Review Article - Imaging in Medicine (2010) Volume 2, Issue 2
Color Doppler sonography: characterizing breast lesions
Atif Hashmi, Susan Ackerman† and Abid Irshad
Department of Radiology, Medical University of South Carolina, PO Box 250322, 169 Ashley Avenue, Charleston, SC 29425, USA
- *Corresponding Author:
- Susan Ackerman
Department of Radiology, Medical University of South Carolina
PO Box 250322, 169 Ashley Avenue Charleston
SC 29425, USA
Tel: +1 843 792 1957
Fax:+1 843 792 9503
E-mail: ackerman@musc.edu
Abstract
This article discusses the utilization of Doppler sonography in the breast. The article encompasses the use of Doppler sonography to differentiate benign from malignant lesions to assess neovascularity in tumors to assess the response after chemotherapy and to differentiate benign from malignant lymph nodes based on various blood-flow patterns. The discussion also incorporates the recent advances in the current literature regarding the value of techniques such as sonoelastography, 3D Doppler imaging and the use of tissue harmonics. While mammography remains the prime modality to evaluate breast lesions, advances in the field of ultrasound demonstrate promising results and future hope for a more specific role of ultrasound in this cause.
Keywords
3D Doppler; breast ultrasound; Doppler sonography; duplex ultrasound; power Doppler
Breast ultrasound
Breast ultrasound is an extremely useful tool to evaluate breast lesions. Ultrasound of the breast can be categorized as either a screening or a diagnostic examination. The goal of screening is to detect breast abnormalities in a large population of asymptomatic patients, whereas the goal of a diagnostic ultrasound is to perform a focused examination to either evaluate a sign or symptom, such as a palpable mass, or an abnormality noted on a previous imaging modality.
The following are the American College of Rheumatology (ACR) practice guidelines for the performance of a breast ultrasound examination [101]:
• Evaluation and characterization of palpable masses and other breast-related signs and/or symptoms;
• Evaluation of suspected or apparent abnormalities detected in other imaging studies, such as mammography or MRI;
• Initial imaging evaluation of palpable masses in women under 30 years of age and in lactating and pregnant women;
• Evaluation of problems associated with breast implants;
• Evaluation of breasts with microcalcifications and/or architectural distortion suspicious for malignancy or highly suggestive of malignancy in a setting of dense fibroglandular tissue, for detection of an underlying mass that may be obscured on the mammogram;
• Guidance of breast biopsy and other interventional procedures;
• Treatment planning for radiation therapy
Screening breast ultrasound is only recommended in selected populations owing to low specificity, high false-positive results and limited cost–effectiveness. Currently, ultrasound may be helpful in mamographically dense breasts, in cases of silicone breast implants, in pregnant patients and in intermediate- to high-risk patients, especially if they are unable to tolerate breast MRI.
In practice, ultrasound is mostly used as a diagnostic modality as studies have proven it to be less effective than mammography as a screening tool. If used according to the recommendations, diagnostic breast ultrasound prevents unnecessary negative biopsies, detects cancer that was missed or under-classified by mammography, stages cancers and determines the extent of malignant disease [1].
Doppler sonography
Continuous-wave Doppler sonography was the first development of its kind to investigate the vascularity of breast tumors. In 1977, Wells et al. published the first study demonstrating continuous- wave Doppler blood-flow signals associated with malignant tumor neovascularization [2]. This technique could be used to record vascular signals from a palpable breast mass, but this signal was difficult to observe in nonpalpable lesions [3]. Pulsed-Doppler techniques were introduced for the evaluation of these tumors. Tumors with grossly visible vessels are visualized by duplex ultrasound. However, not all tumor vessels are accessible by B‑mode imaging. Color Doppler sonography was accessible by the 1990s, allowing a combination of grayscale and Doppler technique to effectively locate tumor vessels. Technical parameters to optimize Doppler imaging include a low pulsatile-repetition frequency, ideally not exceeding 1 kHz, use of appropriate filters and amplification (slightly over the noise threshold of each ultrasonographic system). It is important that the cursor size is appropriately adjusted to vessel lumen in order to reduce the flow artifacts and augment the sensitivity [4,5]. Power Doppler allows a better detection of small vessels with slow blood circulation, but is much more sensitive to flow artifacts.
Uses of color Doppler sonography
Color Doppler sonography serves as a useful tool to differentiate cystic lesions from their solid counterparts, to distinguish benign nodules from malignant ones, to determine the aggressiveness of a suspicious lesion, to evaluate inflammatory conditions of the breast, to identify vascular abnormalities and to evaluate response to tumor therapy. Although Doppler sonography is currently considered as a useful adjunct to grayscale ultrasound to differentiate benign from malignant lesions, the definite role of Doppler sonography in this respect remains to be established. There are many new studies performed using various models to identify neovascularity that have demonstrated its promising role. However, further work is required to establish its efficacy to differentiate benign from malignant tumors.
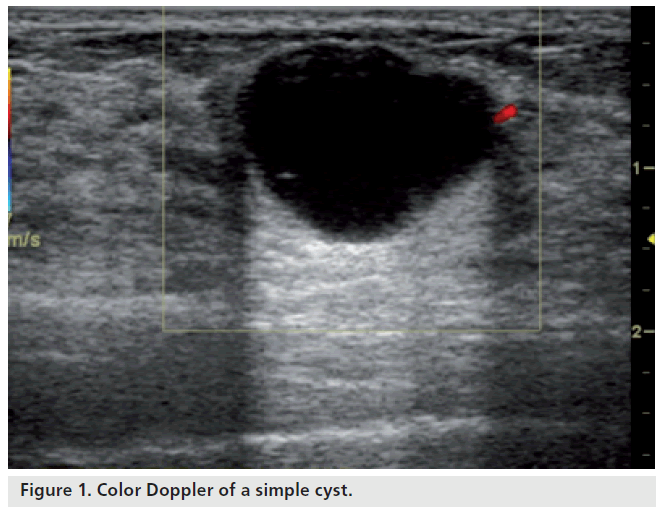
Distinguishing solid nodules from complex cysts with low-level internal echoes
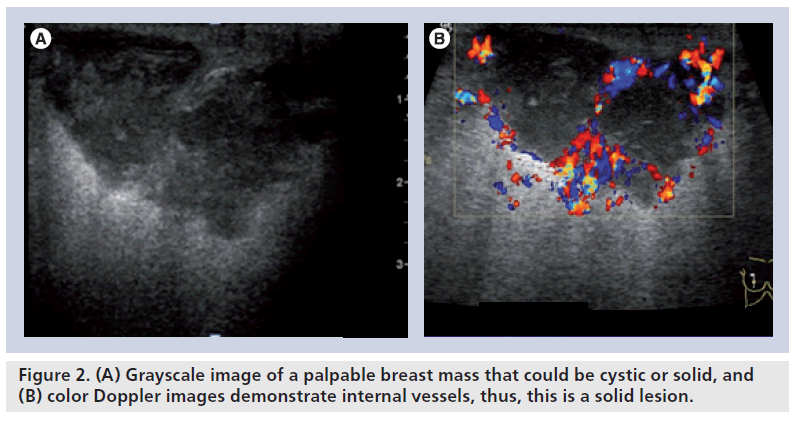
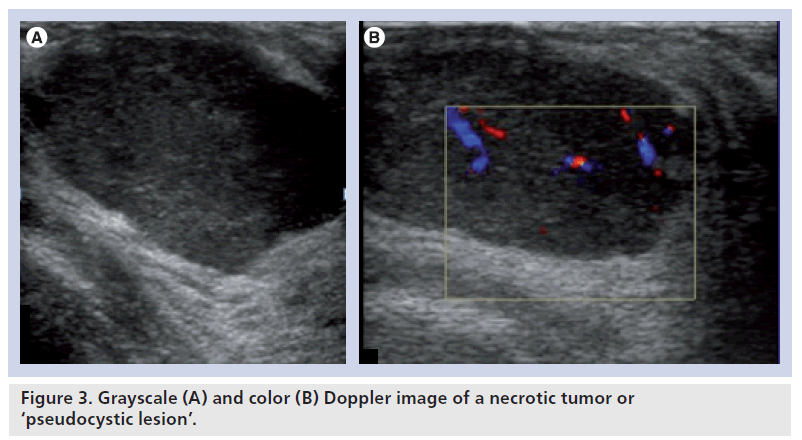
One of the most important contributions of breast ultrasound is the confident diagnosis of a simple cyst. Simple cysts have no internal blood flow (Figure 1). Some lesions detected on ultrasonography cannot be classified as either solid or cystic with certainty. These include complex benign cysts, cystic cancers and fibroadenomas. Color Doppler sonography can demonstrate an internal vessel, indicating that a lesion is solid (Figure 2). Pseudocystic lesions are solid lesions that are markedly hypoechoic and have a pseudocystic appearance. These are often misdiagnosed as cysts. Some of the tumors that may present as pseudocystic lesions include high-grade ductal carcinoma, medullary carcinoma, metastasis and lymphoma. Color Doppler sonography can demonstrate the presence of blood vessels within these pseudocystic lesions (Figure 3). False negatives can be seen in necrotic tumors presenting as pseudocystic lesions.
Figure 3. Grayscale (A) and color (B) Doppler image of a necrotic tumor or ‘pseudocystic lesion’.
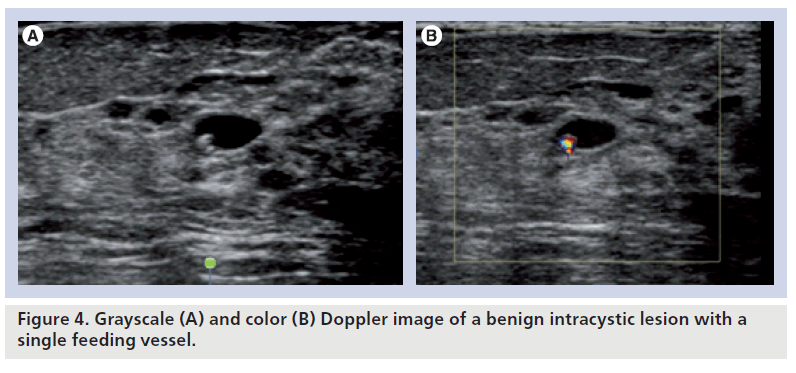
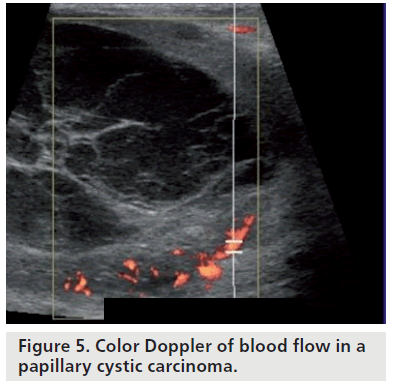
Another function of color Doppler sonography in the assessment of intracystic lesions is to evaluate fibrovascular stalks in these lesions. Benign intracystic lesions usually have a single feeding vessel within the stalk (Figure 4), while malignant intracystic lesions usually have multiple feeding vessels (Figure 5) [1]. Assessing the role of color Doppler in intracystic breast lesions, Catalano et al. analyzed the flow differences between carcinomas and papillomas. The color Doppler imaging showed a lack of flow in four papillomas and subtle flow in the remaining four papillomas. All 13 carcinomas showed diffuse vascularity, with multiple sparse vessels and multiple vascular poles. The average resistive index (RI), measurable in four out of eight papillomas, was 0.43. Spectral analysis was measured in all carcinoma cases, yielding a mean RI of 0.71 [6].
Distinguishing benign from malignant lesions
There have been numerous studies performed utilizing the color Doppler, power Doppler and spectral waveform with qualitative, quantitative and distribution analysis of the tumor vascularity to see its usefulness for the purpose of differentiating benign from malignant lesions. Some have demonstrated promising results while others have found Doppler sonography not very helpful in this regard. Among the initial studies performed for this purpose, Cosgrove et al. found that almost 99% of malignant lesions presented a color Doppler signal, whereas only 4% of benign lesions were found to have a detectable vascularization [7]. Sehgal et al. demonstrated that benign lesions were found to be 2.2-times more vascularized than normal breast parenchyma, whereas malignancies were five-times more vascularized [8]. Vessel distribution was found to be equivalent for the core and periphery of the lesion in case of benignity, whereas malignant lesions had greater vascularization towards their center. A higher number of penetrating vessels and higher values of pulsatility index (PI), RI and maximum velocity were considered as indicators of malignancy [8]. In another study, Raza et al. obtained a sensitivity of 68% and a specificity of 85% in distinguishing benign nodules from malignant nodules [9].
However, in the study of Ozdemir et al., neither morphologic nor spectral Doppler analysis proved to be successful on its own, but the information obtained could increase the diagnostic certainty of grayscale ultrasound and mammography [10]. Similar results were obtained in the study by Buadu et al., who concluded that even the combination of color and spectral Doppler analysis does not appear to contribute significantly to the differentiation between benign and malignant breast lesions [11]. Currently, color Doppler imaging holds a complementary role in the evaluation of breast lesions.
Utilizing differences in blood-flow physiology
Blood flow to a tissue correlates with the level of its metabolic activity. Most benign lesions have low metabolic demands and, therefore, require a relatively low blood supply. Malignant lesions usually have high metabolic demands and necessitate a greater amount of blood flow.
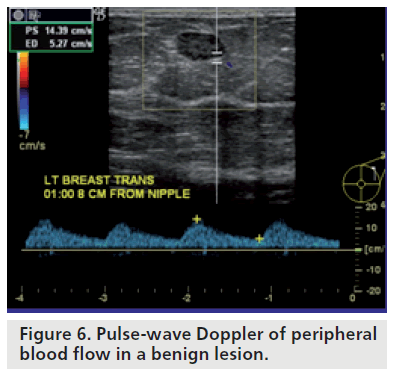
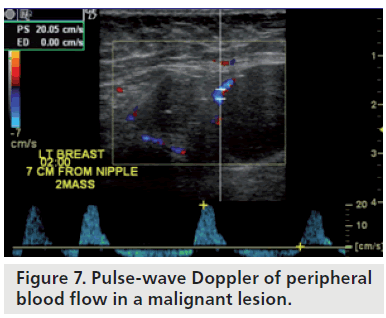
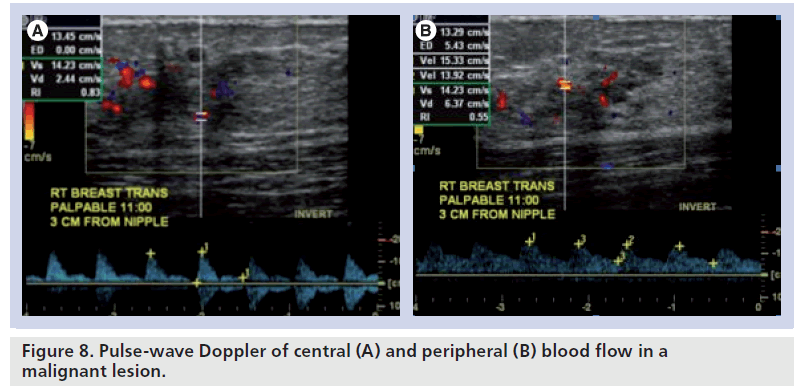
Doppler sonography is able to detect neovascularity based on a number of factors. Tumors are neovascular and, thus, have an increased number of vessels supplying them. Often, these tumors are surrounded by vessels and this is referred to as a pattern of peripheral net of vessels. Normal capillary beds are usually absent in tumors and thus the sinusoidal spaces serve as arteriovenous connections. This results in blood flow with a higher velocity than normal and a resistance that is lower than normal. Pulsed Doppler waveforms may help to distinguish benign lesions from malignant lesions by flow pattern (peripheral vs central blood flow), peak systolic velocity and RI. Benign lesions exhibit central and peripheral blood flow in equal amounts, have relatively low peak systolic velocity, have a rounded systolic peak and have lower RI (Figure 6). Malignant lesions exhibit differences in blood flow centrally and peripherally. Peripheral blood flow has lower systolic velocity, round systolic peak and lacks a diastolic notch (Figure 7). Central blood flow has high systolic velocity, a sharp systolic peak and a high RI. A noticeable difference in waveform patterns between central and peripheral vessels is a strong predictor of malignancy (Figure 8). Nevertheless, it should be kept in mind that overlap may exist between benign and malignant lesions.
In a recent study, del Cura et al. suggested diagnostic criteria for Doppler sonography of breast lesions [12]. They performed power and duplex color Doppler sonography in breast lesions to evaluate lesion vascularity, PI and RI of the vessels, and correlated with histologic results. Although color flow was more frequently seen in malignant than in benign lesions, the sensitivity, specificity, and positive- and negative-predictive values for this sign were low (68, 64, 58 and 73%, respectively). The RI and PI values were appreciably higher in cancers. Although an overlap in these values between benign and malignant lesions was observed, no noteworthy relationship was found between PI, RI or flow visualization on power Doppler sonography and tumor grade or lymph node involvement in cancers. Power Doppler sonography pointed out a higher likelihood of malignancy but did not turn out to be valuable as a key sign for malignancy.
Kwak et al. assessed the role of power Doppler sonography in distinguishing benign from malignant solid breast masses when used in conjunction with grayscale sonography and the relationship with an axillary lymph node metastasis [13]. On power Doppler, the presence of identified or penetrating vessels within the mass was evaluated. Among malignant cases, tumor vascularity was correlated with lymph node involvement. Controlling the size of the masses, the identified or penetrating vessels were considerably more numerous in malignant lesions. They used the identified and penetrating vessels in the mass as one of the diagnostic criteria for malignancy and demonstrated that the diagnostic accuracy was improved. Therefore, they concluded that identified or penetrating vessels within the mass on power Doppler sonography can be used as one of the malignant criterion. Yang et al. showed a significant correlation between color power Doppler sonographic measurement of tumor vascularity and microvessel density by immunohistochemical analysis [14].
Using computer-aided systems & 3D models
While trying to obtain additional information regarding the present diagnostic approaches in breast tumor diagnosis via the investigation of tumor vessels, Du et al. presented a new technique that helps to capture the morphological features from power Doppler sonography images using a computer-aided system facilitating pixel relation analysis techniques in the region of interest [15]. The use of color range was statistically significant to make a distinction between benign lesions and malignant lesions by counting more pixels in malignant cases. Deyle et al. utilized a computer-aided system facilitating pixel relation in their study to evaluate the morphological features from power Doppler sonography images [16]. Various histologically proven solid breast tumors were studied using quantitative and qualitative parameters, with extracted 3D diagrams focused on pixel counting and on physiologic and pathophysiologic vascular analysis over time. The color range demonstrated a statistical significance for distinguishing between benign and malignant lesions by counting more pixels in malignant cases. They detected differences in the blood flow dynamics with a characteristic flow texture in 89% of benign lesions and periodic oscillations that were identified with a diagnostic accuracy of 78% in malignant cases [16].
Hsiao et al. proposed a decision model to distinguish benign from malignant tumors, assessing tumor vascularity utilizing 3D power Doppler breast ultrasound [17]. They used the Virtual Organ Computer-Aided Analysis (VOCAL) imaging program to analyze the stored volume. Histogram indices of the vascularization index (VI), flow index (FI) and vascularization–flow index (VFI) demonstrated that for both intratumor and shells with a thickness of 3 mm surrounding the breast lesion, the malignant lesions had a higher VI, FI and VFI compared with the benign lesions. They demonstrated that the model (including patient age, volume and intratumor FI in 3D power Doppler vascularity) was the best choice for malignant breast tumor characterization. In another study, Hsiao et al. compared the diagnostic performance of nonharmonic ultrasound and tissue harmonic imaging using 3D power Doppler sonographic technique to classify benign and malignant breast tumors by vascularization. They found that the performance of 3D power Doppler sonography with respect to the characterization of solid breast masses as benign or malignant was not significantly improved with tissue harmonic imaging [18].
LeCarpentier et al. also used 3D quantitative models of color Doppler analysis and found that the quantitative Doppler sonography vascularity measurements contribute considerably to malignant breast tissue identification beyond subjective grayscale evaluation alone [19].
Contrast-enhanced sonography
The second-generation contrast agents intensively increase the signal level of breast lesions and new sequences, such as coherence pulse sequencing, might be accurate to detect malignant vessels in breast lesions. This can be helpful to characterize and assess the extent of infiltrative breast carcinoma or to evaluate the tumor response after chemotherapy. Another possible clinical application is the differentiation between postoperative changes and recurrences [20]. In malignant lesions, enhancement is fast, starting with less than 20 s. Compared with MRI, enhancement may be more rapid [20]. Balleyguier et al. found that the malignant vessels were predominant in the external ring of the nodule; conversely vessels were seen in the center of the lesion in benign nodules. Malignant vessels were also seen outside the lesion [20].
Forsberg et al. used contrast-enhanced (Levovist®) color flow ultrasound images of the breast lesions to compare quantifiable measures of vascularity by ultrasound with pathologic vascularity [21]. From the ultrasound images, the number of color pixels before and after contrast administration, relative to the total area of the breast mass, was calculated. There was a significant increase in flow visualization from pre- to post-contrast injection (p = 0.001), but no differences were found between the 11 benign and the eight malignant lesions (p > 0.35). They concluded that contrast-enhanced color flow imaging provides a noninvasive measure of breast tumor neovascularity, corresponding mainly to vessels 20–39 μm in diameter, when used in a typical clinical setting [21].
Sonoelastography
Intrinsic elastic properties of a tissue can be illustrated by sonoelastography. Thomas et al. presented a study to investigate the combined effect of B‑mode imaging with tissue Doppler imaging and offline analysis of tissue strain imaging on the specificity of the B‑mode ultrasound [22]. They performed B‑mode ultrasound on an equal number of benign and malignant focal breast lesions and the images were overlaid with color-coded tissue Doppler imaging information. Area quotients:

were calculated and tissue strain imaging views were reconstructed offline from the source data. Tissue elasticity was evaluated using a scale of 1–5 corresponding to the Breast Imaging- Reporting and Data System (BI‑RADS) categories. Maximum strain factor was determined in the region of interest. Sensitivities and specificities were calculated and statistical analysis was performed. Sensitivity and specificity turned out to be 96 and 68% for B‑mode ultrasound, 100 and 40% for combined B‑mode ultrasound and mammography and 96 and 80% for tissue strain imaging. There was an improvement in the characterization of focal breast lesions by evaluating elasticity based on the quantification of strain factors. The simple technique of tissue Doppler imaging showed a lower tissue displacement in malignant cases.
Combining color Doppler flow & imaging changes of breast fascia & ligament
Zeng et al. tried to explore the value of a combined use of color Doppler flow and imaging changes of breast fascia and ligament in the differential diagnosis of small breast neoplasms [23]. They performed color Doppler flow on benign and malignant lesions and analyzed the blood flow and Doppler image both in and around the breast lesions. The infiltration of breast fascia and ligament was also taken into account. The color Doppler flow imaging results were compared with the results of a pathologic examination. Color Doppler flow imaging correctly diagnosed benign and malignant small breast lesions (90.4 and 84.5% correct diagnosis rate, respectively). Zeng et al. also noted that there were observable differences in blood flow grading, systolic maximum velocity, RI and imaging changes of superficial fascia, deep fascia and Cooper’s ligament between benign and malignant small breast lesions. This combination may turn out to be valuable in clinical diagnosis and differential diagnosis of small breast neoplasms.
Utilizing neural network models
Using a neural network model as a classifier, Kuo et al. evaluated the role of 3D power Doppler sonography in the differential diagnosis of solid breast tumors [24]. 3D power Doppler sonography was used to calculate three indices (VI, FI and VFI) for the tumor itself and for the tumor plus a 3 mm shell surrounding it. The data were applied to a multiplayer perception neural network model and the model was evaluated as a classifier to assess the capability of 3D power Doppler sonography to distinguish between benign and malignant solid breast tumors. The accuracy of the multilayer perceptron model for classifying malignancy was 84.6%, the sensitivity was 90.3%, the specificity was 79.4%, the positive predictive value was 80.0% and the negative predictive value was 90.0%. They also used neural network to combine the three 3D power Doppler indices and demonstrated that the area under the receiver-operating characteristics curve was 0.89. Therefore, it can be concluded that the capability of 3D power Doppler sonography may be increased by using an multilayer perceptron neural network model as a classifier.
The study by Chen et al. used logistic regression analysis, a support vector machine and a neural network on a database that contained 3D power Doppler imaging pairs of nonharmonic and tissue-harmonic images for 97 benign and 86 malignant solid tumors. They found the Az values of six receiver-operating characteristic curves for the use of logistic regression analysis, support vector machine and neural network for nonharmonic or harmonic 3D power Doppler imaging to be similar. In their view, depending on user emphasis for the use of receive-operating characteristic curve findings, the use of logistic regression analysis appears to provide better sensitivity compared with the other statistical models [25].
Neovascularization provides blood supply for tumor expansion. It also increases the likelihood of tumor cells entering the blood or lymphatic circulation. Proangiogenic factors such as VEGF and angiopoietin are implicated in the growth of endothelial cells. Tumor vasculature is highly disorganized, tortuous and dilated, with an uneven diameter and excessive branching. Chang et al. presented a new method that helps to capture different morphologic features from 3D power Doppler sonography images [26]. They narrowed down the vessels into their skeletons using a 3D-thinning algorithm and extracted seven features, including vessel-to-volume ratio, number of vascular trees, number of bifurcation, mean of radius and three tortuosity measures from the skeleton. They applied a neural network to classify the tumors by using these features. The accuracy, sensitivity, specificity and positive- and negative-predictive values were 80.09, 80.18, 80.00, 80.18 and 80.00%, respectively, with an Az value of 0.89. The results were in agreement with the pathologic diagnosis. They found that 3D power Doppler sonography images and neural network analysis of features can aid in the classification of breast tumors as benign or malignant [27].
Limitations
One should be aware that there are certain limitations of Doppler sonography. Low cellular malignant lesions, particularly the ones composed of desmoplastic tissue, typically have less vascularity; hence, they have the potential to be missed on color Doppler sonography. Nevertheless, benign tumors containing cellular stroma can be more vascular than low-grade cancers. Some examples of benign lesions exhibiting high flow on color Doppler sonography include juvenile fibroadenoma and lactating adenoma. The use of color Doppler in demonstrating the peripheral vascular pattern around malignant tumors is limited, depending on the lesion cellularity. Low-grade invasive ductal carcinoma may show little blood flow, while high-grade invasive ductal carcinoma usually exhibits a large peripheral vascular pattern.
Assessing the aggressiveness of a suspicious lesion
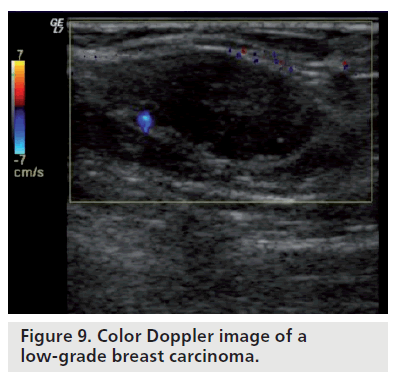
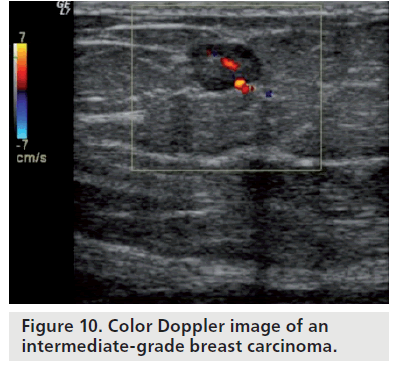
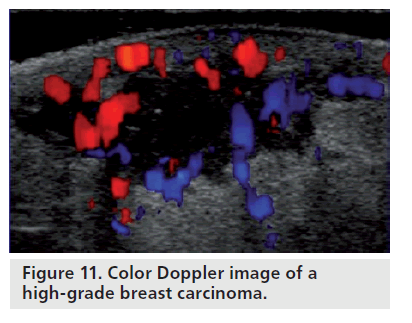
Color Doppler sonography can be used to determine the aggressiveness of a suspicious lesion. Neovascular lesions usually have a higher grade and may be associated with lymph node involvement and metastasis. Lower grade, less cellular lesions have fewer angiogenesis factors and, therefore, have less neovascularity. On color Doppler, low-grade invasive carcinomas may have little or no internal vascularity; only feeding vessels are usually identified (Figure 9). Intermediate-grade invasive cancers may have internal and peripheral flow on color Doppler (Figure 10). High-grade invasive tumors may have a pattern of a peripheral net of vessels (Figure 11). In a study performed by Svensson et al., the observed benign vascular patterns were avascularity, vascularity in the periphery and peripheral marginal vessels connecting with internal vascularity. The observed malignant vascular patterns were radially aligned external vessels with internal vessels being more numerous than external vessels that connected to radial vessels. According to their findings, any breast lesion with radial rather than marginal connecting vessels should be regarded with suspicion [28].
Use of color Doppler to demonstrate inflammatory hyperemia
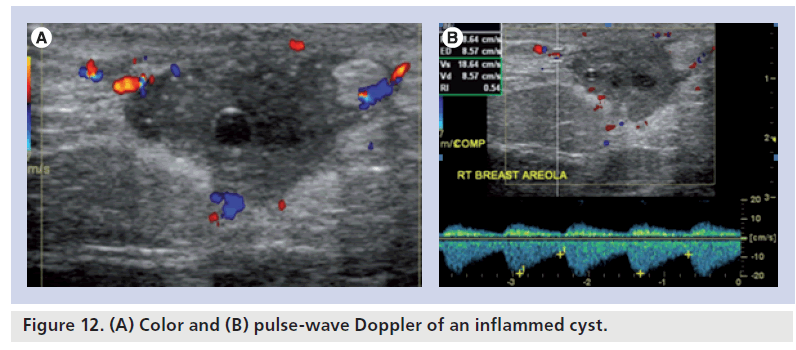
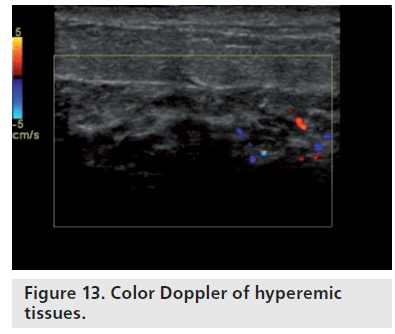
On color Doppler sonography, acutely inflamed cysts exhibit wall thickening, fluid-debris levels and hyperemia within the cyst wall (Figure 12). Breast cysts that are not inflamed exhibit an echogenic, thin outer wall. Usually, blood flow cannot be detected within the wall of noninflammed cysts. Superficial cellulitis (Figure 13) shows hyperemia of subcutaneous fat, skin and fibrous elements. Comparison with the contralateral mirror image may be helpful in differentiating mild edema.
Use of color Doppler to evaluate the lumpectomy site
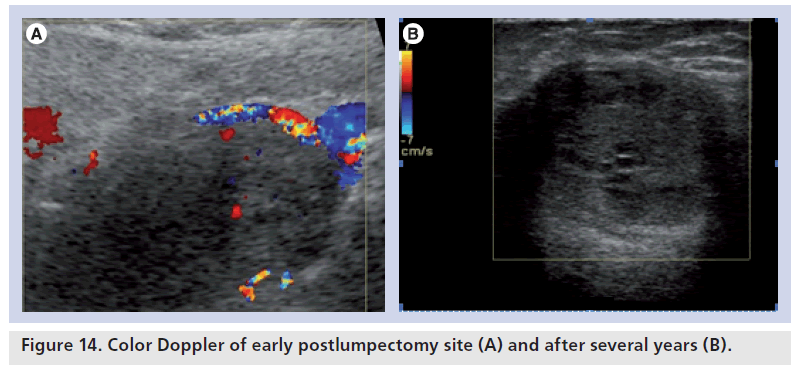
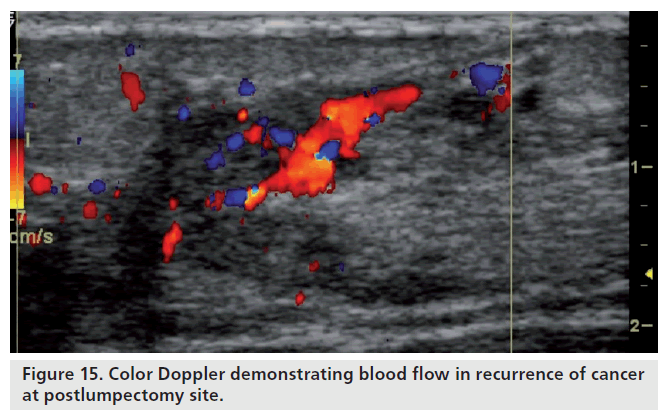
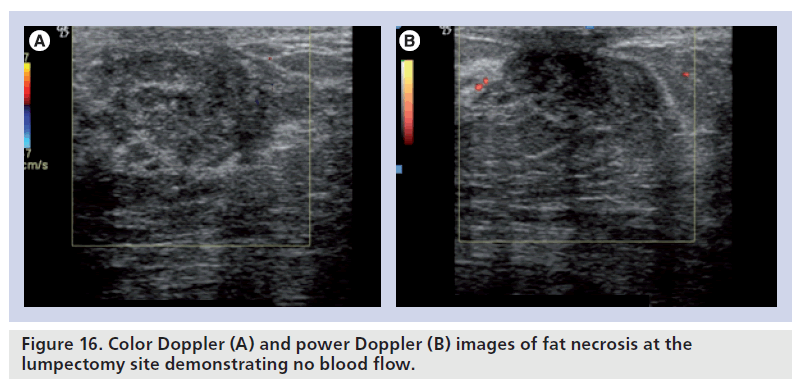
In the early postoperative period, lumpectomy cavities demonstrate flow on color Doppler sonography, indicating a healing response (Figure 14). After 6 months, very little blood flow is noted in the lumpectomy scar. Newly detected blood flow in a lumpectomy site several years later is likely to be a recurrent breast cancer (Figure 15). On grayscale, fat necrosis may show suspicious appearance and mimic breast cancers. On color Doppler, it may show some peripheral vascularity in acute phase and may resemble breast cancer, requiring a biopsy to establish tissue diagnosis. However, fat necrosis usually exhibits no significant blood flow (Figure 16). In general, a positive study showing blood flow is a better positive predictor of recurrent cancer, than lack of blood flow is a negative predictor.
Figure 15.Color Doppler demonstrating blood flow in recurrence of cancer at postlumpectomy site.
Assessing vascular conditions in the breast
Vascular conditions of the breast, for example, superficial venous thrombosis, venous malformations and cavernous hemangiomas can be detected by Doppler sonography of the breast. Thrombosed veins do not show any blood flow on Doppler and tend to be incompressible. Venous malformations may show dilated venous channels and fibrous elements.
Assessing acoustic vibratory response to vocal fremitus
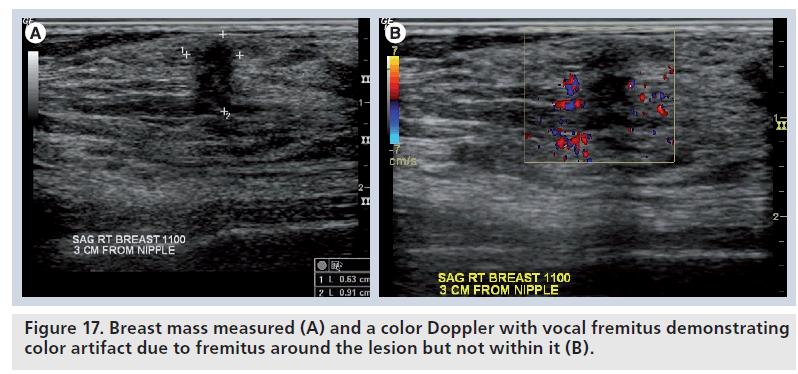
Acoustic vibratory response to vocal fremitis was first described by Sohn et al. in Germany [29]. The underlying mechanism is that when a patient speaks, vibrations from the chest wall cause color artifacts in normal tissue, but not tumors. Even though it is not useful to distinguish benign from malignant lesions, it may be helpful in distinguishing normal from abnormal tissue and differentiating isoechoic tissue from tumor tissue (Figure 17). Power Doppler is considered more effective than color Doppler to assess acoustic vibratory response to vocal fremitus.
Kim et al. conducted a study to illustrate the method and use of power Doppler vocal fremitus sonography to detect and diagnose breast lesions [30]. They evaluated and compared various breast conditions, including normal anatomic structures and abnormal lesions with B‑mode and power Doppler vocal fremitus sonography. They were successful in differentiating between isoechoic tumors and isoechoic glandular tissues, and in discriminating entrapped fat lobules from isoechoic tumors with power Doppler vocal fremitus sonography. They were also able to determine if intracystic echoes are attached to the cyst wall. Hence, power Doppler vocal fremitus imaging may prove to be a valuable adjunct tool to B‑mode sonography to evaluate breast lesions.
Monitoring response to tumor therapy
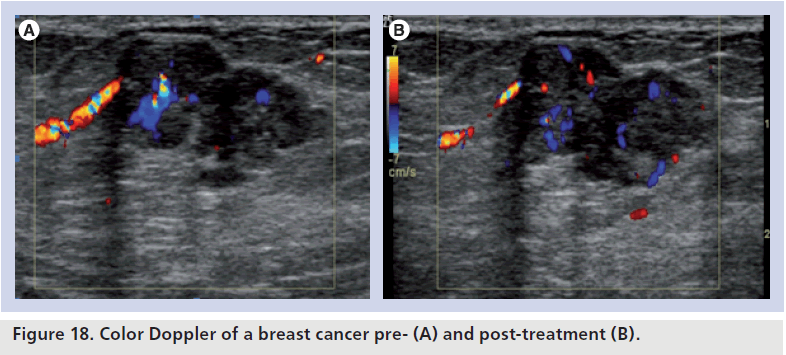
The use of color Doppler to monitor the response to tumor therapy is based on the principle that tumor vascularity may decrease before tumor volume after undergoing tumor therapy, including chemotherapy, radiotherapy, antiangiogenesis and immunotherapy. It is of particular use in antiangiogenesis therapy as a decrease in blood supply could be immediately picked up by Doppler sonography (Figure 18), before a reduction in tumor size is recognized on ultrasound.
Figure 18.Color Doppler of a breast cancer pre- (A) and post-treatment (B).
Neoadjuvant chemotherapy improves local control and surgical outcome in advanced breast cancer with severe local disease (stage III/IV). However, approximately 20–30% of these cancers either do not respond or show poor response to chemotherapy. Hence, it is necessary to predict clinical response to neoadjuvant chemotherapy to avoid unnecessary treatment. In a study conducted by Kuo et al., two parameters – VI increment greater than 5% and peak VI greater than 10% – appeared to be the potential early predictors for good responses to neoadjuvant chemotherapy within 1 month in patients with advanced breast cancer [31]. Singh et al., in their study, observed that the patients with an intratumoral blood flow velocity increase following chemotherapy had a greater likelihood of local recurrence and metastasis compared with patients in whom blood flow velocity decreased following chemotherapy [32]. The study also confirmed a greater correlation between Doppler peak systolic velocity and clinical assessment. Tumor blood flow velocity measured by Doppler sonography can be used as an independent marker of disease- free survival in patients with breast cancer [32].
Differentiating benign from malignant lymph nodes
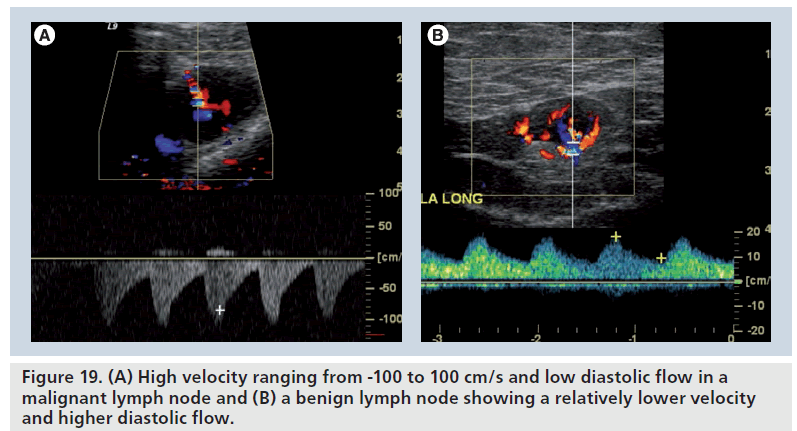
Approximately 85% of the time, breast cancer metastases to lymph nodes have the same histiologic type as the primary tumor [1]. If the primary tumor is hypervascular, the lymph node metastases are usually hypervascular as well. In cases where Doppler waveforms of the primary tumors are characterized by high peak systolic flows and resistivity indices, the same findings will likely be seen in malignant lymph nodes (Figure 19A). Benign lymph nodes usually exhibit lower systolic velocities, rounded systolic peaks and lower reistivity indices (Figure 19B).
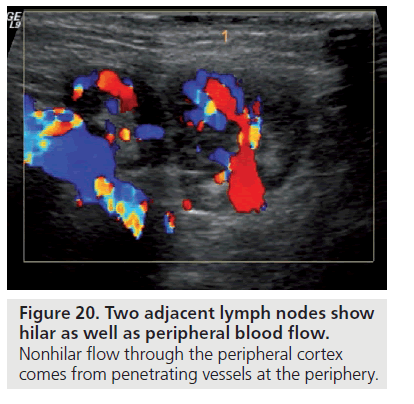
The vascular supply of a normal lymph node enters and exits from the hilum and there are no vessels transversing the capsule. Neoplastic nodes may have multiple feeding vessels and one of the most specific findings for lymph node metastases is presence of transcapsular feeding vessels (Figure 20).
Power Doppler is more sensitive than color Doppler and may be better for demonstrating transcapsular vessels. A recent article by Kwak et al. evaluated solid breast lesions and correlated the incidence of malignancy with lymph node metastasis evaluating penetrating vessels using power Doppler techniques [13]. Santamaria et al. prospectively compared power Doppler sonographic findings of invasive breast carcinoma with histiopathologic and immunohistochemical parameters to see whether arterial vascular pattern of the primary tumor can predict the nodal status [33]. They concluded that while Doppler findings and tumor size are independent predictors of axillary nodal status, these variables can contribute to the reliable prediction of the absence of axillary nodal involvement on the basis of a mathematical model.
Recommendation
Color Doppler sonography is best used in conjunction with grayscale imaging. It should be used with other modalities for the final assessment of breast lesions. However, it should be noted that negative results should not be used to negate tissue sampling of a suspicious lesion.
Future perspective
A significant portion of current research in the field of ultrasound imaging is devoted to the use of microbubble techniques in evaluating the vascularity of breast tumors and angiogenesis effects. Microbubbles serve as echo enhancers by expanding and contracting to generate backscatter when exposed to an ultrasound beam [34]. At low ultrasound beam power, the bubbles oscillate in a linear manner and the frequency of the scattered signal is not altered. At a higher power the microbubbles behave in a nonlinear fashion as they resist contraction under positive pressures more than expansion under negative pressures. This nonlinear response resulting in the emission of harmonics, which are specific to the microbubbles and range between 1 and 20 MHz, needs to be selectively displayed by novel techniques, such as pulse inversion harmonic imaging [34]. As an increasing number of harmonic and phase inversion techniques are being developed, the use of microbubble technique in evaluating the vascularity of breast tumors and angiogenesis effects is likely to be increased.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
*of interest
* of considerable interest
References
- Stavros AT: Breast Ultrasound (1st Edition). Lippincott Williams & Wilkins, PA, USA (2004).
- Wells PT, Halliwell M, Skidmore R, Webb AJ, Woodcock JP: Tumour detection by ultrasonic Doppler blood-flow signals. Ultrasonics 15, 231–232 (1977).
- Minasian H, Bamber JC: A preliminary assessment of an ultrasonic Doppler method for the study of blood flow in human breast cancer. Ultrasound Med. Biol. 8, 357–364 (1982).
- Giuseppeti GM, Baldassarre S, Marconi E: Color Doppler sonography. Eur. J. Radiol. 27, S254–S258 (1998).
- Athanasiou A, Tardivon A, Ollivier L et al.: How to optimize breast ultrasound. Eur. J. Radiol. 69(1), 6–13 (2009).
- Catalano O, Raso MM, D’Aiuto M et al.: Additional role of colour Doppler ultrasound imaging in intracystic breast tumours. Radiol. Med. 114(2), 253–266 (2009).
- Cosgrove D, Kedar R, Bamber JC et al.: Breast diseases: color Doppler US in differential diagnosis. Radiology 189, 99–104 (1993). & One of the first works regarding the use of color Doppler in breast.
- Sehgal C, Arger P, Rowling S et al.: Quantitative vascularity of breast masses by Doppler imaging: regional variations and diagnostic implications. J. Ultrasound Med. (19), 427–440 (2000).
- Raza S, Baum J: Solid breast lesions: evaluation with power Doppler US. Radiology (203), 164 (1997). nn One of the first articles regarding the use of power Doppler in breast lesions.
- Ozdemir A, Ozdemir H, Maral I et al.: Differential diagnosis of solid breast lesions: contribution of Doppler studies to mammography and gray scale imaging, J. Ultrasound Med. 20(10), 1091–1101 (2001).
- Buadu L, Murakami J, Murayama S et al.: Colour Doppler sonography of breast masses: a multiparameter analysis. Clin. Radiol. 52(12), 917–923 (1997).
- del Cura JL, Elizagaray E, Zabala R et al.: The use of unenhanced Doppler sonography in the evaluation of solid breast lesions. Am. J. Roentgenol. 184(6), 1788–1794 (2005).
- Kwak JY, Kim EK, Kim MJ et al.: Power Doppler sonography: evaluation of solid breast lesions and correlation with lymph node metastasis. Clin. Imaging 32(3), 167–171 (2008).
- Yang WT, Tse GM, Lam PK et al.: Correlation between color power Doppler sonographic measurement of breast tumor vasculature and immunohistochemical analysis of microvessel density for the quantitation of angiogenesis. J. Ultrasound Med. 21(11), 1227–1235 (2002).
- Du J, Li FH, Fang H et al.: Microvascular architecture of breast lesions: evaluation with contrast-enhanced ultrasonographic micro flow imaging. J. Ultrasound Med. 27(6), 833–842 (2008).
- Deyle S, Jörn H, Schmid-Schönbein H, Rath W: Quantitative and qualitative analysis of the blood flow in breast tumors using power Doppler ultrasound. Ultraschall Med. 5, 233–238 (2008).
- Hsiao YH, Kuo SJ, Liang WM et al.: Intra-tumor flow index can predict the malignant potential of breast tumor: dependent on age and volume. Ultrasound Med. Biol. 34(1), 88–95 (2008).
- Hsiao YH, Huang YL, Liang WM et al.: Characterization of benign and malignant solid breast masses: harmonic versus nonharmonic 3D power Doppler imaging. Ultrasound Med. Biol. 35(3), 353–359 (2009).
- LeCarpentier GL, Roubidoux MA, Fowlkes JB et al.: Suspicious breast lesions: assessment of 3D Doppler US indexes for classification in a test population and fourfold cross-validation scheme. Radiology 249(2), 463–470 (2008). & More recent work using 3D Doppler and computer models.
- Balleyguier C, Opolon P, Mathieu MC et al.: New potential and applications of contrast-enhanced ultrasound of the breast: own investigations and review of the literature. Eur. J. Radiol. 69 (1), 14–23 (2009). & More recent work and review regarding use of contrast agent in breast lesions.
- Forsberg F, Kuruvilla B, Pascua MB et al.: Comparing contrast-enhanced color flow imaging and pathological measures of breast lesion vascularity. Ultrasound Med. Biol. 34(9), 1365–1372 (2008).
- Thomas A, Warm M, Hoopmann M et al.: Tissue Doppler and strain imaging for evaluating tissue elasticity of breast lesions. Acad. Radiol. 14(5), 522–529 (2007).
- Zeng H, Zhao YL, Huang Y et al.: Values of color Doppler flow imaging and imaging changes of breast fascia and ligament in differential diagnosis of small breast neoplasms. Ai Zheng 25(3), 339–342 (2006).
- Kuo SJ, Hsiao YH, Huang YL et al.: Classification of benign and malignant breast tumors using neural networks and threedimensional power Doppler ultrasound. Ultrasound Obstet. Gynecol. 32(1), 97–102 (2008).
- Chen ST, Hsiao YH, Huang YL et al.: Comparative analysis of logistic regression, support vector machine and artificial neural network for the differential diagnosis of benign and malignant solid breast tumors by the use of three-dimensional power Doppler imaging. Korean J. Radiol. 10(5), 464–471 (2009).
- Chang RF, Huang SF, Moon WK et al.: Computer algorithm for analysing breast tumor angiogenesis using 3-D power Doppler ultrasound. Ultrasound Med. Biol. 32(10), 1499–1508 (2006).
- Chang RF, Huang SF, Moon WK et al.: Solid breast masses: neural network analysis of vascular features at three-dimensional power Doppler US for benign or malignant classification. Radiology 243(1), 56–62 (2007). & Recent work with computer models and neural network.
- Svensson WE, Pandian AJ, Hashimoto H et al.: The use of breast ultrasound color Doppler vascular pattern morphology improves diagnostic sensitivity with minimal change in specificity. Ultraschall Med. (2010) (Epub ahead of print).
- Sohn C, Baudendistel A, Bastert G: Diagnosis of the breast tumor entity with “vocal fremitus” in ultrasound diagnosis. Bildgebung 61(4), 291–294 (1994).
- Kim MJ, Kim EK, Youk JH et al.: Application of power Doppler vocal fremitus sonography in breast lesions. J. Ultrasound Med. 25(7), 897–906 (2006).
- Kuo WH, Chen CN, Hsieh FJ et al.: Vascularity change and tumor response to neoadjuvant chemotherapy for advanced breast cancer. Ultrasound Med. Biol. 34(6), 857-866 (2008).
- Singh G, Kumar P, Parshad R et al.: Role of color Doppler indices in predicting disease-free survival of breast cancer patients during neoadjuvant chemotherapy. Eur. J. Radiol. (2010) (Epub ahead of print).
- Santamaria F, Velasco M, Farre X et al.: Power Doppler sonography of invasive breast carcinoma: does tumor vascularization contribute to prediction of axillary status? Radiology 234, 374–380 (2005). & Interesting work regarding the predictability of lymph-node status based on Doppler findings of the primary tumor.
- Stewart VR, Sidhu PS: New directions in ultrasound: microbubble contrast. Br. J. Radiol. 79(939), 188–194 (2006).