Review Article - Imaging in Medicine (2009) Volume 1, Issue 1
Ultrasound imaging of the early fetus: is it safe?
Jacques S Abramowicz*Department of Obstetrics & Gynecology and Fetal & Neonatal Medicine Program, Rush University Medical Center, Chicago, IL, USA
- Corresponding Author:
- Jacques S Abramowicz
Department of Obstetrics & Gynecology and Fetal & Neonatal Medicine Program
Rush University Medical Center, Chicago, IL, USA
Tel: +1 312 942 9428
Fax: +1 312 942 9477
E-mail: jacques_abramowicz@ rush.edu
Abstract
Keywords
bioeffect ▪ Doppler ▪ fetus ▪ first trimester ▪ obstetrics ▪ safety
Diagnostic ultrasound has been in use for over 50 years in obstetrics and gynecology. Its record of safety is excellent, with no epidemiological studies demonstrating harmful effects in human fetuses. However, a very important fact to keep in mind when considering bioeffects and safety of ultrasound for the fetus is that all epidemiological studies published so far are based on information obtained with pre-1992 machines, a time when estimated in situ intensity for fetal use was allowed to be increased from 94 to 720 mW/cm2, a factor of almost eight [1]. Furthermore, while B-mode gray scale continues to be the main examination mode, newer technologies and applications of these technologies have been introduced over the years. Moreover, with advancements in technology and resolution, many fetuses are examined very early in pregnancy, for example for nuchal translucency (NT) screening [2] or, for fetal anatomy survey starting around 11–13 weeks, at the time of the NT screening [3]. An additional opportunity for early exposure is the performance of nonmedically indicated ultrasound (also known as entertainment ultrasound), a trend that has recently increased, particularly in the USA [4]. Doppler ultrasound, in particular, needs to be attentively examined, in terms of bioeffects and safety to the fetus. Specifically, color and spectral Doppler evaluation of the fetal heart valves, heart function and ductus venosus is advocated by some for early assessment in the first trimester [5–7]. One needs to ponder whether there is enough evidence to validate the use of ultrasound imaging in general and Doppler in particular in the first trimester and whether ultrasound can have detrimental effects on the fetus in the first trimester? The answer to the first question is well beyond the realm of this article, which will discuss the mechanisms for effects of ultrasound in tissues, experimental evidence, clinical relevance and, in particular, ways to limit the possible hazards of exposure of the fetus at early stages of gestation.
Bioeffects of ultrasound
Two major mechanisms are operative in any tissue traversed by ultrasound: thermal and nonthermal. Local tissue heating is an indirect effect due to acoustic energy being transformed into thermal energy, thus it is called a thermal effect [8], while nonthermal effects (also known as mechanical) are a direct response, consisting of tissue reaction to altering positive and negative pressure. Mechanical effects also include effects that are not purely mechanical, such as chemical or physical [9,10].
Thermal effects
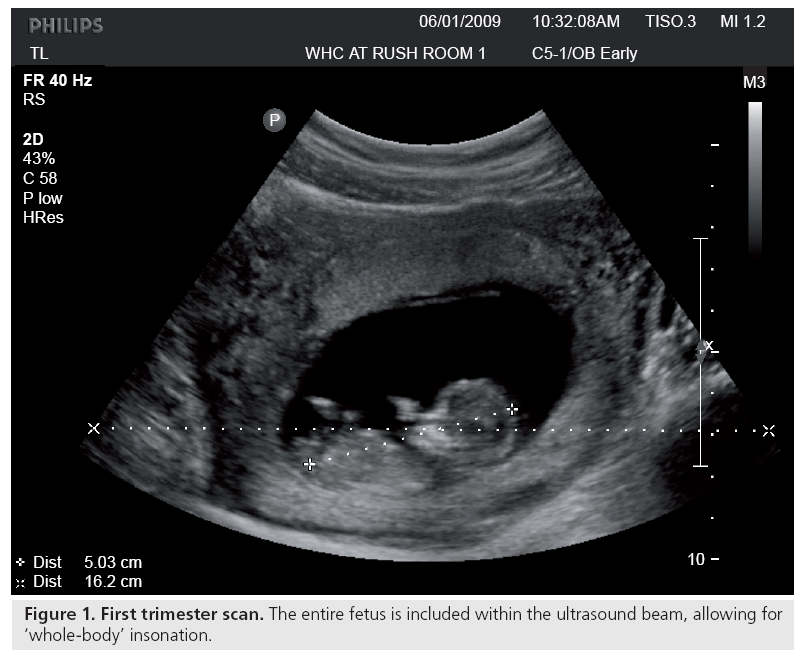
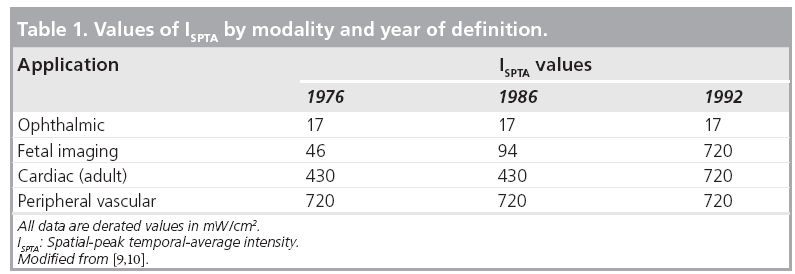
Ultrasound may induce a rise in temperature in insonated tissues. This is important because specific structural abnormalities have been shown to be induced by increased temperature in many animal studies, as well as several controlled human studies [9]. Elevated maternal temperature in early gestation has been associated with a higher than expected incidence of congenital anomalies [11]. Such teratogenic effects do not refer to ultrasound- induced temperature elevation, unless so indicated. Edwards and others have demonstrated that hyperthermia is teratogenic for numerous animal species, including humans [12], and suggested a 1.5°C temperature elevation above the normal value as a universal threshold [13]. This acceptance of a threshold forms the basis for the as low as reasonably achievable (ALARA) principle: keep the exposure as low as possible, for the least amount of time possible, but yet enough to get adequate diagnostic images. It has even been proposed that there may be no thermal threshold for hyperthermia-induced birth defects [14,15]. Any temperature increment for any period of time has some effect, the higher the temperature differential or the longer the temperature increment, the greater the likelihood of producing an effect. Since two accepted facts are that ultrasound has the potential to elevate the temperature of the tissues being scanned [9,16–18] and elevated maternal temperature, whether from illness or exposure to heat, can produce teratologic effects [11,12,15,19–21], the obvious question is whether diagnostic ultrasound can induce a harmful/teratological rise in temperature in the fetus [8,22,23]. Some believe that this temperature rise is, in fact, a major mechanism for ultrasound bioeffects [9,15]. For prolonged exposures, temperature elevations of up to 5°C have been obtained [22]. Temperature change in insonated tissues depends on the balance between heat production and heat loss. A particular tissue property that strongly influences the amount of heat transported is local perfusion, which very clearly diminishes the risk, if present. It is worth noting here that in early pregnancy, under 6–8 weeks, there appears to be minimal maternal–fetal circulation, that is, minimal fetal perfusion, which may potentially reduce heat dispersion [24]. The lack of perfusion is one reason why the spatial-peak temporal-average intensity (ISPTA) for ophthalmic applications has been kept very low, in fact, much lower than peripheral vascular, cardiovascular and even obstetric scanning, despite the general increase in acoustic power that was allowed after 1992 (Table 1). There are some similarities in physical characteristics between the early, first-trimester embryo and the eye. Neither is perfused, they can be of similar size and protein is present (in an increasing proportion in the fetus). At approximately weeks 4–5 (throughout this article, as accepted in the obstetrical literature, gestational age is equivalent to menstrual age which is 2 weeks more than fertilization age), the gestational sac is approximately the size of the eye (2.5 cm in diameter), and by week 8, it is approximately 8 cm in diameter. Ultrasound imaging in these early stages of gestation involves ‘wholebody’ scanning since the fetal size is less than the cross-section of the beam (Figure 1). In addition, there is no or minimal perfusion in very early gestation. Only at about weeks 10–11 does the embryonic circulation actually link up with the maternal circulation [25]. Thus, there may be some underestimation of the actual ultrasound-induced temperature in early gestation, mainly because of the absence of perfusion. The perfusion issue is in addition to modifications of tissue temperature due to ambient maternal and fetal temperatures. Furthermore, motions (even very small) of the examiner’s hand as well as the patient’s breathing and body movements (in the case of obstetric ultrasound, both the mother and the fetus) tend to spread the region being heated. The highest potential for heating is always when the beam is stationary. With beam movement, previously heated tissue quickly cools, when not being exposed to ultrasound. However, early in gestation, as described earlier, the fetus is very small and the amount of perfusion and circulation are low and how much heat is being dispersed is next to impossible to evaluate. Furthermore, for spectral (pulsed) Doppler studies, conditions may be different (see later). As mentioned earlier, there is a mathematical/physical relationship between temperature elevation and several beam characteristics. The final temperature rise depends primarily on acoustic power and beam size, as well as the acoustic absorption and thermal properties of the tissue. This acoustic power may be altered by changes in wave amplitude (controlled by the machine output), pulse length and pulse-repetition frequency. Hence, manipulating any of these via instrument controls will alter the in situ conditions. Temperature increases of 1°C or more can easily be reached in routine scanning [26].
Nonthermal effects
Ultrasound bioeffects also occur through mechanical processes [27,28]. These are interactions between the ultrasound wave and the tissue that do not cause a significant degree of temperature increase (<1°C above physiologic temperature). These include acoustic cavitation, as well as radiation torque and force, and acoustic streaming, secondary to propagation of the ultrasound waves. While included in this category, some effects are, in fact, the result of the mechanical interaction but are actually physical (shock wave) or chemical (release of free radicals) effects. Cavitation seems to be the major factor in mechanical effects [29] as it has been demonstrated to occur in living tissues (e.g., lungs and intestines) under ultrasound insonation [30,31]. Two types of cavitation can be described: stable and inertial (previously defined as transient). To occur, both need the presence of gas bubbles in insonated tissues. Stable cavitation corresponds to vibrations or small repeatable oscillation of the bubble diameter without leading to bubble collapse (see later). It occurs at relatively lower acoustic intensities and is more long-lived than inertial cavitation with possible resulting microstreaming. Inertial cavitation indicates expansion and reduction in volume of the bubbles, secondary to alternating positive and negative pressures generated by the ultrasound wave. Expansion can be to an unstable size followed by collapse of the bubble with production of very high pressure (hundreds of atmosphere) and very elevated temperature (thousands of degrees) but on such a small area and for such a brief time that it will not be felt and is very hard to measure (adiabatic reaction = occurring without the gain or loss of heat). This can, however, produce microstreaming (a phenomenon that has also been described with no clear involvement of bubbles [32–34]) or even release of free radicals [35,36]. Biological effects, such as local intestinal [37], renal [38] and pulmonary [39] hemorrhages, of ultrasound in animals have been attributed to mechanical effects, although cavitation could not always be implicated. Furthermore, since there does not seem to be gas bubbles in fetal lungs or bowels (where effects have been described in neonates or adult animals), the risk to the fetus from mechanical effects appears to be minimal [10,40]. Another described result of mechanical energy is hemolysis [41]. Again, it is evident, however, that the presence of some cavitation nuclei is necessary for hemolysis to occur. Ultrasound contrast agents can be a source of such cavitation nuclei, when injected into the body before ultrasound examination. But, at present, there is no clear clinical indication for the use of ultrasound contrast agents in fetal ultrasound [42] and, to date, no studies have investigated specifically the interaction of ultrasound and microbubble contrast agents in fetal tissues in vivo. It should, nevertheless, be noted that in the presence of such contrast agents, fetal red blood cells are more susceptible to lysis from ultrasound exposure in vitro [43]. In addition to the above, fetal stimulation caused by ultrasound (Doppler) insonation has been described, with no apparent relation to cavitation [44]. This effect may be secondary to radiation forces associated with ultrasound exposures. These forces were suspected at the earliest stages of ultrasound research [45] and are known to possibly stimulate auditory [46], sensory [47] and cardiac tissues [48]. No harmful effects of diagnostic ultrasound, secondary to nonthermal mechanisms, have been reported in human fetuses.
Output Display Standard
The Standard for Real-Time Display of Thermal and Mechanical Indices on Diagnostic Ultrasound Equipment, generally known as the Output Display Standard or ODS, dates from 1992 when the US FDA yielded to pressure from ultrasound clinical users and manufacturers to increase the power output of instruments. Higher outputs were assumed to generate better images and, thus, improve diagnostic accuracy. To allow clinical users of ultrasound to use their instruments at higher powers than originally intended and to reflect the two major potential biological consequences of ultrasound (mechanical and thermal, see earlier), the American Institute of Ultrasound in Medicine (AIUM), the National Electrical Manufacturers’ Association (NEMA) and the FDA (with representatives from the Canadian Health Protection Branch, the National Council on Radiation Protection and Measurements [NCRP] and 14 other medical organizations [9]) developed a standard related to the potential for ultrasound bioeffects. This was an attempt to provide quantitative safetyrelated information. This information was to appear onscreen during an examination so that the end-users would be able to see how manipulation of the instrument controls during an examination causes alterations in the output and thus on the exposure. As previously noted, the acoustic intensity for fetal use, as expressed by the estimated in situ ISPTA, went from a previous value of 94 to 720 mW/cm2 (Table 1). The indices to appear were the thermal index (TI), to provide some indication of potential temperature increase, and the mechanical index (MI), to provide indication of potential for nonthermal (i.e., mechanical) effects [9,49,50]. The TI had three variants: for soft tissue, to be used mostly in early pregnancy when ossification is low; for bones, to be used when the ultrasound beam impinges on bone, at or near the beam focus, such as late second and third trimesters of pregnancy; and for transcranial studies, when the transducer is essentially against bone, mostly for examinations in adult patients. These indices were required to be displayed if equal to or over 0.4. It needs to be made very clear that TI does not represent an actual or an assumed temperature increase. It bears some correlation with temperature rise in degrees Celsius, such that a higher TI can be assumed to be associated with a higher temperature rise than a lower TI, but does not allow an estimate or a guess as to what that temperature change actually is in the tissue. The MI represents the potential for cavitation in tissues but is not based on actual in situ measurements. It is a theoretical formulation of the ratio of the pressure to the square root of the ultrasound frequency (hence, the higher the frequency, the lesser risk of mechanical effect). Both the TI and MI can and should be followed as an indication of change in output during the clinical examination. A clear extension of the above statements is that education of the end-user is a major part in the implementation of the indices.
Unfortunately, this aspect of the ODS does not seem to have succeeded as end-users’ knowledge of bioeffects, safety and output indices is lacking [51,52]. Both in Europe [52] and the USA [51], approximately 70% of clinicians (physicians and sonographers, including nurses who perform ultrasound) show very poor or no knowledge of bioeffects and safety issues, do not know what TI and MI represent and do not even know that these appear onscreen during clinical ultrasound examinations. Furthermore, several assumptions were made when formulating the indices, which bring questions on their clinical value. The most significant (from a clinical aspect) is the choice of the homogeneous attenuation path model (defined as the H3 model), with an attenuation coefficient of 0.3 dB/cm/MHz, which is supposed to be equivalent to the attenuation occurring under worst-case conditions when the ultrasound wave traverses the maternal abdomen and uterus, on its way to the fetus. This may be an overestimation of the attenuation in many clinical scenarios, a situation which would underestimate the actual exposure. In NCRP report number 140 [9], there is a whole chapter indicating conditions where both indices may be inaccurate, for example long fluid path (full bladder, amniotic fluid, ascites or hydrocephalus) or path through increased amounts of soft tissue (e.g., obese patients). Based on mechanisms involved, as far as is known, nonthermal effects of ultrasound are probably negligible, if they exist at all, in the fetus, as described earlier, since no naturally occurring gas bodies are present in the fetal lungs and bowels. It should be noted, however, that some nonthermal effects have been described in animals but at exposures well above the upper limit (MI = 1.9) imposed by the FDA [28]. There is, in fact, little information on energy output and exposure in clinical obstetrical ultrasound. Only relatively recently has it been shown that, if one considers TI and MI to be some indication of acoustic output, then the levels are low in the first [53], second and third trimester [54], and even in Doppler studies [55] – although higher levels of TI can be reached in this modality – as well as 3D/4D examinations [56]. The aforementioned studies should be viewed with some caution since they were performed in units where end-users were knowledgeable of bioeffects and safety. It should also be noted that in some countries, the number of prenatal ultrasound examinations has reached ten per pregnancy and it is presently unknown whether there is a cumulative dose effect to exposure [57].
Fetal susceptibility to external insults
This is, of course, the other side of the equation. The growing fetus is very sensitive to external influences. Known teratological agents include, for instance, certain medications or drugs of abuse taken by the pregnant woman, exposure to x-rays and elevated temperature, secondary to infectious diseases. This is especially true in the first 10–12 weeks of gestation [58–60]. Gestational age is thus a vital factor when dealing with possible bioeffects: milder exposure during the preimplantation period can have similar consequences to more severe exposures during embryonic and fetal development and can result in prenatal death and abortion or a wide range of structural and functional defects. Most at risk is the CNS owing to a lack of compensatory growth of undamaged neuroblasts. In experimental animals, the most common defects are of the neural tube as well as microphthalmia, cataract and microencephaly, with associated functional and behavioral problems [12]. More subtle effects are possible, such as abnormal neuronal migration, with unclear potential results [61]. Other prominent defects are seen in craniofacial development (more specifically facial clefts [62]), the skeleton [63], the body wall, teeth and the heart [64]. Hyperthermia in utero (due to maternal influenza for instance) was long known to potentially induce structural anomalies in the fetus [19,21,63,65–70] but, relatively recently, it has been described as an environmental risk factor for psychological/behavioral disturbances [71] and, more particularly, schizophrenia [72]. It is stressed that these are not ultrasound-induced hyperthermia effects and it is suggested that temperature elevation under 38.9°C is probably not harmful. Yet, ultrasound has been shown to induce temperature increase in vivo [12,15,73–79], albeit not in humans. There is, however, a serious lack of data examining the effects of ultrasound while rigorously excluding other confounding factors. If one considers together the facts that hyperthermia is potentially harmful to the fetus and that ultrasound may, under certain circumstances, elevate tissue temperature, then precaution has to be recommended, particularly in early gestation and especially with modes known to emit higher acoustic energy levels (e.g., pulsed Doppler).
Ultrasound in early gestation
There are many valid medical indications to perform ultrasound in early gestation [201]. These include, among others, bleeding, accurate gestation dating, confirmation of viability and verification of the number of fetuses. All of these examinations are performed with B-mode, a mode with relatively low acoustic output. However, more recently, screening for genetic abnormalities and early assessment of structural abnormalities are described in the literature in early (11–15 weeks) pregnancy [2,3,80–82]. While most of these are also performed with B-mode, often Doppler is used to detect blood vessels and/or to visualize and analyze cardiac valves, potentially exposing the fetus to much higher energy levels (see later). One needs to keep in mind that, even with B-mode, dwell time is important since prolonged examination can result in higher exposure levels.
Is Doppler different & can it have detrimental effects on the fetus in the first trimester?
Several applications of the Doppler principle are in clinical use: color Doppler, power (also known as ‘energy’) Doppler and spectral (pulsed) Doppler. Most of the discussion on Doppler refers to pulsed Doppler, which is the one with the potential highest acoustic power. Color and power Doppler are somewhat in the middle of the spectrum between B-mode and pulsed Doppler. In addition, they are scanned modes (as opposed to pulsed Doppler), meaning the beam is not immobile but scans through the region of interest, therefore, exposing each segment for much shorter periods. While a variety of movements intervene during B-mode imaging, such as fetal body motion, observer’s hand movements and maternal breathing, it is necessary to have the transducer as steady as possible during a Doppler examination. This is because, in general, blood vessels or heart valves are small in comparison to the general organ or body size being scanned and even small movements will have more undesired effects on the resulting image. As described later, the maximal intensity (ISPTA) and acoustic power associated with Doppler ultrasound are the highest of all the general-use categories, 1180 mW/cm2 for pulsed Doppler as opposed to 34 mW/cm2 for B-mode. These numbers do not necessarily indicate the actual exposure levels and, because of the way they are obtained, cannot be easily compared with the FDA limits, as previously discussed. However, they express the fact that, in general, acoustic exposure can be much higher during Doppler examination. As mentioned, dwell time (duration of exposure) is also of major importance: Ziskin reported that among 15,973 Doppler ultrasound examinations, the average duration was 27 min (and the longest 4 h) [83]. Hearing the fetal heart beat is certainly a very satisfying experience for the expecting parents, and often this is accomplished by using pulsed Doppler. In fact, using Doppler to ‘listen’ to the fetal heart is not new [84–86]. This should be discouraged and replaced by m-mode assessment. If Doppler is used, it is sufficient to ‘hear’ three to four heart beats and thus limit the exposure. It is important to point out that fetal heart monitoring is also performed with a Doppler instrument but with continuous wave (as opposed to pulsed in diagnostic ultrasound), with extremely low outputs and, to the best of our knowledge, with no thermal risk to the fetus. Evidently, one of the major uses of ultrasound is the prenatal detection of fetal abnormalities. The organ most commonly affected by major genetic disorders is the heart, hence extensive research in imaging and functional assessment of the heart. Doppler (pulsed [spectral] and color) are the ideal techniques to examine the heart. Extensive research has been published on the value of ultrasound examination of the fetal heart, including Doppler analysis of flow across the cardiac valves and Doppler velocimetry of various fetal vessels. The vast majority of published reports were, until recently, on B-mode examinations performed at 18–20 weeks. However, several authors have demonstrated the feasibility of examining the heart much sooner in pregnancy, beginning at 10 or 11 weeks [87–91]. Doppler analysis has long been a tool to study cardiac function, although mostly in the placenta, umbilical or uterine arteries [92]. Studies have been published of Doppler study of flow through cardiac valves, beginning at 6 weeks [93,94]. It should be noted that it is technically extremely difficult to obtain these tracings and, thus, very prolonged dwell times are necessary. Some have described performing a measurement of the heart diameter, heart rate and inflow and outflow waveforms after 5 weeks [95]. It should also be remembered that, at these early stages of pregnancy, fetuses measure 1–2 cm in length and therefore, as described earlier, ultrasound scanning causes total body exposure in B-mode. This is necessary to position the Doppler gate. Analysis of ductus venosus flow as well as characteristics of flow across the tricuspid valve have been shown to be helpful in screening for chromosomal anomalies in the first trimester of pregnancy, as an adjunct to measurement of the NT. Waveform analysis of the ductus venosus reduces the false-positive rate of the screening test [96,97].
For example, in fetuses from 11–13 6/7 weeks with increased NT but with normal karyotype, absent or reversed A-wave (atrial contraction) in the ductus venosus is associated with a threefold increase in the likelihood of a major cardiac defect, whereas normal ductal flow is associated with a 50% reduction in the risk for such defects [7,98,99]. It is interesting to note that abnormal findings in the ductus venosus associated with an increased risk of chromosomal anomalies (reverse diastolic velocity) are similar to those described many years ago for fetuses at risk of hypoxia, in cases of intrauterine growth restriction [100].
Acoustic output
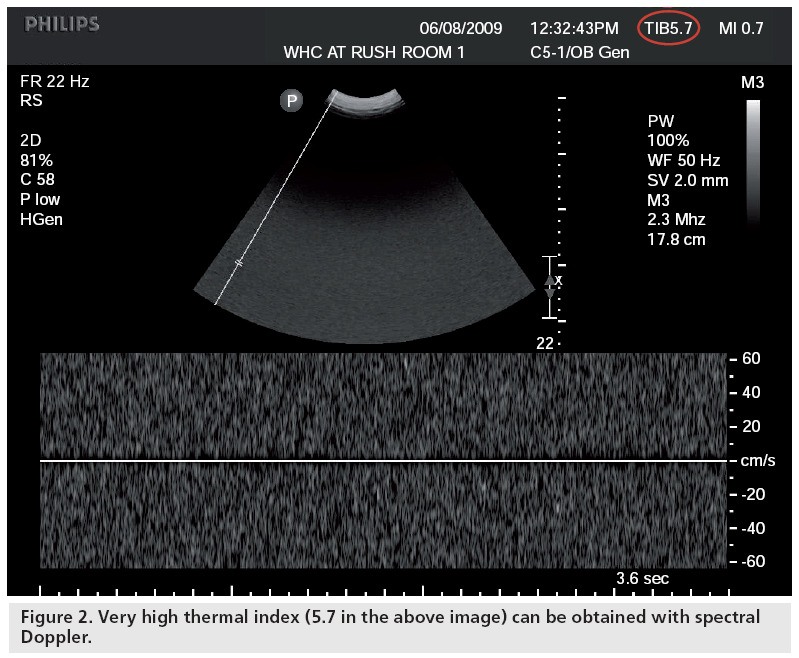
Based on various sources, it appears that acoustic output (as expressed by various intensities) can be much higher in Doppler mode: for instance, 34 mW/cm2 for the ISPTA in B-mode versus 1180 mW/cm2 for spectral Doppler [101]. If one compares outputs (as expressed by TI and MI, a clinically easy-to-use but somewhat remote expression of output) between first and second–third trimesters, differences are not major [53], but higher TI values are obtained when switching to Doppler mode [55]. The increase in TI is generally small but with some new machines, TI’s of up to 5 and 6 are displayed in the Doppler mode (Figure 2). Concerns about the fact that outputs are much higher in Doppler applications were expressed in three editorials [102–104]. The question was raised whether research involving Doppler in the first trimester should even be considered for publication [103]. Despite this, as detailed earlier, there has been a major recrudescence in the usage of Doppler in the first trimester in recent years. Unfortunately, one of the reasons for this is the complete ignorance of most end-users of potential bioeffects, based on the ‘nothing has been shown’ principle. Therefore, the risk is that this will become a routine standard, secondary to the push by certain individuals who are experts in these examinations, and that inexperienced end-users, wishing to imitate and adulate these experts, will attempt to perform these examinations for extremely extended period of time at pregnancy stages that are very susceptible to external insults [Brezinka C, Pers. Comm.]. Indeed, a major issue is the lack of knowledge of ultrasound clinical users on output, bioeffects and safety, both in the USA [51] and abroad [52]. It is important to note that the above is not a condemnation of the use of Doppler technology when indicated. Furthermore, excellent diagnostic accuracy can be obtained when using Doppler with very low outputs (as expressed by TI’s well below 1), which should be used as the default and increased only if necessary, as opposed to blindly using the technology without taking minimal precautions. It should be noted that many countries in the world have no regulations on training of individuals performing diagnostic ultrasound examinations, nor standards or guidelines for the examinations or quality control for the instrumentation used.
Safety statements
Some scientists have clearly stated that Doppler should be avoided in the first trimester. Several ultrasound organizations (American Institute of Ultrasound in Medicine [AIUM], International Society of Ultrasound in Obstetrics and Gynecology [ISUOG] and World Federation of Ultrasound in Medicine and Biology [WFUMB]) are studying the issue with the intent of publishing statements and/or guidelines specific for first trimester ultrasound, with a particular emphasis on the use of Doppler in early pregnancy.
How to limit fetal exposure?
The answer is very simple: perform ultrasound only with a clear indication, keep exposure to a minimum power and time, compatible with an adequate diagnosis (application of the ALARA principle), watch the TI and, to a lesser degree, the MI onscreen and do not perform examinations with new techniques ‘simply because you can’, if they have not been scientifically shown to afford diagnostic advantages. In general, begin your examination with a low power output and increase only if necessary [202].
Conclusion
Ultrasound may, arguably, be the most important technology in the last 50 years in obstetrical clinical practice. Its advantages are numerous and its use has expanded from simply measuring a biparietal diameter to 3D study of the brain or heart anatomy or ‘real-time 3D’ (also known as 4D) evaluation of fetal behavior. Not only is structural analysis possible, but functional assessment of cardiac function is achievable with the use of Doppler applications. The fact this can be done is not a blanket permission to perform it with no control or limits, particularly in early pregnancy – a time when the fetus is very susceptible to external insults. Indications to perform an examination should be clear and the lowest possible acoustic output power should be used, for the shortest possible time.
Future perspective
Prenatal diagnosis may progress over the next few years to the point of routine fetal DNA analysis in the blood of the future mother. In the meantime, prenatal diagnosis will continue to be based both on maternal biochemistry and diagnostic ultrasound in early pregnancy. Given the noninvasiveness of ultrasound and the immediate availability of results, it is safe to assume this modality will continue to be the primary form of imaging throughout pregnancy. Besides improvements in image quality, it is expected that miniaturization of instruments and cost reduction will permit introduction to a larger number of practitioners in various countries, thus exposing an ever-increasing number of fetuses to this modality. Therefore, education of the end-users on safety and bioeffects needs to continue to be a vital part of their training.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Abramowicz JS, Fowlkes JB, Skelly AC, Stratmeyer ME, Ziskin MC: Conclusions regarding epidemiology for obstetric ultrasound. J. Ultrasound Med. 27(4), 637–644 (2008).
- Nicolaides KH: First-trimester screening for chromosomal abnormalities. Semin. Perinatol. 29(4), 190–194 (2005).
- Timor-Tritsch IE, Fuchs KM, Monteagudo A, D’Alton ME: Performing a fetal anatomy scan at the time of first-trimester screening. Obstet. Gynecol. 113(2 Pt 1), 402–407 (2009).
- Abramowicz J, Brezinka C, Salvesen K, ter Haar G: ISUOG Statement on the non-medical use of ultrasound, 2009. Ultrasound Obstet. Gynecol. 33(5), 617 (2009).
- Vinals F, Ascenzo R, Naveas R, Huggon I, Giuliano A: Fetal echocardiography at 11 + 0 to 13 + 6 weeks using four-dimensional spatiotemporal image correlation telemedicine via an Internet link: a pilot study. Ultrasound Obstet. Gynecol. 31(6), 633–638 (2008).
- Russell NE, McAuliffe FM: First-trimester fetal cardiac function. J. Ultrasound Med. 27(3), 379–383 (2008).
- Teixeira LS, Leite J, Viegas MJ et al.: Ductus venosus Doppler velocimetry in the first trimester: a new finding. Ultrasound Obstet. Gynecol. 31(3), 261–265 (2008).
- Abramowicz JS, Barnett SB, Duck FA, Edmonds PD, Hynynen KH, Ziskin MC: Fetal thermal effects of diagnostic ultrasound. J. Ultrasound Med. 27(4), 541–559; quiz 560–563 (2008).
- National Council on Radiation Protection and Measurements (NCRP): Exposure criteria for medical diagnostic ultrasound: II. Criteria based on all known mechanisms. Report no. 140. Contract no. 140. Bethesda, MD, USA (2002).
- Stratmeyer ME, Greenleaf JF, Dalecki D, Salvesen KA: Fetal ultrasound: mechanical effects. J. Ultrasound Med. 27(4), 597–605; quiz 606–609 (2008).
- Shaw GM, Todoroff K, Velie EM, Lammer EJ: Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology 57(1), 1–7 (1998).
- Edwards MJ, Saunders RD, Shiota K: Effects of heat on embryos and foetuses. Int. J. Hyperthermia 19(3), 295–324 (2003).
- Edwards MJ: Hyperthermia as a teratogen: a review of experimental studies and their clinical significance. Teratog. Carcinog. Mutagen. 6, 563–582 (1986).
- Miller MW, Miller HE, Church CC: A new perspective on hyperthermia-induced birth defects: the role of activation energy and its relation to obstetric ultrasound. J. Therm. Biol. 30, 400–409 (2005).
- Miller MW, Nyborg WL, Dewey WC, Edwards MJ, Abramowicz JS, Brayman AA: Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int. J. Hyperthermia 18(5), 361–384 (2002).
- Abraham V, Ziskin MC, Heyner S: Temperature elevation in the rat fetus due to ultrasound exposure. Ultrasound Med. Biol. 15(5), 443–449 (1989).
- Duck FA, Starritt HC: A study of the heating capabilities of diagnostic ultrasound beams. Ultrasound Med. Biol. 20(5), 481–492 (1994).
- Nyborg WL, Steele RB: Temperature elevation in a beam of ultrasound. Ultrasound Med. Biol. 9(6), 611–620 (1983).
- Layde PM, Edmonds LD, Erickson JD: Maternal fever and neural tube defects. Teratology 21(1), 105–108 (1980).
- Milunsky A, Ulcickas M, Rothman KJ, Willett W, Jick SS, Jick H: Maternal heat exposure and neural tube defects. JAMA 268(7), 882–885 (1992).
- Moretti ME, Bar-Oz B, Fried S, Koren G: Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology 16(2), 216–219 (2005).
- Miller MW, Ziskin MC: Biological consequences of hyperthermia. Ultrasound Med. Biol. 15(8), 707–722 (1989).
- Barnett SB: Can diagnostic ultrasound heat tissue and cause biological effects? In: Safety of Diagnostic Ultrasound. Barnett SB, Kossoff G (Eds). Parthenon Publishing, Canforth, UK 30–31 (1998).
- Jauniaux E, Gulbis B, Burton GJ: The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus – a review. Placenta 24(Suppl. A), S86–S93 (2003).
- Makikallio K, Tekay A, Jouppila P: Uteroplacental hemodynamics during early human pregnancy: a longitudinal study. Gynecol. Obstet. Invest. 58(1), 49–54 (2004).
- O’Brien WD, Siddiqi TA: Obstetric sonography: the output display standard and ultrasound bioeffects. In: Sonography in Obstetrics and Gynecology: Principles and Practice (6th Edition). Fleischer AC, Manning FA, Jeanty P, Romero R (Eds). McGraw-Hill, NY, USA 29–48 (2001).
- Fowlkes JB, Holland CK: Mechanical bioeffects from diagnostic ultrasound: AIUM consensus statements. American Institute of Ultrasound in Medicine. J. Ultrasound Med. 19(2), 69–72 (2000).
- Dalecki D: Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 6, 229–248 (2004).
- Carstensen EL: Acoustic cavitation and the safety of diagnostic ultrasound. Ultrasound Med. Biol. 13(10), 597–606 (1987).
- Holland CK, Deng CX, Apfel RE, Alderman JL, Fernandez LA, Taylor KJ: Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med. Biol. 22(7), 917–925 (1996).
- Kimmel E: Cavitation bioeffects. Crit. Rev. Biomed. Eng. 34(2), 105–161 (2006).
- Rooney JA: Shear as a mechanism for sonically induced biological effects. J. Acoust. Soc. Am. 52, 1718–1724 (1972).
- Nyborg WL: Ultrasonic microstreaming and related phenomena. Br. J. Cancer 5, 156–160 (1982).
- Zauhar G, Starritt HC, Duck FA: Studies of acoustic streaming in biological fluids with an ultrasound Doppler technique. Br. J. Radiol. 71(843), 297–302 (1998).
- Kondo T, Kano E: Effects of free radicals induced by ultrasonic cavitation on cell killing. Int. J. Radiat. Biol. 54, 475–486 (1988).
- Riesz P, Kondo T: Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 13, 247–270 (1992).
- Dalecki D, Raeman CH, Child SZ, Carstensen EL: Intestinal hemorrhage from exposure to pulsed ultrasound. Ultrasound Med. Biol. 21(8), 1067–1072 (1995).
- Wible JH Jr, Galen KP, Wojdyla JK, Hughes MS, Klibanov AL, Brandenburger GH: Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats. Ultrasound Med. Biol. 28(11–12), 1535–1546 (2002).
- Child SZ, Hartman CL, Schery LA, Carstensen EL: Lung damage from exposure to pulsed ultrasound. Ultrasound Med. Biol. 16(8), 817–825 (1990).
- Carstensen EL, Gates AH: The effects of pulsed ultrasound on the fetus. J. Ultrasound Med. 3(4), 145–147 (1984).
- Dalecki D, Raeman CH, Child SZ et al.: Hemolysis in vivo from exposure to pulsed ultrasound. Ultrasound Med. Biol. 23(2), 307–313 (1997).
- Abramowicz JS: Ultrasonographic contrast media: has the time come in obstetrics and gynecology? J. Ultrasound Med. 24(4), 517–531 (2005).
- Miller MW, Brayman AA, Sherman TA, Abramowicz JS, Cox C: Comparative sensitivity of human fetal and adult erythrocytes to hemolysis by pulsed 1 MHz ultrasound. Ultrasound Med. Biol. 27(3), 419–425 (2001).
- Fatemi M, Ogburn PL Jr, Greenleaf JF: Fetal stimulation by pulsed diagnostic ultrasound. J. Ultrasound Med. 20(8), 883–889 (2001).
- Harvey EN, Loomis AL: High frequency sound waves of small intensity and their biological effects. Nature 121, 622–624 (1928).
- Siddiqi TA, Plessinger MA, Meyer RA, Woods JR Jr: Bioeffects of diagnostic ultrasound on auditory function in the neonatal lamb. Ultrasound Med. Biol. 16(6), 621–625 (1990).
- Dalecki D, Child SZ, Raeman CH, Carstensen EL: Tactile perception of ultrasound. J. Acoust. Soc. Am. 97(5 Pt 1), 3165–3170 (1995).
- Dalecki D, Raeman CH, Child SZ, Carstensen EL: Effects of pulsed ultrasound on the frog heart: III. The radiation force mechanism. Ultrasound Med. Biol. 23(2), 275–285 (1997).
- Abbott JG: Rationale and derivation of MI and TI – a review. Ultrasound Med. Biol. 25(3), 431–441 (1999).
- AIUM/NEMA; American Institute of Ultrasound in Medicine and the National Electrical Manufacturers’ Association: Standard for Real-Time Display of Thermal and Mechanical Acoustic Output Indices on Diagnostic Ultrasound Devices. AIUM Publications, Rockville, MD, USA (1992).
- Sheiner E, Shoham-Vardi I, Abramowicz JS: What do clinical users know regarding safety of ultrasound during pregnancy? J. Ultrasound Med. 26(3), 319–325; quiz 326–327 (2007).
- Marsal K: The output display standard: has it missed its target? Ultrasound Obstet. Gynecol. 25(3), 211–214 (2005).
- Sheiner E, Shoham-Vardi I, Hussey MJ et al.: First-trimester sonography: is the fetus exposed to high levels of acoustic energy? J. Clin. Ultrasound 35(5), 245–249 (2007).
- Sheiner E, Freeman J, Abramowicz JS: Acoustic output as measured by mechanical and thermal indices during routine obstetric ultrasound examinations. J. Ultrasound Med. 24(12), 1665–1670 (2005).
- Sheiner E, Shoham-Vardi I, Pombar X, Hussey MJ, Strassner HT, Abramowicz JS: An increased thermal index can be achieved when performing Doppler studies in obstetric sonography. J. Ultrasound Med. 26(1), 71–76 (2007).
- Sheiner E, Hackmon R, Shoham-Vardi I et al.: A comparison between acoustic output indices in 2D and 3D/4D ultrasound in obstetrics. Ultrasound Obstet. Gynecol. 29(3), 326–328 (2007).
- Bellieni CV, Buonocore G, Bagnoli F et al.: Is an excessive number of prenatal echographies a risk for fetal growth? Early Hum. Dev. 81(8), 689–693 (2005).
- Brent RL, Beckman DA, Landel CP: Clinical teratology. Curr. Opin. Pediatr. 5(2), 201–211 (1993).
- Allen VM, Armson BA, Wilson RD et al.: Teratogenicity associated with pre-existing and gestational diabetes. J. Obstet. Gynaecol. Can. 29(11), 927–944 (2007).
- Webster WS, Abela D: The effect of hypoxia in development. Birth Defects Res. C Embryo Today 81(3), 215–228 (2007).
- Ang ES Jr, Gluncic V, Duque A, Schafer ME, Rakic P: Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc. Natl Acad. Sci. USA 103, 12903–12910 (2006).
- Toneto AD, Lopes RA, Oliveira PT, Sala MA, Maia Campos G: Effect of hyperthermia on rat fetus palate epithelium. Braz. Dent. J. 5(2), 99–103 (1994).
- Martinez-Frias ML, Garcia Mazario MJ, Caldas CF, Conejero Gallego MP, Bermejo E, Rodriguez-Pinilla E: High maternal fever during gestation and severe congenital limb disruptions. Am. J. Med. Genet. 98(2), 201–203 (2001).
- Tikkanen J, Heinonen OP: Maternal hyperthermia during pregnancy and cardiovascular malformations in the offspring. Eur. J. Epidemiol. 7(6), 628–635 (1991).
- Acs N, Banhidy F, Puho E, Czeizel AE: Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res. 73(12), 989–996 (2005).
- Graham JM Jr, Edwards MJ, Edwards MJ: Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology 58(5), 209–221 (1998).
- Halperin LR, Wilroy RS Jr: Maternal hyperthermia and neural-tube defects. Lancet 2(8082), 212–213 (1978).
- Kleinebrecht J, Michaelis H, Michaelis J, Koller S: Fever in pregnancy and congenital anomalies. Lancet 1(8131), 1403 (1979).
- Li Z, Ren A, Liu J et al.: Maternal flu or fever, medication use, and neural tube defects: a population-based case-control study in Northern China. Birth Defects Res. 79(4), 295–300 (2007).
- Shiota K: Induction of neural tube defects and skeletal malformations in mice following brief hyperthermia in utero. Biol. Neonate 53(2), 86–97 (1988).
- Dombrowski SC, Martin RP, Huttunen MO: Association between maternal fever and psychological/behavior outcomes: a hypothesis. Birth Defects Res. 67(11), 905–910 (2003).
- Edwards MJ: Hyperthermia in utero due to maternal influenza is an environmental risk factor for schizophrenia. Congenit. Anom. 47, 84–89 (2007).
- Bosward KL, Barnett SB, Wood AK, Edwards MJ, Kossoff G: Heating of guinea-pig fetal brain during exposure to pulsed ultrasound. Ultrasound Med. Biol. 19(5), 415–424 (1993).
- Tarantal AF, O’Brien WD, Hendrickx AG: Evaluation of the bioeffects of prenatal ultrasound exposure in the cynomolgus macaque (Macaca fascicularis): III. Developmental and hematologic studies. Teratology 47(2), 159–170 (1993).
- Duggan PM, Liggins GC, Barnett SB: Ultrasonic heating of the brain of the fetal sheep in utero. Ultrasound Med. Biol. 21(4), 553–560 (1995).
- Horder MM, Barnett SB, Vella GJ, Edwards MJ, Wood AK: Ultrasound-induced temperature increase in guinea-pig fetal brain in utero: third-trimester gestation. Ultrasound Med. Biol. 24(9), 1501–1510 (1998).
- Ziskin MC, Petitti DB: Epidemiology of human exposure to ultrasound: a critical review. Ultrasound Med. Biol. 14, 91–96 (1988).
- Barnett SB, Rott HD, ter Haar GR, Ziskin MC, Maeda K: The sensitivity of biological tissue to ultrasound. Ultrasound Med. Biol. 23(6), 805–812 (1997).
- WFUMB: WFUMB Symposium on Safety of Ultrasound in Medicine: conclusions and recommendations on thermal and nonthermal mechanisms for biological effects of ultrasound. Ultrasound Med. Biol. 24, S1–S55 (1998).
- Becker R, Wegner RD: Detailed screening for fetal anomalies and cardiac defects at the 11–13-week scan. Ultrasound Obstet. Gynecol. 27(6), 613–618 (2006).
- Cedergren M, Selbing A: Detection of fetal structural abnormalities by an 11–14-week ultrasound dating scan in an unselected Swedish population. Acta Obstet. Gynecol. Scand. 85(8), 912–915 (2006).
- Ndumbe FM, Navti O, Chilaka VN, Konje JC: Prenatal diagnosis in the first trimester of pregnancy. Obstet. Gynecol. Surv. 63(5), 317–328 (2008).
- Ziskin MC: Intrauterine effects of ultrasound: human epidemiology. Teratology 59, 252–260 (1999).
- Resch B, Herczeg J, Altmayer P, Sztano P: The efficiency of Doppler-technique in the first trimester of pregnancy. Ann. Chir. Gynaecol. Fenn. 60(2), 85–88 (1971).
- Jouppila P, Piironinen O: Ultrasonic diagnosis of fetal life in early pregnancy. Obstet. Gynecol. 46(5), 616–620 (1975).
- Resch BA, Papp JG, Herczeg J: Normal values of fetal heart rate in the 5th to 18th weeks of pregnancy (in vivo and in vitro studies). Zentralbl. Gynakol. 101(1), 29–34 (1979).
- Achiron R, Rotstein Z, Lipitz S, Mashiach S, Hegesh J: First-trimester diagnosis of fetal congenital heart disease by transvaginal ultrasonography. Obstet. Gynecol. 84(1), 69–72 (1994).
- Carvalho JS: Early prenatal diagnosis of major congenital heart defects. Curr. Opin. Obstet. Gynecol. 13(2), 155–159 (2001).
- DeVore GR: First-trimester fetal echocardiography: is the future now? Ultrasound Obstet. Gynecol. 20(1), 6–8 (2002).
- Gembruch U, Knopfle G, Chatterjee M, Bald R, Hansmann M: First-trimester diagnosis of fetal congenital heart disease by transvaginal two-dimensional and Doppler echocardiography. Obstet. Gynecol. 75(3 Pt 2), 496–498 (1990).
- Marques Carvalho SR, Mendes MC, Poli Neto OB, Berezowski AT: First trimester fetal echocardiography. Gynecol. Obstet. Invest. 65(3), 162–168 (2008).
- Dillon EH, Case CQ, Ramos IM, Holland CK, Taylor KJ: Endovaginal pulsed and color Doppler in first-trimester pregnancy. Ultrasound Med. Biol. 19(7), 517–525 (1993).
- Leiva MC, Tolosa JE, Binotto CN et al.: Fetal cardiac development and hemodynamics in the first trimester. Ultrasound Obstet. Gynecol. 14(3), 169–174 (1999).
- Makikallio K, Jouppila P, Rasanen J: Human fetal cardiac function during the first trimester of pregnancy. Heart 91(3), 334–338 (2005).
- Wloch A, Rozmus-Warcholinska W, Czuba B et al.: Doppler study of the embryonic heart in normal pregnant women. J. Matern. Fetal Neonatal Med. 20(7), 533–539 (2007).
- Borrell A, Gonce A, Martinez JM et al.: First-trimester screening for Down syndrome with ductus venosus Doppler studies in addition to nuchal translucency and serum markers. Prenat. Diagn. 25(10), 901–905 (2005).
- Matias A, Gomes C, Flack N, Montenegro N, Nicolaides KH: Screening for chromosomal abnormalities at 10–14 weeks: the role of ductus venosus blood flow. Ultrasound Obstet. Gynecol. 12(6), 380–384 (1998).
- Maiz N, Plasencia W, Dagklis T, Faros E, Nicolaides K: Ductus venosus Doppler in fetuses with cardiac defects and increased nuchal translucency thickness. Ultrasound Obstet. Gynecol. 31(3), 256–260 (2008).
- Mavrides E, Sairam S, Hollis B, Thilaganathan B: Screening for aneuploidy in the first trimester by assessment of blood flow in the ductus venosus. BJOG 109(9), 1015–1019 (2002).
- Hofstaetter C, Gudmundsson S, Dubiel M, Marsal K: Ductus venosus velocimetry in high-risk pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 70(2), 135–140 (1996).
- Duck FA, Henderson J: Acoustic output of modern ultrasound equipment: is it increasing? In: Safety of Diagnostic Ultrasound. Barnett SB, Kossoff G (Eds). Parthenon Publishing, Canforth, UK 15–25 (1998).
- Chervenak FA, McCullough LB: Research on the fetus using Doppler ultrasound in the first trimester: guiding ethical considerations. Ultrasound Obstet. Gynecol. 14(3), 161 (1999).
- Campbell S, Platt L: The publishing of papers on first-trimester Doppler. Ultrasound Obstet. Gynecol. 14(3), 159–160 (1999).
- Duck FA: Is it safe to use diagnostic ultrasound during the first trimester? Ultrasound Obstet. Gynecol. 13(6), 385–388 (1999).
- AIUM practice guideline for the performance of obstetric ultrasound examinations (2007). www.aium.org/publications/guidelines.aspx
- Abramowicz JS, Lewin PA, Goldberg BB: Ultrasound bioeffects for the perinatologist (2008). www.glowm.com/index.html?p=glowm.cml/ print&articleid=204#r22
• • Very thorough analysis of what is known regarding epidemiology of safety and bioeffects of ultrasound in human.
• • One of the chapters of the consensus report of a major American Institute of Ultrasound in Medicine (AIUM) 2008 symposium on bioeffects. Other chapters deal with mechanical effects in fetuses as well as thermal and mechanical effects in patients other than fetuses.
• • One of the most complete and readable (basic sciences oriented) books on exposure.
• Important study on the role of hyperthermia in congenital abnormalities.
• • Major study on the relation between temperature increase and time of exposure. This is the basis of all the modern theoretical literature on fetal thermal exposure.
• Depicts the lack of knowledge of professionals performing obstetric ultrasound on safety and bioeffects, in the USA and the rest of the world.
• Depicts the lack of knowledge of professionals performing obstetric ultrasound on safety and bioeffects, in the USA and the rest of the world.
• A recent study on the effect of hyperthermia on intellectual function.
• A study clearly demonstrating that ultrasound may cause a rise of temperature in the brain of insonated animals.
• • Important paper that raises the question of safety of ultrasound, specifically in early gestation.
Websites