Research Article - Diabetes Management (2017) Volume 7, Issue 5
Effects of the dietary approaches to stop hypertension (DASH) diet on glucose variability in youth with Type 1 diabetes
- *Corresponding Author:
- Abigail D. Peairs
Department of Rehabilitation Exercise, and Nutritional Sciences
University of Cincinnati, USA
E-mail: peairsal@ucmail.uc.edu
Abstract
Objective: Glucose variability (GV) independently increases risk for vascular events in patients with diabetes. The Dietary Approaches to Stop Hypertension (DASH) dietary pattern emphasizes fruits, vegetables, whole grains, lean meats, and low fat dairy and has the potential to reduce postprandial blood glucose (BG) excursions, however, its effect on GV is not known. The purpose of this work was to assess feasibility and collect preliminary data on the efficacy of the DASH diet on GV in adolescents with type 1 diabetes (T1D). Methods: Twenty one adolescents recruited from the Diabetes Center of Cincinnati Children’s Hospital Medical Center with T1D (11-17y) participated in one of two phases of a controlled feeding study. The first phase tested the acceptability and blood glucose response to a traditional DASH diet (DASH) and the second phase tested a DASH diet specifically modified for diabetes (DASH-D) to improve glucose response to meals. For each phase, participants consumed first their usual diet, and then a controlled DASH diet while wearing continuous glucose monitoring (CGM) systems for 3 days of each diet. All foods were provided to the patients during the DASH dietary periods and 24 h dietary recalls were conducted during the usual diet periods to assess daily intake. Results: Sixteen participants (14.1 +/- 2.2y) were included in final analyses (DASH n=7, DASH-D n=9). Both DASH diets were significantly higher in fruits, vegetables, fiber, vitamin A, and % energy from protein than usual intakes. DASH was higher in carbohydrate (CHO) (60 vs. 50%) and lower in fat (21 vs. 36%) than usual intake, resulting in higher GV (Standard Deviation and Lability Index) and more low BG excursions (3 ± 2.8 vs. 7.1 ± 3.3, p=0.024). DASH-D was modified to better match CHO and fat content of patients’ usual intakes in phase 1 (50/30/20 for CHO/fat/pro respectively, which resulted in no difference in GV between DASH-D and usual intake. There were also trends for lower average BG (144.1 vs. 168.9, p=0.072) and less percentage of time spent in the hyperglycemic range (39.3 ± 25.5 vs. 54.1 ± 19.4, p=0.07) on DASH-D compared to usual intake. Conclusion: The DASH dietary pattern tended to result in less hyperglycemia and an overall lower BG compared to usual care. Modifying a traditional DASH diet by increasing heart healthy fats improves glycemic response to DASH and may bebeneficial for long term cardiovascular benefits in youth with T1D.
Keywords
insulin, nutrition, hyperglycemia, type 1 diabetes, blood sugar
Introduction
Adolescents with type 1 diabetes (T1D) frequently have suboptimal glycemic control, which deteriorates with increasing duration of the disease [1]. Large-scale epidemiologic and clinical data show average hemoglobin A1c (HbA1c) values between 8.5 and 9.0% [1- 3] while the American Diabetes Association’s practice guidelines recommend an A1c target of 7.5% or lower for adolescents with T1D [4]. While the HbA1c value is the primary indicator of diabetes control, glycemic variability (GV), or the degree and extent of postprandial surges and between meal drops in BG, also imparts risk. Notably, GV is associated with diabetic complications, such as coronary artery disease, retinopathy, and nephropathy [5,6]. It has even been suggested that the root of macro and micro-vascular complications in diabetes is oxidative stress induced by glucose variability [7]. In addition, poor glycemic control in T1D may lead to adverse cardiovascular consequences by chronic low grade inflammation associated with recurrent hyperglycemia [8].
Evidence-based recommendations from the American Diabetes Association (ADA) for youth with T1D incorporate specific needs for glycemic control and management of lipids, blood pressure and weight, along with nutrition needs to support healthful growth and development. Thus, guidelines on medical nutrition therapy (MNT) in T1D advocate a diet that includes carbohydrate (CHO) from fruits, vegetables, whole grains, legumes and low fat milk and is low in saturated fat (SFA) and cholesterol [9]. These nutritional guidelines closely align with the tenets of the Dietary Approaches to Stop Hypertension (DASH) diet which emphasizes the same food groups and promotes heart health, ideal for any group at increased risk for cardiovascular disease.
The DASH diet is an eating plan that has been shown to reduce BP and presence of metabolic syndrome in other populations of youth at risk for future CVD (hypertensive and metabolic syndrome) [10,11]. It is likely that the DASH diet would also be beneficial for patients with diabetes. In fact, epidemiological data from the SEARCH for Diabetes in Youth Study show that among adolescents with T1D, those whose diets were closer to a DASH dietary pattern had lower odds of hypertension, better lipid profiles, and better glucose control than those diets less similar to DASH [12,13]. Further, a longitudinal analysis showed that an increase in DASH diet score was related to a decrease in HbA1c and blood pressure in youth with diabetes [14]. These studies indicate that the DASH diet may be beneficial as an approach to manage blood sugar as well as preventing longer term complications associated with diabetes.
Reducing GV, which primarily reflects large swings in BG in the postprandial (after meal) state, has been associated with better glucose control overall as measured by HbA1c levels in T1D [15]. While many factors can influence GV, diet composition plays a key role in postprandial glucose surges as some dietary factors increase (e.g. sugars) while some reduce (e.g. fiber) the rate of glucose release into the bloodstream. The DASH diet contains CHO sources rich in fiber, fructose, and lactose, which tend to be lower “glycemic index” (GI) foods (the ability of a food to raise blood sugar levels). The DASH diet has the potential to reduce postprandial glucose responses and overall GV, and could thus prove useful as an eating plan for patients with diabetes. While several meta-analyses have reported that consuming diets with a lower GI may help to reduce postprandial hyperglycemia and improve glycemic control in patients with diabetes [16-18], the effects of the DASH diet on BG excursions in patients with diabetes are not known. Since the GV response to the DASH diet may influence the adherence and usefulness of the diet for youth with T1D, these short term controlled feeding studies were conducted to determine feasibility and acceptability of the diet and to collect preliminary data on the efficacy of the DASH diet on GV in adolescents with type 1 diabetes.
Methods
▪ Subjects
Adolescents (aged 11-17 years) with T1D on continuous insulin infusion (CSII) therapy were recruited from the Diabetes Center at Cincinnati Children’s Hospital Medical Center (CCHMC) by direct mailings and follow-up phone calls. Patients were included if they were diagnosed with T1D for at least one year, on CSII for at least 6 months, taking insulin of at least 0.5 units/ kg/d, and had HbA1c level between 7-11%, and a body mass index <95th percentile. Patients were excluded for lactose intolerance, refusal to eat fruits and/or vegetables, extensive food allergies, taking medications that could impact appetite or BG, or having any other health condition known to influence BG levels or that required dietary restriction or modification. Based on these criteria, ten patients were enrolled in the first phase (DASH), and then eleven were enrolled in the second (DASH-D) phase. Written parental consent and child assent were obtained prior to any study procedures. Following consent, patients were further screened by checking current HbA1c levels and body weight and were excluded if HbA1c was >11% or BMI was >95th percentile.
▪ Study design
We conducted two feeding studies sequentially in adolescents with T1D. The first (DASH) compared the glucose response of a traditional DASH diet to patients’ usual diets (Table 2).
Then, adjustments were made to the DASH diet to improve glucose control (DASH-D), new patients were recruited, and the same procedures were performed as in DASH and glucose response to DASH-D was compared to usual diets. All procedures took place in the Clinical Translational Research Center (CTRC) at CCHMC. For each study, patients visited the CTRC five times. The first visit was for consent, screening, and education on participating in 24 h food recalls. Then patients wore continuous glucose monitors (CGM) for three days while consuming first their usual diet and then for three days while consuming a controlled DASH diet thereby serving as their own control. Usual dietary intake was based on the patients’ use of the typical CHO counting approach to diabetes management and assessed by daily 24h dietary recalls. During the controlled DASH diet periods, all DASH diet foods were provided to patients to ensure that the diet was properly followed and standardized across patients. Patients followed the prescribed diet for 2-3 days prior to having the CGM inserted to help familiarize them with the foods and the protocol that they were to follow while being monitored. The usual dietary periods were always monitored first, to prevent the controlled diets from influencing patients’ self-selected “usual” intake. The DASH diet foods were then provided to the patients the following week (or later).
▪ Laboratory studies
A fingerstick was used to obtain a small amount of blood during the initial visit for immediate laboratory analysis of Hemoglobin A1c for screening purposes using a DCA analyzer (Siemens Analyzers DCA Vantage).
▪ Height/weight
Weight was determined with light clothing and without shoes, at each visit using a Scaletronix 5002 scale (Welch-Allyn, Skaneateles Falls, NY) and height was measured during the first visit using a Harpenden wall-mount stadiometer (Holtain Ltd, Crymych, UK) in the CTRC by a trained member of the CTRC staff.
▪ Usual dietary intake assessment
Dietary intake during the usual intake periods was assessed via dietary recall interviews conducted by the CTRC bionutrition staff using the USDA multiple-pass method [19,20]. The first recall interview was done in-person at the time of the initial study visit and the following 3 dietary recalls were done by telephone during the usual diet CGM monitoring period with participants using 2-dimensional portion model handouts to assist them with estimating portion sizes. The 24-hour dietary recall multiple-pass interview method of estimating dietary intake has been validated in children against doubly-labeled water [21] and weighed diet diaries [22]. Dietary intake data collected during telephone-administered dietary recall multiplepass interviews have been shown to be similar to data collected in-person [23,24]. Dietary recall interviews were analyzed using Nutrition Data System for Research software (NDSR, Nutrition Coordinating Center, Minneapolis, MN).
▪ DASH diets and controlled feeding
The traditional DASH meal plans (phase 1) were developed based on the American Diabetes Association medical nutrition therapy (MNT) guidelines [25,26] for diabetes and the DASH for Teens Diet Manual [10]. In an attempt to improve glucose control, we then developed the DASH for Diabetes Diet (DASH-D) for the second phase by increasing the percentage of energy from fat on the DASH diet from 20% up to 30% and utilizing a macronutrient distribution of 50% of calories from CHO/30% of calories from fat/20% of calories from protein, which still complies with the American Diabetes Association guidelines for diabetic meal planning [26,27] but more closely matched the macronutrient distribution of patients’ usual diets. All DASH and DASH-D meals were prepared in the CTRC Metabolic Research Kitchen by CTRC bionutrition staff. Participant menu preferences and energy (kcal) needs (to ensure weight maintenance) were used to calculate the appropriate nutrient and food group distribution in concordance with DASH recommendations. All foods were weighed for accurate portion size determination and five to six days of meals were provided to each participant along with CHO counts for foods/meals to ensure consistent insulin dosing. CHO counts were determined using ESHA (DASH) and NDSR (DASH-D) software programs and provided to the participants. For meals containing >5 g of fiber, the grams of fiber were removed from the total CHO count and patients were provided with the “available carbohydrates” for foods on which to base their insulin dosing before meals in an effort to prevent “over” provision of pre-meal insulin. Participants recorded the time in which they consumed each food item on the provided check-sheets and all food containers with any foods not consumed were returned along with the intake check-sheets to assess diet compliance. Dietary non-compliance was considered if a patient consumed additional foods outside of the provided DASH foods, except in the case where a reasonable addition was needed to correct a low BG reading.
▪ Blood Glucose (BG) monitoring
Continuous glucose monitoring (CGM) systems: iPro and iPro2 systems (Medtronic) were used in these studies. The transmitter in these FDAapproved devices continuously records readings from an interstitial sensor every 10 seconds and averages these values into 5 minute intervals. Patients wore CGMs while consuming 3 days of each diet. On the first day, the sensor was inserted by a trained nurse and patients were instructed to perform at least 4 self-BG checks each day before meals in order to retroactively calibrate the CGM. Also, as glucose readings were not visible on the CGM, participants continued their prescribed method of monitoring and treating BG during the studies.
▪Measures of glycemic variability and postprandial glucose excursions
Daily glucose variation values used in these studies include the standard deviation (SD), mean amplitude of glucose excursions (MAGE), percentage of time spent within, above (>140) and below (<70) the normal glucose range, and mean of daily differences (MODD), which are all well recognized measures of glycemic variability [28]. As this was an exploratory analysis, the lability index (LI; an average of how much BG values vary over a given time period) was also included. To standardize the time frame in which patients were analyzed for the glycemic variables calculated over more than one day, the 48 hours in the middle of the 3 day monitoring period was used for all patients. The period included was 6 a.m. to 6 a.m., 48 hours later. Blood glucose excursions (hyper- or hypoglycemic episodes) were assessed across the entire monitoring period (approximately 72 h). Additional measures of glycemic control included BG levels at specific time points postprandial (every 30 minutes up to 4 h), the peak postprandial BG achieved, and time to reach peak BG. These postprandial assessments were averaged for each participant for each dietary period.
▪ Statistics
Differences between diets (DASH vs. usual care) within each phase were calculated using paired t-tests, with significance set at p<0.05. The characteristics of participants in DASH vs. DASH-D studies were compared by independent t-test. Data were analyzed using SPSS v20.
Results
From the original 21 patients that participated in these studies, data from three patients were excluded from DASH and two from DASH-D due to CGM issues, (non-recording of data (n=1)), insulin pump malfunction (n=1), illness (fever, stomach virus, n=2), and dietary non-compliance (n=1).
Characteristics of the participants included were similar for each study, except that BMI was slightly lower in the DASH1 vs. DASH-D study (Table 1). Overall, participants were highly compliant and the DASH diets were well tolerated. For example, in DASH-D, participants’ energy intake was within 5% of the prescribed diet and for those patients who needed to consume extraneous foods/beverages while on the DASH-D diet for treatment of low blood sugar (n=7), the average was only 77 kcals/day. Overall, patients consumed the vast majority (87-98%) of food provided to them on DASH and DASH-D respectively (data reported elsewhere).
The plant based DASH diets both resulted in higher fruit and vegetable intake, and therefore higher fiber, vitamin A, and vitamin E and lower saturated fat intake than usual diets (Table 2). Of note, while both DASH diets were slightly higher in percentage of protein, DASH also had a higher percentage of energy from CHO than participants’ usual intake and was lower in fat (Table 2). As this difference in macronutrient distribution may influence GV, it was corrected in DASH-D to achieve a macronutrient composition more in line with usual intake by significantly increasing levels of unsaturated fats including nuts/seeds in the diet. Patients consuming DASH-D also had higher magnesium, potassium, and vitamin C and lower sodium intake compared to their usual diets.
| Age Mean ± SD |
Gender (M,F) | Height (cm) | Weight (kg) | BMI % | HbA1c | |
|---|---|---|---|---|---|---|
| Combined | 14.1 ± 2.2 | 6,10 | 165.0 ± 8.5 | 59.5 ± 13.4 | 64.8 ± 26.7 | 8.8 ± 0.8 |
| DASH phase | 14.0 ± 2.4 | 3, 4 | 162.1 ± 7.9 | 52.74 ± 13.4 | 8.0 ± 24.4 | 9.0 ± 1.0 |
| DASH-D phase | 14.2 ± 2.1 | 3, 6 | 167.2 ± 8.0 | 64.8 ± 11.5 | 76.0 ± 22.9 | 8.7 ± 0.6 |
Table 1. Demographics of participants in the two phases. SD = standard deviation, HbA1c = hemoglobin A1C, BMI = body mass index percentile, DASH phase = traditional DASH diet, DASH-D phase = DASH modified for Diabetes phase.
| Usual | DASH | p-value | Usual | DASH-D | p-value | |
|---|---|---|---|---|---|---|
| Energy (kcals) | 2273 | 1956 | 0.118 | 2142 | 1973 | 0.579 |
| Carbohydrate grams | 283 | 297 | 0.558 | 276 | 255 | 0.531 |
| Carbohydrate as % of energy | 50 | 60 | 0.003* | 52 | 52 | 0.998 |
| Fat grams | 91 | 46 | 0.003* | 87 | 68 | 0.235 |
| Fat as % of energy | 36 | 21 | 0.000* | 36 | 31 | 0.08 |
| Protein grams | 87 | 100 | 0.115 | 77 | 104 | 0.064 |
| Protein as % of energy | 15 | 21 | 0.001* | 15 | 21 | <0.001* |
| Monounsaturated fat grams | 31.8 | 13.3 | 0.001* | 28.7 | 28.9 | 0.85 |
| Polyunsaturated fat grams | 19.5 | 7.5 | 0.024* | 19.6 | 15.1 | 0.269 |
| Saturated fat grams | 39.7 | 25.1 | 0.01* | 39.6 | 24.1 | 0.03* |
| Fiber grams | 20.3 | 29.1 | 0.035* | 15.4 | 29.8 | 0.002* |
| Micronutrients (mg/1000kcals) | ||||||
| Total Calcium (mg) | 537.0 | 651.3 | 0.051 | 558.6 | 672.9 | 0.213 |
| Total Potassium (mg) | 1105.4 | 1186.4 | 0.442 | 1091.3 | 1622.5 | 0.002* |
| Total Sodium (mg) | 1606.5 | 1668.6 | 0.586 | 1558.0 | 1311.7 | 0.008* |
| Total Magnesium (mg) | 130.9 | 114.7 | 0.207 | 131.9 | 214.4 | <0.001* |
| Total Vitamin A (IU) | 3212.6 | 8430.5 | 0.001* | 2973.6 | 6990.9 | 0.003* |
| Total Vitamin C (mg) | 48.7 | 47.8 | 0.96 | 25.5 | 45.9 | 0.02* |
| Total Vitamin E (mg) | 4.1 | 2.4 | 0.034* | 3.8 | 7.1 | 0.003* |
Table 2. Nutrient intake comparisons for each phase of the study. Each DASH diet was compared to the corresponding usual dietary intake for patients during each phase. DASH= traditional DASH diet, DASH-D= DASH for Diabetes Diet, Usual = patients’ self-selected dietary intake. Data were analyzed by paired t-tests. *p<0.05 considered significant difference between the DASH (or DASH-D) diet and the usual diet in the same phase.
▪ Glycemic variability measures
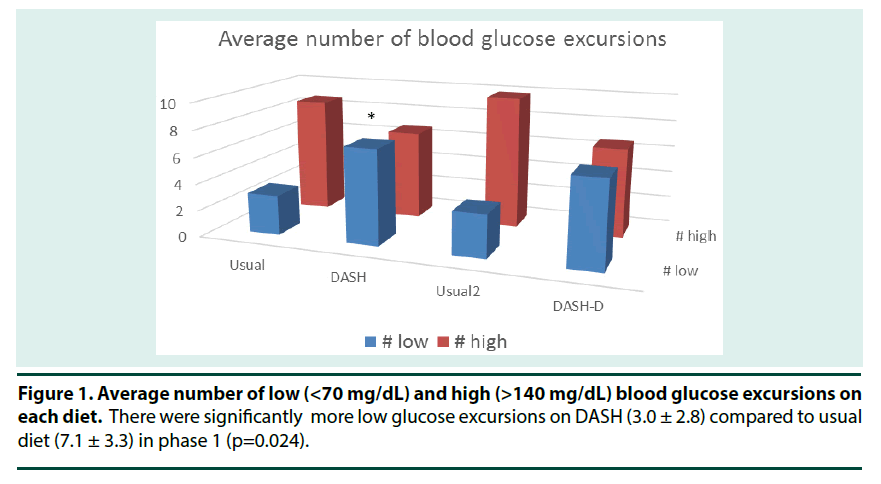
Since there is no gold standard for reporting glycemic variability, and this was a preliminary analysis, we included several measures of GV to explore which would be most responsive to dietary change. Two measures of glycemic variability (Standard Deviation, SD and lability index, LI) were significantly higher on DASH compared to when participants consumed their usual diet (Table 3). This was due to participants having more low glucose excursions (p=0.024) and tending to spend more time with BG <70 mg/dL (p=0.065) while on DASH compared to their usual diets (Figures 1 & 2). Although there was also 20% less time spent in the hyperglycemic range on DASH compared to usual diet, this difference was not statistically significant (p=0.136).
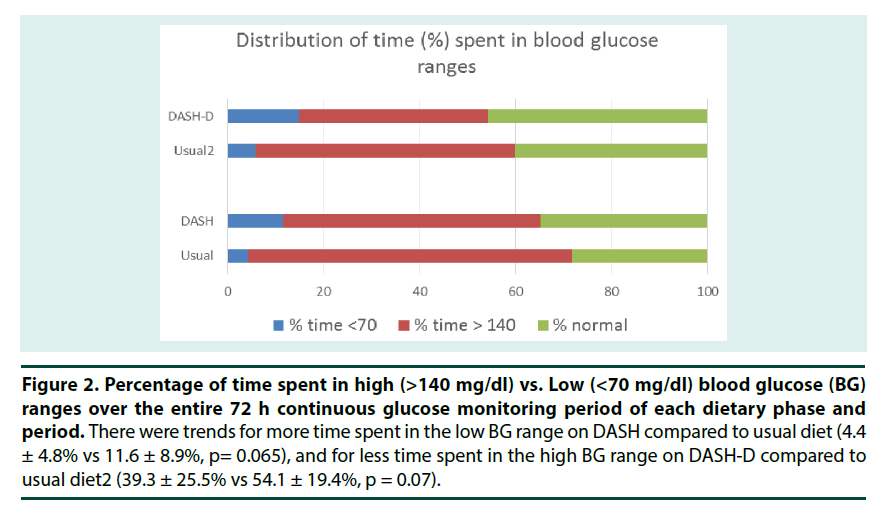
Figure 2. Percentage of time spent in high (>140 mg/dl) vs. Low (<70 mg/dl) blood glucose (BG) ranges over the entire 72 h continuous glucose monitoring period of each dietary phase and period. There were trends for more time spent in the low BG range on DASH compared to usual diet (4.4 ± 4.8% vs 11.6 ± 8.9%, p= 0.065), and for less time spent in the high BG range on DASH-D compared to usual diet2 (39.3 ± 25.5% vs 54.1 ± 19.4%, p = 0.07).
While there was a trend (p=0.072) for participants to have a lower overall BG level (23% lower) when consuming DASH-D compared to their usual diets, the measures of glycemic variability did not differ (Table 3). Again, participants tended to spend less time in the hyperglycemic range (30%) while on DASH-D (p=0.07) compared to usual diet (Figure 2). There was no difference in average peak BG achieved during the 4 hour postprandial period between DASH and usual diet, and the only time point that was different during this period was at 180 minutes postprandial where patients had lower BG on DASH than their usual diet. Likewise, in phase 2 when patients consumed DASH-D, the average postprandial BG and peak BG were similar to the usual diet except at 120 minutes postprandial where they had lower BG on DASH-D than the usual diet.
| Diet | Mean | Stdev | Conga | LI | MODD | MAGE |
|---|---|---|---|---|---|---|
| Usual | 180.1 | 73.0 | 173.0 | 55.5 | 87.1 | 124.2 |
| DASH | 173.7 | 86.2 | 170.5 | 73.3 | 106.8 | 143.7 |
| p-value | 0.598 | 0.039* | 0.831 | 0.044* | 0.113 | 0.574 |
| Diet | Mean | Stdev | Conga | LI | MODD | MAGE |
| Usual | 168.86 | 70.158 | 162.78 | 58.7 | 75.22 | -1885 |
| DASH-D | 144.11 | 61.354 | 140.11 | 41.5 | 67.394 | -1925.5 |
| p-value | 0.072 | 0.454 | 0.125 | 0.302 | 0.525 | 0.990 |
Table 3. Glycemic variability measures obtained by continuous glucose monitoring. SD=the standard deviation, MAGE=mean amplitude of glucose excursions, MODD=mean of daily differences, LI=lability index. *p<0.05 considered significant. To standardize the time frame in which patients were analyzed for the glycemic variables calculated over more than one day, the 48 hours in the middle of the 3 day monitoring period was used for all patients. The period included was 6 a.m. to 6 a.m., 48 hours later. Glucose variability was higher on DASH than usual diet in phase 1 according to SD and LI measures. The average BG tended to be lower (p=0.072) on DASH-D than usual diet during phase 2.
Discussion
Hyperglycemia and increased glycemic variability have been determined to be risk factors of adverse cardiovascular events in TID [29]. The present study is the first to directly evaluate the effect of a DASH dietary pattern on blood glucose control and glycemic variability in adolescents with T1D. In this controlled feeding study, we showed that adherence to a traditional DASH diet tended to lower blood glucose levels more than usual intake in adolescents with T1D. With the addition of healthy fats at the expense of CHO, we showed that glycemic variability was also lowered compared to the traditional DASH diet. As cardiovascular events are more common and occur earlier in patients with T1D [30,31] and atherosclerosis and vascular dysfunction begin in childhood, it is imperative to find strategies to reduce CV risk factors before complications develop. Based on findings from the present investigation, a DASH dietary pattern may improve glycemic control and thereby lower CVD risk in an at risk population.
The first DASH diet we tested was directly in line with the food serving recommendations of the traditional DASH diet e.g., high in fruits, vegetables and low fat dairy foods, moderate in protein, and relatively low in fat. This resulted in a macronutrient distribution that was much different than patients’ usual intake and the metabolic response to this diet in youth with T1D was an increase in SD, which is considered one of the most useful measures of GV [32]. Given that oscillations in blood glucose correlate strongly with oxidative stress and endothelial dysfunction [33] and the SD over a period of time reflects this occurrence, an increase in SD was not considered a favorable response. Therefore, the traditional DASH diet was reformulated to create the DASH for Diabetes Diet (DASH-D). DASH-D was closer in macronutrient distribution to patients’ usual diets yet still compliant with the ADA guidelines for diabetic meal planning. The amounts of mono- and polyunsaturated fats were increased, and not saturated fats, in order to maintain the heart-healthy characteristics of the DASH diet. DASH-D was acceptable to patients (even greater compliance) and no difference was found in measures of GV between DASH-D and usual care. Further, when compared across trials, DASH-D resulted in significantly less GV than the traditional DASH diet suggesting that DASH-D may be cardioprotective in adolescents with T1D.
The DASH diets were very different in composition than the usual dietary intake of adolescents in our study, which was not surprising. While medical nutrition therapy for T1D advocates a diet that includes CHO from fruits, vegetables, whole grains, legumes and low fat milk and is low in saturated fat and cholesterol [25] dietary intake data from the large epidemiological SEARCH for Diabetes in Youth Study paints a very different picture. In this study, it was shown that fewer than 20% of youth with diabetes met ADA recommendations for fruit, vegetable and whole grain consumption. In addition, over 90% were above recommended saturated fat goals and less than 10% met the fiber recommended levels [34,35] Since high saturated fat is associated with suboptimal HbA1c and therefore CVD risk [29-36], the identification of dietary patterns that acutely improve BG responses and have long term favorable effects on cardiovascular health in youth with T1D is clearly warranted. While originally intended and now well accepted for reducing HTN and CVD risk [37] the DASH diet may also be beneficial as an approach to help adolescents increase compliance with ADA nutrition recommendations, improve glucose control, and prevent longer term CV complications associated with T1D.
Based on the frequency of hypoglycemic episodes when consuming the DASH diet, small adjustments in insulin may be necessary in youth with T1D when following this diet. Diets high in complex CHO with fiber from fruits, vegetables, and whole grains appear to lower BG, likely because only half (at most) of dietary fiber is metabolized to glucose [38]. Thus, high fiber meals raise BG to a lower extent and may therefore require less insulin to promote clearance. Similar to our results, a previous study in youth with T1D consuming low glycemic meals (higher in fiber and similar in composition to the DASH diet) reported a greater frequency of mild hypoglycemia [39]. Further, a short term continuous glucose monitoring study of adolescents with T1D recently showed that out of all dietary components, soluble fiber and protein were positively associated with hypoglycemic events [40]. This could be problematic for dietary adherence in this population as fear of hypoglycemia is prevalent and reports indicate that many patients with T1D would sacrifice optimal glucose control in order to avoid feeling the negative effects of low blood sugar [41,42]. Thus, although current CHO counting guidelines for adolescents with T1D are without regard to the “type” of CHO consumed and do not recommend any adjustment for fiber, it appears prudent for practitioners to monitor BG in patients making dietary changes that involve including more fruits, vegetables, and whole grains as adjustments to their insulin dosing regimen may be necessary. The addition of unsaturated dietary fat in exchange for carbohydrate in DASH-D remediated the frequency of mild hypoglycemic episodes to some extent, although there may be further room for improvement.
Our data support the fact that DASH diets can be effective in lowering blood glucose in adolescents with T1D, although our studies also indicate that BG monitoring is advised and individualized adjustments to insulin dosing may be necessary in order to prevent episodes of mild hypoglycemia. While important, the DASH diet has other advantages for cardiovascular health in diabetes in addition to its effects on blood glucose. It is prescriptive in nature and straightforward to implement with daily food group goals. Clinicbased approaches to educating adolescents with T1D to follow DASH-D on their own and best strategies for insulin dosing adjustments when consuming a diet naturally rich in fiber and low in added sugars are warranted.
▪ Strengths
We utilized a controlled feeding study design and by providing all of the DASH foods for patients, we were able to optimize compliance to the diet in a free living situation. Also, using the continuous glucose monitoring systems allowed us to obtain a broader view of the BG responses to the diets. We were able to gather both short term and long term (over the 72 h) postprandial data, which enabled us to observe the carryover effects of the diet on BG during the overnight fasting hours.
▪ Limitations
These were small pilot studies with relatively few subjects that were not powered to detect small differences between treatments or differences by age and sex. We acknowledge that having a small sample size may have resulted in a wider than usual variability in responses to the diet. We also did not interfere with the patients’ usual methods of treating low BG events. Perhaps, we could have required only “DASH” appropriate foods/drinks for treatment of hypoglycemia, however, this was not feasible with this population in the at home setting. As patients were free living, we were unable to monitor or completely control their intake. However, overall, patients were very compliant and reported enjoying the experience of having foods provided to them and learning new ways to incorporate fruits and vegetables into the diet.
Conclusions
The modified DASH diet was well received by adolescents with T1D and appears to lower overall BG and minimize high BG excursions, which may have long term cardiovascular benefits. These preliminary results are promising and provide valuable information regarding the usefulness of DASH for adolescents with T1D. However, further research is needed to investigate the impact of specific dietary components on blood glucose in order to reduce the potential for low glucose excursions.
Acknowledgements
The authors would like to acknowledge Meghan McNeill and the rest of the bionutrition staff, the CTRC nursing staff, and the patients and their families for participating in our studies, and Medtronic for providing Continuous Glucose Monitors and materials for these studies.
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Petitti DB, Klingensmith GJ, Bell RA et al. Glycemic control in youth with diabetes: The SEARCH for diabetes in youth study. J. Pediatr. 155(5),668–672 (2009).
- Svoren BM, Volkening LK, Butler DA et al. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J. Pediatr. 150(3),279–285 (2007).
- Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. the hvidore study group on childhood diabetes. Diabetes. Care. 20(5) 714–720 (1997).
- Silverstein J, Klingensmith G, Copeland K et al. Care of children and adolescents with type 1 diabetes: A statement of the american diabetes association. Diabetes. Care. 28(1),186–212 (2005).
- Prince CT, Becker DJ, Costacou T et al. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: Findings from the pittsburgh epidemiology of diabetes complications study (EDC). Diabetologia. 50(11),2280–2288 (2007).
- Kilpatrick ES, Rigby AS, Atkin SL et al. A1C variability and the risk of microvascular complications in type 1 diabetes: Data from the diabetes control and complications trial. Diabetes. Care. 31(11),2198–2202 (2008).
- Brownlee M, Hirsch IB. Glycemic variability: A hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 295(14),1707–1708 (2006).
- Rosa JS, Flores RL, Oliver SR et al. Resting and exercise-induced IL-6 levels in children with type 1 diabetes reflect hyperglycemic profiles during the previous 3 days. J. Appl. Physiol. 108(2),334–342 (2010).
- Bantle JP, Wylie-Rosett J, Albright AL et al. Nutrition recommendations and interventions for diabetes: A position statement of the american diabetes association. Diabetes. Care. 31 (1),61–78 (2008).
- Couch SC, Saelens BE, Levin L et al. The efficacy of a clinic-based behavioral nutrition intervention emphasizing a DASH-type diet for adolescents with elevated blood pressure. J. Pediatr. 152 (4),494–501(2008).
- Saneei P, Hashemipour M, Kelishadi R et al. Effects of recommendations to follow the dietary approaches to stop hypertension (DASH) diet v. usual dietary advice on childhood metabolic syndrome: A randomised cross-over clinical trial. Br. J. Nutr. 110(12),2250–2259 (2013).
- Gunther AL, Liese AD, Bell RA et al. Association between the dietary approaches to hypertension diet and hypertension in youth with diabetes mellitus. Hypertension. 53(1),6–12 (2009).
- Liese AD, Bortsov A, Gunther AL et al. Association of DASH diet with cardiovascular risk factors in youth with diabetes mellitus: The SEARCH for diabetes in youth study. Circulation. 123(13),1410–1417 (2011).
- Barnes TL, Crandell JL, Bell RA et al. Change in DASH diet score and cardiovascular risk factors in youth with type 1 and type 2 diabetes mellitus: The SEARCH for diabetes in youth study. Nutr. Diabetes. 14(9), (2013).
- Heptulla RA, Allen HF, Gross TM et al. Continuous glucose monitoring in children with type 1 diabetes: Before and after insulin pump therapy. Pediatr. Diabetes. 5(1),10–15 (2004)
- Greenwood DC, Threapleton DE, Evans CE et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose-response meta-analysis of prospective studies. Diabetes. Care. 36(12),4166–4171(2013).
- Brand-Miller J, Hayne S, Petocz P et al. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes. Care. 26(8),2261–2267 (2003).
- Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br. J. Nutr. 104(6),797–802 (2010)
- Blanton CA, Moshfegh AJ, Baer DJ et al. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J. Nutr. 136(10),2594–2599 (2006).
- Moshfegh AJ, Rhodes DG, Baer DJ et al. The US department of agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 88(2),324-332 (2008).
- Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J. Am. Diet. Assoc. 96(11),1140–1144 (1996).
- Klesges RC, Klesges LM, Brown G et al. Validation of the 24-hour dietary recall in preschool children. J. Am. Diet. Assoc. 87(10),1383–1385 (1987).
- Brustad M, Skeie G, Braaten T et al. Comparison of telephone vs face-to-face interviews in the assessment of dietary intake by the 24 h recall EPIC SOFT program--the norwegian calibration study. Eur. J. Clin. Nutr. 57(1),107–113 (2003).
- Tran KM, Johnson RK, Soultanakis RP et al. In-person vs telephone-administered multiple-pass 24-hour recalls in women: Validation with doubly labeled water. J. Am. Diet. Assoc. 100(7),777–783 (2000).
- Bantle JP, Wylie-Rosett J, Albright AL et al. Nutrition recommendations and interventions for diabetes: A position statement of the american diabetes association. Diabetes. Care. 31(1),61–78 (2008).
- Franz MJ, Bantle JP, Beebe CA et al. Nutrition principles and recommendations in diabetes. Diabetes. Care. 27(1),36–46 (2004).
- Holler HJ. Understanding the use of the exchange lists for meal planning in diabetes management. Diabetes. Educ. 17(6),474–484 (1991).
- Rausch JR. Measures of glycemic variability and links with psychological functioning. Curr Diab Rep. 10(6),415-421 (2010).
- Diabetes Control and Complications Trial (DCCT)-Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Nathan DM, Bebu I, et al. Risk factors for cardiovascular disease in type 1 diabetes. Diabetes. 2016.
- Ferranti SD, Boer IH, Fonseca V et al. Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the american heart association and american diabetes association. Diabetes. Care. 37(10),2843–2863 (2014).
- Soedamah-Muthu SS, Fuller JH, Mulnier HE et al. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: A cohort study using the general practice research database. Diabetes. Care. 29(4),798–804 (2006).
- Rodbard D. Interpretation of continuous glucose monitoring data: Glycemic variability and quality of glycemic control. Diabetes. Technol. Ther. 11(1),55–67 (2009).
- Ceriello A, Esposito K, Piconi L et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 57(5),1349–1354 (2008).
- Mayer-Davis EJ, Nichols M, Liese AD et al. Dietary intake among youth with diabetes: The SEARCH for diabetes in youth study. J. Am. Diet. Assoc. 106(5),689–697 (2006).
- Bortsov AV, Liese AD, Bell RA et al. Sugar-sweetened and diet beverage consumption is associated with cardiovascular risk factor profile in youth with type 1 diabetes. Acta. Diabetol. 48(4),275–282 (2011).
- Delahanty LM, Nathan DM, Lachin JM et al. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the diabetes control and complications trial. Am. J. Clin. Nutr. 89(2),518–524 (2009).
- Siervo M, Lara J, Chowdhury S et al. Effects of the dietary approach to stop hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 113(1),1–15 (2015).
- Wheeler ML, Pi-Sunyer FX. Carbohydrate issues: Type and amount. J. Am. Diet. Assoc. 108 (4),34-49 (2008).
- Nansel TR, Gellar L, McGill A. Effect of varying glycemic index meals on blood glucose control assessed with continuous glucose monitoring in youth with type 1 diabetes on basal-bolus insulin regimens. Diabetes. Care. 31(4),695-697 (2008).
- Zhong VW, Crandell JL, Shay CM, et al. Dietary intake and risk of non-severe hypoglycemia in adolescents with type 1 diabetes. J. Diabetes. Complications. 31(8),1340–1347 (2017).
- Driscoll KA, Raymond J, Naranjo D. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr. Diab. Rep. 16(8),77 (2016).
- Peveler RC, Davies BA, Mayou RA et al. Self-care behaviour and blood glucose control in young adults with type 1 diabetes mellitus. Diabe . Med. 10(1),74–80 (1993).