Research Article - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 5
The effect of bone growth stimulators on venous congestion in osteoarthritis of the knee
- Corresponding Author:
- Kenneth Willeford MD
Coastal Carolinas Integrated Medicine, North Carolina, USA
E-mail: Willefordmd@yahoo.com
Abstract
Background: The aim of the present study was to determine if there is an effect to decrease intraosseous venous congestion in osteoarthritis of the knee with the use of bone growth stimulators. This is based on the understanding that osteoarthritis of the knee is primarily a disease of subchondral bone and the joint changes are secondary. Bone growth stimulators are a promising future treatment modality because of the effects of bone remodeling. Methods: WOMAC scores, RAND health survey scores, and intraossous venous congestion were measured prior to and after treatment with noninvasive bone growth stimulators utilizing ultrasound technology on twenty patients with osteoarthritis of the knee. There were twenty participants in the treatment group and ten in the control group. Results: There was a 100% response rate with a high level of statistical significance with decreased venous congestion. The mean intraosseous pressure before treatment was 29.48 mm Hg and after treatment was 15.13 mm Hg. There was also a high level of significance for all portions of the two independent quality of life scales. Conclusions: This is the second study in the world’s literature of treatment of subchondral bone in osteoarthritis of the knee. Intraosseous venous congestion is discussed as initiating a cascade of events culminating in the constellation of molecular, biochemical and structural changes and is the unifying factor leading to impaired nutrition of the subchondral bone and cartilage, micro-fractures and alterations of trabecular microarchitecture, subchondral bone stiffening from intraosseous hypertension and fibrosis, culminating in the loss of the articular cartilage and in osteophyte formation.
Keywords
osteoarthritis • knee • treatment • bone growth stimulators • ultrasound • PEMF’s • exogen
List of Abbreviations
PEMF: Pulsed electromagnetic fields; OA: Osteoarthritis; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; RQ: Rand Quality of Life; RP: RP: Rand Pain; RC: Rand Composite; WP: WOMAC Pain; WS: WOMAC Stiffness; WF: WOMAC Function; WC: WOMAC Composite
Introduction
Significance and innovation
• This is the second study in the world’s literature of treatment of subchondral bone in osteoarthritis of the knee in humans.
• This is quite surprising since it is well established that osteoarthritis of the knee is primarily a disease of subchondral bone and the joint changes are secondary.
• A discussion is also presented that provides the basis for understanding intraosseous venous congestion as the unifying factor in the pathophysiology of osteoarthritis of the knee.
• The role of subchondral bone health in the pathophysiology of osteoarthritis of the knee makes bone growth stimulators which affect bone remodeling a promising treatment modality.
• These results confirm the effect for the reduction of venous congestion with the use of bone growth stimulators for osteoarthritis of the knee and verify subjective improvement.
Introduction
The continuing quest for a complete understanding of the pathophysiology of osteoarthritis of the knee is developing into an intriguing testament to the process of scientific discovery in general, which in this case has only progressed with the collaborative efforts of a variety of sub disciplines. The classic hallmark diagnostic criteria of joint space narrowing have led to decades of treatment directed solely to the cartilage and synovial fluid. A breakthrough in understanding came with the discovery of the contributions of the health of the subchondral bone in the onset and progression of osteoarthritis of the knee. We now are confident that that this is primarily a disease of subchondral bone and the joint changes are secondary. Despite the undisputed confirma-tion of this truth, to date there has only been one article of treatment of subchondral bone in osteoarthritis of the knee in humans. This utilized bone growth stimulators with several signal modalities including ultrasound, and this revealed improved pain and function in osteoarthritis of the knee [1].
There is an important distinction between other previous uses of ultrasound for osteoarthritis of the knee. There have been many investigations using therapeutic ultrasound and these are distinct from bone growth stimulators that use ultrasound technology. Therapeutic ultrasound uses signal characteristics and devices that are approved by the FDA under the Code of Federal Regulations Part 890, subpart F, section 890.5860 to generate deep heat within body tissues for the treatment of selected medical conditions such as the relief of pain, muscle spasms, joint contractures and increase local circulation, and these are class II devices. Bone growth stimulators use different ultrasound signal characteristics, have unique effects, and are class III medical devices.
The focus of this article is intraosseous venous congestion which is unquestionably present in and associated with osteoarthritis of the knee with a myriad of deleterious effects including impaired nutrition of the subchondral bone and cartilage, alterations of trabecular microarchitecture, development of fibrosis, and induced periosteal bone formation. There is an abundance of evidence in the literature of venous stasis and intraosseous hypertension in osteoarthritis of the knee [2].
The interesting history begins with the development of advanced imaging techniques which led to observations of radiologists who are credited with advancing the concept that the subchondral bone and hyaline cartilage of the knee is a functional unit [3-5]. This functional unit has since been confirmed and efforts to understand the biochemical and molecular signaling are ongoing [6-11]. It can be demonstrated that the unifying factor in the disease of osteoarthritis of the knee with consideration of this functional unit is intraosseous venous congestion.
Intraosseous venous congestion has been known to be associated with osteoarthritis of the knee since the early 1960’s and at that time was treated with osteotomy to relieve venous congestion [12-14]. In the 1970’s this was described as the intraosseous engorgement-pain syndrome, and even at that time it was known that intraosseous pressure was mainly determined by venous congestion and that these changes occurred prior to secondary structural damage in osteoarthritis of the knee [14-16]. Investigations in the 1980’s utilized intraosseous phlebography with intraosseous pressure measurements and scintigraphy to confirm venous congestion and stasis with increased intraosseous pressure [17,18]. In the 1990’s investigations continued to delineate the venous congestion and included imaging the morphology [19,20]. In the early 2000’s further studies confirmed that venous stasis and intraosseous pressure correlate with the radiographic stage of osteoarthritis of the knee such that the more severe the venous congestion and the higher intraosseous pressure, the more advanced the stage [21,22]. Increased intramedullary pressure continues to be cited as one of the main causes of knee osteoarthritis [23], and surprisingly, tibial osteotomy is still a current treatment option effecting vascular decompression of intraosseous venous hypertension [22,24-26].
An inevitable outcome of venous congestion is decreased perfusion which manifests with metabolic and structural derangements. The health of all cells is dependent upon the delivery of nutrients and oxygen and the disposal of metabolic waste products and these processes are mediated through the vascular system. In this way venous congestion in a broad sense creates a nutritional deficiency in both the bone and cartilage [2,19,27,28]. Venous congestion has specifically been identified as the source of hypoxia, hypercapnia, and acidity in both synovial fluid and subchondral bone [18,29,30]. The hyaline cartilage is particularly susceptible because of the reliance on subchondral bone to supply nutrition to the avascular articular cartilage [31]. The metabolic changes associated with the intrameduallary microcirculatory failure caused by venous congestion include altered coagulability which leads to micro-hemorrhage, and decreased oxygenation and perfusion which lead to micro-necrosis [2,19,32]. Osteocytes are particularly susceptible and die without nutrient exchange for 4 h and bone ischaemia for more than 6 h causes significant osteonecrosis [33,34]. Osteocytes also lose their ability to detect loading from the loss of fluid flow in osteocyte lacunae with venous congestion and intraosseous hypertension which diminishes their viability [35,36].
The loss of the articular cartilage can be understood as the culmination of events which occur subsequent to venous congestion. In the normal condition the shock absorbing property of the subchondral bone results from the microarchitecture of trabecular orientation which is uniquely adapted to the mechanical forces imposed across the joint [37]. This structure provides for the function of the bone as the primary shock absorber in the healthy state which limits forces that are transmitted to the articular cartilage. In the disease state of osteoarthritis of the knee a sentinel event occurs as the subchondral bone becomes stiffened and results in increased forces subjected to the articular cartilage leading to a cascade of inflammatory events combined with mechanical forces deteriorating the joint space [37-40]. This sequence of events is supported by observations of variations in the trabecular microstructure in osteoarthritis of the knee which affects the biomechanical competence of bone [2,41,42]. One of these events is the development of microfractures which result from venous congestion [19]. Another event is the intraosseous hypertension which stiffens the subchondral bone [29].
The development of intraosseous fibrosis is also a result of venous congestion and is an independent contributor to the increased bone stiffness. The common pathophysiology of venous congestion leading to fibrosis is well known and has been reported in the heart, lung, liver, kidney, skin, and uterus, and in cystic fibrosis [43-50]. In osteoarthritis of the knee histopathology has identified predominantly fibrous tissue replacing the fatty marrow [51]. Studies have mechanistically linked congestion to fibrosis not through an inflammation mediated pathway, but rather through sinusoidal thrombosis and mechanical strain. Sinusoidal dilation leads to stretch of adjacent cells and stasis promotes intravascular thrombosis with fibrin clot formation. Cyclic mechanical stretch induces release of fibronectin and both fibrin and stretch stimulate fibronectin fibril assembly through a β-1 actin-dependent mechanism [46]. These changes result in intraosseous fibrosis as a result of venous congestion in osteoarthritis of the knee.
Another structural condition of osteoarthritis of the knee that is a result of venous congestion is osteophytes. Osteophytes are independently associated with structural progression of osteoarthritis of the knee [52], and venous congestion is included as one of the many causes of osteophytes [53,54]. The development of extensive lower extremity periosteal reaction secondary to venous insufficiency is well known on radiography [55]. It has long been known that proliferative bone changes result from experimentally induced venous congestion [56]. The relationship of increased venous pressure to formation of periosteal new bone has been studied [57]. The results of this and other studies verify that venous stasis stimulates periosteal bone growth [30]. The mechanism involves a generalized periosteal reaction that initially can cause the separation of the periosteum from the cortex [58,59]. The precise mechanism remains unclear but factors associated with the pressurization and stretching deformation of the periosteum due to the increased intramedullary pressure and/or the metabolic changes due to venous congestion, such as oxygen tension, carbon dioxide tension, and local pH value, may be the signal that triggers the cellular activity that results in periosteal bone formation [30].
This summary provides the basis for understanding intraosseous venous congestion as the unifying factor in the pathophysiology of osteoarthritis of the knee. Intraosseous venous congestion creates impaired nutrition of the subchondral bone and cartilage, micro-fractures and alterations of trabecular microarchitecture, subchondral bone stiffening from intraosseous hypertension and fibrosis, culminating in the loss of the articular cartilage and in osteophyte formation. This role of subchondral bone in the pathophysiology of osteoarthritis of the knee makes bone growth stimulators which affect bone remodeling a promising treatment modality. The purpose of this investigation was to determine if bone growth stimulators decrease intraosseous venous congestion in osteoarthritis of the knee.
Methods
This study was performed with Investigational Review Board oversight and ethic committee approval, was published on ClinicalTrials.gov, and adheres to CONSORT guidelines. Patient consent was obtained for the off label use of bone growth stimulators for the treatment of osteoarthritis of the knee. Thirty patients with confirmed osteoarthritis of the knee were included in this study, twenty in the treatment group and ten in the control group. Nineteen patients used ultrasound technology for bone growth stimulation and one patient used Pulsed Electromagnetic Fields (PEMF) technology for bone growth stimulation. The control group did not receive active bone growth stimulator treatment. Sample size was based on a previous report using same treatment with statistically significant results [1].
Each patient’s pain and quality of life were assessed on two independent scales before and after treatment with noninvasive bone growth stimulators utilizing ultrasound technology. Clinical assessments were made at the start of the study, and at completion of treatment. Duration of treatment was 14 weeks at 20 minutes per day. The Exogen 2000+ was used and placed over the tibial tubercle and a calendar of patient use was recorded.
Ultrasound frequency specifications were 1.5 +/- 5% MHz Modulating signal burst width 200+/- 10% microsecond (μs), Repetition Rate 1.0+/- 10% kilohertz(kHz), Duty Factor 20%, Temporal average power 117 +/- 30% milliwatts (mW), Spatial avg.-temporal avg. (SATA) 30+/- 30% mW/cm2, Beam Non-uniformity Ratio (BNR) 4.0 maximum, and collimated beam type. Patients used the device for 20 min daily [60].
Pulsed electromagnetic fields were generated by a control unit powered by a 9 V direct current power source. The magnetic field waveform consists of bursts of triangular (saw-tooth) pulses having a pulse frequency of 3.8 kHz, a burst duration of 5.56 ms, and a burst on-off period of 67 ms. The resulting burst on-off frequency is 1.5 Hz. The maximum amplitude of the magnetic field was approximately 2 mT (20 G) [61].
All patients had radiologically confirmed osteoarthritis of the knee. Patients were excluded from the study if they had received intra-articular injections in the joint and/ or attended physiotherapy sessions for the affected knee, within the 6 months prior to the study. Patients were also excluded if they had a known or suspected joint infection or a specific condition (neoplasm, diabetes mellitus, paresis, osteonecrosis, or recent trauma) that would interfere with the functional assessments during the study. The use of NSAIDs was not permitted during the study period; any pretreatment with NSAIDs had to be discontinued 15 days before the start of the study.
Efficacy parameters
The efficacy criterion utilized the validated and reliable clinical assessment tools of the WOMAC scores and RAND health survey [62,63]. These included WOMAC scores for joint pain (WP), joint stiffness (WS), physical function (WF), and total (WC). The RAND Short Form 36 (SF- 36) health survey questionnaire was utilized to generate the Rand Quality of life score (RQ), Rand Pain score (RP), and Rand composite score (RC).
Intraossous venous congestion was measured by Intraosseous Pressure (IOP) with a 15 gauge intraosseous infusion needle placed adjacent to the tibial tubercle under fluoroscopic guidance with local anesthesia. Intraosseous pressure was measured with sterile arterial pressure tubing connected to a pressure transducer. This measurement was obtained prior to treatment and at the conclusion of the study protocol.
Statistical analyses
SPSS Statistics was utilized to generate paired t-tests for the 10 measurements. Paired samples correlations produced correlation and significance values, and paired samples tests produced mean, standard deviation, standard error mean, 95% confidence intervals, t-values, degrees of freedom, and significance (2-tailed). The p-value was truncated at 3 decimal points.
Results
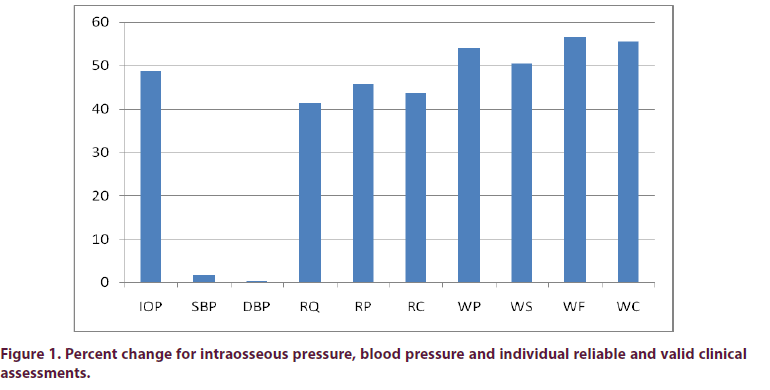
The intraosseous pressure measurement and all 7 quality of life scales were highly significant as seen in Figure 1 and Table 1. The mean intraosseous pressure before treatment was 29.48 mm Hg and after treatment was 15.13 mm Hg, representing a 49% decrease in venous congestion (p=<0.001), and there was 100% response rate with decreased intraosseous pressure in all treated participants.
Table 1. Paired t-test with N=23 and 22 degrees of freedom for Ultrasound Treatment for intraosseous pressure measurement. Paired t-test with N=18 and 17 degrees of freedom for Ultrasound Treatment on 7 quality of life scales and blood pressure measurements.
| Test | t- test | p- value |
|---|---|---|
| Intraosseous Pressure | 5.24 | <0.001 |
| Systolic Blood Pressure (SBP) | -0.617 | 0.545 |
| Diastolic Blood Pressure (DBP) | -0.131 | 0.898 |
| Rand Quality of Life (RQ) | -6.693 | <0.001 |
| Rand Pain (RP) | -7.77 | <0.001 |
| Rand Composite (RC) | -9.351 | <0.001 |
| WOMAC Pain (WP) | 6.714 | <0.001 |
| WOMAC Stiffness (WS) | 6.153 | <0.001 |
| WOMAC Function (WF) | 9.475 | <0.001 |
| WOMAC Composite (WC) | 9.228 | <0.001 |
Ten participants were in the control group and 3 measured bilaterally for 13 control knees. The control and treatment groups did not differ in initial IOP measurements (p=0.97). The control and treatment groups were different after treatment using the difference score Independent t-test (p=0.002, t=3.442). The initial mean intraosseous pressure in the control group was 23.6 mm Hg and was 25.2 mm Hg 14 weeks later.
There was no significant change in SBP or DBP. The mean SBP before treatment was 132.89 mm Hg and after treatment 135.00 mm Hg or 1.6% increase. The mean DBP before treatment was 75.56 mm Hg and after treatment 75.78 mm Hg or 0.3% decrease.
There were no adverse events. One participant was severely injured in an auto accident and did not complete the trial. Of the 18 participants who completed the trial who were treated with ultrasound technology 5 were treated bilaterally for a total of 23 treatment knees. One participant was treated with PEMF’s and completed the trial. For the participant treated with pulsed electromagnetic fields the intraosseous pressure decreased from 23 mm Hg to 13 mm Hg with treatment.
Discussion
We are entering an exciting time with high expectations of new treatments which will be directed to the health of subchondral bone in osteoarthritis of the knee. In 2004 the subchondral bone was identified as a particular interest in any attempt to determine the nature of the factors initiating osteoarthritis [64-68]. The evidence for targeting subchondral bone for treating osteoarthritis was later summarized in 2010, and identified as a key target for future treatments in 2012 [69,70]. In 2014 modalities specifically targeting bone remodeling for treatment of osteoarthritis of the knee was suggested [9]. Research confirming the concept that poor subchondral bone quality is associated with osteoarthritis continued in 2015, and further calls were made for treatments of subchondral bone which may serve as a potential therapeutic target for osteoarthritis interventions [71,72]. In 2017 the current understanding of the connection of subchondral bone pathology in OA was reviewed and the subchondral bone again identified as a key target for OA of the knee [73,74].
The method to measure venous congestion is the measurement of intraosseous pressure. This is because intraosseous pressure is determined by venous congestion and is due to poor venous drainage from the marrow [14,18,19,22-25,30,64,65]. Venous congestion increases the pressure in the system which causes the extravascular pressure to rise because bone is a relatively rigid compartment [2]. Pressure in the marrow cavities rises when venous congestion impedes blood flow away from the bone and always falls when venous congestion is alleviated [66]. Bone marrow and medulllary venous pressure have been determined to be nearly equal and mutually interdependent and venous congestion is the primary determinant of bone marrow pressure [67]. The normal intraosseous pressure has been reported as 15 mm Hg compared to 44 mm Hg in osteoarthritis of the knee [17]. The SBP increased 1.6% and the DBP decreased 0.3% in this study and these were not statistically significant, so the intraosseous pressure is a valid measurement of venous congestion.
Our previous study on the use of bone growth simulators for osteoarthritis of the knee provided subjective evidence of benefit (1). The present study confirms these results and adds objective measurements. It also responds well to specific recommendations in 2017 to address what is described as an urgent clinical need to develop effective regimens to alleviate intraosseous hypertension and venous congestion for the treatment of osteoarthritis of the knee (23). These results confirm the effect for the reduction of venous congestion with the use of bone growth stimulators for osteoarthritis of the knee. It is intuitively obvious that alleviating venous congestion would be effective with the discussion of venous congestion as the unifying factor in the pathophysiology of osteoarthritis of the knee which initiates a cascade of events that result in subchondral bone stiffening, the loss of the articular cartilage and in osteophyte formation.
Video evidence was obtained of pre and post treatment intraosseous pressure measurements to verify lack of bias and future studies are planned with an independent assessor.
Conclusions
We found the mean intraosseous pressure before treatment to be 29.48 mm Hg after treatment was 15.13 mm Hg, representing a 49% decrease in venous congestion, with a 100% response rate with decreased venous congestion in all treated participants. The small increase in venous congestion in the control group is felt to be due to progression of the disease.
This is the second study in the world’s literature of treatment of subchondral bone in osteoarthritis of the knee in humans. This is quite surprising since it is well established that osteoarthritis of the knee is primarily a disease of subchondral bone and the joint changes are secondary. The role of subchondral bone health and an understanding of intraosseous venous congestion as the unifying factor in the pathophysiology of osteoarthritis of the knee makes bone growth stimulators which affect bone remodeling a promising treatment modality. A third study is in progress recruiting 180 veterans in a double-blind, randomized, sham-controlled trial using the Exogen 2000+ Bone Growth Stimulator.
Modalities of bone growth stimulators that are approved by the FDA include PEMF’s, Capacitively Coupled Electric Fields, Combined Magnetic Fields, Direct Current, and Ultrasound. We found decreased venous congestion using PEMF’s as well, so these results do not appear to be specific to ultrasound as the technology for bone growth stimulation.
Ethics approval and consent to participate New England IRB oversaw the protocol and included ethics committee approval and written informed consent which were completed before study initiation. New England IRB Protocol Number: 001 120180024.
Conflict of Interest
KW is owner of US Patent # 8,972,019 Method and device for treating osteoarthritis noninvasively.
Funding
Coastal Carolinas Integrated Medicine in Supply, NC funded the study by supplying the facility, staff and supplies.
Acknowledgements
Robert A Wilson Ph.D. Professor Emeritus Marshall University performed the statistical analysis.
References
- Willeford B, Willeford S. The use of bone growth stimulators for osteoarthritis of the knee. Int. J. Clin. Rheumatol. 12(3), 50-58 (2017).
- Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford). 46(12), 1763-1768 (2007).
- Imhof H, Breitenseher M, Kainberger F et al. Importance of subchondral bone to articular cartilage in health and disease. Top. Magn. Reson. Imaging. 3, 180-192 (1999).
- Felson DT, Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis. Rheum. 50(2), 341-344 (2004).
- Imhof H, Sulzbacher I, Grampp S et al. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest. Radiol. 35(10), 581-588 (2000).
- Pan J, Zhou X, Li W et al. In Situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 27(10), 1347–1352 (2009).
- Lories, RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 7(1), 43–49 (2011).
- Bellido M, Lugo L, Roman-Blas JA et al. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthr. Cartilage. 19, 1228–1236 (2011).
- Yuan X, Meng H, Peng J et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthr. Cartilage. 22(8), 1077-1089 (2014).
- Sharma AR, Jagga S, Lee SS et al. Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 14(10), 19805-19830 (2013).
- Huebner JL, Bay-Jensen AC, Huffman KM et al. ALPHA-CTX is associated with subchondral bone turnover and predicts progression of joint space narrowing and osteophytes in osteoarthritis. Arthritis. Rheumatol. 66(9), 2440–2449 (2014).
- Helal B. The pain in primary osteoarthritis of the knee. Its causes and treatment by osteotomy. Postgrad. Med. J. 41(474), 172–181 (1965).
- Brookes M, Helal B. Primary osteoarthritis, venous engortement and osteogenesis. J. Bone. Joint. Surg. Br. 50(3), 493-504 (1968).
- Lemperg R, Arnoldi CC. The significance of intraosseous pressure in normal and diseased states with special reference to the intraosseous engorgement-pain syndrome. Clin. Orthop. Relat. Res. 136, 143-156 (1978).
- Arnoldi C, Linderholm H, Mussbichler H. Venostasis and intramedullary hypertension in patients with arthrosis in knee and hip joint. Nord. Med. 25(8), 257 (1971).
- Arnoldi CC, Lemperg K, Linderholm H. Intraosseous hypertension and pain in the knee. J. Bone. Joint. Surg. Br. 57(3), 360-363 (1975).
- Arnoldi CC, Djurhuus JC, Heerfordt J et al. Intraosseous phlebography, intraosseous pressure measurements and 99mTC-polyphosphate scintigraphy in patients with various painful conditions in the hip and knee. Acta. Orthop. Scand. 51(1), 19-28 (1980).
- Kofoed H. Hemodynamics and metabolism in arthrosis. Studies in the rabbit knee. Acta. Orthop. Scand. 57(2), 119-122 (1986).
- Imhof H, Breitenseher M, Kainberger F et al. Degenerative joint disease: cartilage or vascular disease? Skeletal. Radiol. 26(7), 398-403 (1997).
- He S, Xiu Z, Hansen E et al. Microvascular morphology of bone in arthrosis. Scanning electron microscopy in rabbits. Acta. Orthop. Scand. 61(3), 195-200 (1990).
- Willeford B. Orthostatic hypotension in the anesthetized rabbit in the sitting position exceeds cerebral autoregulation. J. Basic. Clin. Physiol. Pharmacol. 23(1), 11-15 (2011).
- Uchio Y, Ochi M, Adachi N et al. Intraosseous hypertension and venous congestion in osteonecrosis of the knee. Clin. Orthop. Relat. Res. 384, 217-223 (2001).
- Yan W, Zhifeng L, Faliang L. Clinical application of bone decompressing needle in knee osteoarthritis. Biomed. Res. Pp, S279-S285 (2017).
- Dey A, Sarma U, Dave P. Effect of high tibial osteotomy on upper tibial venous drainage: study by intraosseous phlebography in primary osteoarthritis of knee joint. Ann. Rheum. Dis. 48(3), 188-193 (1989).
- Waldman S. Pain Management, 2nd Ed. Philadelphia PA. (2011).
- DiStefano V, Cohen J. Incomplete nondisplaced tibial osteotomy for treatment of osteoarthritic knee pain. Contemp. Orthop. 23(5), 455-468 (1991).
- Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J. Bone. Miner. Res. 16(9), 1583-1585 (2001).
- Kiaer T, Dahl B, Lausten G. The relationship between inert gas wash-out and radioactive tracer microspheres in measurement of bone blood flow: effect of decreased arterial supply and venous congestion on bone blood flow in an animal model. J. Orthop. Res. 11(1), 28-35 (1993).
- Wang L, Fritton SP, Weinbaum S et al. On bone adaptation due to venous stasis. J. Biomech. 36(10), 1439-1451 (2003).
- Kofoed H. Synovitis causes hypoxia and acidity in synovial fluid and subchondral bone. Injury. 17(6), 391-96 (1986).
- Link TM, Steinbach LS, Ghosh S et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 226(2), 373-381 (2003).
- Bullough P, DiCarlo E. Subchondral avascular necrosis: a common cause of arthritis. Ann. Rheum. Dis. 49(6), 412-420 (1990).
- James J, Steijn-Myagkaya G. Death of osteocytes. Electron microscopy after in vitro ischaemia. J. Bone. Joint. Surg. Br. 68(4), 620-624 (1986).
- Catto M. Ischaemia of bone. J. Clin. Pathol. 11, 78-93 (1977).
- Han Y, Cowin SC, Schaffler MB et al. Mechanotransduction and strain amplification in osteocyte cell processes. Proceedings of the National Academy of Sciences of the United States of America. 101(47), 16689-16694 (2004).
- Bakker A, Klein-Nulend J, Burger E. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem. Biophys. Res. Commun. 320(4), 1163-1168 (2004).
- Li G, Yin J, Gao J et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis. Res. Ther. 5(6), 223 (2013).
- Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol. Clin. North. Am. 42(1), 1-9 (2004).
- Weinstein AM, Rome BN, Reichmann WM et al. Estimating the burden of total knee replacement in the United States. J. Bone. Joint. Surg. Am. 95(5), 385-392 (2013).
- Bastick AN, Runhaar J, Belo JN et al. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis. Res. Ther. 17, 152 (2015).
- Kamibayashi L, Wyss UP, Cooke TD et al. Trabecular microstructure in the medial condyle of the proximal tibia of patients with knee osteoarthritis. Bone. 17(1), 27-35 (1995).
- Patel V, Issever AS, Burghardt A et al. MicroCT evaluation of normal and osteoarthritic bone structure in human knee specimens. J. Orthop. Res. 1, 6-13 (2003).
- Ziarkiewicz-Wróblewska B, Górnicka B, Wróblewski T et al. Idiopathic portal hypertension: a case report. Med. Sci. Monit. 10(11), CS69-CS72 (2004).
- Petersen SE, Jerosch-Herold M, Hudsmith LE et al. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 115(18), 2418-2425 (2007).
- Sudo N, Nambu A, Yamakawa T et al. Pulmonary focal fibrosis associated with microscopic arterio-venous fistula manifesting as focal ground-glass opacity on thin-section CT. BMC. Pulmonary. Med. (2013).
- Simonetto DA, Yang H, Yin M et al. Chronic Passive Venous Congestion drives Hepatic Fibrogenesis via Sinusoidal Thrombosis and Mechanical Forces. Hepatol. 61(2), 648–659 (2015).
- Tanaka M, Yoshida H, Furuhashi M et al. Deterioration of renal function by chronic heart failure is associated with congestion and oxidative stress in the tubulointerstitium. Intern. Med. 50(23), 2877-2887 (2011).
- Nicholls SC. Sequelae of Untreated Venous Insufficiency. Semin. Intervent. Rad. 22(3), 162–168 (2005).
- Durham JD, Machan L. Pelvic Congestion Syndrome. Semin. Intervent. Rad. 30(4), 372–380 (2013).
- Rodriguez-Miguelez P, Thomas J, Seigler N et al. Evidence of microvascular dysfunction in patients with cystic fibrosis. Am. J. Physiol. Heart. Circ. Physiol. 310(11), H1479-1485 (2016).
- Bergman A, Willen H, LIndstrand A et al. Osteoarthritis of the knee: correlation of subchondral MR signal abnormalities with histopathologic and radiographic features. Skeletal. Radiol. 23(6), 445-448 (1994).
- Barr AJ, Campbell TM, Hopkinson D et al. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis. Res. Ther. 17(1), 228 (2015)..
- Levine S, Lambiase R, Petchprapa C. Cortical Lesions of the Tibia: Characteristic Appearances at Conventional Radiography. RadioGraphics. 23(1), 157–177 (2003).
- Trout N. Venous congestion is a source of Hypertrophic Osteopathy. Handbook of Small Animal Practice (Fifth Edition). (2008).
- Gensburg R, Kawashima A, Sandler C. Scintigraphic Demonstration of Lower Extremity Periostitis Secondary to Venous Insufficiency. J. Nuc. Med. 29(7), 1279-1282 (1988).
- Bernstein M. Experimental production of arthritis by artificially produced passive congestion. J. Bone. Joint. Surg. 15, 661-663 (1933).
- Kelly P, Bronk J. Venous pressure and bone formation. Microvasc. Res. 39(3), 364-375 (1990).
- Serdar A, Tayfun U, Ömer E et al. Generalized periosteal reaction and tissue swelling secondary to prolonged prostaglandin E1 infusion and venous stasis: a case report. Turkish. J. Pediatr. 55(5), 543-545 (2013).
- Rana RS, Wu JS, Eisenberg RL. Periosteal reaction. AJR. 193, 259-272 (2009).
- Biglari B, Yildirim TM, Swing T et al. Failed treatment of long bone nonunions with low intensity pulsed ultrasound. Arch. Orthop. Traum. Su. 136, 1121–1134 (2016).
- Midura RJ, Ibiwoye MO, Powell KA et al. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J. Orthop. Res. 23(5), 1035–1046.
- Collins NJ, Misra D, Felson DT et al. Measures of Knee Function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis. Care. Res. 63(11), S208–S228 (2011).
- Bellamy N. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 15(12), 1833-1840 (1988).
- Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthr. Cartil. 12, S10-S19 (2014).
- Brooks M, Revell W. Blood Supply of Bone. (1998).
- Bourne GH. The Biochemistry and Physiology of Bone Volume IV Calcification and Physiology, 2nd Ed. New York: Academic Press. (1976).
- Wilkes C, Visscher M. Some Physiological Aspects of Bone Marrow Pressure. J. Bone. Joint. Surg. 57(1), 49–57 (1975).
- Bailey AJ, Mansell JP, Sims TJ et al. Biochemical and mechanical properties of subchondral bone in osteoarthritis. Biorheology. 41(3-4), 349-358 (2004).
- Kwan Tat S, Lajeunesse D, Pelletier JP et al. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best. Pract. Res. Clin. Rheumatol. 24(1), 51-70 (2010).
- Castañeda S, Roman-Blas JA, Largo R et al. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol. 83(3), 315-323 (2012).
- Sepriano A, Roman-Bias J, Little R et al. DXA in the assessment of subchondral bone mineral density in knee osteoarthritis--A semi-standardized protocol after systematic review. Semin. Arthritis. Rheum. 45(3), 275-283 (2015).
- Chang G, Xia D, Chen C et al. 7T MRI detects deterioration in subchondral bone microarchitecture in subjects with mild knee osteoarthritis as compared with healthy controls. J. Magn. Reson. Imaging. 41(5), 1311-1317 (2015).
- Hugle T, Geurts J. What drives osteoarthritis? – synovial versus subchondral bone pathology. Rheumatology (Oxford). 56(9), 1461-1471 (2016).
- Wang CJ, Cheng JH, Huang CY et al. Medial tibial subchondral bone is the key target for extrocorporal shockwave therapy in early osteoarthritis of the knee. Am. J. Transl. Res. 9(4), 1720-1731 (2017).