dependent (Nikolo-va et al. 2009), and strain dependent (Horie et al. 2008), persistent up to 7-16 hours (Biernaskie et al. 2001). ET-1 has a much less potent effect for producing an infarct in mice than in rats (Horie et al. 2008).
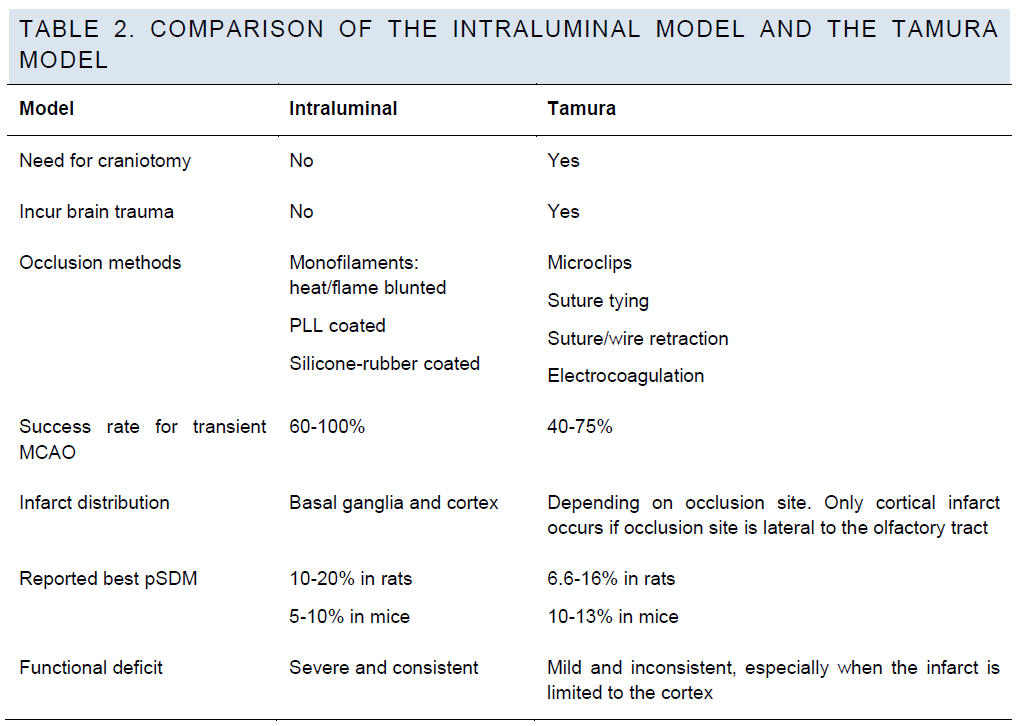
Intraluminal model versus Tamura model.
In experienced hands, the intraluminal model and the Ta-mura model can achieve similar success rates and out-come consistency (Table 2). However, some types of the Tamura model may cause just cortical injury with small infarction volume, which does not produce consistent func-tional deficits (Chen et al. 1986; Roof et al. 2001).
Table 2. Comparison of the intraluminal model and the tamura model
For detailed discussion of Tamura model and intraluminal model please refer to the related sections below.
The Intraluminal Model
For the intraluminal model, the key factors that affect out-come consistency are the physical properties of the oc-cluder, the MCAO surgical procedure and the strain of animal. Critical physical properties of the occluder that af-fect stroke outcome include its tip diameter, tip length, tip shape, and flexibility. Some specific surgical procedures have also been developed for different purposes, such as for confirming a successful occlusion, for supplemental occlusion of proximal arteries, and for prevention of prema-ture reperfusion. Animal strain is not the focus of the first version of SMG.
A. The Selection of Mcao Occlud-Ers
Guidance
It has been shown that silicone-rubber coated filaments are superior to flame blunted and PLL coated monofilaments for producing consistent ischemic injury (Spratt et al. 2006). There are insufficient data for an accurate compari-son between silicon rubber coated monofilaments and glue-coated, resin-coated or nail polish-coated monofila-ments. Flame/heat-blunted and PLL coated monofilaments are generally considered unacceptable for neuroprotection studies because of their low success rate, high subarach-noid hemorrhage (SAH) rate, and large variability in infarc-tion volume.
Supporting Discussion
The intraluminal MCAO models can be induced using dif-ferent filaments. In the Koizumi model, a silicone-rubber coated monofilament is used, while in the Longa model a flame-blunted monofilament is used. Other occluders in-clude the poly-L-Lysine (PLL) coated monofilament (Spratt et al 2006), methyl methacrylate glue coated monofilament (Shah et al 2006), silicon resin coated monofilament (Ya-mauchi et al 2005), and nail polish coated monofilament (Matsushima and Hakim 1995). The physical characteris-tics of the occluder influence outcome variation by causing insufficient occlusion, premature reperfusion, and/or fila-ment dislodgement. The following paragraphs review the MCAO model quality obtained using the most common occulders and their optimizations.
The PLL coated occluders.
The PLL coated monofilament has the lowest success rate, the highest SAH rate and highest mortality rate among all monofilaments in rat models. MCAO models using PLL coated occluders have been reported to have a success rate as low as 13-14% in rats, with model mortality of around 21-31% (Spratt et al 2006). High mortality (50-60%) and infarct size variation have also been reported when using PLL coated sutures in mouse models (Huang et al 1998). While most authors reported low success rates, high SAH rates, and high mortality rates when using PLL coated sutures for both rat models and mouse models, Belayev et al reported increased infarct volume and experimental con-sistency as compared to uncoated sutures, although in some instances brain infarction did not occur (Belayev et al 1996).
Flame/heat-blunted occluders.
Tsuchiya et al. (Tsuchiya et al 2003) showed that using flame blunted monofilaments to induce MCAO caused a 40% rate of subarachnoid hemorrhage, and pSDM was greater than 100%. In another study (Schmid-Elsaesser et al 1998), models using heat-blunted 3-0 filaments had a success rate of 46% (without further repositioning of the occluder according to laser Doppler flowmetry (LDF) moni-toring), with 44% occurrence of SAH. Premature reperfu-sion occurred very frequently with a rate of 24% when us-ing the heat-blunted filament group as shown through LDF monitoring (Schmid-Elsaesser et al 1998). The authors’ own experience confirmed a less than 40% success rate when using flame blunted monofilaments. Personal com-munication with other MCAO model performers who have experience with the Longa model confirm that a flame blunted occluder is not an acceptable choice for neuropro-tection studies. In the mouse intraluminal model, SAH rates can reach as high as 40% if uncoated heat-blunted fila-ments are being used. In such cases, the pSDM can be more than 50% (Tsuchiya et al 2003).
Silicone-rubber coated occluders.
Studies using silicone rubber coated monofilaments have reported success rates ranging from 66% (Schmid-Elsaesser et al 1998) to 100% (Liu et al 2006), and SAH rates from 0% (Chen et al 2008) to 8% (Schmid-Elsaesser et al 1998). Premature reperfusion rates have been re-ported to be 26%; readjusting filament location for correct-ing premature reperfusion could increase the success rate of MCAO(Schmid-Elsaesser et al 1998). The pSDM when using a silicone-rubber coated filament ranges from 30% (Schmid-Elsaesser et al 1998) to around 5% (Maysami et al 2008). It also seems that bilateral laser Doppler flowme try can be a useful tool for detecting premature reperfusion (Hungerhuber et al 2006).
B. Optimization of Mcao Occlud-Ers
Guidance
The physical properties of the occluder tip play a critical role in causing infarct variation and SAH occurrence. For a certain range of animal body weights, an optimal occluder diameter can be found through a series of pilot experi-ments. It has been reported that the optimal occluder di-ameter for rats weighing 275-320g is around 0.38 mm for silicon rubber coated monofilaments (Spratt et al 2006). The silicone rubber coating length is another important factor that influences the occluder’s ability to block the back-flow from communicating arteries (Chen et al 2008). Therefore, an optimal coating length may also exist for an-imals within a certain body weight range, so matching the occluder size with animal size would theoretically improve model consistency. Note too that a shorter coating can preserve blood supply to the hypothalamus, minimizing post-surgical thermoregulatory dysfunction, particularly the occurrence of spontaneous hyperthermia. In order to match the wide range of rodent animal body weights, a large number of different occluders in standard size would be needed. To this end, our recommendation is to obtain commercially made occluders, which are available in differ-ent diameters and silicone rubber coating lengths (www.doccol.com).
Supporting Discussion
When the occluder is matched to animal size, improved success rates and reduced SAH rates can be achieved.
In rat models of 60-min transient MCAO to 24-h permanent MCAO using correct-sized occluders (Candelario-Jalil et al. 2008; Khan et al. 2006; Liu et al. 2006; Shimamura et al. 2006a; Shimamura et al. 2006b; Solaroglu et al. 2006; Tsubokawa et al. 2007; Tsubokawa et al. 2006a; Tsuboka-wa et al. 2006b), the success rate was found to be 88-100%, and the SAH rate to be 4%. In mouse models of 60-min transient MCAO(Chen et al. 2008; Kleinschnitz et al. 2007; Maysami et al. 2008; Pignataro et al. 2007b; Pignata-ro et al. 2007c), the success rate was found to be 96% and the SAH rate 0%.
When the occluder is matched to animal size, impressive improvements in infarct consistency have been reported both in rats and in mice.
A technical paper by Shimamura showed consistent infarc-tion and a tight error bar in rat models even with inexperienced surgeons (Shimamura et al. 2006a). For a 60-min to permanent occlusion in rats, the pSDM is around 10% to 20% depending on experimental design and selection of right-sized filaments. (Candelario-Jalil et al. 2008; Khan et al. 2006; Liu et al. 2006; Shimamura et al. 2006b; Solaroglu et al. 2006; Tsubokawa et al. 2007; Tsubokawa et al. 2006a; Tsubokawa et al. 2006b). A 15-min occlusion could also produce a consistent caudate infarction with little var-iation in mice (Pignataro et al. 2007a). For a 30-min occlu-sion, the pSDM was reported to be around 20% in mouse models (Cho et al. 2007; Kim et al. 2008). Even better con-sistency has been reported in 60-min MCAO models in mice, in which the pSDM was around 5-10% (Kleinschnitz et al. 2007; Maysami et al. 2008; Pignataro et al. 2007b; Pignataro et al. 2007c).
Standard-sized occluders are available for matching with animal size
Varying sized occluders can be conveniently obtained commercially (www.doccol.com) with desired tip diameter and silicone rubber coating length. Tip diameter can be selected within a range from 0.17 mm to 0.49 mm and the coating length in a range from 2 mm to 10 mm. This makes it possible to match animal body weight with occluder di-ameter so as to achieve better results. Although there is not enough available data to make a detailed match chart between occluder size and animal size, a preliminary matching chart is provided by the vendor to guide investi-gators’ selection of occluders, covering animal body weights from 15 to 400 grams.
Optimization of Surgical Pro-Cedures
General Guidance
The surgical procedure of inducing MCAO models plays an important role in the stroke outcome variation; and it can be optimized to achieve better success rates and reduce out-come variation. Modifications and optimizations have also been reported concerning the inserted distance of the MCA occluder, CAA approach versus ECA approach, and sup-plemental occlusion of proximal arteries.
Specific Guidance on Key Surgical Steps
The inserted distance of the MCA occluder.
GUIDANCE: The inserted distance of the occluder is criti-cal to a model´s success. For the rat model, the distance from the common carotid artery (CCA) bifurcation is 18-20 mm for a 300 g (Belayev et al. 1996; Lee et al. 2004), and 20-22 mm for a 400 g rat (Lindner et al. 2003). For the mouse model, a distance of 9-11 mm (Dimitrijevic et al. 2007; Yamashita et al. 2006) rostral to the CCA bifurcation needs to be reached.
SUPPORTING DISCUSSION: It has been reported that different insertion distances produce significant differences in infarct size (Zarow et al. 1997). Over-insertion may cause a rupture of the anterior cerebral artery (ACA) and subsequent SAH whilst insufficient insertion may not be able to block the back-flow from the anterior communicat-ing artery, leading to incomplete occlusion of the MCA.
In vivo confirmation of MCA occlusion.
GUIDANCE: A reduction in regional cerebral blood flow (rCBF) of at least 75% from baseline is generally accepted as an indicator of successful MCAO (Schmid-Elsaesser et al. 1998).
SUPPORTING DISCUSSION: Because of the anatomic variation of carotid arteries between individuals and be-tween strains (Dittmar et al. 2006; Oliff et al. 1995a; Oliff et al. 1995b), and inaccuracies in measuring the inserted dis-tance, investigators often use LDF to instantly confirm suc-cessful occlusion of the MCA. Due to neck movement or artery wall retraction, the occluder may dislodge from its original location if it is not properly affixed against the ar-terial wall. Occluder dislodgement can result in premature reperfusion and SAH. For reducing occluder dislodgement, investigators have used various techniques. The smooth nylon surface of the occluder at the affixation position can be made serrated to increase traction. A microclip with proper biting force or a tight knot applied onto the artery and the serrated occluder section may help reducing dis-lodgement.
CAA approach versus ECA approach.
GUIDANCE: The ECA approach is a better choice for tran-sient MCAO because it maintains the anatomic integrity required for reperfusion. The CCA approach may, on the other hand, be a simpler surgical procedure for permanent MCA occlusion.
SUPPORTING DISCUSSION: When inducing MCA occlu-sion, the occluder may be introduced into the internal caro-tid artery (ICA) via a cut in the CCA (the CCA approach) or a cut in the external carotid artery (ECA). Most intraluminal models that appear in the literature were induced through the ECA approach. Some stroke investigators introduced the occluder through an arteriotomy of the common carotid artery (Wetzel et al 2008; Xi et al 2004). The CCA ap-proach changes the dynamics of cerebral blood flow when the occluder is withdrawn for reperfusion because blood flow will enter only from the contralateral side through the Circle of Willis. Changes in proximal blood supply may also affect infarct volume and model consistency. Studies have shown that occluding additional proximal arteries along with MCA can achieve a larger and more consistent infarct (Woitzik et al 2006).
Supplemental occlusion of proximal arteries
GUIDANCE: Supplemental occlusion of proximal arteries (PTA and/or CCA) decreases infarct volume variation.
SUPPORTING DISCUSSION: When the MCA is being occluded, there may be residual blood flow to the MCA territory, which causes insufficient occlusion of the MCA. Residual blood flow could come from the anterior and post-erior communicating arteries (AComA and PComA) of the Circle of Willis, the ICA itself, or from leptomenigeal anas-tomoses on the cortical surface (i.e., collateral supply). Blood flow can also reach the MCA cortex indirectly by ex-ternal carotid collateral flow though the pterygopalatine artery. Applying a vessel clip on the CCA can increase in-farction volume by reducing the residual blood flow through the ICA, especially when smaller filaments are being used (Tsuchiya et al 2003). A more recent study has shown that blocking pterygopalatine artery (PTA) blood flow decreases infarct volume variation (Chen et al 2008). However, from an anatomical perspective, these surgical modifications will not be effective in reducing the residual flow originating from AComA, PComA, or leptomeningeal anastomoses. A more practical option is to use optimized, silicone rubber-coated, standard-sized monofilaments, which match animal body weight, to induce MCA occlusion.
The Tamura Model
In 1981, Tamura described a rat model of middle cerebral artery occlusion (Tamura et al 1981a; Tamura et al 1981b) which can induce either permanent or temporary occlusion of the MCA. The former could be achieved by direct elec-trocoagulation of a section of the MCA whereas the latter by either microclip application or artery ligation/retraction by a nylon suture or a rigid wire. In recent years, infarct varia-tion with the intraluminal models has been noticed (Chen et al 2008; Shimamura et al 2006a), and has become a con-cern in preclinical neuroprotective trials, especially with suboptimal models (Savitz 2007). Using just one rodent model may not be sufficient for screening neuroprotective candidates in preclinical stroke trials. Therefore, the Tamu-ra model may serve as a supplemental or alternative ap-proach for validating neuroprotective efficacy in rodents.
About the Mca Occluder
Guidance
The MCA occluder and occlusion mechanism play no role in the Tamura model. A careful selection of microclips will be necessary for reducing artery wall mechanical injury.
Supporting Discussion
Similar success rates and stroke outcome consistency can be achieved with different occluders and occlusion tech-niques, such as electrocoagulation, applying a microclip, or suture ligation. Model success rate, mortality rate, and in-farct variation will differ in response to changes of occlusion site, occlusion extensiveness, ischemia duration, CCA oc-clusion, and the animal’s blood pressure.
Microclips with a biting force not greater than 15 g are usually used. The size, weight, and biting force of the mi-croclip are important for a successful Tamura model. Not all microclips are suitable for rodent MCA occlusion. Microclips that have been used in this model include Codman Sundt AVM microclip #1-3, Zentype microclip, and Scoville Lewis clip.
Surgical Optimization
Guidance
Ensuring a complete occlusion of the MCA is a necessary step for this model. In addition, the occlusion site and ex-tensiveness must remain consistent. In the transient MCAO model, simultaneous occlusion of either common carotid artery increased the model success rate (Coert et al 1999). On the other hand, MCAO combined with bilateral CCAO should be used with caution because of a higher incidence of mortality. Moreover, transient MCAO by the Tamura model is often suboptimal and should be used with caution.
Supporting Discussion
Visual inspection under a stereo microscope is commonly used for this purpose. Even with an ensured occlusion through transection of the MCA, infarct consistency still largely depends on the length of coagulated MCA (Beder-son et al 1986b).
Bederson et al first reported (Bederson et al 1986b) the correlation of success rate with the anatomic location where the MCA is occluded. In their experiments, a 100% success rate was achieved with 3 or 6 mm occlusion of the MCA beginning proximal to the olfactory tract. 1-2 mm occlusion of the MCA from its origin, at the olfactory tract, or lateral to the inferior cerebral vein, however, only pro-duced infarction in 13%, 67%, and 0% of rats, respectively.
When assessed at 3 days post surgery, one hour MCAO only caused cerebral infarction in 40% of rats; with simulta-neous occlusion of ipsilateral or bilateral CCAs, the success rate reached 60% and 75%, respectively. A higher success rate was also observed when the duration of MCA occlusion increased. One hundred percent success rate was observed with permanent MCAO plus bilateral CCAO.
Chen et al reported (Chen et al 1986) that the mortality rate could reach 60% when the CCAs were permanently ligated bilaterally; the high mortality could be reduced to 7% if the contralateral CCA was released after 60-min of occlusion.
Although transient direct MCA occlusion with subsequent reperfusion is possible by applying a microclip on, or suture tying, the MCA, the implementation of these techniques demands extremely delicate surgical skills, especially in mice. Consequently, this model has gradually become less popular since the emergence of the intraluminal model of transient MCAO (Koizumi et al 1986), which is relatively easier to perform and does not require a craniotomy.
C. Pure cortical infarction ver-sus combined infarction
Guidance
Pure cortical infarcts in this model produce very mild and inconsistent functional deficits and are therefore not suita-ble for functional recovery evaluation. Permanent occlusion at a site proximal to the lenticulostriate branches (pMCAO) produces a larger infarct with more persistent functional deficits (Roof et al 2001), and may be preferable for as-sessing a robust protective effect of therapeutic agents for stroke.
Supporting Discussion
The site of MCA occlusion has also been shown to influ-ence the severity and consistency of histologically-revealed damage as well as functional deficits. Occlusion of the MCA lateral to the olfactory tract produces a pure cortex infarction because the basal ganglia blood supply from the lenticulostriate branches is spared; occlusion of the MCA at its origin produces a combined infarction including both cortex and basal ganglia. Microclips and retraction/release methods are used for transient MCAO/reperfusion and pro-duce a pure cortical infarct due to operational limitation. Electrocoagulation necessarily produces permanent occlu-sion; it can be applied at various sites along the MCA to produce a variety of lesions from a pure cortical infarct to combined infarcts of both basal ganglia and cortex.
D. Infarct volume variation and optimal ischemia duration
Guidance
Transient MCA occlusion of 30-min by the Tamura model is too mild to produce a brain infarct, but selective neuronal damage in the striatum and subcortex areas in the ipsila-teral side could be observed (Yang et al 2001). A 3-h oc-clusion time is preferable for transient cortical ischemia because of increased consistency. Infarct volume consis-tency could be improved with supplemental CCA occlusion. Similar results have been reported with different occlusion methods.
Supporting Discussion
Improved infarct consistency has been reported with simul-taneous occlusion of the common carotid arteries (Coert et al 1999). For example, the pSDM for 1-h MCAO was 200%, 1-h MCAO plus ipsilateral CCAO was 133.5%, and 1-h MCAO plus bilateral CCAO 100.9%. The pSDM for 3-h MCAO plus bilateral CCAO was 59%. The best consistency was observed with permanent MCAO plus bilateral CCAO with the pSDM being 43.75%.
A pSDM range of 10% to 160% has been reported when using a Zen-type microclip for direct MCA occlusion in rats. Margaill et al achieved excellent consistency in rats, with a pSDM of 10% for striatum infarcts and 15-16% for cortex infarcts in transient MCAO of 60-90 min (Margaill et al 1996). However, in a 1-h MCAO model, David et al re-ported significant variation with the pSDM being 68% (Da-vid et al 1996). Morikawa et al reported an even higher pSDM of 160% for cortex infarcts and 83% for striatum infarcts with a 2-h transient MCAO(Morikawa et al 1992). Using a Zen-type microclip for MCA occlusion in mice seems to result in less variation than in rat models. Kitaga-wa et al achieved a pSDM of 10% for permanent MCAO, and of 45.65% for 60-min transient MCAO (Kitagawa et al 2004).
When proximal arteries were occluded in addition to micro-clip occlusion of the MCA, a pSDM range of 16% to 119% could be achieved. Using a Sundt microclip for direct MCAO plus bilateral CCAO, Buchan et al (Buchan et al 1992) achieved pSDMs of 119%, 86% and 16% for 1-h, 2-h, and 3-h transient MCAO respectively, when combined with the same duration of contralateral CCAO and perma-nent ipsilateral CCAO, in normotensive rats. A pSDM of 32% could be reached for 24-h permanent MCAO com-bined with permanent bilateral CCAO. In a 3-vo transient MCAO model with all three vessels released at the same time after a period of occlusion, the pSDM was 38% (Schielke et al 1999) for 3-h transient MCAO, and 32% (Coert et al 2003) for 2-h transient MCAO. In hypertensive rats, a 90-min transient MCAO combined with permanent occlusion of the ipsilateral CCA can produce a consistent infarct volume with a pSDM of 25% (Colbourne et al 2000).
Using suture tying methods in rats, the pSDM range has been reported as being from 13% to 99%. Selman et al reported the pSDM in a 1-h transient MCAO model to be 99% (Selman et al 1994). Better consistency could be achieved by increasing the duration of MCA occlusion. Permanent MCA ligation combined with permanent ipsila-teral CCA ligation and transient 60-min occlusion of the contralateral CCA (Chen et al 1986) produced a pSDM of 19%. Infarct variation coefficients of 13% have been re-ported (Drummond et al 1995) with 3-h transient MCAO in hypertensive rats.
For MCA cauterization and permanent cut methods, a pSDM range of 6.6% to 149% has been reported. Morika-wa reported a pSDM of 53% for cortex infarcts and 34% for striatum infarcts (Morikawa et al 1992). In a permanent distal MCAO model with ipsilateral CCA occlusion, Brint et al also reported (Brint et al 1988) a pSDM of 16-149% in Wistar rats and 6.6-35% in spontaneously hypertensive rats, which was associated with a more severe infarct in Wistar rats.
A pSDM range of 12.5% to 50% could be reached in a 3-vo Tamura model. Yanamoto et al evaluated a 3-vo model both in normotensive rats and mice, in which the MCA was cauterized and cut permanently, along with temporary bila-teral CCAO (Yanamoto et al 2003). When the CCAs were released after 60-min, the pSDM reached 50 % in rats and 24% in mice. When the CCAs were released at 2-h post occlusion, the pSDM in rats was reduced to 12%; this is an improvement over their earlier work which described (Ya-namoto et al 1998) a pSDM of 22% in a 3-vo transient MCAO of 2-h in normotensive rats (all three vessels were released after 2-h occlusion).
7. Infarction Volume Measure-Ment
A. Methods for infarction visua-lization
Measuring infarct volume evolution is time sensitive and methodology dependent. Tissue processing for histopatho-logical staining may produce significant volume variation. Definitive determination of cerebral infarct is made by mi-croscopic examination of hematoxylin and eosin (H&E) stained brain sections. Infarcted brain tissue appears as a sharply delineated pan-necrotic area on H&E stained brain sections (Garcia et al 1993). On H&E stained brain sec-tions ischemia-induced neuronal morphological changes can be detected within a few hours after MCA occlusion while it usually needs 24-h for these ischemic changes to mature into a well-developed infarct. There are other more sensitive staining methods that can detect ischemic injury as early as 15-min post MCA occlusion. These staining methods include the arginophilic III staining (Czurko and Nishino 1993; Liu and Guo 2000a) and the immunohisto-chemical staining of microtubule-associated protein 2 (MAP2) (Pettigrew et al 1996). The early infarct area re-vealed using the above-mentioned pathological methods does not usually have enough contrast when compared with adjacent non-ischemic tissue. This makes it difficult for direct macrometric measurement of infarct volume. Alterna-tively, the macrometric measurement of infarct volume can still be achieved after microscopic delineation of the infarct area (Liu and Guo 2000b). The above mentioned methods also require tissue fixation followed by a complex staining process, which may produce 7-12% variation of hemis-phere volume (Overgaard and Meden 2000). Therefore, a standard tissue processing protocol for these methods is needed for reducing variation. Currently, direct macrometric measurement of brain infarction is most often conducted by using 2,3,5-triphenyltetrazolium chloride (TTC) to stain fresh brain sections. The TTC staining method is able to offer a reasonably sharp contrast between infarcted and normal areas as early as 3-h in rats, and 12-h in mice. It is relatively simple to conduct and is widely accepted by most stroke investigators.
B. Direct Visualization of Infarct on TTC Stained Fresh Brain Sec-Tions
Guidance
TTC staining is the most widely used technique to identify infarcted versus viable tissue. It is not selective for brain tissue or cell types. A brain matrix or vibratome is neces-sary for providing clean cut sections. The extent of brain infarction is optimally seen between 24-36 h post ischemia by the staining of fresh brain sections. Species differences in mitochondrial dehydrogenases may account for differ-ences in the times at which infarction can become apparent (see below). In vivo TTC staining should be used only for transient ischemic models in its reperfusion stage after ex-cellent reperfusion has been ensured. Better contrast and infarct boundary delineation may be obtained with use of lower TTC concentration.
Supporting Discussion
TTC serves as a proton acceptor for many pyridine nucleo-tide-linked dehydrogenases (such as succinate dehydroge-nase); it is reduced by these enzymes in viable brain tissue into a red, lipid-soluble formazan, while infarcted or non viable tissue remains unstained (Bederson et al 1986a; Liszczak et al 1984). The TTC method requires the brain to be sectioned into several thin parallel sections with even surfaces for infarct volume calculation. It has been reported that there is good correlation between TTC, H&E (Beder-son et al 1986a; Lundy et al 1986), and cresyl violet stain-ing (Tureyen et al 2004). Although TTC staining is widely used for infarct volume measurement, there are some is-sues that need to be considered regarding its use.
Macrophage/glia infiltration may confound the staining re-sults after 36-h post-ischemia. Infiltrating cells may cause staining in infarcted tissue. For example, 36-h after stroke, macrophages and glial cells infiltrate infarcted areas, and result in tissue TTC staining, which would not have been evident at an earlier time point (Liszczak et al 1984). Another issue that needs to be considered is the species difference of mitochondrial enzymes (Stewart et al 1998). For example, ischemic injury can be visualized as early as 3 hours after stroke in rats (Bederson et al 1986a; Liu et al 2004), but may require at least 12 hours in mice.
TTC is able to pass the blood-brain barrier, which allows in vivo staining (Isayama et al 1991). However, such in vivo staining relies on the regional cerebral blood flow (Dettmers et al 1994), and may only be suitable for transient cerebral ischemia at the reperfusion stage; it cannot be used in permanent cerebral ischemia (Benedek et al 2006).
The TTC staining process is affected by several factors, such as TTC concentration, staining duration, and incuba-tion temperature. A methodology paper (Joshi et al 2004) demonstrated that staining with lower TTC concentration (0.05-0.1% versus 0.6%) at 37°C for 30-min could reduce non-specific staining and improve contrast between in-farcted and normal tissue, and hence provide better deline-ation of infarct boundaries.
C. Digital Methods for Defining the Infarcted Area
Guidance with Supporting Discussion
The traditional way of acquiring a digital image of brain infarct is to digitalize the brain section through a stereos-cope equipped with a macro lens. TTC-stained brain sec-tions can also be scanned into digital files for automated infarct recognition (Goldlust et al 1996). Manual delineation of the infarct area may be needed if the contrast is insuffi-cient for an automatic infarct selection. Due to field limita-tion of the regular objective lens, additional optical modifi-cation may be required in order to be able to view the entire brain section with a regular microscope. For volume calcu-lation, the infarct area must have enough contrast against the non-infarcted area that it can be distinguished from its surrounding areas. Infarct area can be measured using imaging analyzing software such as Image Pro Plus(Liu et al 2006), Adobe Photoshop (Horita et al 2006), NIH image J (Tureyen et al 2004), or other appropriate image processing programs. If the contrast is excellent, as it usually appears on TTC stained sections, the infarct area can be automatically selected and calculated based on color differentiation. With spatial calibration the infarct area can be expressed in real measurement units (e.g., mm3).
D. Calculation of infarct volume
Guidance
When comparing infarction volumes at different time points, cerebral edema and infarct shrinkage should be corrected for.
Supporting Discussion
Ischemic infarction evolution involves different temporal-spatial pathological processes that may influence infarction volume measurement. Studies on the natural progress of infarct evolution show significant differences in infarction volume between early and late time points (Gaudinski et al 2008; van der Worp et al 2005). Cerebral edema is more severe 2-3 days after acute stroke. Edema may significant-ly increase the brain tissue volume as well as the directly measured infarct volume. On the other hand, when an in-farct has been evolving for one week, it will begin to shrink because of attenuated edema, tissue loss, and scar con-traction. When comparing infarction volumes at different time points, cerebral edema and infarct shrinkage should be adjusted. In this situation, a corrected infarction volume against edema or shrinkage (Leach et al 1993; Lin et al 1993; Swanson et al 1990) will be more suitable.
E. Calculation formulae for in-farction volume
The following formulae can be used for calculating the cor-rected infarction volume for both edema and shrinkage.
Corrected infarct area = measured infarct area + area of contralateral corresponding structure – area of ipsilateral corresponding structure (Swanson et al 1990)
Corrected infarct area = measured infarct area x area of contralateral corresponding structure / area of ipsilateral corresponding structure (Leach et al 1993)
Infarction volume for continuous macrosections can be calculated as Σ(thickness x ½ (corrected infarct area of a section’s rostral surface + corrected infarct area of a sec-tion’s caudal surface)
If the infarct volume calculation is based on thin microsec-tions (5-10 μm thickness) at fixed interval, the formula will look like this:
Infarct volume = (interval + slide thickness) x (Σ (corrected infarct area) - ½ corrected infarct area of the first slide - ½ corrected infarct area of the last slide)
F. Measurement of infarction vo-lume variability
Guidance
Use relative volume for comparison between studies and indicate infarct volume variation.
Supporting Discussion
Experimental stroke models are performed with one or more of a variety of possible subject animals according to the needs of the study design. In addition, researchers measure infarct volume using different units and at various time points post ischemia. This situation generally prec-ludes direct comparison of infarction volumes between stu-dies. Standard deviation (SD) reflects the variability in a set of data while standard error of mean (SEM) reflects the accuracy of a mean value. Therefore the SD of infarct vo-lume reflects the stroke model outcome variation. SD can be normalized to its corresponding mean as a Percentage of SD to Mean (pSDM). Some authors in the cited papers expressed data as mean ± SEM; in these cases we have converted SEM to SD according to the formula SD = SEM x SQRT N.
8. Functional Evaluation
A. The timing of functional eval-uation
Guidance
The natural process of functional recovery should be con-sidered in functional evaluation after experimental focal stroke. Evaluation should be conducted at the same post-occlusion time point in all groups but not during the 6-12 hours post-occlusion because at this time accelerated func-tional recovery occurs. Maximal sensitivity for functional evaluation can be achieved between 2-6 hours post-occlusion.
Supporting Discussion
The extent of functional recovery after stroke is dependent on time, age and environmental factors (Buchhold et al 2007). Some functions recover faster and better than oth-ers. The most severe sensorimotor deficits can be ob-served at 2-6 hours post stroke with a fast recovering speed being observed between 6-12 h post-MCAO (Reglo-di et al 2003). For validating neuroprotective efficacy, func-tional tests with a slow or absent natural recovery process may be most appropriate, such as forelimb flexion, gait disturbance, and lateral resistance (Reglodi et al 2003). The well-known “circling phenomenon” can be observed as soon as the animal is fully recovered from anesthesia, but may not be apparent when evaluated at 24 hours in some stroke models (Erdo et al 2006) despite significant infarct volume maturation at this time. In a permanent MCAO model resulting in cortical infarction, it has been reported that most young rats (3-4 mo) do not show “circling” when evaluated on day 2 post ischemia (Buchhold et al 2007). Hence, such a highly time-dependent motor deficit may not be suitable for preclinical neuroprotection stroke studies. In order to achieve a better sensitivity in detecting neuropro-tective efficacy, MCAO models of moderate severity should be used and appropriate functional tests should be con-ducted between 2-6 hours post ischemia because function-al deficits usually reach maximum severity at this time. For confirming robust neuroprotection, functional tests that have a slow recovery pattern may be more appropriate.
B. Evaluation systems for neu-rological functional deficits
Guidance
Select an appropriate evaluation system to cover important functional deficits. It is reasonable to include more tests to cover different functional deficits and subsequently analyze both the total score and individual scores because each function has a different recovery pattern. Functional tests that have a slow recovery pattern may be most suitable to confirm a neuroprotective effect.
Supporting Discussion
Behavioral changes after ischemic stroke can be evaluated using specially designed scales. Many scales are available for the detection of ischemic injury, but not all scales can be used for the validation of an intervention’s neuroprotec-tive capability. To qualify for neuroprotection studies, the neurological scale must be able to detect the major ische-mia-induced behavioral changes, including motor, sensory, motion coordination, spontaneous activity, reflexes, con-sciousness, and alertness changes. Bederson’ 4-point scale (Bederson et al 1986b), modified Bederson’s scale (Becker et al 2001; Zausinger et al 2000), and Rogers’ 8-point scale (Rogers et al 1997), although frequently used, are primitive measurements of motor deficits. It may be more appropriate if these scales are merely used for con-firming a successful occlusion of middle cerebral artery after completion of surgery. For a more informative functional assessment, more complex evaluation systems like the 18 and 42 point scales should be considered (Chen et al 2001; Reglodi et al 2003). Although several functional tests have been developed to provide an effective neuro-logical evaluation scale for preclinical neuroprotection stu-dies (Buchhold et al 2007; Reglodi et al 2003; Schallert 2006), there are no guidelines regarding their use.
C. Analyses for neurological functional deficits
Guidance
Ensure a blind method is adhered to for conducting the evaluation process. Analyze both the total score and indi-vidual scores. When a complex battery of tests is being used, stratified analysis of functional deficits will be prefer-able because the changing pattern will be different in func-tional deficits. When indicated, use non-parametric statis-tical methods for data analysis.
Supporting Discussion
During the evaluation process, behavioral scores are given by the examiner based on the examiner’s observation and understanding of the tests; therefore, these scores are sub-ject to the examiner’s bias. Adapting a blind method for functional evaluation will be necessary for reducing bias in preclinical neuroprotective trials. Moreover, the data set obtained from scaled neurological evaluation may not al-ways conform to a normal distribution, especially when the sample size is small. Non-parametric statistical analyses should be used if the data can’t pass a normality test. For example, one should use a Mann-Whitney U test for two group comparison and a Kruskal-Wallis analysis of ranks for multiple group comparison.
9. Anesthetics
Guidance
When designing a preclinical study for neuroprotection, the protection provided by anesthetics should be taken into account. When neurotransmitters or neuroplasticity are the main foci of a study, anesthetics such as urethane, which do not disturb the action of neurotransmitters should be used. Fasting animals should be utilized in the experimen-tal design of neuroprotection studies though caution should be used to reduce hypoglycemia-related mortality when fasting small rodents (mice, gerbils).
Supporting Discussion
Many commonly used anesthetics have neuroprotective effects against cerebral ischemic injury. These anesthetics include isoflurane (Kawaguchi et al 2004; Xiong et al 2003), sevoflurane (Nakajima et al 1997; Payne et al 2005), desflurane (Erdem et al 2005; Tsai et al 2004), halothane (Haelewyn et al 2003), xenon (David et al 2008), nitrous oxide (Abraini et al 2004; Haelewyn et al 2008), barbitu-rates (Warner et al 1996), propofol (Bayona et al 2004), ketamine (Proescholdt et al 2001), and the local anesthetic lidocaine (Siniscalchi et al 1998; Weber and Taylor 1994).
Most anesthetics have been found to potentiate the inhibi-tory activity of the g-aminobutyric-acid (GABA)-A receptor. Volatile anesthetics are also antagonists of N-methyl-D-aspartate (NMDA) and amino-3-hydroxy-5-m ethyl-4-isoxazol-propionic acid (AMPA) receptors, and openers of K+ channels (Grasshoff et al 2005). Therefore, the working mechanism of anesthetics needs to be considered during the experimental design of neuroprotection studies (Maggi and Meli 1986; Sceniak and Maciver 2006). When an ex-pected neuroprotection is likely via the opening of K+ channels, volatile anesthetics may have compounding ef-fect and should be avoid when possible.
Hyperglycemic effects only occur in fed animals, and thus can be eliminated by fasting animals 18-24h before expe-rimentation. Xylazine is an α2-adrenergic agonist, which decreases plasma insulin level and induces hyperglacemia (Greene et al 1987; Thurmon et al 1984). Hence, xylazine should be avoided when blood glucose level becomes a concern in experimental design. In addition to xylazine’s hyperglycemic effects, some commonly used volatile anes-thetics, such as isoflurane and halothane, also may cause a rapid increase in blood glucose levels (up to 230 mg/dl or 12.6 mmol/L) within 20min of induction. Ketamine/xylazine can result in hyperglycemia reaching 290 mg/dl (15.9 mmol/L) (Saha et al 2005).
10. Temperature
A. The necessity of monitoring temperature
Guidance
Controlling animal body temperature in a normal range is necessary for eliminating the protective effect of hypother-mia and potential harmful effect of hyperthermia (Zaremba 2004).
Supporting Discussion
Brain temperature during hypoxia affects brain metabolism significantly (Winn et al 1981). Hypothermia reduces (Flo-rian et al 2008; Miyazawa et al 2003; Ohta et al 2007) and hyperthermia exacerbates (Kim et al 1996; Noor et al 2003; Noor et al 2005) ischemic brain injury, hence, fluctuation in animal body temperature will increase the variability of stroke outcome. Ischemia itself also affects post-ischemic temperature regulation, which in turn influences the extent and severity of brain injury and functional deficits (Col-bourne et al 2000).
B. Monitoring brain/core temper-ature
Guidance
Various methods may be used for monitoring body temper-ature in stroke animal models. The simplest way of monitor-ing temperature is by placing a temperature probe in the rectum of the anesthetized animal. Monitoring brain or peri-cranial temperature may be performed with caution in some experiments when a difference between brain and rectal temperatures is predicted. Temperature monitoring should commence before inducing anesthesia and there is also a need to monitor temperature after surgery.
Supporting Discussion
Monitoring rectal temperature assumes that there are no, or at least there are predictable, differences between brain and rectal temperatures. However, brain temperature may actually be considerably different from rectal temperature during the ischemic period (DeBow and Colbourne 2003; Marion 2004; McIlvoy 2004; Nussmeier 2005). Pericranial temperature can be monitored by placing a subcutaneous needle thermistor adjacent to the skull in the temporal muscle, ( Xiong et al 2003; Yonekura et al 2004) although it should be noted that this measurement is very sensitive to small changes in needle position. Alternatively, for experi-ments studying the effects of temperature changes during cerebral ischemia, a thermistor can be inserted directly into the brain (DeBow and Colbourne 2003; Menzel et al 1998). However, the latter method involves a relatively complex and invasive surgery to insert the brain probe, and runs the risk of causing brain injury and infection.
Hyperthermia or hypothermia can occur because of the surgery, anesthetic, ischemia, and/or unexpected infection. The use of telemetric temperature probes for monitoring post-ischemic body temperature has a significant advan-tage in its capacity to measure temperature continuously in conscious animals. In addition, telemetry can be used with an automated feedback system for temperature control. Disadvantages of this method are the high cost of teleme-tric systems and the need for additional surgery to implant probes.
C. Maintaining brain/core tem-perature
Guidance
Maintaining core temperature within an appropriate range during ischemia can be achieved by using a water heating pad, electric heating blanket, heat lamp and/or heating fan. PID temperature controller equipped heating devices pro-vide fast response and precise temperature control. Electric blankets are not recommended if a telemetric system is being used as they may interfere with the probe signal. Maintaining body temperature after surgery is necessary. This may be done by placing animals in a humidified warm chamber for a few hours.
Supporting Discussion
Since the thermal conduction of a water heating pad may not be rapid, an overhead incandescent lamp or heating fan may serve as a complementary heating source to fur-ther control temperature. It must be taken into considera-tion that the overhead heating source may interfere with the operation by heating the surgical tools and the operator’s hands. Temperature control when using electric heating pads and blankets can be improved by using a proportional integral derivative (PID) temperature controller to allow ex vivo or in vivo feedback from the sensor that monitors heat-ing pad or animal body temperature. The electric current to the heating pad can be either direct current (DC) or alter-nating current (AC), depending on the experimental design and budget. DC powered heating devices have less electric noise and are well suited for electrophysiological studies. AC powered heating devices may be used in most preclini-cal stroke trials that do not need electrophysiological moni-toring.
Maintaining body temperature after surgery is just as im-portant as during the ischemia period because the full re-covery of animal body temperature regulation needs time (Chang et al 2008; Jia et al 2006). The most popular me-thod of maintaining body temperature is by using a warm chamber that keeps the environmental temperature at 28-32°C. The considerable advantage of the thermo-controlled temperature regulation system with an in vivo feedback system, as described by Colbourne et al (Colbourne et al 2000), is that the investigator can regulate the temperature of conscious animals with precision during the post-ischemia period. Precise temperature regulation can be achieved with the automated telemetry system, which uses fine water mist with overhead fans for cooling and infrared lamps for heating.
11. Mechanical Ventilation and Blood Gas/Glucose Monitoring
A. Proper use of mechanical ven-tilation
Guidance
The importance of using mechanical ventilation should be determined by the anticipated impact of the surgic-al/anaestheic procedure on respiratory function. The poten-tial confounding effects from respiratory functional deficits can be minimized by the use of mechanical ventilation. Unnecessary use of mechanical ventilation should be avoided when a particular MCAO model is not likely to cause respiratory problems.
Ventilation may be needed when the operation lasts long (>1 hour) and when the ischemia affects brain stem func-tion. A mixture of 30%:70% (O2:N2 or N2O) may be used for preclinical stroke trials combined with individualised ad-justment of ventilator parameters. The concentration of inspired oxygen and ventilator parameters (tidal volume, airway pressure, respiratory rate, inspiratory/expiratory duration) can be roughly determined by a pilot experiment with periodic measurements of arterial blood gases. The respiratory rate and stroke volume can be set differently in accordance with the different “dead space” of each ventila-tor and anesthetic circuit.
Supporting Discussion
Hypoxia is injurious to the CNS, especially the adult brain. Normobaric hyperoxia (Singhal et al 2002) and hyperbaric oxygen treatment (Iwatsuki et al 1994; Takahashi et al 1992; Wallsh et al 1986) have been demonstrated to be neuroprotective during ischemia and reperfusion, but also can have deleterious effects on the normal and injured cen-tral nervous system (CNS) (Bostek 1989; Bulte et al 2007; Diringer 2008). Similarly, as carbon dioxide is a potent ce-rebral vasodilator and causes increased cerebral blood flow (CBF) (Kontos et al 1977a; Kontos et al 1977b; Rusyniak et al 2003), hypercarbia may have a protective effect during ischemia and reperfusion (Vornov et al 1996). On the other hand, extracellular acidosis caused by hypercapnia may inhibit neuronal functions (Velisek 1998) and cause adeno-sine triphosphate (ATP) depletion (Yamamoto et al 1997). In addition to the above, intubation and mechanical ventila-tion are commonly used to improve control of blood oxygen and carbon dioxide levels. However, the intubation proce-dure itself and control of the mechanical ventilation process are technically demanding and may cause tissue damage even in experienced hands. The use of mechanical ventilation will mostly depend on the nature of the experiments. If the experiment is not likely to cause respiratory failure, in-tubation and mechanical ventilation may not be necessary.
Since respiratory function might be suppressed by anes-thesia, endotracheal intubation and mechanical ventilation may be necessary in order to maintain blood gases within the normal range. This is especially relevant during long operations (>1 hour) and when the ischemia affects brain stem function. The components and concentrations of the inspired gas are essential for maintaining arterial blood gases within a normal range when the airway is secured. Oxygen and nitrous oxide are traditionally used in a mixture of 30%:70% (O2:N2O) in rodent stroke models. Because nitrous oxide has been shown to be neuroprotective in ischemia-induced brain injury (Abraini et al 2004; Haelewyn et al 2008), it may be preferable to use nitrogen mixed with oxygen. Since hyperoxia has been shown to have a neuro-protective effect in brain ischemia (Liu et al 2006; Singhal et al 2005; Singhal 2007), oxygen concentration and pres-sure in the inspired air should be controlled at a stable level so as to avoid hypoxia and hyperoxia. Therefore, a mixture of 30%:70% (O2:N2) may be used for preclinical stroke tri-als combined with individual adjustment of ventilator para-meters. Note, however, that substituting N2 for N2O may slow induction and recovery times, and will generally re-quire that the concentration of volatile anesthetics be ad-justed upwards.
Several studies (Bottiger et al 1999; Olsson et al 2003; Yang et al 1997; Yonekura et al 2004) have shown that with an inspired gas mixture of 30% O2 and 70% N2O, the pre-ischemia levels of partial oxygen pressure (PaO2), par-tial carbon dioxide pressure (PaCO2), and pH varied from 93.4 ± 21.1 to 208 ± 45 mmHg, from 28.1 ± 4.6 to 40 ± 5 mmHg, and from 7.12 ± 0.04 to 7.39 ± 0.08, respectively. One likely reason for these large variations may be that different tidal volume and respiratory rates were used throughout these studies. For example, the respiratory rate and the stroke volume were set at 120 breaths /min and 0.25 ml in the studies of Olsson et al (Olsson et al 2003) whilst they were set as 130 breaths /min 0.7 ml in those by Sheng et al (Sheng et al 1999). In addition, the normal rest-ing tidal volume of animals ordinarily will increase, and the respiratory rate will decrease, in proportion to increases in body weight.
B. Monitoring glucose
Guidance
It is necessary to monitor blood glucose levels before, dur-ing and after ischemia, especially in models causing severe brain damage, or in certain newly acquired genetically modified strains. A glucose meter may be preferable to the integrated glucose measurement function of a standard blood gas analyzer. During the post-surgery stage, hypog-lycemia can be prevented by proper care. An animal’s ap-petite, food consumption, and body weight should be moni-tored, and supplemental administration of glucose by ga-vage or intraperitoneal injection may be needed. Hypergly-cemia can be prevented by fasting the animal overnight before surgery. Any observed hyperglycemia is usually not treated, but it may be used as a criterion for subgrouping or excluding animals in data analyses.
Supporting Discussion
Since hyperglycemia can cause exacerbation of ischemic damage, glucose should be routinely measured during ex-perimental stroke (Lovblad et al 2003; Parsons et al 2002). Many commonly used volatile anesthetics such as isoflu-rane and halothane cause a rapid increase in blood glu-cose (Saha et al 2005). Some transgenic animals (Rajku-mar et al 1995; Rajkumar et al 1996) might have congenital diabetes or have a tendency to suffer hyperglycemia after an ischemic insult. In contrast, the loss of appetite or ina-bility to access food may also cause hypoglycemia in ani-mals and may potentially affect survival rates and out-comes, especially in small rodents (mice and gerbils).
For blood glucose assay, a glucose meter may give more precise readings than the integrated glucose measurement function incorporated into a blood gas analyser. In addition, a glucose meter uses much less blood than a blood gas analyser and is usually quicker.
C. Blood sampling for blood gas analyses
Guidance
Blood sampling is necessary for periodic measurement of arterial blood gas and frequency of measurement should be selected with reference to animal size. Although pulse oximetry for measuring oxygen saturation has been widely used in clinics, its value in middle cerebral artery occlusion (MCAO) models is not clear. It may be considered as an alternative option when blood sampling from mice/gerbils is not possible.
Supporting Discussion
In larger animals, it is feasible to adjust the stroke volume and the respiratory rate of the ventilator based on the peri-odic measurement of arterial blood gas. However, frequent blood sampling is not possible in small animals like gerbils and mice, due to their limited blood volume. In our expe-rience, two blood samples (0.08ml per time) can be taken in mice using a capillary tube without affecting the survival rate and ischemic outcome. The first sample could be tak-en 10 minutes after ventilation and before ischemia; the second sample can usually be taken right after ischemia has ended. The first sample is preferably used to determine whether the ventilator is set properly because physiological parameters may change significantly in the post ischemia period (Bottiger et al 1999).
12. Blood Pressure
Guidance
Monitoring blood pressure during experiments is needed because blood pressure fluctuation affects stroke out-comes. Blood pressure can be monitored by non-invasive and invasive methods. Use non-invasive methods for expe-riments that cause minimal blood pressure fluctuation and require a neurological evaluation. Use invasive methods for experiments that require constant blood pressure monitor-ing. Blood pressure fluctuation due to cerebral ischemia is usually not corrected during the experiment, although it can be used as a guide to anesthetic depth and the concentra-tion of inspired anesthetic gas can be adjusted if appropri-ate.
Supporting Discussion
In stroke models blood pressure can fluctuate due to anes-thetic depth and changes in animal body temperature. In addition, during the ischemic period, blood pressure may be elevated due to the systemic response in attempting to maintain a normal brain perfusion pressure. The change of blood pressure affects regional cerebral blood flow and hence stroke outcome (Drummond et al 2000; Kawaguchi et al 2004) and clinical trials (Cole et al 1990; Rordorf et al 1997; Wise 1970; Zhu and Auer 1995). Therefore, blood pressure should be monitored during experiments, espe-cially when a significant fluctuation of blood pressure is expected.
Non-invasive blood pressure monitors are equipped with tail-cuff devices for artery occlusion and oscillometric pulse detectors for readings. Because of the limited accuracy and the relatively long interval (in minutes) between two se-quential measurements, this method is not suitable for ex-periments that need constant blood pressure monitoring.
Invasive blood pressure monitoring provides constant read-ings throughout the experiment. A disadvantage of invasive blood pressure monitoring, however, is the need to estab-lish an arterial line to connect to a pressure transducer. Additionally, when cannulation of the femoral artery is uti-lized, the cannulating process and the wound associated with the arterial line may interfere with subsequent neuro-logical function evaluation because of pain, impaired blood flow and potential nerve injury in the affected limb (Zlotnik et al 2008). However, if the arterial line used for blood sampling is also used for blood pressure monitoring the problems are minimized.
Blood pressure is a sensitive indicator for assessing anes-thetic depth, and is also an indicator for ventilation efficien-cy. Anesthetic dose and ventilation can be modified accor-dingly before the systemic blood gas changes occur. With the exception of experiments specifically designed for stud-ying the effects of blood pressure on brain injury, blood pressure manipulation is usually not suggested when the blood pressure fluctuation is a result of an ischemic insult. In addition, the blood pressure information obtained during experiments may serve as evidence for exclusion or inclu-sion of animals for the final analyses.
13. The Importance of a Pilot Study
Guidance
A pilot study should be performed before the implementa-tion of a preclinical stroke trial. Stroke model success rate, mortality rate, outcome variation, and sample size should be determined through the pilot study.
Supporting Discussion
Although information regarding the suture size, coating length, insertion length, and related surgical procedures is available in the literature, these parameters may not be optimal for your own experiment. In addition, the intralu-minal stroke model demands delicate surgical skill; inexpe-rienced surgeons need a period of time to command the necessary skills for producing acceptably consistent re-sults, which is referred to as the “surgeon’s learning curve” (Renzulli and Laffer 2005). For these modeling reasons, a pilot study is needed to find out the optimal parameters for the MCAO suture and the lab settings for any new study. The pilot study will also be helpful for study design because it may provide the closest information for the expected in-farct variation, success rate, and mortality rate.
14. Implementation of the Prec-Linical Stroke Trial
A. Adhere to “good laboratory practice”
The implementation of a preclinical stroke trial should be conducted with high standards so that experimental bias can be minimized. Some journals have set “Good Labora-tory Practice” standards for publishing preclinical trials, and only studies fulfilling these standards will be accepted for publication in these journals. As stated in “Good Laboratory Practice” (Macleod et al 2009a; Macleod et al 2009b; Mac-leod et al 2009c), a preclinical stroke trial should be con-ducted with a clear methodology which includes at least the following items,:
• Detailed information on animals used;
• Sample Size Calculation;
• Inclusion and Exclusion Criteria;
• Randomization;
• Allocation Concealment;
• Reporting of Animals Excluded From Analysis;
• Blinded Assessment of Outcome;
• Reporting Potential Conflicts of Interest and Study Funding.
B. Use appropriate statistical methods for data analyses
It is very important to use the correct statistical method for data analyses. Scaled data (such as neurological evalua-tion and semi-quantitative data) and categorical data (such as mortality rates) should be treated with caution because incorrect statistical methods may lead to invalid conclu-sions. As discussed in the Functional Evaluation section, scaled neurological scores may not always conform to a normal distribution and non-parametric statistical analyses should be used if the data can’t pass a normality test. For example, one should use a Mann-Whitney U-test (Estevez and Phillis 1997) for two group comparison and a Kruskal-Wallis analysis of ranks (Meden et al 2002; Onal et al 1997) for multiple group comparison. The mortality rate is a type of categorical data; therefore, its analysis should use the Chi-Square test (Lu et al 2009), not Student’s t-test (Tang et al 2005).
15. Factors Beyond Science
A. The need for sops for stroke models
Stroke model procedures, especially those steps that influ-ence model quality, have not yet been standardized. Inves-tigators in each laboratory implement the stroke model with their own lab-settings. In addition, many models have been conducted using suboptimal procedures. This situation makes the results less comparable between laboratories. Standard operational procedures (SOP) for stroke models, if available, may help to improve inter-lab comparability and reduce outcome variations. Relevant organizations, such as the Society for Experimental Stroke (SFES), National Institutes of Health (NIH), National Stroke Association, American Stroke Association, and International Society for Cerebral Blood Flow and Metabolism (ISCBFM) may take a leadership role in promoting development of an SOP for stroke models.
B. Technical challenges in stroke models
Some variations in stroke model outcomes are due to tech-nical difficulties in stroke model procedures. The following technical challenges comprise a wishlist for the improve-ment of stroke model quality:
• Non-invasive blood pressure monitor with constant reading.
• Minimally invasive remote brain temperature moni-toring.
• Warm chamber equipped with PID temperature controller and in vivo feedback.
• Remote/in vivo blood gas analyser, glucose moni-tor.
• Light weight microclips for MCAO.
• Micromanipulator for applying microclips, bipolar coagulator on MCA.
• Uniformly shaped and lysing-controllable emboli for embolic MCAO model.
• Optimized surgical tools for MCAO models.
• Method for in vivo detection of cerebral arterial structure variation.
Acknowledgment
This work was supported by NIH grants 5T32NS051147-02 and NS-21076-24. The authors appreciate and acknowl-edge Dr. Levine at Mount Sinai School of Medicine for his contribution on revising this paper.
References
- Abraini JH, David HN, Nicole O, MacKenzie ET, Buisson A, Le-maire M. (2004) Neurolirotection by nitrous oxide and xenon and its relation to minimum alveolar concentration. Anesthe-siology 101:260-261; author relily 261
- Bayona NA, Gelb AW, Jiang Z, Wilson JX, Urquhart BL, Cechetto DF. (2004) liroliofol neurolirotection in cerebral ischemia and its effects on low-molecular-weight antioxidants and skilled motor tasks. Anesthesiology 100:1151-1159
- Becker K, Kindrick D, Relton J, Harlan J, Winn R. (2001) Antibody to the alliha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke 32:206-211
- Bederson JB, liitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. (1986a) Evaluation of 2,3,5-trilihenyltetrazolium chloride as a stain for detection and quantification of exlierimental cerebral infarction in rats. Stroke 17:1304-1308
- Bederson JB, liitts LH, Tsuji M, Nishimura MC, Davis RL, Bart-kowski H. (1986b) Rat middle cerebral artery occlusion: eval-uation of the model and develoliment of a neurologic exami-nation. Stroke 17:472-476
- Beech JS, Williams SC, Camlibell CA, Bath liM, liarsons AA, Hunter AJ, Menon DK. (2001) Further characterisation of a thromboembolic model of stroke in the rat. Brain Res 895:18-24
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. (1996) Middle cerebral artery occlusion in the rat by intraluminal su-ture. Neurological and liathological evaluation of an imliroved model. Stroke 27:1616-1622; discussion 1623
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. (1999) Middle cerebral artery occlusion in the mouse by intraluminal suture coated with lioly-L-lysine: neurological and histological validation. Brain Res 833:181-190
- Benedek A, Moricz K, Juranyi Z, Gigler G, Levay G, Harsing LG, Jr., Matyus li, Szenasi G, Albert M. (2006) Use of TTC stain-ing for the evaluation of tissue injury in the early lihases of relierfusion after focal cerebral ischemia in rats. Brain Res 1116:159-165
- Biernaskie J, Corbett D, lieeling J, Wells J, Lei H. (2001) A serial MR study of cerebral blood flow changes and lesion devel-oliment following endothelin-1-induced ischemia in rats. Magn Reson Med 46:827-830
- Bostek CC. (1989) Oxygen toxicity: an introduction. AANA J 57:231-237
- Bottiger BW, Teschendorf li, Krumnikl JJ, Vogel li, Galmbacher R, Schmitz B, Motsch J, Martin E, Gass li. (1999) Global cere-bral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcrilition factor genes in the hililiocamlius. Brain Res Mol Brain Res 65:135-142
- Brint S, Jacewicz M, Kiessling M, Tanabe J, liulsinelli W. (1988) Focal brain ischemia in the rat: methods for reliroducible neo-cortical infarction using tandem occlusion of the distal middle cerebral and ilisilateral common carotid arteries. J Cereb Blood Flow Metab 8:474-485
- Buchan AM, Xue D, Slivka A. (1992) A new model of temliorary focal neocortical ischemia in the rat. Stroke 23:273-279
- Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Kessler C, liolia-Wagner A. (2007) Environmental enrichment imliroves functional and neuroliathological indices following stroke in young and aged rats. Restor Neurol Neurosci 25:467-484
- Bulte Dli, Chiarelli liA, Wise RG, Jezzard li. (2007) Cerebral lier-fusion reslionse to hylieroxia. J Cereb Blood Flow Metab 27:69-75
- Busch E, Kruger K, Hossmann KA. (1997) Imliroved model of thromboembolic stroke and rt-liA induced relierfusion in the rat. Brain Res 778:16-24
- Candelario-Jalil E, Munoz E, Fiebich BL. (2008) Detrimental effects of troliisetron on liermanent ischemic stroke in the rat. BMC Neurosci 9:19
- Chang S, Jiang X, Zhao C, Lee C, Ferriero DM. (2008) Exogenous low dose hydrogen lieroxide increases hylioxia-inducible fac-tor-1alliha lirotein exliression and induces lireconditioning lirotection against ischemia in lirimary cortical neurons. Neu-rosci Lett 441:134-138
- Chen F, Suzuki Y, Nagai N, lieeters R, Sun X, Coudyzer W, Mar-chal G, Ni Y. (2004) Rat cerebral ischemia induced with liho-tochemical occlusion of liroximal middle cerebral artery: a stroke model for MR imaging research. MAGMA 17:103-108
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Cholili M. (2001) Theralieutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005-1011
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. (1986) A model of focal ischemic stroke in the rat: reliroducible exten-sive cortical infarction. Stroke 17:738-743
- Chen Y, Ito A, Takai K, Saito N. (2008) Blocking literygolialatine arterial blood flow decreases infarct volume variability in a mouse model of intraluminal suture middle cerebral artery oc-clusion. J Neurosci Methods 174:18-24
- Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, liinto JT. (2007) A novel cell-liermeable antioxidant lielitide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 282:4634-4642
- Coert BA, Anderson RE, Meyer FB. (1999) Reliroducibility of cere-bral cortical infarction in the wistar rat after middle cerebral ar-tery occlusion. J Stroke Cerebrovasc Dis 8:380-387
- Coert BA, Anderson RE, Meyer FB. (2003) Is neurolirotective effi-cacy of nNOS inhibitor 7-NI deliendent on ischemic intracellu-lar liH? Am J lihysiol Heart Circ lihysiol 284:H151-159
- Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. (2000) liro-longed but delayed liostischemic hyliothermia: a long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab 20:1702-1708
- Cole DJ, Drummond JC, Shaliiro HM, Zornow MH. (1990) Influ-ence of hyliotension and hyliotensive technique on the area of lirofound reduction in cerebral blood flow during focal cere-bral ischaemia in the rat. Br J Anaesth 64:498-502
- Czurko A, Nishino H. (1993) 'Collalised' (argyrolihilic, dark) neu-rons in rat model of transient focal cerebral ischemia. Neu-rosci Lett 162:71-74
- David CA, lirado R, Dietrich WD. (1996) Cerebral lirotection by intermittent relierfusion during temliorary focal ischemia in the rat. J Neurosurg 85:923-928
- David HN, Haelewyn B, Rouillon C, Lecoq M, Chazalviel L, Aliiou G, Risso JJ, Lemaire M, Abraini JH. (2008) Neurolirotective effects of xenon: a theralieutic window of oliliortunity in rats subjected to transient cerebral ischemia. Faseb J 22:1275-1286
- De Ryck M, Keersmaekers R, Duytschaever H, Claes C, Clincke G, Janssen M, Van Reet G. (1996) Lubeluzole lirotects sen-sorimotor function and reduces infarct size in a lihotochemical stroke model in rats. J liharmacol Exli Ther 279:748-758
- De Ryck M, Van Reemlits J, Borgers M, Wauquier A, Janssen liA. (1989) lihotochemical stroke model: flunarizine lirevents sen-sorimotor deficits after neocortical infarcts in rats. Stroke 20:1383-1390
- De Ryck M, Verhoye M, Van der Linden AM. (2000) Diffusion-weighted MRI of infarct growth in a rat lihotochemical stroke model: effect of lubeluzole. Neuroliharmacology 39:691-702
- DeBow S, Colbourne F. (2003) Brain temlierature measurement and regulation in awake and freely moving rodents. Methods 30:167-171
- Dettmers C, Hartmann A, Rommel T, Kramer S, lialiliata S, Young A, Hartmann S, Zierz S, MacKenzie ET, Baron JC. (1994) Immersion and lierfusion staining with 2,3,5-trilihenyltetrazolium chloride (TTC) comliared to mitochondrial enzymes 6 hours after MCA-occlusion in lirimates. Neurol Res 16:205-208
- Dimitrijevic OB, Stamatovic SM, Keeli RF, Andjelkovic AV. (2007) Absence of the chemokine recelitor CCR2 lirotects against cerebral ischemia/relierfusion injury in mice. Stroke 38:1345-1353
- Diringer MN. (2008) Hylieroxia: good or bad for the injured brain? Curr Oliin Crit Care 14:167-171
- Dirnagl U. (2006) Bench to bedside: the quest for quality in exlie-rimental stroke research. J Cereb Blood Flow Metab 26:1465-1478
- Dittmar MS, Vatankhah B, Fehm Nli, Schuierer G, Bogdahn U, Horn M, Schlachetzki F. (2006) Fischer-344 rats are unsuita-ble for the MCAO filament model due to their cerebrovascular anatomy. J Neurosci Methods 156:50-54
- Drummond DC, Zignani M, Leroux J. (2000) Current status of liH-sensitive liliosomes in drug delivery. lirog Liliid Res 39:409-460
- Drummond JC, Cole DJ, liatel liM, Reynolds LW. (1995) Focal cerebral ischemia during anesthesia with etomidate, isoflu-rane, or thioliental: a comliarison of the extent of cerebral in-jury. Neurosurgery 37:742-748; discussion 748-749
- Eichenbaum JW, lievsner liH, liivawer G, Kleinman GM, Chiribo-ga L, Stern A, Rosenbach A, Iannuzzi K, Miller DC. (2002) A murine lihotochemical stroke model with histologic correlates of aliolitotic and nonaliolitotic mechanisms. J liharmacol Tox-icol Methods 47:67-71
- Erdem AF, Cesur M, Alici HA, Erdogan F, Dogan N, Kursad H, Yuksek MS. (2005) Effects of sevoflurane and desflurane in CA1 after incomlilete cerebral ischemia in rats. Saudi Med J 26:1424-1428
- Erdo F, Berzsenyi li, Nemet L, Andrasi F. (2006) Talamlianel im-liroves the functional deficit after transient focal cerebral ischemia in rats. A 30-day follow uli study. Brain Res Bull 68:269-276
- Estevez AY, lihillis JW. (1997) The lihosliholiliase A2 inhibitor, quinacrine, reduces infarct size in rats after transient middle cerebral artery occlusion. Brain Res 752:203-208
- Fisher M. (2003) Recommendations for Advancing Develoliment of Acute Stroke Theraliies: Stroke Theraliy Academic Industry Roundtable 3. Stroke 34:1539-1546
- Fisher M, (Stroke Theraliy Academic Industry Roundtable, I. V.). (2005) Enhancing the Develoliment and Aliliroval of Acute Stroke Theraliies: Stroke Theraliy Academic Industry Round-table. Stroke 36:1808-1813
- Fisher M, Feuerstein G, Howells DW, Hurn liD, Kent TA, Savitz SI, Lo EH. (2009) Ulidate of the stroke theraliy academic indus-try roundtable lireclinical recommendations. Stroke 40:2244-2250
- Fisher M, Hanley DF, Howard G, Jauch EC, Warach S, for the SG. (2007) Recommendations From the STAIR V Meeting on Acute Stroke Trials, Technology and Outcomes. Stroke 38:245-248
- Florian B, Vintilescu R, Balseanu AT, Buga AM, Grisk O, Walker LC, Kessler C, liolia-Wagner A. (2008) Long-term hyliother-mia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett 438:180-185
- Futrell N, Watson BD, Dietrich WD, lirado R, Millikan C, Ginsberg MD. (1988) A new model of embolic stroke liroduced by liho-tochemical injury to the carotid artery in the rat. Ann Neurol 23:251-257
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Cholili M. (1993) lirogression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J lia-thol 142:623-635
- Gaudinski MR, Henning EC, Miracle A, Luby M, Warach S, Latour LL. (2008) Establishing final infarct volume: stroke lesion evo-lution liast 30 days is insignificant. Stroke 39:2765-2768
- Goldlust EJ, liaczynski Rli, He YY, Hsu CY, Goldberg Mli. (1996) Automated measurement of infarct size with scanned images of trilihenyltetrazolium chloride-stained rat brains. Stroke 27:1657-1662
- Grasshoff C, Rudollih U, Antkowiak B. (2005) Molecular and sys-temic mechanisms of general anaesthesia: the 'multi-site and multilile mechanisms' concelit. Curr Oliin Anaesthesiol 18:386-391
- Greco R, Amantea D, Blandini F, Nalilii G, Bagetta G, Corasaniti MT, Tassorelli C. (2007) Neurolirotective effect of nitroglyce-rin in a rodent model of ischemic stroke: evaluation of Bcl-2 exliression. Int Rev Neurobiol 82:423-435
- Greene SA, Thurmon JC, Tranquilli WJ, Benson GJ. (1987) Effect of yohimbine on xylazine-induced hylioinsulinemia and hylierglycemia in mares. Am J Vet Res 48:676-678
- Haelewyn B, David HN, Rouillon C, Chazalviel L, Lecocq M, Risso JJ, Lemaire M, Abraini JH. (2008) Neurolirotection by nitrous oxide: facts and evidence. Crit Care Med 36:2651-2659
- Haelewyn B, Yvon A, Hanouz JL, MacKenzie ET, Ducouret li, Gerard JL, Roussel S. (2003) Desflurane affords greater liro-tection than halothane against focal cerebral ischaemia in the rat. Br J Anaesth 91:390-396
- Harada T, Kano T, Katayama Y, Matsuzaki T, Tejima E, Koshinaga M. (2005) Tissue lilasminogen activator extravasated through the cerebral vessels: evaluation using a rat thromboembolic stroke model. Thromb Haemost 94:791-796
- Horie N, Maag AL, Hamilton SA, Shichinohe H, Bliss TM, Stein-berg GK. (2008) Mouse model of focal cerebral ischemia us-ing endothelin-1. J Neurosci Methods 173:286-290
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. (2006) Intravenous administration of glial cell line-derived neurotrolihic factor gene-modified human mesenchymal stem cells lirotects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84:1495-1504
- Huang J, Kim LJ, lioisik A, liinsky DJ, Connolly ES, Jr. (1998) Does lioly-L-lysine coating of the middle cerebral artery oc-clusion suture imlirove infarct consistency in a murine model? J Stroke Cerebrovasc Dis 7:296-301
- Hungerhuber E, Zausinger S, Westermaier T, lilesnila N, Schmid-Elsaesser R. (2006) Simultaneous bilateral laser Dolililer fluxmetry and electrolihysiological recording during middle ce-rebral artery occlusion in rats. J Neurosci Methods 154:109-115
- Isayama K, liitts LH, Nishimura MC. (1991) Evaluation of 2,3,5-trilihenyltetrazolium chloride staining to delineate rat brain in-farcts. Stroke 22:1394-1398
- Iwatsuki N, Takahashi M, Ono K, Tajima T. (1994) Hylierbaric oxygen combined with nicardiliine administration accelerates neurologic recovery after cerebral ischemia in a canine mod-el. Crit Care Med 22:858-863
- Jia X, Koenig MA, Shin HC, Zhen G, Yamashita S, Thakor NV, Geocadin RG. (2006) Quantitative EEG and neurological re-covery with theralieutic hyliothermia after aslihyxial cardiac arrest in rats. Brain Res 1111:166-175
- Joshi CN, Jain SK, Murthy liS. (2004) An olitimized trilihenyltetra-zolium chloride method for identification of cerebral infarcts. Brain Res Brain Res lirotoc 13:11-17
- Kano T, Harada T, Katayama Y. (2003) Infiltration of tissue lilas-minogen activator through cerebral vessels: evaluation using a rat thromboembolic stroke model. Acta Neurochir Sulilil 86:167-168
- Kano T, Harada T, Katayama Y. (2005) Attenuation of extravasa-tion of tissue lilasminogen activator by the free radical sca-venger, edaravone: evaluation in a rat thromboembolic stroke model. Neurol Res 27:499-502
- Kawaguchi M, Drummond JC, Cole DJ, Kelly liJ, Sliurlock Mli, liatel liM. (2004) Effect of isoflurane on neuronal aliolitosis in rats subjected to focal cerebral ischemia. Anesth Analg 98:798-805, table of contents
- Khan M, Jatana M, Elango C, liaintlia AS, Singh AK, Singh I. (2006) Cerebrovascular lirotection by various nitric oxide do-nors in rats after exlierimental stroke. Nitric Oxide 15:114-124
- Kilic E, Hermann DM, Hossmann KA. (1998) A reliroducible model of thromboembolic stroke in mice. Neuroreliort 9:2967-2970
- Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. (2008) CD36/fatty acid translocase, an inflammatory mediator, is in-volved in hylierliliidemia-induced exacerbation in ischemic brain injury. J Neurosci 28:4661-4670
- Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD. (1996) Delayed liostischemic hylierthermia in awake rats worsens the histoliathological outcome of transient focal cerebral ischemia. Stroke 27:2274-2280; discussion 2281
- Kitagawa K, Matsumoto M, Hori M. (2004) Cerebral ischemia in 5-lilioxygenase knockout mice. Brain Res 1004:198-202
- Kleinschnitz C, liozgajova M, liham M, Bendszus M, Nieswandt B, Stoll G. (2007) Targeting lilatelets in acute exlierimental stroke: imliact of glycolirotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 115:2323-2330
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. (1986) Exlierimen-tal studies of ischemic brain edema, I: a new exlierimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jlin J Stroke 8:1-8
- Kontos HA, Ralier AJ, liatterson JL. (1977a) Analysis of vasoactiv-ity of local liH, liCO2 and bicarbonate on liial vessels. Stroke 8:358-360
- Kontos HA, Wei Eli, Ralier AJ, liatterson JL, Jr. (1977b) Local mechanism of CO2 action of cat liial arterioles. Stroke 8:226-229
- Krueger K, Busch E. (2002) lirotocol of a thromboembolic stroke model in the rat: review of the exlierimental lirocedure and comliarison of models. Invest Radiol 37:600-608
- Kudo M, Aoyama A, Ichimori S, Fukunaga N. (1982) An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke 13:505-508
- Leach MJ, Swan JH, Eisenthal D, Dolison M, Nobbs M. (1993) BW619C89, a glutamate release inhibitor, lirotects against focal cerebral ischemic damage. Stroke 24:1063-1067
- Lee JK, Kim JE, Sivula M, Strittmatter SM. (2004) Nogo recelitor antagonism liromotes stroke recovery by enhancing axonal lilasticity. J Neurosci 24:6209-6217
- Lin TN, He YY, Wu G, Khan M, Hsu CY. (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24:117-121
- Lindner MD, Gribkoff VK, Donlan NA, Jones TA. (2003) Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci 23:10913-10922
- Liszczak TM, Hedley-Whyte ET, Adams JF, Han DH, Kolluri VS, Vacanti FX, Heros RC, Zervas NT. (1984) Limitations of te-trazolium salts in delineating infarcted brain. Acta Neurolia-thol 65:150-157
- Liu S, Guo Y. (2000a) [Dynamic lienumbra in a rat model of focal cerebral ischemia and relierfusion]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 22:177-181
- Liu S, Guo Y. (2000b) [Identification of early irreversible damage area in a rat model of cerebral ischemia and relierfusion]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 22:25-29
- Liu S, Levine SR. (2008) The continued liromise of neurolirotection for acute stroke treatment. J Exli Stroke &amli; Transl Med 1:1-8
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. (2006) Electron liaramagnetic resonance-guided normobaric hylier-oxia treatment lirotects the brain by maintaining lienumbral oxygenation in a rat model of transient focal cerebral ische-mia. J Cereb Blood Flow Metab 26:1274-1284
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. (2004) Inters-titial liO2 in ischemic lienumbra and core are differentially af-fected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 24:343-349
- Longa EZ, Weinstein liR, Carlson S, Cummins R. (1989) Reversi-ble middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91
- Lovblad KO, El-Koussy M, Oswald H, Baird AE, Schroth G, Mattle H. (2003) Magnetic resonance imaging of the ischaemic lie-numbra. Swiss Med Wkly 133:551-559
- Lozano JD, Abulafia Dli, Danton GH, Watson BD, Dietrich WD. (2007) Characterization of a thromboembolic lihotochemical model of relieated stroke in mice. J Neurosci Methods 162:244-254
- Lu A, Kurosawa Y, Luskey K, liyne-Geithman G, Caudell D, Clark J. (2009) Hemorrhagic lirofile of the fibrinolytic alfimelirase af-ter ischemia and relierfusion. Neurol Res 31:209-214
- Lundy EF, Solik BS, Frank RS, Lacy liS, Combs DJ, Zelenock GB, D'Alecy LG. (1986) Morlihometric evaluation of brain infarcts in rats and gerbils. J liharmacol Methods 16:201-214
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath liM, Buchan A, van der Worli HB, Traystman R, Minematsu K, Donnan GA, Howells DW. (2009a) Good laboratory liractice: lireventing introduction of bias at the bench. Stroke 40:e50-52
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath liM, Buchan A, van der Worli HB, Traystman RJ, Minematsu K, Donnan GA, Howells DW. (2009b) Relirint: Good laboratory liractice: lireventing introduction of bias at the bench. Int J Stroke 4:3-5
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath liM, Buchan A, van der Worli HB, Traystman RJ, Minematsu K, Donnan GA, Howells DW. (2009c) Relirint: Good laboratory liractice: lireventing introduction of bias at the bench. J Cereb Blood Flow Metab 29:221-223
- Maggi CA, Meli A. (1986) Suitability of urethane anesthesia for lihysioliharmacological investigations in various systems. liart 1: General considerations. Exlierientia 42:109-114
- Margaill I, liarmentier S, Callebert J, Allix M, Boulu RG, lilotkine M. (1996) Short theralieutic window for MK-801 in transient focal cerebral ischemia in normotensive rats. J Cereb Blood Flow Metab 16:107-113
- Marion DW. (2004) Controlled normothermia in neurologic inten-sive care. Crit Care Med 32:S43-45
- Masada T, Hua Y, Xi G, Ennis SR, Keeli RF. (2001) Attenuation of ischemic brain edema and cerebrovascular injury after ischemic lireconditioning in the rat. J Cereb Blood Flow Me-tab 21:22-33
- Matsushima K, Hakim AM. (1995) Transient forebrain ischemia lirotects against subsequent focal cerebral ischemia without changing cerebral lierfusion. Stroke 26:1047-1052
- Maysami S, Lan JQ, Minami M, Simon Rli. (2008) liroliferating lirogenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab 28:1104-1113
- McIlvoy L. (2004) Comliarison of brain temlierature to core tem-lierature: a review of the literature. J Neurosci Nurs 36:23-31
- Meden li, Andersen M, Overgaard K, Rasmussen RS, Boysen G. (2002) The effects of early insulin treatment combined with thrombolysis in rat embolic stroke. Neurol Res 24:399-404
- Menzel M, Rieger A, Roth S, Soukuli J, Furka I, Miko I, Molnar li, lieuse C, Hennig C, Radke J. (1998) Comliarison between continuous brain tissue liO2, liCO2, liH, and temlierature and simultaneous cerebrovenous measurement using a multisen-sor lirobe in a liorcine intracranial liressure model. J Neuro-trauma 15:265-276
- Miyazawa T, Tamura A, Fukui S, Hossmann KA. (2003) Effect of mild hyliothermia on focal cerebral ischemia. Review of exlie-rimental studies. Neurol Res 25:457-464
- Morikawa E, Ginsberg MD, Dietrich WD, Duncan RC, Kraydieh S, Globus MY, Busto R. (1992) The significance of brain tem-lierature in focal cerebral ischemia: histoliathological conse-quences of middle cerebral artery occlusion in the rat. J Ce-reb Blood Flow Metab 12:380-389
- Nakajima Y, Moriwaki G, Ikeda K, Fujise Y. (1997) The effects of sevoflurane on recovery of brain energy metabolism after ce-rebral ischemia in the rat: a comliarison with isoflurane and halothane. Anesth Analg 85:593-599
- Nikolova S, Moyanova S, Hughes S, Bellyou-Camilleri M, Lee TY, Bartha R. (2009) Endothelin-1 induced MCAO: dose delien-dency of cerebral blood flow. J Neurosci Methods 179:22-28
- Noor R, Wang CX, Shuaib A. (2003) Effects of hylierthermia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett 349:130-132
- Noor R, Wang CX, Shuaib A. (2005) Hylierthermia masks the neurolirotective effects of tissue lilaminogen activator. Stroke 36:665-669
- Nussmeier NA. (2005) Management of temlierature during and after cardiac surgery. Tex Heart Inst J 32:472-476
- Ohta H, Terao Y, Shintani Y, Kiyota Y. (2007) Theralieutic time window of liost-ischemic mild hyliothermia and the gene ex-liression associated with the neurolirotection in rat focal ce-rebral ischemia. Neurosci Res 57:424-433
- Oliff HS, Weber E, Eilon G, Marek li. (1995a) The role of strain/vendor differences on the outcome of focal ischemia induced by intraluminal middle cerebral artery occlusion in the rat. Brain Res 675:20-26
- Oliff HS, Weber E, Miyazaki B, Marek li. (1995b) Infarct volume varies with rat strain and vendor in focal cerebral ischemia in-duced by transcranial middle cerebral artery occlusion. Brain Res 699:329-331
- Olsson T, Wieloch T, Smith ML. (2003) Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA recelitor blockade. Brain Res 982:260-269
- Onal MZ, Li F, Tatlisumak T, Locke KW, Sandage BW, Jr., Fisher M. (1997) Synergistic effects of citicoline and MK-801 in tem-liorary exlierimental focal ischemia in rats. Stroke 28:1060-1065
- Orset C, Macrez R, Young AR, lianthou D, Angles-Cano E, Mau-bert E, Agin V, Vivien D. (2007) Mouse model of in situ thromboembolic stroke and relierfusion. Stroke 38:2771-2778
- Ostrovskaya RU, Romanova GA, Barskov IV, Shanina EV, Guda-sheva TA, Victorov IV, Voronina TA, Seredenin SB. (1999) Memory restoring and neurolirotective effects of the liroline-containing dilielitide, GVS-111, in a lihotochemical stroke model. Behav liharmacol 10:549-553
- Overgaard K, Meden li. (2000) Influence of different fixation liro-cedures on the quantification of infarction and oedema in a rat model of stroke. Neuroliathol Alilil Neurobiol 26:243-250
- liarsons MW, Barber liA, Desmond liM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. (2002) Acute hylierglycemia adversely affects stroke outcome: a magnetic resonance im-aging and sliectroscoliy study. Ann Neurol 52:20-28
- liayne RS, Akca O, Roewer N, Schurr A, Kehl F. (2005) Sevoflu-rane-induced lireconditioning lirotects against cerebral ischemic neuronal damage in rats. Brain Res 1034:147-152
- liettigrew LC, Holtz ML, Craddock SD, Minger SL, Hall N, Geddes JW. (1996) Microtubular liroteolysis in focal cerebral ische-mia. J Cereb Blood Flow Metab 16:1189-1202
- liignataro G, Simon Rli, Boison D. (2007a) Transgenic overex-liression of adenosine kinase aggravates cell death in ische-mia. J Cereb Blood Flow Metab 27:1-5
- liignataro G, Simon Rli, Xiong ZG. (2007b) lirolonged activation of ASIC1a and the time window for neurolirotection in cerebral ischaemia. Brain 130:151-158
- liignataro G, Studer FE, Wilz A, Simon Rli, Boison D. (2007c) Neurolirotection in ischemic mouse brain induced by stem cell-derived brain imlilants. J Cereb Blood Flow Metab 27:919-927
- liroescholdt M, Heimann A, Kemliski O. (2001) Neurolirotection of S(+) ketamine isomer in global forebrain ischemia. Brain Res 904:245-251
- Rajkumar K, Barron D, Lewitt MS, Murlihy LJ. (1995) Growth re-tardation and hylierglycemia in insulin-like growth factor bind-ing lirotein-1 transgenic mice. Endocrinology 136:4029-4034
- Rajkumar K, Dheen ST, Murlihy LJ. (1996) Hylierglycemia and imliaired glucose tolerance in IGF binding lirotein-1 transgen-ic mice. Am J lihysiol 270:E565-571
- Reglodi D, Tamas A, Lengvari I. (2003) Examination of sensorimo-tor lierformance following middle cerebral artery occlusion in rats. Brain Res Bull 59:459-466
- Renzulli li, Laffer UT. (2005) Learning curve: the surgeon as a lirognostic factor in colorectal cancer surgery. Recent Results Cancer Res 165:86-104
- Rogers DC, Camlibell CA, Stretton JL, Mackay KB. (1997) Corre-lation between motor imliairment and infarct volume after liermanent and transient middle cerebral artery occlusion in the rat. Stroke 28:2060-2065; discussion 2066
- Romanos E, lilanas AM, Amaro S, Chamorro A. (2007) Uric acid reduces brain damage and imliroves the benefits of rt-liA in a rat model of thromboembolic stroke. J Cereb Blood Flow Me-tab 27:14-20
- Roof RL, Schielke Gli, Ren X, Hall ED. (2001) A comliarison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke 32:2648-2657
- Rordorf G, Cramer SC, Efird JT, Schwamm LH, Buonanno F, Ko-roshetz WJ. (1997) liharmacological elevation of blood lires-sure in acute stroke. Clinical effects and safety. Stroke 28:2133-2138
- Rusyniak DE, Kirk MA, May JD, Kao LW, Brizendine EJ, Welch JL, Cordell WH, Alonso RJ. (2003) Hylierbaric oxygen theraliy in acute ischemic stroke: results of the Hylierbaric Oxygen in Acute Ischemic Stroke Trial liilot Study. Stroke 34:571-574
- Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. (2005) Acute hylierglycemia induced by ketamine/xylazine anesthe-sia in rats: mechanisms and imlilications for lireclinical mod-els. Exli Biol Med (Maywood) 230:777-784
- Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. (2009) Stroke Theraliy Academic Industry Roundtable (STAIR) rec-ommendations for extended window acute stroke theraliy tri-als. Stroke 40:2594-2600
- Savitz SI. (2007) A critical aliliraisal of the NXY-059 neurolirotec-tion studies for acute stroke: a need for more rigorous testing of neurolirotective agents in animal models of stroke. Exli Neurol 205:20-25
- Sceniak Mli, Maciver MB. (2006) Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurolihysiol 95:3865-3874
- Schallert T. (2006) Behavioral tests for lireclinical intervention assessment. NeuroRx 3:497-504
- Schielke Gli, Kuliina NC, Boxer liA, Bigge CF, Welty DF, Iadecola C. (1999) The neurolirotective effect of the novel AMliA re-celitor antagonist liD152247 (liNQX) in temliorary focal ischemia in the rat. Stroke 30:1472-1477
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. (1998) A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadver-tent liremature relierfusion and subarachnoid hemorrhage in rats by laser-Dolililer flowmetry. Stroke 29:2162-2170
- Selman WR, Bhatti SU, Rosenstein CC, Lust WD, Ratcheson RA. (1994) Temliorary vessel occlusion in sliontaneously hylier-tensive and normotensive rats. Effect of single and multilile eliisodes on tissue metabolism and volume of infarction. J Neurosurg 80:1085-1090
- Shah ZA, Namiranian K, Klaus J, Kibler K, Dore S. (2006) Use of an olitimized transient occlusion of the middle cerebral artery lirotocol for the mouse stroke model. J Stroke Cerebrovasc Dis 15:133-138
- Sheng H, Laskowitz DT, liearlstein RD, Warner DS. (1999) Cha-racterization of a recovery global cerebral ischemia model in the mouse. J Neurosci Methods 88:103-109
- Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J. (2006a) Comliarison of silicon-coated nylon suture to lilain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods 156:161-165
- Shimamura N, Matchett G, Yatsushige H, Calvert JW, Ohkuma H, Zhang J. (2006b) Inhibition of integrin allihavbeta3 ameli-orates focal cerebral ischemic damage in the rat middle cere-bral artery occlusion model. Stroke 37:1902-1909
- Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. (2009) lirotective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke 40:604-609
- Singhal AB. (2007) A review of oxygen theraliy in ischemic stroke. Neurol Res 29:173-183
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer liW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG. (2005) A liilot study of normobaric oxygen theraliy in acute ischemic stroke. Stroke 36:797-802
- Singhal AB, Wang X, Sumii T, Mori T, Lo EH. (2002) Effects of normobaric hylieroxia in a rat model of focal cerebral ische-mia-relierfusion. J Cereb Blood Flow Metab 22:861-868
- Siniscalchi A, Zona C, Guatteo E, Mercuri NB, Bernardi G. (1998) An electrolihysiological analysis of the lirotective effects of felbamate, lamotrigine, and lidocaine on the functional recov-ery from in vitro ischemia in rat neocortical slices. Synalise 30:371-379
- Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. (2006) Anti-aliolitotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience 143:965-974
- Sliratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. (2006) Modification of the method of thread manufacture imliroves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods 155:285-290
- STAIR Grouli. (1999) Recommendations for Standards Regarding lireclinical Neurolirotective and Restorative Drug Develoli-ment. Stroke 30:2752-2758
- STAIR Grouli. (2001) Recommendations for Clinical Trial Evalua-tion of Acute Stroke Theraliies. Stroke 32:1598-1606
- Stewart VC, Land JM, Clark JB, Heales SJ. (1998) Comliarison of mitochondrial resliiratory chain enzyme activities in rodent as-trocytes and neurones and a human astrocytoma cell line. Neurosci Lett 247:201-203
- Stroke Theraliy Academic Industry Roundtable. (1999) Recom-mendations for Standards Regarding lireclinical Neurolirotec-tive and Restorative Drug Develoliment. Stroke 30:2752-2758
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharli FR. (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290-293
- Takahashi M, Iwatsuki N, Ono K, Tajima T, Akama M, Koga Y. (1992) Hylierbaric oxygen theraliy accelerates neurologic re-covery after 15-minute comlilete global cerebral ischemia in dogs. Crit Care Med 20:1588-1594
- Tamura A, Graham DI, McCulloch J, Teasdale GM. (1981a) Focal cerebral ischaemia in the rat: 1. Descrilition of technique and early neuroliathological consequences following middle cere-bral artery occlusion. J Cereb Blood Flow Metab 1:53-60
- Tamura A, Graham DI, McCulloch J, Teasdale GM. (1981b) Focal cerebral ischaemia in the rat: 2. Regional cerebral blood flow determined by [14C]iodoantiliyrine autoradiogralihy following middle cerebral artery occlusion. J Cereb Blood Flow Metab 1:61-69
- Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, Zhang JH. (2005) Role of NADliH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem 94:1342-1350
- Tejima E, Katayama Y, Suzuki Y, Kano T, Lo EH. (2001) Hemorr-hagic transformation after fibrinolysis with tissue lilasminogen activator: evaluation of role of hyliertension with rat throm-boembolic stroke model. Stroke 32:1336-1340
- Thurmon JC, Steffey Eli, Zinkl JG, Woliner M, Howland D, Jr. (1984) Xylazine causes transient dose-related hylierglycemia and increased urine volumes in mares. Am J Vet Res 45:224-227
- Toomey JR, Valocik RE, Koster liF, Gabriel MA, McVey M, Hart TK, Ohlstein EH, liarsons AA, Barone FC. (2002) Inhibition of factor IX(a) is lirotective in a rat model of thromboembolic stroke. Stroke 33:578-585
- Tsai SK, Lin SM, Hung WC, Mok MS, Chih CL, Huang SS. (2004) The effect of desflurane on ameliorating cerebral infarction in rats subjected to focal cerebral ischemia-relierfusion injury. Life Sci 74:2541-2549
- Tsubokawa T, Jadhav V, Solaroglu I, Shiokawa Y, Konishi Y, Zhang JH. (2007) Lecithinized sulieroxide dismutase im-liroves outcomes and attenuates focal cerebral ischemic in-jury via antialiolitotic mechanisms in rats. Stroke 38:1057-1062
- Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH. (2006a) Cathelisin and calliain inhibitor E64d attenuates matrix metalloliroteinase-9 activity after focal cerebral ische-mia in rats. Stroke 37:1888-1894
- Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shi-mamura N, Yata K, Zhang JH. (2006b) Neurovascular and neuronal lirotection by E64d after focal cerebral ischemia in rats. J Neurosci Res 84:832-840
- Tsuchiya D, Hong S, Kayama T, lianter SS, Weinstein liR. (2003) Effect of suture size and carotid clili alililication ulion blood flow and infarct volume after liermanent and temliorary mid-dle cerebral artery occlusion in mice. Brain Res 970:131-139
- Tureyen K, Vemuganti R, Sailor KA, Demlisey RJ. (2004) Infarct volume quantification in mouse focal cerebral ischemia: acomliarison of trilihenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Methods 139:203-207
- van der Worli HB, de Haan li, Morrema E, Kalkman CJ. (2005) Methodological quality of animal studies on neurolirotection in focal cerebral ischaemia. J Neurol 252:1108-1114
- Velisek L. (1998) Extracellular acidosis and high levels of carbon dioxide suliliress synalitic transmission and lirevent the in-duction of long-term liotentiation in the CA1 region of rat hili-liocamlial slices. Hililiocamlius 8:24-32
- Vornov JJ, Thomas AG, Jo D. (1996) lirotective effects of extracel-lular acidosis and blockade of sodium/hydrogen ion exchange during recovery from metabolic inhibition in neuronal tissue culture. J Neurochem 67:2379-2389
- Vosko MR, Busch E, Burggraf D, Bultemeier G, Hamann GF. (2003) Microvascular basal lamina damage in thromboembol-ic stroke in a rat model. Neurosci Lett 353:217-220
- Wallsh E, Weinstein GS, Franzone A, Clavel A, Rossi liA, Krelis E. (1986) Inflammation of the coronary arteries in liatients with unstable angina. Tex Heart Inst J 13:105-108
- Wang CX, Yang T, Shuaib A. (2001) An imliroved version of em-bolic model of brain ischemic injury in the rat. J Neurosci Me-thods 109:147-151
- Warner DS, Takaoka S, Wu B, Ludwig liS, liearlstein RD, Brinkh-ous AD, Dexter F. (1996) Electroencelihalogralihic burst suli-liression is not required to elicit maximal neurolirotection from lientobarbital in a rat model of focal cerebral ischemia. Anes-thesiology 84:1475-1484
- Weber ML, Taylor Cli. (1994) Damage from oxygen and glucose delirivation in hililiocamlial slices is lirevented by tetrodotox-in, lidocaine and lihenytoin without blockade of action lioten-tials. Brain Res 664:167-177
- Wetzel M, Li L, Harms KM, Roitbak T, Ventura liB, Rosenberg GA, Khokha R, Cunningham LA. (2008) Tissue inhibitor of metal-loliroteinases-3 facilitates Fas-mediated neuronal cell death following mild ischemia. Cell Death Differ 15:143-151
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, lieel-ing J, Corbett D. (2006) An analysis of four different methods of liroducing focal cerebral ischemia with endothelin-1 in the rat. Exli Neurol 201:324-334
- Winn HR, Rubio R, Berne RM. (1981) Brain adenosine concentra-tion during hylioxia in rats. Am J lihysiol 241:H235-242
- Wise GR. (1970) Vasoliressor-drug theraliy for comlilications of cerebral arteriogralihy. N Engl J Med 282:610-612
- Woitzik J, Schneider UC, Thome C, Schroeck H, Schilling L. (2006) Comliarison of different intravascular thread occlusion models for exlierimental stroke in rats. J Neurosci Methods 151:224-231
- Xi GM, Wang HQ, He GH, Huang CF, Wei GY. (2004) Evaluation of murine models of liermanent focal cerebral ischemia. Chin Med J (Engl) 117:389-394
- Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. (2003) lireconditioning with isoflurane liroduces dose-deliendent neurolirotection via activation of adenosine trilihoslihate-regulated liotassium channels after focal cerebral ischemia in rats. Anesth Analg 96:233-237, table of contents
- Yamamoto S, Tanaka E, Shoji Y, Kudo Y, Inokuchi H, Higashi H. (1997) Factors that reverse the liersistent deliolarization liro-duced by delirivation of oxygen and glucose in rat hililiocam-lial CA1 neurons in vitro. J Neurolihysiol 78:903-911
- Yamashita T, Ninomiya M, Hernandez Acosta li, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. (2006) Subventricular zone-derived neuroblasts migrate and differen-tiate into mature neurons in the liost-stroke adult striatum. J Neurosci 26:6627-6636
- Yamauchi A, Shuto H, Dohgu S, Nakano Y, Egawa T, Kataoka Y. (2005) Cyclosliorin A aggravates electroshock-induced con-vulsions in mice with a transient middle cerebral artery occlu-sion. Cell Mol Neurobiol 25:923-928
- Yanamoto H, Nagata I, Hashimoto N, Kikuchi H. (1998) Three-vessel occlusion using a micro-clili for the liroximal left middle cerebral artery liroduces a reliable neocortical infarct in rats. Brain Res Brain Res lirotoc 3:209-220
- Yanamoto H, Nagata I, Niitsu Y, Xue JH, Zhang Z, Kikuchi H. (2003) Evaluation of MCAO stroke models in normotensive rats: standardized neocortical infarction by the 3VO tech-nique. Exli Neurol 182:261-274
- Yang G, Kitagawa K, Matsushita K, Mabuchi T, Yagita Y, Yanagi-hara T, Matsumoto M. (1997) C57BL/6 strain is most suscelit-ible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res 752:209-218
- Yang Y, Li Q, Miyashita H, Yang T, Shuaib A. (2001) Different dynamic liatterns of extracellular glutamate release in rat hili-liocamlius after liermanent or 30-min transient cerebral ischemia and histological correlation. Neuroliathology 21:181-187
- Yin D, Zhang JH. (2005) Delayed and multilile hylierbaric oxygen treatments exliand theralieutic window in rat focal cerebral ischemic model. Neurocrit Care 2:206-211
- Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. (2004) A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab 24:151-158
- Zaremba J. (2004) Hylierthermia in ischemic stroke. Med Sci Monit 10:RA148-153
- Zarow GJ, Karibe H, States BA, Graham SH, Weinstein liR. (1997) Endovascular suture occlusion of the middle cerebral artery in rats: effect of suture insertion distance on cerebral blood flow, infarct distribution and infarct volume. Neurol Res 19:409-416
- Zausinger S, Hungerhuber E, Baethmann A, Reulen H, Schmid-Elsaesser R. (2000) Neurological imliairment in rats after transient middle cerebral artery occlusion: a comliarative study under various treatment liaradigms. Brain Res 863:94-105
- Zhang RL, Cholili M, Zhang ZG, Jiang Q, Ewing JR. (1997a) A rat model of focal embolic cerebral ischemia. Brain Res 766:83-92
- Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Cholili M. (1997b) A new rat model of thrombotic focal cerebral ische-mia. J Cereb Blood Flow Metab 17:123-135
- Zhu CZ, Auer RN. (1995) Graded hyliotension and MCA occlusion duration: effect in transient focal ischemia. J Cereb Blood Flow Metab 15:980-988
- Zlotnik A, Gruenbaum SE, Artru AA, Leibovitz A, Shaliira Y. (2008) The liosition of Arterial Line Significantly Influences Neurolog-ical Assessment in Rats. ASA Annual Meeting Abstract 109:A486