Research Article - Imaging in Medicine (2016) Volume 8, Issue 3
The role of radiofrequency ablation of hepatocellular carcinoma in cirrhosis: from guidelines to real practice - a literature review
- *Corresponding Author:
- Mauro Borzio
Department of Gastroenterology and Hepatology “G. da Saliceto” Hospital
Piacenza, Italy
E-mail: mauro.borzio@gmail.com
Abstract
The epidemiologic features of hepatocellular carcinoma (HCC) in cirrhosis have been changing over the last decades as documented in field cohorts where the prevalence of elderly patients with severe comorbidities is progressively increasing. This may account, at least in clinical practice, for changing of treatment modalities for liver-confined HCC and for the worldwide increased popularity of percutaneous ablation which is undoubtedly more easily applied to weak patients. Radiofrequency ablation (RFA) is the standard reference technique for percutaneous ablation and it is currently included among the curative therapies of early HCC in cirrhosis by international guidelines. In particular, for single HCC<2 cm, RFA should be the first choice therapy, being effective as surgery but less invasive and less expensive. For larger HCCs (not exceeding 4 cm), RFA competes with resection in terms of survival benefit even though the latter provides better local disease control and longer disease-free survival. For non-resectable HCC greater than 4 cm, a treatment combining RFA and transarterial chemoembolization (TACE) can be performed to expand the ablated area. However, this approach needs to be standardized. Microwave (MWA) is a new ablative procedure potentially able to overcome some technical limits of RFA. The effectiveness of MWA is still under investigation even though preliminary results are encouraging. Emerging data from clinical practice outline the increasing role of ablative procedures for treatment of HCC in cirrhosis in the near future.
Keywords
transarterial chemoembolization, microwave, hepatocellular carcinoma, barcelona clinic liver cancer, liver transplantation
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh in women. It is diagnosed annually in more than half a million people worldwide [1] and is the third most common cause of cancer-related death. Besides cancer, another life-threatening condition, liver cirrhosis, is present in more than 80% of patients with HCC. According to Barcelona Clinic Liver Cancer (BCLC) stage system, HCC can be classified into 5 stages based on tumour burden, liver function and health status [1,2]. Patients with early stage disease (BCLC 0-A) are candidates for receiving curative therapies such as surgical resection, liver transplantation and percutaneous ablation offering an expected 40-70% 5 year survival. Patients with intermediate-stage disease (BCLC-B) are candidates for transarterial chemoembolisation (TACE) with a mean overall survival of 20 months (range 14-45). About 40% of patients belong to advanced stage (C) (portal invasion, N1, M1, PS 1-2) with a poor prognosis (overall mean survival 11 months; range 6-14). In this group of patients when a good hepatic function is preserved, sorafenib is currently the standard of care and new systemic target therapies are under clinical investigation [2,3]. Although the extensive application of regular screening-surveillance with ultrasound allowed an increased number of HCC patients to be diagnosed at an early stage, when curative treatments could be offered, the proportion of patients submitted to liver transplantation or surgical resection did not increase in the last years. On the other hand, the use of percutaneous ablation techniques has been increasing worldwide [4-7]. In a US population study [4] based on SEER registries data, the authors reported that between 2002 and 2005 there was an increase in RFA use, with a concurrent decrease in resection, among patients with solitary lesions. In particular, RFA use increased by 26% in patients with very early HCCs and 73% in patients with solitary tumors measuring 2 to 5 cm, respectively. In this study, the use of RFA increased 15.5 fold and accounted for the 43% of the overall increase of any intervention offered to the patients [4]. These data were confirmed in a more recent analysis on SEER data [5] showing that the use of surgical therapies for early tumors (within Milan Criteria) reached the steady state from 2000 to 2010 whereas the use of ablation techniques continued to increase becoming the second most common modality in the second half of the decade. The increasing rate of HCC patients undergoing percutaneous ablation was confirmed in other observational cohort studies [6,7].
Several reasons may account for the increasing popularity of percutaneous ablation since it has been validated among the curative therapies of HCC: chronic organ shortage for OLT on one hand and the progressive ageing of cirrhotic population with frequent concomitance of severe comorbidities on the other hand, making surgery less feasible [8,9]. Percutaneous ethanol injection (PEI) was the first percutaneous ablation treatment introduced in the clinical practice, able to achieve a 5 year survival rate higher than 60% in patients with a single tumor<3 cm in diameter [10,11]. Radiofrequency ablation (RFA) was introduced in clinical practice at the mid of 1990s [12-14] and, today, it is recommended as the main ablative treatment for tumours <5 cm, being superior to PEI in terms of better local disease control and greater survival benefit [2]. In addition, percutaneous RFA proved to be safe with post-procedure mortality ranging from 0 to 0.88% and a rate of major complications of 4.1% [15]. However, to be accepted as a real competitor to surgery, RFA has to confront with the modern standards of hepatic resection in cirrhotic patients calling for a peri-operative mortality <3% and an expected 5 years survival rate above 60%. Most of the uncertainties about the efficacy of ablation techniques depend on the fact that response is strongly influenced by tumour size and location. In addition, patients allocated to ablation tend to suffer from a more advanced degree of liver dysfunction in comparison to those undergoing surgery, and this can bias the observed results. In this review we discuss on the efficacy, advantages and limits of RFA as compared to surgery and to other ablative procedures as PEI with particular attention to what is recommended by the international guidelines for HCC management (mainly AASLD and EORTC-EASL) and what is applied in clinical practice.
Results and prognostic factors of radiofrequency ablation of hepatocellular carcinoma
In TABLE 1 we report and remark the results of percutaneous RFA of HCC obtained in the largest series. In a French series of 235 consecutive patients [16] with Child-Pugh- Turcotte (CPT) A-B cirrhosis, who received RFA as first line treatment for HCC inside the Milan criteria (single nodule <5 cm or up to three nodules <3 cm), complete ablation was obtained in 94% of cases with an overall 5 year survival and recurrence-free survival rates of 40% and 17% respectively. Independent prognostic factors were prothrombin activity and serum level of AFP. Tumour size was associated with local recurrence but not with overall and tumour-free survival. In the retrospective Italian series of Rossiet al.[17], collecting 706 patients, with HCC<35 mm in diameter (1-2 nodule/s per patient), cumulative incidence of first recurrences at 3 and 5 years were 70.8 and 81.7%. RFA demonstrated high repeatability and efficacy also for controlling intrahepatic recurrences. The authors concluded that RFA should be recommended as the firstline treatment for patients with one or two small HCCs whereas surgical resection can be reserved for patients with good liver function whose tumours cannot be treated with RFA or in which RFA did not produce complete response. Shina et al. in Japan, performed 2,982 RFA treatments in 1,170 HCCs: the 5 and 10 year survival rates were 60.2 and 27.3%; at multivariate analysis, age, anti-HCV, CPT class, tumour size, number of lesions, DCP and serum AFP-L3 were significantly related to survival [18]. Kim et al. evaluated 10 year follow-up results in 1305 patients with 1502 early HCCs treated with percutaneous RFA as a first line option. In this series, the cumulative local tumour progression rates were 27 % and 36.9 % at 5 and 10 years with a corresponding overall survival rate of 59.7% and 32.3%. Poor survival was associated with old age and Child- Pugh class B [19]. The long-term effectiveness of RFA for solitary small HCC was assessed also in a retrospective Italian analysis of 363 patients [20]. The 3 and 5 year overall survival rates were 80% and 64%; at multivariate analysis only age, presence of ascites and CPT score >B8 were independent predictors for overall survival.
| Author (year) | No. of patients (age mean- years) | Inclusion criteria | Follow-up (months) | OS (%) | DFS (%) | ||
|---|---|---|---|---|---|---|---|
| 3 year | 5 year | 3 year | 5 year | ||||
| N'Kontchou 2009 [16] |
235 (65)* | CP A-B criteria Milan in |
27 (mean) | 60 | 40 | 37 | 18 |
| Rossi 2011 [17] |
706 (68.2) | CP >B7 1-2 nod<30 mm |
29 (median) | 67 | 40,1 | 68 | 38 |
| Shiina 2012 [18] |
1170 (68.3) |
Max 3 nodules | 38,2 (median) | 80,5 | 60,2 | nr | nr |
| Kim 2013 [19] |
1305 (58.4) |
BCLC-A | 33,4 (median) | 59,7 | 32,3 | 50 | 41,8 |
| Francica 2013 [20] |
365 (67) |
CP A-B single<3 cm |
37 (median) | 80 | 64 | 50 | 41,8 |
| Abbreviations: RFA:radiofrequency ablation; OS: overall survival, DFS: disease free survival; CP: Child-Pugh; nr: not reported *No. of patients aged >75 years: 51 (21.5%) |
|||||||
Table 1. Outcome of percutaneous RFA of HCC in larger published series.
Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma
According to North American and European guidelines, hepatic resection is the first-line treatment for patients with solitary HCCs and very well-preserved liver function (evidence 2A; recommendation 1B) [1,2]. This statement however has been extensively debated and even confuted since the introduction in clinical practice of percutaneous ablation. There are more studies, published in the literature, comparing resection to percutaneous ablation when early HCC (namely within Milan Criteria) was selected as an inclusion criterion. In a recently published systematic literature revision, Cuchetti et al. retrieved 19 studies that directly compared resection and radiofrequency ablation [21]. However, only three of them were RCTs and all of them were from Eastern countries. These three studies are not perfectly comparable given the differences in most clinical and tumor variables that may confound results. Therefore, they were unable to demonstrate a clear superiority of surgery over ablation in terms of survival rate. Thus they leave the question regarding the best therapeutic approach to be adopted still unsolved [22-24]. Data from these studies are further discussed in the following sections.
RFA has a number of clear advantages over surgical resection in the setting of small HCCs. First, it is much less invasive and is associated with a lower complication rate. Second, RFA significantly reduces treatment costs, hospital stay and need for blood transfusions. Third, nowadays, the progressive ageing of patients with newly diagnosed HCC with multiple comorbidities [5,7] frequently represents a contraindication to surgery independently from the stage of HCC and residual liver function. Fourth, RFA can be performed by nonsurgical and/or non-oncologic practitioners at non referral centers.
In TABLE 2 and 3 we summarized the results of more recent and larger studies comparing liver resection and radiofrequency in very-early and early HCC.
Very early HCC (single nodule<2 cm, BCLC stage 0)
Livraghi et al. [25] in a seminal study carried out in 218 patients with small HCC showed that RFA was able to achieve a sustained complete response in 97.2% of cases, after a median follow-up of 31 months. Perioperative mortality, major complications and 5 year survival rates were 0, 1.8 and 68.5%, respectively. In the subgroup of 100 patients potentially resectable, the 3 and 5 year survival rates (89 and 68%) were significantly better than those for inoperable patients (75 and 47%). In multivariate analysis, appearance of new lesions was the only factor significantly associated with survival. The authors concluded that RFA can be considered the treatment of choice for patients with very early HCC (single<2 cm) even when surgical resection is feasible. In a large retrospective comparative study, Peng et al. [26] included 145 patients 66 of which had a central HCC, 71 cases submitted to RFA as initial treatment and 74 to surgical resection. In patients with HCC measuring 2 cm or smaller, the authors showed a 1-3 and 5 year overall survival of 98.5, 87.7 and 71.9% for those treated with RFA and 90.5, 70.9 and 62.1% for those treated with resection. Remarkably, in the subgroup of patients with central HCC the difference in the 5-year survival rates between RFA and surgical resection was more significant (79,9% vs. 61,5%). Moreover, the corresponding percentages of recurrence free-survival were similar in the two subgroups of treatment. Major complications were significantly more frequent in the surgical group (38 cases vs. 71 cases in RFA patients). The authors concluded that in patients with very early HCC, percutaneous RFA is superior to surgery because of its safety, efficacy and low costs; in particular, patients with a central very early HCC are the best candidates to receive ablative treatment. A recent meta-analysis [27] comparing resection and RFA and evaluating 3 randomized (RCTs) and 25 non randomized controlled trials (NRCTs), showed that, in the subset of patients with solitary HCC smaller than 2 cm, there was no significant difference in 1, 3 and 5 year survival and in 1, 3 year recurrence rates between the two groups.
All these data have been fully considered by AASLD and EASL guidelines and in the last version the algorithms have been modified accordingly, the authors endorsing RFA as the first-line treatment for very early HCC in cirrhosis [28].
| Author (year) | No. of patients (mean-age years) |
Follow-up (months) (median) |
OS (%) | DFS (%) | ||
|---|---|---|---|---|---|---|
| 3 year | 5 year | 3 year | 5 year | |||
| Livraghi (2008) [25] | 218 (68) * |
31 | 76 | 65 | 26 | 20 |
| Peng (2012) [26] RFA RES |
71 (53.1) 74 (51.5) |
59 57.5 | 87.7 70.9 | 71.9 62.1 | 65.2 56.1 | 59.8 51.3 |
| Wang (2012) [29] RFA ** RES *** |
91 (nr) 52 (nr) | 30 27.6 | 80.3 98 | 72 91.5 | 39.8 62.1 | 29.3 49.7 |
| Abbreviations: RFA: Radiofrequency Ablation; RES: Surgical Resection; nr: not reported * No. of patients aged >75 years: 47 (21.5%) ** 40 patients (44%) with age <60 years *** 35 patients (67.3%) with age <60 years |
||||||
Table 2. Radiofrequency ablation (RFA) versus surgical resection (SR) for very early HCC (BCLC-0): results from more recent and larger studies.
| Author (year) | No. of patients (Mean age years) |
InclusionCriteria | OS (%) | DFS (%) | ||
|---|---|---|---|---|---|---|
| 3 year | 5 year | 3 year | 5 year | |||
| RCTs studies | ||||||
| RFA Chen (2006) [22] SR |
71 (51.9) 90 (49.4) | single =5 cm | 71.4 73.4 | 67.9* 64* | 64.1 69 | 46.4** 51.6** |
| RFA Huang (2010) [23] SR |
115 (56.7) 116 (55.9) | Single =5 cm or up to 3 nodules <3 cm | 69.6 92.2 | 54.8 75.7°° | 46.1 60.9 | 28.7 51.3°° |
| RFA Feng (2012) [24] SR |
84 (51) 84 (47) | 1-2 nodules <4 cm | nr nr | 67.2 74.8 | nr nr | 49.6 61.1 |
| Retrospective studies | ||||||
| RFA Wang (2012) [29] SR |
254 (nr) 208 (nr) |
Not specified | 73,5 87.8 |
57.4 77.2 |
28.3 59.9 |
14.1 50.8 |
| RFA Hasegawa (2013) [30] SR |
5548 (69) 5361 (66) | National database | 81 85.3 | 61.1 71.1 | 63.8 71.7 | 43.3 52.7 |
| RFA Pompili (2013) [31] SR |
298 (68) 246 (67) | Single<3 cm | 80.9 81.9 | 66.2* 74.4* | 48.9 54.1 | 42.9** 44** |
| RFA Ueno (2015) [32] SR |
160 (71) 136 (71) | Single<5 cm | 70.1 69.8 | 58.4° 60.9° | ||
| Abbreviations: OS: Overall Survival; DFS: Disease Free Survival *OS at 4 years; ** DFS at 4 years; ° DFS at 2 years, °° p<0.05; nr: not reported |
||||||
Table 3. Radiofrequency ablation (RFA) versus surgical resection (SR) for early HCC (BCLC-A): Results from recent and larger RCTs or retrospective studies.
Early HCC (single nodule or up to 3 nodules <3 cm, BCLC stage A)
To date, surgery is recommended as the firstline treatment for single, resectable HCC arising in well-compensated cirrhosis. Radiofrequency ablation is recommended in small multifocal HCC (maximum 3 nodules) or in single nodules not resectable. In the last decades however, several studies have been carried out in an attempt to see whether or not RFA could compete with surgery also in early stage of HCC.
Only three RCTs comparing resection and RFA in early HCC are so far available. In two of these studies [22,23] the authors provided outcome information not only on the whole series but also on the subgroups of patients stratified according to the dimension and number of HCCs (≤ 3 cm, 3.1-5 cm, single, multiple up to 3 nodules). The first RCT included 71 patients submitted to ablation and 90 submitted to resection. The main findings were that the 3-year OS was 71.4% after ablation and 73.4% after surgery. The corresponding DFS were 64.1% and 69.0%, respectively. No statistical difference was observed when tumor size was taken into account [22]. In the second RCT, including 115 patients undergoing ablation and 115 resections, Huang et al. reported 5-year OS rates of 54.8% after ablation and 75.7% after surgery (P=0.001). The corresponding RFS rates were 28.7% and 51.3%, respectively (P=0.017). The superiority of resection was maintained when patients were stratified by tumor size and number [23]. In the third RCT, Feng et al. compared 84 patients submitted to RFA with 84 patients submitted to surgical resection. This study collected only small HCCs (1-2 nodules <4 cm in diameter) arising in well compensated cirrhosis (CPT class A-B). The 3 year survival rate was 74.8% for surgery and 67.2% for RFA and the corresponding recurrence-free survival 61.1% and 49.6%, respectively. The authors pointed out the difficulty of RFA to achieve a complete necrosis in nodules larger than 3 cm, as foci of residual tumour or peripheral satellitosis are left in place [24]. Data from these RCTs were not able to draw conclusion on which treatment, resection or ablation, is superior even if surgery seems to offer a better DFS. In a non-randomized study Wang et al. [23] enrolled 143 very early (52 treated with surgical resection) and 462 early stage (208 of which underwent surgery) HCC. Statistically no significant differences of overall survival between surgery and RFA emerged from this study. Although the authors reported a higher risk of recurrence after RFA both in very early and in early stage, the possibility to repeat local ablation to treat recurrences, might explain why the overall survival was similar in both groups. An increased local recurrence after RFA was observed also by. Contrasting results emerged from a Japanese nationwide survey on 28,510 HCCs belonging to different stages and treated with surgical resection, PEI and RFA between 2000 and 2005 [30]. A sub-analysis on 12,968 patients fulfilling the Milan Criteria revealed that the overall survival at 5 years was 71.1%, 61.1% and 56.3% in surgical, RFA and PEI groups, respectively. The corresponding rates of recurrence were 63.8%, 71.7% and 76.9% in the same groups. By multivariate analysis, the hazard ratio for mortality and for recurrence was significantly lower in patients treated with surgery than in RFA and PEI group. This finding may be explained by the evidence that patients eligible for surgery were younger, with better liver function, without portal hypertension, with less number of nodules and lower AFP levels. A large retrospective study, comparing RFA and liver resection in a western series, was conducted by Pompili et al. [31]. This observational cooperative Italian study involved 544 cirrhotic patients CPT class A with a single HCC<3 cm: 246 treated with resection and 298 with RFA. Four year overall survival and recurrence rates were 74.4% and 56% in the resection group and 66.2% and 57.1% in the RFA group respectively. The rate of major complications was 4.5% in resected patients and 2% in patients undergoing RFA. Older age and higher AFP levels were independent predictors of poor outcome.
Ueno et al. addressed the prognostic role of three tumour markers: AFP, AFP-L3 and DCP in cirrhotic patients with single HCC<5 cm, treated with surgery or RFA. In patients positive for all three neoplastic markers, five year overall survival was 75.9% for those treated with surgery and 47.6% for patients submitted to RFA These findings further suggest that the better results obtained by surgery were largely explained by the superiority of resection to achieve a radical tumour control, particularly in patients harbouring more aggressive tumours [32]. Conversely, a non-superiority of surgery emerged from the study by Osaki et al. who analysed a cohort of 4165 patients with HCC within Milan criteria, diagnosed between 1981 and 2013 in the Osaka Red Cross Hospital. The 3 and 5 year overall survival rates were 76.3% and 55.8% in the resection group and 77.2% and 55.5% in the RFA group [33]. Similar results were reported by Kim et al. in a retrospective study enrolling 604 patients with a single HCC nodule <3 cm, 273 of which were submitted to liver resection and 331 to RFA. During a followup of 10 years, the overall survival after hepatic resection was 59% and 61.2% after RFA. The recurrence-free survival rates at 5 and 10 year were 60.6% and 37.5% after surgery and 39.4% and 25.1% after RFA [34].
Several systematic reviews and meta-analyses have been recently dedicated to clarify the role of RFA versus hepatic resection in the treatment of small HCC [21,35-38]. The conclusions of these studies are controversial. Even though the level of evidence was low, a marginal superiority of surgery over RFA, particularly for tumours larger than 3 cm, emerged. A more recent comparative meta-analysis [27] pooling data from 6,094 patients treated with RFA and 5,779 with surgery, observed in 3 randomized and 25 non-randomized controlled trials, concluded that in HCC sized between 3 and 5 cm, 5 year survival rate and the 5 year disease-free survival rate was lower in the RFA group. In summary, in the setting of early HCC, available data from large observational series and meta-analyses seem to suggest to limit RFA to multinodular HCC (max 3 nodules) smaller than 3 cm, or to single HCC not exceeding 4 cm. Indeed, beyond this maximum diameter, the possibility to obtain a local complete control of the tumour is low by RFA. The acquisition of a sufficient safety margin seems to be critical to avoid an in loco early recurrence after RFA. For lesions less than 4 cm, the optimal choice between the two treatments should emerge from a multidisciplinary discussion by balancing the expected perioperative mortality and morbidity of surgery, local experience on RFA, residual liver function and the presence of relevant comorbidities.
Radiofrequency ablation versus combined treatment with TACE and versus other ablative techniques such as ethanol injection (PEI) and microwave ablation (MWA) or percutaneous cryoablation
According to BCLC staging system, tumours sized 4-5 cm not resectable, still belong to early stage and, theoretically, they could be treated by RFA. In an attempt to overcome the above discussed limited efficacy of RFA alone to achieve a complete necrosis in large HCC, several authors worldwide recommended for these lesions combined treatment schedules in which RFA is coupled with transcatheter arterial embolization (TAE) or transcatheter arterial chemoembolization (TACE).
Rossi et al. performed RFA of non-resectable HCCs after occlusion of the main feeding artery (TAE) in 62 patients with HCC nodules 3.5-8.5 cm in diameter, obtaining a 100% complete necrosis of the tumour, with a 1 year local recurrence of 19% [39]. In a randomized controlled trial conducted on 189 patients with HCC less than 7cm, the TACE-RFA group had better overall survival and recurrence-free survival than patients in the RFA group [40]. In a meta-analysis on eight RCTs including 306 patients treated with RFA plus TACE and 292 with RFA alone, the former treatment was associated with a significantly higher 1, 2 and 3 year overall survival rate than the later. However, there was no significant difference between these two treatments as to 5 year overall survival [41]. In a more recent meta-analysis, Wang et al. collected 21 studies (six RCTs) with 3073 patients and showed a higher 3 year and 5 year overall survival with RFA compared with TACE alone. In this study the combination of RFA and TACE was associated with a significantly higher 1, 3 and 5 year survival compared with RFA or TACE alone [42]. These data seem to indicate that, in large-size HCC (diameter>4-5 cm) the combination of RFA plus TACE is superior to the sole RFA in improving survival. To date, combined therapy is considered a valid approach to treat large non resectable HCCs and its popularity is progressively increasing worldwide. However, literature data are not yet considered sufficiently robust to include combination of TACE and RFA among curative therapies endorsed by international algorithms for treatment of early HCC. In addition, some issues on its correct utilization are still unresolved. First, it has not been agreed yet which one between RFA and TACE should be performed first. Second, the best interval between the two procedures: should they be performed simultaneously or at a two to three days interval? This is not a trivial issue since in most centres the possibility to perform both procedures on the same day is almost impossible. Third, how many sessions should be needed before deciding suspension because of non-response. Fourth, which procedure should be performed to retreat a partially responsive nodule? Unfortunately, all these aspects have not been fully addressed yet and no clear and accepted recommendations are available so far.
Percutaneous RFA versus PEI
In a meta-analysis of six studies collecting 787 patients and comparing RFA versus PEI, the former resulted more frequently in complete nodule necrosis, with a less local tumour recurrence in a minor number of sessions. With HCC nodules <2 cm in diameter there was no significant difference between RFA and PEI as to mortality and local recurrence [43]. In a more recent systematic review of the literature on RFA versus PEI, collecting four RCTs with 766 patients, Shen et al. confirmed that RFA was significantly better than PEI with respect to a 3 year overall survival for patients with HCCs<3 cm. Necrotic effect of RFA was more predictable as compared to PEI and the risk of local recurrence in the RFA group was lower [44]. In a recent retrospective multi-centric Italian survey including 244 cirrhotic Child A patients with single HCC<2 cm in diameter, Pompili et al. showed that a five year survival was not significantly different comparing PEI (64,7%) versus RFA (72,9%) even though the 5 year recurrence was higher in PEI group than in RFA group (73,3% vs. 49%) [45]. These studies seem to indicate that RFA is superior to PEI in terms of better outcome and local disease control. One of the most relevant advantages of RFA over PEI relies on the evidence that heat may diffuse very quickly unlike ethanol penetration which may be hampered by the presence of the fibrous septa and this would limit the necrotic capacity of alcohol injection. Despite these limitations, PEI, has not be abandoned at all, and it still remains a valid alternative to RFA in some clinical situations like in patients with severely impaired clotting parameters or whose HCC nodules are located in sites too dangerous for thermal ablation or close to large intrahepatic vessels which reduce thermal damage due to the well-known heat-sink effect.
Percutaneous RFA versus other ablative procedures (microwave ablation and cryoablation)
Microwaves (MW) utilize electromagnetic energy and therefore a more homogeneous and larger ablation zone may be obtained without the well-known negative heat-sink effect typical of RFA. In addition, the time needed for ablation by MW is shorter than that required by RF. In a recent comparison of clinical published series of HCC patients treated with MWA or RFA, Poulou et al. concluded that MWA seems to overcome size limitation of RFA in the treatment of lesions >5 cm in diameter with comparable results in terms of overall survival, local recurrence and complications rates [46]. However, to date, there is no solid evidence to support the advantage of one technique over the other.
Finally, in a multicentre randomized controlled trial comparing percutaneous cryoablation versus RFA in patients with CP class A or B and 1 or 2 HCC lesions <4 cm, cryoablation resulted in a significantly lower local tumour progression than RFA. Cryoablation and RFA were equally safe and effective with a similar 5 year survival rate [47]. Although these encouraging positive results need to be confirmed in series other than those collected in far east countries, percutaneous cryoablation should be included among the standard local ablation modalities in patients with HCC.
From guidelines to clinical practice
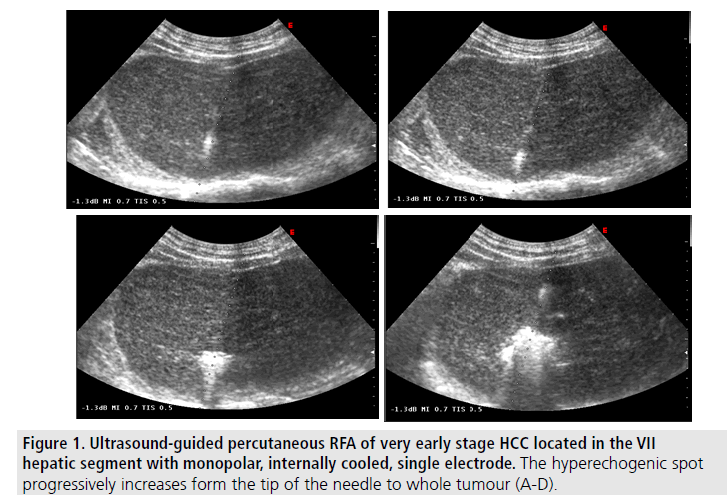
Although it is often very difficult to reach a consensus on therapeutic approach to HCC in cirrhosis, given the extremely limited availability of high quality trials, data analysed in this review constitute the rationale for guidelines recommendations in this field. For very early HCC (≤ 2 cm) resection and RFA result equally effective as curative treatments but ablation is less expensive, and may ensure less posttreatment complications, less pain, and a shorter in-hospital stay. Thus, it seems reasonable to offer RFA to patients with very small HCC with no technical contraindications. In this setting RFA should be considered as the first choice therapy [28] (FIGURE 1).
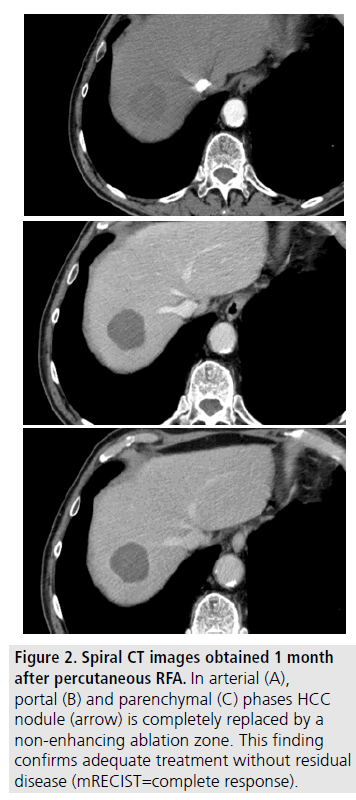
Uncertainties remain for HCC sized up to 3 cm. (FIGURE 2). In this setting, surgery, if non contraindicated, is to be preferred, given its superiority over RFA in terms of better longterm local disease control; therefore RFA should be employed as an “alternative option” [28]. For HCC 3 to 4 cm and not resectable, RFA should not be employed as a single therapy taking into account its limitation to ensure complete tumour necrosis with a safety margin. For these lesions a combined approach (RFA and TACE) or perhaps, MW ablation may be attempted [28].
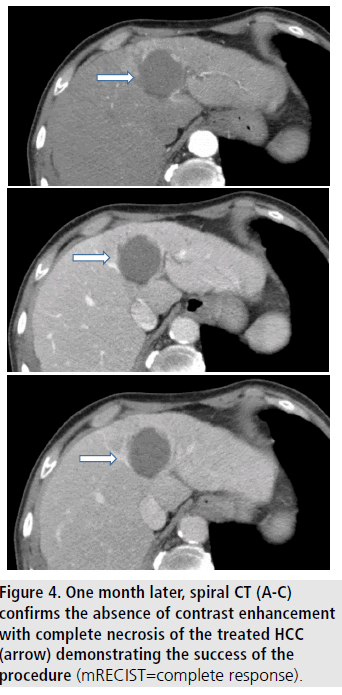
In the “real world”, however, therapeutic approach to HCC may remarkably differ from that recommended by international guidelines. Recent in field cohorts from different countries, reported a poor adherence to treatment algorithms endorsed by guidelines [8,48,49]. Progressive ageing of HCC population at first diagnosis and the frequent occurrence of extra hepatic illnesses represent the most relevant barriers against the full adherence to treatment recommendations. Ageing and comorbidities are indeed two of the most important epidemiologic hallmarks of HCC in the third millennium. These two factors, together with the marginal allocation to OLT and the low rate of patients allocated to surgical treatment, shift to less invasive procedures as percutaneous ablation. In a recent systematic literature revision emerged that RFA was effective and safe in old patients [50]. Comorbidities do not seem to negatively affect clinical benefit of RFA in the elderly. Data from studies comparing the effectiveness of RFA in young and old patients [51-55] are reported in TABLE 4. In four of these studies the overall survival offered by RFA did not significantly differ in elderly in comparison with younger patients [51-54]. Conversely Nishikawa observed a longer OS and DFS after RFA in younger than in older patients [55]. Although the question whether or not ablative therapies are able to achieve a comparable clinical outcomes in elderly as well as in younger patients remains controversial, the authors agree that RFA in aged patients is a safe procedure and can represent a valid curative alternative to surgery. These findings further stress the future role of RFA in the “real world” of HCC, considering that in most weak cirrhotic patients with early HCC it remains the only treatment feasible (FIGURES 3 and 4).
| Author (year) | No. of patients | Age upper limit (years) | OS (%) | DFS (%) | |||
|---|---|---|---|---|---|---|---|
| 3 year | 5 year | 3 year | 5 year | ||||
|
MiriciCappa(2010) [51] |
E Y |
195 230 |
70 | 53.4 52.9 |
29 35.1 |
nr nr |
nr nr |
|
Hiraoka (2010) [52] |
E Y | 63 143 | 75 | 83 78 | 50 58 | nr nr | nr nr |
|
Takahashi (2010) [53] |
E Y | 107 354 | 75 | 82 80 | 62 63 | 49 56 | nr nr |
|
Kao (2012) [54] |
E Y | 158 100 | 65 | nr nr | 81.3 65.4 | nr nr | nr nr |
|
Nishikawa (2012) [55] |
E Y | 130 238 | 75 | 64.1 83.7 | 44.8 64* | 21.3 40 | 19 19.5 |
| Abbreviations: E: Elderly; Y: Young; OS: Overall Survival; DFS: Disease Free Survival; nr: not reported *P<0.001 | |||||||
Table 4. Clinical outcomes of percutaneous RFA of HCC in elderly and young patients.
Conclusive remarks
Changing on the main characteristics of HCC population, namely ageing and fragility, will greatly influence the therapeutic approach to this tumor in the near future. Although resection still remains the first-line option for early, resectable, HCC, percutaneous ablation is expected to assume a relevant role. Among the different ablative procedures, RFA is currently considered the standard reference even though, given the rapid evolution of technology, new and more potent ablation techniques are expected to emerge. In our opinion therefore, the presence of an expert on interventional ultrasonography within the multidisciplinary team for HCC management is highly recommended: “the right man at the right place.”
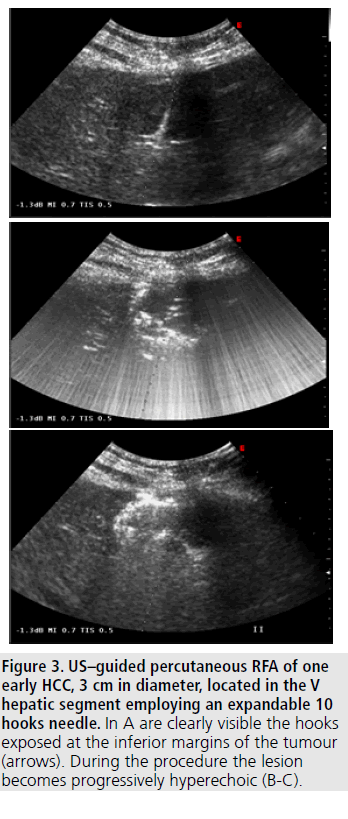
Figure 4. US–guided percutaneous RFA of one early HCC, 3 cm in diameter, located in the V hepatic segment employing an expandable 10 hooks needle. In A are clearly visible the hooks exposed at the inferior margins of the tumour (arrows). During the procedure the lesion becomes progressively hyperechoic (B-C).
References
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 53, 1020–1022 (2011).
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 56, 908-943 (2012).
- Lope CR, Tremosini S, Forner A et al. Management of HCC. J. Hepatol. 56, S75-S87 (2012).
- Massarweh NN, Park JO, Farjah F et al. Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. Am. Coll. Surg.210, 441–448 (2010).
- Ulahannan SV, Duffy AG, McNeel TS et al.Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology. 60, 1637-1644 (2014).
- Meer S, de Man R, Coenraad MJ et al.Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J. Hepatol.63, 1156-1163 (2015).
- Borzio M, Dionigi E, Rossini A et al.Trend of improving prognosis of hepatocellular carcinoma in clinical practice: An Italian in-field experience. Dig.Dis.Sci. 60, 1465-1473 (2015).
- Borzio M, Fornari F, De Sio I et al. Adherence to American Association for the Study of Liver Diseases guidelines for the management of hepatocellular carcinoma; results of an Italian field practice multicenter study. Fut. Oncol. 9, 283-294 (2013).
- Santi V, Buccione D, Di Micoli A et al.The changing scenario of hepatocellular carcinoma over the last two decades in Italy. J. Hepatol. 56, 397-405 (2012).
- Livraghi T, Bolondi L, Lazzaroni S et al.Percutaneos ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 15, 925-929 (1992).
- Livraghi T, Giorgio A, Marin G et al.Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology.197, 101-108 (1995).
- Rossi S, Fornari F, Pathies C et al.Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori.76, 54-57 (1990).
- Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J. Intervent. Radiol. 8, 97-103 (1993).
- Buscarini L, Rossi S, Fornari F et al. Laparoscopic ablation of liver adenoma by radiofrequency electrocauthery. Gastrointest. Endosc. 41, 68-70 (1995).
- Lahat E, Eshkenazy R, ZendelAet al. Complications after percutaneous ablation of liver tumors: A systematic review. Hepatobil. Surg. Nutr.3, 317-323 (2014).
- N’Kontchou G, Mahamoudi A, Aout M et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 western patients with cirrhosis. Hepatology. 50, 1475-1483 (2009).
- Rossi S, Ravetta V, Rosa L et al.Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 53, 136-147 (2011).
- Shina S, Tateishi R, Arano T et al. Radiofrequency ablation for hepatocellular carcinoma: 10year outcome and prognostic factors. Am. J. Gastroenterol. 107, 569-577 (2012).
- Kim Y, Lim HK, Rhim H et al. Ten-year outomes of percutaneous radiofrequency ablation as first-line therapy of early heaptocellular carcinoma: Analysis of prognostic factors. J.Hepatol. 58, 89-97 (2013).
- Francica G, Saviano A, De Sio I et al. Long-term effectiveness ofradiofrequency ablation for solitary small hepatocellular carcinoma: A retrospective analysis of 363 patients. Dig. Liver. Dis. 45, 336-41 (2013).
- Cucchetti A, Piscaglia F, Cescon M et al.Systematic review of surgical resection vs. radiofrequency ablation for hepatocellular carcinoma. World. J. Gastroenterol.14, 4106-4118 (2013).
- Chen MS, Li JQ, Zheng Y et al.A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg.243, 321-328 (2006).
- Huang J, Yan L, Cheng Z et al. A randomized trial comparingradiofrequency ablation and surgicalresection for HCC conforming to the Milancriteria. Ann. Surg.252, 903-912 (2010).
- Feng K, Yan J, Li X et al.A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 57, 794-802 (2012).
- Livraghi T, Meloni F, Di Stasi M et al.Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 47, 82-89 (2008).
- Peng ZW, Lin XJ, Zhang YJ et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: A retrospective comparative study. Radiology. 262, 1022-1033 (2012).
- Wang Y, Luo GL, Li Y et al.Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: A meta-analysis of randomized and non-randomized controlled trials. Plos. One. 9(1), e84484 (2014).
- Bruix J, Reig M, Sherman M Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 150, 835–853 (2016).
- Wang JH, Wang CC, Hung CH et al.Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 56, 412-418 (2012).
- Hasegawa K, Kokudo N, Makuuchi M et al.Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J Hepato. 58, 724-729 (2013).
- Pompili M, Saviano A, de Matthaeis N et al.Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma <3 cm. Results of a multicenter Italian survey. J. Hepatol. 59, 89-97 (2013).
- Ueno M, Hayami S, ShigekawaYet al. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC<5 cm: Cohort study based on seruma HCC markers. J. Hepatol. 63, 1352-1359 (2015).
- Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol. Res. 45, 59-74.
- Kim GA, Shim JH, Kim MJ et al.Radiofrequencyablation as analternativetohepaticresectionfor single smallhepatocellular carcinoma. Br. J. Surg. 103, 126-135 (2016).
- Zhou Y, Zhao Y, Li B et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC. Gastroenterol. 10, 78-84 (2010).
- Cho YK, Rhim H, Noh S (2011) Radiofrequency ablation versus surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: A systematic review. J.Gastroenterol. Hepatol. 26, 1354-1360 (2011).
- Li L, Zhang J, Lui X et al.Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: A meta-analysis. J. Gastroenterol. Hepatol. 27, 51-58 (2012).
- Duan C, Liu M, Zhang Z et al. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World. J. Surg. Oncol. 11, 190-198 (2013).
- Rossi S, Garbagnati F, Lencioni R et al.Percutaneous radio-frequency thermal ablation of non-resectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 217, 119-126 (2000).
- Peng ZW, Zhang YJ, Chen MS et al. Radiofrequency ablation with and without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J. Clin. Oncol. 31, 426-432 (2012).
- Ni JY, Lui SS, Xu LF, et al. Meta-analysis of radiofrequency ablation in combinationwithtransarterialchemoembolization for hepatocelluarcarcinoma. World. J. Gastroenterol. 19(24), 3872-3882 (2013).
- Wang Y, Deng T, Zeng L et al.Efficacy and safety of radiofrequency ablation and transcatheterarterialchemoembolization for treatment of hepatocellularcarcinoma: A meta-analysis. Hepatol. Res. 46, 58-71 (2016).
- Germani G, Pleguezuelo M, Gurusamy K et al.Clinicaloutcomes of radiofrequency ablation, percutaneousalcohol and aceticacid injection for hepatocellularcarcinoma: A meta-analysis. J. Hepatol. 52, 380-388 (2010).
- .Shen A, Zhang H, Tang C et al. A systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J. Gastroenterol. Hepatol. 28, 793-800 (2013).
- Pompili M, De Matthaies N, Saviano A et al. Single hepatocellular carcinoma smaller than 2 cm: Are ethanol injection and radiofrequency ablation equally effective? Antican. Res. 35, 325-332 (2015).
- Poulou L, Botsa E, Thanou I et al. Percutaneous microwave ablation vs. radiofrequency ablation in the treatment of hepatocellular carcinoma. World. J. Hepatol. 7, 1054-1063 (2015).
- Wang C, Wang H, Yang W et al.Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 61, 1579-1590 (2015).
- Leoni S, Piscaglia F, Serio I et al.Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: Experience of the Bologna Liver Oncology Group. Dig. Liv. Dis. 46, 549-555 (2014).
- Radu P, Ioan G, Iancu C et al. Treatment of hepatocellular carcinoma in a tertiary Romaniancenter. Deviations from BCLC recommendations and influence on survival rate. J. Gastrointest. In. iver. Dis. 22, 291-297 (2013).
- Borzio M, Dionigi E, Parisi G et al.Management of hepatocellular carcinoma in the elderly. World. J. Hepatol.7, 1521-1529 (2015).
- Mirici-Cappa F, Gramenzi A, Santi V et al.Treatments for hepatocellular carcinoma in elderly patients are as effectiveas in younger patients: A20year multicentre experience. Gut.59, 387-396 (2010).
- Hiraoka A, Michitaka K, Horiike N et al. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J. Gastroenterol. Hepatol. 25, 403-407 (2010).
- Takahashi H, Mizuta T, Kawazoe S et al.Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol. Res. 40, 997-1005 (2010).
- Kao WY, Chiou YY, Hung HH et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofre quency ablation therapy. J. Clin. Gastroenterol. 46, 62-70 (2012).
- Nishikawa H, Osaki Y, Iguchi E et al.Percutaneous radiofrequency ablation for hepatocellular carcinoma: clinical outcome and safety in elderly patients. J. Gastrointest. In. Liver. Dis. 21, 397-405 (2012).