Review Article - Imaging in Medicine (2013) Volume 5, Issue 3
Pre- and intra-operative imaging techniques for sentinel node localization in breast cancer
Sergi Vidal-Sicart*1, Francesco Giammarile2, Giuliano Mariani3 & Renato A Valdés Olmos4,51Nuclear Medicine Department, Hospital Clínic Barcelona, Villarroel 170, 08036, Barcelona, Spain
2Médecine Nucléaire, Hospices Civils de Lyon-EA 3738, Université Claude Bernard Lyon 1, Centre Hospitalier Lyon Sud, 165 Chemin du Grand Revoyet, 69495 Pierre-Bénite Cedex, France
3Regional Center of Nuclear Medicine, University of Pisa, Via Roma 67, I-56126, Pisa, Italy
4Nuclear Medicine Department, Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX, Amsterdam, The Netherlands
5Interventional Molecular Imaging, Radiology Department, Leiden University Medical Center, Leiden, The Netherlands
- Corresponding Author:
- Sergi Vidal-Sicart
Nuclear Medicine Department
Hospital Clínic Barcelona
Villarroel 170, 08036, Barcelona, Spain
Tel: +34 932 275 516
Fax: +34 934 518 137
E-mail: svidal@clinic.ub.es
Abstract
Keywords
fluorescence ▪ lymphoscintigraphy ▪ portable γ camera ▪ radioguided surgery ▪ SPECT/CT ▪ ultrasonography
General considerations
The concept of lymphatic mapping in oncology is based on the fact that lymphatic vessels from a cancer drain to a specific regional lymph node or nodes, called sentinel lymph nodes (SLNs). From these particular nodes, lymph goes through efferent lymph vessels to secondary lymph nodes. In the case of tumor diffusion through the lymphatic route, tumor cells will first be harbored in the SLNs, which are the lymph nodes that are at high risk of tumor cell involvement.
Since the first application in melanoma patients, this technique has rapidly expanded to other types of solid malignancies. Thanks to SLN biopsy, nowadays many patients with breast cancer are spared an unnecessary lymph node dissection and often patients who may benefit from adjuvant systemic therapy are identified. Thus, it can be concluded that lymphatic mapping with SLN biopsy is one of the most interesting and remarkable developments in clinical oncology in recent years [1].
Radioguided lymphatic mapping and SLN biopsy require a close collaboration between nuclear medicine physicians, radiologists, surgeons and pathologists. Preoperative lymphoscintigraphy enables depiction of the nodes receiving lymph from the primary malignancy, and it is important to emphasize that its systematic application has demonstrated that tumors can drain to lymph nodes in previously unsuspected locations. Radioguided surgical procedures are generally less invasive than traditional surgical approaches. In the case of the SLN biopsy in breast cancer, instead of a total ipsilateral axillary lymphadenectomy, most patients now undergo surgical removal of only one (or a few) lymph node(s); axillary lymphadenectomy is then performed only in approximately 30% of the patients – that is, those in whom SLN biopsy has shown the presence of metastasis. This procedure permits more accurate staging as it identifies the nodes at the greatest risk of harboring metastasis. Side effects of the procedure are rare and, above all, much milder than those of conventional lymphadenectomy, thus reducing postsurgical complications, such as lymphedema, motor and sensory nerve damage, and functional impairment of the shoulder/arm [2].
The SLN approach is based on the assumption that lymphatic drainage to a regional lymph node basin follows an orderly, predictable pattern, and those lymph nodes on a direct drainage pathway function as effective filters for tumor cells. Consequently, all lymph nodes with direct drainage from the primary tumor are considered as SLNs. The sentinel node is not necessarily the hottest or the most nearby node, although that is often the case [3].
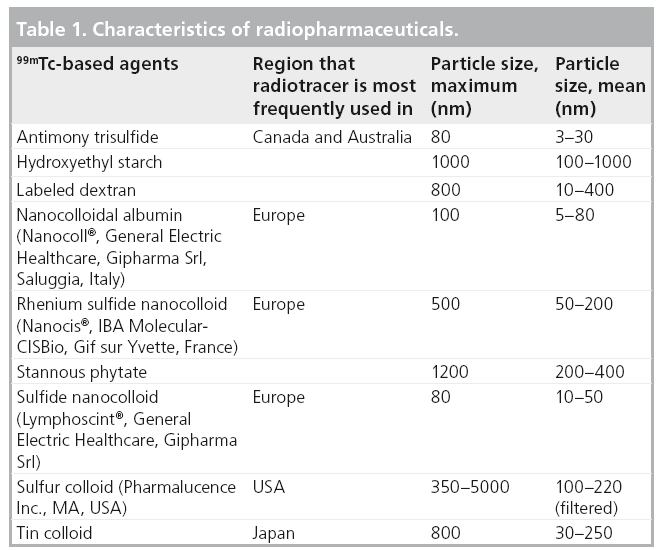
The identification of SLNs is based on the use of a combined approach injecting first a radiotracer (usually labeled with 99mTc; Table 1), with subsequent utilization of an imaging protocol (preoperative lymphoscintigraphy); sometimes a blue dye is used intraoperatively. The nodal field is finally assessed with a handheld g probe, followed by surgical removal of the SLNs that are detected [4].
In general, a radiopharmaceutical is a radioactive compound with chemical, physical and biological characteristics mimicking the in vivo pharmacokinetic pattern of a natural molecule. Thus, the nuclear medicine equipments commonly employed for diagnostic purposes produce functional images that represent the distribution in space and time of the radiopharmaceutical in the different regions of the body.
Some issues regarding how the procedure should be performed are still a matter of discussion. Particularly in breast cancer, controversies exist with regard to the size of the particles of the radiotracer, the optimal route for injection, time to scintigraphy and intraoperative detection, and whether or not extra-axillary lymph nodes should be considered. Choice of the radiocolloid used is usually determined by local availability, and this may influence some of these procedural issues. The optimal injection technique is still a matter of debate. When using superficial (intradermal, periareolar or subdermal) injections, large volumes of colloid may disrupt local lymphatics; therefore, volumes as small as 0.2–0.3 ml should be injected. In deep lesions, a slightly larger volume (0.5 ml) may be used. When large particle radiocolloids were injected around the breast tumor, there was poor migration to the SLNs. Other approaches were used, including superficial injections with smaller particles, all of which enabled axillary SLNs to be identified accurately. The significance of internal mammary chain biopsy remains under debate. There is evidence that mapping its nodes leads to stage migration and to modifications of treatment planning with respect to radiotherapy and systemic therapy, but more evidence is necessary to support the idea that mapping of the internal mammary chain will improve the outcome of treatment and survival [5–9].
Since the introduction of this technique for breast cancer in 1994, preoperative lymphoscintigraphy has been an essential component for SLN identification in the majority of the protocols [10–12], although some groups are reluctant to use it [13].
Preoperative lymphoscintigraphic mapping should be employed whenever possible because of the added benefit in identifying all potential sites of drainage and in providing the surgeon with a map of SLNs. Thus, preoperative lymphatic mapping has the potential both to improve accuracy and to reduce morbidity relative to the use of the handheld g probe alone [14,15]
To perform lymphoscintigraphy, a γ camera with a large field of view is necessary. The γ camera should be equipped with a low-energy, high-resolution collimator. The energy window should be 15% (±5%) centered over the 140 keV photopeak of 99mTc.
The patient lies supine for imaging on the γ camera bed, although there are other possibilities (prone with hanging breast position or upright). Anterior, 45° anterior oblique and lateral imaging can be obtained (at least two of them).
At variance with the protocol for melanoma, in general a dynamic study is not performed in breast cancer. Imaging should be performed within 15–30 min after the injection and 2–4 h, or as needed up to the time of surgery. Delayed images are helpful for detecting SLNs close to the primary site that may have been obscured in the initial acquisitions, and to detect possible drainage to multiple nodal basins.
Planar images are generally acquired for 3–5 min. A 57Co or 99mTc flood source or a 57Co or 99mTc point source can be used for delineation of the patient’s body contour during scintigraphy. The site of any suspected SLN can be localized on the overlying skin projection, using a pointer; the skin can be marked with a small spot of indelible ink [9].
SPECT/CT procedures
Although the use of conventional planar imaging is the basis of lymphoscintigraphy, this technique does not provide the exact preoperative anatomic location of the detected lymph nodes. Hybrid integrated systems (SPECT/CT) fuse tomographic lymphoscintigrams with anatomical data, keeping the patient in the same position during imaging for acquisition of both SPECT and CT. After reconstruction, fusion of the two sets of images is performed easily employing in-built softwares [16].
Currently, with the new-generation γ cameras, SPECT/CT has been incorporated into the SLN procedure. In this way, the added value of the combined use of SPECT/CT is the possibility to visualize the anatomy of the part of the body under examination with a high spatial resolution of the CT and to overlap these morphological images to the relatively low spatial resolution functional images from SPECT. The resulting fused SPECT/CT images depict SLNs in an anatomical environment, thus providing a useful roadmap for surgeons. In recent years, SPECT/CT has been used in breast cancer patients with absent, unusual or complex lymphatic drainage. This is the case in patients with drainage outside the axilla, as well as in obese patients. SPECT/CT may also be performed if the conventional images are difficult to interpret (e.g., suspicion of skin contamination or when a suspicious uptake is located near the injection area) and for SLN depiction when no lymph nodes are visualized on planar imaging [17–19].
SPECT/CT is principally oriented to the anatomical localization of SLNs. This is the main reason why SPECT/CT is acquired using a lowdose CT, although the CT component of the system is usually able to yield optimal anatomical information. Thus, for superficial areas, such as the axilla, a minimum 5‑mm slice thickness is recommended. For more complex anatomical areas (e.g., the head and neck), 2-mm thick slices may be necessary. CT is also used to correct the SPECT signal for tissue attenuation and scattering. After these corrections, SPECT is fused with CT [20].
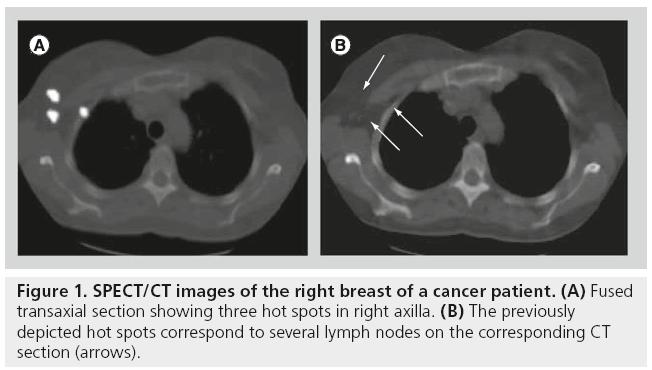
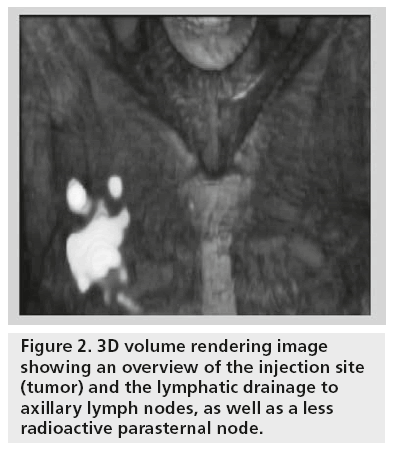
The display of SPECT/CT is similar to that of conventional tomography, the use of cross-reference lines allowing the navigation between axial, coronal and sagittal views. At the same time, this tool allows the correlation of radioactive SLNs seen on SPECT with lymph nodes seen on CT (Figure 1). This information may be helpful for the intraoperative procedure and the postexcision control using portable γ cameras or probes. Using volume rendering leads to better anatomical reference points being obtained, and such displays produce a useful anatomical overview of the anatomo- topographic coordinates of SLNs (Figure 2).
Figure 1: SPECT/CT images of the right breast of a cancer patient. (A) Fused transaxial section showing three hot spots in right axilla. (B) The previously depicted hot spots correspond to several lymph nodes on the corresponding CT section (arrows).
Nevertheless, it should be emphasized that SPECT/CT is not intended to replace planar lymphoscintigraphy, and must rather be considered as a complementary imaging modality. Preoperative radiotracer lymphoscintigraphic mapping is highly recommended because of the potential added benefit in both improving accuracy and reducing morbidity relative to the use of a handheld g probe alone. However, conventional planar imaging does not give an exact preoperative anatomic location of the detected nodes. Thus, SPECT/CT imaging aims to anatomically localize SLNs already visualized on early and delayed planar lymphoscintigraphic images. Importantly, SPECT/CT depicts the SLN distribution in all axillary areas (e.g., three Berg’s levels) and this issue improves the potential information provided by intraoperative blue dye and/or fluorescent tracers (usually useful in level I, but with limited penetration to higher axillary levels).
In current protocols, SPECT/CT is acquired following delayed planar imaging (usually 2–4 h after radiocolloid administration). SPECT/CT allows the identification of the SLNs for their further localization in the operation room, by correlating findings of fused SPECT/CT with those of CT using cross-reference lines. However, in many cases, radioactive SLNs correspond to single lymph nodes, in some cases single radioactive spots on SPECT/CT may correspond to a cluster of lymph nodes on CT [21].
Intraoperative options for SLN localization
Blue dyes
Most surgeons combine preoperative lymphoscintigraphic information (with or without SPECT/ CT) with visual guidance provided by blue dye injected during surgery. Indeed, the blue dye can be a useful adjunct to aid in SLN localization and harvesting. Following interstitial injection, the blue dye migrates to the SLNs, thus staining the lymphatic channels, which sometimes can be followed up to the first-echelon nodes. Direct visualization and dissection of these channels facilitates SLN localization.
Several studies have established the validity of blue dyes as markers for SLN mapping with relatively high detection rates in every clinical situation (ranging from 75 to 100%), although slightly lower than those achieved when radiopharmaceuticals are used [22,23].
Radiocolloid injection with preoperative lymphoscintigraphy and intraoperative g-probe search combined with intraoperative blue dye injection and visual guidance has been shown to be the optimum method for decreasing falsenegative results and for increasing sensitivity [24]. The blue dye is usually injected in the operation room by the surgeon, followed by dissection in search of blue-stained lymphatic channels and blue-stained axillary nodes. For example, in the UK the majority of surgeons (86%) use the recommended technique of preoperative lymphoscintigraphy and intraoperative g-probe search, while 47% continue to use the dual localization method (radioguidance and blue dye). The remaining 14% use either the blue dye or the radiocolloid alone, without previous lymphoscintigraphy.
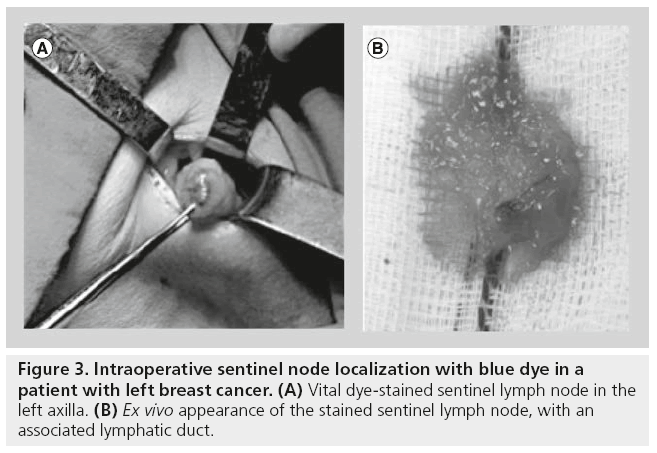
Commonly used dyes are patent blue V, isosulfan blue and methylene blue. The blue dye can be injected in a way similar to that of radiocolloid injections between 10 and 20 min prior to surgery. Implementing 5 min of massage at the injection site enhances movement of the dye through the lymphatics to the SLN. Within 5–15 min, the SLNs are coloured (Figure 3). Washout is evident after approximately 45 min.
In most cases, the same SLNs are detected by the two methods when they are used in combination [24–27].
In a recent study, 84 patients underwent SLN biopsy using isosulfan blue, while methylene blue was used in another group of 110 patients. The clinical and pathological characteristics of the two groups were similar. The SLN identification rate was 100% in the isosulfan blue group and 99.1% in the methylene blue group, without any statistically significant difference. However, more SLNs were identified with methylene blue versus isosulfan blue (mean: 2.7 vs 2.1; p = 0.03) The authors concluded that methylene blue also presents significant advantages with respect to costs, allergenic reactions and surgical problems; although there are some minimal drawbacks (anaphylactic reactions and interference with pulse oximetry) [28].
A notable disadvantage of using blue dyes is that SLN cannot be seen from the skin surface, which makes them impractical as a standalone method, requiring the use of radiocolloids as well; and they are not helpful if involvement of extra-axillary nodes (internal mammary or supraclavicular) should be evaluated. Another disadvantage is temporary blue tattooing of the skin or areola in patients submitted to breast conservation surgery, when the dye is injected superficially. It is also important to be aware of contraindications for the use of blue dyes. Blue dyes may interfere with pulse oximetry readings, so in certain patients, they should be used with caution. Furthermore, blue dyes can induce anaphylactic reactions in 0.15–2% of patients, a non-negligible fraction. Due to this possibility, the blue dye is especially contraindicated in pregnancy. It is also contraindicated in case of an earlier allergic reaction to blue dye and in case of severe impairment of renal function. On the other hand, hypersensitivity reactions to radiopharmaceuticals are rare, but have also been reported [29–33].
Handheld γ probes
During surgery, preoperative radiocolloid lymphatic mapping is complemented with a radiation detector probe. Current handheld g detector probes must be able to detect the SLN from the skin surface, as well as within the exposed surgical cavity. The first task implies that the sensitivity of the detector is sufficient to identify a weakly active SLN when attenuated by the overlying soft tissues. Discriminating activity within the SLN requires the probe to also be well collimated, with a small angle view. The detector should also be constructed to offer a high level of shielding against radiation hitting the side face of the probe assembly. The whole system must be designed and constructed to be suitable for intraoperative use [34].
Detectors for intraoperative probes are further sub-divided into two categories: the first one includes probes based on scintillation detectors (both crystal and plastic types), while semiconductor- based probes form the second group.
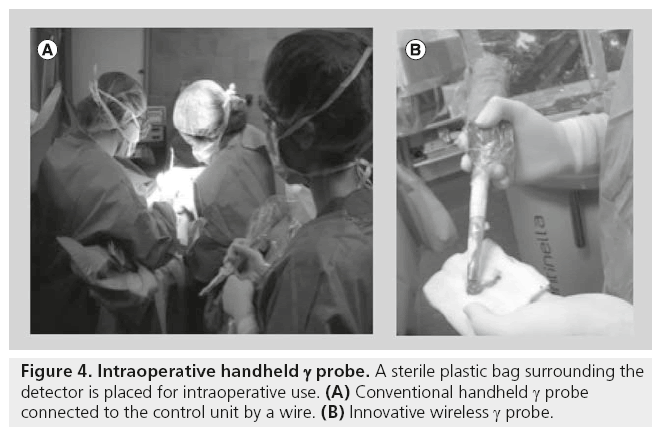
The detector must be ergonomically designed for easy maneuverability, and constructed to be suitable for sterilization. The device is usually placed in a sterile bag for intraoperative use (Figure 4). An increase of loudness or an increase of frequency of the audible signal indicates to the surgeon how to locate the targeted radiolabeled tissue.
γ probes for radioguided SLN surgery require high spatial resolution to allow for a more precise localization of small lymph nodes. On the other hand, γ probes for radioguided surgical resection of tumors require high sensitivity to guide the surgeon to the specific site of the target over a relatively large surgical field. Recent developments of handheld γ probes based on wireless technology eliminate the need for cables that previously connected the probe to the control unit within the surgical field [35–39].
Using the preoperative images and skin markings as guides, the g probe can be used to select the optimum location for incision. The surgeon then uses the probe to guide dissection to the hot SLNs, and explores the surgical bed with the probe after node excision, to confirm removal of the hot nodes.
Thus, after removal of one node, in a limited number of cases another hot node may still be present nearby. The current use of SPECT/CT imaging may identify the presence of a cluster of lymph nodes, especially on CT images.
Deeply located SLNs are difficult to detect because of tissue attenuation and because the large amount of radioactivity at the injection site may hide SLNs located nearby. This situation is frequently observed in tumors of the superior outer quadrant of the breast and in internal mammary chain nodes when the tumor is located in inner quadrants. In the latter instance, it is advisable to use thinner probes (e.g., 10 mm in diameter) in the intercostal areas, in order to better identify the activity point in a reduced surgical space. The use of SPECT/CT images may be especially helpful in these cases [18].
Although all these conventional techniques are accurate, in order to minimize false-negative results the open axilla should be palpated and suspicious lymph nodes harvested, even if they are neither hot nor blue [40]. γ probes can also be used to locate nonpalpable breast cancers based on the detection of radiotracers previously injected into the tumor (radioguided occult lesion localization [ROLL]) or radioactive seeds previously placed into the tumor (radioactive seed localization [RSL]). A signi-cantly higher percentage of patients undergoing ROLL (usually performed with 99mTc-albumin macroaggregates, or sometimes with the same radiocolloid used for SLN mapping) or RSL (usually performed with 125I seeds) achieved negative margins as opposed to those undergoing wire localization procedures, and both approaches can be performed at the same surgical setting as the SLN procedure. If there is enough 125I activity with a photo peak of 27 keV to overcome the down scatter of the 99mTc photons, then both photopeaks of 125I (27 keV) and 99mTc (140 keV) can be detected easily at surgery. RSL is also effective when placed in the breast before neoadjuvant chemotherapy, since post-therapy lesions can be dif-cult to localize surgically. In this setting, achievement of a high percentage of negative margins has been reported, although further studies are necessary to determine RSL usefulness in localizing malignant axillary lymph nodes before neoadjuvant chemotherapy [41–46].
Portable γ cameras
The sensitivity and speci-city of SLN detection with the use of current approaches, such as preoperative g-camera imaging, γ probes, and the ‘blue dye’ technique, are quite high. A meta-analysis of more than 60 published studies found an overall sensitivity of more than 90% and a false-negative rate of 8.4% for detection of such SLNs in breast cancer [47,48]. For preoperative lymphoscintigraphy, recent studies with more than 100 patients showed detection rates of 78–100% [47,48]. SLNs were successfully detected using the intraoperative g probe in 98% of the patients, in whom such nodes were successfully imaged preoperatively, with a false-negative rate of only 7% [48]. In addition, for SLNs not visualized by preoperative lymphoscintigraphy, there was a 90% intraoperative detection rate. However, it should be emphasized that a negative preoperative lymphoscintigraphy often predicted a negative intraoperative g probe search result, and the improvement in the detection rate intraoperatively was primarily due to the use of blue dye [47–50].
The handheld g probe used intraoperatively has only a single detector. In experienced hands, it works well, and it is certainly a valuable tool. However, it presents several limitations: it does not provide imaging documentation for the medical record; its usefulness is highly dependent on correct probe positioning and thus is highly operator-dependent; and its counting rate is highly dependent on the distance between the probe tip and the node. SLNs with ‘ex vivo’ counting rates that are 10% or more of the counting rate of the hottest node may contain metastases even when the hotter nodes are negative, a -nding that supports the standard practice of excising SLNs until the residual bed count is less than 10% of that of the hottest node. When this measurement is made with the handheld probe, if the probe is even a few millimeters away from a residual SLN, it will incorrectly record the activity as being arti-cially less than the true value, thus possibly leaving a true SLN in the surgical field.
On the other hand, surgeons need to translate in their minds the 1D probe readings and the 2D lymphoscintigrams into a 3D image. In addition, the blue lymphatic vessel that guides the surgeon to the SLN is not always easy to find, especially in deep-seated lymph nodes or when a considerable amount of fat lies over the node. For these reasons, several new approaches have recently been developed to overcome some difficulties observed with the conventional procedures described above. As previously mentioned, in these approaches preoperative SPECT/CT plays an essential role [50–52].
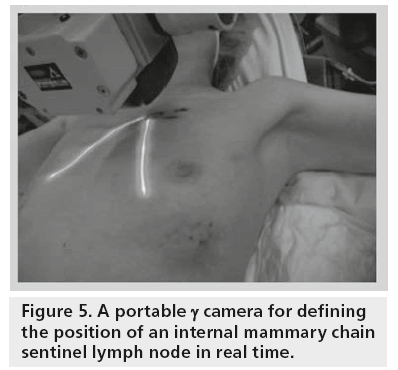
The no-imaging handheld γ probes are the current standard equipment for radioguided surgery; however, they cannot provide specific information about margins or anatomical relationships on the radiolabeled tissue (SLN or tumor). The exact localization of a target (tumor or SLN) can only be performed if the tip is in direct contact with the tissue after dissection. Thus, intraoperative portable γ cameras have recently been developed to obtain preincision imaging of the SLN in order to increase the yield of this procedure (Figure 5). In comparison with γ probes, these devices can generate images, and thus enable a more accurate correlation with the SPECT/CT findings.
The portable γ camera can also be used in conjunction with the γ probes. Imaging devices must meet several requirements to be employed in the intraoperative setting. These include a portable and stable design, no delay between image acquisition and display (real-time imaging), and the possibility for continuous monitoring, spatial orientation on screen and real-time quantification and display of the counts recorded. Finally, they should also have an adequate spatial resolution, sensitivity and field of view. Its position can also be adjusted to show SLNs near the injection area, which can easily be overlooked by using the handheld g probe. Discrimination between a SLN and an upper-tier node is primarily related to the preoperative scintigraphic images and only secondarily determined by the number of counts simultaneously recorded with both cameras and probes [53–56].
Another advantage offered by a portable γ camera is that its sensitivity for the detection of a radioactive signal does not signi-cantly change over the range of node-to-camera distances used in the operating room.
In a preliminary pilot study, Mathelin et al. found that the use of an intraoperative small -eld-of-view γ camera for detection of SLNs in breast cancer was practical. The intraoperative γ camera allowed the detection of an additional SLN (metastatic) that was not visualized by preoperative imaging or detected with the g probe, thus suggesting that intraoperative g-camera imaging may reduce the false-negative detection rate [57,58].
The earliest systems were handheld devices having -elds of view of only 1.5–2.5 cm in diameter and using conventional sodium iodide or cesium iodide activated with thallium scintillation detectors. The main problems with these early units were their very small -eld of view (with the resulting large number of images required to assess the surgical area) and the dif-culty in holding the device still enough for the duration of image acquisition. Later units used 2D arrays of scintillation crystals connected to a position-sensitive photomultiplier and, more recently, semiconductors, such as cadmiun telluride.
A handheld cadmiun zinc telluride-based semiconductor γ camera known as the eZ-SCOPE (Anzai Medical, Tokyo, Japan) was introduced in the market approximately 10 years ago. The device was light enough (820 g) to hold it for a short time. The field-of-view was 3.2 × 3.2 cm. Its collimators were easily changed. Spatial resolution of the system with the low-energy high-resolution collimation was 2.3, 8.0 and 15 mm at 1, 5 and 10 cm, respectively. This camera was able to depict the SLNs intraoperatively but, for a more extensive clinical use, it was necessary to develop an articulating arm. However, the scanning of all fields of view was rather time-consuming.
Another available device is the Sentinella S102 (Oncovision-GEM Imaging, Valencia, Spain), which is equipped with a cesium iodide doped with sodium continuous scintillating crystal readout by position-sensitive photomultipliers and different collimators (pinhole collimators, 2.5 mm and 4 mm in diameter). The pinhole collimator enables visualization of the whole surgical field, depending on the distance between the camera and the source. To obtain high-resolution images, the camera is positioned at 3 cm from the source; this corresponds to a field of view of 4 × 4 cm. At 15 cm and the field of view is 20 × 20 cm. This device has been integrated in a mobile and ergonomic support that is adjustable. The imaging head is located on one arm, which allows positioning on the specific area.
Another approach is based on the use of cadmium zinc telluride as the radiation detector. The head is equipped with a series of interchangeable parallel-hole collimators, to achieve different spatial resolution and/or sensitivity.
A further development of the intraoperative γ camera is the LumaGEM® from Gamma Medica Ideas (CA, USA). Similar to the previous one, it is still based on cadmium zinc telluride technology and was originally developed for g imaging of the breast. The field of view is 13 × 13 cm and the intrinsic spatial resolution is 2 mm. This camera is also equipped with a parallel-hole collimator and is integrated in a workstand articulated arm.
In the future, the photomultiplier-based systems will most likely be replaced by cameras consisting of solid-state photodetectors [54].
All these systems are fully portable and small enough for surgery rooms. However, since nonimaginγ probes are still the standard equipment for intraoperative detection of radiolabeled tissues, the role of intraoperative imaging is generally limited, at least so far, to constituting an additional aid to the surgeon for SLN identification. Some authors have tried to assess the added value of portable γ cameras in clinical practice [55,58,59]. Their usefulness in patients with breast cancer is being established in the following conditions:
▪ When no conventional γ camera is available for preoperative lymphoscintigraphy;
▪ In cases with difficult drainage or extra-axillary drainage (intrammamary or internal mammary chain nodes, see Figure 5);
▪ In cases of only faint lymph nodal radiocolloid uptake;
▪ When the SLN is located very close to the injection site.
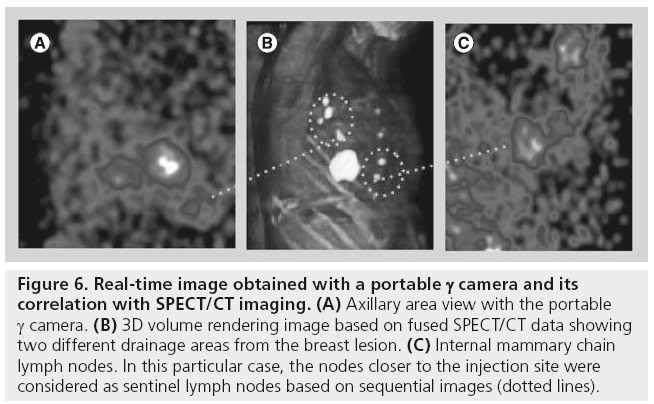
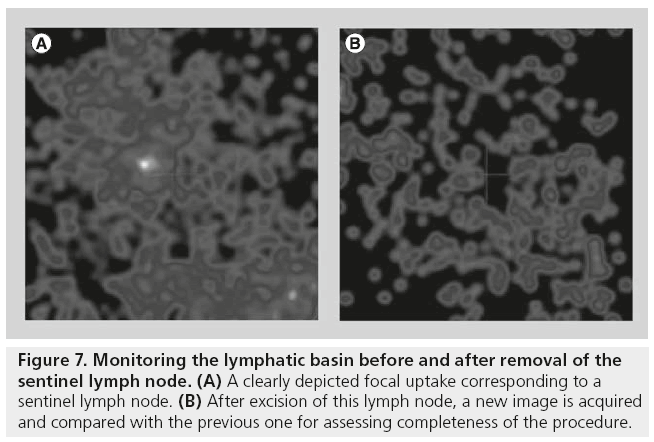
The position of the portable γ camera can be changed and adjusted in order to acquire specialangle views, so that SLNs near the injection area can also be shown (Figure 6). Some studies have reported missing detection of SLNs when only the g probe was used. In this regard, using an intraoperative imaging device implies the possibility of monitoring the lymphatic basin before and after removal of the SLNs, in order to verify the completeness of SLNs excision. After excision of each lymph node, a new image is acquired and compared with the image acquired before excision (Figure 7). If focal radioactivity remains at the same location, it is concluded that another possible SLN is still in place. Removing extra nodes that probably receive direct lymph drainage from the tumor should be weighed up against the fact that the surgical time is prolonged. However, even if additional time is needed, this extra time may be sufficiently useful in the context of SLN procedures that are likely to be difficult, since the use of the γ camera might reduce the possibility of missing a malignant SLN.
Figure 6: Real-time image obtained with a portable g camera and its correlation with SPECT/CT imaging. (A) Axillary area view with the portable g camera. (B) 3D volume rendering image based on fused SPECT/CT data showing two different drainage areas from the breast lesion. (C) Internal mammary chain lymph nodes. In this particular case, the nodes closer to the injection site were considered as sentinel lymph nodes based on sequential images (dotted lines).
Figure 7: Monitoring the lymphatic basin before and after removal of the sentinel lymph node. (A) A clearly depicted focal uptake corresponding to a sentinel lymph node. (B) After excision of this lymph node, a new image is acquired and compared with the previous one for assessing completeness of the procedure.
Discrimination between the true SLN and a second-tier radioactive node may be supported by the radioactive counts simultaneously recorded with the portable γ cameras, and can be related to the preoperative scintigraphic images. Although the majority of these cases can be solved with the presurgical information provided by planar images and SPECT/CT, realtime images acquired with a portable γ camera enhance the reliability of using the g probe, by adding a clear overview of surgical fields. In the operating room, the γ camera can be placed above the previously marked SLN locations using some external point sources (such as 153Gd, 133Ba or 125I); alternatively, in some γ cameras a laser pointer is coupled with to the device. In one of them (Sentinella 102), the laser pointer is displayed as a red cross over the patient’s skin. During surgery, an initial 30–60-s image is acquired with the γ camera, to assess the surgical field and confirm SLN uptake. This time can be longer when the lymph nodes are depicted as areas with faint uptake. Although this device can provide a real-time imaging, it is sometimes preferred to spend a few seconds to obtain a better image quality. After incision, if there is any difficulty in finding the location of the SLN using the g probe, another 30–120-s image is acquired using the portable γ camera. The use of the external point sources facilitates SLN localization, as these sources can be depicted separately on the screen of the portable γ camera, thus functioning as a pointer in the search for the nodes. Matching of two signals (99mTc signal and 153Gd, 133Ba or 125I pointer signals) indicates the correct location of the SLNs. This location is then checked again using the g probe. After SLN harvesting, another set of images is acquired to ascertain the absence of the previously visualized SLNs, as well as the absence of remaining nodes [55].
In breast cancer, there is no consensus about the need for intraoperative imaging to help SLN detection. Some authors believe that the utility of the portable γ cameras in breast cancer remains only when no conventional γ camera is available and in particular in cases with extra-axillary drainage [60].
Difficult situations can include intramammary and internal mammary chain lymph nodes, or when the SLN is located very close to the injection site. The large majority of these cases can be solved with the presurgical information provided by SPECT/CT. However, realtime images acquired with a portable γ camera can be useful in such cases. The appropriate use of a portable or a handheld mini γ camera enhances the reliability of the g probe by adding a clear image of the surgical fields. Using an intraoperative imaging device implies the possibility to better plan the procedure and to monitor the lymphatic basin before and after removal of the hot nodes, so to verify correct and complete lymph node excision. For difficult to retrieve lymph nodes, real-time imaging with such portable γ cameras in combination with the use of a traditional g-counting probe results in a higher intraoperative detection and localization of SLNs, especially for intramammary and internal mammary chain nodes [52,53,55,61].
In other malignancies, portable γ cameras have recently been introduced for intraoperative use based on the anatomical landmarks previously established by the fused SPECT/CT images. In both open surgery and laparoscopy, these devices may be used in combination with a conventional, handheld g probe. This is especially valuable in head and neck malignancies, melanoma and pelvic tumors [62–66].
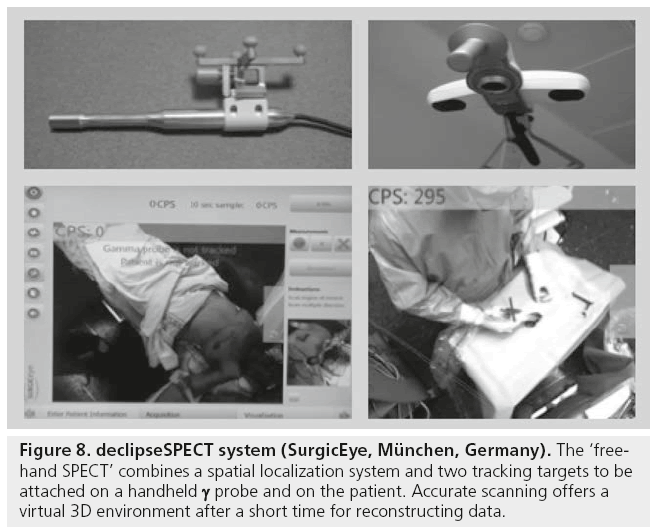
Other equipment for intraoperative purposes has recently become available. Thus, the declipseSPECT (SurgicEye, München, Germany) or so-called ‘free-hand SPECT’ combines a spatial localization system and two tracking targets to be attached on a handheld g probe (Figure 8). This results in a 3D visualization of the traditional acoustic signal of the g probe. This feature, together with the real-time information on depth, expands the applications of radioguided surgery in oncology. The positioning system installed in the operating room is based on a fixed pointing device, on the patient’s body and on the handheld g probe, thus permitting, after a few minutes of manual scanning, a virtual reconstruction in a 3D environment. The position of the g probe relative to the attached device is tracked by infrared technology, and the output of the g probe is coregistered in the surgical field (depicted by a video camera) and displayed on a monitor where the surgeon can easily check the location and depth of the foci of radioactivity accumulation to be resected. This 3D information allows the localization of the SLN and tumoral radiolabeled tissue [67,68].
Figure 8: declipseSPECT system (SurgicEye, München, Germany). The ‘freehand SPECT’ combines a spatial localization system and two tracking targets to be attached on a handheld γ probe and on the patient. Accurate scanning offers a virtual 3D environment after a short time for reconstructing data.
Fluorescent tracers
The combination of radiocolloid and blue dye is a standard method for SLN localization and biopsy. However, during breast cancer surgery, as in other malignancies, positive resection margins and surgical morbidity, as a result of damage to vital structures, are not uncommon. On the other hand, the use of a radiotracer requires some radioprotection measures and, in some countries, is not available on a general scale. Thus, there is a need for new intraoperative imaging modalities that can provide real-time assessment of tumor borders and involved lymph nodes, without the risk of damaging other structures.
Optical imaging using near infrared (NIR) fluorescence is a technique that can be used to visualize structures in real-time during surgery. Advantages of NIR fluorescent light (700–900 nm) include tissue penetration (millimeters deep) and low autofluorescence, thereby providing suf-cient contrast [69]. Since the human eye is not sensitive to NIR wavelengths, the use of these agents does not alter the surgical -eld. The currently developed intraoperative imaging systems are able to provide simultaneous acquisition of surgical anatomy (white light, color video) and NIR fluorescence signal. Therefore, the use of NIR fluorescence imaging could potentially be of great value in the intraoperative detection of anatomical structures and oncologic targets. In addition to NIR fluorescence imaging systems, exogenous fluorescent contrast agents are necessary to visualize speci-c tissues. The most commonly used fluorescent contrast agents are indocyanine green (ICG; Pulsion Medical Systems SE, Fedkirchen, Germany) and fluorescein.
ICG is more suitable for these purposes and it has been largely employed in preclinical investigations using animal models. ICG provides a higher signal-to-background ratio because of lower autofluorescence and increased tissue penetration at 820 nm compared with lower wavelengths and has a greater ‘brightness’ compared with methylene blue [70]. ICG is currently utilized in NIR fluorescence image-guided oncologic surgery for multiple indications. NIR fluorescence imaging has the potential to improve SLN mapping in multiple types of cancer, by real-time transcutaneous and intraoperative visualization of lymphatic channels and subsequent detection of the SLN [70–77].
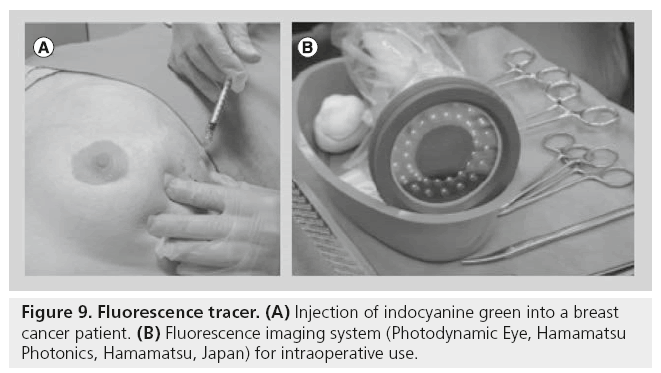
Several NIR fluorescence imaging systems have been described for intraoperative clinical use [78]. Although differing in their technical speci-cations, all of them allow the surgeon to obtain an image of the fluorescence tracer injected. Most of the studies published so far used the Photodynamic Eye (Hamamatsu Photonics, Hamamatsu, Japan; Figure 9). Other available systems are the SPY system (Novadaq Technologies, Concord, Canada), the Fluobeam™ (Fluoptics, Grenoble, France), HyperEye (Kochi Medical School, Kochi, Japan), the FLARE and Mini- FLARE (Beth Israel Deaconess Hospital, MA, USA), the FDP Mimager (Texas Medical Center, TX, USA) and a prototype from the Technical University Munich (Munich, Germany) and SurgOptix, Inc., (CA, USA). However, not all devices provide simultaneous color and f luorescence visualization, a desirable feature to coregister anatomical and fluorescence information.
Figure 9: Fluorescence tracer. (A) Injection of indocyanine green into a breast cancer patient. (B) Fluorescence imaging system (Photodynamic Eye, Hamamatsu Photonics, Hamamatsu, Japan) for intraoperative use.
The allergic reactions reported for ICG are very rare (one case per 10,000 procedures). The dose used for standard diagnostic procedures is between 0.1 and 0.5 mg/kg [79]. Imaging provided by NIR fluorescence may improve and extend some indications of the SLN procedure, especially in those instances when SLN localization can be dif-cult using a handheld g probe and blue dyes cannot be easily seen through the skin and fatty tissue. Imaging with ICG has been shown to visualize super-cial lymphatic channels transcutaneously [80], which could reduce time of surgery, although NIR dye performances are also strongly degraded when imaging through highly scattering tissues, such as fat.
Sixteen studies report the use of ICG as a NIR fluorescent lymphatic tracer for SLN biopsy in more than 800 breast cancer patients [80–95]. With intraoperative NIR fluorescence imaging systems, the SLN identi-cation rates in the axilla vary between 87.5 and 100%, with an average of 3.6 SLNs detected per patient. Two studies performed an axillary dissection irrespective of the SLN status and found a mean false-negative rate of 7.7% [80,91].
ICG offers noninvasive imaging of lymphatic flow due to the ability of the fluorescence light to penetrate tissue. After ICG injection, the tracer reaches the axilla in 1–10 min [96]. The small size of the ICG particle is responsible for this relatively high speed, which offers some advantages compared with larger particle size radiotracers. Hojo et al. found a larger number of SLNs with ICG than with the blue dye. They suggested that this issue could be due to the low molecular weight and high degree of diffusion of the ICG (viewing more secondary nodes) [90]. This potential drawback (i.e., too many lymph nodes identified as SLNs) can be overcome by performing the SLN detection shortly after ICG injection (approximately 10 min). On the other hand, there are currently no clinically approved fluorescent tracers for SLN mapping indications. Most studies previously mentioned are clinical trials with approval for local use out of the intended indication. This is one of the main limitations for the development of NIR fluorescence, as regulatory approval is needed for drugs, which takes takes years and a high monetary budget.
When comparing ICG fluorescence with the radiocolloid approach, several authors found a similar identification rate for the axilla [86,91]. Mieog et al. studied patient groups with escalating ICG concentrations ranging from 0.05 to 0.1 mM diluted in albumin [87]. They showed that the highest SLN brightness was achieved when using a concentration from 0.4 to 0.8 mM ICG. Polom et al. found NIR-guided real-time lymphatic flow in 47 out of 49 patients (96%) [94]. In all cases except one, SLNs detected by the radiocolloid were also detected by ICG.
Furthermore, ICG detected ten additional SLNs in eight patients. Statistical analysis revealed no difference between the numbers of SLNs detected between ICG versus radiotracer. They concluded, in general, that the use of ICG to obtain SLNs seems to be an effective alternative [94].
Finally, Wishart et al. studied 100 women with clinically node-negative breast cancer [95]. They performed SLN biopsy using blue dye, radiocolloid and ICG. Transcutaneous fluorescent lymphography was visible in all 104 procedures (in 99 patients). All 202 true SLNs, defined as blue and/or radioactive, were also fluorescent with ICG. Detection rates were: ICG alone 100%, ICG and blue dye 95.0%, ICG and radiocolloid 77.2%, ICG and blue dye and radiocolloid 73.1%. Metastases were found in 25 of 201 SLNs (12.4%) and all positive nodes were fluorescent, blue and radioactive. The procedural node positivity rate was 17.3% [94]. They concluded that the high sensitivity of ICG fluorescence for SLN detection in early breast cancer is confirmed. Thus, the combination of ICG and blue dye had the highest nodal sensitivity at 95.0%, defining a dual approach to SLN biopsy that avoids the need for radiocolloid mapping.
However, in spite of the advantages of the use of NIR fluorescent tracers, there are some important drawbacks.
As a result of the limited tissue penetration of the fluorescent signal, visualization is limited when ICG has reached the lower part of the axillary fossa, especially when patients have a high BMI [95]. Second, mapping is principally limited to level I of the axilla. Furthermore, after the first SLN is resected, lymph vessels are cut, and ICG leaks out and spreads to the surgical field, thus making it difficult to detect another fluorescent node. The reduction of the ICG dose may help to solve this problem [81]. Major work is currently being performed to design optimized NIR fluorescent contrast agents, with optimized biodistribution and SLN retention.
Hybrid tracers
As previously mentioned, fluorescence-based surgical guidance is a rapidly growing -eld of clinical research, which has been predominantly focused on the NIR ICG [77].
However, even with a 1–1.5 cm penetration of the NIR emission of ICG in tissues, fluorescence guidance is suboptimal for the localization of deeply located lymph nodes. Furthermore, fluorescence imaging does not enable preoperative lymphatic mapping for surgical planning, which helps to minimize the region of exploration [97]. However, as the signal penetration of fluorescent probes is limited by tissue attenuation, radio guidance to the general area of interest is still indispensable.
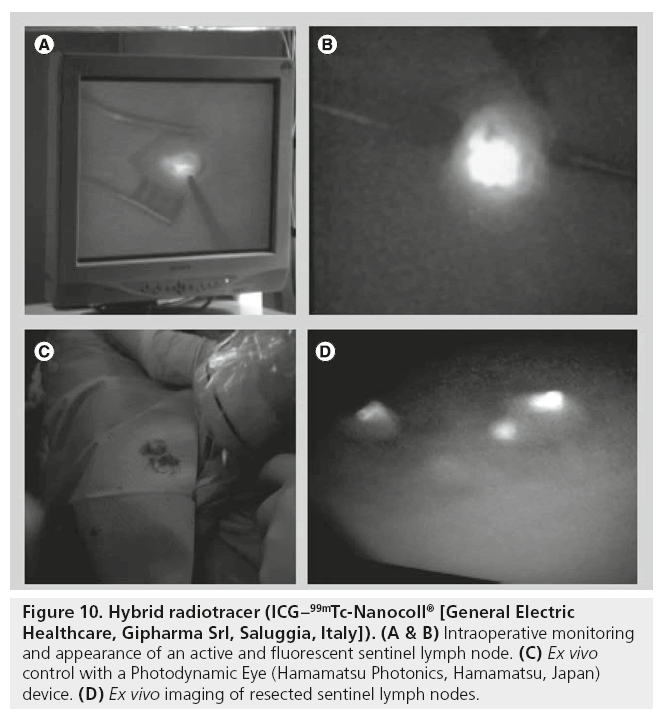
Against this background, the possibility of combining a radiopharmaceutical with fluorescent agents is attractive and opens new fields to explore. Recently, a radiolabeled nanocolloid agent has been combined with ICG for SLN detection (ICG–99mTc-Nanocoll® [General Electric Healthcare, Gipharma Srl, Saluggia, Italy]). In contrast to the use of a single fluorescent agent, this bimodal hybrid tracer allows surgeons to integrate the standard approach based on radioguided detection with a handheld g probe and/or a portable γ camera with a new optical modality based on fluorescent signal detection [98]. Also important is the fact that preoperative lymphoscintigraphy and SPECT/CT acquisition with this hybrid tracer remain unchanged as starting points for surgical planning. Hybrid tracers allow for both surgical planning via 3D SPECT/CT and real-time fluorescence-based surgical guidance. Previous studies have demonstrated that the migration and stability of this hybrid tracer resembles the migration and stability pattern previously described for 99mTc-Nanocoll. This indicates that the hybrid tracer retains the functional properties of the original radiocolloid and depicts the same SLNs [99,100]. Thus, in the study conducted by Brouwer et al. the improved tissue penetration that fluorescence imaging offers over vital blue dyes and the high resolution that can be obtained compared with g-tracing modalities enhance optical identi-cation of the SLNs (Figure 10) [101]. This advantage proved to be especially helpful when high radioactive background signals impeded SLN localization using the probe [101].
Figure 10: Hybrid radiotracer (ICG–99mTc-Nanocoll® [General Electric Healthcare, Gipharma Srl, Saluggia, Italy]). (A & B) Intraoperative monitoring and appearance of an active and fluorescent sentinel lymph node. (C) Ex vivo control with a Photodynamic Eye (Hamamatsu Photonics, Hamamatsu, Japan) device. (D) Ex vivo imaging of resected sentinel lymph nodes.
Importantly, the labeling procedure uses a lower ICG concentration than that previously used for SLN mapping. Thus, 99mTc-nanocolloid is prepared by adding 2 ml of 99mTc-pertechnetate in saline (up to 1400 MBq) to a commercial vial of nanocolloid containing 0.5 mg of albumin colloid. After 30 min of incubation at room temperature, the 99mTc-nanocolloid solution (pH 6–7) is exposed to air via a needle, to remove any excess reactive elements. Subsequently, 0.25‑mg of ICG is added to form hybrid ICG–99mTc-nanocolloid [101]. This hybrid-guidance concept was clinically introduced for the visualization of deeply sited pelvic SLNs in the head and neck region [101–105]. Preclinically, it was shown that this technology can be expanded to the use of radioactive and fluorescent marker seeds and the visualization of speci-c tumor biomarkers [106].
Since cost reduction is a priority for hospitals and healthcare systems, the classical approach, using blue dye only, could be adequate, but its results are far from optimal. Therefore, the addition of other tracers (radiotracers and/or fluorescent), as well as other equipment, refines the technique with moderate costs. In summary, the SLN biopsy approach, involving some expensive techniques, is finally less expensive than conventional lymphadenectomy. The length of hospital stay is the cost driver of these procedures. There are heterogeneous practices for this validated and largely diffused technique. Although we can combine several technical choices and they have an impact on the cost of SLN procedure, we know that intraoperative specimen analysis allows us to reduce rehospitalization rates for secondary lymphadenectomy, suggesting possible savings on hospital resources.
Other intraoperative possibilities
The ultrasonographic evaluation of axillary lymph nodes in combination with biopsy has been reported to increase the preoperative identification of axillary node metastasis, thus allowing a reduction in the number of unnecessary SLN procedures, with considerable savings of time and costs [107,108].
A review by Alvarez et al. concluded that axillary ultrasound should not be used as a unique modality for deciding whether or not to perform complete axillary dissection [109]. On the other hand, the use of contrast-enhanced ultrasound is well established in clinical practice. Ultrasound contrast agents are composed of a dispersion of micro bubbles (mean diameter of 2.5 μm) that act as reflectors of the ultrasound beam [109]. Sever et al. investigated this microbubble approach in 80 patients [110]. Conventional gray-scale ultrasound examination of the axilla was performed before injection of the contrast agent, and the position of imaged lymph nodes was noted. After injection of contrast agent, lymphatic channels were visualized immediately on contrast pulse sequencing and were followed into the axilla. When no obvious lymphatic filling was obtained, the areola area was massaged for 10–30 s and imaging was repeated. In the patients whose nodes were successfully identified, the transit time from injection to arrival in the axillary node was 15–45 s. The contrast agent remained in the SLN for 1–3 min. Repeating the areola massage often produced a refill of the SLN without the need for a second injection. In 71 out of 80 patients (89%), contrast-enhanced ultrasound images revealed visualization of lymphatic vessels, starting from the site of injection and traced to the level of axilla [110,111]. Although SLN identification under ultrasound in the axilla holds the possibility of performing a biopsy, we know that fine-needle aspiration biopsy of nodes has a recognized false-negative rate resulting from sampling error [112]. The next stage in this approach is to harvest the SLNs depicted by microbubbles to permit detailed histologic analysis.
Much more recently, the application of intraoperative probe detection after a preoperative same-day injection of [18F]-fluorodeoxyglucose (FDG) has been reported in a limited number of selected cases [113]. Other new possibilities combine the preoperative use of [18F]-FDG and other conventional techniques. Thus, in one study Garcia et al. showed a mixed approach in 12 patients with suspected tumor recurrence detected by [18F]-FDG PET/CT considered resectable. In five patients with accessible lesions, the ROLL technique was performed after injection of 99mTc-nanocolloid inside the lesion under ultrasound or CT guidance and the subsequent use of a conventional g probe [114]. In the remaining seven patients with nonaccessible lesions or multiple lesions, about 370 MBq [18F]-FDG was injected 3–5 h before radioguided surgery using a PET-dedicated probe. All lesions with ROLL technique were resected and recurrence was histologically confirmed. In the PET probe group, 14 out of 16 lesions detected on the PET/ CT were resected. Histological recurrence was confirmed in 12 out of 14 lesions, although information was not provided about the margins involvement of the resected tissue. This approach opens new perspectives in radioguided surgery, because [18F]-FDG PET/CT can be fundamental in deciding surgical management and appropriate radioguided protocol. On the other hand, new portable breast-dedicated PET devices (positron emission mammography) would be implemented in the intraoperative setting, although this approach is still cumbersome at this moment [115]. Finally, some difficult clinical approaches in breast cancer could be solved with radioguided surgery. Thus, it is feasible to apply the ROLL technique with a 99mTc-nanocolloid in locally advanced breast cancers after neoadjuvant chemotherapy [114]. The placement of a 125I seed previously to neoadjuvant chemotherapy could be a good alternative to this approach to overcome the short half-life of 99mTc [116].
Conclusion
Radioguided surgery and, in particular, SLN biopsy is now the accepted standard of care in various clinical conditions, in particular for patients with early-stage breast cancer or cutaneous melanoma. Lymphoscintigraphy, a mandatory preoperative step of the SLN biopsy procedure, is normally performed with conventional γ cameras. When the γ camera is combined with x-ray CT to constitute a hybrid SPECT/CT tomograph, the fused images obtained are highly useful, providing the surgeon with a morphologic and functional roadmap for planning the procedure with minimal surgical access and operating time, especially in higher axillary levels and extra-axillary locations. For immediate decision-making during surgery, intraoperative exploration of the surgical field is performed with a handheld g probe with or without the aid of vital dyes. The recently developed portable γ cameras enable real-time scintigraphic imaging of the surgical field. On the other hand, the use of fluorescent tracers is currently increasing. Image navigation with hybrid (radioactive and fluorescent) compounds appears to be technically feasible. This approach makes optimal use of all available preoperative data and may be a next step to refine the surgical guidance process.
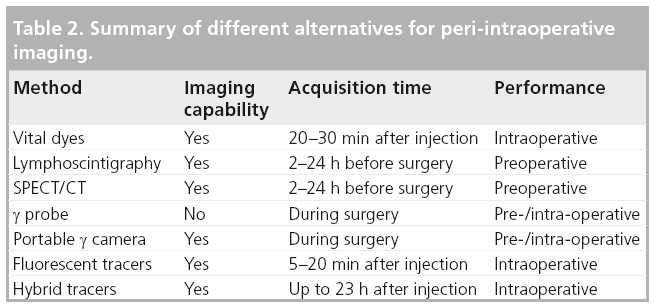
In summary, it is relevant to note that, despite all the variations encountered worldwide, the benefits of the SLN biopsy method seem overwhelming, whatever the approach taken. However, the interaction between technologies and medical disciplines permits continuous refinement of the methodology and improves the outcomes (Table 2).
Future perspective
Since radioguided surgery is a comprehensive team approach, establishing successful radioguided surgery programmes needs great collaboration and coordinated efforts of the different specialties involved. In the coming years there will be a higher utilization of technology and the research in this field will lead to new strategies for radioguided surgery. In this setting, the use of minimally invasive surgery approaches benefit from the development of new devices and procedures, to expand existing applications. Thus, the so-called ‘virtual surgery’ (using radionuclide and/or optical imaging) is becoming essential for optimizing surgical approaches.
The above considerations highlight the needs to combine such techniques in a manner that will assure high quality in the management of cancer patients with a reasonable cost.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Tanis PJ, Nieweg OE, Valdés Olmos RA et al. History of sentinel node and validation of the technique. Breast Cancer Res. 3, 109–112 (2001).
- Nieweg OE, Estourgie S, Valdés Olmos RA. Lymphatic mapping and sentinel node biopsy. In: Nuclear Medicine in Clinical Diagnosis and Treatment (3rd Edition). Ell PJ, Gambhir SS (Eds). Churchill Livingstone, Edinburgh, UK, 229–260 (2004).
- Morton DL, Wen DR, Wong JH et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 127, 392–399 (1992).
- Keshtgar, MRS, Ell, PJ. Sentinel lymph node detection and imaging. A comprehensive review of conventional technique for sentinel node identification. Eur. J. Nucl. Med. 26, 57–67 (1999).
- Uren RF, Howman-Giles RB, Thompson JF et al. Mammary lymphoscintigraphy in patients with breast cancer – mapping lymph drainage patterns and locating the sentinel nodes. J. Nucl. Med. 36, 1775–1780 (1995).
- Noguchi M, Inokuchi M, Zen Y. Complement of peritumoral and subareolar injection in breast cancer sentinel lymph node biopsy. J. Surg. Oncol. 100, 100–105 (2009).
- Pelosi E, Bello M, Griors M et al. Sentinel lymph node detection in patients with earlystage breast cancer: comparison of periareolar and subdermal/peritumoral injection techniques. J. Nucl. Med. 45, 220–225 (2004).
- Chakera AH, Friis E, Hesse U et al. Factors of importance for scintigraphic non-visualization of sentinel nodes in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 32, 286–293 (2005).
- Buscombe J, Paganelli G, Burak ZE et al. Sentinel node in breast cancer procedural guidelines. Eur. J. Nucl. Med. Mol. Imaging 34, 2154–2159 (2007).
- Albertini JJ, Lyman GH, Cox C et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 276, 1818–1822 (1996).
- Alazraki NP, Eshima D, Eshima LA et al. Lymphoscintigraphy, the sentinel node concept, and the intraoperative gamma probe in melanoma, breast cancer, and other potential cancers. Semin. Nucl. Med. 27, 55–67 (1997).
- Pijpers R, Meijer S, Hoekstra OS et al. Impact of lymphoscintigraphy on sentinel node identification with technetium-99m-colloidal albumin in breast cancer. J. Nucl. Med. 38, 366–368 (1997).
- McMasters KM, Wong SL, Tuttle TM et al. Preoperative lymphoscintigraphy for breast cancer does not improve the ability to identify axillary sentinel lymph nodes. Ann. Surg. 231, 724–731(2000).
- Wang L, Yu JM, Wang YS et al. Preoperative lymphoscintigraphy predicts the successful identification but is not necessary in sentinel lymph nodes biopsy in breast cancer. Ann. Surg. Oncol. 14, 2215–2220 (2007).
- Dordea M, Colvin H, Cox P et al. Clinical and histopathological factors affecting failed sentinel node localization in axillary staging for breast cancer. Surgeon 11(2), 63–66 (2013).
- Lerman H, Metser U, Lievshitz G et al. Lymphoscintigraphic sentinel node identification in patients with breast cancer: the role of SPECT-CT. Eur. J. Nucl. Med. Mol. Imaging 33, 329–337 (2006).
- Lerman H, Lievshitz G, Zak O et al. Improved sentinel node identification by SPECT/CT in overweight patients with breast cancer. J. Nucl. Med. 48, 201–206 (2007).
- van der Ploeg IM, Nieweg OE, Kroon BB et al. The yield of SPECT/CT for anatomical lymphatic mapping in patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging 36, 903–909 (2009).
- Vermeeren L, van der Ploeg IM, Valdés Olmos RA et al. SPECT/CT for preoperative sentinel node localization. J. Surg. Oncol. 101, 184–190 (2010).
- Delbeke D, Coleman RE, Guiberteau MJ et al. Procedure guidelines for SPECT/CT imaging. J. Nucl. Med. 47, 1227–1234 (2006).
- Orsini F, Guidoccio F, Vidal-Sicart S, Valdés Olmos RA, Mariani G. General concepts on radioguided sentinel lymph node biopsy: preoperative imaging, intraoperative gammaprobe guidance, intraoperative imaging, and multimodality imaging. In: Atlas of Lymphoscintigraphy and Sentinel Node Mapping. Mariani G, Manca G, Orsini F, Vidal-Sicart S, Valdés Olmos RA (Eds). Springer-Verlag, Milan, Italy, 95–110 (2012).
- Pesek S, Ashikaga T, Krag LE et al. The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J. Surg. 36, 2239–2251 (2012).
- Keshtgar M, Aresti N, Macneil F. Establishing axillary sentinel lymph node biopsy (SLNB) for early breast cáncer in United Kingdom: a survey of the national training program. Eur. J. Surg. Oncol. 36, 393–398 (2010).
- Rodier JF, Velten M, Wilt M et al. Prospective multicentric randomized study comparing periareolar and peritumoral injection of radiotracer and blue dye for the detection of sentinel lymph node in breast sparing procedures: FRANSENODE trial. J. Clin. Oncol. 25, 3664–3669 (2007).
- Anan K, Mitsuyama S, Kuga H et al. Double mapping with subareolar blue dye and peritumoral green dye injections decreases the false negative rate of dye only sentinel node biopsy for early breast cancer: 2 site injection is more accurate than 1-site injection. Surgery 139, 624–629 (2006).
- Chintamani, Tandon M, Mishra A et al. Sentinel lymph node biopsy using dye alone method is reliable and accurate even after neoadjuvantchemotherapy in locally advanced breast cancer – a prospective study. World J. Surg. Oncol. 9, 19–25 (2011).
- Rodier JF, Velten M, Wilt M et al. Prospective multicentric randomized study comparing periareolar and peritumoral injection of radiotracer and blue dye for the detection of sentinel lymph node in breast sparing procedures: FRANSENODE trial. J. Clin. Oncol. 25, 3664–3669 (2007).
- Varghese P, Abdel-Rahman AT, Akberali S et al. Methylene blue dye – a safe and effective alternative for sentinel lymph node localization. Breast J. 14, 61–67 (2008).
- Shirah GR, Bouton ME, Komenaka IK. Occurrence of prolonged injection site mass with methylene blue but not isosulfan blue after the sentinel node procedure. Arch. Surg. 146, 137–141 (2011).
- Wong A, Spillane A; Breast Surgeons of Australia and New Zealand Incorporated (BreastSurgANZ). Patent Blue V dye anaphylaxis: experience of Australian and New Zealand surgeons. ANZ J. Surg. doi:10.1111/j.1445-2197.2012.06277.x (2012) (Epub ahead of print).
- Raut CP, Hunt KK, Akins JS et al. Incidence of anaphylactoid reactions to isosulfan blue dye during breast carcinoma lymphatic mapping in patients treated with preoperative prophylaxis: results of a surgical prospective clinical practice protocol. Cancer 104, 692–699 (2005).
- Scherer K, Studer W, Figueiredo V et al. Anaphylaxis to isosulfan blue and crossreactivity to patent blue V: case report and review of the nomenclature of vital blue dyes. Ann. Allergy Asthma Immunol. 96, 497–500 (2006).
- Joshi M, Hart M, Ahmed F et al. Adverse reaction; patent blue turning patient blue. BMJ Case Rep. doi:10.1136/bcr-2012-007339 (2012) (Epub ahead of print).
- Barthelmes L, Goyal A, Newcombe RG et al. NEW START and ALMANAC study groups. Adverse reactions to patent blue V dye – the NEW START and ALMANAC experience. Eur. J. Surg. Oncol. 36, 399–403 (2010).
- Schneebaum S, Even-Sapir E, Cohen M et al. Clinical applications of gamma-detection probes-radio-guided surgery. Eur. J. Nucl. Med. 26, S26–S35 (1999).
- Tiourina T, Arends B, Huysmans D et al. Evaluation of surgical gamma probes for radioguided sentinel node localisation. Eur. J. Nucl. Med. 25, 1224–1231 (1998).
- Zanzonico P. The intra-operative gamma probe: design, operation, and safety. In: Sentinel Lymph Node Biopsy. Cody HS (Ed.). Informa Health Care, London, UK, 45–68 (2002).
- Mariani G, Vaiano A, Nibale O et al. Is the ‘ideal’ gamma-probe for intra-operative radioguided surgery conceivable? J. Nucl. Med. 46, 388–390 (2005).
- Moffat FL Jr. Targeting gold at the end of the rainbow: surgical gamma probes in the 21st century. J. Surg. Oncol. 96, 286–289 (2007).
- Povoski SP, Neff RL, Mojzisik CM et al. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J. Surg. Oncol. 7, 11–73 (2009).
- Serrano Vicente J, Infante de la Torre JR, Domínguez Grande ML et al. Optimization of sentinel lymph node biopsy in breast cancer by intraoperative axillary palpation. Rev. Esp. Med. Nucl. 29, 8–11 (2010).
- Postma EL, Verkooijen HM, van Esser S et al. Efficacy of radioguided occult lesion localisation (ROLL) versus wire-guided localization (WGL) in breast conserving surgery for non-palpable breast cancer: a randomized controlled multicentre trial. Breast Cancer Res. Treat. 136, 469–478 (2012).
- Donker M, Straver ME, Rutgers EJ et al. Radioguided occult lesion localisation (ROLL) in breast-conserving surgery after neoadjuvant chemotherapy. Eur. J. Surg. Oncol. 38, 1218–1224 (2012).
- Sajid MS, Parampalli U, Haider Z et al. Comparison of radioguided occult lesion localization (ROLL) and wire localization for non-palpable breast cancers: a meta-analysis. J. Surg. Oncol. 105, 852–858 (2012).
- Hughes JH, Mason MC, Gray RJ et al. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. Breast J. 14, 153–157 (2008).
- van Riet YE, Jansen FH, van Beek M et al. Localization of non-palpable breast cancer using a radiolabelled titanium seed. Br. J. Surg. 97, 1240–1245 (2010).
- Cheng G, Kurita S, Torigian DA et al. Current status of sentinel lymph-node biopsy in patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging 38, 562–575 (2011).
- Newman EA, Newman LA. Lymphatic mapping techniques and sentinel lymph node biopsy in breast cancer. Surg. Clin. North Am. 87, 353–364 (2007).
- Goyal A, Newcombe RG, Mansel RE et al. Role of routine preoperative lymphoscintigraphy in sentinel node biopsy for breast cancer. Eur. J. Cancer 41, 238–243 (2005).
- Goyal A, Mansel RE. Does imaging in sentinel node scintigraphic localization add value to the procedure in patients with breast cancer? Nucl. Med. Commun. 26, 845–847 (2005).
- Vidal-Sicart S, Valdés Olmos R. Sentinel node mapping for breast cancer: current situation. J. Oncol. 2012, 361341 (2012).
- Vidal-Sicart S, Brouwer OR, Valdés-Olmos RA. Evaluation of the sentinel lymph node combining SPECT/CT with the planar image and its importance for the surgical act. Rev. Esp. Med. Nucl. 30, 331–337 (2011).
- Valdés Olmos RA, Vidal-Sicart S, Nieweg OE. Technological innovation in the sentinel node procedure: towards 3-D intraoperative imaging. Eur. J. Nucl. Med. Mol. Imaging 37, 1449–1451 (2010).
- Cardona-Arboniés J, Mucientes-Rasilla J, Moreno Elola-Olaso A et al. Contribution of the portable gamma camera to detect the sentinel node in breast cancer during surgery. Rev. Esp. Med. Nucl. Imagen Mol. 31, 130–134 (2012).
- Heller S, Zanzonico P. Nuclear probes and intraoperative gamma cameras. Semin. Nucl. Med. 41, 166–181 (2011).
- Vidal-Sicart S, Paredes P, Zanón G et al. Added value of intraoperative real-time imaging in searches for difficult-to-locate sentinel nodes. J. Nucl. Med. 51, 1219–1225 (2010).
- Vermeeren L, Klop WM, van den Brekel MW et al. Sentinel node detection in head and neck malignancies: innovations in radioguided surgery. J. Oncol. 2009, 681746 (2009).
- Mathelin C, Salvador S, Bekaert V et al. A new intraoperative gamma camera for the sentinel lymph node procedure in breast cancer. Anticancer Res. 28 (5B), 2859–2864 (2008).
- Mathelin C, Salvador S, Croce S et al. Optimization of sentinel lymph node biopsy in breast cancer using an operative gamma camera. World J. Surg. Oncol. 17, 132–137 (2007).
- Vidal-Sicart S, Vermeeren L, Solà O et al. The use of a portable gamma camera for preoperative lymphatic mapping: a comparison with a conventional gamma camera. Eur. J. Nucl. Med. Mol. Imaging 38, 636–641 (2011).
- Duch J. Portable gamma cameras: the real value of an additional view in the operating theatre. Eur. J. Nucl. Med. Mol. Imaging 38, 633–635 (2011).
- Paredes P, Vidal-Sicart S, Zanón G et al. Radioguided occult lesion localisation in breast cancer using an intraoperative portable gamma camera: first results. Eur. J. Nucl. Med. Mol. Imaging 35 230–235 (2008).
- Vermeeren L, Valdés Olmos RA, Meinhardt W et al. Intraoperative imaging for sentinel node identification in prostate carcinoma: its use in combination with other techniques. J. Nucl. Med. 52, 741–744 (2011).
- Stoffels I, Poeppel T, Boy C et al. Radio-guided surgery: advantages of a new portable γ-camera (Sentinella) for intraoperative real time imaging and detection of sentinel lymph nodes in cutaneous malignancies. J. Eur. Acad. Dermatol. Venereol. 26, 308–313 (2012).
- Brouwer OR, Valdés Olmos RA, Vermeeren L et al. SPECT/CT and a portable gammacamera for image-guided laparoscopic sentinel node biopsy in testicular cancer. J. Nucl. Med. 52, 551–554 (2011).
- Vermeeren L, Valdés Olmos RA, Meinhardt W et al. Intraoperative radioguidance with a portable gamma camera: a novel technique for laparoscopic sentinel node localisation in urological malignancies. Eur. J. Nucl. Med. Mol. Imaging 36, 1029–1036 (2009).
- Wendler T, Hartl A, Lasser T et al. Towards intra-operative 3D nuclear imaging: reconstruction of 3D radioactive distributions using tracked gamma probes. Med. Image. Comput. Comput. Assist. Interv. 10, 909–917 (2007).
- Wendler T, Herrmann K, Schnelzer A et al. First demonstration of 3-D lymphatic mapping in breast cancer using freehand SPECT. Eur. J. Nucl. Med. Mol. Imaging 37, 1452–1461 (2010).
- Frangioni JV. New technologies for human cancer imaging. J. Clin. Oncol. 26, 4012–4021 (2008).
- Matsui A, Tanaka E, Choi HS et al. Real-time intraoperative near-infrared fluorescence identi-cation of the extrahepatic bile ducts using clinically available contrast agents. Surgery 148, 87–95 (2010).
- Crane LM, Themelis G, Arts HJ et al. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: first clinical results. Gynecol. Oncol. 120, 291–295 (2011).
- Rossi EC, Ivanova A, Boggess JF. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: a feasibility study. Gynecol. Oncol. 124, 78–82 (2012).
- Holloway RW, Bravo RA, Rakowski JA et al. Detection of sentinel lymph nodes in patients with endometrial cancer undergoing roboticassisted staging: a comparison of colorimetric and fluorescence imaging. Gynecol. Oncol. 126, 25–29 (2012).
- van der Vorst JR, Schaafsma BE, Verbeek FP et al. Dose optimization for near-infrared fluorescence sentinel lymph node mapping in melanoma patients. Br. J. Dermatol. 168, 93–98 (2013).
- Stoffels I, von der Stück H, Boy C et al. Indocyanine green fluorescence-guided sentinel lymph node biopsy in dermato-oncology. J. Dtsch Dermatol. Ges. 10, 51–57 (2012).
- Hirche C, Mohr Z, Kneif S et al. Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int. J. Colorectal Dis. 27, 319–324 (2012).
- Yuasa Y, Seike J, Yoshida T et al. Sentinel lymph node biopsy using intraoperative indocyanine green fluorescence imaging navigated with preoperative CT lymphography for superficial esophageal cancer. Ann. Surg. Oncol. 19, 486–493 (2012).
- Polom K, Murawa D, Rho YS et al. Current trends and emerging future of indocyanine green usage in surgery and oncology: a literature review. Cancer 117, 4812–4822 (2011).
- Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 9, 237–255 (2010).
- Schaafsma BE, Mieog JS, Hutteman M et al. The clinical use of indocyanine green as a nearinfrared fluorescent contrast agent for imageguided oncologic surgery. J. Surg. Oncol. 104, 323–332 (2011).
- Hirche C, Murawa D, Mohr Z et al. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res. Treat. 121, 373–378 (2010).
- Hirano A, Kamimura M, Ogura K et al. A comparison of indocyanine green fluorescence imaging plus blue dye and blue dye alone for sentinel node navigation surgery in breast cancer patients. Ann. Surg. Oncol. 19, 4112–4116 (2012).
- van der Vorst JR, Schaafsma BE, Verbeek FP et al. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99m technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann. Surg. Oncol. 19, 4104–4111 (2012).
- Aoyama K, Kamio T, Ohchi T et al. Sentinel lymph node biopsy for breast cancer patients using fluorescence navigation with indocyanine green. World J. Surg. Oncol. 9, 157 (2011).
- Kitai T, Kawashima M. Transcutaneous detection and direct approach to the sentinel node using axillary compression technique in ICG fluorescence-navigated sentinel node biopsy for breast cancer. Breast Cancer 19, 343– 348 (2012).
- Nagao T, Hojo T, Kurihara H et al. Sentinel lymph node biopsy in breast cancer patients with previous breast augmentation surgery. Breast Cancer doi:10.1007/s12282-011-0280-7 (2011) (Epub ahead of print).
- Mieog JS, Troyan SL, Hutteman M et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 18, 2483–2491 (2011).
- Hutteman M, Mieog JS, van der Vorst JR et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res. Treat. 127, 163–170 (2011).
- Abe H, Mori T, Umeda T et al. Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg. Today 41, 197–202 (2011).
- Tagaya N, Aoyagi H, Nakagawa A et al. A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J. Surg. 35, 154–158 (2011).
- Hojo T, Nagao T, Kikuyama M et al. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast 19, 210–213 (2010).
- Murawa D, Hirche C, Dresel S et al. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br. J. Surg. 96, 1289–1294 (2009).
- Troyan SL, Kianzad V, Gibbs-Strauss SL et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 16, 2943– 2952 (2009).
- Polom K, Murawa D, Nowaczyk P et al. Breast cancer sentinel lymph node mapping using near infrared guided indocyanine green and indocyanine green-human serum albumin in comparison with gamma emitting radioactive colloid tracer. Eur. J. Surg. Oncol. 38, 137–142 (2012).
- Wishart GC, Loh SW, Jones L et al. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur. J. Surg. Oncol. 38, 651–656 (2012).
- Ogasawara Y, Ikeda H, Takahashi M et al. Evaluation of breast lymphatic pathways with indocyanine green fluorescence imaging in patients with breast cancer. World J. Surg. 32, 1924–1929 (2008).
- Sevick-Muraca EM, Sharma R, Rasmussen JC et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: Feasibility study. Radiology 246, 734–741 (2008).
- Chin PT, Buckle T, Aguirre de Miguel A et al. Dual-emissive quantum dots for multispectral intraoperative fluorescence imaging. Biomaterials 31, 6823–6832 (2010).
- Buckle T, van Leeuwen AC, Chin PT et al. A self-assembled multimodal complex for combined pre- and intraoperative imaging of the sentinel lymph node. Nanotechnology 21(35), 355101 (2010).
- Buckle T, Chin PT, van Leeuwen FW. (Nontargeted) radioactive/fluorescent nanoparticles and their potential in combined pre- and intraoperative imaging during sentinel lymph node resection. Nanotechnology 21, 482001 (2010).
- Brouwer OR, Buckle T, Vermeeren L et al. Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 53, 1034–1040 (2012).
- van der Poel HG, Buckle T, Brouwer OR et al. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur. Urol. 60, 826–833 (2011).
- Brouwer OR, Buckle T, Bunschoten A et al. Image navigation as a means to expand the boundaries of fluorescence-guided surgery. Phys. Med. Biol. 57, 3123–3136 (2012).
- van den Berg NS, van Leeuwen FW, van der Poel HG. Fluorescence guidance in urologic surgery. Curr. Opin. Urol. 22, 109–120 (2012).
- Brouwer OR, Klop WM, Buckle T et al. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann. Surg. Oncol. 19, 1988– 1994 (2012).
- van den Berg NS, Brouwer OR, Klop WM et al. Concomitant radio- and fluorescenceguided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG–99mTc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 39, 1128–1136 (2012).
- Buckle T, Chin PT, van den Berg NS et al. Tumor bracketing and safety margin estimation using multimodal marker seeds: a proof of concept. J. Biomed. Opt. 15, 056021 (2010).
- Susini T, Nori J, Olivieri S et al. Predicting the status of axillary lymph nodes in breast cancer: a multiparameter approach including axillary ultrasound scanning. Breast 18, 103–108 (2009).
- Alvarez S, Añorbe E, Alcorta P et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am. J. Roentgenol. 186, 1342–1348 (2006).
- Sever AR, Mills P, Jones SE et al. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am. J. Roentgenol. 196, 251–256 (2011).
- Cosgrove D. Ultrasound contrast agents: an overview. Eur. J. Radiol. 60, 324–330 (2006).
- Sever AR, Mills P, Weeks J et al. Preoperative needle biopsy of sentinel lymph nodes using intradermal microbubbles and contrastenhanced ultrasound in patients with breast cancer. AJR Am. J. Roentgenol. 199, 465–470 (2012).
- Britton PD, Provenzano E, Barter S et al. Ultrasound guided percutaneous axillary lymph node core biopsy: how often is the sentinel lymph node being biopsied? Breast 18, 13–16 (2009).
- García JR, Fraile M, Soler M et al. PET/CTguided salvage surgery protocol. Results with ROLL Technique and PET probe. Rev. Esp. Med. Nucl. 30, 217–222 (2011).
- Hall NC, Povoski SP, Murrey DA, Knopp MV, Martin EW. Combined approach of perioperative 18F-FDG PET/CT imaging and intraoperative 18F-FDG handheld gamma probe detection for tumor localization and verification of complete tumor resection in breast cancer. World J. Surg. Oncol. 5, 143 (2007).
- Donker M, Straver ME, Rutgers EJ et al. Radioguided occult lesion localisation (ROLL) in breast-conserving surgery after neoadjuvant chemotherapy. Eur. J. Surg. Oncol. 38, 1218–1224 (2012).
• Comprehensive review of conventional techniques for sentinel node identification.
• European Association of Nuclear Medicine guidelines for sentinel lymph node biopsy technique.
• • Enhances and warrants the use of preoperative SPECT/CT.
• • Very comprehensive review regarding radioguided surgery in all potential applications.
• • General review of the technology currently available in this field.
• Introduces this innovative approach (3D freehand SPECT) in breast cancer.
• Extensive review regarding indocyanine green fluorescence tracer use.
• • Demonstrates the similar characteristics of hybrid tracers against conventional nanocolloid radiotracers.