Research Article - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 3
Diagnostic and prognostic values of serum HSP70 and YKL-40 in patients with rheumatoid arthritis
- *Corresponding Author:
- Abdelhafeez Moshrif
Rheumatology and Rehabilitation Department
Al-Azhar University
Assiut, Egypt
E-mail: dr.moshrif@live.com
Abstract
Objective: We aimed to study the applicability of heat shock protein 70 (HSP70) and YKL-40 serum levels as diagnostic and prognostic factors in patients with RA. Subjects and Methods: Sixty RA patients with 60 matched healthy persons were included in this study. Disease activity levels of RA were assessed by diseases activity score-28 (DAS28). Complete Blood Count (CBC), Erythrocytes Sedimentation Rate (ESR), serum C-reactive Protein (CRP), Rheumatoid Factor (RF), Anticyclic Cetrolinated Peptide (Anti- CCP), HSP70 and YKL-40 were measured. Results: HSP70 level were significantly higher in patients with severe activity (0.922 ± 0.103 ng/mL) compared to other groups (p<0.001). Interestingly, HSP70 levels in patients within remission phase were still higher than control subjects (0.372 ± 0.176 vs. 0.173 ± 0.11 ng/mL, p<0.05). Also, there was a significant difference in HSP70 level between patients in remission and patients with mild (0.478 ± 0.255 ng/mL) or moderate (0.619 ± 0.207 ng/mL) disease activity. YKL-40 also has a similar pattern of HSP70 in different grades of RA activity. It was significantly higher in patients with severe activity of RA (386.85 ± 22.61 ng/mL) compared to other groups (p<0.001) and its level in patients within remission phase was still higher than control subjects (179.0 ± 73.19 vs. 50.26 ± 11.18 ng/mL, p<0.05), with a significant difference in YKL-40 level between patients in remission and patients with mild (230.0 ± 116.15 ng/mL) or moderate (256.74 ± 89.92 ng/mL) disease activityHSP70 predicted RA at a cutoff ≥ 0.50 ng/mL with 76.09% sensitivity and 87.84 % specificity while YKL-40 predicted those having RA in levels more than 100 ng/mL with 100% sensitivity and 78.38% specificity. Conclusion: Both HSP70 and YKL-40 can be used as markers for diagnosis and monitoring of disease activity in patients with RA.
Keywords
anti-CCP, C-reactive protein, HSP70, rheumatoid arthritis, YKL-40
Introduction
Rheumatoid Arthritis (RA) is a systemic autoimmune disease presented by symmetrical inflammatory polyarthritis affecting predominantly smaller joints such as hands and feet but can also affect large joints [1]. It is the most common cause of inflammatory arthritis affecting 0.8% of the world’s population [2].
Lots of researches are made to identify a biomarker, which alone or in combination with other conventional markers could be useful for diagnosis and monitoring of RA [3]. The 70 kDa heat shock protein (HSP70) resembles one of these markers. It is released from damaged cells after stress and has been found in the bloodstream of patients suffering from autoimmune diseases [4] including RA [5,6]. Furthermore, HSP70 could alter immune cells activity through changes in secretion of inflammatory cytokines such as tumor necrosis alpha (TNF-alpha) and interleukin-10 (IL-10), hence protecting joints from progressive destruction [7].
YKL-40, another potential biomarker of inflammatory processes, is a chitinase-like protein that binds chitin with no chitinase activity. The protein was named YKL-40 based on its three N-terminal amino acids tyrosine (Y), lysine (K) and leucine (L), and its molecular mass of 40 kDa [8].
Several studies indicate that secretion levels of YKL-40 are elevated in patients with diseases characterized by inflammation, increased extracellular remodeling and ongoing fibrosis [9] such as cancer [10], coronary artery disease [11], heart failure [12], ischemic cerebrovascular disease [13], diabetes [14], asthma [15], Chronic Obstructive Pulmonary Disease (COPD) [16], pneumonia [17], sepsis [18], osteoarthritis [19] and RA [20,21]. However, there is still a paucity of studies about the potential diagnostic and prognostic role of HSP70 and YKL-40 in RA.
The aim of our study was to investigate whether HSP70 and YKL-40 might be useful biomarkers for diagnosis and monitoring of disease activity in RA.
Subjects and methods
Sixty RA patients (19 men and 41 women) with a mean age of 38.61 ± 11.00 years were recruited from Rheumatology Clinic of Al-Azhar University Hospital (Assiut), between November 2015 and October 2016. They were classified as having RA according to ACR/EULAR 2010 criteria [22].
Patients with acute or chronic renal failure, glomerulonephritis, congestive heart failure, acute infections, pregnancy, thyroid disorders, bronchial asthma, diabetes and those admitted to hospital during the last 3 months were excluded from this study. None of the studied women were on hormonal therapy.
The age-matched control sera were obtained from 60 healthy persons; 14 men and 26 women with a mean age of 36.78 ± 12.47 years. None of them were taking any medicine and none had any signs or clinical symptoms of cancer, joint or liver disease.
The study protocol was approved by the local ethics committee of Al-Azhar University, and all patients gave an informed consent.
Examination of patients
Complete history taking including general and demographic data as age, sex, age of disease onset and disease duration were recorded. General and local examination including total number of swollen joints, joints with tenderness and extra-articular involvement were recorded. Disease activity score (DAS-28) was adopted for assessment of disease activity [23]. Drug history of patients also was recorded.
Biochemical analysis
Blood samples were collected from patients and controls (five milliliters of venous blood) using a 22G disposable syringe under aseptic technique. These specimens were divided to three aliquots: First aliquot; 1.6 ml of blood was added to a tube containing 0.4 sodium citrate for ESR measurement by Westergren method. Second aliquot; about 1.5 ml of blood was added to a tube containing k3EDTA for CBC measurement on automated cell counter Drew system (Erba® Diagnostics INC, USA). For the third aliquot, the remaining part of specimen was placed in a sterile plane tube and allowed to clot, and then serum was separated by centrifugation at 2500 rpm for 15 min. The sera were stored at -10°C until used for the following tests:
• CRP and RF were assayed by Reactivos GPL TURBILATEX® kit, Spain) using Photometer 5010 system (Robert Riele, Berlin, Germany).
• Anti- CCP levels were determined by ELISA kit (CCP31-K01, Eagle Biosciences®, USA), which measures human IgG antibody against CCP.
• Serum HSP70 was measured using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) (ADIEKS- 750, Enzo®, Switzerland). The intraand inter-assay coefficient of variation (CV) <10% with sensitivity up to 6.79 ng/L.
• Serum YKL-40 was measured using a Human Chitinase-3-like 1(YKL-40) ELISA kit (Aviscera Bioscience, INC®, USA). The intra-assay CV ranged between 4.0 and 6.0% with a sensitivity of 50 ng/L.
• ELISA technique was done on Stat Fax system (Awareness® Technology Inc. USA).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM, Chicago, IL). Data are presented as mean ± SD, or numbers as appropriate. Parametric values were analyzed using the student’s t-test. Non-parametric values were studied using the Chi-square test or Fisher’s exact test. Mann-Whitney test was used for unpaired differences. Correlations between the different parameters were calculated by the Spearman test and p values of 0.05 were considered significant.
Results
Baseline characteristics of healthy controls and patients with rheumatoid arthritis are summarized in Table 1. Patients were more likely to have a higher average of conventional markers, Anti-CCP, HSP70 and YKL-40 levels than the control group with p<0.01.
| Groups | Parameters | Control group (no=60) | Patient group (no=60) | t test | P value |
|---|---|---|---|---|---|
| Mean ± SD No or % |
Mean ± SD No or % |
||||
| Age (years) | 36.78 ± 12.47 | 38.61 ± 11.00 | 1.003 | 0.3199 | |
| Sex (Male: female) | 14:46 | 19:41 | 0.669 | 0.413* | |
| Age of onset (years) | - | 28.28 ± 7.48 | - | - | |
| Disease duration (years) | - | 10.31 ± 6.21 | - | - | |
| Extra-articular manifestations | - | 23 (38.3 %) | - | - | |
| DAS28-28: Remission | - | 8 (13.3 %) | - | - | |
| Low activity | - | 6 (10.0 %) | - | - | |
| Moderate activity | - | 39 (65.0 %) | - | - | |
| High activity | - | 7 (11.7 %) | - | - | |
| Hb g/dL | 12.71 ± 0.98 | 11.43 ± 1.49 | 5.658 | <0.0001 | |
| RBCs × 106/ml | 4.72 ± 0.19 | 4.65 ± 0.59 | 0.834 | 0.407 | |
| Platelet × 103/ml | 287.35 ± 75.86 | 252.75 ± 62.76 | 3.040 | 0.0035 | |
| WBCs × 103/ml | 8.01 ± 2.01 | 9.41 ± 3.09 | 3.085 | 0.0031 | |
| ESR mm/h | 9.06 ± 4.32 | 36.75 ± 19.24 | 11.015 | <0.0001 | |
| CRP mg/L | 3.52 ± 1.43 | 19.23 ± 13.49 | 9.065 | <0.0001 | |
| RF IU/mL | 3.17 ± 2.73 | 9.51 ± 6.51 | 6.359 | <0.0001 | |
| Anti-CCP IU/mL | 6.01 ± 2.60 | 29.62 ± 13.41 | 12.992 | <0.0001 | |
| HSP70 ng/mL | 0.173 ± 0.11 | 0.608 ± 0.24 | 12.740 | <0.0001 | |
| YKL-40 ng/mL | 50.26 ± 11.18 | 258.88 ± 99.61 | 16.253 | <0.0001 | |
*Tested by Chi-square test; DAS28: Disease Activity Score; Hb: Hemoglobin; RBCs: Red Blood Cells; WBCs: White Blood Cells; ESR: Erythrocytic Sedimentation Rate; CRP: C-reactive Protein, RF: Rheumatoid Factor; Anti-CCP: Anticitrullinated Cyclic Peptide; HSP70: Heat Shock Proteins-70; YKL-40: Tyrosine, Lysine and Leucine with Molecular Mass 40 kDa
Table 1. Baseline characteristics of healthy controls and patients with rheumatoid arthritis.
Statistical comparison of ESR, CRP, RF and Anti-CCP between patients with various disease activity levels revealed highly significant difference in ESR, CRP, RF and Anti-CCP markers in patients with severe form of RA compared to other groups. They were still higher in patients in remission stage of RA compared to healthy control group but there were no significant differences in their levels between patients with remission compared to those with mild activity of RA (Table 2).
| Groups | Parameters | Control No=60 |
Remission No=8 |
Mild No=6 |
Moderate No=39 |
Severe No=7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR mm/h | 9.06 ± 4.32 | 22.5 ± 7.58 | 25.0 ± 20.87 | 37.05 ± 12.81 | 60.71 ± 31.31 | ||||||||
| CRP mg/L | 3.52 ± 1.43 | 10.13 ± 7.02 | 10.58 ± 6.06 | 18.65 ± 9.61 | 40.21 ± 20.42 | ||||||||
| RF IU/mL | 3.17 ± 2.73 | 6.06 ± 2.99 | 8.11 ± 4.69 | 9.19 ± 6.09 | 16.42 ± 8.87 | ||||||||
| Anti-CCP IU/mL | 6.01 ± 2.60 | 18.51 ± 3.04 | 20.66 ± 3.20 | 28.37 ± 8.92 | 56.97 ± 10.81 | ||||||||
| HSP70 ng/mL | 0.173 ± 0.11 | 0.372 ± 0.176 | 0.478 ± 0.255 | 0.619 ± 0.207 | 0.922 ± 0.103 | ||||||||
| YKL-40 ng/mL | 50.26 ± 11.18 | 179.0 ± 73.19 | 230.0 ± 116.15 | 256.74 ± 89.92 | 386.85 ± 22.61 | ||||||||
| P value | |||||||||||||
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | ||||
| <0.001 | <0.001 | <0.001 | <0.001 | 0.757 | 0.003 | 0.005 | 0.056 | 0.037 | 0.001 | ||||
| <0.001 | <0.001 | <0.001 | <0.001 | 0.902 | 0.022 | 0.001 | 0.053 | 0.005 | 0.0001 | ||||
| 0.007 | 0.0002 | <0.001 | <0.001 | 0.336 | 0.165 | 0.081 | 0.680 | 0.064 | 0.010 | ||||
| <0.001 | <0.001 | <0.001 | <0.001 | 0.224 | 0.003 | <0.001 | 0.043 | <0.001 | <0.001 | ||||
| <0.001 | <0.001 | <0.001 | <0.001 | 0.373 | 0.003 | <0.001 | 0.138 | 0.001 | 0.0005 | ||||
| <0.001 | <0.001 | <0.001 | <0.001 | 0.332 | 0.026 | <0.001 | 0.522 | 0.004 | 0.0005 | ||||
P1: Patients with remission versus healthy control; P2: Patients with mild activity versus healthy control; P3: Patients with moderate activity versus healthy control; P4: Patients with severe activity versus healthy control; P5: Patients with mild activity versus those with remission; P6: Patients with moderate activity those with remission; P7: Patients with severe activity those with remission; P8: Patients with moderate activity versus those with mild activity; P9: Patients with severe activity versus those with mild activity; P10: Patients with severe activity versus those with moderate activity; ESR: Erythrocytic Sedimentation Rate; CRP: C-reactive Protein, RF: Rheumatoid Factor; Anti-CCP: Anti-citrullinated Cyclic Peptide; HSP70: Heat Shock Proteins-70; YKL-40: Tyrosine, Lysine and Leucine with Molecular Mass 40 kDa
Table 2. Studied parameters in different stages of rheumatoid arthritis activity.
HSP70 level were significantly higher in patients with severe activity (0.922 ± 0.103 ng/mL) compared to other groups (p<0.001). Interestingly, HSP70 levels in patients within remission phase were still higher than control subjects (0.372 ± 0.176 vs. 0.173 ± 0.11 ng/mL, p<0.05). Also, there was a significant difference in HSP70 level between patients in remission and patients with mild (0.478 ± 0.255 ng/mL) or moderate (0.619 ± 0.207 ng/mL) disease activity (Table 2)
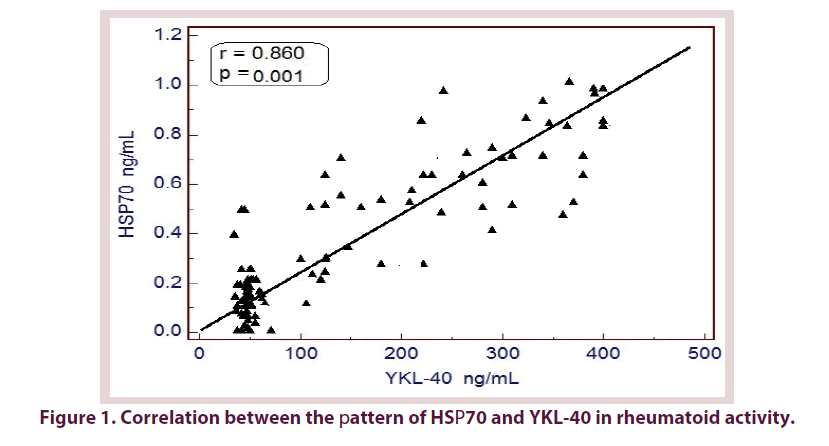
YKL-40 also has a similar pattern of HSP70 in different grades of RA activity. It was significantly higher in patients with severe activity of RA (386.85 ± 22.61 ng/mL) compared to other groups (p<0.001) and its level in patients within remission phase was still higher than control subjects (179.0 ± 73.19 vs. 50.26 ± 11.18 ng/mL, p<0.05), with a significant difference in YKL-40 level between patients in remission and patients with mild (230.0 ± 116.15 ng/mL) or moderate (256.74 ± 89.92 ng/mL) disease activity (Figure 1 and Table 2).
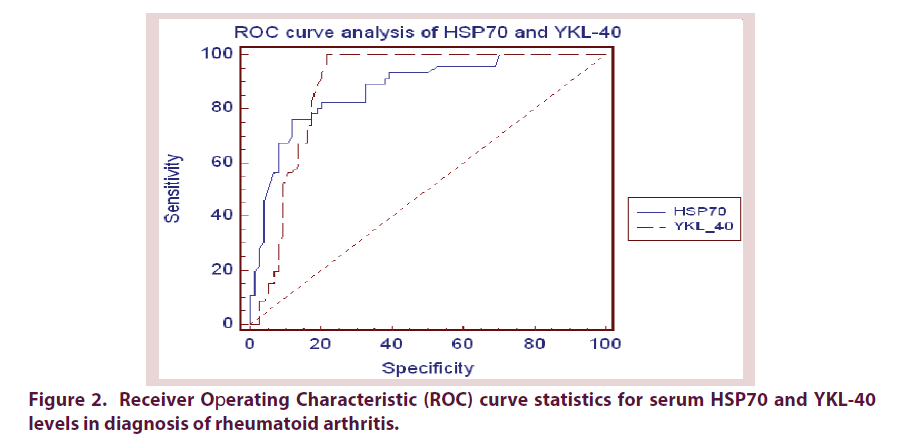
Receiver operating characteristic (ROC) curve analysis was performed to evaluate diagnostic accuracy of HSP70 and YKL-40 in discriminating patients with RA from control subjects. HSP70 tended to be a more specific (AUC was 0.873 with 87.84% specificity) than YKL-40 (AUC was 0.883 and 78.38% specificity), ESR (AUC was 0.805 and 66.22% specificity), CRP (AUC was 0.775 and 71.62% specificity) and RF (AUC was 0.864 and 85.10% specificity) tests but less specific than Anti-CCP test (AUC was 0.907 and 89.73% specificity) in diagnosing RA. Next, we investigated cut-offs in which these markers predicts state of having RA. Statistical analysis of our results presume the 10 mm/h, 5.6 mg/L, 8.2 IU/mL, 10.1 IU/ml, 0.50 ng/mL and 100 ng/mL as the optimum cut-off points of ESR, CRP, RF, Anti-CCP, HSP70 and YKL-40 respectively in diagnosis of RA (Figure 2 and Table 3).
| AUC | P value | Cut off | Sensitivity | Specificity | +LR | -LR | |
|---|---|---|---|---|---|---|---|
| ESR mm/h | 0.805 | 0.0001 | 10 | 97.83 | 66.22 | 2.90 | 0.03 |
| CRP mg/L | 0.775 | 0.0001 | 5.6 | 69.57 | 71.62 | 2.45 | 0.42 |
| RF IU/mL | 0.864 | 0.0001 | 8.2 | 84.78 | 85.10 | 5.70 | 0.18 |
| Anti-CCP IU/mL | 0.907 | 0.0001 | 10.1 | 100.0 | 89.73 | 4.93 | 0.00 |
| HSP70 ng/mL | 0.873 | 0.0001 | 0.50 | 76.09 | 87.84 | 6.26 | 0.27 |
| YKL-40 ng/mL | 0.883 | 0.0001 | 100 | 100.0 | 78.38 | 4.62 | 0.00 |
+LR: Positive Likelihood Ratio; -LR: Negative Likelihood Ratio; ESR: Erythrocytic Sedimentation Rate; CRP: C-reactive Protein; RF: Rheumatoid Factor; Anti-CCP: Anti-citrullinated Cyclic Peptide; HSP70: Heat Shock Proteins-70; YKL-40: Tyrosine, Lysine and Leucine with Molecular Mass 40 kDa
Table 3. ROC curve analysis of studied parameters of rheumatoid arthritis.
Statistically significant positive correlations were found between HSP70 and DAS28, ESR, CRP, RF and Anti-CCP. The same correlations were found between YKL-40 and these parameters. Moreover, HSP70 in serum was significantly positively correlated with serum YKL-40 (Table 4).
| HSP70 | YKL-40 | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.084 | 0.362 | 0.102 | 0.267 |
| Disease duration | 0.004 | 0.975 | 0.028 | 0.834 |
| DAS28 score | 0.457 | 0.000 | 0.382 | 0.002 |
| ESR | 0.761 | 0.000 | 0.777 | 0.000 |
| CRP | 0.717 | 0.000 | 0.699 | 0.000 |
| RF | 0.543 | 0.000 | 0.569 | 0.000 |
| Anti-CCP | 0.879 | 0.000 | 0.890 | 0.000 |
| YKL-40 | 0.860 | 0.000 | - | - |
r: Pearson Correlation Coefficient; p: Significance Level; ESR: Erythrocytic Sedimentation Rate; CRP: C-reactive Protein; RF: Rheumatoid Factor; Anti-CCP: Anti-citrullinated Cyclic Peptide; HSP70: Heat Shock Proteins-70; YKL-40: Tyrosine, Lysine and Leucine with Molecular Mass 40 kDa
Table 4. Correlation between studied parameters.
Regarding the treatment, all patients were on methotrexate or leflunamide as disease modifying anti-rheumatic drugs in addition to hydrocxychloroquine and low dose prednisolone. No one of our patients was receiving biologic treatment.
Discussion
Chronic active inflammation of the rheumatoid joint often leads to irreversible destruction of articular cartilage and subchondral bone. Measurements of acute-phase reactants as ESR and CRP are considered to be suitable biochemical markers for long-term monitoring of disease activity in RA. However, differences exist between clinical disease activity and the level of these markers [20].
Measurement of RF is not specific for diagnosis of RA and it can be found in other autoimmune diseases, non-autoimmune conditions and in 3-5% of the healthy population [24].
In our study, ESR, CRP, RF and Anti-CCP markers were higher in patients with severe RA compared to other groups of disease activity. These markers also found to be higher in patients with remission state compared to healthy control group. These results were in agreement with Serdaroğlu et al. [25] and Vanichapuntu et al. [26] studies. They found that none of these markers have definitely allowed for accurate monitoring of RA activity.
The emergence of the Anti-CCP Antibody assay, as a new serological marker for RA, helped in earlier diagnosis and changed treatment decisions of RA [27]. Anti-CCP test in conjunction with RF gives a more reliable early diagnosis of RA and found to have a prognostic value. Moreover, this biomarker was reported to be correlated with radiographic damage in RA [28].
To the best of our knowledge, few studies to date have experimentally tested the serum levels of HSP70 in patients with RA. In our study, HSP70 levels were significantly higher in patients with severe activity of RA compared to other groups of disease activity. Interestingly, HSP70 levels in remission phase were still higher than control subjects. Also, there was a significant difference in its level between patients in remission and patients with mild or moderate disease activity.
In agreement with our results, Hayem et al. [29] showed increased concentration of HSP70 in patients with RA, which was correlated with RA activity. This is contrary to a study done by Sedlackova et al. [6] showed insignificant difference in serum HSP70 levels in patients with RA compared to control subjects. However, the participants in their study were only RFpositive; while in our study both RF-positive and RF-negative patients were enrolled.
We observed that serum HSP70 levels are elevated in patients with RA and could be used as a diagnostic test for RA. Increased serum levels of HSP70 in patients with RA were independent of serum RF and anti-CCP status. ROC curve analysis revealed that HSP70 is less specific and less sensitive than anti-CCP. Nevertheless, it tends to be a specific test, rather than being a sensitive one for diagnosis of RA.
On the other hand, we demonstrated significant increase in YKL-40 serum levels in patients with high RA activity compared to those in remission, or those with low/moderate RA activity. Interestingly, patients in remission still had significant higher serum YKL-40 levels compared to control subjects. These results support the relative specificity of YKL40 in distinguishing high disease activity from other severity grades and patients in remission from healthy subjects.
The results of this study showed no correlation of serum YKL-40 with age or disease duration. This is in agreement with the finding of Vos et al. [30], Peltomaa et al. [31], Kassem et al. [20] and Kazakova et al. [32]. On the other hand, we found significant positive correlation between serum YKL-40 and ESR, CRP, RF and anti-CCP. This is in agreement with Vos et al., [30], Syversen et al. [33], Kassem et al. [20] and Kazakova et al. [32].
In this study, serum YKL-40 levels were influenced by the disease activity as evaluated by DAS-28. There was a statistically significant increase in serum YKL-40 in active RA patients in comparison to inactive patients. This finding coincided with Johansen et al. [34] and Kassem et al. [20] who confirmed that serum YKL-40 was increased in patients with active disease. Also, Syversen et al. [33] found an association between YKL-40 and DAS28 in their RA patients.
In our study, a statistically significant correlation was found between serum YKL- 40 and clinical parameters and disease activity markers. This consistent with Volck et al. [35] who also found significant correlation between serum and synovial fluid concentrations of YKL- 40 in RA patients with ESR and CRP. The same was reported by Johansen et al. [34], Vos et al. [30] and Matsumoto & Tsurumoto [36]. In another study, Johansen et al. [37] stated that approximately 70% of their RA patients with elevated serum YKL-40 had also high ESR or serum CRP indicating that ESR, serum CRP and YKL-40 levels reflect inflammation of RA patients, differently. Also, Syversen et al. [33] stated that YKL-40 is a marker of joint inflammation in RA.
We suppose that YKL-40 could provide a different and a more local view of the inflammatory process than conventional biomarkers such as ESR, CRP and RF. The explanation may lie in the fact that YKL-40 is secreted by activated macrophages in the inflamed synovial membrane [38] or released by exocytosis from specific granules of neutrophils [39] and by articular chondrocytes [40]. Inspite of the treatment, 39 (65%) of our patients presented with active RA which indicates that they could be regarded as nonresponders to the applied therapy. A similar phenomenon was recorded by Knudsen et al. [41], Takahashi et al., [42] and Zyvanovic et al. [43].
The relationship between the concentration of HSP70 and YKL-40 levels with conventional parameters and DAS28 score of disease activity indicates that both markers are associated with progression of inflammatory processes.
Conclusion
We concluded that serum levels of HSP70 and YKL-40 can be used as diagnostic tests in RA. We found a significant positive correlation between these markers and severity of RA. Positive correlation of these markers with other well-established RA markers point to the importance of HSP70 and YKL-40 as serum makers in predicting the outcome of RA.
Conflicts of interest
All authors declare no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Kazakova M, Sarafian V. YKL-40 in health and disease: a challenge for joint inflammation. Biomed. Rev. 24, 49–56 (2013).
- Trese MG, Yonekawa Y, Thomas BJ et al. Vasculitic central retinal vein occlusion: The presenting sign of seronegative rheumatoid arthritis. Am. J. Ophthalmol. Case Rep. 2, 26–29 (2016).
- Al-Salih MWA, Selman AN. Evaluation of heat shock proteins 70 (HSP70) and some risk factors in sera of rheumatoid arthritis patients in Thi-Qar province. J. Al-Nahrain Univ. 18(1), 123–129 (2015).
- Cwiklinska H, Mycko MP, Szymanska B et al. Aberrant stress-induced Hsp70 expression in immune cells in multiple sclerosis. J. Neurosci. Res. 88(14), 3102–3110 (2010).
- Rice JW, Veal JM, Fadden RP et al. Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity inmodels of rheumatoid arthritis. Arthritis Rheum. 58(12), 3765–3775 (2008).
- Sedlackova L, Sosna A, Vavrincova P et al. Heat shock protein gene expression profile may differentiate between rheumatoid arthritis, osteoarthritis, and healthy controls. Scand. J. Rheumatol. 40(5), 354–357 ( 2011).
- Luo X, Zuo X, Zhou Y et al. Extracellular heat shock protein 70 inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res. Ther. 10(2), R41 (2008).
- Tang H, Fang Z, Sun Y et al. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur. Respir. J. 35, 757–760 (2010).
- Schultz NA, Johansen JS. YKL-40—a protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers . 2, 1453–1491 (2010).
- Kastrup J. Can YKL-40 be a new inflammatory biomarker in cardiovascular disease? Immunobiology. 217(5), 483–491 (2012).
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 53, 172–209 (2006).
- Harutyunyan M, Christiansen M, Johansen J et al. The inflammatory biomarker YKL-40 as a new prognostic marker for all-cause mortality in patients with heartfailure. Immunobiology. 217, 652–656 (2012).
- Kjaergaard AD, Bojesen SE, Johansen JS, Nordestgaard BG. Elevated plasma YKL-40 levels and ischemic stroke in the general population. Ann. Neurol. 68, 672–680 (2010).
- Rathcke CN, Persson F, Tarnow L et al. YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 32, 323–328 (2009).
- Specjalski K, Jassem E. YKL-40 Protein is a Marker of Asthma. J. Asthma. 48, 767–72 (2011).
- Park J, Drazen J, Tschumperlin D. The chitinase-like protein YKL-40 is secreted by airway epithelial cells at baseline and in response to compressive mechanical stress. J. Biol. Chem. 285, 29817–29825 (2010).
- Kronborg G, Ostergaard C, Weis N et al. Serum level of YKL- 40 is elevated in patients with Streptococcus pneumonia bacteremia and is associated with the outcome of the disease. Scand J. Infect. Dis. 34, 323–326 (2002).
- Hattori N, Oda S, Sadahiro T et al. YKL-40 identified by proteomic analysis as a biomarker of sepsis. Shock. 32, 393–400 (2009).
- Gungen G, Ardic F, Findikoglu G et al. The effect of mud pack therapy on serum YKL-40 and hsCRP levels in patients with knee osteoarthritis. Rheumatol. Int. (2009).
- Kassem E, Mahmoud L, Salah W. Study of Resistin and YKL-40 in Rheumatoid Arthritis. J Am Sci. 6(10), 1004–1012 (2010).
- Nielsen K, Steffensen R, Boegsted M et al. Promoter polymorphisms in the chitinase3-like1gene influence the serum concentration of YKL-40 in Danish patients with rheumatoid arthritis and in healthy subjects. Arth. Res. Ther. (2011).
- Aletaha D, Neogi T, Silman AJ et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
- Van Gestel AM, Haagsmac J, Van Riel PLCM. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 41, 1845–1850 (1998).
- Koivula MK. Autoantibodies binding citrullinated type I and II collagens in rheumatoid arthritis. Acta. Univer. Ouluensis D Med. BBI. 19–38 (2006).
- Serdaroğlu M, Cakirbay H, Değer O et al. The association of Anti-CCP Antibodies with disease activity in rheumatoid arthritis. Rheumatol. Int. 28(10), 965–970 (2008).
- Vanichapuntu M, Phuekfon P, Suwannalai P. Are Anti-citrulline autoantibodies better serum markers for rheumatoid arthritis than rheumatoid factor in Thai population? Rheumatol. Int. J.30(6), 755–759 (2009).
- Eltokhy HM, Ali ST, Abdrabo SA et al. Relationship between anti-cyclic citrullinated peptide antibodies and disease activity and extra-articular manifestations of rheumatoid arthritis in Egyptian patients. AAMJ. 9(1), 21–35 (2011).
- Cojocaru M, Cojocaru IM, Silosi I. Autoimmunity to cyclic citrullinated peptide in rheumatoid arthritis. J. Clin. Med. 4(3), 216–220 (2009).
- Hayem G, De BandtM, Palazzo E et al. Anti-heat shock protein 70 kDa and 90 kDa antibodies in serum of patients with rheumatoid arthritis. Ann. Rheum. Dis. 58(5), 291–296 (1999).
- Vos K, Steenbakkers P, Miltenburg AMM et al. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann. Rheum. Dis. 59, 544–548 (2000).
- Peltomaa R, Paimela L, Harvey S et al. Increased level of YKL-40 in sera from patients with early rheumatoid arthritis: a new marker for disease activity. Rheumatol. Int. 20, 192–196 (2001).
- Kazakova M, Batalov A, Deneva T et al. Relationship between sonographic parameters and YKL-40 levels in rheumatoid arthritis. Rheumatol. Int. 33, 341–346 (2013).
- Syversen S, Haavardsholm E, Bøyesen P et al. (2010) Biomarkers in early rheumatoid arthritis: longitudinal associations with inflammation and joint destruction measured by magnetic resonance imaging and conventional radiographs. Ann. Rheum. Dis. 16, 1223–1225 (2010).
- Johansen JS, Olee T, Price PA et al. Regulation of YKL-40 production by human articular chondrocytes. Arthritis Rheum. 44, 826–837 (2001).
- Volck B, Johansen JS, Stoltenberg M et al. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthritis Cartilage. 9, 203–214 (2001).
- Matsumoto T, Tsurumoto T. Serum YKL-40 levels in rheumatoid arthritis: correlation between clinical and laboratory parameters. Clin. Exp. Rheumatol. 19, 655–600 (2001).
- Johansen JS, Stoltenberg M, Hansen M et al. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology. 38, 618–626 (1999).
- Kirkpatrick RB, Emery JG, Connor JR et al. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inXammatory and peripheral blood monocyte-derived macrophages. Exp. Cell. Res. 237, 46–54 (1997).
- Volck B, Price PA, Johansen JS et al. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Phys. 110(4), 351–360 (1998).
- Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 268, 25803–25810 (1993).
- Knudsen LS, Hetland ML, Johansen JS et al. Changes in plasma IL-6, plasma VEGF and serum YKL-40 during treatment with etanercept and methotrexate or etanercept alone in patients with active rheumatoid arthritis despite methotrexate therapy. Biomarker Insights. 4, 91–95 (2009).
- Takahashi M, Naito K, Abe M et al. Relationship between radiographic grading of osteoarthritis and the biochemical markers for arthritis in knee osteoarthritis. Arthritis Res. Ther. 6, 208–212 (2004).
- Zyvanovic S, Rackov LP, Vojvodic D et al. Human cartilage glycoprotein 39-biomarker of joint damage in knee osteoarthritis. Int. Orthop. 33, 1165–1170 (2009).