Review Article - Imaging in Medicine (2013) Volume 5, Issue 2
Hybrid techniques for intraoperative sentinel lymph node imaging: early experiences and future prospects
Daphne DD Rietbergen1, Nynke S van den Berg1, Fijs WB van Leeuwen1 & Renato A Valdés Olmos*1,21Interventional Molecular Imaging Laboratory & Section of Nuclear Medicine, Department of Radiology, Leiden University Medical Hospital, Albinusdreef 2, PO Box 9600, 2300 RC, Leiden, The Netherlands
2Department of Nuclear Medicine, The Netherlands Cancer Institute – Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX, Amsterdam, The Netherlands
- Corresponding Author:
- Renato A Valdés
Olmos
Interventional Molecular Imaging Laboratory & Section of Nuclear Medicine
Department of Radiology
Leiden University Medical Hospital
Albinusdreef 2, PO Box 9600, 2300 RC
Leiden, The Netherlands
E-mail: r.valdes@nki.nl
Abstract
Keywords
breast cancer n fluorescence imaging n head and neck cancer n image-guided surgery n indocyanine green n melanoma n prostate carcinoma n radioguided surgery n sentinel lymph node biopsy
Nodal status remains the single most important prognostic variable in the management of cancer. Cabañas was the first person who proposed the significance of cancer treatment management in relation to the sentinel lymph node (SLN) [1]. SLNs are defined as the first lymph nodes (LNs) in the regional lymphatic basins that receive lymph flow from the primary tumor. Assuming the orderly spread of metastasis through the lymphatic system, SLNs will be the first nodes to contain metastasis and their biopsy will accurately predict regional node status. SLN biopsy (SLNB) is accompanied by a low morbidity and for many patients with malignancies, such as breast cancer and melanoma, complete regional lymphadenectomy has been replaced by SLNB.
Currently, SLNB is widely accepted as an accurate predictor of regional nodal status, and, as such, in determining prognosis and devising therapeutic strategies in a variety of malignancies, such as breast cancer and melanoma. It has also been increasingly used as a staging procedure for head and neck cancer, and gynecological and urogenital malignancies.
In spite of these achievements, the evolution of the SLNB demands new technological improvements. The SLN concept assumes the function of LNs as effective tumor cell filters on direct drainage pathways. Therefore, all LNs with direct drainage from the site of the primary tumor are considered to be SLNs. In many cases, this factor does increase the complexity of the procedure because of the existence of aberrant routes of lymphatic drainage or due to the direct drainage from the primary tumor to multiple LN basins. For instance, in breast cancer SLNB, areas of alternative drainage, such as the internal mammary chain or around the clavicle, often require a different approach compared with the axilla. In addition, in melanoma, the routes of lymphatic drainage are frequently multidirectional and sometimes unpredictable in areas such as the head, neck and trunk. The need to localize SLNs in complex anatomical areas has significantly increased with the introduction of the SLNB in head and neck cancer, prostate cancer, cervical cancer and other malignancies.
To facilitate the procedure adequately, in recent years preoperative lymphatic mapping has significantly improved with the introduction of SPECT/CT. Using this technique in addition to lymphoscintigraphy, surgeons may receive 2D and 3D information about the anatomical SLN localization. This is important, not only to adequately plan operations in the expected areas of drainage, but also to include other draining lymphatic basins to the operative strategy. Another significant practical advantage of SPECT/CT is that no additional tracer injections are required.
The necessity to preserve preoperative mapping led to the development of hybrid tracers for SLNB. Due to the radioactive component of these tracers, preoperative lymphoscintigraphy and SPECT/CT remains unmodified. In addition, the intraoperative SLNB procedure can significantly be improved by the incorporation of high-resolution images based on the fluorescent component of the tracer. Moreover, in the operating room, hybrid tracers are able to facilitate the combination of γ-probes and/or portable γ-cameras with devices adjusted to detect the fluorescent SLN signals.
One important objective of the use of hybrid tracers is to enable SLNB in complex areas of drainage such as the parotid/periauricular region (periauricular melanomas), the submandibular LNs (oral cavity cancer, melanomas of the cheek/skin) or in pelvic LN stations (prostate cancer). Failure to find the SLN in these areas may lead to an increase in the false-negative rate and clinical recurrences. For instance, in head and neck melanomas, a median false-negative rate of 20.4% has been determined in a meta-analysis of 32 studies and 3442 patients [2]. In another study concerning oral cavity cancer, the negative predictive value for carcinoma of the floor of the mouth was lower than for other malignancies (88.5 vs 95.4%); this was due to the difficulties in identifying SLNs in levels I and IIa of the neck [3].
The background of the hybrid approach, as well as its initial clinical validation in various malignancies, will be discussed in the present review. In order to better understand the properties and function of hybrid tracers, the application of single fluorescent tracers for SLNB will also be discussed. Furthermore, the hybrid approach will be evaluated in light of other technological advances for intraoperative procedures, such as mobile γ-cameras and sophisticated fluorescence cameras.
■Preoperative SNL mapping
■Lymphoscintigraphy
Almost 20 years after its introduction for the SLNB procedure, lymphoscintigraphy still remains the standard for preoperative lymphatic mapping. Based on its ability to identify LN basins at high risk for metastases, lymphoscintigraphy became a useful roadmap for surgeons, leading to both the improvement of the accuracy and the reduction of morbidity related to the use of handheld γ-probes for SLNB in the operating room.
Based on the use of large-field γ-cameras, lymphoscintigraphy is usually performed following the injection of radiocolloids (diameter ranging between 10 and 600 nm) in or surrounding the tumor. For breast cancer, tracer injection may be related to the skin (subcutaneous, intradermal), the aureola (periaurelar, subaureolar) or the tumor (around or into the tumor). For melanoma, head and neck cancers and other malignancies (e.g., penile, vulva and cervix), several injections are given, tracer dose is divided in three to four injections intradermally around the tumor. In deeply located malignancies (e.g., prostate), or when tumors are not palpable (e.g., breast), multiple injections are usually guided by ultrasound.
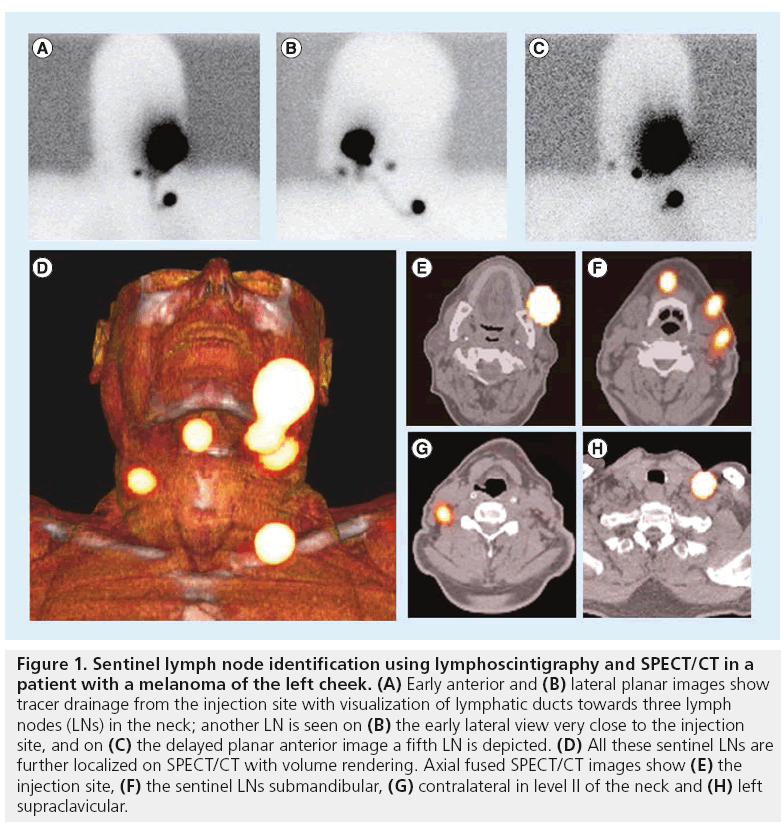
In line with the SLN concept, an important aspect of lymphoscintigraphy relies on its ability to detect LNs directly draining from the injection site. This is accomplished by the visualization of the lymphatic vessels. For this purpose, image acquisition must be sequential and, in cases with expected fast drainage (e.g., melanoma, penile cancer and head and neck cancer), a dynamic study, started as soon as possible after tracer administration, is mandatory. Current protocols are further based on the acquisition of early (in the first 30 min after injection) and late (mostly after 2 h) static planar images. This sequential protocol frequently leads to differentiate early draining LNs (the SLNs) from other higher echelon nodes, as is illustrated in Figure 1.
Figure 1: Sentinel lymph node identification using lymphoscintigraphy and SPECT/CT in a patient with a melanoma of the left cheek. (A) Early anterior and (B) lateral planar images show tracer drainage from the injection site with visualization of lymphatic ducts towards three lymph nodes (LNs) in the neck; another LN is seen on (B) the early lateral view very close to the injection site, and on (C) the delayed planar anterior image a fifth LN is depicted. (D) All these sentinel LNs are further localized on SPECT/CT with volume rendering. Axial fused SPECT/CT images show (E) the injection site, (F) the sentinel LNs submandibular, (G) contralateral in level II of the neck and (H) left supraclavicular.
■SPECT/CT
The standard conventional 2D lymphoscintigraphy does not always define the exact anatomical location of the SLNs. In particular, in head and neck and intra-abdominal areas, localization of SLN is more difficult than elsewhere due to the complex anatomy and the presence of several vital structures which must be spared. With the advent of SPECT/CT cameras, these problems can be fixed. SPECT is a tomographic version of conventional lymphoscintigraphy and the images have better contrast and resolution. When fused with CT, it provides anatomical information facilitating the preoperative planning of the surgical approach. In addition, SLNs close to the injection site, where high signal in the injection depots can hamper SLN detection on lymphoscintigraphy, SPECT/CT can help [4].
SPECT/CT is principally orientated to the anatomical localization of SLNs. This is the principal reason why SPECT/CT is acquired using a low-dose CT. The use of a diagnostic high-dose CT, with or without intravenous contrast, is not necessary because the SLNB procedure primarily aims to detect subclinical metastases in unenlarged LNs. This category mainly includes small macrometastases (>2 and <5 mm) and micrometastases (>0.2 and ≤2 mm).
However, for SLN localization, the CT component of the SPECT/CT must be able to provide optimal anatomical information. This became possible with the second generation of SPECT/CT cameras and it enables the evaluation of the LNs corresponding with the radioactive nodes on fused SPECT/CT images with a low-dose (40 mAs) CT. For superficial areas such as the groin and the axilla, 5-mm slides are recommended. For more complex anatomical areas (e.g., head and neck, pelvis and abdomen) 2-mm slides may be necessary. With this approach SPECT/CT can accurately localize SLNs in relation to the vascular structures in deep anatomical areas.
The CT is also used to correct the SPECT signal for tissue attenuation and scattering. After these corrections, SPECT is fused with CT. A gray scale is used to display the morphology in the background image (CT), whereas a color scale is used to display the SLN in the foreground image (SPECT) [5].
The display of SPECT/CT is similar to that of conventional tomography. Multiplanar reconstruction enables 2D display of fusion images in relation to CT and SPECT. The use of cross-reference lines allows the navigation between axial, coronal and sagital views. At the same time, this tool correlates radioactive SLNs seen on fused SPECT/CT with LNs seen on CT. This information may be helpful for the intraoperative procedure and the postexcision control using portable γ-cameras or probes.
Fused SPECT/CT images may also be displayed using maximum intensity projection (MIP). MIP is a specific type of rendering in which the brightest voxels are projected into a 3D image, allowing improvement of anatomical SLN localization and recognition by the surgeon. One limitation of MIP is that the presence of other high-attenuation voxels on CT may obscure the recognition of the vasculature and other anatomical structures. Another limitation is that the MIP display is a 2D representation that cannot accurately depict the actual relationships of the vessels and other structures [6].
Using volume rendering for 3D display, different colors are assigned to anatomical structures such as vessels, muscle, bone and skin. This allows better anatomical reference points to be obtained and an additional dimension in the recognition of SLNs to be incorporated. By incorporating a color display, volume rendering improves the visualization of complex anatomy and 3D relationships (Figure 1).
■Comprehensive image interpretation in preoperative lymphatic mapping
SPECT/CT does not replace lymphoscintigraphy and is mostly performed following delayed planar images (in general 2 h following tracer administration). SPECT/CT aims to anatomically localize SLNs already visualized on planar images. In some cases, SPECT/CT can detect additional SLNs. van der Ploeg et al. found that up to 14% of SLNs were only detected by SPECT/CT [7]. To understand the combined use of lymphoscintigraphy and SPECT/CT, it is necessary to specify the criteria for SLN identification on preoperative images. The major criteria in identifying LNs as SLNs are the visualization of lymphatic ducts, the time of appearance, the LN basin and the intensity of LN uptake [8,9].
Following these criteria, visualized radioactive LNs may be classified in three categories: definitely SLNs (all LNs draining from the site of the primary tumor through an own lymphatic vessel or a single radioactive LN in a LN basin); highly probable SLNs (LNs appearing between the injection site and a first draining node, or nodes with increasing uptake appearing in other LN stations); and less probable SLNs (all higher echelon nodes – in trunk and extremities – or lower echelon nodes – head and neck).
Early planar images of lymphoscintigraphy are essential to identify first-draining LNs as SLNs by the visualization of lymphatic ducts. First-draining nodes (category one) can be distinguished from secondary LNs (category three), which are mostly appearing on delayed planar images. In other cases, a single LN is seen on early and/or delayed images. This node is also considered as a definitively SLN (category one). However, in some cases, SPECT/CT can detect additional LNs in other basins. These nodes can be considered as definitively (category one) or highly probable (category two) SLNs. Less frequently, a radioactive LN may appear between the injection site and a first draining node; its increasing uptake can confirm this node as a highly probable SLN (category two), and it helps to differentiate this node from prolonged valve activity in a lymphatic duct.
The clinical relevance of SPECT/CT has recently been evaluated in melanoma. Among patients with clinically LN-negative melanoma, the use of SPECT/CT for SLNB was associated with a significantly higher frequency in the detection of metastatic SLN involvement and a higher rate of disease-free survival when compared with SLNB without SPECT/CT [10]. In addition, Covarelli et al. and Klode et al. previously demonstrated that SPECT/CT will significantly reduce operating time and costs [11,12].
SPECT/CT in breast cancer is indicated in cases with no SLN visualization on planar images of lymphoscintigraphy, and also to anatomically localize SLN in localizations (intramammary, interpectoral, intercostal and periclavicular) other than level I of the axilla [13]. In melanoma, SPECT/CT was also demonstrated to localize SLN in cases with aberrant drainage, as seen on lymphoscintigrapy or in cases with no visualization on planar images [14]. However, Stoffels et al. extended the indication to all patients receiving SLNB, due to the benefit in disease-free interval observed when SPECT/CT is incorporated into the protocol [10]. For head and neck malignancies, prostate cancer, cervical cancer and other malignancies, the use of SPECT/CT for SLN localization is always indicated due to the essential anatomical information obtained by this modality [15].
Conventional intraoperative SLN localization
Over the past few decades, the SLNB procedure in the operating room has been based on the combination of visual and acoustic signals. Since the introduction of SLNB, the visual component is represented by the use of blue dyes, which enables both lymph duct visualization and the identification of the SLN. The acoustic component is the contribution of the g-probe, which is able to instantaneously transform count-rates in auditory signals.
■Blue dye
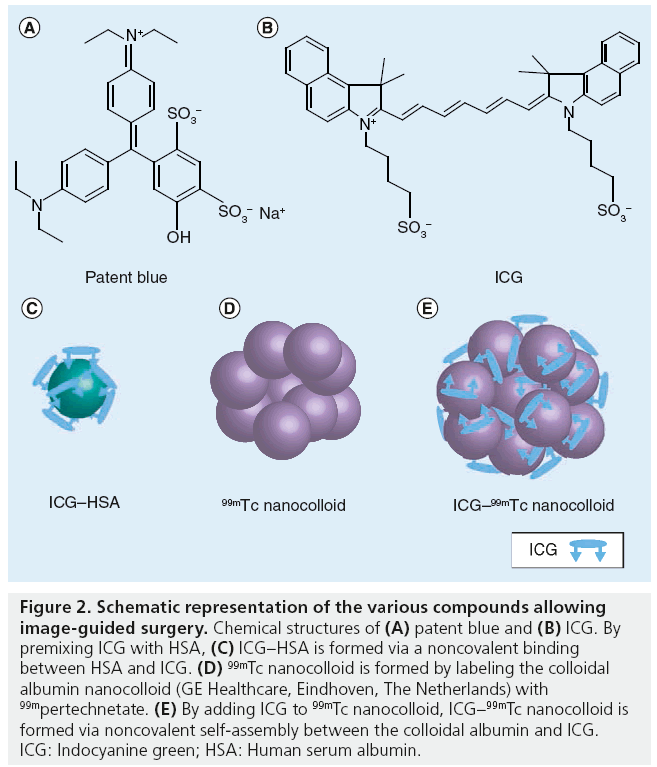
Blue dyes are able to anatomically display lymphatic ducts and LNs. Following injection, blue dyes migrate from the injection site by diffusion. The chemical structure of methylene blue is illustrated in Figure 2. The transport through the lymphatic system can be accelerated by massaging the injection site. Depending on the distance between the injection site and the draining LN basin, SLNs are colored within 5–15 min. SLN washout often occurs after 30–45 min. In some centers without nuclear medicine facilities available for SLN detection with radioactive tracers, intraoperative SLN detection is based exclusively on vital blue dyes. In hospitals where radioactive tracers are available, intraoperative SLNB is mostly performed using the combination of blue dye and a g-probe. Over the recent years, many studies demonstrated that the combined use of blue dye and radiocolloid is more accurate compared with the dye method alone. Radovanovic et al. found a sensitivity of 83% for SLNs found with blue dye, with respect to radiocolloid, with a sensitivity of 95% [16]. The prediction of SLNs using combined techniques had a significantly higher accuracy and facilitated quicker identification of the SLN intraoperatively [5,16,17]. A 10-year study of SLNB in head and neck tumors also showed a complementary success of the combined approach for SLNB; however, the radiolocalization-based biopsy alone had the highest success rate (95%) [18].
Figure 2: Schematic representation of the various compounds allowing image-guided surgery. Chemical structures of (A) patent blue and (B) ICG. By premixing ICG with HSA, (C) ICG–HSA is formed via a noncovalent binding between HSA and ICG. (D) 99mTc nanocolloid is formed by labeling the colloidal albumin nanocolloid (GE Healthcare, Eindhoven, The Netherlands) with 99mpertechnetate. (E) By adding ICG to 99mTc nanocolloid, ICG–99mTc nanocolloid is formed via noncovalent self-assembly between the colloidal albumin and ICG. ICG: Indocyanine green; HSA: Human serum albumin.
The use of blue dyes has some limitations. For instance, the synchronization between injection and SLNB identification may be critical, often depending on the experience of the surgeon. In addition, blue staining of the skin can occur; although mostly temporary, it can lead to a cosmetic limitation, principally in the head and neck. Furthermore, in a small number of patients, blue dye was found to be associated with the occurrence of anaphylactic reactions; this is also the reason for its contraindication in pregnancy. Finally, blue dyes appear to be of limited value in identifying aberrant-draining SLNs.
γ-probes
Intraoperative real-time g-probe use allows acoustic detection of the tracer accumulated in the SLN. Radioactive tracers have no problems with tissue penetration because of their low tissue attenuation. In line with these properties, γ-probes must be able to combine high spatial resolution, to more precisely localize small LNs, with high sensitivity, to promptly guide surgeons to the SLN location in the surgical field. However, this technique can suffer from background signals from the injection site, especially when the SLN is located close to the injection site [19,20]. Vidal- Sicart et al. demonstrated that the conventional g-probe sometimes failed to detect the SLN because of weak uptake of the tracer in the SLNs close to the injection site, SLNs at unclear locations and in case of a cluster of SLNs [21].
Advances in intraoperative SLN imaging
The introduction of SPECT/CT further improved preoperative SLN mapping and SLN identification. SPECT/CT is of great value in difficult anatomic areas such as the neck and abdomen and in patients with no visualization of a SLN on the planar lymphoscingtigraphy. However, the localization of SLNs in complex anatomical areas requires real-time imaging technologies in the operating room to enable surgical navigation. In this context, there is an increasing use of intraoperative devices for image-guided surgery [22].
■Intraoperative portable γ-cameras
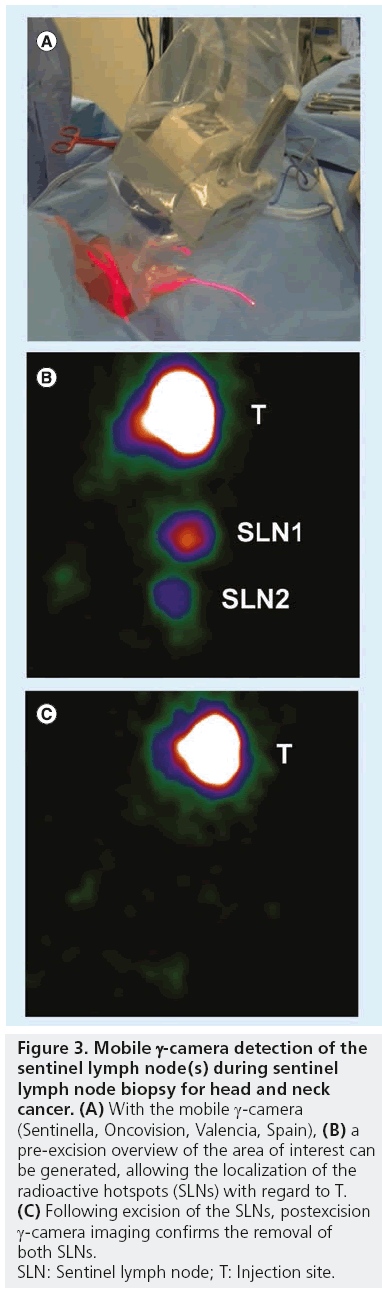
The incorporation of portable γ-cameras (Figure 3) for the SLNB procedure is principally based on the need to facilitate real-time imaging and navigation during surgery by correlating portable g-camera findings with those of preoperative lymphoscintigraphy and SPECT/CT. This enables surgeons to more accurately localize LNs previously identified as SLNs. By using a portable g-camera, it has become possible to reproduce the findings of preoperative SLN mapping in the operation room, giving not only a general orientation of the field to be operated, but also of the target (SLN) within that field. In light of this, portable γ-cameras are more complementary to the g-probe than its alternative.
Figure 3: Mobile g-camera detection of the
sentinel lymph node(s) during sentinel
lymph node biopsy for head and neck
cancer. (A) With the mobile g-camera
(Sentinella, Oncovision, Valencia, Spain), (B) a
pre-excision overview of the area of interest can
be generated, allowing the localization of the
radioactive hotspots (SLNs) with regard to T. (C) Following excision of the SLNs, postexcision
g-camera imaging confirms the removal of
both SLNs.
SLN: Sentinel lymph node; T: Injection site.
Vidal-Sicart et al. preoperatively tested a portable g-camera for the detection of the SLN(s) in patients with breast cancer [23]. The portable g-camera showed less SLNs than the conventional g-camera, but when they used a lead shield to mask the injection area, the detection rate raised to 88% for the portable g-camera versus 95% for the conventional one, although this was not statistically significant [23]. The findings of Vidal-Sicart et al. led to the an important software adjustment in the new version of the used portable device (Sentinella, Oncovision, Valencia, Spain); currently it is possible to electronically mask the injection site in order to obtain a better, high-resolution, image of the radioactive SLNs. Dengel et al. reported a SLN detection sensitivity of 90% using a mobile g-camera versus 97% with the fixed g-camera [24]. A study by Kerrou et al. showed a good prediction and localization of SLNs when using a handheld g-camera in breast cancer patients, it was found to be not inferior to the standard procedure based on conventional lymphoscintigraphy [25].
Another study by Vidal-Sicart et al. studied the value of intraoperative real-time imaging using a portable g-camera in comparison with the conventional g-probe [21]. They evaluated 20 patients with various malignancies (e.g., melanoma, breast and gynecologic cancers) with SLNs which were difficult to localize due to, for example, weak tracer uptake or being located near the injection site or at unclear locations. They found a higher intraoperative detection rate of the SLN in patients where the combination of both techniques was used. The portable g-camera also helped to find the precise location of the SLN in four out of 20 patients [21].
The portable g-camera can also be used to check the excision site for remaining radioactivity after the excision of the SLNs. Vermeeren et al. found a 100% identification rate of all preoperatively identified SLNs in five patients with head and neck melanoma or oral cavity carcinoma. Intraoperatively, they found nine additional SLNs at difficult sites after excision of the primary tumor, of which one SLN contained tumor mass [4]. A feasibility study by Brouwer et al. also showed additional value of the intraoperative use of a portable g-camera, in which the portable g-camera identified additional SLNs in two patients compared with preoperative SLN mapping with lymphoscintigraphy and SPECT/CT [26]. With these results, it can be concluded that a portable g-camera can find additional LNs, which are not found by the g-probe; therefore, this technique can reduce false-negative LNs. False-negative rates may be reduced by the detection of additional SLNs during operations [27,28].
Vermeeren et al. also showed that a laparoscopic g-probe in combination with a portable g-camera enabled adequate real-time SLN identification in 90% of the patients with urological malignancies. They used an I-125 seed fixed on the tip of the laparoscopic g-probe as a pointer. By matching the signals of the seed and the portable g-camera, the exact location of the SLN was found. The portable g-camera allowed the surgeon to check the removal of the radioactive SLNs and was found to be complementary to the intraoperative laparoscopic g-probe [27,29].
In summary, the intraoperative use of a portable g-camera can facilitate the SLNB procedure in all cases, but especially for SLNs at more difficult sites, such as near the injection area, next to another SLNs or deeper-lying SLNs which gives attenuation of the g-signal.
■Freehand SPECT (3D)
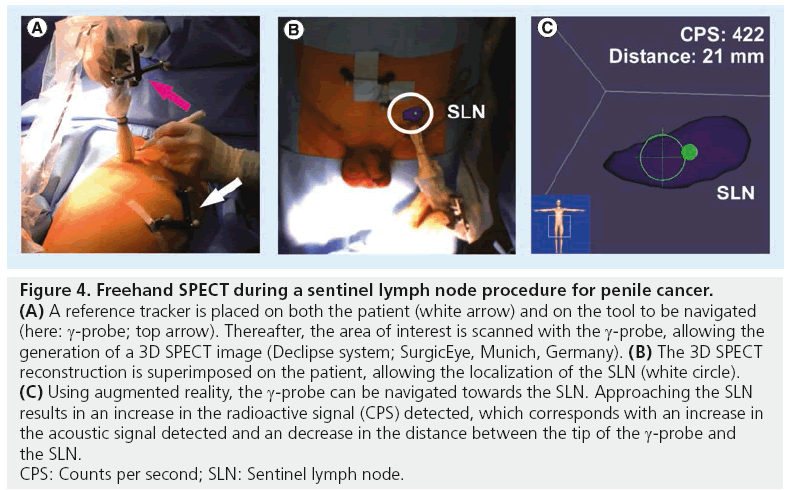
The need to generate imaging in the operating room led to the development of other alternative devices to portable γ-cameras, such as a freehand SPECT probe in combination with a tracking system (Figure 4). From this, 3D reconstructions of radioactivity distributions are possible, which enables 3D imaging and navigation in the operating room. For this objective, surgeons need to move the g-probe around the structure of interest (tumor or SLN). This movement of the probe is tracked by the tracking system. 3D data are acquired and allow display of depth of the target structure [29,30]. Owing to this 3D feature, which acquires display of depth, this camera has advantages in comparison with planar imaging generated by the portable g-camera. A study by Wendler et al. showed that freehand SPECT can be integrated into the operating room without considerably changing the standardized workflow of finding the SLN with radiocolloid [31]. Second, after SLN excision, this technique could be used to confirm that all structures were resected. They found an accuracy of freehand SPECT of 77.8% (seven out of nine nodes) in scans with good quality, while for intermediate and poor quality scans, the accuracy reduced to 34.3 and 12.8%, respectively, when using SPECT/CT as reference. False-negative findings were related to insufficient scanning time, insufficient coverage of the axillary region, close proximity of the SLN to the injection site and low tracer uptake in the SLNs [31].
Figure 4: Freehand SPECT during a sentinel lymph node procedure for penile cancer. (A) A reference tracker is placed on both the patient (white arrow) and on the tool to be navigated
(here: g-probe; top arrow). Thereafter, the area of interest is scanned with the g-probe, allowing the
generation of a 3D SPECT image (Declipse system; SurgicEye, Munich, Germany). (B) The 3D SPECT
reconstruction is superimposed on the patient, allowing the localization of the SLN (white circle). (C) Using augmented reality, the g-probe can be navigated towards the SLN. Approaching the SLN
results in an increase in the radioactive signal (CPS) detected, which corresponds with an increase in
the acoustic signal detected and an decrease in the distance between the tip of the g-probe and
the SLN.
CPS: Counts per second; SLN: Sentinel lymph node.
■Single near-infrared dyes
In some circumstances, the search for the SLN is challenging and time-consuming when using radiocolloids and/or blue dyes. Sometimes the SLN is difficult to localize, for example in head and neck cancer and melanoma where the SLN can be located close to the injection site where background activity may interfere with the tracer signal in the SLN. New techniques have been developed to deal with the limitations of planar lymphscintigraphy and g-probe, especially for these challenging cases, and to deal with the disadvantage tattooing effect of the blue dyes. The introduction of hybrid imaging, such as SPECT/CT, and intraoperative portable devices are a step in the direction aiming to provide a better anatomical mapping for surgeons. For a similar objective, recently single fluorescent agents such as indocyanine green (ICG) and hybrid tracers such as ICG–99mTc nanocolloid were recently introduced.
Motomura et al. was the first group who described the technique near-infrared fluorescence imaging with ICG for detection of the SLN in breast cancer patients with an identification rate of 73.8% [17]. Once injected, the fluorophore ICG is strongly bound to serum proteins and takes several minutes to be transferred by the lymphatic vessels to the SLN. Owing to the small size of the particle, ICG has a velocity of several minutes.
The fluorophore ICG has a tissue penetration of <1 cm and, in the case of superficial laying SLNs, it enables a real-time transcutaneous and intraoperative visualization of both lymphatic vessels and the SLN(s). Owing to the dye is invisible to the naked eye, the surgical field will not be altered and the tattooing effect seen with the blue dye does not occur [32–34]. However, a dedicated imaging system (a fluorescence camera) is required to visualize the near-infrared fluorescence signal [35].
A disadvantage of ICG is its limited tissue penetration to a maximum of approximately 1 cm. This depth is further diminished in patients with a high BMI; Polom et al. found only adequate percutaneous SLN visualization in patients with a BMI below 23 [32]. A second disadvantage is its poor retention in the SLNs because of its small hydrodynamic diameter. As such, ICG can easily pass through to second echelon nodes [33]. Hojo et al. compared two methods for SLNB: the combination of ICG and radioisotope and the combination of ICG and blue dye [36]. They demonstrated that when using radiocolloid and ICG, 93.1% of SLNs were fluorescent, whereas 100% were radioactive. When comparing blue dye and ICG, 100% of nodes were fluorescent. Only 92.9% of these nodes were blue. From this study, the authors concluded that fluorescence was particularly beneficial when SLNs could not be appropriately identified with blue dye. However, on a per-patient basis, a larger number of SLNs were identified with ICG (3.8 vs 2.0 and 1.9 for ICG, radiocolloid and blue dye, respectively). Although this was suggested as a limitation of ICG, the minimal invasive nature of the SLNB was not affected. The authors did not report on statistical significance of their findings.
■Single fluorescent agents in combination with other techniques
The use of ICG alone has the disadvantage of a lack of preoperative visualization of SLNs in different basins as it is possible with lymphoscintigraphy; this may lead to larger incisions in order to gain access to the affected area, and also to the inability to visualize the affected SLN in the presence of more than 1 cm of soft tissue coverage [37].
Motomura et al. compared the use of ICG with the use of a combination of ICG and radioactivity in two separate injections [17]. The study showed a significantly higher success rate in SLNB when using the combination of both techniques compared with ICG alone, with a detection rate of 94.9 versus 83.9% when only ICG was used [17]. Several studies followed to optimize the ICG concentration for its use in SLNB, in order to achieve a better retention in the SLNs. Preclinical work demonstrated that adsorption of ICG to human serum albumin (HSA; ICG–HSA) increased fluorescence intensity and hydrodynamic diameter, therefore, providing improved detection in signal-to-background ratio (SBR) and better retention in the SLN [30,34,35,38].
Polom et al. compared ICG and ICG–HSA with the standard of care and radiolabelled colloid, in patients with breast cancer [32]. In the patients who received ICG alone, ten more SLNs were compared with the standard. It is important to note is that this increase in the amount of SLNs was not associated with upstaging of the disease. When using ICG–HSA only, three more SLNs found compared with the standard. Binding ICG with HSA increased the molecular weight and, therefore, improved retention in the SLNs, which will reduce the number of unnecessarily removed second echelon nodes during surgical procedure. Furthermore, they found less interfering spread of ICG in the surgical field (on gloves and surrounding the resection area) when using ICG–HSA compared with ICG alone [32]. The group of Gioux et al. confirmed the improved kinetics with ICG bound to HSA [38]. By contrast, a study by Hutteman et al. did not find any significant improvement in the SBR of ICG–HSA compared with ICG alone (SBR: 8.4 vs 11.3, respectively; p = 0.18) [39].
Mieog et al. searched for the optimal concentration of ICG–HSA in patients with breast cancer and found a optimal concentration between 400 and 800 μM. There was a significantly lower SBR for concentrations below 400 μM and above 800 μM [34]. Similar findings were shown for melanoma [40].
In a randomized study by van der Vorst et al., ICG and 99mTc nanocolloid were compared with patent blue in 24 patients with breast cancer [41]. The surgeon did not use the g-probe for the first 15 min. After 15 min, the g-probe was used to identify the SLN(s) if the SLN was not localized, using only ICG or ICG in combination with patent blue. In 25% of patients, the g-probe was needed to identify the SLN. The average BMI of patients in whom the g-probe was needed was significantly higher than in patients in whom the probe could be omitted. No benefit of using patent blue for SLN mapping was found owing to a low detecting rate for SLNs of only 84% [41]. A recent review by Schaafsma et al. reported similar findings when comparing ICG (with radiocolloid) with blue dye [33].
■Near-infrared fluorescence cameras for intraoperative imaging
Near-infrared fluorescence is invisible to the naked human eye and, therefore, a dedicated near-infrared fluorescence camera is required. The camera excites the fluorophore with a onewavelength light and, the fluorophore emits a different near-infrared wavelength in response, which is detected by the intensifier and displayed on the screen.
However, due to the interference between light and picture quality (and visualization ability), operating room lighting has to be dimmed as the near-infrared light is present in the operating theatre lights [1,32]. This can be overcome by using special light-emitting diode lights in the operating room, for example, the light-emitting diode Twin Beam (designed for Vision Inc., Ronkonkoma, NY, USA), which can be placed on the head of the surgeon to allow visualization of the operation field, while performing near-infrared fluorescence imaging.
There are several fluorescence cameras on the market (e.g., [mini-]FLARE™ [Beth Israel Deaconess Hospital, MA, USA], Fluobeam® [Fluoptics, Grenoble, France], SPY™ [Novadaq Technologies, ON, Canada], Storz™ [Karl Storz Endoskopes, Tuttlingen, Germany] and PDE™ [Photonics, Hamamatsu, Japan]), which can differ in the spectrally resolved light source and transfer camera and can serve for different types of open and laparoscopic surgeries (Figure 5) [35].
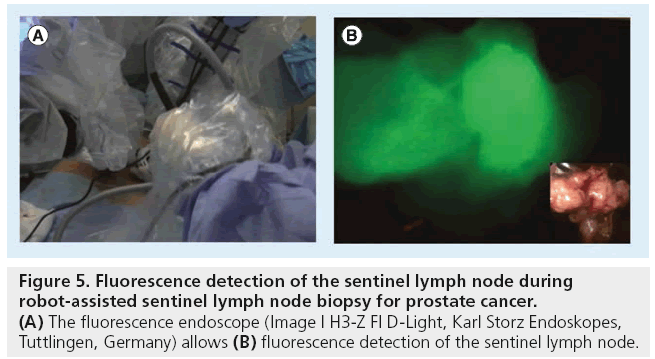
Figure 5: Fluorescence detection of the sentinel lymph node during robot-assisted sentinel lymph node biopsy for prostate cancer. (A) The fluorescence endoscope (Image I H3-Z FI D-Light, Karl Storz Endoskopes, Tuttlingen, Germany) allows (B) fluorescence detection of the sentinel lymph node.
In addition, intraoperative navigation based on the optical tracking of a fluorescence endoscope may help to improve the accuracy of hybrid surgical guidance. Rieger et al. used reference targets fixed on the patient and on a endoscope and to generate a 3D view of the SPECT/CT data from the perspective of the endoscope, which allowed the endoscope to be navigated to the target lesions identified by SPECT/CT [42]. Although near-infrared fluorescence is only suboptimal for the localization of deep lesions, the hybrid navigation approach aims to combine navigation based on preoperative SPECT/CT data with real-time intraoperative near-infrared fluorescence detection.
The hybrid approach
One important shortcoming concerning the use of single fluorescent agents is the lack of preoperative mapping. The need to preserve preoperative mapping as an essential part of the SLN procedure led to the design of hybrid tracers for SLNB. Due to the radioactive component of these tracers, preoperative lymphoscintigraphy and SPECT/CT are still possible. Additionally, the fluorescent component of the tracer enables high-resolution images in the operating room. Moreover, during the surgical act, hybrid tracers facilitate the combination of γ-probes and/or imaging portable g-devices with fluorescence cameras. All these aspects led to the validation of a hybrid approach for SLNB.
■The hybrid tracer ICG–99mTc nanocolloid
Small dyes such as ICG (ICG–HSA) and blue dyes do not accumulate in SLNs or have a very short lymph node retention; hence, these compounds can only be used to visualize real-time lymphatic transport at the operation room [43]. To overcome this shortcoming, the authors developed a multimodal colloid called ICG–99mTc nanocolloid. To form this compound, ICG was noncovalently bound to 99mTc nanocolloid, resulting in a complex that is both radioactive and fluorescent ( Figure 2). This hybrid complex allows SLN detection using preoperative visualization with lymphoscintigraphy or SPECT/CT images, and intraoperative radiotracing using a g-probe, as well as intraoperative visualization with a fluorescence camera in just one injection. Enhanced signal intensity, with an 86-fold increase in SBR for the multimodal imaging agent over ‘free’ ICG was found in preclinal studies [25,30,44]. Moreover, preoperative SPECT/CT imaging demonstrated the same lymphatic dynamics, distribution and specificity as found with 99mTc nanocolloid alone. The preclinical results were recently confirmed in a clinical study by Brouwer et al. comparing drainage patterns of this hybrid tracer ICG–99mTc nanocolloid with the current standard tracer in Europe, 99mTc nanocolloid, in 25 patients with melanoma or penile carcinoma [45]. On the first day, standard SLN identification with 99mTc nanocolloid (dynamic study and planar images at 10 min and 2 h after injection followed by SPECT/CT) was performed. In the same afternoon, or the following day, the procedure was repeated after injection of the hybrid tracer ICG–99mTc nanocolloid. The same SLNs were identified during the second scintigraphic procedure, yielding a high correlation between the radioactive counting rates in the SLNs of both scintigraphic studies. Intraoperatively, all preoperatively identified SLNs were localized using radio and fluorescence guidance. Of all SLNs, only 54% of the SLN were stained blue [45].
Van der Poel et al. published the first human study with the integrated multimodal method in 11 patients with prostate carcinoma with an increased risk of nodal metastasis, who underwent robot-assisted laparoscopic prostatectomy [19] . During surgery, the prostate and SLNs were excised followed by a retroperitoneal LN dissection. At 15 min and 2 h after the tracer injection, static planar g-camera images were acquired, followed by SPECT/CT 2 h postinjection. At 15 min after injection, only 55% of the SLNs were visualized. The visualization rate of the SLNs increased to 91% after 2 h. During the surgical resection, the SLNs were identified in real time using a combination of a g-probe and a near-infrared optimized f luorescence laparoscope. In this study, SLNs located in the prostatic fossa were hard to find owing to the high background signal of the radioactivity from the injection site, which prevented accurate g-probe-based navigation. Those SLNs could, however, be identified via near-infrared fluorescence imaging. In areas where there was a less prominent radioactive signal, the fluorescent signal provided guidance during the last centimetres of surgical exploration. Due to the limited tissue penetration of the fluorescent signal, fluorescence imaging did not directly identify the SLNs in 15% of the cases. Here, g-tracing helped to find these SLNs [19,46].
In two other studies by Brouwer et al. and van den Berg et al., the hybrid tracer was evaluated in the head and neck region during open SLNB [26,47]. Here, the addition of fluorescence imaging proved most valuable for SLNs located near the injection site; these nodes could not be accurately identified via g-tracing because of the shine-through effect of the radioacitive signal. However, the limited tissue penetration of the fluorescent signal of <0.5-cm blocks background signals and, thus, the SLN could accurately be identified. These studies also underlined the additional value of the use of a portable g-camera next to the g-probe; additional, deeper lying SLNs that were not instantly picked up by fluorescence imaging could be detected. Ex vivo, those SLNs were also fluorescent [26,47]. Table 1 gives an overview of the studies reported with the hybrid tracer ICG–99mTc nanocolloid in various malignancies.
Conclusion
Both the incorporation of SPECT/CT for preoperative lymphatic mapping and portable imaging devices for intraoperative surgical navigation have contributed to the extension of the SLN procedure to other malignancies. In many cases, SLNs must be excised in complex anatomical areas. To improve identification of these SLNs, single fluorophore agents and fluorescence imaging devices have been developed and validated in various malignant tumors. The need to preserve preoperative mapping as an essential part of the SLN procedure led to the design of hybrid tracers for SLNB. Preclinical and clinical studies have demonstrated that these tracers have a similar pattern of distribution and SLN uptake as conventional radiocolloids. This approach maintains the preoperative lymphoscintigraphy and SPECT/CT at the nuclear medicine department. During surgery, the combination of γ-probes and/or imaging portable g-devices with fluorescence cameras has been shown to be feasible and particularly useful when the SLNs are close to the injection site. In other areas, such as intra-abdominal, fluorescence helps to identify the exact location of the SLNs with a higher precision. Following this initial clinical study, further clinical validation of hybrid tracers must be extended to larger series of patients on the basis of a protocol combining preoperative mapping (lymphoscintigraphy and SPECT/CT) with intraoperative hybrid signal detection using separate imaging devices for detection of radioactive and fluorescent SLN signals. It is expected that the use of hybrid tracers will lead to improved SLN detection, principally in complex anatomical areas or when SLNs are localized close to primary lesions. This will help to reduce false-negative rates and the number of recurrences without an extension of the procedure.
Future perspective
Hybrid tracers using both acoustic and visual modalities will improve intraoperative imaging for SLNB. The technique is especially valuable in areas where the SLN is located near the primary tumor (the injection site) or when the SLN is deeply embedded within the tissue. With the development of hybrid tracers, better imaging systems have to be developed in order to improve intraoperative detection. The present available systems are limited to the separate imaging of each component of the tracer. Fusing of imaging modalities that can detect radioactivity besides fluorescence can be a significant contribution. Another interesting tool would be the use of integrated imaging systems, which can measure both near-infrared fluorescence and radioactivity. The integration of preoperative SPECT/CT by means of augmented or mixed reality protocols will facilitate 3D anatomical navigation in operating rooms.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Cabañas RM. An approach for the treatment of penile carcinoma. Cancer 39, 456–466 (1977).
- de Rosa N, Lyman GH, Silbermins D et al. Sentinel node biopsy for head and neck melanoma: a systematic review. Otolaryngol. Head Neck Surg. 145(3), 375–382 (2011).
- Coughlin A, Resto WA. Oral cavity squamous cell carcinoma and the clinically n0 neck: the past, present, and future of sentinel lymph node biopsy. Curr. Oncol. Rep. 12(2), 129–135 (2010).
- Vermeeren L, Valdés Olmos RA, Klop WM, Balm AJ, van den Brekel MW. A portable gamma-camera for intraoperative detection of sentinel nodes in the head and neck region. J. Nucl. Med. 51(5), 700–703 (2010).
- Varghese P, Mostafa A, Abdel-Rahman AT et al. Methylene blue dye versus combined dye – radioactive tracer technique for sentinel lymph node localisation in early breast cancer. Eur. J. Surg. Oncol. 33(2), 147–152 (2007).
- Fishman EK, Ney DR, Heath DG, Corl FM, Horton KM, Johnson PT. Volume rendering versus maximum intensity projection in CT angiography: what works best, when, and why. Radiographics 26(3), 905–922 (2006).
- van der Ploeg IM, Valdés Olmos RA, Kroon BB, Nieweg OE. The hybrid SPECT/CT as an additional lymphatic mapping tool in patients with breast cancer. World J. Surg. 32(9), 1930–1934 (2008).
- Vidal-Sicart S, Brouwer OR, Valdés-Olmos RA. Evaluation of the sentinel lymph node combining SPECT/CT with the planar image and its importance for the surgical act. Rev. Esp. Med. Nucl. 30(5), 331–337 (2011).
- Valdés Olmos RA, Vidal-Sicart S. SPECT/CT image generation and criteria for sentinel node mapping. In: Atlas of Lymphoscintigraphy and Sentinel Node Mapping. Mariani G, Manca G, Orisini F, Vidal-Sicart S, Valdés Olmos RA (Eds). Springer-Verlag, Milan, Italy, 111–119 (2012).
- Stoffels I, Boy C, Pöppel T et al. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA 308(10), 1007–1014 (2012).
- Covarelli P, Tomassini GM, Simonetti S et al. The single-photon emission computed tomography/computed tomography: a new procedure to perform the sentinel node biopsy in patients with head and neck melanoma. Melanoma Res. 17(5), 323–328 (2007).
- Klode J, Poeppel T, Boy C et al. Advantages of preoperative hybrid SPECT/CT in detection of sentinel lymph nodes in cutaneous head and neck malignancies. J. Eur. Acad. Dermatol. Venereol. 25(10), 1213–1221 (2011).
- van der Ploeg IM, Nieweg OE, Kroon BB et al. The yield of SPECT/CT for anatomical lymphatic mapping in patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging 36(6), 903–909 (2009).
- van der Ploeg IM, Valdés Olmos RA, Kroon BB et al. The yield of SPECT/CT for anatomical lymphatic mapping in patients with melanoma. Ann. Surg. Oncol. 16(6), 1537–1542 (2009).
- Vermeeren L, van der Ploeg IM, Olmos RA et al. SPECT/CT for preoperative sentinel node localization. J. Surg. Oncol. 101(2), 184–190 (2010).
- Radovanovic Z, Golubovic A, Plzak A, Stojiljkovic B, Radovanovic D. Blue dye versus combined blue dye – radioactive tracer technique in detection of sentinel lymph node in breast cancer. Eur. J. Surg. Oncol. 30(9), 913–917 (2004).
- Motomura K, Inaji H, Komoike Y et al. Combination technique is superior to dye alone in identification of the sentinel node in breast cancer patients. J. Surg. Oncol. 76(2), 95–99 (2001).
- James A. The application of sentinel node radiolocalization to solid tumors of the head and neck: a 10-years experience. Laryngoscope 114, 2–19 (2004).
- van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, van Leeuwen FW. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur. Urol. 60(4), 826–833 (2011).
- Brouwer OR, Buckle T, Bunschoten A et al. Image navigation as a means to expand the boundaries of fluorescence-guided surgery. Phys. Med. Biol. 57(10), 3123–3136 (2012).
- Vidal-Sicart S, Paredes P, Zanón G et al. Added value of intraoperative real-time imaging in searches for difficult-to-locate sentinel nodes. J. Nucl. Med. 51(8), 1219–1225 (2010).
- Keereweer S, Sterenborg HJ, Kerrebijn JD, van Driel PB, Baatenburg de Jong RJ, Löwik CW. Image-guided surgery in head and neck cancer: current practice and future directions of optical imaging. Head Neck 34(1), 120–126 (2012).
- Vidal-Sicart S, Vermeeren L, Solà O, Paredes P, Valdés-Olmos RA. The use of a portable gamma camera for preoperative lymphatic mapping: a comparison with a conventional gamma camera. Eur. J. Nucl. Med. Mol. Imaging 38(4), 636–641 (2011).
- Dengel LT, More MJ, Judy PG et al. Intraoperative imaging guidance for sentinel node biopsy in melanoma using a mobile gamma camera. Ann. Surg. 253(4), 774–778 (2011).
- Kerrou K, Pitre S, Coutant C et al. The usefulness of a preoperative compact imager, a hand-held gamma-camera for breast cancer sentinel node biopsy: final results of a prospective double-blind, clinical study. J. Nucl. Med. 52(9), 1346–1353 (2011).
- Brouwer OR, Klop WM, Buckle T et al. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann. Surg. Oncol. 19(6), 1988–1994 (2012).
- Vermeeren L, Valdés Olmos RA, Meinhardt W et al. Intraoperative radioguidance with a portable gamma camera: a novel technique for laparoscopic sentinel node localisation in urological malignancies. Eur. J. Nucl. Med. Mol. Imaging 36(7), 1029–1036 (2009).
- Vermeeren L, Valdés Olmos RA, Klop WM et al. SPECT/CT for sentinel lymph node mapping in head and neck melanoma. Head Neck 33(1), 1–6 (2011).
- Vermeeren L, Meinhardt W, Bex A et al. Paraaortic sentinel lymph nodes: toward optimal detection and intraoperative localization using SPECT/CT and intraoperative real-time imaging. J. Nucl. Med. 51(3), 376–382 (2010).
- van Leeuwen AC, Buckle T, Bendle G et al. Tracer-cocktail injections for combined preand intraoperative multimodal imaging of lymph nodes in a spontaneous mouse prostate tumor model. J. Biomed. Opt. 16(1), 016004 (2011).
- Wendler T, Herrmann K, Schnelzer A et al. First demonstration of 3-D lymphatic mapping in breast cancer using freehand SPECT. Eur. J. Nucl. Med. Mol. Imaging 37(8), 1452–1461 (2010).
- Polom K, Murawa D, Nowaczyk P, Rho YS, Murawa P. Breast cancer sentinel lymph node mapping using near infrared guided indocyanine green and indocyanine green – human serum albumin in comparison with gamma emitting radioactive colloid tracer. Eur. J. Surg. Oncol. 38(2), 137–142 (2012).
- Schaafsma BE, Mieog JS, Hutteman M et al. The clinical use of indocyanine green as a nearinfrared fluorescent contrast agent for imageguided oncologic surgery. J. Surg. Oncol. 104(3), 323–332 (2011).
- Mieog JS, Troyan SL, Hutteman M et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 18(9), 2483–2491 (2011).
- van den Berg NS, van Leeuwen FW, van der Poel HG. Fluorescence guidance in urologic surgery. Curr. Opin. Urol. 22(2), 109–120 (2012).
- Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast 19(3), 210–213 (2010).
- Bredell MG. Sentinel lymph node mapping by indocyanine green fluorescence imaging in oropharyngeal cancer – preliminary experience. Head Neck Oncol. 2, 31 (2010).
- Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol. Imaging 9(5), 237–255 (2010).
- Hutteman M, Mieog JS, van der Vorst JR et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res. Treat. 127(1), 163–170 (2011).
- van der Vorst JR, Schaafsma BE, Verbeek FP et al. Dose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanoma. Br. J. Dermatol. 168(1), 93–98 (2013).
- van der Vorst JR, Schaafsma BE, Verbeek FP et al. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99(m) technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann. Surg. Oncol. 19(13), 4104–4111 (2012).
- Rieger A, Saeckl J, Belloni B et al. First experiences with navigated radio-guided surgery using freehand SPECT. Case Rep. Oncol. 4(2), 420–425 (2011).
- Buckle T, Chin PT, van Leeuwen FW. (Non-targeted) radioactive/fluorescent nanoparticles and their potential in combined pre- and intraoperative imaging during sentinel lymph node resection. Nanotechnology 21(48), 482001 (2010).
- Buckle T, van Leeuwen AC, Chin PT et al. A self-assembled multimodal complex for combined pre- and intra-operative imaging of the sentinel lymph node. Nanotechnology 21(35), 355101 (2010).
- Brouwer OR, Buckle T, Vermeeren L et al. Comparing the hybrid fluorescent-radioactive tracer indocyanine green–99mTc-nanocolloid with 99mTc nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 53(7), 1034–1040 (2012).
- Buckle T, Brouwer OR, Valdés Olmos RA, van der Poel HG, van Leeuwen FW. Relationship between intraprostatic tracer deposits and sentinel lymph node mapping in prostate cancer patients. J. Nucl. Med. 53(7), 1026–1033 (2012).
- van den Berg NS, Brouwer OR, Klop WM et al. Concomitant radio- and fluorescenceguided sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity using ICG–99mTc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 39(7), 1128–1136 (2012).
• Describes the use of indocyanine green for sentinel lymph node (SLN) mapping in breast, gastric, colorectal, anal and lung cancer.
• Discusses the additional value of SPECT/CT compared with the conventional lymphoscintigraphy. Where lymphoscintigrams are not always clear, SPECT/CT allows anatomical localization of the SLNs. SPECT/CT also allows the identification of sentinel lymph nodes missed with lymphoscintigraphy.
• Following the preclinical papers of van Leeuwen et al. [30] and Buckle et al. [44], this is the first clinical paper describing the use of the hybrid tracer indocyanine green–99mTc nanocolloid for the detection of SLNs during robot-assisted laparoscopic prostatectomy.
• Introduces near-infrared imaging using indocyanine green and its application for SLN mapping in breast, skin and gastrointestinal cancer, and also for tumor imaging, flap reconstruction and angiography. 34 Mieog JS, Troyan SL, Hutteman M et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 18(9), 2483–2491 (2011).
• Provides an overview of the available camera systems for (near-infrared) fluorescence imaging.
• Discusses the ‘ideal’ near-infrared contrast agent and near-infrared camera system. In addition, the authors discuss the indications that could benefit from near-infrared imaging, such as SLN biopsy, bile duct visualization and flap reconstructions.
• • Describes the various types of radioactive- and fluorescence-labeled nanoparticles that have been developed in a preclinical setting for image-guided surgery.
• • The application of indocyanine green–99mTc nanocolloid for SLN biopsy was studied and compared with ‘free’ indocyanine green in an orthotopic mouse model for breast cancer.
• • Compared the hybrid tracer indocyanine green–99mTc nanoclloid with the conventional 99mTc nanocolloid and showed that the drainage pattern of both is similar. By using indocyanine green–99mTc nanocolloid, preoperative SLN mapping can be combined with intraoperative radio- and fluorescence-guided SLN biopsy.
• • In 14 patients, the use of indocyanine green–99mTc nanocolloid for sentinel lymph node biopsy of oral cavity carcinoma was evaluated. The use of the fluorescent signature of the hybrid tracer was found to be especially valuable when nodes were located close to the injection site.