Research Article - Diabetes Management (2018) Volume 8, Issue 6
Lysulin̨̉: A double-blind placebo controlled pilot study of daily oral supplementation in people with type 2 diabetes
Abstract
Objective: This Pilot Study is aimed at evaluating the effect of daily oral supplementation with Lysulin® on glycemic control as assessed by measurement of Hemoglobin A1c (A1c), cardiometabolic and anthropometric parameters as compared to Placebo in people with Type 2 diabetes. This is a Pilot Study and it is intended to be used to design a further confirmatory clinical study by providing information on the feasibility of recruitment, randomization, sample size, retention, and assessment of procedures, methods and implementation of a novel intervention: Lysulin. Methods: A randomized, double-blind placebo-controlled pilot study was conducted over a 12-week period in 67 subjects. Subjects enrolled in the study had average an A1c 8.5 (2.2) and range 5.1%-12.9% at baseline. The inclusive range of low, medium and high in A1c levels was chosen to evaluate if there was a biased response to a specific A1c level. Subjects of ages 49 (9) years with a range of 33-74 years and both sexes (31 women and 36 men) were included in the study. Subjects were randomized 1:1:1 into groups based on oral supplementation: Group A Placebo, Group B 2.22 g/day Lysulin Group, Group C 3.33 g/day Lysulin. Subjects were evaluated at Baseline and at 4, 8 and 12 weeks. The primary endpoint of this pilot study changed in A1c from baseline to week 12. For observation purposes, Groups B and C were followed to 26 weeks. The data will be presented as Average followed by Standard Deviation in parenthesis where appropriate. Results: The results revealed a statistically significant reduction in A1c in the two groups taking Lysulin (Group B p<0.02 and Group C p<0.004) as compared to the placebo group. Changes in A1c were observed for some subjects in as little as 4 weeks after initiation of Lysulin supplementation. At 12 weeks, the change in A1c for Group A was 0.03%, the change for Group B was -0.30% and the change for Group C was -0.97%. For Group C, 65% of the subjects responded to Lysulin with a change of -1.64% A1c in 12 weeks and change of -1.91% in 26 weeks. The results also revealed a statistically significant reduction in Systolic (p<0.04) and Diastolic (p<0.05) Blood Pressure for study subjects in Group C. Clinically significant improvement was also seen in triglycerides in Group C. Conclusion: Daily oral supplementation with Lysulin has a statistically significant reduction in A1c in subjects diagnosed with Type 2 diabetes. Statistically significant improvement in systolic/diastolic blood pressure was also observed. The study revealed that the optimum dose of Lysulin is 3.33 g/day. Further confirmatory clinical studies are planned to verify the results observed in this study.
Keywords
type 2 diabetes, lysulin, HbA1c, glycemic control, pilot study, blood pressure, triglycerides
Introduction
Diabetes and its associated health complications are an increasing global health problem [1,2]. Maintaining good glycemic control is an essential part of routine diabetes care and a major contributor to minimizing future complications [3]. The current clinical practice uses the A1c test to monitor how well diabetes is being managed. The National Institute for Health and Clinical Excellence (NICE) in the United Kingdom (UK) currently recommend monitoring of glycated haemoglobin (HbA1c) every 2-6 months in people with type 2 diabetes [4]. The American Diabetes Association (ADA) guidelines [5,6] recommend performing the HbA1c test at least two times a year in subjects who are meeting treatment goals (and who have stable glycemic control) and performing HbA1c tests quarterly in subjects whose therapy has changed or who are not meeting glycemic goals. The causes of type-2 diabetes are multi-factorial, and the diet plays an important role in it’s incidence, severity and management [7]. Hence studies have frequently focused on dietary components beneficial in the prevention and treatment of diabetes. Recent studies have demonstrated that numerous herbal and nutraceutical products have beneficial effects in subjects by improving glucose and lipid metabolism, antioxidant status, disease progression and capillary function [8]. Lysulin is a unique nutritional supplement that contains three carefully balanced essential nutrients: Lysine, Zinc and Vitamin C [9]. In over 20 years of independent research, these three essential nutrients have shown to reduce blood glucose and Hemoglobin A1c (A1c) levels while improving the lipid profile. Lysulin is manufactured in the US (Lysulin, Inc., San Diego, CA) and is characterized as a dietary supplement under Dietary Supplement Health and Education Act of 1994 of the US National Institute of Health (NIH) Food and Drug Regulatory Authority (FDA) [10]. This Pilot Study is aimed at evaluating the effect of daily oral supplementation with Lysulin™ on glycemic control as assessed by measurement of Hemoglobin A1c (A1c), cardiometabolic and anthropometric parameters when compared to Placebo in people with Type 2 diabetes. This is a Pilot Study and it is intended to be used to design a further confirmatory clinical study by providing information on the feasibility of recruitment, randomization, sample size, retention, and assessment of procedures, methods and implementation of a novel intervention: Lysulin. Lysine is an essential amino acid that plays a major role in calcium absorption, building muscle protein, and the body’s production of hormones, enzymes, and antibodies. It has also shown numerous beneficial effects in the treatment/prevention of diabetes and/or it’s complications in in vivo animal and human studies. In diabetes-induced animal models, Lysine has shown a beneficial effect in lowering blood glucose as well as acting as an inhibitor of protein glycation [11]. Furthermore, the ability of Lysine to reduce the formation of glycated proteins in diabetes-induced rats, have also shown to delay the appearance of the late pathologies associated with protein glycation [12]. Lysine is known to react with glucose with the glycated amino acid being excreted in urine and it has been shown to markedly attenuate the glucose response to ingested glucose without a change in insulin response in humans [11]. Glycated proteins are known to be involved in the pathogenesis of several chronic diabetes complications, including nephropathy leading to chronic kidney disease, neuropathy, and retinopathy, as well as in other macrovascular complications [13]. Hence, it is evident that Lysine may have the potentially beneficial effect on the reduction of blood glucose as well as on the progression of diabetes and its complications [14,15]. Zinc is involved in numerous metabolic pathways as a cofactor for more than 300 enzymes [16]. Insulin, which contains a variable number of Zinc atoms, are stored in β-cells of the pancreas and released into the portal venous system at the time of β-cells degranulation. It is evident that Zinc plays an important role in insulin action, carbohydrate and protein metabolism [15]. In addition, there is particular interest in the idea that oxidative stress is relevant in the pathogenesis of diabetes and its’ complications. Impaired synthesis of enzymes, such as superoxide dismutase and glutathione peroxidase, where Zinc is a part of these enzymes’ structures and its deficiency may impair their synthesis and associated with increased oxidative stress [16]. It has been long known that diabetes is accompanied by hypozincemia [17] and hyperzincuria [18]. In Korea, 76 diabetic subjects and 72 normal subjects were supplemented with 50 mg Zinc daily as Zinc gluconate for 4 weeks. The results showed that significant improvement of fasting glucose, as well as HbA1c, was observed in zinc supplemented diabetic subjects with shorter diabetic duration, poorer glycemic control, and marginal Zinc status [19]. A recent double-blind placebo-controlled on 200 patients with prediabetes demonstrated that zinc supplementation helped to reduce blood glucose and insulin resistance while improving beta-cell function [20]. Furthermore, disease progression to Type 2 diabetes was also reduced and beneficial effects of zinc supplementation were also noted on total and LDL cholesterol [20]. Ascorbic acid (vitamin C), an antioxidant vitamin, plays an important role in protecting free radical-induced damage. Previous studies have shown a decrease in basal vitamin C level in type 2 diabetes [21]. Furthermore, randomized controlled studies have shown that supplementation of Vitamin C reduces blood glucose, serum lipids and improves HbA1c in type 2 diabetes [22]. Hence, we postulate that Lysulin which contains an optimized formulation of Lysine, Zinc and Vitamin C will have beneficial effects on glycemic control in those with diabetes and help to reduce disease progression in subjects with pre-diabetes, and those at risk of developing diabetes.
Methods
Reporting on this study is done in accordance with the NIH Policy on Good Clinical Practice (GCP) [22,23]. The data will be presented as Average followed by Standard Deviation in parenthesis where appropriate. Subjects were recruited to the present randomized (1:1:1) double-blind placebo-controlled Pilot Study between April 2018 and November 2018. The duration of the intervention was 12 weeks in Part 1 of this study. At the end of Part 1 of the study, subjects in groups B and C could volunteer to continue the intervention to 26 weeks (Part 2). We recruited subjects with a medical diagnosis of type 2 diabetes of at least 3 months duration who had not had a change in diabetes treatment or medication in the prior 2 months as deemed necessary by their General Practitioner (GP). All subjects gave written informed consent prior to enrollment in the study. Enrolled subjects were randomized 1:1:1 into groups based on oral supplementation: Group A Placebo, Group B 2.22 g/day Lysulin, and Group C 3.33 g/day Lysulin. Subjects were provided with white bottles labelled A, B and C, representing the three groups and instructed to start taking their supplement the following day. Subjects were instructed to report any change in lifestyle, diet or diabetes medication or therapy. At the baseline visit, subjects were evaluated by demographics and anthropometry parameters including measurement of height, weight, waist circumference, BMI and Blood Pressure Table 1. At the baseline visit, all subjects provided a non-fasting urine sample and venous blood sample for measurement of A1c and thirty chemistry tests including but not limited to lipids panel, liver panel, and cardiovascular biomarkers. A summary of these tests is provided in Table 2. Non-fasting venous blood samples were taken for A1c measurement at four, eight and twelve weeks after the introduction of either Lysulin or Placebo intervention. All Laboratory testing was done at an independent ISO/IEC 17025 and ISO 9001 accredited laboratory (CERTUS Lab). A1c was measured by High-Performance Liquid Chromatography (HPLC) Bio-Rad A1c System.
| Group A | Group B | Group C | All per Group | Each Group | |
|---|---|---|---|---|---|
| Average (SD) | Average (SD) | Average (SD) | Average (SD) | Within 1 SD | |
| n baseline | 25 | 23 | 19 | 22 (3) | Yes |
| n Completed | 19 | 20 | 17 | 19 (2) | Yes |
| % Dropout | 24% | 13% | 11% | 16% (7) | No* |
| Age | 48 (10) | 48 (8) | 49 (10) | 49 (9) | Yes |
| Female | 15 | 8 | 8 | 8 | NA |
| Male | 10 | 15 | 11 | 13 | NA |
| Weight | 186 (48) | 214 (62) | 188 (40) | 196 (52) | Yes |
| Height | 5.3 (0.4) | 5.5 (0.4) | 5.5 (0.3) | 5.4 (0.4) | Yes |
| Waist | 41 (7) | 44 (8) | 40 (5) | 42 (7) | Yes |
| Hip | 43 (5) | 43 (5) | 42 (3) | 43 (4) | Yes |
| Waist/Hip Ratio | 0.96 (0.08) | 1.01 (0.09) | 0.96 (0.08) | 0.98 (0.08) | Yes |
| BMI | 32 (8) | 34 (8) | 31 (5) | 32 (7) | Yes |
*The placebo had the largest drop-out rate. But still within the estimated 34-40%. Reason unknown.
Table 1. Baseline anthropometric summary
| Parameter | Average at Baseline | SD | Normal lab values | Units |
|---|---|---|---|---|
| Hematocrit | 40.6 | -4.1 | >36F >41M | % |
| CRP* | 7.2 | -11 | <5 | mg/L |
| Glucose** | 195 | -116 | 70-100 | mg/dL |
| BUN | 14 | -5.7 | 7-18.7 | mg/dL |
| Creatinine | 0.72 | -0.24 | 0.4-1.2 | mg/dL |
| Total protein | 7.25 | -0.52 | 6.3-8.3 | g/dL |
| Albumin | 4.3 | -0.4 | 3.5-5 | g/dL |
| AST | 23.2 | -11.7 | Feb-50 | U/L |
| ALT | 33.6 | -23.8 | Feb-60 | U/L |
| ALP | 79.5 | -35.4 | 40-150 | U/L |
| Bilirubin | 0.48 | -0.2 | 0.1-1.4 | mg/dL |
| Triglycerides** | 236 | -168 | 50-150 | mg/dL |
| Cholesterol | 197 | -47 | 50-200 | mg/dL |
| HDL | 44.5 | -11.3 | >40 | mg/dL |
| LDL | 124 | -31.5 | <129 | mg/dL |
| Ur-Protein** | 20.4 | -71.4 | 0-10 | mg/dL |
| Ur- Glucose** | 348.5 | -456.9 | 0-15 | mg/dL |
*Above Normal Lab Values ** Above Normal Lab Values may be due to non-fasting sample
Table 2. Baseline clinical chemistry results summary
„„ Study sample size
Though sample size statistical criteria are not required in a Pilot Study, the sample size was calculated to meet the requirement of demonstrating binary outcome superiority of the treatment groups as compared to the placebo group with a 90% power and 95% confidence interval. The sample size requirement was 16 subjects per group or a total sample size of 48 subjects. The drop-out rate was estimated to be around 35%-40%. Thus, a total of 67 subjects were recruited to ensure that there would be at least 16 subjects in each group at the completion of Part 1 of the study (12 weeks). The drop-out rate for Group A was largest at 24% but still within the estimated 34%-40%.
„„ Inclusion and exclusion criteria
Subjects were eligible if they had previously been diagnosed as having Type 2 Diabetes by their healthcare professional (>3 months) and that had no changes to diabetes medication or therapy for at least 2 months prior to enrolling. The inclusive range of low, medium and high in A1c levels was chosen to evaluate if there was a biased response at a specific A1c level. Subjects enrolled in the study had average an A1c 8.5 (2.2) and range 5.1%-12.9% at baseline. The baseline A1c levels for each group were within 1 SD of the average A1c (6.3%-10.7%) for the total enrolled subjects: Group A 7.5%, Group B 8.1% and C 9.4%. Subjects of ages 49 (9) years with a range of 33-74 years and both sexes (31 women and 36 men) were included in the study. Subjects were excluded from the study if they were pregnant or breastfeeding had a life-threatening illness or were unable to give informed consent. Additional exclusion criteria were people with Type 1 diabetes, advanced kidney or liver disease, any known medical condition that in the judgement of the principal investigator might interfere with the completion of the study.
„„ Study masking
The study was blinded to the principal investigator, the independent laboratory, and to the subjects. The subjects received their assigned supplement in white plastic bottles designated as Group A, B or C representing the three groups. Randomization was performed by the study sponsor. The treatment code for any subject could be broken in a medical emergency; however, the treatment code was not broken for any subject during this study.
„„ Study intervention and monitoring
The subjects were given uniform instructions regarding their scheduled visits, how to take the supplements and how to contact the study administrator with any questions or concern. The visit schedule was provided in writing plus the study administrator contacted each of the subjects a few days prior to the day of the visit (Table 3). The subjects were informed that they could chew, swallow or crush the supplement tablets and that they could take the tablets altogether or spread out during the day. All Laboratory and Anthropometric analysis were performed by licensed laboratory or medical technologist at an independent ISO/IEC 17025 and ISO 9001 accredited laboratory (CERTUS Lab)
| 4 | 8 | 12 | 26 | ||
|---|---|---|---|---|---|
| Base line | Weeks | Weeks | Weeks | Weeks | |
| Informed consent form |  |
||||
| Socio-demographic data |  |
||||
| Clinical/Anthropometric Parameters1 |  |
 |
 |
||
| A1C |  |
 |
 |
 |
 |
| Lipid Biochemical |  |
 |
 |
||
| Parameters2 | |||||
| Liver Biochemical Parameters3 |  |
 |
 |
||
| Blood pressure |  |
 |
 |
Body weight, height, waist circumference and hip circumference;
Total cholesterol, triglyceride, LDL cholesterol, and HDL cholesterol;
Creatinine and blood urea nitrogen (BUN)
Table 3. Study schedule
„„ Statistical analysis
Data were analyzed using parametric and nonparametric tests in SPSS version 16 (SPSS Inc., Chicago, IL, USA) and Microsoft® Excel® MSO Version 1810 Statistical Analysis Tool Pac and XLSTAT 365. Summary statistics were calculated and are presented as the Average (SD). Baseline and end-of-study characteristics, as well as laboratory findings, were compared using twosample and paired t-tests, with one-sided P<0.05 considered significant.
Results
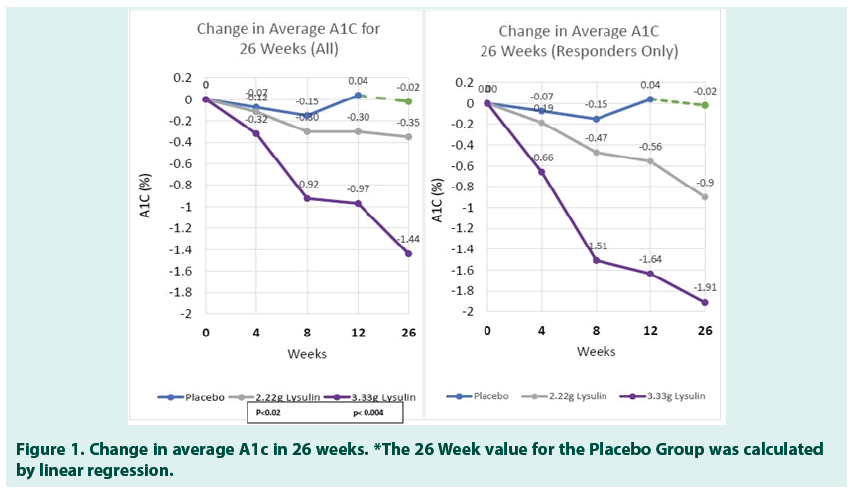
HbA1c test results are presented in % HbA1c as reported in the USA. To convert to the IFCC mmol/mol unit, the following equation can be used: mmol/mol=(% A1c - 2.15) x 10.929. As shown in Tables 4-6, the results revealed a statistically significant reduction in A1c in the two groups taking Lysulin at 12 weeks, Group B p<0.02, Group C p<0.004; 26 weeks, Group B p<0.02, Group C p<0.004) as compared to the placebo group. In Table 7 changes in A1c were observed for some subjects in as little as 4 weeks after initiation of Lysulin supplementation. At 12 weeks the change in A1c for the placebo Group A was 0.03%, the change for Group B was -0.30% and the change for Group C was -0.97%. At 26 weeks the reduction in A1C for Group B was -0.35% and for Group C was -1.44%. In Tables 5 and 6, a responder is defined as a study subject with an observed drop in A1c. For Group B, 14 of 20 subjects responded to Lysulin with a drop of -0.35% A1c in 12 weeks and -0.9 in 26 Weeks. For Group C, 11 of 17 subjects responded to Lysulin with a drop of -1.64% A1c in 12 weeks and -1.91 in 26 Weeks (Figures 1-3).
| % A1c | % A1c | % A1c | % A1c | Absolute Change | Absolute Change | Absolute Change |
% Change from Day 0 |
% Change from Day 0 |
% Change from Day 0 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 8 | Week 12 | Week 4 | Week 8 | Week 12 | Week 4 | Week 8 | Week 12 | |
| AVERAGE | 7.5 | 7.4 | 7.0 | 7.3 | -0.07 | -0.15 | 0.04 | -1.0% | -2.7% | 0.6% |
| STDEV | 1.7 | 1.8 | 1.8 | 1.5 | 0.25 | 0.30 | 0.28 | 0.03 | 0.04 | 0.04 |
Table 4. Average changes in A1C for group A (Placebo) n=19
| % A1c | % A1c | % A1c | % A1c | Absolute Change | Absolute Change | Absolute Change | % Change from Day 0 |
% Change from Day 0 |
% Change from Day 0 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 |
Week 4 |
Week 8 |
Week 12 |
Week 4 | Week 8 | Week 12 | Week 4 | Week 8 | Week 12 |
|
| AVERAGE | 8.6 | 8.5 | 8.2 | 8.1 | -0.19 | -0.47 | -0.56 | -2.3% | -5.6% | -6.5% |
| STDEV | 2.3 | 2.3 | 2.3 | 2.2 | 0.30 | 0.51 | 0.52 | 3.3% | 5.9% | 5.4% |
Table 5. Average changes in A1C for group B (2.22 g/day Lysulin)5a. Responders n=14
| AVERAGE | 6.9 | 7.0 | 7.0 | 7.2 | 0.05 | 0.10 | 0.30 | 0.7% | 0.6% | 3.6% |
| STDEV | 1.9 | 1.9 | 2.3 | 2.3 | 0.10 | 0.40 | 0.46 | 1.6% | 4.1% | 4.4% |
5b. Non-responders n=6
| AVERAGE | 8.13 | 8.02 | 7.83 | 7.83 | -0.12 | -0.30 | -0.30 | -1.4% | -3.7% | -3.5% |
| STDEV | 2.26 | 2.24 | 2.26 | 2.21 | 0.27 | 0.54 | 0.63 | 3.2% | 6.1% | 6.9% |
5c. All n=20
| %A1C | %A1C | %A1C | %A1C | Absolute Change | Absolute Change | Absolute Change | % Change from Day 0 |
% Change from Day 0 |
% Change from Day 0 |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Week |
4 Weeks |
8 Weeks |
12 Weeks |
4 Weeks | 8 Weeks | 12 Weeks |
4 Weeks |
8 Weeks |
12 Weeks |
||
| AVERAGE | 9.51 | 9.21 | 8.00 | 7.87 | -0.66 | -1.51 | -1.64 | -7.0% | -15.2% | -16.6% | |
| STDEV | 2.0 | 1.9 | 1.7 | 1.8 | 0.8 | 1.1 | 1.2 | 7.7% | 11.0% | 11.3% | |
| 6b. Non- Responders |
n=6 | ||||||||||
| AVERAGE | 9.0 | 8.9 | 8.1 | 8.1 | 0.25 | 0.15 | 0.25 | -2.9% | -8.5% | -9.0% | |
| STDEV | 2.5 | 2.4 | 2.4 | 2.4 | 0.7 | 1.0 | 1.0 | 8% | 13% | 14% | |
Table 6. Average changes in A1C for group C (3.33g/day Lysulin) 6a. Responders n=11
| AVERAGE | 9.0 | 8.9 | 8.1 | 8.1 | 0.25 | 0.15 | 0.25 | -2.9% | -8.5% | -9.0% |
| STDEV | 2.5 | 2.4 | 2.4 | 2.4 | 0.7 | 1.0 | 1.0 | 8% | 13% | 14% |
6b. Non-Responders n=6
| AVERAGE | 9.41 | 9.3 | 8.5 | 8.44 | -0.32 | -0.92 | -0.97 | -3.4% | -9.3% | -9.7% |
| STDEV | 2.0 | 2.0 | 1.9 | 2.0 | 0.8 | 1.2 | 1.4 | 8.4% | 12.4% | 13.5% |
6c. All n=17
| t-Test: Two-Sample Assuming Unequal Variances | t-Test: Two-Sample Assuming Unequal Variances | ||||
|---|---|---|---|---|---|
| At 12 Weeks | Placebo | 2.22 g Lysulin | At 12 Weeks | Placebo | 3.33 g Lysulin |
| Average | 0.038889 | 0.3 | Average | 0.0388889 | -0.9705882 |
| Variance | 0.080163 | 0.4 | Variance | 0.0801634 | 1.8459559 |
| Observations | 18 | 20 | Observations | 18 | 17 |
| P(T<=t) one-tail | 0.019607 | P(T<=t) one-tail | 0.004006 | ||
Table 7. Statistical analysis of average A1C results at 12 weeks
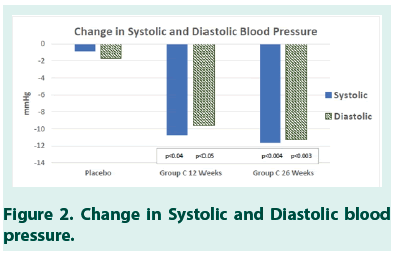
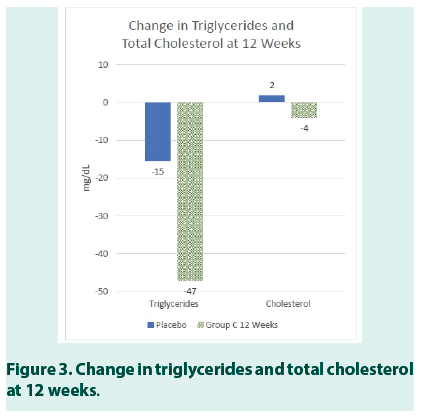
The results also revealed a statistically significant reduction in Systolic (p<0.04) and Diastolic (p<0.05) Blood Pressure for study subjects in Group C (Tables 8 AND 9). In Group C, the moderate improvement was also seen in triglycerides (Table 10).
| Anthropometric Parameters | Baseline Placebo | 12 Weeks Placebo | Baseline Group C | 12 Weeks Group C | 26 Weeks Group C |
|---|---|---|---|---|---|
| Average | Average | Average | Average | Average | |
| Weight (lbs) | 185.7 (48) | 181.8 (44.3) | 187.7 (39.9) | 185.3 (38.8) | 190.1 (40.2) |
| Waist (in) | 41.1 (7) | 40.7 (6.3) | 40.3 (4.8) | 41.2 (4.8) | 42.0 (4.8) |
| Hip (in) | 42.5 (5) | 42.7 (5.4) | 41.8 (3.3) | 43.1 (3.7) | 43.1 (3.6) |
| Waist/ Hip Ratio (NA) | 0.96 ( 0 ) | 0.95 ( 0.1 ) | 0.96 ( 0.1 ) | 0.96 ( 0.1 ) | 0.97 ( 0.07 ) |
| BP Systolic (mmHg) | 128.6 ( 21 ) | 122.5 ( 15.8 ) | 144.9 ( 21.5 ) | 134.2 ( 19.2 ) | 133.3 ( 10.3 ) |
| BP Diastolic (mmHg) | 79.8 ( 14 ) | 74.2 ( 8.4 ) | 89.7 ( 14.2 ) | 80.1 ( 13.6 ) | 78.4 ( 11.3 ) |
| BMI (NA) | 31.7 ( 8 ) | 32.1 ( 6.9 ) | 30.9 ( 5.3 ) | 31.6 ( 5.3 ) | 32.8 ( 5.2 ) |
Table 8. Baseline and 12 weeks anthropometric results summary
| 12 Weeks Placebo | 12 Weeks Group C | 26 Weeks Group C |
|---|---|---|
| Change | Change | Change |
| 4.79 | -2.4 | 2.39 |
| 0.73 | 0.98 | 1.71 |
| 0 | 1.32 | 1.32 |
| 0.02 | 0 | 0.02 |
| -0.89 | -10.76 | -11.65 |
| -1.72 | -9.56 | -11.28 |
| 1.19 | 0.72 | 1.91 |
Table 9. Statistical analysis of diastolic and systolic blood pressure from baseline to 12 Weeks for Placebo and Group C; and from baseline to 26 weeks for group C
| Baseline | 12 Weeks | 12 Weeks | Baseline | 12 Weeks | 12 Weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Placebo | Placebo | Group C | Group C | Group C | |||||||
| Analyte | Normal Range | Units | Average | ±SD | Average | ± SD | Change | Average | ± SD | Average | ± SD | Change |
| Hematocrit | >36F >41M | % | 40 | 5 | 41 | 5 | 1 | 40 | 4 | 40 | 5 | 0 |
| CRP | >5 | mg/L | 8 | 16 | 4 | 4 | -4 | 8 | 8 | 5 | 5 | -2 |
| BUN | 7-18.7 | mg/dL | 13 | 7 | 15 | 7 | 2 | 15 | 6 | 17 | 6 | 1 |
| Creatinine | 0.4-1.2 | mg/dL | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| T-protein | 6.3-8.3 | g/dL | 7 | 1 | 7 | 1 | 0 | 7 | 1 | 7 | 0 | 0 |
| Albumin | 3.5-5 | g/dL | 4 | 0 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 0 |
| AST | Feb-50 | U/L | 25 | 14 | 24 | 14 | -1 | 22 | 13 | 22 | 11 | 0 |
| ALT | Feb-60 | U/L | 34 | 31 | 30 | 22 | -4 | 36 | 24 | 29 | 19 | -7 |
| ALP | 40-150 | U/L | 80 | 28 | 92 | 32 | 12 | 76 | 19 | 100 | 30 | 25 |
| Bilirubin | 0.1-1.4 | mg/dL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tryglicerides | 50-150 | mg/dL | 248 | 212 | 232 | 92 | -15 | 244 | 175 | 197 | 76 | -47 |
| Cholesterol | 50-200 | mg/dL | 195 | 62 | 197 | 33 | 2 | 201 | 41 | 197 | 44 | -4 |
| HDL | >40 | mg/dL | 44 | 15 | 42 | 13 | -3 | 43 | 8 | 39 | 9 | -3 |
| LDL | <129 | mg/dL | 117 | 37 | 127 | 23 | 9 | 130 | 28 | 128 | 31 | -3 |
Table 10. Baseline and 12 weeks clinical chemistry results summary
„„ Adverse effects and safety
There were no serious adverse effects noted and no subject was hospitalized due to adverse effects during the 6- month follow-up period. Biochemical assessments evaluating potential target organ toxicity (liver enzymes, serum bilirubin, and serum creatinine) remained normal throughout. None of the subjects experienced any form of hypersensitivity during the study (immediate and/or delayed).
Conclusions
This double-blind, placebo-controlled study with people with Type 2 diabetes has clearly shown that Lysulin supplementation has a dramatic improvement in glycemic control. The observed improvement in A1c and blood pressure compared to placebo all meet statistical significance. The optimum dose of Lysulin is 3.33 g/day. Further confirmatory clinical studies are planned to verify the results observed in this study.
Acknowledgements
The authors thank all the subjects, investigators, CERTUS lab personnel and study managers who were involved in the conduct of the study.
Disclosures
The principal investigator, Dr Francisco Alberto Alvarez Melero and the clinical site and the laboratory had no conflict of interest. The other three authors are employees of Lysulin, Inc. The study was funded by Lysulin, Inc.
References
- Danaei G, Finucane MM, Lu Y et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million subjects. Lancet. 378(9785), 31-40 (2011).
- Kanavos P, van den Aardweg S. Diabetes expenditure, burden of disease and management in 5 EU countries. LSE Health, London School of Economics. (2012).
- Stratton IM, Adler AI, Neil HA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 321, 405-412 (2000).
- NICE Type 2 diabetes: National clinical guideline for management in primary and secondary care (update). NICE clinical guideline 66: Royal College of Physicians. (2008).
- American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes. Care. 36, S11-66 (2013).
- Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes. Care. 30 (S1), S48-65 (2013).
- Bailey CJ, Kodack M. Subject adherence to medication requirements for therapy of type 2 diabetes. Int. J. Clin. Pract. 65(3), 314-322 (2011).
- Dietary Supplement Health and Education Act of 1994. National Institutes of Health. US Department of Health & Human Services (1994).
- Burd JF. Lysulin™, a new supplement for Nutritional Support for People with Diabetes and Pre-diabetes (those at risk of developing diabetes). We have presented data which illustrates that Lysulin does lower blood glucose and HbA1c after just one month of use. Diab. Manag. 8(2), 38-40 (2018).
- Dietary Supplement Health and Education Act of 1994. National Institutes of Health. US Department of Health & Human Services (1994).
- Kalogeropoulou D, LaFave L, Schweim K et al. Lysine ingestion markedly attenuates the glucose response to ingested glucose without a change in insulin response. Am. J Clin. Nutr. 90(2), 314-320 (2009).
- Sensi M, De Rossi MG, Celi FS et al. d-Lysine reduces the non- enzymatic glycation of proteins in experimental diabetes mellitus in rats. Diabetologia. 36(9), 797-801 (1993).
- Singh VP, Bali A, Singh N et al. Advanced Glycation End Products and Diabetic Complications. Korean. J. Physiol. Pharmacol. 18(1), 1-14 (2014).
- Zimmerman GA, Meistrell M, Bloom O et al. Neurotoxicity of advanced glycation end products during focal stroke and neuroprotective effects of aminoguanidine. PNAS. 92 (9), 3744-3748 (1995).
- Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes. Res. Clin. Pract. 67(1), 3-21 (2005).
- Jayawardena R, Ranasinghe P, Galappatthy P et al. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 4(1), 13 (2012).
- Garg VK, Gupta R, Goyal RK. Hypozincemia in diabetes mellitus. J. Assoc. Physicians. India. 42, 720-721 (1994).
- Piducck HG, Wren PJ, Evans DA. Hyperzincuria of diabetes mellitus and Possible genetical implications of this observation. Diabetes. 19, 240-247 (1970).
- Oh HM, Yoon JS. Glycemic control of type 2 diabetic patients after short-term zinc supplementation. Nutr. Res. Pract. 2, 283-288 (2008).
- Ranasinghe P, Wathurapatha WS, Galappatthy P et al. Zinc supplementation in prediabetes: A randomized double blind placebo controlled clinical trial. J. Diabetes. 10, 386-397 (2017).
- Kotb A, Azzam KMA. Effect of Vitamin C on Blood Glucose and Glycosylated Hemoglobin in Type II Diabetes Mellitus. World. J. Analytic. Chem. 3(1A), 6-8 (2015).
- Dakhale GN, Chaudhari HV, Shrivastava M. Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: A randomized, double-blind study. Adv. Pharmacol. Sci. 2011, 5 (2011).
- Policy on good clinical practice training for NIH awardees involved in NIH-funded clinical trials. National Institutes of Health. (2016).