Review Article - Diabetes Management (2018) Volume 8, Issue 3
Perioperative diabetes management
- *Corresponding Author:
- Tracy L Setji
Department of Medicine
Division of Endocrinology
Duke University Medical Center
Durham, North Carolina
E-mail: tracy.setji@duke.edu
Abstract
Uncontrolled diabetes increases the risk of postoperative complications including surgical site infection, acute renal failure, and mortality. Further, the stress of surgery and anesthesia increase counter regulatory hormones which contribute to an insulin resistant state and can lead to hyperglycemia, even in patients without known diabetes. Hyperglycemia impairs neutrophil function, and increases inflammatory cytokines and formation of reactive oxygen species. These changes lead to cellular damage and dysfunction of the immune and vascular systems. Recognizing patients at risk for perioperative hyperglycemia during the preoperative assessment provides an opportunity to intervene on glycemic control. Further, attention to glycemic control on the day of surgery and postoperatively with active management of blood glucoses with insulin has been shown to improve outcomes. This article reviews perioperative complications associated with diabetes and hyperglycemia, and provides guidelines for assessment and management of the patient preoperatively, on the day of surgery and postoperatively. It also provides a protocol for treating hypoglycemia in the hospital setting.
Introduction
Diabetes mellitus affects 30.3 million people in the United States [1], and an estimated 25% of patients with diabetes will require surgery [2]. Diabetes is a risk factor for postoperative complications including surgical site infections, postoperative myocardial ischemia, acute renal failure, ileus, and increased length of hospitalization [3-7]. Patients with diabetes are 1.5 times more likely to develop a surgical site infection [8]. Each surgical site infection is estimated to cost $7,000 to $38,000 and extend the length of stay by 10 days [9].
In addition to patients with known diabetes, up to 10% of patients presenting for surgery may have previously undiagnosed diabetes [10,11]. This may lead to higher perioperative glucoses [10]. Moreover, these patients have a higher risk of mortality in the perioperative period than patients with known diabetes [11]. Hyperglycemia noted in surgical or critically ill patients without a known diagnosis of diabetes it is often referred to as stress hyperglycemia [12].
Patients with newly diagnosed hyperglycemia in the hospital have a higher in-hospital mortality rate (16%) compared with patients with known diabetes (3%) and patients with normal glucoses (1.7%; both p<0.01) [13].
The pathophysiology behind hyperglycemia and poor surgical outcomes is complex. The stress of surgery and anesthesia increase the levels of counter regulatory hormones, which subsequently cause a state of insulin resistance and raise glucose level. Hyperglycemia has been shown to impair neutrophil function as well as lead to overproduction of inflammatory mediators and reactive oxygen species leading to cellular damage, and subsequent immune and vascular dysfunction [14,15].
Controlling blood glucoses in the perioperative setting improves surgical outcomes. Cardiac surgery patients have decreased mortality when glucoses are controlled with continuous intravenous (IV) insulin infusion [16]. Further, a randomized multicenter trial demonstrated improvement in the composite outcome of wound infection, pneumonia, bacteremia, and respiratory failure, and acute renal failure [17]. This review discusses the pathophysiology and resulting complications of perioperative hyperglycemia, and the management of diabetes in the perioperative period including preoperative evaluation, day of surgery management, and postoperative care.
Pathophysiology of perioperative hyperglycemia
There are many factors that contribute to hyperglycemia in the perioperative setting. Surgical stress increases secretion of counter regulatory hormones including cortisol, catecholamines, growth hormone and glucagon. The impact of these excess hormones is widespread. Cortisol stimulates gluconeogenesis and protein catabolism. Catecholamines inhibit insulin secretion and increase glucagon secretion. Counter regulatory hormones also increase lipolysis resulting in increased levels of free fatty acids. Free fatty acids further impair glucose uptake in skeletal muscle. Thus, the cumulative result is an increase in hepatic glucose production, decrease in glucose uptake and utilization, and inhibition of insulin secretion from the pancreas resulting in hyperglycemia and insulin resistance [14,18].
In addition to the surgery and anesthesia, dexamethasone is frequently given during surgery to help with postoperative nausea and pain and to decrease length of stay [19,20]. However, dexamethasone may also raise blood glucose and the potential complications related to steroidinduced hyperglycemia in this population has not been well studied. Only 3 of 45 studies in a meta-analysis evaluating the effects of dexamethasone measured perioperative glucose. Further, none of the studies evaluated the effects of dexamethasone on glucose in patients with diabetes [21]. Thus, further research is needed to help delineate the risks and benefits of perioperative dexamethasone use in patients with diabetes.
The combination of the surgical stress, anesthesia and steroids may result in hyperglycemia. Hyperglycemia leads to the production of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6, interleukin-1beta). These cytokines further contribute to insulin resistance, potentially by interfering with signaling of the insulin receptor and/or the glucose transporter-4 receptor [22]. Further, hyperglycemia impairs neutrophil function by limiting adherence, chemotaxis, phagocytosis, and bactericidal destruction [15]. The increase in reactive oxygen species from hyperglycemia may result in damage at the cellular level, and immune, and vascular dysfunction [22]. The combination of these changes likely contributes to the poor wound healing and increased risk of surgical site infection in patients with uncontrolled diabetes.
Preoperative evaluation and treatment
▪ A. Role of measuring A1c
Poor glycemic control is associated with postoperative complications and increased mortality. However, the impact of glucose control in the preoperative period versus the intra- and postoperative setting has been less clear. Several studies have shown an association between elevated preoperative A1c and postoperative complications and/or mortality [23,24]. Additional studies have found an association between elevated perioperative blood glucose levels and mortality [25]. Further investigation into the effects of preoperative A1c and perioperative glucose levels on postoperative mortality demonstrated that preoperative A1c is indeed an excellent predictor of perioperative glucose control. However, when controlling for each other, perioperative glucose control was clearly associated with postoperative mortality whereas preoperative A1c was not [26]. Thus, an elevated A1c can best help by identifying patients that need focused attention on glycemic control in the perioperative period. Of note, an A1c of 6.5% or higher is diagnostic for diabetes [27] and the target A1c for most nonpregnant adults with diabetes is less than 7 to 8% depending on their concomitant comorbidities [28]. Providers should consider checking an A1c on any surgical patient with a history of diabetes if the A1c has not been assessed within the last 90 days. Screening criteria for diabetes in patients without known diabetes are listed in (Table 1) [27].
| All patients age 45 years; if normal repeat at least every 3 years |
| Patients with BMI >25 kg/m2 (>23 in Asian Americans) and one additional risk factor* |
| Patients with history prediabetes should be tested annually |
| Women with history of gestational diabetes should be tested every 3 years |
*Risk factors: first degree relative with diabetes; high-risk race/ethnicity; history of cardiovascular disease; polycystic ovary syndrome or other conditions associated with insulin resistance; hypertension; low HDL and/or high triglycerides; physical inactivity.
Table 1. American Diabetes Association Screening Criteria for Diabetes (27)
There have been no prospective randomized trials that have evaluated the impact of controlling blood glucose prior to surgery. However, large clinical trials have demonstrated reduction in micro vascular and macro vascular complications with improved glycemic control in patients with type 1 and type 2 diabetes [29- 32]. Patients with type 1 diabetes randomized to intensive insulin therapy (resultant A1c 7.2%) had a reduction in the incidence of retinopathy by 76%, neuropathy by 69%, and micro albuminuria by 34% compared with patients with type 1 diabetes randomized to conventional treatment group (resultant A1c 9.1%). Furthermore, intensive insulin therapy decreased progression of all three micro vascular complications in patients that had baseline abnormalities [29]. Follow up 17 years after the intervention demonstrated 42% reduction in cardiovascular disease, and 57% reduction in the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease [30]. Similarly, patients with type 2 diabetes randomized to intensive glucose control with oral medications or insulin (resultant A1c 7%) experienced a 25% risk reduction in micro vascular endpoints compared to patients in the conventional group (resultant A1c 7.9%) [31]. Follow up ten years later demonstrated additional risk reductions for myocardial infarction (15%, p=0.01) and death from any cause (13%, p=0.007), with even more substantial reductions in the group treated with metformin (33% reduction in myocardial infarction, p=0.005; 27% reduction in death from any cause, p=0.002) [32]. Thus, identifying patients with poorly controlled diabetes and aiming to improve glucoses prevents micro and macro vascular complications. Based on this reasoning and the opportunity to identify patients preoperatively, some institutions have programs to identify and treat poorly controlled diabetes preoperatively [22,33,34]. Although the long term benefits of these programs remain to be seen, the proportion of patients presenting on the day of surgery with a blood glucose level>200 mg/dl decreased from 33% to 20% at one institution after implementation of their preoperative program [34]. In addition, early results from patients going through a similar process resulted in a median glucose on day of surgery of 151 mg/dl [35].
▪ B. Carbohydrate loading before surgery
In recent years, enhanced recovery after surgery has allowed clear fluids including carbohydrate (CHO)-rich beverages a couple of hours prior to surgery. By altering the catabolic response to surgery that normally occurs in the fasting state, the preoperative consumption of CHO-rich beverages may reduce insulin resistance. Wang et al. found that colorectal patients randomized to preoperative CHO had lower postoperative insulin resistance compared to those who were fasting or received placebo, and this appeared to be from stimulation of the phosphatidylinositol 3-kinase/protein kinase B signaling pathway [36]. CHO-rich beverages have also been shown to improve patient well-being before surgery by reducing thirst, hunger and anxiety [37]. It is less clear whether patients with diabetes will have similar benefits. There is concern about potential delay in gastric emptying in patients with diabetes which may increase aspiration risk, as well as hyperglycemia from the CHO load. A small study comparing 25 patients with type 2 diabetes to healthy controls did not find a difference in gastric emptying, however, there was transient hyperglycemia noted in the patients with diabetes that resolved by 180 minutes [38]. These findings are overall reassuring; however, further study is needed to clarify the risks and benefits of CHO loading in patients with diabetes.
▪ C. Preoperative medication recommendations
Patients with diet controlled diabetes do not generally require medications for their diabetes before surgery, but should have close postoperative monitoring to evaluate for hyperglycemia related to the surgery, steroids, etc. For patients with type 2 diabetes treated with oral medications or non-insulin injection therapy, medications can be given as usual the day prior to surgery, but held on the morning of surgery [39]. It is notable that recent evidence demonstrates that dipeptidyl peptidase 4 (DPP4) inhibitors are safe in patients undergoing noncardiac surgeries [40]. Further studies including cardiac and general surgery patients are underway.
For patients with either type 1 and type 2 diabetes, basal insulin therapy (glargine, levemir, degludec) is continued but at a reduced dose (often 75-80% of normal dose starting the night prior to surgery) [22]. Prandial insulin therapy (lispro, aspart, glulisine) is given as normal the day prior to surgery but held on the day of surgery if the patient is fasting. Patients treated with NPH or mixed insulin should consider reducing the dose the night prior to surgery by 20-25% as well as the day of surgery by 50% [22,39]. The treatment of patients that use continuous subcutaneous insulin infusion (CSII) pumps to control their glucoses is discussed in a separate section.
Day of Surgery
The American Diabetes Association recommends target blood glucose levels of 80 to 180 mg/dl in the perioperative setting. If insulin is used to treat hyperglycemia, the glucose treatment goal for most patients is 140 to 180 mg/ dl, and 110 to 140 mg/dl for select patients if this can be achieved without significant risk for hypoglycemia [39]. These treatment recommendations are fairly consistent with those of the Joint British Diabetes Societies who also recommend initiating insulin therapy if glucoses are >80 mg/dl and targeting glucoses between 108 to 180 mg/dl in most patients [41,42].
Blood glucose testing should be completed preoperatively and every 2 hours intraoperatively for patients with diabetes [22,33]. If patient experiences hypoglycemia, intravenous dextrose should be administered with reassessment of glucose, more frequent glucose monitoring, and consideration of a continual dextrose-containing IV solution during surgery (see Hypoglycemia section for more details).
Patients with hyperglycemia (glucose >180 mg/dl) should be treated with subcutaneous or intravenous insulin infusion during surgery [39,42]. Patients undergoing short procedures can often be treated with a single subcutaneous dose of correction insulin, preferably rapid acting insulin analogs (lispro, aspart, glulisine) over regular insulin because rapid acting analogs work faster to bring the glucose down and are less likely to cause hypoglycemia [22]. Critically ill patients and those undergoing long and complex procedures should be treated with IV insulin. IV insulin has a short half-life which allows for rapid titration to achieve control of glucoses. There is a number of paper IV insulin protocols published. Alternatively, computer based algorithms may more rapidly control glucoses with less variability and hypoglycemia in glycemic control than paper protocols [43] and have been shown to be safe in the operating room [44]. However, the cost of these programs and available resources need to be weighed when considering implementation. Patients treated with IV insulin need to have glucoses monitored at least every 1-2 hours depending on the IV insulin protocol, and perhaps more frequently if the rate of blood glucose fall is rapid or glucose is in the low end of the target range.
Postoperative Care
Patients that have required IV insulin infusions can be transitioned to subcutaneous insulin once infusion rates are stable and glucoses controlled, particularly if a diet has been initiated. Because IV insulin has a very short half-life, the subcutaneous insulin should be administered prior to discontinuation of IV insulin [45]. The basal infusion rate during fasting is a good predictor of basal subcutaneous insulin requirements, however, providers often reduce the amount by 20% upon transition [46]. For instance, if a patient required 1.5 unit/hour of IV insulin overnight, this would suggest a basal need of approximately 36 units of insulin daily. However, reducing this by 20% would result in a starting basal dose of 30 units of insulin daily. Some institutions have dedicated pharmacists or diabetes providers to help with the transition of IV insulin to subcutaneous regimens. Inpatient diabetes management by a specialized team has been shown to reduce 30-day readmission rates, reduce inpatient diabetes cost, and improve adherence to follow up and transition of care. Further, length of stay is significantly shortened if the diabetes team is consulted during the first 24 hours of admission [47].
Patients not requiring IV insulin infusion but whose diabetes has been treated with subcutaneous insulin, oral medications or non-insulin injectable therapy often require basal insulin therapy in the hospital to control glucoses. If baseline basal insulin requirements are unknown, initiating basal insulin therapy at 0.1 to 0.25 unit/kg/day is a good place to start [22]. Patients that are insulin sensitive (type 1, thin body habitus), elderly, or have impaired renal function should be started on the lower end of the range. Patients that are obese or insulin resistant often need higher doses of basal insulin.
Prandial insulin (starting at 0.1 to 0.25 unit/ kg/day, thus 0.03 to 0.08 unit/kg/meal) is often required when patients are eating well [22]. A randomized controlled trial demonstrated that basal bolus (basal insulin plus bolus prandial insulin) therapy reduces postoperative complications including wound infections compared to those treated with only sliding scale [17]. Alternatively, basal insulin plus correction insulin (the Basal Plus approach) can effectively control glucoses in surgical patients in whom it is less certain how well they will eat [48]. In addition, a recent prospective randomized trial demonstrated that basal insulin plus a DPP-4 inhibitor can be effective in noncardiac surgical patients with type 2 diabetes treated at home with diet, oral medications, or low dose subcutaneous insulin (total daily dose of 0.4 unit/kg/day or less) [40]. Although not demonstrated in this small study, it seems plausible that a combination of basal insulin and DPP4 inhibitor therapy may have less hypoglycemia and improved patient and staff satisfaction compared to basal bolus therapy. Larger studies are ongoing to further evaluate the role of DPP4 inhibitors in surgical patients.
Continuous subcutaneous insulin infusion (CSII) therapy
Hospitals should have standardized procedures for patients that use subcutaneous insulin (CSII) pump therapy [49]. Patients can either be transitioned to an off pump plan by their primary diabetes provider prior to surgery or can continue pump therapy until they present for their surgery. Depending on the baseline level of glycemic control, the basal rate the night prior to surgery may need a reduction in rates [39], particularly if blood glucose trends down overnight at baseline or if glucoses in the mornings are in the low to low-normal range. However, many patients with appropriate basal rates in their CSII do not need to decrease their basal rate the night prior surgery. If their procedure is not compatible with CSII (surgery is long and complex, radiology exposure incompatible with the pump), the patient can be transitioned to intravenous insulin therapy for the procedure with resultant resumption of CSII once the patient is able to manage their pump. Alternatively, a basal bolus subcutaneous regimen calculated by their current pump settings and prandial requirements can be initiated in the perioperative setting. However, it is important to remember that patients with type 1 diabetes always need some form of basal insulin on board.
Diabetic emergencies in the perioperative period
▪ A. Hypoglycemia
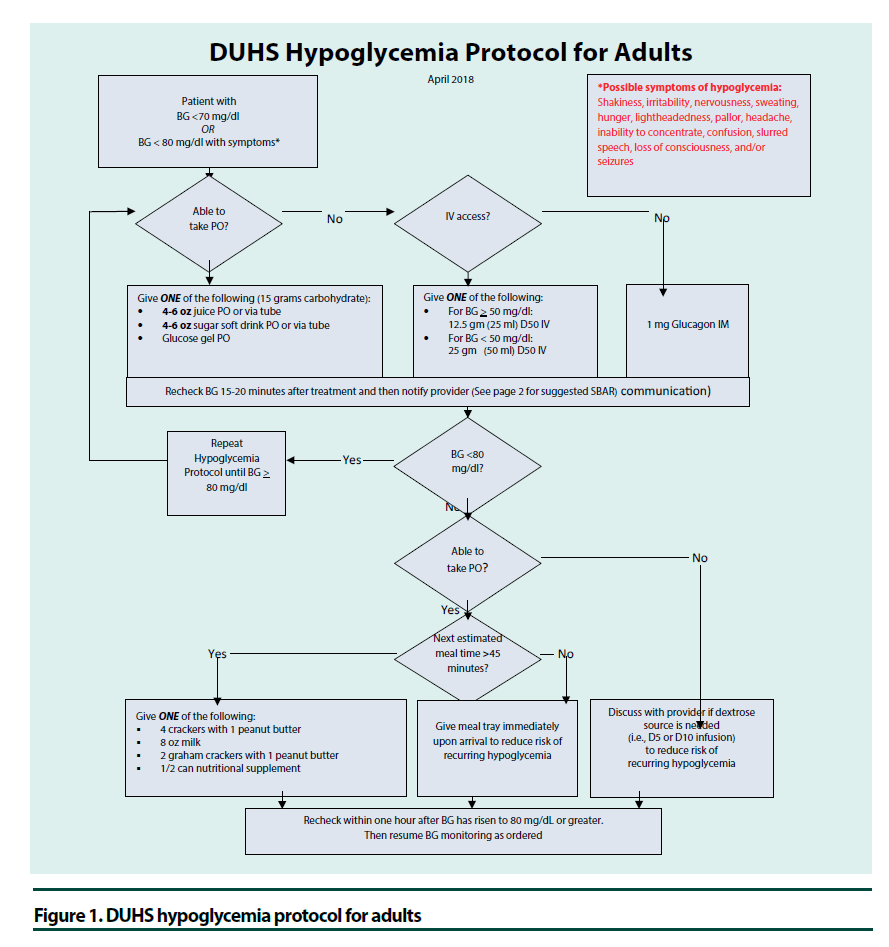
Historically, hypoglycemia has been defined as glucose<70 mg/dl and severe hypoglycemia as glucose<40 mg/dl or evidence of severe cognitive impairment requiring assistance. It has recently been recognized that glucoses<54 mg/ dl are associated with increased mortality and thus considered serious, clinically important hypoglycemia [50]. Although hypoglycemia is uncommon intraoperatively, glucoses should be monitored since hypoglycemia may not be recognized while under anesthesia or immediately postoperatively when the patient is still experiencing the effects of anesthesia. If hypoglycemia is not recognized and treated, it could lead to seizures, arrhythmias, and death [51]. In the preoperative and intraoperative setting, patients with glucose <80 mg/dl should receive a dextrose-containing IV solution and this should continue until the patient is able to eat and drink [22,33]. The percentage of dextrose and rate per hour should be dictated by the clinical situation. Postoperatively, hospitals should have nurse driven protocols to treat glucoses<70 mg/dl that includes notification of providers (Figure 1).
Figure 1. DUHS hypoglycemia protocol for adults
▪ B. Diabetic ketoacidosis or hyperosmolar non-ketotic state
Patients presenting for surgery with evidence of severe dehydration, diabetic ketoacidosis, or hyperosmolar hyperglycemic non-ketotic state should have their surgery postponed if possible [52]. Further, patients scheduled for elective surgeries that have presence of ketosis or symptomatic hyperglycemic non-ketotic state may benefit from delaying surgery in order to provide treatment of hyperglycemia [22]. Patients without these findings who present with hyperglycemia can be treated with IV insulin infusion or subcutaneous insulin, however, if glucoses remain persistently elevated, postponing the surgery could be considered depending on the urgency of surgery.
Conclusion
Diabetes and hyperglycemia are significant risk factors for postoperative complications. However, recognition and treatment of hyperglycemia in the perioperative setting has been shown to improve outcomes. Although current trials are evaluating the role of DPP4 inhibitors, insulin is most often used to control glucoses perioperatively. Subcutaneous and IV insulin protocols can help patients reach their glycemic goals in the hospital and close follow up after discharge should be arranged to ensure continued glycemic control for optimal wound healing.
References
- American Diabetes Association. www.diabetes.org/assets/pdfs/basics/cdc-statistics-report-2017.pdf.
- Jacober S, Sowers J. An update in perioperative management of diabetes. Arch. Intern. Med. 159, 2405–2411 (1999).
- Schuster J, Rechtime G, Norvell D et al. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: A systematic review. Spine. 35(9), S125–S127 (2010).
- Godoy D, Di Napoli , Biestro A, et al. Perioperative glucose control in neurosurgical patients. Anesthesiol Res Pract 690362, 2012.
- Kohl B, Schwartz S. How to manage perioperative endocrine insufficiency. Anesthesiology. Clin. 28, 139–155 (2010).
- Han H, Kang S. Relations between long-term glycemic control and postoperative wound and infectious complications after total knee arthroplasty in type 2 diabetics. Clin. Orthoped. Surg. 5, 118–123 (2013).
- Hollenberg M, Mangano D, Browner W et al. The study of perioperative ischemia research group. Predictors of postoperative myocardial ischemia in patients undergoing noncardiac surgery. JAMA. 268(2), 205–209 (1992).
- Martin E, Kaye K, Knott C et al. Diabetes and risk of surgical site infection: A systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 37(1), 88–99 (2016).
- de Lissovoy G, Fraeman K, Hutchins V et al. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am. J. Infect. Control. 37(5), 387–397 (2009).
- Abdelmalak B, Abdelmalak J, Knittel J et al. The prevalence of undiagnosed diabetes in non-cardiac surgery patients, an observational study. Can. J. Anesth. 57(12), 1058–1064 (2010).
- Lauruschkat A, Arnich B, Albert A et al. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation. 112(16), 2397–2402 (2005).
- Dungan K, Braithwaite S, Preiser J. Stress hyperglycemia. Lancet 373(9677), 1798–1807 (2009).
- Umpierrez G, Isaacs S, Bazargan N. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J. Clin. Endocrinol. Metab. 87(3), 978–982 (2002).
- Duggan E, Carlson K, Umpierrez G. Perioperative hyperglycemia management. Anestthesiology 126(3), 547–560 (2017).
- Delamaire M, Maugendre D, Moreno M et al. Impaired leucoyte functons in diabetic patients. Diabet. Med. 14(1), 29–34 (1997).
- Furnary A, Gao G, Grunkemeier G et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 125(5), 1007–1021 (2003).
- Umpierrez G, Smiley D, Jacobs S et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 Surgery). Diabetes. Care. 34, 256–261 (2011).
- Farrokhi F, Smiley D, Umpierrez G. Glycemic control in non-diabetic critically ill patients. Best. Pract. Res. Clin. Endocrinol. Metab. 25(5), 813–824 (2011).
- Backes J, Bentley J, Politi J et al. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: A prospective, randomized controlled trial. J. Arthroplasty. 28(8), 11–17 (2013).
- Elsamadicy A, Wang T, Back A et al. Impact of intraoperative steroids on postoperative infection rates and length of hospital stay: A study of 1200 spine surgery patients. World. Neurosurg. 96, 429–433 (2016).
- Waldron N, Jones C, Gan T et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: Systematic review and meta-analysis. Br. J. Anaesth. 110(2), 191–200 (2013).
- Duggan E, Klopman M, Berry A et al. The Emory University perioperative algorithm for the management of hyperglycemia and diabetes in non-cardiac surgery patients. Curr. Diab. Rep. 16(3), 34 (2016).
- Underwood P, Askari R, Hurwitz S et al. Preoperative A1c and clinical outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes. Care. 37(3), 611–616 (2014).
- Kuhl J, Sartipy U, Eliasson B et al. Relationship between preoperative hemoglobin A1c levels and long-term mortality after coronary artery bypass grafting in patients with type 2 diabetes mellitus. Int. J. Cardiol. 202, 291–296 (2016).
- Kwon S, Thompson R, Dellinger P et al. Importance of perioperative glycemic control in general surgery. Ann. Surg. 257(1), 8–14 (2013).
- Vanden W, Schroeder R, Manning M et al. Effect of A1c and glucose on perioperative mortality in noncardiac and cardiac surgeries. Diabetes. Care. 41, 782–788 (2018).
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care 2018. Diabetes. Care. 41(1), 13–27 (2018).
- American Diabetes Association. Glycemic targets: Standards of medical care. Diabetes. Care. 2018 41(1), 55–64 (2018).
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes.
- United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33). Lancet 352(9131), 837–853 (1998).
- Holman R, Paul S, Bethel M et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
- Setji T, Hopkins T, Jimenez M et al on behalf of the Duke Perioperative Enhancement Team (POET). Rationalization, development, and implementation of a preoperative diabetes optimization program designed to improve perioperative outcomes and cost. Diabetes. Spectr. 30(3), 217–223 (2017).
- Underwood P, Seiden J, Carbone K et al. Early identification of individuals with poorly controlled diabetes undergoing elective surgery: Improving A1c testing in the preoperative period. Endocrine. Pract. 21(3), 231–236 (2015).
- Aronson S, Westover J, Guinn N et al. A perioperative medicine model for population health: An integrated approach for an evolving clinical science. Anesth. Analg. 126(2), 682–690 (2018).
- Wang Z, Wang Q, Wang W et al. Randomized clinical trial to compare the effects of pre-operative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br. J. Surg. 97(3), 317–327 (2010).
- Hausel J, Nygren J, Lagerkranser M et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth. Analg. 93(5), 1344–1350 (2001).
- Gustafsson U, Nygren J, Thorell A et al. Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta. Anaesthesiol. Scand. 52(7), 946–951 (2008).
- American Diabetes Association. Diabetes care in the hospital: Standards of medical care in diabetes. Diabetes. Care. 41(1), 144–151(2018).
- Pasquel F, Gianchandani R, Rubin D et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): A multicentre, prospective, open-label, non-inferiority randomised trial. Lancet. Diabetes. Endocrinol. 5(2), 125–133 (2017).
- Demma L, Carlson K, Duggan E et al. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J . Clin. Anesth. 36, 184–188 (2017).
- Dhatariya K, Levy N, Kivert A et al. Joint British Diabetes Societies: NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet. Med. 29, 420–433 (2012).
- Newton C, Smiley D, Bode B et al. A comparison study of continuous insulin infusion protocols in the intensive care unit: Computer-guided vs. standard column-based algorithms. J. Hosp. Med. 5(8), 432–437 (2013).
- Davidson P, Steed R, Bode B. Glucommander: A computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes. Care. 28(10), 2418–2423 (2005).
- Umpierrez G, Hellman R, Korytkowski M et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 97, 16–38 (2012).
- Kreider K, Lien L. Transitioning Safely from Intravenous to Subcutaneous Insulin. Curr. Diab. Rep. 15, 23 (2015).
- Bansal V, Mottalib A, Pawar T et al. Inpatient diabetes management by specialized diabetes team versus primary service team in non-critical care units: Impact on 30-day readmission rate and hospital cost. BMJ. Open. Diab. Res. Care. 6(1), 1–8 (2018).
- Umpierrez G, Smiley D, Hermayer K et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes. Diabetes. Care. 36(8), 2169–2174 (2013).
- Thompson B, Korytkowski M, Klonoff D et al. Consensus statement on use of continuous subcutaneous insulin infusion therapy in the hospital. J. Diabetes. Sci. Tech. 1–10 (2018).
- International Hypoglycemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: A joint position statement for the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes. Care. 40(1), 155–157 (2017).
- Tsujimoto T, Yamamoto-Honda R, Kajio H et al. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes. Care. 37(1), 217–225 (2014).
- Joshi G, Chung F, Vann M et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth. Analg. 111(6), 1378–1387 (2010).