Research Article - Journal of Experimental Stroke & Translational Medicine (2022) Volume 14, Issue 5
Hematoma Volume Alters Efficacy of Progesterone Treatment for Preclinical Intracerebral Hemorrhage
Beilei Lei1,2, Ramona Rodriguiz M3,4, Talagnair Venkatraman1,5, Haichen Wang1,6, Christopher Lascola D1,5, Jay Lusk B8,9, Anna Covington10, Daniel Laskowitz T1,6,11,12, David Warner S1,7,11,12, William Wetsel C3,4,11,13, Michael James L1,6,7,12*
1Multidisciplinary Neuroprotection Laboratories, Duke University, Durham
2Department of Medicine, Duke University, Durham
3Department of Psychiatry and Behavioral Sciences, Duke University, Durham
4Mouse Behavioral and Neuroendocrine Analysis Core Facility, Duke University, Durham
5Department of Radiology, Duke University, Durham
6Department of Neurology, Duke University, Durham
7Department of Anesthesiology, Duke University, Durham
8School of Medicine, Duke University, Durham
9Fuqua School of Business, Duke University, Durham
10Department of Surgery, Duke University, Durham
11Department of Neurobiology, Duke University, Durham
12Duke Institute for Brain Sciences, Duke University, Durham
13Department of Cell Biology, Duke University, Durham
Received: 02-Sep-2022, Manuscript No. JESTM-22-73509; Editor assigned: 05-Sep-2022, PreQC No. JESTM-22-73509 (PQ); Reviewed: 19- Sep-2022, QC No. JESTM-22-73509; Revised: 23-Sep-2022, Manuscript No. JESTM-22-73509 (R); Published: 30-Sep-2022, DOI: 10.37532/ jestm.2022.14(5).85-96
Abstract
Hematoma volume after intracerebral hemorrhage (ICH) affects recovery in humans but has been inadequately examined in preclinical models. Recovery after preclinical ICH improves with progesterone treatment, but the effects of hematoma volumes are unknown. Thus, mice were subjected to 0.03U or 0.1U intrastriatal collagenase injections followed by concealed vehicle or progesterone treatment and assessed using neurobehavioral tests over 28 days. 24 hours after injury, hematoma volume by MRI increased with collagenase dose; perfusion and diffusion were unaffected. Open field testing did not distinguish ICH condition from sham injury. Both collagenase doses increased foot-fault errors, decreased latencies on rotarod, and perturbed prepulse inhibition relative to sham controls. Notably, mice injured with 0.1U collagenase were deficient in short- and long-term memory on novel object recognition tasks compared to sham and 0.03U collagenase injured animals. Progesterone treatment did not affect these assessments. Morris water maze performance was similar among ICH groups. However, vehicle-treated mice injured with 0.1U collagenase failed to distinguish target quadrants on probe trials. Progesterone treatment resulted in partial recovery for this group to identify the target quadrant. Findings that hematoma volume and progesterone treatment variably affect neurobehavioral tests after intrastriatal collagenase injection in mice could have implications for the translation of progesterone into acute ICH therapeutics.
Keywords
progesterone • Intracerebral hemorrhage • Preclinical • Rodent model • Stroke severity
Introduction
Intracerebral hemorrhage (ICH) is a devastating stroke subtype without proven efficacious therapy. Translational failure of promising therapeutic candidates is multifactorial, but insufficient completion of Stroke Treatment Academic Industry Roundtable (STAIR) [1] criteria using RIGOR guidelines [2] is likely contributory as preclinical studies vary in their ability to reproducibly define dose response and time windows and assess functional outcomes [3]. Diseaserelevant paradigms in preclinical efficacy analysis should improve potential translational success and may inform subsequent clinical design.
Hematoma volume correlates with ICH injury severity in humans and is a major predictor of outcomes after ICH [4]. Relevance of hematoma volume to ICH severity and prognosis is so highly correlated that clinical trials often set inclusion/exclusion parameters around high and low hematoma volumes. In theory, excluding low hematoma volumes avoids enrolling patients likely to have good recovery regardless of treatment. Conversely, excluding high hematoma volumes avoids enrolling patients likely to have poor recovery. Despite this issue, testing ICH therapeutics using different hematoma volumes is extremely limited.
Based on emerging results, one potentially translatable therapeutic strategy for acute ICH is the administration of exogenous progesterone [5-8]. Progesterone has been extensively investigated as a neurotherapeutic for other forms of acute brain injury, such as trauma and ischemia [9-11], but has been unable to translate to proven therapy in clinical trials [12,13]. Prior work with progesterone in ICH models has demonstrated improvement in early and longterm neurobehavioral recovery in young and old male and ovariectomized female mice, as well as in spontaneously hypertensive rats [6,8]. Furthermore, efficacious effects of progesterone have been replicated in other independent laboratories [5,7]. While progesterone has been evaluated in multiple pre-clinical ICH paradigms, the potential effects of hematoma volume on progesterone efficacy have not been systematically assessed, which may impact its translatability.
The goal of the current study was to expand preclinical testing of progesterone efficacy as a therapeutic for acute ICH, especially pertaining to effects of hematoma volume on recovery. Progesterone was hypothesized to ameliorate neurobehavioral deficits after ICH to varying degrees depending upon hematoma volumes.
Methods
Duke University is an AAALAC-accredited institution. All experiments were performed within the guidelines as outlined for mice in the National Research Council “Guide for the Care and Use of Laboratory Animals” [14] with an approved protocol from the Duke University Institutional Animal Care and Use Committee.
Animals
12 to 14 week-old male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed in standard acrylic cages with ad libitum access to food (Lab Diet 5001; PMI Nutrition International, St. Louis, MO) and water throughout the study. After computergenerated randomization of study groups before ICH injury, mice received sham or blinded intrastriatal injection of one of two collagenase doses (0.03 units [U] or 0.1 U) and concealed subcutaneous injection of either 4 milligram (mg)/kilogram (kg) progesterone (BHR-100) or a volume-equivalent vehicle. Individuals assessing mice were blinded to the treatment and injury conditions of the animals. Following completion of injury, treatment, and 72-hour recovery, mice were transferred to the Mouse Behavioral Core Facility and allowed to acclimate to the new environment for one day prior to the start of testing. All testing and husbandry rooms were maintained on a 14:10 hour light: dark cycle (light onset: 0700 hours). Mice were examined in behavioral tests between 0900 and 1500 hours.
Experimental Paradigm
Two cohorts of mice were used: one for imaging at 2 and 5 hours after injury with 0.03 U or 0.1 U of intrastriatal collagenase injection and treatment with BHR-progesterone or vehicle (Figure 1) and a second for behavioral assessments. The experimental groups of the second cohort (Table 1) and experimental paradigm for behavioral assessments (Table 2) are presented.
Table 1. Experimental Groups.Mice were randomized to sham injury or collagenase induced ICH groups. Mice receiving collagenase were further randomized to 0.03 U or 0.1 U collagenase intrastriatal injection. All cohorts were blindly treated with BHR-progesterone or vehicle (BHR suspension formulation without progesterone). Table 1 reports the number of mice randomized to each condition. Five vehicle-treated 0.1 U collagenase injected mice and three BHR-treated 0.1 U collagenase injected mice died 24 hours after injury. Two vehicle-treated 0.1 U collagenase mice died before or during before the completion of testing. Note: U, units.
| Vehicle Treatment | Progesterone Treatment | Total | |
|---|---|---|---|
| Sham Injury | 8 | 7 | 15 |
| 0.03 U Collagenase Intrastriatal Injection | 15 | 15 | 30 |
| 0.1 U Collagenase Intrastriatal Injection | 15 | 15 | 20 |
| Total | 31 | 34 | 65 |
Table 2. Experimental Paradigm.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|
| Week 1 | Injury | Mice arrive at Core | Rotorod, Accelerating, 5 trials | Open Field | Foot-Fault Day 1 | Foot-Fault Day 2 |
|
| Week 2 | Foot-Fault Day 3 |
Forced Gait | Novel Object Recognition | ||||
| Week 3 | Morris Water Maze Acclimation | Water Maze | Water Maze | Water Maze | Water Maze | Water Maze | Water Maze |
| Week 4 | Pre-Pulse Inhibition |
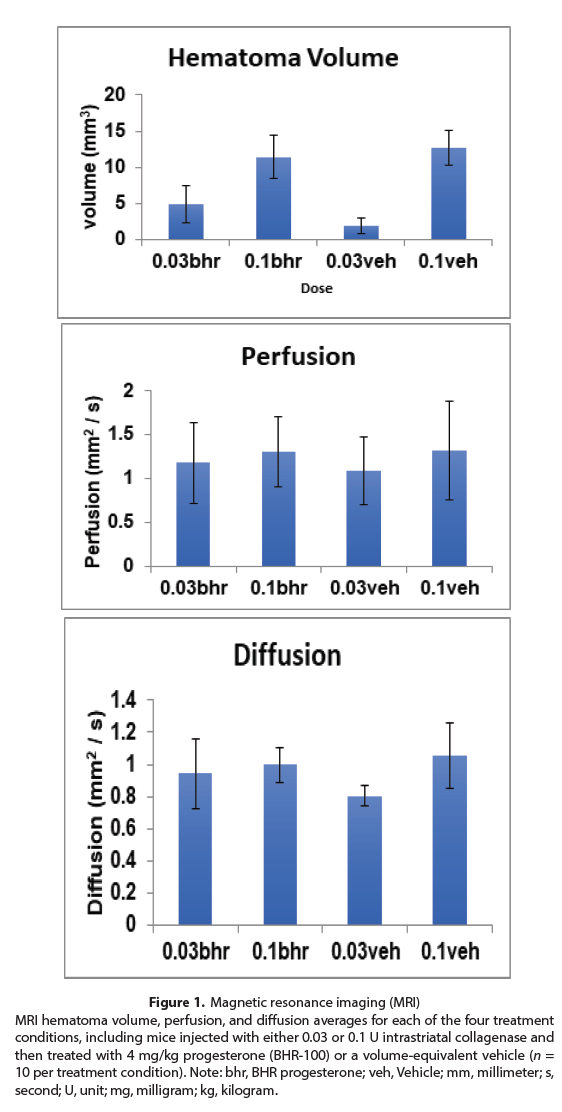
Figure 1: Magnetic resonance imaging (MRI) MRI hematoma volume, perfusion, and diffusion averages for each of the four treatment conditions, including mice injected with either 0.03 or 0.1 U intrastriatal collagenase and then treated with 4 mg/kg progesterone (BHR-100) or a volume-equivalent vehicle (n = 10 per treatment condition). Note: bhr, BHR progesterone; veh, Vehicle; mm, millimeter; s, second; U, unit; mg, milligram; kg, kilogram.
Preparation and Administration of Progesterone
Administration BHR-100 (2 mg/milliliter (ml) progesterone; BHR Pharma, Herndon, VA, USA) was used for experiments. BHR-100 is the proprietary ready-to-use sterile emulsion, containing soybean oil and progesterone, used in the SyNAPSe clinical trial (NCT 01143064) for acute traumatic brain injury. To achieve a 4 mg/kg dose of progesterone, 50–70 microliters (μL; based on animal weight) of BHR-100 was administered subcutaneously. Volume-equivalent vehicle groups were given the same lipid emulsion BHR formulation without progesterone. Initial dosing was determined by the range of efficacy demonstrated in preclinical models of traumatic brain injury [9-10] and ICH [6, 8]. Progesterone or volume-equivalent vehicle (50–70 μL) was subcutaneously injected in neck depots at 2 and 5 hours after intrastriatal collagenase injection, and then every 24 hours for subsequent 2 days. The progesterone administration regimen was chosen based upon the impracticality of administering progesterone to patients with ICH 2 hours prior to injury in the clinical setting.
ICH Model
Intrastriatal collagenase injection was used to induce ICH in mice as previously described [15]. Briefly, mice were intubated after anesthesia induction with 4.6% isoflurane and mechanically ventilated with 1.5% isoflurane in a mixture of 30%/70% O2/N2 mixture. Core body temperature, monitored by rectal probe, was maintained at 37±0.2 °C by circulating warm water in an underbody waterbed. The animal’s head was secured in a stereotactic frame, and a midline scalp incision was made. After exposing the skull, a burr hole was created 2.2 millimeters (mm) left-lateral to bregma, and a 0.5 μL syringe needle (Hamilton, Reno, NV, USA) was advanced to a depth of 3 mm from the cortex. Type IV-S Clostridial collagenase (Sigma, St. Louis, MO, USA) was injected over 2 minutes (0.03 or 0.1 U in 0.4 μL normal saline).
After immediately withdrawing the needle at 1 minute (min) and closing the incision, animals were allowed to recover spontaneous ventilation and were then extubated and provided access to food and water. Sham injured animals were exposed to the above procedure without injection of collagenase.
Magnetic Resonance Imaging (MRI)
MR images were collected using a Bruker 7.0T MRI scanner (Bruker Biospin, Billerica, MA, USA) operating with Para vision 5.1 as previously described [15]. Animals were anesthetized with 1.5% isoflurane in room air. Core body temperature, monitored by rectal probe, was maintained at 37±0.5 °C by a circulating water bath, and respiratory rates were maintained at 50–70 respirations per second (sec). Susceptibility Weighted Images (SWI) were collected using fast low angle shot sequence with the following parameters: Time to Echo/Time to Repetition=9/700 milliseconds (msec), Flip Angle=40, Field of View=3 centimeters (cm) x 3 cm, Matrix size = 256 x 256, thickness = 0.5 mm, slices = 32; Total scan time = 27 min. SWI images were reconstructed using Paravison5.1 (Bruker Biospin Billerica MA USA). The Digital Imaging and Communications in Medicine images were post-processed off-line using OSIRIX imaging software to quantitate ICH volumes.
Neurobehavioral Assessments
Open Field
Mice were housed in the test room 24 hours prior to the experiment. Motor activity was assessed in a Versamax open field (Omnitech Electronics, Columbus, OH) as previously described [16]. Mice were individually placed into a 21 x 21 x 30 cm arena where 8 infrared diodes monitored activity for over an hour. Test arenas were interfaced with Fusion software (Omnitech Electronics). Locomotor (distance traveled) and center zone activities were examined.
Foot-fault
Motor performance was assessed by evaluating the number of foot-slips in the parallel bar task as described previously [17]. Mice were given free access to a test arena (15 x 15 x 20 cm) for 5 min. Arenas consisted of a floor with 16 parallel stainless-steel rods (3 mm in diameter) spaced 6 mm apart and located 10 mm above a touch sensor (Clever Sys Inc., Reston, VA). When a mouse’s foot slipped between the bars, the paw would touch sensors, and this response was recorded as a “foot-fault.” A digital camera (DCR-SR45; Sony Instruments, Laredo, TX) was positioned 30 cm over each arena to record motor activity. Test arenas and sensors were interfaced with FootFaultScan software (Clever Sys Inc., Reston, VA). The number of foot-slips and distance moved were scored.
Rotarod
Balance and coordination were assessed using an accelerating (4-40 rotations per min over 5 min) rotarod (Med-Associates, St. Albans, VT) as previously described [18]. Mice were administered five successive 5-min trials, separated by a 20 min inter-trial interval. Trials were terminated and recorded as latency to fall when animals fell from the rod, or at 300 sec.
Pre-pulse Inhibition (PPI)
PPI was evaluated using the SRL-Lab startle response system (San Diego Instruments, San Diego, CA) [16]. Three types of trials were administered: null trials were composed only of background white noise (64 decibels [dB]); pulsealone trials consisted of a 40 msec 120 dB whitenoise pulse or startle stimulus; and prepulsepulse trials were comprised of onset of pulse stimulus preceded by 100 msec with 20 msec prepulse stimuli that were 4, 8, or 12 dB above the 64 dB white-noise background. Individual mice were placed into a Plexiglas® holder and habituated to the apparatus for 10 min prior to testing. Each test consisted of 42 trials with 18 pulse-alone trials, 6 null trials, and 18 prepulsepulse trials with 6 trials at each intensity. The test consisted of 6 pulse-alone trials followed by combinations of the prepulse-pulse, pulse-alone, and null trials and was terminated with 6 pulsealone trials. Activity in the tubes prior to each trial was assessed as null activity (AU, arbitrary units) measured in the first 100 msec of each trial, prior to the onset of auditory stimuli. The pulse-alone response was measured as the peak startle response (AU) to this stimulus. PPI for each prepulse intensity was calculated as a ratio of responses on prepulse-pulse trials to pulsealone trials subtracted from 1 and expressed as a percent [1-(prepulse-pulse trials/pulse-alone trials)*100].
Novel Object Recognition Memory
Mice were examined for short- (STM) and longterm memory (LTM) as previously described [19]. In this task, mice were presented with two identical LEGO® objects for 5-min. Following training, mice were placed into their home-cage and returned to test arenas after short (20 min) and long intervals (24 hours). At each test-time, one of the familiar objects was replaced with a novel object. All tests were scored using Noldus Ethovision 9 (Noldus Information Technologies, Leesburg, VA) with three-point tracking for the nose relative to the center of the body and tail. The time that the nose was oriented towards and within 3 cm of an object was scored as the duration (sec) spent exploring the object. Preference for the novel object was expressed as a ratio of time spent exploring the novel object minus time spent exploring the familiar object, divided by the total time spent with both objects.
Positive values reflected a preference for the novel object, negative values indicated a preference for the familiar object, and scores approaching zero indicated no preference for either object.
Morris Water Maze
As previously described (Taylor et al., 2008), the pool consisted of quadrants: northeast (NE), northwest (NW), southeast (SE), and southwest (SW). In the week prior to testing, all animals were handled for 5-10 min per day, terminating in a 1 min period when mice were acclimated to standing in water. Following handling on the day prior to testing, mice were trained to sit on the hidden platform in the NE quadrant for 20 sec and then permitted to swim freely for 30 sec before being returned to the platform for 15 sec. If a mouse returned to the hidden platform before the 30 sec elapsed, it was immediately removed from the pool and placed into its home-cage. The next day, mice were given two sets of paired trials per day, separated by 60 min, for a total of 4 trials per day. Release points were randomized across trials and days. One hour after completing acquisition trials on days 2, 4, and 6, a single probe trial was administered. During probe trials, the hidden platform was removed and mice were released from the southern-most point in the maze. All trials were recorded with highresolution cameras suspended 167 cm above pool center. Acquisition and probe trial performance was analyzed for swim distance. For probe trials, distance swam in each quadrant relative to the target quadrant (i.e., NE) was assessed as index of learning.
Statistical Analyses
Statistical analyses of the imaging results were performed using StatView (SAS Institute Inc., Cary, N.C.), while analyses for behavioral experiments were calculated with SPSS 25 (IBM SPSS Statistics, Chicago, IL). Hematoma, perfusion, and diffusion MRI volumes (Figure 1) were analyzed by two-way analysis of variance (ANOVA), comparing vehicle and treatment groups at each collagenase dose with posthoc analyses by Scheffé tests. Based on known variance within this model [20-23], a sample size of 10 mice per group was calculated to demonstrate a 50% treatment effect in water maze latencies over 28-31 days post-injury for a 4-group repeated measures ANOVA (RMANOVA). All results were expressed as means ± standard error of the mean. Behavioral data were analyzed with either ANOVA or repeated measures ANOVA (RMANOVA) with time as the repeated measure. Between subject effects were designated as treatment (i.e., vehicle or BHR) and injury condition effects (i.e., sham, 0.03 U or 0.1 U intrastriatal collagenase injury). Post-hoc statistics were by Bonferroni corrected pair-wise comparisons or with Dunnett comparisons where vehicle or sham groups were the control conditions. In all cases, p<0.05 was considered statistically significant.
Results
Imaging analyses revealed that larger hematoma volumes were produced overall after intrastriatal injection of 0.1 U compared to 0.03 U collagenase (p<0.001). There were no statistical differences in brain MR perfusion and diffusion indices between doses of collagenase regardless of post-injury treatment (Figure 1). One mouse receiving BHR-progesterone died postcollagenase injection before imaging.
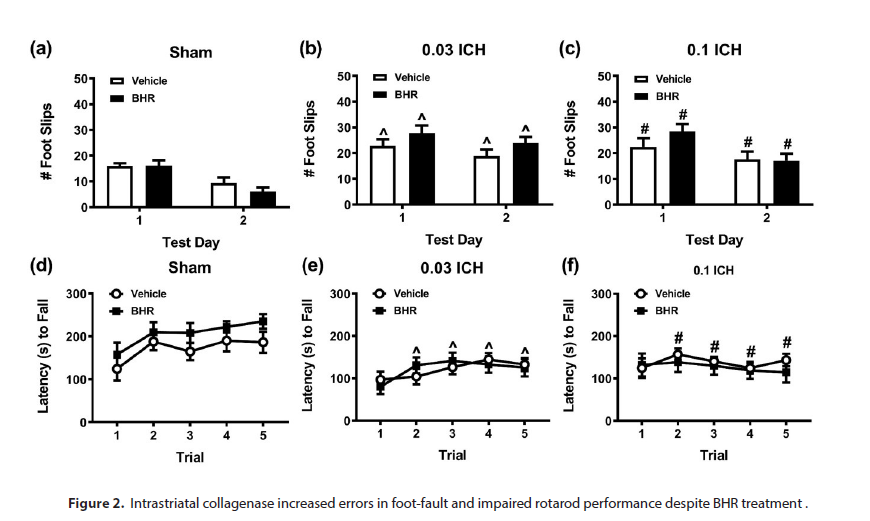
Behavioral responses were analyzed for motor performance according to effects of intrastriatal collagenase dose and for BHR-100 treatment. Open field testing found no significant differences among different injury and treatment conditions in cumulative distance traveled or in cumulative center zone activity over 1 hour (Supplemental Table 1). In foot-fault tasks, foot-slips were higher on day 1 than day 2 (p<0.001) and increased overall for both ICH injured groups compared to sham controls (p- ≤0.002; Figure 2a-c). Progesterone treatment did not affect performance on the foot-fault task, regardless of injury condition. As a control, distance moved was not significantly different among groups; however, overall locomotion was higher on day 1 than on day 2 (p<0.001; Supplemental Table 2). On rotarod, latency to fall across trials 2-5 was reduced overall for both ICH injured groups compared to sham control (p≤0.046; Figure 2d-f). In addition, sham and 0.03 U collagenase groups both demonstrated motor learning, whereas the 0.1 U collagenase group was deficient. Hence, relative to trial 1, performance on trials 2, 4, and 5 were superior for sham controls (p≤0.043), while that from the 0.03 U collagenase group increased on trials 3 and 4 compared to trial 1 (p≤0.033). Progesterone treatment did not affect trial performance.
Supplemental Table 1. Effects of ICH injury and progesterone treatment on motor activity in the open field. Mice numbers in each injury condition and treatment are presented in Table 1. Data are presented as means ± standard errors of the mean. Two-way ANOVA for locomotion revealed no significant main effects for the injury condition or treatment; the injury condition by treatment interaction was also not significant. Two-way ANOVA for center zone activity observed none of the statistics to be significant. Note: BHR, BHR progesterone; cm, centimeter; U, unit.
Injury Condition |
Treatment | Cumulative Distance Traveled (cm) | Cumulative Center Distance (cm) |
|---|---|---|---|
| Sham | Vehicle | 2114.98 ±241.99 | 1406.17 ±165.74 |
| BHR-100 | 2236.36 ±282.32 | 1563.67 ±228.24 | |
| 0.03 U Collagenase | Vehicle | 2001.05 ±128.37 | 1384.23 ±115.53 |
| BHR-100 | 2174.61 ±156.21 | 1533.74 ±106.69 | |
| 0.1 U Collagenase | Vehicle | 2174.52 ±256.16 | 1347.48 ±113.70 |
| BHR-100 | 2608.54 ±245.73 | 1659.33 ±178.47 |
Supplemental Table 2. Effects of ICH injury and progesterone treatment on distance moved in foot-fault test. Mice numbers in each injury condition and treatment are presented in Table 1. Data are presented as means ± standard errors of the mean. Repeated measures analysis of variance for distance moved revealed a significant within-subjects effect of test-day [F(1,59)=55.665, p<0.001} and near significance for the between-subjects effect of injury condition. [F(2,59)=3.002, p=0.057]. Note: BHR, BHR progesterone; mm, millimeter; U, unit.
Injury Condition |
Treatment | Day 1 Cumulative Distance Moved (mm) | Day 2 Cumulative Distance Moved (mm) |
|---|---|---|---|
| Sham | Vehicle | 3231.96 ±301.13 | 2222.36 ±323.31 |
| BHR-100 | 3584.86 ±232.92 | 1928.21 ±243.91 | |
| 0.03 U Collagenase | Vehicle | 3052.85 ±253.08 | 2080.11 ±216.59 |
| BHR-100 | 3265.80 ±297.18 | 2589.00 ±217.90 | |
| 0.1 U Collagenase | Vehicle | 2319.64 ±242.13 | 1940.23 ±361.78 |
| BHR-100 | 2857.75 ±305.08 | 1808.64 ±246.72 |
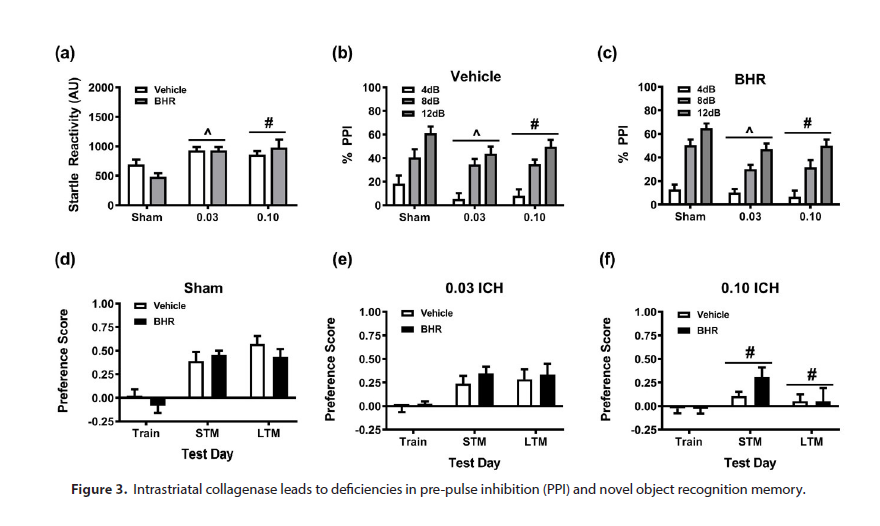
In addition to motor tasks, effects of ICH injury on cognitive function were assessed. Animals were first evaluated for acoustic sensorimotor gating (PPI). Null activities were not differentiated among the groups (Supplemental Table 3). By comparison, pulse-alone activity was augmented overall in both ICH injured groups relative to sham controls (p≤0.003; Figure 3a). PPI responses were deficient in both ICH groups relative to sham controls, regardless of treatment group (p≤0.033; Figure 3b-c). In the novel object recognition memory test, no group distinctions were noted for training. However, mice subjected to 0.1U intrastriatal collagenase were deficient overall in short- (STM) and long-term (LTM) memory compared to sham controls (p≤0.038). Analyses of responses within treatment revealed that novel object preference significantly increased for sham and 0.03 U collagenase groups during STM and LTM tests relative to training (p≤0.008), but scores in the 0.1U collagenase group were not significantly different among the three phases of testing.
Supplemental Table 3. Effects of ICH injury and progesterone treatment on null activity in prepulse inhibition test. Mice numbers in each injury condition and treatment are presented in Table 1. Data are presented as means ± standard errors of the mean. Two-way analysis of variance for null activity found no significant main effects for the injury condition or treatment; injury condition by treatment interaction was not significant. Note: AU, arbitrary unit; BHR, BHR progesterone; U, unit.
Injury Condition |
Treatment | Null Activity (AU) |
|---|---|---|
| Sham | Vehicle | 6.09 ±0.65 |
| BHR-100 | 5.51±0.80 | |
| 0.03 U Collagenase | Vehicle | 7.45 ±0.67 |
| BHR-100 | 7.11 ±0.62 | |
| 0.1 U Collagenase | Vehicle | 6.94 ±0.97 |
| BHR-100 | 6.48 ±0.43 |
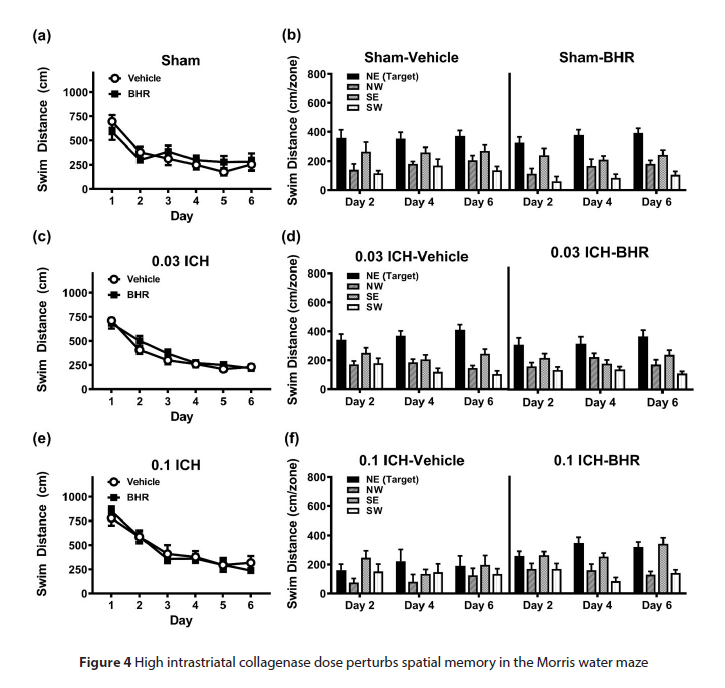
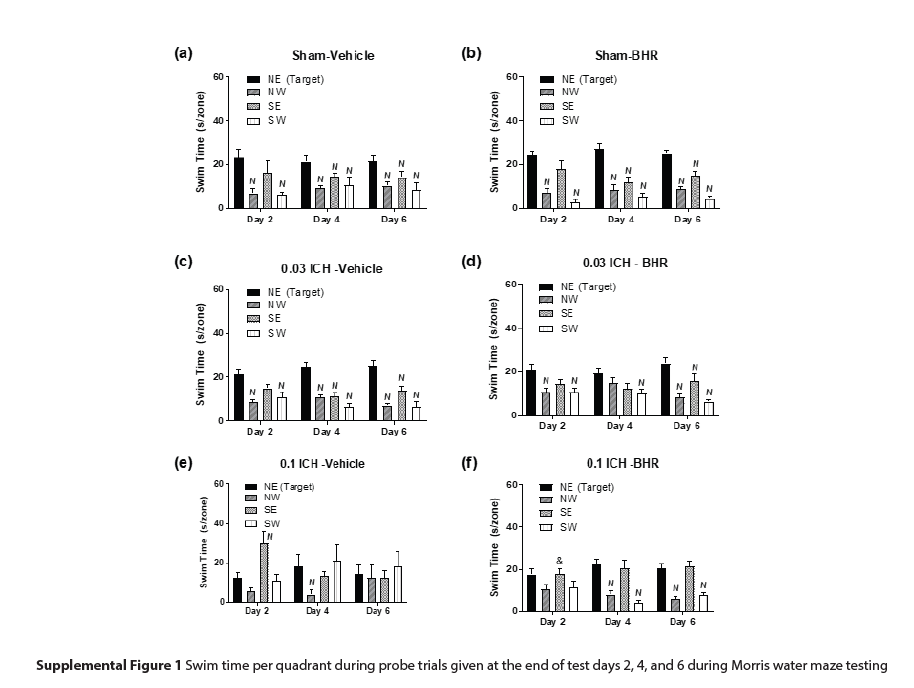
Notably, all groups interacted with the objects for similar amounts of time (Supplemental Table 4); thus, deficiencies in STM and LTM in the 0.1 U collagenase group cannot be attributed to failure to engage with objects. In Morris water maze testing, all groups located hidden platforms across trials (Figure 4 a,c,e). Overall swim distances declined across days (p<0.001). However, the 0.1 U collagenase group swam longer distances overall compared to sham control and 0.03 U collagenase groups (p=0.004). Analysis of probe trials found that overall sham control and 0.03 U collagenase groups swam longer distances in the target quadrant containing the hidden platform (i.e., NE quadrant) than the 0.1 U collagenase group (p≤0.024; Figure 4 b,d,f). No differences were observed between vehicle and BHR-100 treatments in sham and 0.03 U collagenase groups, but swim distances were augmented with progesterone in the 0.1 U collagenase group (p=0.018). Overall swim distances were longer in vehicle-treated sham and 0.03 U collagenase groups than in the vehicle-treated 0.1 U collagenase group (p≤0.021), and no distinctions were found among the three injury groups after BHR-100 administration. Analyses of swim times found comparable results (Supplemental Figure 1).
Supplemental Table 4. Effects of ICH injury and progesterone treatment on duration of time spent with objects in novel object recognition memory test. Mice numbers in each injury condition and treatment are presented in Table 1. Data are presented as means ± standard errors of the mean. Repeated measures analysis of variance for novel object recognition observed only the within-subject main effect of test-phase to be significant [F(2,118)=22.014, p<0.001]. Note: BHR, BHR progesterone; U, unit.
Injury Condition |
Treatment | Training | Short-Term Memory | Long-Term Memory |
|---|---|---|---|---|
| Sham | Vehicle | 38.38 ±4.13 | 51.00 ±6.38 | 63.98 ±10.01 |
| BHR-100 | 42.93 ±3.85 | 56.97 ±9.56 | 52.84 ±4.27 | |
| 0.03 U Collagenase | Vehicle | 32.93 ±3.75 | 43.93 ±5.61 | 52.35 ±6.51 |
| BHR-100 | 35.73 ±4.33 | 49.59 ±6.26 | 48.99 ±4.33 | |
| 0.1 U Collagenase | Vehicle | 35.95 ±5.10 | 48.74 ±7.99 | 52.75 ±11.65 |
| BHR-100 | 36.34 ±4.53 | 51.80 ±5.30 | 50.64 ±7.73 |
Figure 4: High intrastriatal collagenase dose perturbs spatial memory in the Morris water maze
Discussion
This study was designed to assess progesterone effects across an array of neurological tests after different hematoma volumes in a preclinical ICH model. First, different intrastriatal collagenase doses resulted in differential brain hematoma volumes. Since hematoma volume correlates with human ICH outcome, preclinical testing of potential therapeutics should include different hematoma volumes. Second, preclinical ICH injury results in differential effects across a battery of behavioral assessments. For example, ICH injury had negligible effect in open field testing but exerted differential effects on other motor responses and cognition, based on intrastriatal collagenase dosing. Thus, administering a multitude of neurobehavioral tests provides neurobehavioral batteries. Consistent with our prior findings [6,8], progesterone administration after ICH injury marginally improved water maze performance compared to vehicle treatment in the 0.1 U collagenase group but had no effect on other assessments. Finally, diffusion and perfusion imaging did not vary with different collagenase treatments or hematoma volumes, and further work should be conducted to define relevance of these radiographic markers in preclinical testing for development of potential therapeutics.
The failure of potential therapeutic translation is due to many factors [3] but, unfortunately, is rooted in a lack of thorough and rigorous preclinical testing in disease-relevant paradigms. Previous preclinical ICH studies have ignored variability in hematoma volume, despite its strong correlation with human outcomes. Accordingly, testing potential therapeutic responses across a variety of hematoma volumes is a critical consideration in preclinical modeling and seems likely to impact acute ICH clinical trial design. In this context, preclinical findings might inform early phase human trials regarding dosing regimens for patients within distinctive hematoma volume ranges. The present study manipulated hematoma volume by adjusting intrastriatal collagenase injection dose. Hematoma volume, as dictated by collagenase dose, differentially affected several, but not all, behavioral outcomes. For example, larger volumes resulted in worse footfault and water maze performance than smaller ones. Interestingly, though, outcomes for several other behavioral assessments lacked variation between different collagenase doses. These results may suggest that collagenase dosing resulted in insufficiently precise hematoma volumes, negligible differences in hematoma volumes, or that timing of assessments did not capture peak deficits for a given test. Notably, a substantial number of behavioral tests were performed in a brief amount of time in the sub-acute phase of injury and recovery. Thus, future evaluation of behavioral test performance across multiple hematoma volumes at various time-points may be important.
Presently, outcome measures commonly used in preclinical modeling are not recapitulated in clinical trials. Potential therapeutics could be screened across multiple behavioral assessments at specified times to identify maximum efficacy of treatment, which may then inform intermediate and primary outcomes for subsequent clinical trials of potential therapeutics. Interestingly, progesterone may have fallen victim to a lack of concordant outcomes measures from preclinical testing to Phase III trials in acute severe traumatic brain injury [24]. In the current study, progesterone partially ameliorated ICH effects of 0.1 U collagenase in probe trials only in the Morris water maze but improved performance in other neurobehavioral assessments at the time points and different collagenase doses tested. These results may provide proof-of-principle for future experimental designs and lend credence to assessing multiple relevant behavioral outcomes across various hematoma volumes beginning in the preclinical phase of drug development.
To this end, progesterone as a potential neurotherapeutic has undergone extensive preclinical testing, especially in acute brain injury models [25-26]. However, its effects at different hematoma volumes and across different behavioral assessments after preclinical ICH have not previously been tested. Similar to our prior work [8], water maze performance improved with progesterone administration, though in the present study only in mice with greater collagenase dosing (i.e., larger hematoma volume). Interestingly, prior experience with progesterone demonstrated increasing rotarod latency through serial testing after preclinical ICH [6,8,12,24]. However, in the current study, progesterone administration did not improve coordination and balance, as assessed by rotarod, regardless of hematoma volume. Given the multitude of testing in the present study, rotarod performance was measured on only a single day. Thus, rotarod has previously been analyzed similarly to water maze in the present study, attempting to find differences in rate of recovery and recovery trajectories over several days. Experimental paradigms and testing schedules should always be kept in mind. Without complete time course testing for recovery patterns in each assessment, comparing across studies may be difficult to interpret.
Taken together and at face value, the case could be made for testing progesterone in an acute ICH trial for patients with larger hematoma volumes and using longer term cognitive testing. More likely, the converse may be true: progesterone effects are unlikely to improve recovery in patients with small hemorrhage and modest neurological deficits; thus, these patients might be excluded from ICH trials. In addition, longer-term outcome metrics (e.g., 6-month) may be more applicable to ICH therapeutic trials than earlier outcomes (e.g., 30-day). However, considerable ongoing work is required for successful translation, including characterization of relevant pathophysiological biomarkers that are strongly tied to important neurological outcomes in both humans and preclinical models; further definition of optimal dosing regimens, particularly as it pertains to duration; obtaining clearer understanding of gonadal hormone interactions and their immunomodulatory functions after ICH; and defining timing of and relevance of neurobehavioral outcomes to use in clinical trials.
The preliminary nature and limitations of this study should be noted. At present, few experiments utilize assessments under the condition of different hematoma volumes. Further, many of the behavioral tests used in the present study, while thoroughly utilized in other neurological injury models, have not been fully characterized in ICH models. The testing regimen time course was required to accommodate the variety of behavioral assessments; however, this extent of testing and handling may have modified the behavioral phenotype beyond the effects of injury and progesterone treatment alone, despite rigorous methodology. Finally, optimal dosing regimens for progesterone in these conditions have not been defined and results may vary if true dose-finding studies were performed for each. While informative, dose-response studies for each specific behavioral test require sizeable experimental design and are not practical in the current study.
In conclusion, progesterone may continue to emerge as a potential therapeutic for patients suffering acute ICH, but multiple injury-relevant testing paradigms are required. Future studies should seek to refine dosing parameters and define appropriate biomarkers and neurological outcomes for translation into clinical trials.
Funding
Provided by the Duke University School of Medicine Department of Anesthesiology. There are no other financial disclosures from any author.
Competing interests
The authors have no competing interests to report.
Ethical Approval
Duke University is an AAALAC-accredited institution.
Animal Ethics
All experiments were performed within the guidelines as outlined for mice in the National Research Council “Guide for the Care and Use of Laboratory Animals” [14] with an approved protocol from the Duke University Institutional Animal Care and Use Committee.
Availability of supporting data
Supporting data for this manuscript are available upon reasonable request to the corresponding author.
Acknowledgement
The authors would like to thank Mr. Christopher Means for his assistance in testing the mice in the behavioral assays and conducting the pilot studies.
Authors’ contributions
Dr. Lei, Dr. Lascola, Dr. Laskowitz, Dr. Warner, Dr. Wang, Dr. James, Dr. Rodriguez, Dr. Venkatraman, and Dr. Wetsel conceptualized this project; Dr. James acquired funding; Dr. Lascola, Dr. Wang, Dr. Rodriguiz, and Dr. Venkatraman contributed to methodology creation; Dr. Lei, Dr. Wang, Dr. Rodriguiz, and Dr. Venkatraman carried out study investigation procedures, including data collection, under the supervision of Dr. Lascola, Dr. Warner, and Dr. Wetsel. Ms. Covington, Dr. Lei, Dr. Wang, Mr. Lusk, Dr. Rodriguiz, and Dr. Venkatraman curated the data; Dr. Rodruiguiz carried out formal statistical analyses; Ms. Covington and Mr. Lusk transformed the data and results into writing, under the supervision of Dr. James, though all authors contributed to the writing to some extent and reviewed the manuscript.
References
- Fisher M, Feuerstein G, Howells DW et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 40, 2244-2250 (2009).
- Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 4, 279-285 (2013).
- Warner DS, James ML, Laskowitz DT et al. Translational research in acute central nervous system injury: lessons learned and the future. JAMA Neurol. 71, 1311-1318 (2014).
- Broderick JP, Brott TG, Duldner JE et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 24, 987-993 (1993).
- Chen Z, Xi G, Mao Y et al. Effects of progesterone and testosterone on ICH-induced brain injury in rats. Intracerebral Hemorrhage Research. 111, 289-293(2011).
- Hsieh JT, Lei B, Sheng H et al. Sex-Specific Effects of Progesterone on Early Outcome of Intracerebral Hemorrhage. Neuroendocrinol. 103, 518-530 (2016).
- Jiang C, Zuo F, Wang Y et al. Progesterone exerts neuroprotective effects and improves long-term neurologic outcome after intracerebral hemorrhage in middle-aged mice. Neurobiol Aging. 42, 13-24 (2016).
- Lei B, Wang H, Jeong S et al. Progesterone Improves Neurobehavioral Outcome in Models of Intracerebral Hemorrhage. Neuroendocrinol. 103, 665-677 (2016).
- Cutler SM, Cekic M, Miller DM et al. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 24, 1475-1486 (2007).
- Grossman KJ, Goss CW, Stein DG. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain research. 1008, 29-39 (2004).
- Lei B, Mace B, Dawson HN et al. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS One. 9, e103969 (2014).
- Skolnick BE, Maas AI, Narayan RK et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 371, 2467-2476 (2014).
- Wright DW, Yeatts SD, Silbergleit R et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 371, 2457-2466 (2014).
- National Research Council. Guide for the Care and Use of Laboratory Animals. In: Council NR, ed., 8 ed. Washington (DC): National Academies Press (US) (2011).
- Lei B, Sheng H, Wang H et al. Intrastriatal injection of autologous blood or clostridial collagenase as murine models of intracerebral hemorrhage. J Vis Exp. 89, e51439 (2014).
- Pogorelov VM, Rodriguiz RM, Cheng J et al. 5-HT2C Agonists Modulate Schizophrenia-Like Behaviors in Mice. Neuropsychopharmacol. 42, 2163-2177 (2017).
- Kamens HM, Crabbe JC. The parallel rod floor test: a measure of ataxia in mice. Nat Protoc. 2, 277-281 (2007).
- Ribar TJ, Rodriguiz RM, Khiroug L et al. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J Neurosci. 20, RC107 (2000).
- Taylor GA, Rodriguiz RM, Greene RI et al. Behavioral characterization of P311 knockout mice. Genes Brain Behav. 7, 786-795 (2008).
- James ML, Sullivan PM, Lascola CD et al. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 40, 632-639 (2009).
- James ML, Wang H, Venkatraman T et al. Brain natriuretic peptide improves long-term functional recovery after acute CNS injury in mice. J Neurotrauma. 27, 217-228 (2009).
- Lei B, Mace B, Bellows ST et al. Interaction between Sex and Apolipoprotein E Genetic Background in a Murine Model of Intracerebral Hemorrhage. Transl Stroke Res. 3, 94-101 (2012).
- Sheng SP, Lei B, James ML et al. Xenon neuroprotection in experimental stroke: interactions with hypothermia and intracerebral hemorrhage. Anesthesiol. 117, 1262-1275 (2012).
- Stein DG, Sayeed I. Repurposing and repositioning neurosteroids in the treatment of traumatic brain injury: A report from the trenches. Neuropharmacol. 147, 66-73 (2008).
- De Nicola AF, Garay LI, Meyer M et al. Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J Neuroendocrinol. 30, e12502 (2018).
- Guennoun R, Zhu X, Fréchou M et al. Steroids in Stroke with Special Reference to Progesterone. Cell Mol Neurobiol. 39, 551-568 (2018).
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref