Research Article - Imaging in Medicine (2022) Volume 14, Issue 9
Early detection of left atrial dysfunction in hypertensive patients with normal left atrial size: Role of speckle tracking imaging
Taamallah K*, Jabloun Y, Haggui A, Hajlaoui N, Lahidheb D, Fehri W
Department of cardiology, Military hospital of Tunis
Department of cardiology, Military hospital of Tunis
E-mail: cardiokarima@yahoo.fr
Received: 05-Jan-2021, Manuscript No. FMIM-21-24198; Editor assigned: 07-Jan-2022, PreQC No. FMIM-22-24198 (PQ); Reviewed: 01-Feb-2021, QC No. FMIM-22-24198; Revised: 22-Feb-2021, Manuscript No. FMIM-22-24198 (R); Published: 28-Sep-2022, DOI: 10.37532/1755-5191.2022.14(9).01-10
Abstract
Background: Systemic hypertension is a major health problem worldwide; it is associated with impaired left atrial (LA) function. Myocardial deformation analysis using speckle-tracking echocardiography has emerged as a promising tool to evaluate atrial deformation and function. This study aimed to evaluate early changes in left atrial longitudinal strain based on speckle tracking echocardiography in patients with hypertension.
Methods: LA strain was studied using speckle-tracking echocardiography in 109 hypertensive patients without LA enlargement and 50 age-matched controls. Conventional and bidimensional strain echocardiographic assessments were performed and the following parameters were measured: peak atrial longitudinal strain and strain rate during the reservoir, conduit, and contractile periods in four and two-chambers views and time to peak atrial longitudinal strain/strain rate measured in the three phases of LA function.
Results: LA area and anteroposterior diameter were within the normal range, no difference between the hypertensive patients and controls was noted. LA maximum, minimum and pre-atrial volumes were higher in hypertensive patients, and impaired reservoir and conduit functions were noted in hypertensive patients compared to normotensive patients. During the contractile period, peak strain and strain rate were higher in hypertensive patients without reaching the level of significance. Time to peak strain and strain rate and duration of diastole were significantly higher in hypertensive patients compared to controls. A significant relationship between the parameters of the volumetric study and those of the bidimensional strain/strain rate study was noted.
Conclusion: Left atrial longitudinal strain during the reservoir and conduit periods is impaired in patients with hypertension despite normal cavity size and before the detection of other echocardiographic changes. Speckle-tracking echocardiography may be considered a promising tool for the early detection of LA strain abnormalities in these patients.
Keywords
echocardiography • speckle tracking • left atrium • hypertension • diastolic function • atrial fibrillation • ischemic stroke
Introduction
Hypertension (HTN) is a major health problem worldwide, and its prevalence is increasing. Unfortunately, its pathophysiology and clinical course are not yet clearly established. Several studies demonstrated a negative influence of HTN on target organ damage explaining the unfavorable prognosis of hypertensive patients and increased cardiovascular morbidity and mortality including atrial fibrillation, stroke, myocardial infarction, and sudden cardiac death in this population [1].
In hypertensive patients, comprehensive assessment of left atrial (LA) phasic function may be of clinical importance and might be helpful in the risk stratification in these patients. Indeed, LA enlargement and LA functional abnormalities may predict the occurrence of atrial fibrillation and cerebrovascular strokes in these patients [2,3]. Although structural and functional changes in the left ventricle during hypertension are well-known, relatively little is known about the effect of hypertension on LA functions and its prognostic impact, because they were insufficiently studied [3]. Some studies suggested that left atrial (LA) dysfunction and left ventricular (LV) diastolic dysfunction occur before structural changes of the LA and the LV even in patients with well-controlled HT. Atrial structural and functional changes caused by hypertension can be evaluated with several conventional techniques such as echocardiography, computed tomography, magnetic resonance imaging. Two-dimensional (2DE) and, recently, three-dimensional (3DE) echocardiography are the most commonly employed noninvasive imaging techniques to evaluate LA size and function
Speckle tracking echocardiography (STE) is an imaging technique that can be applied to the analysis of left atrial function. It allows direct and angle-independent analysis of myocardial deformation, thus providing sensitive and reproducible indices of myocardial fiber dysfunction that overcome most of the limitations of Doppler-derived strain measures, so, a more precise evaluation of the myocardium in patients with HTN, what is required to provide effective diagnosis and management of cardiac dysfunctions in patients with hypertension.
The assessment of LA function using bidimensional (2D) strain may be of particular interest in patients with no evidence of LA enlargement, because it may provide additional information for the early detection of LA abnormalities at the very early stage of the disease, which would improve clinicians’ understanding and management of hypertensive patients and help to identify patients at high risk for adverse events especially heart failure with preserved ejection fraction, and to predict atrial fibrillation (AF) and cardiac events. There have been few studies using STE to assess LA deformation in hypertensive patients and its prognostic impact [5].
So, we aimed in this current study to evaluate early changes in the left atrial function during the three mechanical LA phases by using two-dimensional speckle tracking echocardiography (2-DSTE) in hypertensive patients with HTN and normal LA size
Methods
We conducted a prospective cross-sectional study at our department of Cardiology between February 2015 and June 2020 including 159 subjects divided into two groups: The hypertensive group (HTN group) consisted of 109 patients with primary hypertension, and the control group comprised 50 healthy subjects. The two groups were age- and gender-matched and were comparable for most clinical variables. The study was conducted following the principles of the declaration of Helsinki and was approved by the local ethics committee. All participates gave informed consent to participate in the study.
Hypertension was defined as systolic blood pressure (BP)≥ 140 mmHg and/or diastolic BP ≥90 mmHg or antihypertensive treatment with a documented history of hypertension. Blood pressure was measured before echocardiography, on the non-dominant arm by a sphygmomanometer in a sitting position according to current guidelines for the management of hypertension [1]. For each patient in both groups, anamnestic and clinical data were collected. Were included in this study, patients with an established diagnosis of hypertension and well-controlled by antihypertensive drugs, with normal BP (BP ≤140 mmHg and/or diastolic BP ≤90 mmHg) measured just before the practice of the echocardiography and with normal heart structure and functions: Normal LA size (LA anteroposterior diameter ≤ 40 mm on the parasternal long-axis view), and volume (LA maximum volume index< 34 ml/m2), normal left ventricular (LV) ejection fraction (EF) and without LV hypertrophy (LVH) ( LVEF ≥50% and normal LV mass <105g/ m2 in male and <95g/ m2 in female) [6].
Exclusion criteria
were excluded from this study, patients with conditions that affect LA size and function such as patients aged over 70 years, patients with secondary hypertension, with a history of ischaemic heart disease or angiographically documented coronary artery disease, conduction abnormalities, sinus bradycardia and tachycardia, atrial and ventricular arrhythmias documented by electrocardiogram (ECG) or 24-h Holter ECG, permanent cardiac pacemaker implantation, bundle branch block, LV systolic dysfunction, and abnormal LV wall motion, aortic and mitral valvulopathy, previous cardiac surgery, diabetes mellitus, chronic obstructive pulmonary disease (COPD), obstructive pulmonary sleep apnea (OSA), and patients with poor echocardiographic window.
Echocardiography: All patients underwent an echocardiographic examination using the ultrasound machine General Electric Vivid9, with a 3.5-MHz transducer. The data were analyzed offline using EchoPAC (GE Vingmed Ultrasound AS). A standard echocardiographic study using 2D, M-mode, and Doppler techniques were performed in addition to speckle tracking for LA. All echocardiograms were performed by an experienced cardiologist, he was the same operator. The transthoracic echocardiography was performed with simultaneous recording of a stable and a good quality electrocardiogram trace. Cardiac dimensions and volumes were measured according to the American Society of Echocardiography’s Guidelines [6].
Standard assessment of left atrium: LA diameter was derived from parasternal long-axis B-mode view, and LA volumes were calculated from apical four-chamber and two-chamber views using the biplane Simpson’s method. All volumes were indexed to body surface area. LA maximum volume (LAVmax) was measured just before mitral valve opening, at the end of the T wave on systole, and LA minimum volume (LAVmin) was measured at mitral valve closure at the beginning of the QRS wave on the end of diastole. LA pre-atrial volume (LAVp) was measured just before the beginning of the P wave on the electrocardiogram.
The following parameters of the LA function were calculated:
•Total LA stroke volume (LASV): Calculated as the difference between maximal and minimal LA volumes.
•LA expansion index (LAEI): Calculated as the ratio of total LASV to minimum LAvolume x 100.
•LA active emptying volume and LA passive emptying volume were calculated using (LAVp - LAVmin) and (LAVmax -LAVp) formulas, respectively.
•LA contractile function was assessed by calculating the LA active emptying fraction (LAAEF) that could be obtained as the ratio of LA active emptying volume to LAVp x 100.
•LA reservoir function was assessed by calculating LA ejection fraction (LAEF) that could be obtained as the ratio of LASV to LAVmax x 100.
•LA conduit function was assessed by calculating the LA passive emptying fraction (LApEF) that could be obtained as the ratio of LA passive emptying volume to LAVmax x 100.
•LA stiffness index (LASi) is calculated as the ratio of Em/Ea to PS-S.
Left ventricular end-diastolic diameter (LVEDd), the interventricular septal (IVS), and LV posterior wall thickness in diastole (LVPW) in diastole were measured using parasternal long-axis view, left ventricular ejection fractions (LVEF) were calculated by Simpson’s method. LV mass (LVM, in grams) was calculated using the Penn formula and was subsequently indexed to body surface area (BSA) to obtain LV mass index.
The classification of the LV geometry was based on the relative wall thickness. (RWT=2PW/EDD) and the LV mass : normal geometry (Normal LVM and a RWT < 0.42) and concentric remodelling (normal LVM and RWT> 0.42). Patients with LVH were excluded.
Diastolic parameters, including E/A and Em/Ea, were assessed using pulsed-wave Doppler at the tips of the mitral leaflets and from tissue Doppler imaging at the level of the lateral annulus, respectively. The ratio of Em/Ea was calculated using the average Ea value.
Longitudinal Strain acquisition
Speckle tracking echocardiography recordings were processed using acoustic-tracking software (EchoPAC PC, Version113; GE Health VIVID 9), allowing off-line semi-automated analysis of speckle-based strain. Different strain parameters were acquired according to current recommendations [7].
Four and two apical-chambers views were acquired, using a conventional 2D gray-scale echocardiography, over three consecutive cardiac cycles at a rate of 70 images per second by asking the patient to perform an apnea for better image quality. A small sector angle (30°) was chosen to acquire the maximum frame rate, the software is applied in harmonic imaging. Care was taken to optimize visualization of the LA cavity and to maximize the LA area in apical views and avoid foreshortening of the left atrium. In the apical four-chamber and two-chamber views, the LA endocardial border was manually traced at end-systole in apical four-chamber and two-chamber views by a point-and-click approach. The system generated epicardial surface tracing automatically using the software automatically for each view. After manual adjustment of the region of interest (ROI) width and shape, the software divided the ROI into 6 segments for each view, and the resulting tracking quality for each segment was automatically scored as either acceptable or non-acceptable. Inadequately tracked segments were either corrected manually or rejected from the analysis.
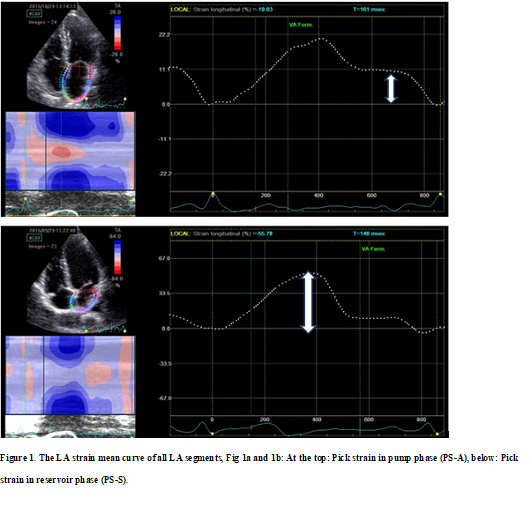
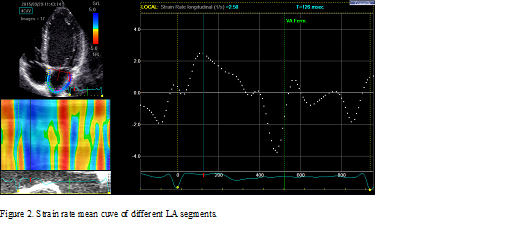
The LA walls were divided into 12 segments, in subjects with adequate image quality, a total of 12 segments were then analyzed. Lastly, the software generated the longitudinal strain curves for each segment and a mean curve of all segments that reflect the pathophysiology of atrial function. Setting zero strain at LV end-diastole, the LA strain pattern is characterized by a predominant positive wave that peaks at the end of ventricular systole, followed by two distinct descending phases in early diastole and late diastole. From apical four-chamber and two-chamber views, peak longitudinal LA strain (PS-S) and strain rate during ventricular systole (PSR-S) reflecting LA reservoir function was obtained just before the mitral valve opening. Peak strain (PS-E) and strain rate (PSR-E) are measured during early diastole and reflect LA conduit function during the conduit phase. Peak negative strain-A wave (PS-A) and peak strain rate-A wave (PSR-A) during late diastole reflecting pump function during LA contractile phase was obtained at the onset of the P-wave on electrocardiography. Peak atrial longitudinal strain during a different phase of LA function was calculated by averaging values obtained from all LA segments from apical four-chamber and two-chamber views. Figure 1 illustrates the LA strain mean curve of all LA segments and Figure 2 illustrates the LA strain rate mean curve of all LA segments.
In the same manner, time to peak strain and strain rate were measured manually by using the time curser of 2-DSTE. They were measured from the R wave of the electrocardiogram to the peak strain/strain rate during the reservoir, conduit, and contraction phases.
Duration of reservoir, conduit, and contractile phases were measured in hypertensive patients and controls, mean duration was calculated for each LA phase function.
Intraobserver and interobserver variability study
Intraobserver and interobserver variability were determined by repeating a Two-dimensional speckle-tracking study after three months for 30 randomly selected patients by the same observer and by a second independent observer.
Correlation study
The correlation between parameters measured by the conventional echocardiography and parameters measured by Speckle tracking imaging was studied. Absolute values of PSR-E and PSR-E were used for the determination of the correlation between these two parameters and conventional parameters.
Statistical study
SPSS for Windows software was used for statistical analysis (SPSS Inc. Chicago, USA). Peak strain and strain rate, SR, time to the peak strain, and strain rate of 12 LA wall during LA relaxation, conduit, and contraction were compared between hypertensive and normotensive subjects. The homogeneity and normal distribution of continuous data were tested through the Leneve’s test and Shapiro–Wilk test, respectively. A p-value of more than 0.05 was considered significant for the homogeneity and normal distribution values.
Categorical variables were expressed as absolute frequencies and percentages, Continuous non-parametric variables were expressed as the median value, and 25th–75th percentile and continuous parametric variables were expressed as the mean and standard deviation.
Categorical variables were compared by the likelihood-ratio x 2 test. Comparison of parametric values between the two groups was performed by using a two-tailed Student’s t-test. All values were obtained with 95% confidence intervals. A p-value of less than 0.05 was considered statistically significant.
Intraobserver and interobserver analyses were performed using Bland–Altman to evaluate the standard deviation and 95% limits of agreement.
Pearson's correlation coefficient (r) was used to determine a correlation between parameters of LA function from standard echocardiography and the 2D strain study.
Results
General characteristics of the population
The mean age in the HTN group was 58.86 ±11.61. The average duration of hypertension was 8.36 ± 6.37 years. As expected, systolic and diastolic blood pressure were significantly higher in the hypertensive group than those in the control group (Table 1).
| HTN (+) (n=109) | HTN (-) (n=50) | P-value | |
|---|---|---|---|
| Age (years) | 58.86 ± 11.61 | 54.84 ± 13.54 | 0.36 |
| Men n (%) | 58 (53%) | 27 (54%) | 0.49 |
| Body surface area (m2) | 1.76±0.2 | 1.74±0.2 | 0.20 |
| Body mass index (BMI) kg/m2 | 27.2±1.3 | 26.9±1.1 | 0.82 |
| Systolic blood pressure (mmHg) | 149.5±24.9 | 121.3±10.5 | <0.001 |
| Diastolic blood pressure (mmHg) | 90.1±11.0 | 74.8±6.7 | <0.001 |
| Heart rate (beats/min) | 68.2±8.8 | 62.4±10.2 | 0.37 |
| Duration of hypertension (years) | 8.36 ± 6.37 | - |
Table 1. Clinical Characteristics of Hypertensive and Normotensive Subjects.
Conventional echocardiographic characteristics
There was no difference between the two groups concerning LVEF, LVED, and LVES diameter, and ascending aortic diameter. Hypertensive patients had also greater septal and posterior wall thicknesses and LV mass, 69.7% (n=76) of patients have normal LV geometry. A significant difference was noted between the two groups for all diastolic function parameters (except Em /Ea ratio and DTm). Systolic pulmonary artery pressure (SPAP) was significantly increased in the hypertensive group (p=0.02) (Table 2).
| HTN (+) | HTN (-) | P-value | |
|---|---|---|---|
| LV EDD (mm) | 48.12± 5.94 | 46.76 ± 4.07 | 0.82 |
| LV ESD (mm) | 30.28 ±4.64 | 29.46 ±4.86 | 0.44 |
| IVS (mm) | 9.58±1.8 | 8.48±1.6 | 0.02 |
| PW (mm) | 9.10±1.53 | 8.16±1.31 | 0.05 |
| LVM indexed to body surface area (g/m2 ) | 91.28±9.47 | 75.98±12.23 | 0.04 |
| LVEF (%) | 66.2 ±4.71 | 68.21 ±5.22 | 0.25 |
| ascending aortic diameter (mm) | 26.32±5.43 | 24.97±6.73 | 0.07 |
| LA Diameter (mm) | 34.35 ±4.91 | 31.82 ±4.87 | 0.16 |
| LA area (cm²) | 16.22±3.66 | 13.96±2.74 | 0.02 |
| LA maximum volume (ml) | 41.78±10.29 | 47±13.21 | 0.01 |
| LA maximum volume indexed to body surface area (ml/m²) | 28.60 ±5.4 | 27.5 ±6.17 | 0.01 |
| LA minimum volume (ml) | 23.95±12.18 | 16.94±7.91 | 0.001 |
| LA minimum volume indexed to body surface area (ml/m²) | 14.38±7.22 | 10±4.71 | 0.001 |
| LA stroke volume (ml/m²) | 20.36±4.21 | 24.34±2.18 | 0.001 |
| LA stroke volume indexed to body surface area (ml/m²) | 14.3±6.1 | 15.9±5.8 | 0.02 |
| LAVp (ml) | 14.83±9.5 31 | 16.42±6.02 | 0.03 |
| LAVp indexed to body surface area (ml/m²) | 26.41±10.42 | 28.4±3.8 | 0.04 |
| LA ejection fraction (%) | 62.53±9.12 | 66.8±1.10 | 0.05 |
| LA expansion index (%) | 1.7±0.83 | 2.0±0.9 | 0.03 |
| LA stiffness index (%) | 0.25±0.35 | 0.28±0.17 | 0.01 |
| LApEF (%) | 24.70±14.38 | 34.12±12 | 0.01 |
| LAAEF (%) | 48.65±17.28 | 44.10±13 | 0.06 |
| Em (cm/s) | 71.19±18.11 | 85.00±18.40 | 0.02 |
| Am (cm/s) | 85.56±18.23 | 66.83±16.77 | 0.001 |
| Em/Am | 0.94±0.33 | 1.35±0.46 | 0.000 |
| DTm (ms) | 218.53±57.68 | 205.00±47.52 | 0.06 |
| Ea lateral (cm/s) | 10.01±3.22 | 13.88±4.49 | 0.000 |
| Ea average septal and lateral (cm/s) | 9.86±2.47 | 11.74±3.08 | 0.049 |
| Em /Ea | 7.69±3.06 | 7.86±6.38 | 0.74 |
| PSAP | 29.85±5.11 | 23.07±08.74 | 0.02 |
Table 2. Conventional echocardiographic data of the two groups.
Left atrium volume and emptying fraction
Although all subjects in the hypertensive and control groups had normal LA size and volume, LA area, LAVmax, LAVmin, and LAVp were significantly higher in the hypertensive group than those in the control group (p<0.05). LA anteroposterior diameters were similar in the two groups. Hypertensive patients had lower LASV, LAEF, LAEI, LApEF, and LASi than controls (p<0.05). There was no difference between the two groups concerning LAAEF.
Left atrial 2D strain/strain rate
Left atrial 2D strain: In the hypertensive group, Peak strain (PS) measured in apical 4c and 2c views during reservoir phase (PS-S) and conduit phase (PS-E) were significantly lower with values of 31.23 ±9.93 versus 46.43 ±11.06 (p=0.000), and 14.26±2.91 % versus 21.41±2.8 % (p=0.000) respectively.
Although higher in hypertensive patients, there was no significant difference between the two groups regarding Peak strain obtained from both apical four-chamber and two-chamber views images during contractile phase (PS-A), with a value of 16.73 ±3.84% versus 15.29±2.75% (p=0.07).
Comparison of LA longitudinal strain values between the two groups is shown in Table 3.
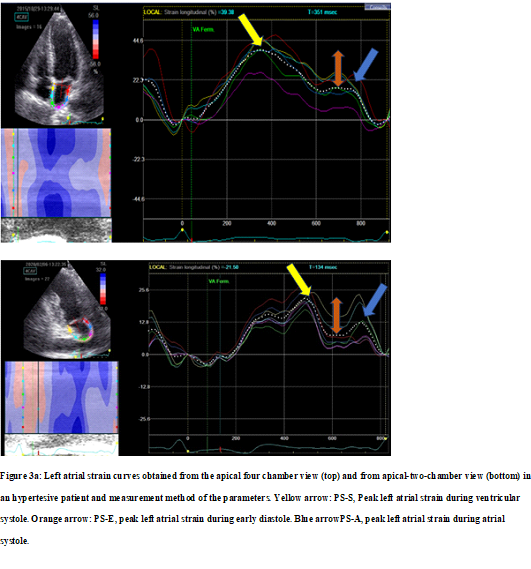
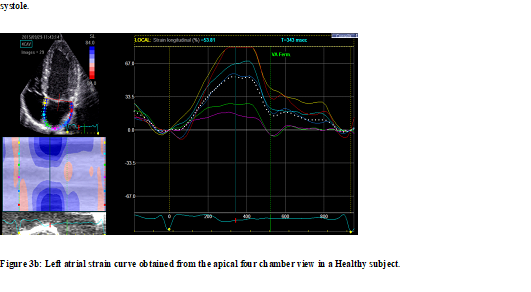
Figure 3 illustrates examples of LA longitudinal strain patterns taken from the hypertensive patient (Figure 3a) and healthy subjects (Figure 3b).
Figure 3a: Left atrial strain curves obtained from the apical four chamber view (top) and from apical-two-chamber view (bottom) in an hypertesive patient and measurement method of the parameters. Yellow arrow: PS-S, Peak left atrial strain during ventricular systole. Orange arrow: PS-E, peak left atrial strain during early diastole. Blue arrowPS-A, peak left atrial strain during atrial systole.
Left atrial 2D strain rate
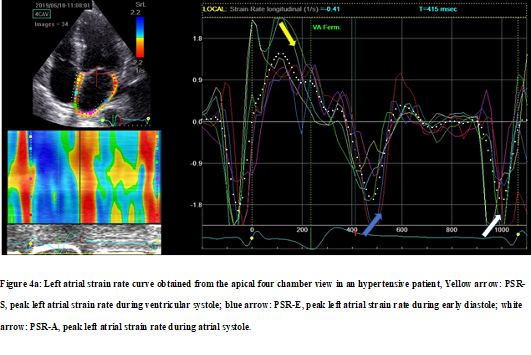
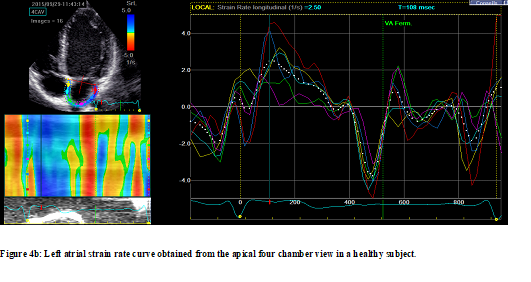
Strain rate values were significantly impaired in the LA walls in hypertensive patients measured during reservoir (PSR-S) and conduit phases (PSR-E) from apical four-chamber et two-chamber views as well as their averages. The strain rate value measured during the contractile phase (PSR-A) was higher in hypertensive patients but this increase did not reach statistical significance. Global longitudinal peak strain rate values in the hypertensive group compared with the control group for reservoir, conduit, and contractile phases are shown in Table 4. Figure 4 illustrates examples of LA strain rate patterns taken from hypertensive (Figure 4a) and normotensive subjects (Figure 4b).
Figure 4a: Left atrial strain rate curve obtained from the apical four chamber view in an hypertensive patient, Yellow arrow: PSR-S, peak left atrial strain rate during ventricular systole; blue arrow: PSR-E, peak left atrial strain rate during early diastole; white arrow: PSR-A, peak left atrial strain rate during atrial systole.
Duration of different phases of LA function
Compared to normal subjects, the mean duration of reservoir period (D-S) and contractile period (D-A) duration were significantly higher in hypertensive patients, but the mean duration of conduit period (D-E) was lower in hypertensive patients compared with healthy subjects, this decrease in D-E was not statistically significant (Table 3).
LA time to peak strain/strain rate
Mean LA time to peak strain/strain rate the reservoir, conduit, and contractile phases were significantly higher in the hypertensive group except T-PSA which was higher in hypertensive patients without reaching the level of significativity (p=0.14). (Table 3 and Table 4).
| HTN (+) | HTN (-) | P-value | |
|---|---|---|---|
| PS-S A4C (%) | 30.22±9.96 | 45.16±10.11 | 0.000 |
| PS-S A2C (%) | 32.76±10.91 | 48.36±13.90 | 0.000 |
| PS-S (%) | 31.23 ±9.93 | 46.43 ±11.06 | 0.000 |
| T-PS S (ms) | 405.02±55.51 | 387.13±47.48 | 0.05 |
| PS-E A4C (%) | 14.69±2.79 | 21.13±3.4 | 0.000 |
| PS-E A2C (%) | 14.09±2.88 | 21.24±1.7 | 0.000 |
| PS-E (%) | 14.26±2.91 | 21.41±2.8 | 0.000 |
| T-PS E (ms) | 596±49 | 629±74 | 0.001 |
| PS-A A4C (%) | 16.46±3.12 | 15.31±2.75 | 0.08 |
| PS-A A2C (%) | 16.89±4.93 | 15.10±2.75 | 0.07 |
| PS-A (%) | 16.73 ±3.84 | 15.29±2.75 | 0.07 |
| T PS-A(ms) | 686±70 | 692±96 | 0.14 |
| Reservoir phase duration (ms) | 560±23 | 522±31 | 0.02 |
| Conduit phase duration (ms) | 68±13 | 71±16 | 0.06 |
| Contraction phase duration (ms) | 163±26 | 146±24 | 0.04 |
Table 3. Global longitudinal peak strain values in the hypertensive group compared with the control group for the reservoir, conduit, and contractile phases.
| HTN (+) | HTN (-) | P value | |
|---|---|---|---|
| P-SR S A4c(%) | 0.98±0.14 | 1.36±0.48 | 0.04 |
| P-SR S A2c(%) | 0.99±0.18 | 1.37±0.37 | 0.03 |
| P-SR S(%) | 0.99±0.12 | 1.37±0.19 | 0.03 |
| P-SR E A4c(%) | -0.98±0.3 | -1.58±0.6 | 0.001 |
| P-SR E A2c(%) | -0.98±0.3 | -1.51±0.7 | 0.002 |
| P-SR E(%) | -0.98±0.3 | -1.56±0.4 | 0.001 |
| P-SR A A4c(%) | -1.87±0.19 | -1.92±0.11 | 0.56 |
| P-SR A A2c(%) | -1.9±0.24 | -1.72±0.87 | 0.60 |
| P-SR A(%) | -1.89±0.16 | -1.82±0.21 | 0.54 |
| T P-SR S(ms) | 215±28 | 199±13 | 0.02 |
| TP-SR E(ms) | 498±11 | 436±24 | 0.01 |
| TP-SR A(ms) | 741±16 | 739±28 | 0.05 |
Table 4. Global longitudinal peak strain rate values in the hypertensive group compared with the control group for reservoir, conduit, and contractile phases.
Intra-and interobserver variability
Intra-and interobserver variability of PS-S measurements were 5.8% and 6.6%, respectively, of PS-E measurements were 7.2% and 8.7%, respectively, and of PS-A measurements were 7.4% and 7.9%, respectively.
Intra-and interobserver variability of PSR-S measurements were 5.3% and 7.6%, respectively, of PSR-E measurements were 6.2% and 8.1%, respectively, and of PSR-A measurements were 6.9% and 8.7%, respectively.
Correlation analysis between 2D strain and standard echocardiography parameters
No correlation was found between LA diameter and peak strain and strain rate in the reservoir, conduit, and contractile phases. LA area was correlated only with pic strain and strain rate in the contractile phase.
All echocardiographic parameters of the volumetric study, except LASV and LAAEF, correlated significantly with PS-S. A significant correlation was observed between PS-E and IVS, Em/Am, E/Ea, LAV max, LAV min, LApV, LASV, LAEI, LAEF, LApEF, and SPAP.
LAVmin, LAV max, LAEF, and LAEI, were significantly correlated with PS-A, no statistically significant correlation was noted between PS-A and Em/Am, Em/Ea, LApV, LASV, LAEI, LApEF.
Correlation between 2D strain rate and standard echocardiography parameters
PSR-S was significantly correlated with LAVmax, LASV, LAEF, LApEF, Em/Am, Em/Ea, and SPAP.
LAVp, LApEF, Em/Am, Em/Ea, and SPAP were correlated with PSR-E.
Except for a correlation between PSR-A and LAAEF and LA minimum volume, no significant correlation existed between PSR-A and Em/Am, Em/Ea, LAV max, LApV, stroke volume, LAEI, LAEF, and LApEF.
The correlation between standard echocardiographic parameters and 2D strain and strain rate study is shown in Table 5.
| PS-S | PS-E | PS-A | PSR-S | PSR-E | PSR-A | |
|---|---|---|---|---|---|---|
| LA Diameter | r=-0.49 p=0.12 |
r=-0.49 p=0.16 |
r=-0.287 p=0.26 |
r=-0.49 p=0.18 |
r=-0.49 p=0.27 |
r=-0.287 p=0.34 |
| LA area | r=-0.18 p=0.08 |
r=-0.08 p=0.09 |
r=-0.143 p=0.01 |
r=-0.13 p=0.12 |
r=-0.22 p=0.32 |
r=-0.143 p=0.01 |
| LA maximum volume | r=-0.651 p=0.002 |
r=-0.64 p=0.002 |
r=-0.67 p=0.03 |
r=-0.54 p=0.04 |
r=-0.11 p=0.06 |
r=-0.12 p=0.42 |
| LA minimum volume | r=-0.69 p=0.001 |
r=-0.67 p=0.002 |
r=-0.61 p=0.03 |
r=-0.013 p=0.32 |
r=-0.34 p=0.09 |
r=-0.53 p=0.01 |
| Pre-atrial contraction volume | r=-0.64 p=0.002 |
r=-0.68 p=0.002 |
r=-0.15 p=0.30 |
r=-0.22 p=0.47 |
r=-0.48 p=0.04 |
r=-0.28 p=0.37 |
| Stroke volume | r=0.15 p=0.19 |
r=0.43 p=0.01 |
r=0.13 p=0.12 |
r=0.41 p=0.04 |
r=0.18 p=0.06 |
r=0.28 p=0.34 |
| LAEF | r=0.27 p=0.04 |
r=0.63 p=0.02 |
r=0.69 p=0.01 |
r=0.52 p=0.04 |
r=0.14 p=0.13 |
r=0.23 p=0.38 |
| LAEI | r=0.62 p=0.00 |
r=0.52 p=0.03 |
r=0.38 p=0.00 |
r=0.21 p=0.06 |
r=0.19 p=0.07 |
r=0.12 p=0.52 |
| LAAEF | r=0.21 p=0.06 |
r=0.16 p=0.07 |
r=0.12 p=0.6 |
r=0.31 p=0.07 |
r=0.24 p=0.08 |
r=0.63 p=0.001 |
| LAEF | r=0.72 p=0.01 |
r=0.62 p=0.03 |
r=0.09 p=0.72 |
r=0.70 p=0.01 |
r=0.69 p=0.01 |
r=0.09 p=0.72 |
| IVS | r=-0.62 p=0.02 |
r=-0.62 p=0.02 |
r= -0.39 p=0.19 |
r=-0.09 p=0.08 |
r=-0.15 p=0.14 |
r=-0.17 p=0.82 |
| E/A | r=0.27 p=0.04 |
r=0.27 p=0.04 |
r=0.288 p=0.24 |
r=0.62 p=0.03 |
r=0.54 p=0.03 |
r=0.15 p=0.12 |
| E/e’ | r=-0.31 p=0.02 |
r=-0.31 p=0.03 |
r=0.28 p=0.35 |
r=-0.68 p=0.02 |
r=-0.61 p=0.02 |
r=-0.22 p=0.32 |
| SPAP | r=-0.51 p=0.001 |
r=-0.34 p=0.001 |
r=-0.39 p=0.34 |
r=-0.53 p=0.01 |
r=-0.44 p=0.03 |
r=-0.17 p=0.26 |
Table 5. Correlations between standard echographic parameters and atrial 2d strain parameters.
Discussion
In our study, we used 2D strain and strain rate imaging to assess left atrial deformation in hypertensive patients with normal LA size. Despite normal LA size assessed by conventional 2D echocardiography, an impaired reservoir and conduit function in hypertensive patients compared to normotensive patients was noted. Besides, the correlation analysis indicated a significant relationship between parameters of the 2D strain/strain rate study and those of the volumetric study. During the contractile period, PS and PSR were higher in hypertensive patients without reaching the level of significance. Time to PS, T-PSR, and the duration of diastole were significantly higher in hypertensive patients compared to controls.
Physiopathology
Normal LA structure and function are essential for cardiac function with its three functions: Reservoir, conduct, and pump. Several studies suggest that LA structural remodeling and/or functional impairment might be involved in the pathogenesis and development of ventricular disorders, such as heart failure with reduced or preserved ejection fraction [8].
In hypertensive patients, complex morphological changes of LA and LV is the result of the heart adapting to the increased left ventricular workload. The LA enlargement is the result of forceful atrial contraction compensating for the reduction in early diastolic emptying due to diastolic dysfunction. Besides, the intermittent or permanent elevation of LV filling pressures leads to over LA filling leading to atrial fibrosis predisposing to atrial remodeling and dysfunction and, and consequently to arrhythmia [9].
Speckle Tracking imaging
Advanced echocardiographic tools, such as two-dimensional (2DE) and, recently, three-dimensional (3DE) Speckle Tracking echocardiography, allow a better understanding of different cardiac chamber’s function. It is used to measure both global and regional strain, defined as the degree of displacement of a region over the cardiac cycle, through tracking acoustic markers generated by the effect of ultrasound on the myocardium. Clinical implications of STE are various, it has shown to be sensitive for the detection of subclinical disease, including hypertensive heart disease (2). It was suggested that speckle-tracking echocardiography is more accurate than LA size or volume to detect an early LA dysfunction [10-12].
The interest of speckle tracking in hypertensive patients
Our findings extend previous reports describing LA deformation during the three phases of LA function. Several studies have shown that abnormal LA deformation precedes LA enlargement and LVH. It was demonstrated that in hypertensive patients with normal LA size, peak strain, and strain rate were significantly lower during reservoir and conduit phases [13-16].
An increased LASi was proposed by some authors as a marker of early target organ damage in hypertension [16], this parameter is decreased in our patients. Influence of left ventricular geometry on the left atrial phasic function was reported by several authors [16-19]. The decrease in reservoir and conduct functions are more pronounced in hypertensive patients with concentric LV than in hypertensive patients with normal geometry or concentric remodeling. Several studies have demonstrated that the decrease of different indices of LA function was significantly pronounced in the hypertensive patients in the presence of diabetes mellitus, a higher grade of LV diastolic dysfunction, higher age, and obesity [5,20].
In opposition to our results, an impairment of atrial function during the contraction phase in addition to the decreased strain during reservoir and conduit has been demonstrated by several authors [9]. According to these authors, an impairment of the LA contractile function, even before LA enlargement develops was noted. Dernellis have demonstrated an increase in reservoir and pump booster function which was in contrast with our results, in this study conduit function was decreased, the alteration of LA conduit function would be related to the increase in LV filling pressure during diastole.
In our study, strain and strain rate parameters were increased during the contraction phase in hypertensive patients without reaching the level of significance, a significant increase in LA booster pump function was revealed by several studies [9,11]. It was suggested that LA function during atrial contraction is a potential non-invasive indicator of left ventricular end-diastolic compliance [7]. According to some authors, the increase in LA contraction function was pronounced in patients with concentric LVH compared to subjects with normal LV geometry.
Several studies suggest that the decreased LA strain is a good sign of LV diastolic dysfunction, they have shown that there is a good correlation between PS-S and LV diastolic function as well as increased LV filling pressures. According to some authors, LA strain measurements allow accurate diagnosis and categorization of diastolic dysfunction. Since LA conduit and reservoir functions decrease earlier than the diagnosis of LV diastolic dysfunction, some authors have proposed these parameters in the decisional algorithm to study filling pressures. Left atrial dysfunction was also demonstrated in patients with masked hypertension. Soe M. Aung have demonstrated the utility of 2D strain to detect masked hypertension in patients with hypertensive response to exercise. A decreased reservoir and pump function in these patients was noted. The same results were demonstrated by Tadic, who suggest that 24-hour systolic blood pressure increment was closely related to LA remodeling.
A disturbance in LA function was also noted in patients with white coat hypertension.Tadic have demonstrated that patients with white coat hypertension have a reduced conduit function and an increased pomp function, however, reservoir function was normal. These authors have compared LA function in patients with sustained hypertension, white coat hypertension, and controls, the LA function’ changes mentionned above, were more pronounced in sustained hypertensive patients. In this same study, LA stiffness, as well as aortic stiffness, gradually increases from controls to sustained hypertensives.
A Lower peak strain and strain rate during reservoir, conduit, and contraction phase have been reported in hypertensive patients with obstructive sleep apnea (OSA) compared to hypertensive patients without OSA, highlighting, according to some authors, the potential role of OSA in increasing the risk of atrial fibrillation in these patients.
In patients with non-dipper hypertension, LA function is more impaired compared to dipper hypertensive patients. It was demonstrated that the raised LV pressure secondary to the nocturnal systemic pressure overload was strongly associated with LA deformation.
A decrease in LA strain was also found in cases of gestational hypertension. Andrea Sonaglioni et al. [5] reported an impaired LA function in women with new-onset gestational hypertension, these authors have demonstrated an incremental prognostic value of global left atrial peak strain in these patients.
Gee Hee Kim revealed that compared with normotensive subjects, never treated early hypertensive patients and without clinically apparent target organ damage, had significantly increased plasma aldosterone concentration, and decreased atrial reservoir pump strain, and atrial systolic strain rate. LA pump strain was independently associated with nighttime systolic BP. Plasma aldosterone concentration was correlated with LV deformation No correlation was found between LA deformation and plasma aldosterone level. Recently, in hypertensive and diabetic patients, Kalaycıoğlu have demonstrated that higher serum osteoprotegerin level was associated with impaired LA function assessed by speckle tracking. These authors suggested that serum osteoprotegerin may be used as a risk predictor for LA mechanical dysfunction.
Speckle tracking echocardiography is useful as a monitoring tool predicting the response to hypertension treatment and the reversibility of structural anomalies. Dernellis have demonstrated an improvement in atrial function in hypertensive patients who had adequate hypotensive treatment by ACE inhibitors and/ or thiazide diuretics. According to these authors, this improvement in atrial function might be explained by the regression of LV hypertrophy and the contribution of the autonomic nervous system. YA suo have demonstrated that treatment by ACE inhibitors is associated with reduced risk of left atrial appendage thrombosis formation in hypertensive patients with atrial fibrillation. Several other studies have demonstrated a beneficial effect of ACE on left atrial function [19].
In patients with suboptimal blood pressure control, Chen XJ have demonstrated a decreased left atrial myocardial strain. These authors suggest that suboptimal BP control status in hypertensive patients is related to a further reduction of LA myocardial reservoir, conduit, and pump function, they thought that suboptimal BP might be regarded as a composite risk factor and therefore a simplified treatment target. Warita have demonstrated that Pitavastatin had a beneficial effect on LV diastolic function and LA structure and function in elderly patients with HTN. Pitavastatin treatment may be associated with a lower incidence of new-onset atrial fibrillation (AF).
From a prognostic point of view, it is widely known that hypertension is a risk factor for developing AF. In addition to LA enlargement, LA functional abnormalities may also predict the occurrence of atrial fibrillation. According to some authors, LA strain parameters could be useful predictors of AF occurrence in hypertensive patients. So, in patients with impaired atrial function, closer surveillance to detect arrhythmia is necessary. Table 6 summarizes data of different published studies on left atrium deformation assessment in hypertensive patients.
Authors |
Year | Software | The population of the study n patients |
Reservoir Function S (%) SR (L/s) |
Conduit Function S (%) SR (L/s) |
Pump Function S (%) SR (L/s) |
|---|---|---|---|---|---|---|
| T.XU et al.(9) | 2015 | E9, General Electric | 124 HTN | 45.3±7.7 | 20.2±3.8 | 25.0±5.9 |
| 2.3±0.5 | 2.2±0.6 | 2.6±0.5 | ||||
| Sahebjam et al.(13) | 2014 | 7 Vivid 7,GE Healthcare | 75 HTN | 14.98±5.86 | - | - |
| 1.31±0.5 | - | - | ||||
| Liu et al. (14) | 2014 | Vivid 7 (GE Vingmed Ultrasound, Horten, Norway) equipped | 99HTN and 65 HTN +diabetes | 29.2±7.9 | 14.9±5.5 | 14.3±4.1 |
| 1.2±0.3 | 1.1±0.4 | 1.4±0.5 | ||||
| Karakurt et al.(15) | 2019 | Vivid 7,GE Healthcare | 55 HTN | 11.25±5.81 | 2.69±2.79 | 11.63±6.2 |
| 1.22±0.45 | -0.95±0.54 | -1.62±0.8 | ||||
| Kukubu et al. (19) | 2007 | - | 55 HTN without LA dilation | 2.15±0.57 | ||
| Lang et al.(6) | 2018 | Vivid S5 or Vivid 9; GE Medical Systems. A | 50 HTN | 24±6.29 | - | - |
| - | - | - | ||||
| Badano et al.(7) | 2016 | Vivid 9 General electric medical health Echo PAC software |
30 HTN with LVH | 23±6 | 11.3±5 | 12±4 |
| 1.11±0.4 | 0.91±0.4 | 1.47±0.43 | ||||
| Miyoshi et al. (20) | 2014 | Vivid 7, General Electric Healthcare, Milwaukee, WI | 126 HTN | 31.4±8.9 | 16.6±6.2 | 14.8±4.8 |
| 1.4±0.4 | -1.3±0.5 | -1.7±0.6 | ||||
| Tadic et al.(19) | 2020 | CX-50 Philips Healthcare | 65 HTN | 51.2 | 30.9±5.7 | 20.1±5.6 |
| 2.1±0.4 | -2.89±0.7 | -3.12±0.7 | ||||
| Eshoo et al. (17) | 2011 | Vivid E9, GE Medical Systems, Horten, Norway | 71 never treated hypertensive patients | 33.2 ±7 11.42 ± 0.31 |
18.2 ± 6.3 -1.51 ± 0.54 |
14.9 ± 3.8 |
| Yang et al.(15) | 2010 | - | 40 HTN | 63.3±4.1 | - | - |
| 3.1 ±0.2 | - | - | ||||
| Onishi et al.(10) | 2017 | IE 33 Philips med system Qlab 7.0 |
I 279 HTN |
35.9±8 | 18.5±7.1 | 17.8±4.2 |
| - | - | - | ||||
| Miyoshi et al.(20) | 2013 | ACUSON sequoia 512 (Siemens, Mountain View, CA, USA) Normotensive |
163 HTN | 25.5±0.92 | 13.68±0.59 | - |
| 1.18±0.34 | -1.02±0.31 | -1.38±0.42 | ||||
| Our study | Vivid E9, GE Medical Systems, Horten, Norway | 109 HTN with normal LA size and function | 31.23 ±9.93 | 14.26±2.91 | 16.73 ±3.84 | |
| 0.99±0.12 | -0.98±0.3 | -1.89±0.16 |
Table 6. Data of different published studies on left atrium deformation assessment in hypertensive patients.
Limitations
The measurement of LA 2D strain is reproducible and feasible in most cases, but relies very widely upon operator skills and adequate apical views. This modality of echocardiography is less accurate in patients with non-sinus rhythm, requiring the average value of almost five consecutive beats. The disadvantages of LA 2D strain are also the possibility of error in the tracing of endocardial contours and the strict dependence of the image rate. The other pitfall of this technique is that the analysis is performed on the left ventricular strain software because there is no software for atrial strain.
Limitations of the present study are the small sample, explained by the exclusion of patients with diabetes mellitus and/or another disease that may lead to LA dysfunction, and patients with AF, who constitute a significant percentage of hypertensive patients, and the number of patients who had to be excluded because of inadequate image quality for measuring LA 2D strain that is angle independent technique but necessitates a good quality imaging.
It would be interesting to conduct further studies with larger populations and follow‐up data to precise the discriminatory role of LA 2D strain in the AF and heart failure with preserved ejection fraction risk stratification. Indeed, identifying hypertensive patients at risk for developing these complications by early diagnosis of LA dysfunction, and prompt institution of effective treatment should be the goal when considering this patient population.
Conclusion
Our study demonstrated that speckle tracking imaging could be used to detect subtle impairment of LA function in patients with hypertension. Hypertension is associated with impaired reservoir and conduit LA function and higher booster function, which may be compensatory. The clinical usefulness of LA function by STE in these patients merits further investigations to better precision of the role of the LA study in the prediction of atrial fibrillation, and the risk of heart failure with preserved ejection fraction.
Declarations
•The authors have no conflicts of interest to declare.
•The authors declare that this manuscript is not under consideration elsewhere and has not been reported earlier.
•All authors have made significant contributions to the study and have read and approved the content.
•No funding to declare.
Statement of Ethics
This research comply with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. that subjects have given their written informed consent and the study protocol was approved by the committee on human research of the military hospital of Tunis.
Author Contributions:
Conception and design of Study: TAAMALLAH Karima
Acquisition of data: TAAMALLAH Karima, JABLOUN Yassine
Interpretation of data: TAAMALLAH Karima, JABLOUN Yassine
Literature review: HAGGUI Abdeddayem
Drafting of manuscript: TAAMALLAH Karima
Revising and editing the manuscript critically for important intellectual contents: HAJLAOUI N, LAHIDHEB D Supervision of the research: FEHRI Wafa
References
- Williams B, Mancia G, Spiering W et al. 2018 practice guidelines for the management of arterial hypertension of the European society of cardiology and the European society of hypertension ESC/ESH task force for the management of arterial hypertension. J Hypertens. 36, 2284-2309 (2018).
- Rijcken J, Bovenoeerd PHM, Schoofs AJG et al. Optimization of cardiac fiber orientation for homogeneous fiber strain during ejection. Ann Biomed Eng. 27, 289–297 (1999).
- Roşca M, Lancellotti P, Popescu BA et al. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart. 97, 1982–1989 (2011).
- Mondillo S, Cameli M, Caputo ML et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 24, 898–908 (2011).
- Sonaglioni A, Lonati C, Lombardo M et al. Incremental prognostic value of global left atrial peak strain in women with new-onset gestational hypertension. J Hypertens. 37, 1668–1675 (2019).
- Lang RM, Badano LP, Victor MA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 28, 1-39.e14 (2015).
- Badano LP, Kolias TJ, Muraru D et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 19, 591–600 (2018).
- Xu TY, Sun JP, Lee APW et al. Left atrial function as assessed by speckle-tracking echocardiography in hypertension. Med (United States). 94, 1–7 (2015).
- Bello V Di, Talini E, Dell’Omo G et al. Early left ventricular mechanics abnormalities in prehypertension: A two-dimensional strain echocardiography study. Am J Hypertens. 23, 405–412 (2010).
- Onishi N, Kawasaki M, Tanaka R et al. Comparison between left atrial features in well-controlled hypertensive patients and normal subjects assessed by three-dimensional speckle tracking echocardiography. J Cardiol.63, 291–5 (2014).
- Furukawa A, Ishii K, Hyodo E et al. Three-dimensional speckle tracking imaging for assessing left atrial function in hypertensive patients with paroxysmal atrial fibrillation. Int Heart J. 57, 705–11 (2016).
- Sahebjam M, Mazareei A, Lotfi-Tokaldany M et al. Comparison of Left Atrial Function between Hypertensive Patients with Normal Atrial Size and Normotensive Subjects Using Strain Rate Imaging Technique. Arch Cardiovasc Imaging. 2, 1–6 (2014).
- Liu Y, Wang K, Su D et al. Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two-dimensional speckle tracking and volumetric parameters.Echocardiography. 31, 727–735 (2014).
- Karakurt A, Yildiz C, Yildiz A et al. Early detection strain/strain rate and time to strain/strain rate abnormalities for left atrial mechanical function in hypertensive patients. Acta Cardiol. 74, 141–51 (2019).
- Yang Y, Zhang BW, Qi LT et al. Speckle tracking analysis of left atrial phasic function in patients with hypertension. Beijing Da Xue Xue Bao Yi Xue Ban. 46, 596–600 (2014).
- Zhao Y, Sun Q, Han J et al. Left atrial stiffness index as a marker of early target organ damage in hypertension. Hypertens Res. (Lv) (2020).
- Eshoo S, Semsarian C, Ross DL et al. Comparison of left atrial phasic function in hypertrophic cardiomyopathy versus systemic hypertension using strain rate imaging. Am J Cardiol. 107, 290–6 (2011).
- Kokubu N, Yuda S, Tsuchihashi K et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens Res. 30, 13–21 (2007).
- Tadic M, Cuspidi C, Ilic I et al. The relationship between blood pressure variability, obesity and left atrial phasic function in hypertensive population. Int J Cardiovasc Imaging. 32, 603–12 (2016).
- Miyoshi H, Oishi Y, Mizuguchi Y et al. Contribution of obesity to left atrial and left ventricular dysfunction in asymptomatic patients with hypertension: A two-dimensional speckle-tracking echocardiographic study. J Am Soc Hypertens. 8, 54–63 (2014).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref