Review Article - Interventional Cardiology (2012) Volume 4, Issue 3
Challenges in the management of postpartum spontaneous coronary artery dissection
- Corresponding Author:
- Alexander G Truesdell
Division of Cardiovascular Medicine& Surgery,
Warren Alpert Medical School of Brown University
, Providence, Rhode Island, USA
Tel: +1 401 444 5328
Fax: +1 401 444 4652
E-mail: atruesdell@lifespan.org
Abstract
Keywords
coronary artery bypass,gender,myocardial injury,pathophysiology,percutaneous coronary intervention,postpartum,spontaneous coronary artery dissection
Introduction
Spontaneous coronary artery dissection (SCAD) is an infrequent but well-known cause of acute coronary syndrome (ACS) and sudden cardiac death. The first case was described in 1931 [1], and the first angiographically confirmed case reported in 1978 [2]. SCAD is more common in women than in men and postpartum SCAD typically occurs in otherwise healthy women, often with devastating consequences [3–6]. Given the rarity of the disease, its pathophysiology remains poorly understood. The bulk of the available information is derived from case reports and retrospective studies. Long-term outcome data are lacking, treatment decisions are made on a case-to-case basis and the absence of guidelines complicates clinical decision-making. We present five cases of postpartum SCAD treated at our institution over a 4-year period (Table 1). In addition, this article will review the etiology, clinical presentation, outcomes, management options and controversies surrounding SCAD with a focus on the postpartum population.
Case presentations
▪ Case 1
A 34-year-old woman with hypertension, hyperlipidemia and migraine headaches presented 13 days following an uncomplicated delivery, with substernal chest pressure and borderline inferolateral ST depressions on electrocardiogram. Computed tomographic angiography (CTA) excluded both pulmonary emboli and aortic dissection. Coronary angiography demonstrated a localized dissection in the mid-portion of an otherwise normal left circumflex coronary artery (LCX) with Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow; the remainder of the coronary artery tree was normal. A ventriculogram revealed mild inferior hypokinesis and an ejection fraction (EF) of 55%. No intervention was performed.
Postprocedure, the patient experienced recurrent episodes of substernal chest pain without electrocardiogram changes. The symptoms resolved over several days of observation and the patient was discharged to home on hospital day 5. On re-evaluation 4 years later the patient reported no recurrent symptoms or events.
▪ Case 2
A 31 year-old woman with hypertension and migraine headaches was admitted 9 days after a normal delivery for headaches and hypertension concerning for postpartum pre-eclampsia. On hospital day 2 she developed substernal chest pain and anterolateral ST elevations on electrocardiogram. Emergent coronary angiography demonstrated a dissection of the proximal left anterior descending coronary artery (LAD). Adjunctive intravascular ultrasound (IVUS) examination revealed a subintimal hematoma extending from the ostial LAD to the first diagonal branch with minimal luminal encroachment and TIMI grade 3 flow. Contrast ventriculography showed anterior, apical and distal inferior akinesis with an EF of 25%. No intervention was performed.
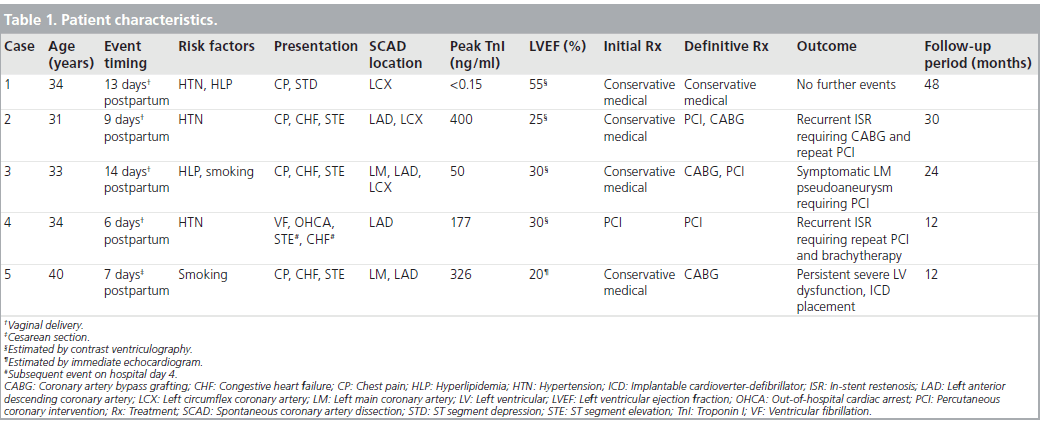
Surveillance angiography undertaken 3 days later demonstrated worsening luminal narrowing (Figure 1A) and percutaneous coronary intervention (PCI) was performed. Following deployment of bare-metal stents (BMS) in the ostial and proximal LAD, poststent IVUS revealed propagation of the dissection into the ostial LCX, necessitating bifurcation stenting (Figures 1B & 1C). She was discharged home on hospital day 12.
Figure 1: Case 2. (A) Coronary angiogram reveals a proximal left anterior descending coronary artery dissection with significant luminal narrowing (white arrow); (B) ‘Kissing’ deployment of stents into proximal left anterior descending coronary artery and left circumflex coronary artery following extension of the dissection into the left circumflex coronary artery; (C) Poststent angiogram; (D) Subsequent focal in-stent restenosis of the ostial left circumflex coronary artery (dashed arrow) and diffuse in-stent restenosis of the left anterior descending coronary artery (solid arrow).
Coronary angiography that was performed 3 months later for recurrent substernal chest pain demonstrated nonobstructive in-stent restenosis (ISR) of the proximal LAD stent and a normalized EF. No intervention was performed. Repeat angiography for angina 4 months later revealed diffuse high-grade restenosis within both the LAD and LCX stents (Figure 1D). Due to limited suitable arterial conduits and concerns regarding long-term vein graft patency in this young patient, after multidisciplinary consultation she underwent a successful hybrid procedure with surgical grafting of the left internal mammary artery (LIMA) to the LAD and repeat stenting of the ostial LCX with a drug eluting stent (DES).
Angina recurred 6 months later and angiography demonstrated a patent LIMA to LAD graft and significant ISR of both the LAD BMS and LCX DES. IVUS demonstrated good stent apposition, significant neointimal hyperplasia within both stents, and a nonhealed dissection with an intimal flap at the distal LCX stent edge. A DES was placed at the site of the LCX ISR and intimal flap complicated by distal extension of the dissection necessitating placement of a second DES. During recent 30-month follow-up she remained symptom-free.
▪ Case 3
A 33-year-old woman with hyperlipidemia, prior tobacco use and remote traumatic subarachnoid hemorrhage presented with acute substernal chest pain and anterolateral ST elevations 14 days following an uncomplicated cesarean section. Emergent cardiac catheterization revealed an ostial left main coronary artery (LM) dissection with subintimal hematoma extending into the LAD with minimal luminal encroachment and TIMI grade 3 flow to the distal vessel. Ventriculogram demonstrated anterior and apical hypokinesis and severe left ventricular dysfunction. No intervention was performed. Postprocedure transesophageal echocardiography and CTA excluded aortic dissection.
On hospital day 7, surveillance angiography showed progression of the LM dissection into the LAD and LCX with significant luminal narrowing and TIMI grade 2 flow. Emergent three-vessel coronary artery bypass grafting (CABG) with LIMA to LAD and vein grafts to the diagonal and obtuse marginal branches were performed. On direct visualization in the operating room, all coronary vessels were noted to be thin-walled and friable. Echocardiography at the time of discharge on postoperative day 7 (hospital day 14) demonstrated persistent severe systolic dysfunction.
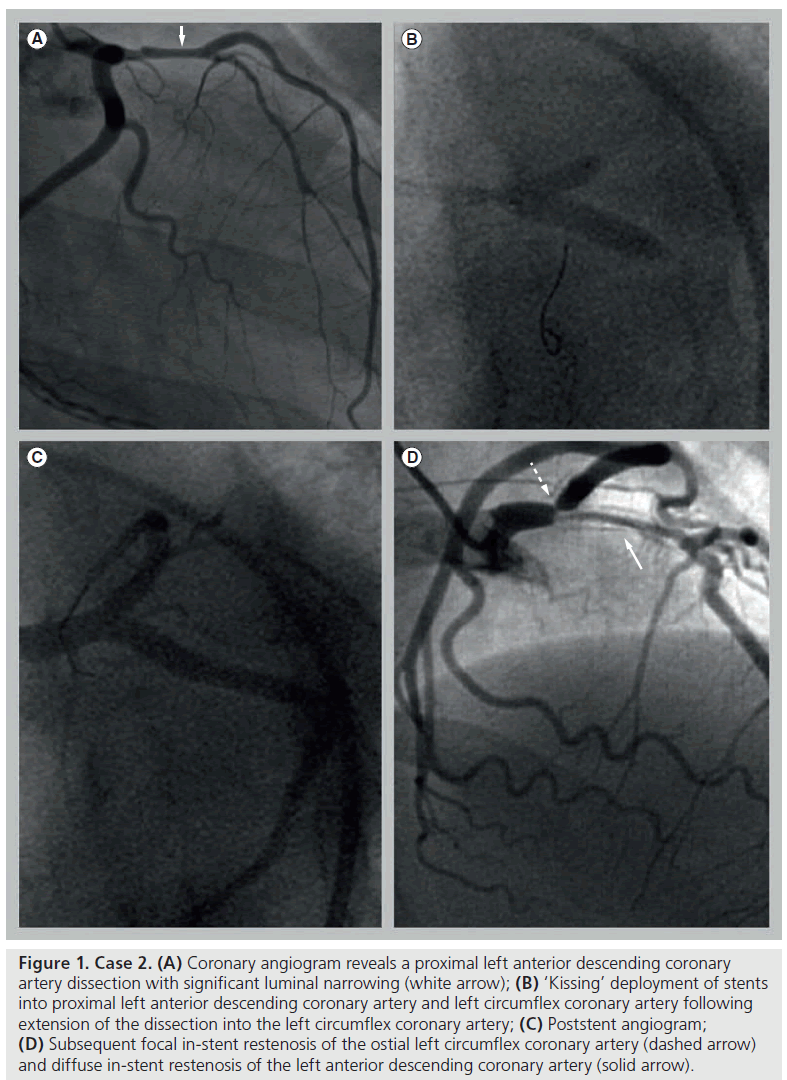
A total of 1 month later, surveillance cardiac CTA demonstrated a 6-mm LM pseudoaneurysm and an occluded diagonal vein graft (Figure 2A). Repeat CTA 6 months later suggested enlargement of the LM pseudoaneurysm. Cardiac catheterization confirmed a large LM pseudoaneurysm (Figures 2B & 2C), occlusion of the diagonal vein graft, a patent but atretic LIMA with competitive flow, and a patent obtuse marginal vein graft with retrograde flow to the LAD. EF had improved to 55%. She underwent successful obliteration of the pseudoaneurysm with IVUS-guided implantation of a covered coronary stent [7]. A total of 3 months later she underwent coronary angiography for recurrent angina demonstrating persistent patency of the LM and obtuse marginal bypass graft. She has had an uneventful clinical course in the 12 months since.
Figure 2: Case 3. (A) Cardiac computed tomographic angiography with volumetric reconstruction reveals a left main coronary artery pseudoaneurysm (solid arrow) and an occluded vein graft to the first diagonal (dashed arrow); (B) Coronary angiogram of the coronary pseudoaneurysm (arrow); (C) Intravascular ultrasound demonstrates the characteristic narrow neck and saccular dilatation (solid arrow) of the coronary pseudoaneurysm and the associated dissection plane and subintimal hematoma (dashed arrow).
▪ Case 4
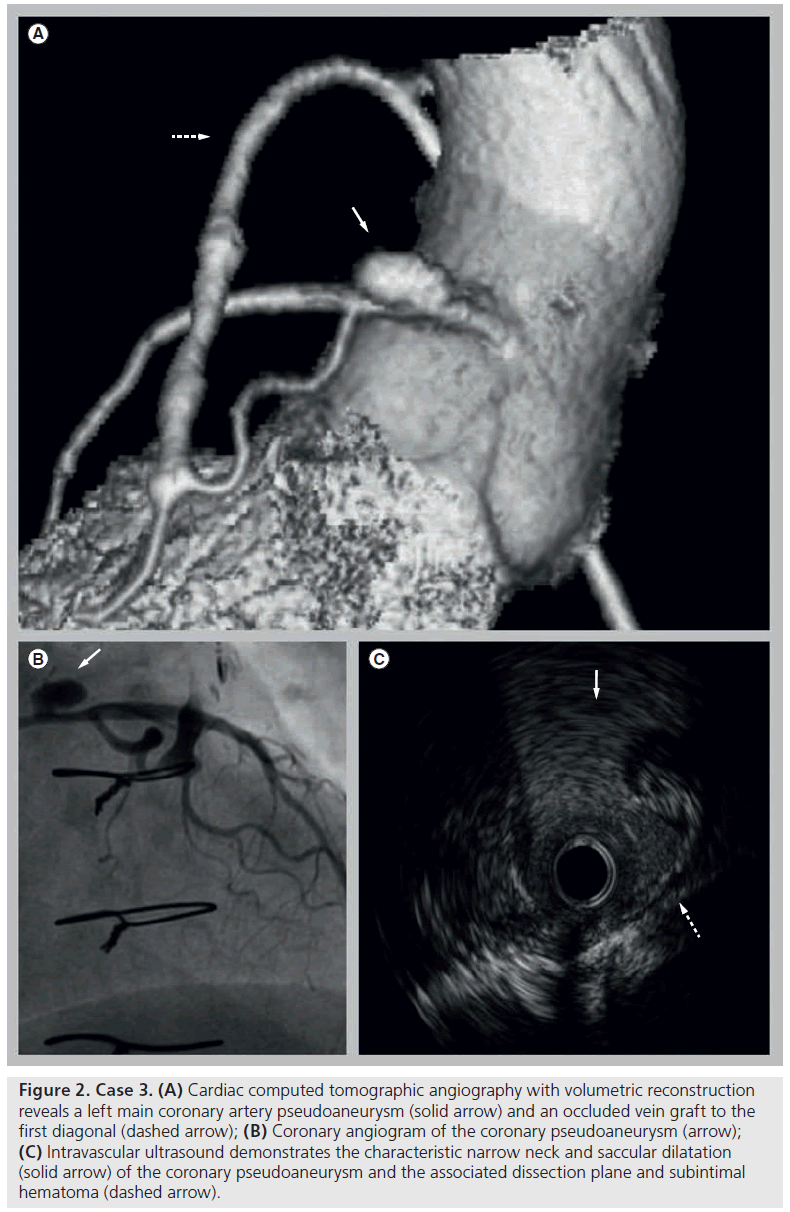
A 34-year-old woman with a past history of hypertension presented 6 days postpartum following ventricular fibrillation out-of-hospital cardiac arrest. She was successfully resuscitated and initiated on an induced hypothermia protocol. Echocardiography demonstrated inferoseptal and apical hypokinesis with an EF of 40%. Following rewarming and extubation on hospital day 3 she experienced substernal chest pain with anterolateral ST elevations. Emergent angiography demonstrated an extensive LAD dissection. IVUS-assisted PCI was performed with stenting of the proximal LAD and diagonal branch with BMS and balloon angioplasty of the mid and distal LAD with restoration of TIMI grade 3 flow. Left ventriculography demonstrated severe anterolateral hypokinesis and apical akinesis with an EF of 30%. Cardiac MRI confirmed a moderate sized transmural infarction in the LM distribution (Figure 3B). The patient was discharged on hospital day 11 with a LifeVest® (Zoll Medical, MA, USA) wearable automatic defibrillator.
Figure 3: Case 4. (A) Coronary angiogram reveals an area of high-grade in-stent restenosis in the left anterior descending coronary artery (solid arrow) and a diffuse segment of in-stent restenosis (dashed arrow) in the first diagonal. (B) Mid-cavity short axis cardiac MRI shows an area of bright myocardium (arrows) consistent with infarction in the left anterior descending coronary artery distribution. (C) Follow-up angiogram after balloon angioplasty demonstrates marked improvement in flow in both the left anterior descending coronary artery and the first diagonal.
A total of 4 months later, coronary angiography for recurrent angina demonstrated significant ISR of the LAD and diagonal necessitating repeat PCI with a DES to the mid-LAD and balloon angioplasty of the diagonal (Figures 3A & 3C). A total of 1 month later, the patient underwent unsuccessful cutting balloon angioplasty for restenosis of the diagonal BMS, followed by successful brachytherapy. In the 6 months since, she has had no recurrent symptoms and recent echocardiography demonstrated near normalization of left ventricular systolic function.
▪ Case 5
A 40-year-old woman with prior tobacco use presented 7 days following an uncomplicated cesarean section with substernal chest pain and anterolateral ST elevations. Emergent coronary angiography revealed a distal LM coronary dissection extending into the proximal LAD with TIMI grade 3 distal flow. No intervention was performed. Postprocedure echocardiography demonstrated severe anterior, lateral and apical hypokinesis with an EF of 20% and an apical thrombus. Cardiac MRI demonstrated a large transmural infarction in the LAD distribution with a large region of microvascular obstruction. A total of 6 days later she developed recurrent substernal chest pressure with anterolateral ST elevations. Angiography revealed a worsening dissection extending from the proximal LM into the LAD and ramus intermedius artery with TIMI grade 0 flow requiring emergent surgical revascularization with grafting to both vessels. Repeat echocardiography on postoperative day 12 (hospital day 18) demonstrated resolution of the left ventricular thrombus and an unimproved EF.
Serial outpatient echocardiography showed persistent systolic dysfunction and she underwent implantable cardioverter-defibrillator insertion. A total of 4 months later, angiography performed for recurrent angina revealed a normal LM, significant narrowing of the proximal LAD and ramus, a patent vein graft to the LAD, and an occluded vein graft to the ramus. No intervention was performed. She has remained symptom-free since.
Epidemiology
Although acute myocardial infarction (MI) is rare in women of reproductive age, pregnancy increases the risk nearly fourfold and is a factor in infarct severity [8,9]. SCAD is an equally infrequent phenomenon, noted in only 0.07–3.5% of ACS cases evaluated by angiography, IVUS or anatomic examination [3,4,10–19]. Historical SCAD data derived primarily from autopsy studies likely underestimated its frequency [11,20]. Recent studies in the era of widespread angiography in ACS and the increased availability of adjunctive intracoronary imaging techniques demonstrate significantly higher rates of SCAD [3,6,15,18,19,21].
The mean age of presentation of SCAD in both sexes is 40 ± 10 years of age [5]. In women below the age of 50 the incidence of SCAD approaches 9% in ACS and 11% for ST elevation MI (STEMI) [22]. Peripartum SCAD typically occurs in relatively older (>30 years of age) and multiparous pregnant women [8]. In a cohort of 58 reported cases of peripartum SCAD between 1952 and 1999, the mean age at presentation was 33 years and the mean parity was 2.4 years [17]. Among 25 cases reported in the literature between 2000 and 2008, the average age and parity were 32.6 and 1.6 years respectively [18]. Among peripartum women, SCAD is present in ≥35% of ACS [5,6,13] with a peak incidence in the first 2 weeks after delivery [17]. The LAD is most commonly affected, involved in 80% of peripartum cases; the LM is involved in 20%; 40% of cases demonstrate multivessel dissection; and 30% involve both the right and left coronary arteries [17,18]. This preponderance of LM, LAD and multivessel involvement places a significant portion of the left ventricle at jeopardy for extensive ischemia and infarction.
Pathophysiology
Coronary ar tery di s sect ions occur spontaneously or result from aortic root dissection, chest trauma or percutaneous or surgical intervention [22]. The precise etiology of spontaneous dissection is unknown. These dissections may occur in patients with pre-existing arteritides and inherited connective tissue disorders or may follow extreme shear stress such as that encountered during childbirth [23–26]. SCAD is distinct from the atherosclerotic plaque rupture responsible for most ACS and 70% of cases occur in women with no known risk factors and no significant coronary artery disease [5,6,8,9]. Recent case series raise the possibility that coronary fibromuscular dysplasia [27] or mechanical stresses resulting from myocardial bridging [28] may contribute to vessel wall weakness, culminating in spontaneous dissection.
Normal hemodynamic consequences of pregnancy include increases in total circulating blood volume, blood pressure, cardiac output and elevation of coronary artery wall tension [29]. Straining during labor also induces shear stresses. Additional hormonal and physiologic changes during pregnancy cause alterations in vessel wall architecture, impaired collagen synthesis, fragmentation of reticulin f ibers, decreases in mucopolysaccharide content and loosening of ground substance, and perhaps primary or reactive eosinophilic inf iltration with enhanced production of proteolytic enzymes [8,30–38]. These pathologic features have been deduced from autopsy cases, explanted hearts and more recently by virtual histology analysis of survivors by IVUS and optical coherence tomography (OCT) [20,30,32,39–42]. Together, these changes predispose vessels to dissection [25,43].
Two mechanisms have been advocated as the initiators of spontaneous dissection. One is an intimal or medial tear with superimposed thrombus. The second, predominant in peripartum SCAD, is the spontaneous rupture of medial vasa vasorum with intramural hematoma formation and luminal compression, similar to aortic intramural hematoma [41,44]. Continued hematoma expansion, usually in the absence of an outlet, leads to distal propagation, true-lumen compression, and flow-limiting obstruction with myocardial ischemia and infarction [45]. The absence of medial atrophy and scarring in young female patients compared with older patients with atherosclerotic and calcific coronary artery disease may also further facilitate dissection propagation [6,46]. In some cases, intimal rupture may provide a protective effect, decompressing a potentially obstructive hematoma, thereby preventing complete arterial occlusion.
Clinical presentation
The presentation of SCAD is variable. Patients may be asymptomatic or present with MI, hemodynamic collapse or sudden cardiac death. SCAD patients more commonly present with STEMI versus other forms of ACS and usually demonstrate larger infarcts [47]. Additionally, in one recent case series of 31 SCAD patients, postpartum patients (n = 7) showed involvement of more proximal coronary segments, larger infarct size, more malignant arrhythmias, greater incidence of cardiogenic shock and lower initial EF compared with both male and nonperipartum female patients [6]. Such significant differences in clinical presentations and angiographic findings between postpartum and nonpostpartum cases may reflect potentially different pathogenesis of SCAD between the two populations.
Diagnosis
Coronary angiography, often with adjunctive IVUS and OCT, is the diagnostic test of choice for SCAD [39,42]. The most common angiographic feature of a classic spontaneous dissection is f low in two separate lumens separated by a radiolucent f lap [47]. In the absence of an intimal tear, a long eccentric narrowing may be the only angiographic clue and IVUS may be required to discriminate between atherosclerotic disease and SCAD [41]. In cases with ambiguous coronary angiography where IVUS fails to identify small entry flaps, OCT provides unprecedented in vivo near-field resolution to more accurately determine the morphology and extent of a dissection [48,49].
Cardiac CTA has slightly poorer spatial resolution than standard angiography but as a true 3D imaging technique can provide information beyond lumenography with relatively modest radiation exposure [50]. However, despite several reports of intimal dissections and hematomas visualized by cardiac CTA, its utility in the initial diagnosis of coronary dissection is unknown [51]. However, recent technological improvements make it an attractive option for noninvasive monitoring of dissection extension or healing and early detection of pseudoaneurysm formation in stable patients after the initial dissection event [52–54].
Management
Optimal management of SCAD is not well defined and treatment decisions are largely empirical. The specific choice of therapy depends on the patient’s hemodynamic stability, site and extent of dissection, adequacy of distal coronary blood flow and amount of myocardium at risk. However, a review of historical cases demonstrates statistically superior outcomes for coronary revascularization with lower mortality rates, longer symptom-free periods, and less need for future interventions compared with conservative medical therapy [4,55]. The decision to pursue PCI versus CABG generally depends on lesion complexity and the number of involved vessels.
Medical management
Studies of iatrogenic dissections following coronary angioplasty demonstrated the success of conservative management [56,57]. In this population, lesions with only minimal flow disruption typically remain open, demonstrate low rates of restenosis and are usually associated with clinical success and favorable outcomes, cautioning against indiscriminate stenting in the absence of vessel occlusion [56,57]. It is unclear if this approach is similarly efficacious in SCAD or peripartum SCAD with its different pathophysiology and vessel wall composition.
Medical management may be appropriate in hemodynamically stable patients with no evidence of active ischemia and a mid-to-distal dissection in a single small-to-medium sized vessel with preserved TIMI grade 2 or 3 flow. Angiographic dissection resolution is typical in nonperipartum SCAD [58] but less commonly observed in pregnancy-related SCAD [3,18,59,60]. Reperfusion therapy is mandated for any evidence of ongoing myocardial ischemia. An analysis of 440 SCAD cases published between 1931 and 2008 revealed that 60% of SCAD lesions either worsened or did not improve with conservative management, often necessitating percutaneous or surgical revascularization, thus recommending more aggressive initial therapy [55].
Although a critical concern in coronary dissections is maintaining the patency of the true lumen, anticoagulation is controversial. Anecdotal reports of success with aspirin and clopidogrel have been reported [26]. While heparin has been used successfully to decrease thrombus formation in the false lumen and permit additional f low through the true lumen, it may also precipitate expansion of the intramural hematoma and flow obstruction of the true lumen [47,61]. Although short-term use of glycoprotein IIb/IIIa inhibitors has also been reported, it is generally not recommended for the same reason [62]. Use of fibrinolytic agents to dissolve compressive clots, relieve true lumen compression and restore vessel patency is also controversial, and may instead increase flow in the false lumen and propagate the dissection, and is probably associated with worse clinical outcomes and best avoided in SCAD [55,63–66].
Dissections managed conservatively also carry a risk of progression to pseudoaneurysm formation and a subsequent increased risk of vessel rupture [43]. Although there are no standard treatment recommendations, cases such as ours with successful obliteration of SCAD-related pseudoaneurysms with covered stents have been reported [7,67].
Percutaneous coronary intervention
While coronary stents represented an important innovation in the management of iatrogenic coronary dissections, technical challenges unique to SCAD make PCI more complex in this population. With intimal rupture, identification of the false and true lumens may be difficult, increasing the risk of passage of the guidewire down the false lumen with subsequent culprit vessel and side branch occlusion. Accurate assessment of stent dimensions may also be difficult in the presence of an intramural hematoma. Finally, stenting may cause further propagation of dissection, necessitating additional stenting, and facilitate late complications of PCI, such as restenosis and stent thrombosis.
IVUS and OCT are useful in the planning of therapeutic strategies and can minimize some of the short- and long-term risks of PCI. Both imaging modalities can be utilized to confirm wire location, aid in assessing reference vessel size and dissection length, localize intimal tears, guide stent positioning and verify full apposition of the stent struts, sealing of the dissection, resolution of intramural hematoma, and obliteration of the false lumen [68,69]. OCT, with its additional near-field resolution, may be even better suited to this particular application in SCAD [42,70]. In a recent prospective study, OCT demonstrated utility in guiding coronary interventions in patients with spontaneous coronary dissections [49]. Unfortunately, even with a meticulous technique, late suboptimal stent apposition still occurs due to healing and reabsorption of intramural hematoma, often requiring postdilatation in a separate procedure, and may also lead to other late clinical events.
Stenting may either be performed preferentially at the suspected dissection entry site or in a distal-to-proximal manner across the entire dissected segment, starting at the nondissected end of the vessel [71–73]. Although the issue is controversial and there are limited data to definitively support either strategy [49], the predominance of intramural hematoma without a dissection flap in the peripartum SCAD population historically recommends the latter technique [68].
It is unclear whether DES or BMS are preferable in peripartum SCAD. The young age of most patients, the frequent need for long or multiple stents, and the presence of systemic inf lammatory mediators stimulating the proliferative response have been cited as reasons to justify the preferential use of DES [31,32,34,74]. Alternatively, the unpredictable impact of DES on healing of nonatheromatous disease in patients with a presumed underlying collagen defect has been cited as a need for BMS use [75].
Studies in the BMS era demonstrated that a longer stented segment is an independent predictor of restenosis [76]. Although later studies following the advent of DES revealed reduced rates of restenosis compared with BMS, increased length and number of stents still correlated with higher rates of restenosis, stent fracture and the need for target vessel revascularization [77,78]. Finally, the potential risk of recurrent spontaneous bleeding in the vasa vasorum with the use of prolonged dual antiplatelet therapy required with DES further complicates decision-making. In the absence of any meaningful comparison in the literature between BMS and DES in SCAD, stent selection in this rare condition remains controversial.
Surgical revascularization
There are no studies comparing revascularization methods in SCAD patients, but CABG is generally reserved for patients with failed PCI or persistent ischemia where the location or extent of disease precludes PCI in accordance with current guidelines: typically significant multivessel or LM coronary artery involvement [31,79,80]. As a group, postpartum women are normally low-risk surgical candidates due to their young age and general absence of significant comorbidities. Internal mammary grafts should be favored because of their higher patency rates in these young patients. Overall, the goals of surgical intervention are to restore flow beyond the obstruction and minimize the likelihood of further dissection [45].
Bypass surgery may be technically challenging due to vessel wall fragility in peripartum SCAD. Identification of the true lumen may be difficult and grafting to the false lumen can be catastrophic, resulting in irreversible myocardial damage or death. With long dissections, if unable to anastomose to a normal vessel segment, the layers of the vessel should be reapproximated and both true and false lumens incorporated into the anastomosis, in order to re-establish true luminal flow [45]. In the past, some surgeons suggested that the vessel be ligated beyond the far edge of the dissection and a vein graft anastomosed distally to minimize this risk. Others later argued that because healing often occurred spontaneously, ligation was unnecessary and would furthermore make the distal circulation entirely dependent on the vein graft [45,79]. The current consensus is that it is indeed unnecessary to ligate the vessel proximal or distal to the dissection [45].
The surgical literature also reveals a variable natural history of conservatively managed dissections with significant percentages both healing and progressing, but no good model to predict which outcome will prevail in an individual patient [81,82]. This uncertainty further complicates the decision-making process as grafts may close or become atretic following reabsorption of intramural hematoma and restoration of native coronary blood flow.
Management trends
Despite the absence of guidelines for treatment of SCAD, current consensus opinion dictates that the decision to pursue conservative management, PCI or surgery should be made in a similar fashion to decision-making for coronary atherosclerosis [79,80]. Urgent diagnosis by coronary angiography is essential and early therapy to restore coronary flow and maintain stable hemodynamics is crucial. Asymptomatic non-LM coronary dissections may be treated conservatively with scheduled follow-up invasive or noninvasive imaging. Symptomatic non-LM, nontriple-vessel dissections are probably best treated with PCI, whereas symptomatic LM or triple-vessel dissections should be treated surgically. Ideally, complicated treatment decisions should be made via multidisciplinary consultation among interventional cardiologists and cardiothoracic surgeons.
Morbidity & mortality
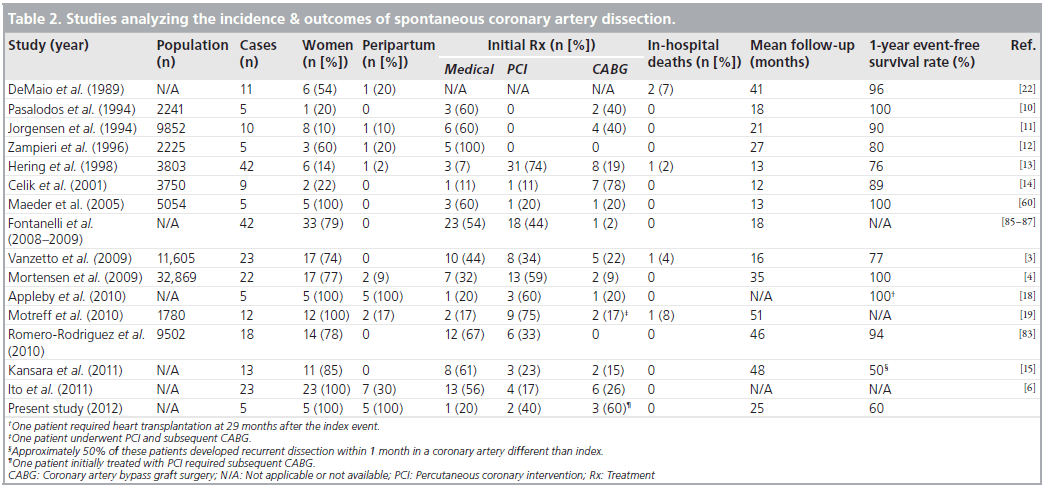
The short-term mortality in SCAD is higher than both ACS and STEMI [22]. According to a recent analysis of gender differences in SCAD, the strongest multivariate predictors of mortality are female sex (odds ratio: 4.27) and absence of early treatment (odds ratio: 35.5) [5]. Prior to widespread coronary angiography, SCAD was treated medically with a near 80% mortality rate [19]. In the interventional era, in-hospital mortality rates dropped significantly, ranging from 0 to 10% in most contemporary series, with 2-year survival rates of nearly 90% for acute-phase survivors [3,4,10–15,19]. Unfortunately, outcomes remain less favorable for peripartum patients, who represent only a small fraction of total SCAD patients in historical series (n = 0–7) (Table 2) [83], and demonstrate a 45% short-term mortality compared with 5% for all-cause MI [5,17], probably due to the greater incidence of left main, proximal vessel and multivessel involvement, longer dissection lengths and the absence of pre-existing collateral circulation.
Table 2: Studies analyzing the incidence & outcomes of spontaneous coronary artery dissection.
Recurrence is also common in SCAD, often in coronary arteries other than the initial culprit and occasionally in noncoronary vessels, advocating for a global arterial weakness [15,28]. One literature review of 152 cases reported a 50% recurrence rate within 2 months, while another recent study of 13 nonperipartum female SCAD patients demonstrated a 50% recurrence rate within the first 2 weeks of the index event [15,84]. Extrapolating from published studies demonstrating uniformly worse outcomes for peripartum patients compared with other SCAD populations, these statistics may be even worse among this patient subset.
Overall there is no long-term clinical data among the peripartum SCAD population, complicating prognostication. Preliminary results from the only multicenter prospective study of SCAD, the DISCOVERY study, demonstrate that SCAD patients exhibit a better long-term prognosis compared with non-SCAD controls [85–87]. However, none of the initial 42 patients in this ongoing registry were peripartum, which may explain why the study’s positive clinical outcomes differ from other series [3–5,18,19]. It is hoped that additional prospective data collection from both this study and an ongoing descriptive database collection by the Mayo Clinic, MN, USA [88] may provide improved data regarding long-term prognosis in SCAD and peripartum SCAD in particular.
Outcomes
In our series of five postpartum cases of SCAD, all occurred within 2 weeks of delivery. One patient presented with unstable angina, four with STEMI and acute systolic heart failure, and one with out-of-hospital cardiac arrest. All STEMI patients underwent immediate coronary angiography. The LM was involved in two cases, the LAD in four, and the LCX in four; no patients demonstrated right coronary artery involvement.
One patient with a limited focal dissection of the mid LCX was successfully managed conservatively with no recurrent symptoms or events at 4-year follow-up. A conservative strategy was pursued in three additional cases following diagnostic coronary angiography while one patient underwent primary PCI during her initial catheterization. Of the four patients initially managed conservatively, three underwent early repeat coronary angiography, one for recurrent symptoms and two for planned surveillance. All three patients demonstrated progression of dissection and two proceeded to urgent CABG. The third was treated with PCI, only to proceed to delayed CABG several months later due to obstructive restenosis of the stented ostial LAD and LCX. Of the four patients with systolic dysfunction at the time of initial hospital discharge, three demonstrated near-total recovery during follow-up, while one evidenced persistent severe left ventricular systolic dysfunction necessitating implantable cardioverter-defibrillator implantation.
Following their initial hospitalizations, four out of five patients underwent an average of 2.5 additional coronary interventions over the course of the next 12 months. Three of these patients demonstrated significant early ISR with both BMS and DES leading to recurrent stenting with DES for two patients and brachytherapy for the third. Two patients suffered iatrogenic dissection extension during their subsequent PCIs requiring additional stent implantation and one underwent covered stent implantation for the treatment of LM coronary pseudoaneurysm. Of the three patients that ultimately underwent CABG, two patients experienced vein graft failure, one suffered LIMA atresia and one demonstrated stenosis at the LIMA anastomotic site. Most of these occurrences occurred early, were symptomatic and necessitated additional interventions.
Future perspective
Postpartum SCAD presents an array of controversies but also numerous opportunities for progress. Enhanced diagnostic and surveillance imaging modalities, new stent technologies designed to mitigate many of the undesired effects of PCI and advances in intracoronary imaging may all improve patient diagnosis and management and increase the technical success of coronary interventions and more importantly, long-term clinical outcomes.
Future improvements in multidetector CTA and cardiac MRI technology are expected to increase the utility of these noninvasive imaging modalities in the diagnosis and surveillance of SCAD and better guide the choice of conservative, endovascular, or surgical therapy. Current coronary CTA details vessel wall composition and has been well-validated against invasive coronary angiography for quantitative assessment of area stenosis [89] while cardiac MRI permits radiationfree visualization of the coronary arteries along with an integrated assessment of cardiac anatomy, perfusion, function and viability [90]. Ongoing innovations in both technologies should dramatically reduce radiation exposure and image acquisition time, and improve spatial resolution and multidimensional image reconstruction. Continued advances may ultimately position these noninvasive imaging techniques ahead of coronary angiography, replacing this technique for the surveillance of the hemodynamically stable patient undergoing serial reassessment after initial diagnosis.
IVUS and OCT have demonstrated utility in optimizing treatment, improving technical success and preventing periprocedural complications [49,91,92]. The emergence of new innovations in these and other ultrasound- and light-based imaging modalities, such as high definition and virtual histology IVUS, OCT and near-infrared spectroscopy, should continue to provide increasingly detailed assessment of vessel wall composition, anatomy and function. Forward-looking IVUS additionally has potential applications to guide vessel wiring in SCAD and avoid guidewire passage down the false lumen. Resolution enhancements, further miniaturization and integration of all of these modalities and others still undiscovered, into a single multimodal catheter should facilitate faster image acquisition, provide better resolution and definition, increase access throughout the coronary tree, guide better stent placement and apposition, reduce overinflation and overcompression of intramural thrombus and dissection extension and improve overall technical success and clinical outcomes.
Bioresorbable stent (BRS) platforms and new antiproliferative agents and delivery mechanisms are exciting innovations with potential in SCAD. BRS may overcome many of the technical and safety concerns associated with bare-metal and DES by maximizing the low-restenosis rates of DES and minimizing the long-term risks of both late-stent thrombosis and impaired healing of abnormal vessel collagen substrate in SCAD. By reabsorbing as the dissection heals, BRS may also reduce the potential for late suboptimal stent apposition and facilitate the restoration of vessel vasomotor tone, adaptive shear stress, late luminal enlargements, and beneficial late expansive remodeling [93]. BRS may also reduce the incidence and consequences of ISR and side-branch coverage in long-stented segments. The ultimate absence of foreign material may also lead to reductions in the duration of dual antiplatelet therapy and its attendant potential risk of continued vasa vasorum bleeding without any risk of late-stent thrombosis. Overall, future BRSs may provide optimal transient scaffolding of the healing coronary artery and improved antiproliferative drug elution to counteract excessive neointimal hyperplasia, and then may be reabsorbed, restoring normal vessel endothelial structure and function.
Conclusion
SCAD is a rare and complex disease that strikes young, typically healthy patients and preferentially women in the peripartum state. Our diverse case series of five postpartum SCAD patients highlights the significant treatment challenges and numerous complications common to the care of these patients. Because there are no randomizedcontrolled trials, no long-term clinical data and only one ongoing prospective trial, optimal treatment of SCAD and peripartum SCAD is controversial and informed only by cumulative experience and literature review [58,62,68,85]. Although there are increasing data from newer and larger reported retrospective series, all of these individual series report very few (n = 0–7) peripartum numbers. Overall, the absence of clinical guidelines and lack of expert consensus merits individualized therapy and multidisciplinary treatment plans. Ultimately, there is great need for systemic acquisition of prospective data, particularly among the peripartum population, which may have a different pathogenesis than other SCAD cohorts. In the future, new noninvasive methods may become suitable for surveillance, diagnosis, and selection of initial therapy, advances in intracoronary imaging may improve PCI techniques and outcomes and the widespread use of BRS may mitigate some of the short- and long-term pitfalls of PCI.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Pathophysiology of postpartum spontaneous coronary artery dissection
▪▪ Hemodynamic, physiologic and histologic changes in pregnancy predispose peripartum women to coronary dissection.
▪▪ Pathology resembles aortic intramural hematoma more than iatrogenic coronary dissection.
Controversies surrounding preferred management strategies
▪▪ Medical management may be appropriate in select stable patients with limited dissections and preserved distal flow.
▪▪ There is no consensus on the preferred mode of coronary revascularization: endovascular or surgical.
Technical aspects of percutaneous coronary intervention
▪▪ Immediate procedural challenges include inconclusive identification of the true lumen, optimal stent deployment and prevention of dissection propagation.
▪▪ Careful use of intravascular ultrasound or optical coherence tomography can minimize many of the short- and long-term risks of percutaneous coronary intervention.
▪▪ It is unclear whether bare-metal stents or drug-eluting stents are preferable in peripartum spontaneous coronary artery dissection (SCAD).
Lack of prospective data, randomized controlled trials, expert consensus & clinical guidelines
▪▪ Optimal treatment is informed primarily by experience and literature review.
▪▪ Most experts recommend approaching the mode of revascularization anatomically according to current guidelines for coronary atherosclerosis.
▪▪ The only multicenter prospective study of SCAD has enrolled no peripartum patients to date.
Poor clinical outcomes in SCAD & peripartum SCAD in particular
▪▪ Morbidity and mortality in SCAD exceeds acute coronary syndrome and ST elevation myocardial infarction.
▪▪ Early recurrence rates approach 50%.
▪▪ Peripartum SCAD patients demonstrate even higher morbidity and mortality than other SCAD cohorts.
Future challenges & opportunities
▪▪ Computed tomographic angiography and MRI demonstrate potential for postangiography surveillance of stable patients.
▪▪ Intravascular ultrasound, optical coherence tomography, and other emerging imaging modalities may improve the procedural success of percutaneous coronary intervention and minimize short- and long-term procedural complications.
▪▪ Bioresorbable stent platforms may be the optimal approach to address the immediate therapeutic requirements of SCAD while avoiding the current complications of percutaneous coronary intervention.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. Br. Med. J. 1, 667 (1931).
- Ciraulo DA, Chesne RB. Coronary arterial dissection: an unrecognized cause of myocardial infarction, with subsequent coronary arterial patency. Chest 73, 677–679 (1978).
- Vanzetto G, Berger-Coz E, Barone-Rochette G et al. Prevalence, therapeutic management andmedium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur.J. Cardiothorac. Surg. 35, 250–254 (2009).
- Mortensen KH, Thuesen L, Kristensen IB et al. Spontaneous coronary artery dissection: awestern Denmark heart registry study. Catheter. Cardiovasc. Interv. 74, 710–717(2009).
- Thompson EA, Ferraris S, Gress T, Ferraris V. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J. Invasive Cardiol. 17, 59–61 (2005).
- Ito H, Taylor L, Bowman M, Fry ET, Hermiller JB, Van Tassel JW. Presentation and therapy of spontaneous coronary artery dissection and comparisons of postpartum versus nonpostpartum cases. Am. J. Cardiol. 107(11), 1590–1596 (2011).
- Boyer WB, Atalay MK, Sharaf BL. Left main pseudoaneurysm after postpartum coronary dissection. Circ. Cardiovasc. Interv. 4(3), 303–305 (2011).
- Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J. Am. Coll. Cardiol. 52, 171–180 (2008).
- James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation 113, 1564–1571 (2006).
- Pasalodos Pita J, Vazquez Gonzalez N, Perez Alvarez L et al. Spontaneous coronary artery dissection. Cathet. Cardiovasc. Diagn. 32, 27–32 (1994).
- Jorgensen MB, Aharonian V, Mansukhani P et al. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am. Heart J. 127, 1382–1387 (1994).
- Zampieri P, Aggio S, Roncon L et al. Follow up after spontaneous coronary artery dissection: a report of five cases. Heart 75, 206–209 (1996).
- Hering D, Piper C, Hohmann C et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous coronary artery dissection diagnosed by coronary angiography. Z. Kardiol. 87, 961–970 (1998).
- Celik SK, Sagcan A, Altintig A et al. Primary spontaneous coronary artery dissections in atherosclerotic patients. Report of nine cases with review of the pertinent literature. Eur. J. Cardiothorac. Surg. 20, 573–576(2001).
- Kansara P, Graham S. Spontaneous coronary artery dissection: case series with extended follow-up. J. Invasive Cardiol. 23(2), 76–80 (2011).
- Kamran M, Guptan A, Bogal M. Spontaneous coronary artery dissection: case series and review. J. Invasive Cardiol. 20(10), 553–559 (2008).
- Koul AK, Hollander G, Moskovits N, Frankel R, Herrera L, Shani J. Coronary artery dissection during pregnancy and the postpartum period: two case reports and review of the literature. Catheter. Cardiovasc. Interv. 52, 88–94 (2001).
- Appleby CE, Barolet A, Ing D et al. Contemporary management of pregnancy-related coronary artery dissection: a single-centre experience and literature review. Exp. Clin. Cardiol. 14, e8–e16 (2009).
- Motreff P, Souteyrand G, Dauphin C, Eschalier R, Cassagnes J, Lusson JR. Management of spontaneous coronary artery dissection: review of the literature and discussion based on a series of 12 young women with acute coronary syndrome. Cardiology 115, 10–18 (2010).
- Basso C, Morgagni GL, Thiene G. Spontaneous coronary artery dissection: a neglected cause of acute myocardial ischaemia and sudden death. Heart 75, 451–454 (1996).
- Desai S, Sheppard MN. Sudden cardiac death: look closely at the coronaries for spontaneous dissection which can be missed. A study of 9 cases. Am. J. Forensic Med. Pathol. 33(1), 26–29 (2012).
- DeMaio SJ Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am. J. Cardiol. 64, 471–474 (1989).
- Aldoboni AH, Hamza EA, Majdi K, Ngibzadhe M, Palasaidi S, Moayed DA. Spontaneous dissection of coronary artery treated by primary stenting as the first presentation of systemic lupus erythematosus.
J. Invasive Cardiol. 14, 694–696 (2002). - Ades LC, Waltham RD, Chiodo AA, Bateman JF. Myocardial infarction resulting from coronary artery dissection in an adolescent with ehlers–danlos syndrome type IV due to a type III collagen mutation.Br. Heart J. 74, 112–116 (1995).
- Sherrid MV, Mieres J, Mogtader A, Menezes N, Steinberg G. Onset during exercise of spontaneous coronary artery dissection and sudden death. Occurrence in a trained athlete: case report and review of prior cases. Chest 108(1), 284–287 (1995).
- Choi JW, Davidson CJ. Spontaneous multivessel coronary artery dissection in a long-distance runner successfully treated with oral antiplatelet therapy. J. Invasive Cardiol. 14, 675–678 (2002).
- Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ. Cardiovasc. Interv. 5, 134–137 (2012).
- De-Giorgio F, Grassi VM, Abbate A, d’Aloja A, Arena V. Causation or coincidence? A case of sudden death due to spontaneous coronary artery dissection in presnece of myocardial bridging. Int. J. Cardiol. doi.org/10.1016/j. ijcard.2011.11.056 (2011) (Epub ahead of print).
- Sibai BM, Frangieh A. Maternal adaptation to pregnancy. Curr. Opin Obstet. Gynecol.7(6), 420–426 (1995).
- Herbst J, Winskog C, Byard R. Cardiovascular conditions and the evaluation of the heart in pregnancy-associated autopsies. J. Forensic Sci. 55(6), 1528–1533 (2010).
- Borczuk AC, van Hoeven KH, Factor SM. Review and hypothesis: the eosinophil and peripartum heart disease (myocarditis and coronary artery dissection) – coincidence or pathogenetic significance? Cardiovasc. Res. 33, 527–532 (1997).
- Briguori C, Bellevicine C, Visconti G, Focaccio A, Aprile V, Troncone G. In vivo histological assessment of a spontaneous coronary artery dissection. Circulation 122, 1044–1046 (2010).
- Rabinowitz M, Virmani R, Mcallister HA Jr. Spontaneous coronary artery dissection and eosinophilic inflammation: a cause-effect relationship. Am. J. Med. 72, 923–928 (1982).
- Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. Obstet. Gynecol. 40, 202–210 (1972).
- Virmani F, Forman MB, Robinowitz M, McAllister HA. Coronary artery dissections. Hum. Pathol. 17, 575–583 (1986).
- Bonnet J, Aumailley JB, Thomas D, Grosgogeat Y, Broustet JP, Bricaud H. Spontaneous coronary artery dissection: case report and evidence for a defect in collagen metabolism. Eur. Heart J. 7, 904–909 (1986).
- Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch. Pathol. 83, 336–341 (1967).
- Dowling, GP, Buja LM. Spontaneous coronary artery dissection occurs with and without periadventitial inflammation. Arch. Pathol. Lab. Med. 111, 470–472 (1987).
- Arnold JR, West NEJ, van Gaal WJ, Karamitsos TD, Banning AP. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc. Ultrasound 6, 240 (2008).
- Cano O, Almenar L, Chirivella M, Martinez L. Idiopathic spontaneous coronary artery dissection: clinical and pathological correlate. Int. J. Cardiol. 133(1), e18–e19 (2009).
- Maehara A, Mintz GS, Castagna MT et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am. J. Cardiol. 89, 466–468 (2002).
- Alfonso F, Canales E, Aleong G. Spontaneous coronary artery dissection: diagnosis by optical coherence tomography. Eur. Heart J. 30, 385 (2009).
- Aqel RA, Zoghbi GJ, Iskandrian A. Spontaneous coronary artery dissection, aneurysms, and pseudoaneurysms: a review. Echocardiography 21(2), 175–182 (2004).
- Yamada T, Tada S, Harada J. Aortic dissection without intimal rupture: diagnosis with MR imaging and CT. Radiology 168, 347–352 (1988).
- Thayer JO, Healy RW, Maggs PR. Spontaneous coronary artery dissection. Ann. Thorac. Surg. 44, 97–102 (1987).
- Isner JM, Donaldson RF, Fortin AH, Tischler A, Clarke RH. Attenuation of the media of coronary arteries in advanced atherosclerosis. Am. J. Cardiol. 58, 937–939 (1986).
- Vrints CJ. Spontaneous coronary artery dissection. Heart 96, 801–808 (2010).
- Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review. J. Am. Coll. Cardiol. Intv. 2, 1035–1046 (2009).
- Alfonso F, Paulo M, Gonzalo N et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. Am. Coll. Cardiol. 59, 1073–1079 (2012).
- Manghat NE, Morgan-Hughes GJ, Roobottom CA. Spontaneous coronary artery dissection: appearance and follow-up on multi-detector CT coronary angiography. Clin. Radiol. 60, 1120–1125 (2005).
- Taylor AJ, Cerqueira M, Hodgson JM et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/ NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 56(22), 1864–1894 (2010).
- Chabrot P, Motreff P, Boyer L. Postpartum spontaneous coronary artery dissection: a case of pseudoaneurysm evolution detected on MDCT. Am. J. Roentgenol. 187(6), W660 (2006).
- Rahman S, Abdul-Waheed M, Helmy T et al. Spontaneous left main coronary arterydissection complicated by pseudoaneurysm formation in pregnancy: role of CT coronary angiography. J. Cardiothorac. Surg. 4, 15 (2009).
- Ohlmann P, Weigold G, Kim SW et al. Spontaneous coronary dissection: computed tomography appearance and insights from intravascular ultrasound examination. Circulation 113, e403–e405 (2006).
- Shamloo BK, Chintala RS, Nasur A et al. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. Invasive. Cardiol. 22, 222–228 (2010).
- Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angiography. Am. J. Cardiol. 68, 467–471 (1991).
- Cappelletti A, Margonato A, Rosano G et al. Short- and long-term evolution of unstented nonocclusive coronary artery dissection after coronary angioplasty. J. Am. Coll. Cardiol. 34(5), 1484–1488 (1999).
- Hoye A. Spontaneous coronary artery dissection: time for a concerted effort to better understand this rare condition. Invasive Cardiol. 22, 229–230 (2010).
- Maeder M, Ammann P, Drack G, Rickli H. Pregnancy-associated spontaneous coronary artery dissection: impact of medical treatment. Case report and systematic review. Z. Kardiol. 94, 829–835 (2005).
- Maeder M, Ammann P, Angehrn W, Rickli
- Idiopathic spontaneous coronary artery dissection: incidence, diagnosis and treatment. Int. J. Cardiol. 101, 363–369 (2005).
- Sarmento-Leite R, Machado PR, Garcia SL. Spontaneous coronary artery dissection: stent it or wait for healing? Heart 89, 164 (2003).
- Cheung S, Mithani V, Watson RM. Healing of spontaneous coronary dissection in the context of glycoprotein IIb/IIIa inhibitor therapy: a case report. Catheter. Cardiovasc. Interv. 51, 95–100 (2000).
- Benham R, Tillinghast S. Thrombolytic therapy in spontaneous coronary artery dissection. Clin. Cardiol. 14, 611–614 (1991).
- Buys EM, Suttorp MJ, Morshuis WJ, Polkker HW. Extension of a spontaneous coronary artery dissection due to thrombolytic therapy. Cathet. Cardiovasc. Diagn. 33, 157–160 (1994).
- Alli O, Jacobs L, Amanullah AM. Acute aortic syndrome: pathophysiology and management. Rev. Cardiovasc. Med. 9(2), 111–124 (2008).
- Vacek JL, McKiernan TL. Intracoronary streptokinase for acute coronary-artery dissection. N. Engl. J. Med. 310, 1187 (1984).
- Dhakam S, Ahmeed H, Jafarani A. Percutaneous coronary intervention of left main pseudoaneurysm with customized covered stents. Catheter. Cardiovasc. Interv.77(7), 1033–1035 (2010).
- Zupan I, Noc M, Trinkaus D, Popovic M. Double vessel extension of spontaneous left main coronary artery dissection in young women treated with thrombolytics. Catheter. Cardiovasc. Interv. 52(2), 226–230 (2001).
- Poon K, Bell B, Raffel OC, Walters DL, Jang IK. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ. Cardiovasc. Interv.4, e5–e7 (2011).
- Vale PR, Baron DW. Coronary artery stenting for spontaneous coronary artery dissection: a case report and review of the literature.Cathet. Cardiovasc. Diagn. 45, 280–286(1998).
- Marti V, Garcia-Picart J, Balcells J. Coronary stenting after failure of conservative treatment for spontaneous coronary artery dissection: usefulness of the intravascular ultrasound. Clin. Ultrasound 39, 175–178 (2011).
- Petronio AS, De Carlo M, Gistri R, Ciabatti N, Mazzoni A, De Viti D. Successful treatment of a spontaneous coronary dissection causing acute myocardial infarction with stenting of the proximal edge of the dissection: a case documented with intravascular ultrasound. Int.Cardiol. 128(2), e74–e76 (2008).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 40, 193–202 (2006).
- Kim MS, Dean LS. In-stent restenosis. Cardiovasc. Ther. 29, 190–198 (2011).
- Kobayashi Y, De Gregorio J, Kobayashi N et al. Stented segment length as an independent predictor of retenosis. J. Am. Coll. Cardiol. 34, 651–659 (1999).
- Sharp AS, Latib A, Ielasi A et al. Long-term follow-up on a large cohort of “full metal jacket” percutaneous coronary intervention procedures. Circ. Cardiovasc. Intervent. 2, 416– 422 (2009).
- Sanchez-Recalde A, Guzman G, Armada E, Moreno R. Multiple spontaneous coronary artery dissection associated with a left main coronary artery lesion treated by stenting. Late multiple stent fractures detected by multislice CT. Rev. Esp. Cardiol. 62, 225–226 (2009).
- Eagle KA, Guyton RA, Davidoff R et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines Committee to update the 1999 guidelines for coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 44(5), e213–e310 (2004).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J. Am. Coll. Cardiol. 58(24), 1–79 (2011).
- Yoshida K, Satsuki M, Tomari S et al. Coronary artery bypass grafting for spontaneous coronary artery dissection: a case report and a review of the literature. Ann. Thorac. Cardiovasc. Surg. 6, 57–60 (2000).
- Thistlethwaite PA, Tarazi RY, Giordano FJ, Jamieson SW. Surgical management of spontaneous left main coronary artery dissection. Ann. Thorac. Surg. 66, 258–260 (1998).
- Romero-Rodriguez N, Fernandez-Quero M, Gil-Ortega MV et al. Spontaneous coronary dissection and its long-term prognostic implications in a cohort of 19 cases. Rev. Esp. Cardiol. 63(9), 1088–1091 (2010).
- Kamineni R, Sadhu A, Alpert JS. Spontaneous coronary artery dissection: report of two cases and a 50-year review of the literature. Cardiol. Rev. 10(5), 279–284 (2002).
- Fontanelli A, Olivari Z, La Vecchia L et al.; DISCOVERY investigators. Spontaneous dissections of the coronary arteries and acute coronary syndromes: rationale and design of the DISCOVERY, a multicenter prospective registry with a case–control group.J. Cardiovasc. Med. 19, 94–99 (2009).
- Fontanelli A, Olivari Z, La Vecchi L et al. ACS in patients with and without spontaneous coronary dissections of coronary arteries: the DISCOVERY study. Circulation 120, S1050 (2009).
- Fontanelli A, Benettin A, Bonanno C et al. Preliminary results of the DISCOVERY-ACS: a multicentre prospective registry with a patients-control group. Circulation 118, S817 (2008).
- Tweet MR, Gulati R, Aase LA, Hayes SN. Spontaneous coronary artery dissection: a disease-specific, social-networking community-initiated study. Mayo Clin. Proc. 86(9), 845–850 (2011).
- Voros S, Rinehart S, Qian Z et al. Coronary atherosclerosis imaging by coronary CT angiography. Current status, correlation with intravascular interrogation and meta-analysis. J. Am. Coll. Cardiol. Img. 4, 537–548 (2011).
- Chiribiri A, Ishida M, Nagel E, Botnar RM. Coronary imaging with cardiovascular magnetic resonance: current state of the art. Prog. Cardiovasc. Dis. 54, 240–252 (2011).
- McDaniel MC, Eshtehardi P, Swaya FJ, Douglas JS Jr, Samady H. Contemporary clinical applications of coronary intravascular ultrasound. J. Am. Coll. Cardiol. Intv. 4(11), 1155–1167 (2011).
- Suter MJ, Nadkarni SK, Weisz G et al. Intravascular optical imaging technology for investigating the coronary artery. J. Am. Coll. Cardiol. Img. 4, 1022–1039 (2011).
- Onuma Y, Ormiston J, Serruys PW. Bioresorbable scaffold technologies. Circ. J. 75, 509–520 (2011).
▪ Review of gender-based differences in morbidity and mortality in spontaneous coronary artery dissection (SCAD).
▪▪ Largest case series of postpartum SCAD patients with a detailed analysis of the clinical characteristics and outcomes of postpartum versus nonpostpartum SCAD cases.
▪▪ Comprehensive review of the anatomy and histopathology of coronary artery dissection based on 53 autopsies.
▪ Review of the use of intravascular ultrasound in the evaluation and percutaneous management of coronary dissections.
▪ Broad overview of the morphology of coronary dissections and pseudoaneurysms.
▪ Contemporary overview of SCAD pathophysiology, diagnosis and management.
▪ Review of therapy and outcomes in 440 historical cases of SCAD demonstrating improved outcomes with early intervention.