Research Article - Diabetes Management (2017) Volume 7, Issue 3
Hypoglycaemic effects of dichloromethane: methanolic leaf and stem bark extracts of Pappea capensis in male Albino rats
- *Corresponding Author:
- David N Ngai
Department of Biochemistry and Biotechnology
School of Pure and Applied Sciences
Kenyatta University
Nairobi, Kenya
E-mail: konjingai@gmail.com
Abstract
Diabetes mellitus is a group of metabolic disorders that result in hyperglycaemia due to reduced insulin production or poor utilization of insulin in the body. The treatment of diabetes mellitus has been confined to the use of oral anti-diabetic agents and insulin which are unaffordable and are known to cause side effects. This has led to increased demand for hypoglycaemic herbal products that are affordable, more readily available and have fewer side effects. The present study was designed to determine the anti-diabetic activity of DCM: Methanolic leaf and stem bark extracts of Pappea capensis on alloxan-induced diabetic male albino rats. The DCM: Methanolic leaf and stem bark extracts of P. capensis demonstrated hypoglycaemic activity by reducing blood sugar levels of alloxan-induced diabetic rats to 6.70 and 6.54 mmol/L respectively. From the histopathological studies of the liver, kidney and pancreas tissues of alloxan-induced diabetic rats, there was evidence of recovery from alloxan-induced damage after administration of leaf and stem bark extracts of P. capensis and this followed a dose-related pattern. These curative effects may be attributed to the presence of phytochemicals such as glycosides, alkaloids, terpenoids, phenolics and flavonoids that are associated with hypoglycaemic activity. The leaf and stem bark extracts of P. capensis, therefore, may be used in the development of herbal products with anti-diabetic activity.
Keywords
Pappea capensis, diabetes mellitus, hypoglycaemic
Introduction
Diabetes mellitus is a metabolic disorder whose main indicator is persistent high levels of glucose in the blood and altered carbohydrates, proteins and lipids metabolism due to diminished secretion and/or activity of insulin [1]. Insulin resistance, which is a feature of diabetes mellitus, can be alleviated by an augmented insulin secretion by the normal β-cells. However, inadequate insulin compensation causes the onset of glucose intolerance. Once hyperglycaemia has developed, β-cell activity continuously deteriorates and glucose-induced secretion of insulin is further curtailed. Degranulation and depopulation of β-cells become apparent after hyperglycaemia persists for long [2].
Compromised metabolism results to excessive release of oxidants through lipid peroxidation. This occurs as a result of the change of functions of various proteins and the subsequent drop in antioxidant immune mechanisms [3]. In persistent hyperglycaemia, oxidative stress is always evident and this is believed to be as a result of increased release of free radicals, reduced antioxidant defenses and tissue antioxidant status, which then leads to the development of diabetic complications [4].
Generally, diabetes mellitus is categorised into two types, namely, type 1 and type 2. Type 1 occurs due to low insulin secretion due to defective insulin gene. Type 2 usually develops later in life and is accompanied by increased insulin resistance by the peripheral tissues. This insulin resistance often results from either an alteration in the structure or a reduction in the number of insulin receptors due to glucotoxicity associated with hyperglycaemia, obesity or genetic predisposition [5].
Currently, treatment of diabetes, in addition to insulin therapy, includes many oral antidiabetic drugs such as sulfonylureas, biguanides, thiazolidines, meglitinides and α-glucosidase inhibitors alongside a suitable lifestyle. These hypoglycaemics used in diabetes management are not perfect remedies due to their side effects and reduced effectiveness after a long time use [6].
In Kenya, Pappea capensis (family; sapindaceae) stem bark, leaf and seed oil are used traditionally against ringworms, nosebleeds, baldness, chest complaints, eye infections, venereal diseases and hyperglycaemia [7,8]. In addition, leaves of P. capensis are potent in killing snails. Maasai warriors in Kenya use the bark infusion to increase libido and as a blood-cleansing tonic [9]. The root of the plant is used as an enema and a purgative for cattle [10].
Medicinal plants are considered as effective and safer alternatives to the conventional medicines in the management of ailments related to oxidative stress such as diabetes [11]. There is a broad variety of antioxidant compounds synthesized by plants including carotenoids, lignans, flavonoids, isoflavones, isocatechins, flavones, ascorbic acid, anthocyanins, coumarins, catechins, polyphenols and α-tocopherol to prevent the oxidation of their susceptible biomolecules [12]. This study, therefore, was designed to determine the antidiabetic activity of DCM: Methanolic leaf and stem bark extracts of P. capensis.
Materials and methods
▪ Sample collection and preparation
Fresh leaves and stem barks of Pappea capensis were collected from their native habitat in Mbeere North Sub-County, Embu County, Kenya with the guidance of a local Traditional Herbal Practitioner (THP). An acknowledged authority authenticating the plant’s botanical identity was obtained and a voucher specimen deposited at National Museums of Kenya herbarium. The study was implemented at the department of Biochemistry and Biotechnology, Kenyatta University. The fresh leaves and stem bark were shade dried separately at room temperature, and then milled into fine a powder using an electric mill. The powdered sample was then packed in sealed, dry plastic bags separately.
▪ Extraction
Five hundred grams of powdered leaf or stem bark material was soaked in two litres of a 1:1 mixture of DCM and methanol for 48 hrs. The extract was then poured and filtered through folded cotton gauze into another clean dry conical flask. The filtrate was then separately concentrated using a rotary evaporator at 40ºC and stored at 4ºC.
▪ Experimental design
Male albino rats (8-9 weeks) weighing between 180 g to 220 g were used in this study. They were maintained at normal environmental conditions of 25ºC temperature with 12 hrs/12 hrs darkness photoperiod. They were fed on rodent pellets diet and water ad libitum. The rats were fasted for 8-12 hrs before being used in bioassay [13], but supplied with water throughout the study period. The standard procedures for handling experimental animals and ethical guidelines were observed throughout the study period [13].
The experimental rats were randomly divided into six groups of five animals each. A volume of 1.5 ml of 10% DMSO was given to the animals in groups one and two daily for one week. Those in group three were given glibenclamide (3 mg/ kg body weight) every day for seven days. Those in groups four, five and six were administered with 1.5 ml of P. capensis leaf or stem bark extract at concentrations of 50, 100 and 150 mg/ kg body weight daily for seven days respectively. This design is summarized in Table 1.
| Group | Treatment |
|---|---|
| I (Normal control) | DMSO |
| II (Negative control) | Alloxan+DMSO |
| III (Positive control) | Alloxan+Glibenclamide (3 mg/kg bwt |
| IV (Experimental group A) | Alloxan+DMSO+P. capensis (50 mg/g bwt) |
| V (Experimental group B) | Alloxan+DMSO+P. capensis (100 mg/g bwt) |
| VI (Experimental group C) | Alloxan+DMSO+P. capensis (150 mg/g bwt) |
| Glibenclamide 3 mg/kg bwt; 10% DMSO | |
Table 1. Treatment schedule.
A stock solution of the leaf or stem bark extract was prepared by dissolving 0.4 g of the extract in 2 ml of Dimethyl Sulfoxide (DMSO) and then the volume was diluted to 20 ml with distilled water. This gave a concentration equivalent to 20 mg/ml. For the diabetic and normal control groups, the stock solution was prepared by diluting 2 ml DMSO to 20 ml with distilled water to give a percentage concentration of 10% (volume by volume). The stock solution for the positive control group was made by dissolving 30 mg of glibenclamide in 15 ml of distilled water to yield a final concentration of 2 mg/ml. Given that the average weight of the rats was 200 g, the various daily dosages were prepared according to the dilution schedule summarized in Table 2.
| Group | Stock solution (ml) |
Water volume (ml) |
Final volume (ml) |
Daily dosage (ml) |
|---|---|---|---|---|
| Normal control | 15 | 0 | 15 | 1.5 |
| Negative control | 15 | 0 | 15 | 1.5 |
| Positive control | 15 | 0 | 15 | 1.5 |
| 50 mg/kg bwt | 5 | 10 | 15 | 1.5 |
| 100 mg/kg bwt | 10 | 5 | 15 | 1.5 |
| 150 mg/kg bwt | 15 | 0 | 15 | 13 |
Table 2. Dilution schedule for the different treatments.
▪ Induction of diabetes and determination of blood sugar levels
The animals were intraperitoneally treated with 186.9 mg/kg body weight of 10% alloxan monohydrate [8]. Seventy-two hrs after the induction, the animals’ blood glucose levels were measured with a glucometer and rats with blood glucose levels ≥11 mmoles/L were described as hyperglycaemic and used in the study. After the seven days treatment period, the final blood sugar levels were measured and recorded. In both cases, blood was obtained by nipping the end of the tail using a sharp sterile pair of scissors and milking the blood from the tail.
▪ Histopathological assessment
After 7 days of plant extract administration, the animals were treated with chloroform and sacrificed. The liver, kidney and pancreas were then quickly excised off and a portion of each organ separately preserved in 10% formalin solution for histological studies.
Excess formalin was removed by washing the tissues in a running stream of water overnight. An automatic tissue processor was used to dehydrate the tissues sequentially with ethanol at various concentrations namely 50%, 80%, 90%, and 96% within intervals of 1 hr. The tissues were then rinsed off ethanol twice in two changes of xylene. The tissues were then infiltrated with paraffin wax in an oven set at 2oC below the melting point of wax for 5 hrs.
The tissues were then fixed in fresh molten paraffin wax and left to solidify. A microtome was used to section embedded tissues at 0.5 μm thicknesses and spread out by floating in warm water. They were then attached to clean dry microscopic slides. The sections were held in a hot oven for 15 min, after which they were dewaxed in xylene. By standard histological protocols, they were stained with haematoxylin and eosin dyes. The stained tissues were cover-slipped with DPX, dried and examined microscopically for any pathological changes.
▪ Qualitative phytochemical screening
The leaf and stem bark extracts were subjected to qualitative phytochemical screening to detect the presence of some selected phytochemicals. Standard phytochemicals screening protocol described by Harbone [14] and Kotake [15] were used. The phytochemicals tested were flavonoids, phenols, saponins, alkaloids, cardiac glycosides, steroids, and terpenoids. These phytochemicals were selected mainly because they are known to possess hypoglycaemic activity.
▪ Data management and statistical analysis
The data on blood glucose levels was tabulated on a spreadsheet in Microsoft excel and then transferred to a Minitab statistical software version 17.0 (State College, Pennsylvania) for analysis. The results were expressed as the mean ± Standard Error of the Mean (SEM). One-way Analysis of Variance (ANOVA) was used to determine the statistical significance of difference among groups. Tukey’s test was used for pairwise comparison of means of specific groups. The values of P ≤ 0.05 were considered significant.
Results
▪ Hypoglycaemic activity
The DCM: Methanolic leaf and stem bark extracts of P. capensis demonstrated hypoglycaemic activities by reducing the blood glucose levels in alloxan-induced diabetic rats within the seven days of the treatment period (Tables 3 and 4). The leaf extract of P. capensis, at doses of 50, 100 and 150 mg/kg body weight, reduced the blood glucose levels of diabetic rats to 7.62, 7.06 and 6.70 mMol/L respectively on the seventh day of the treatment period (Table 3). On the other hand, the stem bark extract of P. capensis at doses of 50, 100 and 150 mg/kg body weight, reduced the blood glucose levels of diabetic rats to 8.26, 7.22 and 6.54 mMol/L respectively (Table 4). Similarly, glibenclamide (reference drug) at a dose of 3 mg/kg body weight reduced the blood glucose levels to 6.92 and 6.82 mMol/L respectively (Tables 3 and 4).
| Group | Treatments | Blood glucose levels(mmol/L) | ||
|---|---|---|---|---|
| Day one | Day seven | |||
| Normal control | DMSO | 5.06=0.191b | 4.98 ± 0.24c | |
| Negative control | Alloxan+DMSO | 21.40 ± 0.822a | 15.36 ± 0.562a | |
| Positive control | Alloxan+Glibenclamide | 21.22 ± 0.893a | 6.92 ± 0.07b | |
| DCM: Me0H | Alloxan+50 mg/kg bw+DMSO | 19.88 ± 0.412a | 7.62 ± 0.18b | |
| Extracts | Alloxan+100 mg/kg bw+DMSO | 20.02 ± 0.663a | 7.06 ± 0.09b | |
| Alloxan+150 mg/kg bw+DMSO | 21.60 ± 0.872a | 6.70 ± 0.28b | ||
| Values expressed as mean ± standard deviation for three replicates. Values with the same letter in the superscript are not significantly different (p>0.05) by one-way ANOVA followed by Tukey's test. | ||||
Table 3. Anti-diabetic activity of leaf extract of P. capensis
| Group | Treatments | Blood glucose levels(mmol/L) | ||
|---|---|---|---|---|
| Day one | Day seven | |||
| Normal control | DMSO | 5.06=0.19b | 4.98 ± 0.24c | |
| Negative control | Alloxan+DMSO | 22.20 ± 1.14a | 14.86 ± 0.81a | |
| Positive control | Alloxan+Glibenclamide | 22.56.44.86a | 6.82 ± -0.15b | |
| DCM: Me0H | Alloxan+50mg/kg bw+DMSO | 21.24+-0.94a | 8.26 ± 0.27b | |
| Extracts | Alloxan+100mg/kg bw+DMSO | 21.72 ± 1.04a | 7.22 ± 0.33b | |
| Alloxan+150mg/kg bw+DMSO | 21.38 ± 0.91a | 6.54 ± 0.16bc | ||
| Values expressed as mean ± standard deviation for three replicates. Values with the same letter in the superscript are not significantly different (p>0.05) by one-way ANOVA followed by Tulcey's test. | ||||
Table 4. Anti-diabetic activity of stem bark extract of P. capensis
There was no significant difference in terms of the antidiabetic activity of leaf and stem bark extracts of P. capensis at the dosage of 50, 100, and 150 mg/kg and they were comparable to reference drug (Glibenclamide) (p>0.05; Tables 3 and 4). The hypoglycaemic activity of leaf and stem bark extracts showed a dose-dependent response as the activity increased with increase in dosage.
▪ Histopathological effects
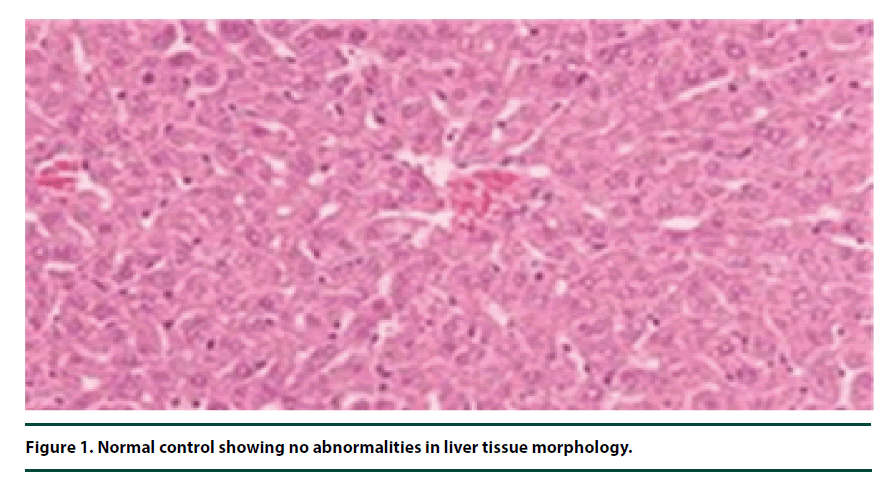
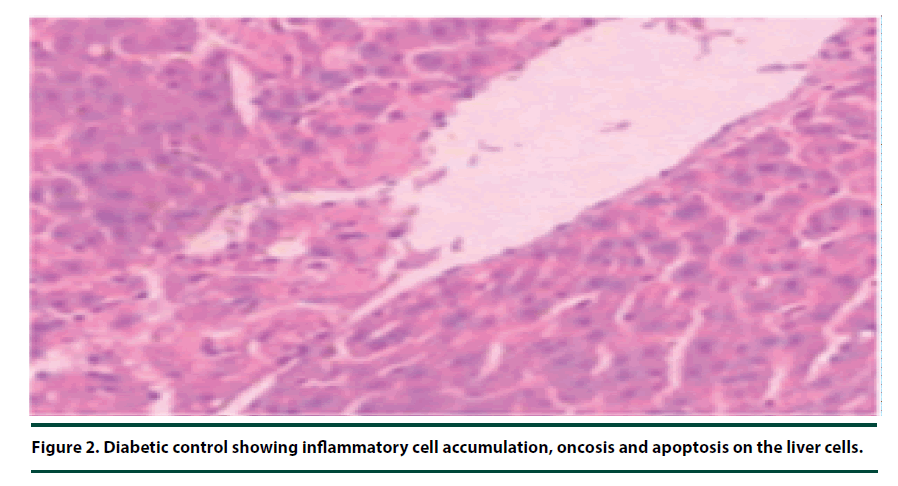
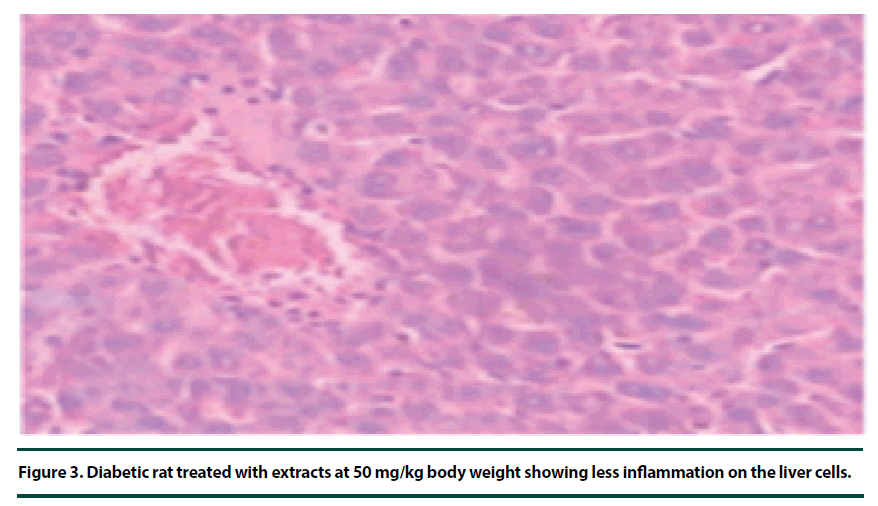
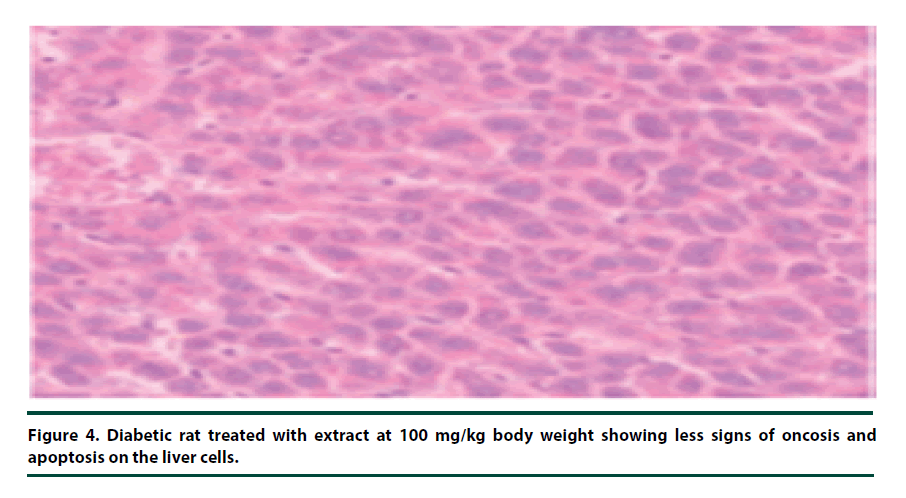
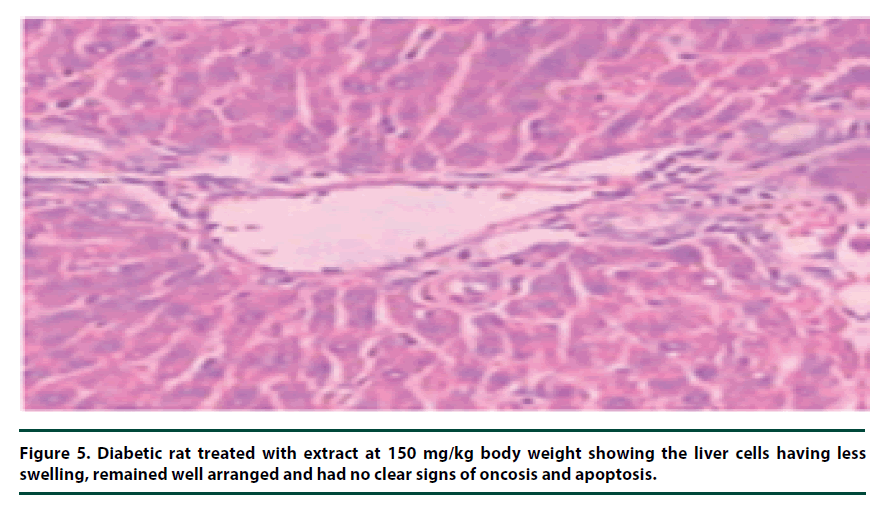
There were no pathological lesions observed in the liver tissue of the normal control group (Figure 1). However, in the negative control group, inflammatory cell accumulation, oncosis and apoptosis of the liver cells were evident (Figure 2). On the other hand, the rats administered with P. capensis leaf and stem bark extracts at a dosage of 50 mg/kg body weight showed relatively fewer signs of inflammation and oncosis on the liver tissue, while the rats that received a higher dosage of 100 mg/kg body weight had only slight signs of inflammation and oncosis (Figures 3 and 4). Furthermore, the liver cells of the rats administered with 150 mg/ kg body weight of P. capensis leaf and stem bark extracts were well arranged and had no signs of oncosis and apoptosis (Figure 5).
Figure 2. Diabetic control showing inflammatory cell accumulation, oncosis and apoptosis on the liver cells.
Figure 3. Diabetic rat treated with extracts at 50 mg/kg body weight showing less inflammation on the liver cells.
Figure 4. Diabetic rat treated with extract at 100 mg/kg body weight showing less signs of oncosis and apoptosis on the liver cells.
Figure 5. Diabetic rat treated with extract at 150 mg/kg body weight showing the liver cells having less swelling, remained well arranged and had no clear signs of oncosis and apoptosis.
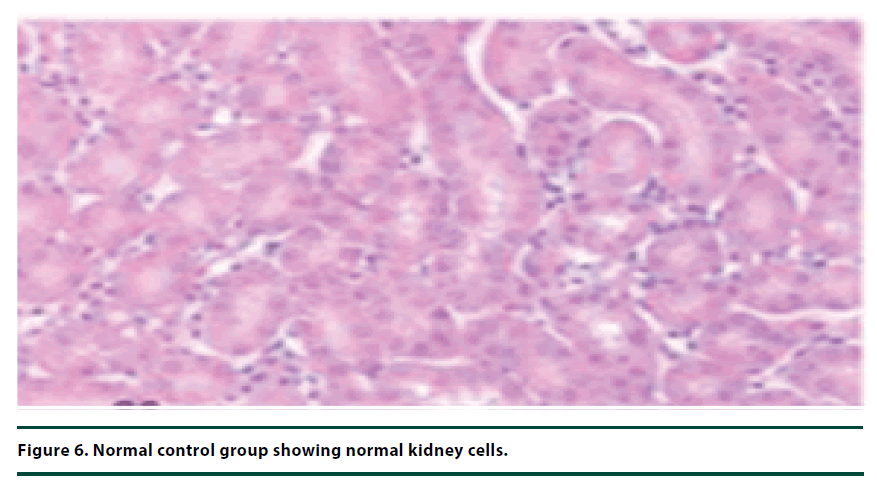
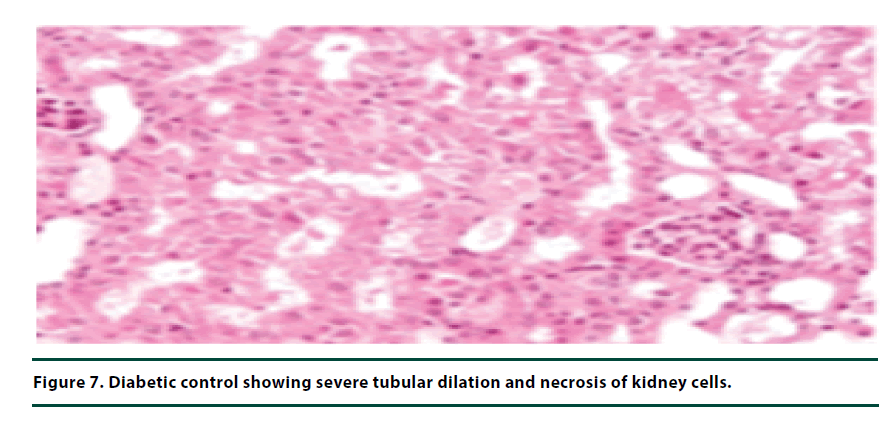
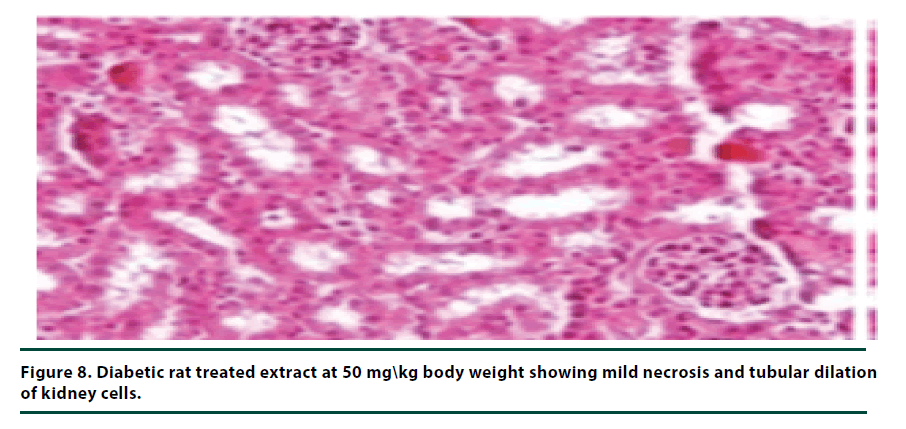
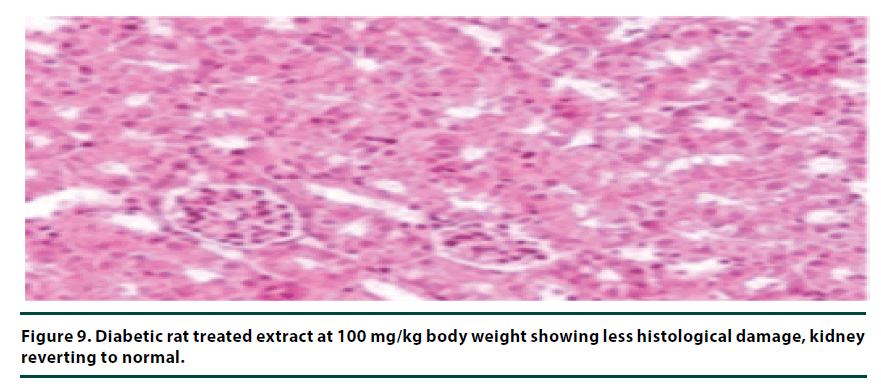
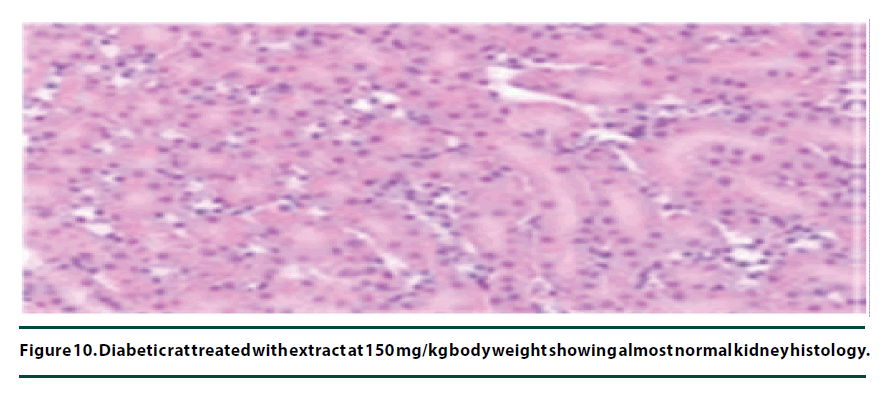
The histological studies of the kidneys from the normal control group showed no tubular dilation or necrosis (Figure 6). However, the diabetic control group showed severe tubular dilation and necrosis (Figure 7). The group of rats administered with P. capensis leaf and stem bark extracts at a dosage of 50 mg/kg body weight showed only mild necrosis and tubular dilation on the kidney tissue (Figure 8). The group of rats administered with extracts at a higher dosage of 100 mg/kg body weight had less histological damage and there was some evidence of the kidney reverting to its normal histology (Figure 9). There were no pathological lesions in the kidneys of the rats administered with 150 mg/kg body weight extracts and they seemed to have had reverted to almost normal kidney histology over the seven days of the treatment period (Figure 10).
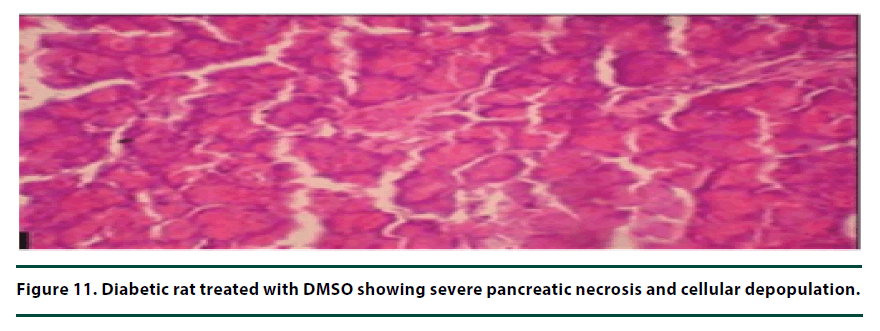
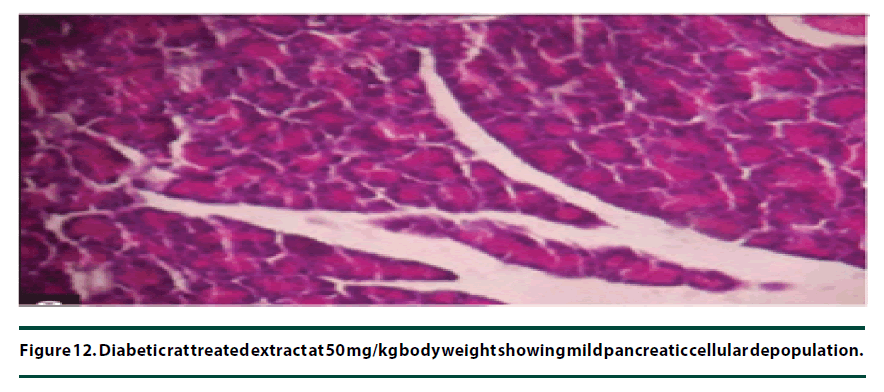
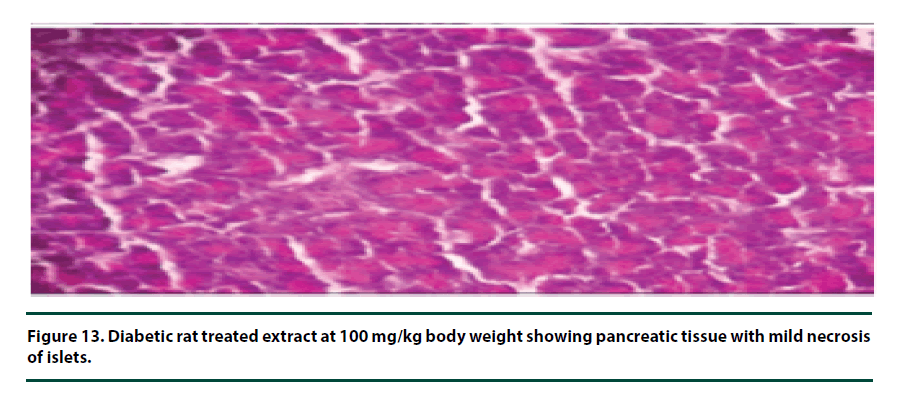
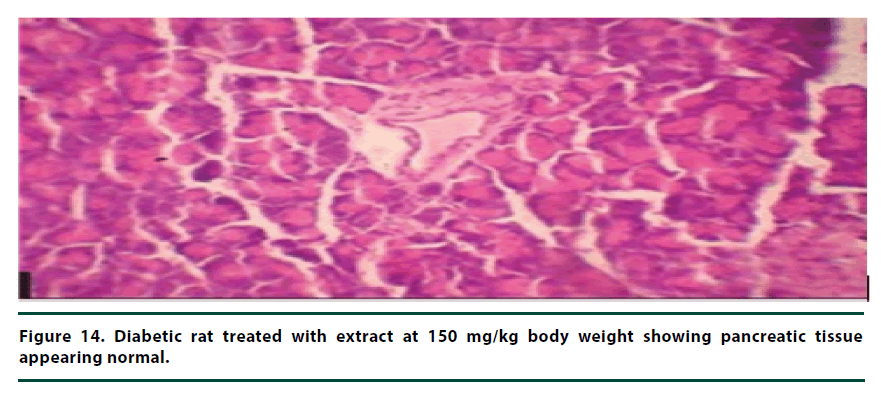
From the histopathological results of the pancreas, the negative control group showed severe necrosis and cellular depopulation (Figure 11). Mild cellular depopulation was evident in the group administered with leaf and stem bark extracts of P. capensis at 50 mg/kg body weight (Figure 12). The pancreas of the rats in the group that was administered with extracts at a dosage of 100 mg/kg body weight had evidence of the pancreas reverting back to normal histology and only mild necrosis of islets was observable (Figure 13). The pancreatic tissue of the group administered with extract at a dose of 150 mg/kg body weight appeared normal with no signs of necrosis or apoptosis (Figure 14).
▪ Qualitative phytochemical screening
The qualitative phytochemical screening of the DCM: Methanolic leaf extract of P. capensis revealed the presence of flavonoids, saponins, cardiac glycosides and phenolics. However, steroids, terpenoids and alkaloids were absent. The DCM: Methanolic stem bark extract of P. capensis indicated the presence of saponins, flavonoids, cardiac glycosides, phenolics, terpenoids, alkaloids and steroids.
Discussion
The present study determined the in vivo hypoglycaemic and qualitatively, the histopathological effects DCM: Methanolic leaf and stem bark extracts of Pappea capensis on alloxan-induced diabetic rats. The leaf and stem bark extracts of P. capensis demonstrated hypoglycaemic activity by reducing the levels of blood glucose within the seven days of treatment period in alloxan-induced diabetic rats. The hypoglycaemic activities of leaf and stem bark extracts of P. capensis were insignificantly different at the dosages of 50, 100 and 150 mg/kg body weight. This could be due to the presence of phenolics, flavonoids, saponins, alkaloids, steroids and terpenoids which are reported to possess hypoglycaemic properties [16].
There are a large number of plants with phytochemicals with hypoglycaemic activity [17]. For example, a fraction guava leaves rich in flavonoids administered at a dose of 7.2-14.4 g daily has demonstrated antidiabetic activity in humans [18]. Phytochemicals such as terpenoids present in leaf extracts of Alisma plantago-aquatica have demonstrated blood glucose reducing activity in rats. In addition, saponins isolated from Panax notoginsen demonstrated antidiabetic potential by lowering levels of blood glucose in alloxan-induced diabetic rats [19]. Therefore, the hypoglycaemic properties of P. capensis extracts may be associated with the flavonoids, terpenoids, saponins or other phytochemicals present in the plant extract.
These phytochemicals lower blood sugar levels by influencing the activities of various enzymes, hormones and other metabolic regulators including the nuclear transcription factors [20]. Through in vivo animal models hypoglycaemic activities of several herbal extracts have been identified and experimentally demonstrated. These plants include Colocynthis citrullus [21], Azadirachta indica [22], Ocimum gratissimum and Zingeber officinale [23] among many others.
The rats in the normal control group had significantly lower blood sugar levels on the seventh day of treatment compared to the rats in experimental groups (50, 100 and 150 mg/ kg body weight). This can be explained by the fact that, unlike the experimental groups, these animals were not administered any alloxan and were relatively stress free as they were subjected to fewer disturbances. The rats in the negative control group also showed some degree of recovery as evidenced by the falling blood sugar levels over the study period. This can be attributed to the normal intrinsic tissue recovery mechanisms of the living systems [24].
Alloxan is a chemical substance that causes specific tissue oncosis and apoptosis in the pancreas hence interfering with insulin production. Alloxan also has an acatalasemic effect as it damages hepatocytes leading to low levels of the enzyme catalase whose deficiency accelerates oxidative stress build up leading to diabetic complications [24].
From the histopathological studies of the liver tissue, there is evidence of some cure as far as recovery from the alloxan-induced damage is concerned and this followed a dose-related pattern. In the negative control group, the rats showed severe inflammatory cell accumulation, oncosis and apoptosis of the liver cells were evident, pointing to the magnitude of hepatic tissue damage that followed alloxan administration. The liver is a common target for most poisons as it is the organ responsible for the detoxification process in the body. It contains antioxidant enzymes such as catalase and nonenzymatic antioxidants including vitamin A, mineral ions and glutathione molecules [25]. The liver damage disrupts both the synthesis and secretion of such metabolic regulators and also their potency as their optimum working environment is affected by the resultant metabolites [26].
The animal models treated with leaf and stem bark extracts of P. capensis at the doses of 50, 100 and 150 mg/kg body weight showed some signs of recovery in the liver tissues as the levels of inflammatory cell accumulation, oncosis and apoptosis declined with increasing dosage. There were no observable differences between the rate of recovery when leaf and stem bark extracts were used, implying that the two extracts had more or less similar curative potentials. The animals treated with leaf and stem bark extracts of P. capensis at a dosage of 150 mg/kg body weight showed almost full recovery over the seven days treatment period.
The histopathological studies of the kidney tissues showed severe tubular dilation and necrosis in the group of rats in negative control group and mild to almost no necrosis and tubular dilation in those administered with leaf and stem bark extracts at 50, 100 and 150 mg/ kg body weight. This trend supports the earlier observation that the curative effects of P. capensis extracts followed a dose-related pattern. The DCM: Methanolic leaf and stem bark extracts of P. Capensis at a dosage of 50 mg/kg body weight were not as effective as the other two higher doses (100 and 150 mg/kg body weight) in reversing the adverse effects of alloxan. This may be attributed to denaturation of the active phytocompound at the lower concentration by the accumulated reactive oxygen species. Besides, there was no much difference between the rates of recovery of the rats treated with the leaf or stem bark extracts of P. capensis.
In the case of histopathological examination of the pancreas, the rats in the negative control group exhibited serious necrosis and cellular depopulation due to apoptosis which plays a major role in the loss of β- cell mass compensation. This was demonstrated by Weir et al. using Zucker diabetic fatty rats, and provided support for the possible involvement of apoptosis in β-cell glucose toxicity [27].
The group of rats administered with 50 mg/ kg body weight extract showed mild cellular depopulation. The pancreas tissue of the group of rats given the extract at the concentration of 100 mg/kg body weight showed recovery from alloxan damaging activity and only little necrosis of islets was evident. The group of rats treated with the extract at the dose of 150 mg/ kg body weight showed a normal pancreas tissue. Therefore, the curative activity of the extracts exhibited a dose-related pattern. These effects of the extracts show that they had the healing effect on the pancreatic tissue and can protect β-cells in diabetic conditions [28].
Conclusion
From the study, it can be concluded that the DCM: Methanolic leaf and stem bark extracts of P. capensis have hypoglycaemic activity. The extracts also possess curative effects on liver, kidney and pancreas of alloxan-induced diabetic rats and contain phytochemicals associated with the hypoglycaemic activity. The DCM: Methanolic leaf and stem bark extracts of P. capensis may therefore, be used in development of antidiabetic agents.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgement
We acknowledge the Department of biochemistry and biotechnology, Kenyatta University, for allowing us access to their facilities during the study.
References
- Valiathan MS. Healing plants. J. Current Sci. 75(2), 1122–1127 (1998).
- Bernard C, Berthault MF, Saulnier C et al. Neogenesis vs. apoptosis as main components of pancreatic β cell mass changes in glucose-infused normal and mildly diabetic adult rats. The FASEB Journal. 13(10), 1195–1205 (1999).
- Basu S. Radioimmunosassay of 8-iso-prostaglandin F 2α: an index for oxidative injury via free radical catalysed lipid peroxidation. PLEFA. 58(4), 319–325 (1998).
- Monnier L, Mas E, Ginet C et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. J. Am. Med. Association. 295(14), 1681–1687 (2006).
- Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. IJMR. 125(3), 451–458 (2007).
- Mishra BB, Tiwari VK. Natural products: An evolving role in future drug discovery. Euro. J. Med. Chem. 46(1), 4769–4807 (2011).
- Koch A, Tamez P, Pezzuto J et al. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharm. 101(1), 95–99 (2005).
- Karau GM, Njagi EN, Machocho AK et al. Hypoglycemic activity of aqueous and ethyl acetate leaf and stem bark extracts of Pappea capensis in alloxan-induced diabetic BALB/c mice. BJPT. 3(1), 251–258 (2012).
- Kloos H, Thiongo FW, Ouma JH et al. Preliminary evaluation of some wild and cultivated plants for snail control in Machakos District, Kenya. The Jour. Tropical Med. Hygiene. 90(4): 197–204 (1987).
- McGaw LJ, Eloff JN. Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. J. Ethnopharmacol. 119(3), 559–574 (2008).
- Solanki R. Some medicinal plants with antibacterial activity. Int. J Comp. Phar. 1(1), 10–15 (2010).
- Middha SK, Mittal Y, Usha T et al. Phyto-mellitus: A phyto-chemical database for diabetes. Bioinfo. 4(2), 78–79 (2009).
- Szkudelski T. The Mechanism of alloxan and streptozotocin action in beta-cells of the rat pancreas. Physiol. Res. 50(6), 536–546 (2001).
- Harbone JB. Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hal Publishers. 3(1): 60–66 (1998).
- Kotake CK. Practical Pharmacognosy. Vallabh Prakashan. 4 (2): 107–111 (2000).
- Sharma S, Chaturvedi M, Edwin E. Evaluation of the phytochemicals and antidiabetic activity of Ficus bengalensis. Int. J. Diab. 27(2), 56–59 (2007).
- Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr. Diab. Rev., 6(4), 247–254 (2010).
- Kimura M, Suzuki J. The pharmacological role of ginseng in the blend effect of traditional Chinese medicines in hyperglycemia. Adv. Chinese Med. Mat. Res. 21(4), 141–143 (1985).
- Yang CY, Wang J, Zhao Y et al. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J. Ethnopharmacol. 130(2), 231–236 (2010).
- Soobrattee MA, Neergheen VS, Luximon-Ramma A et al. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Research Fundamental. 579(4), 200–213 (2005).
- Abdel-Hassan I, Abdel-Barry J, Tariq M. The hypoglycaemic and anti-hyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J. Ethnopharmacol. 71(1), 325–330 (2000).
- Prabbakar, P, Sudha P, Abhay M et al. Antidiabetic activity of alcoholic extract of neem (Azadirachta indica) root bark. Natl. J. Physiol. Pharm. Pharmacol. 3(1), 142–146 (2013).
- Asha B, Krishnamurthy K, Siddappa D. Evaluation of anti-hyperglycaemic activity of Zingiber officinale (Ginger) in albino rats. J. Chem. Pharma. Res. 3(2), 452–456 (2011).
- Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 51(2), 216–226 (2008).
- Zhu R, Wang Y, Zhang L et al. Oxidative stress and liver disease. Hepatol. Res. 42(8), 741–749 (2012).
- Sagir A, Erhardt A, Schmitt M et al. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatol. 47(2), 592–595 (2008).
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. J. Diab. 53(3), 16–21 (2004).
- Coskun O, Kanter M, Korkmaz A et al. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 51(2), 117–123 (2005).