Mini Review - International Journal of Clinical Rheumatology (2018) Volume 13, Issue 3
Rheumatology to hepatology cross talk: An evidence based update on the treat to target strategy in hepatitis-C related extrahepatic autoimmune syndromes
- *Corresponding Author:
- Reem Hamdy A Mohammed
Department of Rheumatology and Rehabilitation
School of Medicine Cairo University, Egypt
E-mail: rmhamdy@yahoo.com
Abstract
Hepatitis C virus (HCV) is a universally identified major epidemiological health problem with an estimated reservoir of almost 200 million or 3% of the global population. In the year 2013, the WHO declared viral hepatitis as “a leading cause of death worldwide 1.46 million deaths, a toll higher than that from HIV, tuberculosis or malaria, and on the increase since 1990” with more than 90% of these deaths being related to the sequelae of the infection with either forms of hepatitis viruses (HBV-HCV). HCV is classically a hepatotropic virus with the liver cells providing the primary bed for viral replication with clinical evidences supporting an additionally significant viral lymphotropism. HCV is a linear single stranded RNA retrovirus, member of the genus hepacivirus of the flaviviridae family. It is a small virus comprised of a positive-sense 9.6 Kb single-stranded RNA genome embodied in a double layered glycosylated protein envelope, the viral genome protein is basically made up of structural and non-structural proteins. The viral enzymes- NS2-3 and NS3-4A proteases, NS3 helicase and NS5BRdRp— are essential contributors for HCV replication, the HCV serine protease NS3, and its cofactor NS4A, constitute a complex that directs polyprotein cleavage. HCV infected individuals are capable of producing 10-13 trillion virions/day with the majority coming primarily from viral replication within the hepatocytes with an unpredictable yet significant extrahepatic contributions that may lead to the development and modulation of systemic extrahepatic disease. The use of conventional antiviral therapy PEGylated interferon (Peg-IFN) and ribavirin (RBV) practically contributed to the disease burden with less than a 50% sustained viral response rates. A major challenge for interferon therapy comes from the Hepatitis C viral genome itself. The challenging draw backs to interferon based regimen in patients with autoimmune extrahepatic disease EHD demanded an evidence based revisit to the classic recommendations on the use of conventional antivirals with immunomodulatory drugs in this indication. It wasn’t until spring of 2011 when the FDA approved the first two directly acting antiviral drugs that the hepatologists experienced a revolutionary shift in Hepatitis C virus (HCV) treatment paradigm. Directly acting antiviral drugs (DAAs are drugs that target some of the main molecular components of HCV, including NS3/4A protease (first and second generation protease inhibitors), NS5B polymerase (nucleoside and non-nucleoside analogs) and NS5A protein. The recent era of DAAs established an additional need to modify treatment regimens in extrahepatic disease. In the year 2017 the international study group of HCV extrahepatic disease published evidence based recommendations on the use of antivirals for control of EHD. The treatment armamentarium in chronic HCV viremia with and without extrahepatic disease has experienced a revolution with the establishment of directly acting antiviral drugs. Interferon free directly acting antiviral drug regimens are currently considered as standard of care in patients with extrahepatic disease. Longitudinal studies are further requested to assess the unmet needs including drugs addressing other potential targets in the viral genome and life cycle.
Keywords
hepatitis c virus, HCV genome, nonstructural proteins, directly acting antiviral drugs, evidence based recommendations-HCV extrahepatic disease
Introduction
Hepatitis C Virus (HCV) is major epidemiological health problem with an estimated worldwide reservoir of almost 200 million or 3% of the global population [1-3]. In the year 2013, the WHO declared viral hepatitis as “a leading cause of death worldwide 1.46 million deaths, a toll higher than that from HIV, tuberculosis or malaria, and on the increase since 1990” with more than 90% of these deaths being related to the sequelae of the infection with either forms of hepatitis viruses (HBV- HCV). The WHO then issued a notification that “In the absence of additional efforts, 19 million hepatitis-related deaths are anticipated from 2015 to 2030” and that “Treatment now can prevent deaths in the short- and medium term.” In 2014 World Health Assembly requested the World Health Organization to examine the feasibility of eliminating hepatitis B and C, and the 2015 Agenda for Sustainable Development commits to combating viral hepatitis. WHO modelled options, and results of the analysis suggest that if the viral hepatitis response reaches five prevention and treatment service coverage targets, hepatitis B and C could be eliminated as a public health threat (i.e. 90% reduction in new chronic infections, 65% reduction in mortality compared with a scenario in which interventions would continue at the current level) [4].
Hepatitis C viral structure
Hepatitis C Virus is a linear single stranded RNA retrovirus, member of the genus hepacivirus of the flaviviridae family that was early identified in 1989. It is a small virus comprised of a positive-sense 9.6 Kb single-stranded RNA genome embodied in a double layered glycosylated protein envelope. The viral genome protein is basically made up of structural and non-structural proteins [5,7]. The structural viral proteins comprise the envelope glycoprotein complex, the core protein, and the P7 protein. Structural proteins are primarily involved in viral endocytosis, antigenic variability, immune evasion and neutralization of antibodies, and metabolic syndromes related to chronic viremia. On the other hand we find that the HCV-RNA genome carries a long open reading frame (ORF) encoding a large non-structural replicative polyprotein comprised of 3010 amino acids that feature a vital role at the translational, post-translational and replicative levels. These are the nonstructural proteins that are potential players in viral replication, resistance to interferon therapy and immune evasion [8-11].
Viral tissue tropism and life cycle
Hepatitis C Virus is classically a hepatotropic virus with the liver cells providing the primary bed for viral replication. Clinical evidences support an additionally significant viral lymphotropism. HCV infected individuals are capable of producing 10-13 trillion virions/day with the majority coming primarily from viral replication within the hepatocytes and a variable yet significant extrahepatic contributions that may lead to the development and modulation of systemic extrahepatic disease [6]. The viral life cycle starts once the virion attaches itself to its specific receptor on the surface membrane of the hepatocytes. HCV enters into host cell via receptor mediated endocytosis which is currently regarded as a slow and complex multistep procedure. The attachment takes place through a number of candidate molecules that contribute to the formation of viral receptor complex with subsequent viral endotropism. These molecules “surface receptors” have included; the CD81 tetraspanin, the scavenger receptor type B class 1 protein (SRB-1), high density lipoprotein binding molecule of the host cell, the HCV low-density lipoprotein (LDL) receptor, the tight junction components claudins (mainly CLDN- 1, CLDN-6 and CLDN-9) and mannose binding proteins (DC-SIGN and L-SIGN) [12-14]. The HCV enzymes- NS2-3 and NS3-4A proteases, NS3 helicase and NS5BRdRp—are essential for HCV replication, the HCV serine protease NS3, and its cofactor NS4A, constitute a complex that directs polyprotein cleavage at the NS3-NS4A, NS4A-NS4B, NS4B-NS5A and NS5A-NS5B junctions via cellular and viral encoded proteases which stratifies them amongst the potential targets for the development of small molecule anti-HCV compounds [5,13-17].
Viral B cell stimulation and Immune Evasion
Hepatitis C Virus envelope glycoproteins and viral genome proteins are key players that interfere with effective viral immune handling by the innate and adaptive immune responses sustaining viremia via a number of mechanisms. First during the acute infection the HCV E2-CD81 receptor interaction suppresses the induction of natural killer cells with upregulation of the inhibitory NK receptors thereby inhibiting cytotoxicity and IFN alpha and beta production by NK cells [5,7,9]. Second HCV infection is sensed in infected hepatocytes by the pattern recognition receptors RIG-I (retinoic acid-inducible gene I) and TLR3 (Toll-like receptor 3). RIG-I and TLR3 signaling are mediated by the adaptor proteins MAVS and TRIF, respectively. Cleavage of adaptor proteins MAVS and TRIF by viral non-structural protein NS3/4A suppresses the transcription of antiviral genes [18-20]. The HCV core protein also interferes with the JAK/STAT pathway that leads to impaired induction of antiviral effector proteins including interferons alpha and beta [21,22]. The observable impaired trafficking of the Dendritic Cells and T lymphocytes insufficient expansion and maturation of HCV specific CD4+ and CD8+ T cell populations [23]. Ongoing Viral mutations affect the virus specific CD8+ T cell responses by decreasing the binding affinity between the epitope and the MHC molecule and impairing proteosomal processing of HCV antigens [24-26]. Mutations within the Foxp3 gene alter CD4+CD25+ regulatory T cell development [5]. The HCV nonstructural (NS) proteins augment the oxidative stresses via the disturbance of mitochondrial metabolism [27,28]. The HCV-Induced B Lymphocyte hyperproliferation via HCV-E2 hypervariable region which is capable of directly interacting with B-lymphocytes through tetraspanin CD 81 receptor lowering the lymphocytes activation threshold with subsequent clonal expansion of the rheumatoid factor (RF) IgM/k producing lymphocytes. HCV possesses the potential of persistent and prolonged stimulation of B lymphocytes passing from a physiologic polyclonal activation to a mono-oligo-clonal expansion characteristic with mixed cryoglobulinemia until the frank monoclonality of B cell lymphoma [29]. The HCV-dependent gene translocation leads to Bcl-2 recombination, activation of this anti-apoptotic protoncogene promotes B cell hyperproliferation [30,31]. HCV, induces expression of Toll like receptors 4(TLR4), through the action of its NS5A protein leading to enhanced IFN-gamma and IL-6 production and secretion. Stimulation of members of the TNF superfamily (B-lymphocyte stimulator- BLyS and APRIL- a proliferation inducing ligand) represents another potential immune-stimulatory pathway, where the binding of BLyS to its receptors (TACI and BLyS-R) induce strong B cells proliferation and extended survival via the strong activation of the antiapoptotic bcl-2 gene [32-35]. Lastly HCV infection of lymphocytes induces sustained clonal somatic hypermutation (SHM) of the B- Lymphocytes generating intraclonal diversity in lymphoid tissue [36].
Viral proteins and resistance to interferon therapy
A major challenge to interferon therapy was the HCV genome itself. HCV had the capacity to resist the INF response with failure of clearance in up to 50% of the treated cases and tendency to recurrence in a significant proportion of responders. The HCV viral non-structural proteins proved to be major contributors to interferon response failure via multiple mechanisms: [3-40] The HCV NS5A plays a central role in escape of antiviral action of interferon via the interferon sensitive region (ISDR). The NS5A also induces IL-8, a chemokine which inhibits the antiviral actions of IFN. Most of the HCV expressed proteins especially the core protein and NS5A seem to impair the mitochondrial respiratory chain through an overproduction of ROS, which alter both mitochondria’s structure and function of infected hepatocytes. HCV infection induced increase in oxidative stress blocks the interferon response via the c-Jun N-terminal Kinase/Signal Transducers and Activator of Transcription (JNK/STAT) pathway required for signaling by interferon. The NS3 serine protease further contributes to defective interferon response by inhibition of RIG-I and TLR3 signaling that leads to defective the interferon response [37- 40].
The treat to target in chronic HCV viremia
Novel insights into the molecular structure and the life cycle of HCV lead to a major advent in the treatment landscape towards effective intervention and targeted approaches to suppress viral replication [41]. It wasn’t until spring of 2011 when the FDA approved the first two directly acting antiviral drugs NS3/4A protease inhibitors (PIs), boceprevir and telaprevir for HCV genotype I that the hepatologists experienced a revolutionary shift in HCV treatment paradigm. However, evidences from clinical trials revealed that monotherapy with either of these two PI resulted in the rapid emergence of resistant variants whereas triple therapy with PEG-IFN/RBV and either of these PIs increased SVR rates to 70–80% with combined adverse events. By then the question was “Is it a tight control strategy or an expansion to the list of adverse events in these patients?” [42].
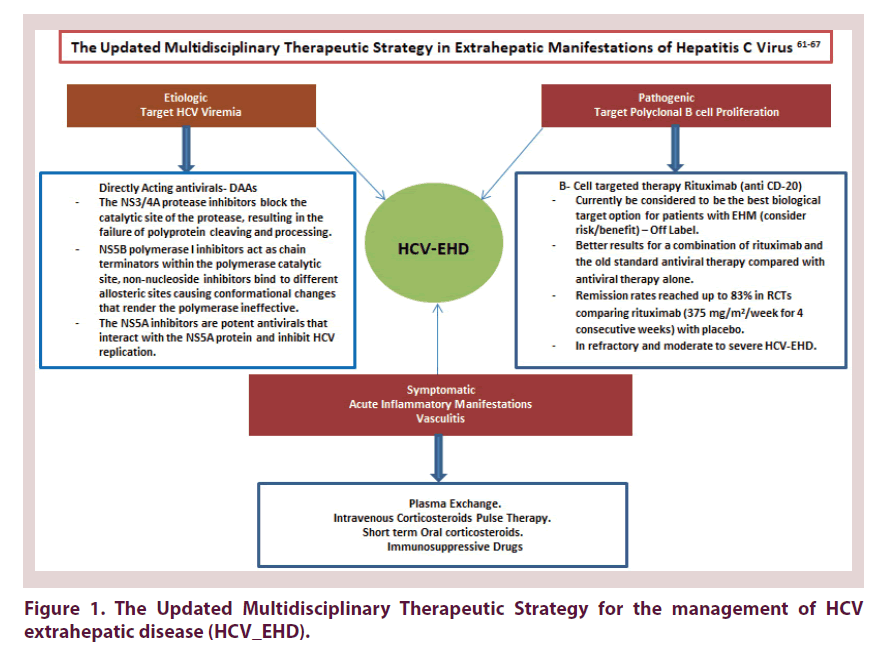
From this point potential efforts have been deployed to establish other forms of DAAs that achieve stringent control of viral replication. Currently a long list of broad spectrum multi-genotypic DAAs exists with barriers to resistance hitting a supreme SVR reaching up to 95% Figure 1.
Directly acting antiviral drugs
Directly acting antiviral drugs (DAAs) are drugs that target some of the main molecular components of HCV, including NS3/4A protease (first and second generation protease inhibitors), NS5B polymerase (nucleoside and non-nucleoside analogs) and NS5A protein. The NS3/4A protease inhibitors (PIs: telaprevir, boceprevir, simeprevir, paritaprevir, voxilaprevir, asunaprevir, grazoprevir, glecaprevir) block the catalytic site of the protease, resulting in the failure of polyprotein cleaving and processing. NS5B polymerase I inhibitors include nucleoside analogs (sofosbuvir) that act as chain terminators within the polymerase catalytic site and non-nucleoside inhibitors (dasabuvir, beclabavir) bind to different allosteric sites causing conformational changes that render the polymerase ineffective. The NS5A inhibitors (daclatasvir, ledipasvir, ombitasvir, velpatasvir, elbasvir, pibrentasvir) are potent antivirals that interact with the NS5A protein and inhibit HCV replication, the mechanism remains unclear [43- 46].
Evidence based recommendations on the use of DAAs and Non antiviral therapy in HCV extrahepatic autoimmune syndromes: The rheumatology to hepatology cross talk
The challenging draw backs to interferon based therapy in patients with autoimmune extrahepatic disease EHD demanded an evidence based revisit to the classic recommendations on the use of conventional antivirals with immunomodulatory drugs in this indication. In the year 2017 the international study group of HCV extrahepatic disease published evidence based recommendations on the use of antivirals for control of EHD. Data were mostly derived from clinical trials in HCV cryoglobulinemic vasculitis because of their higher frequency and potential life threatening manifestations. The panel provided supportive evidences for the value of DAAs in remission of extrahepatic manifestations considering multiple potential confounders in this category of patients [47].
The core tips for the use of antivirals in HCV cryoglobulinemic vasculitis and extrahepatic disease as quoted for these recommendations by the ISG-HCVEHD- 2017 stated that [47,48]
• Antiviral treatment is recommended for all patients with EHM, except those with limited life expectancy due to causes unrelated to HCV or metastatic cancer. These guidelines included patients with CV or renal involvement with the highest priority for treatment due to high risk of life-threatening complications. (Level of evidence: 2 for CV and B cell lymphoma; 3–5 for the remaining EHMs, Strength of recommendation: B)
• When considering a choice between DAA regimens that achieve similar rates of SVR, care providers and clinicians should take into account the potential side effects associated with the regimen in patients with EHMs and not only the cost/effectiveness. RBV-free regimens could be used as the first choice for patients with EHMs presenting with haemoglobin levels\10 g/dl. The reduced length of therapy compared with older options is a strong positive point with respect to safety issues in patients with EHMs. The decision of which DAA regimen to use may involve consideration of drug interactions between DAAs and concomitant medications. (Level of evidence: 3 for CV, 5 for other EHMs, strength of recommendation: C) [48-53].
• If a lack of resources limits the ability to treat all patients with EHM immediately with DAA as recommended, then it is most appropriate to treat those presenting with more severe EHM involvements first. No studies are available that compare the results of current antiviral treatments graded by severity of EHMs. (Level of evidence: 5, strength of recommendation: C) [52,53].
• The efficacy of therapies in EHM patients should be evaluated not only according to the virological response, but also according to the full impact of the other clinical and immunological. The requirement for longer follow-up periods searching for late clinical responses may be recommended for some specific organs (renal or neurological involvements). (Level of evidence: 3, Strength of recommendation: C) [54-57].
• With limited resources, the priority for the immediate initiation of antiviral therapies in the following subset of EHM patients was rated as follows (highest to lowest priority). (Level of evidence: 3, Strength of recommendation: B) [58- 61].
• There is little specific information on the clinical efficacy of antiviral therapies on non-vasculitic autoimmune features (sicca features, arthritis, cutaneous lupus, pulmonary involvement etc.). (Level of evidence: 2 for vasculitic features, 5 for non-vasculitic features, Strength of recommendation: B) [54-60].
• In Patients with non-specific general features (i.e. fatigue, chronic pain, fibromyalgia), evidences found that physical function only improved in patients who achieved SVR with antiviral therapy, other studies found improvements independently of SVR, suggesting that viral clearance alone can achieve significant physiological changes. In the new DAA era, the SF-36 physical status score and the mental status score improved significantly. The use of IFNand RBV-free regimens for HCV showed better patients’ experience and work productivity during treatment. (Level of evidence: 3, Strength of recommendation: C) [60,61].
• Interferon IFN-free antiviral regimens might be less effective than IFNcontaining regimens in some patients with B cell lymphoma, possibly due to the lack of additional anti-proliferative activity of IFN, while the association of rituximab with DAA regimens could be more effective than isolated antiviral therapies. (Level of evidence: 3, Strength of recommendation: C) [62,63].
Recommendations on Non-antiviral therapy considered that:
• Regimens in common use include: gluco-corticosteroids, immuno-suppressant agents, plasma exchange and biological therapies. These non-antiviral approaches were derived primarily from strategies employed in other systemic vasculitides. Non-antiviral therapeutic approaches are recommended for moderate and, especially, for severe organ-specific involvements [54-59].
• Short-term glucocorticoid regimens can be used as a bridge therapy to control mild manifestations and control the acute inflammation in moderate to severe vasculitic manifestations prior to antiviral agents. Methylprednisolone Pulse therapy (0.5–1.0 g/day) for three days followed by prednisone (not exceeding 1 mg/kg/day) may be appropriate for resistant or severe acute life threatening or organ threatening manifestations. • In the current DAA era, the role of immunosuppressive agents (often used in a maintenance therapy regimen) may be marginal. Immunosuppression requires close monitoring of blood counts and other parameters. Patients treated with glucocorticoids and cyclophosphamide should also receive prophylaxis for Pneumocystis pneumonia and surveillance for other opportunistic infections [64,65].
• Plasma exchange may be added to other therapies, especially in patients with severe/ life-threatening manifestations especially cryoglobulinemic vasculitis, coagulopathies. Apheresis techniques can lead to a rebound phenomenon in which cryoglobulin production increases after the cessation of apheresis [66].
• B cell targeted therapy: Rituximab (anti CD-20) RTX is currently be considered to be the best biological target option for patients with EHM (consider risk/ benefit) – Off Label. The level of evidence is the highest of all current therapeutic options for EHMs, both in the number of treated patients and in the data quality (RCTs). Prospective studies found better results for a combination of rituximab and the old standard antiviral therapy compared with antiviral therapy alone. Rituximab showed excellent tolerance in patients with cirrhosis, even with improvement in liver cirrhosis markers. Remission rates reached up to 83% in RCTs comparing rituximab (375 mg/m2/week for 4 consecutive weeks) with placebo. Retention rates were significantly higher in patients with refractory EHD randomized to RTX combination (71% and 61%) compared to Conventional therapy (3%) at 6 and 24 months respectively [50-67].
• In HCV-Cryovasculitis with mild to moderate disease, an optimal antiviral IFN-free treatment should be given alone. Low-dose corticosteroids may help to control inflammatory signs. For patients with severe vasculitis rituximab ± plasmapheresis ± antiviral IFN-free therapy should be started at the same time. A combination of RTX and DAAs gave SVR rates >95% at 24 weeks. In case of persistent Cryovasculitis manifestations in patients with a sustained virologic response (SVR), another underlying condition should be considered, especially B-cell lymphoma. Finally the panel recommended the earlier the better [50-65].
The panel emphasized that the earlier the viral eradication the better the results in terms of sustained and complete clinical response. However, they requested caution while interpreting these data due to the large degree of heterogeneity in patient characteristics, the different DAA regimens used and the uncontrolled designs of the studies [47-48].
Potential future interventional targets
CD81- A co-receptor
The soluble forms of CD81 have the potential to inhibit entry of HCV pseudoparticles to the cell also ectopic expression of CD81 in CD81- negative cells does not permit HCVpp entry indicating that CD81 is a co-receptor. Lately, several host restriction factors that protect cells from viral infection have been identified such as EW1-2wint. EW1- 2wint is a CD81 associated protein which is able to inhibit HCV entry into target cells by blocking the interactions between HCV glycoproteins and CD81. EWI-2wint may interfere with actin- polymerization during viral entry or block signaling pathways necessary for viral entry [68-72].
Targeting host small molecular RNAs
Targeting host micro-RNAs might provide a salvage route to suppress viral replication in patients who fail multiple DAAs. One promising model was the widely used antihistamine chlorcyclizine that was found to block an early stage in the HCV life cycle and showed synergy with DAAs in a humanized mouse model of HCV. However, inhibiting host proteins raises concerns about adverse effects. Addressing host targets need to be carefully assessed in upcoming research [67-70].
Envelope glycoproteins
E2 contains two hypervariable regions (HVR), HVR1 and HVR2, which are under constant selection for mutation probably because they are targets for neutralizing antibodies. Numerous studies have highlighted the genetic heterogeneity of the HVR1, which may enable virus to evade the immune system and facilitate establishment of chronic infection. However, chronic infection has been reported in an experimentally infected chimpanzee even though there was no variation in HVR [67-70].
HCV low density lipoprotein receptor
The low density lipoprotein (LDL) receptor is involved in the process of viral endocytosis. In vitro studies have shown that viral entry could be prevented in a number of cell types using an anti-LDL monoclonal antibody [71-72].
Mannose binding proteins
The mannose binding proteins (DC-SIGN and L-SIGN) have been suggested as to have interactions with E2 but their contribution to viral entry is not known [73].
Conclusion
The treatment armamentarium in chronic HCV viremia with and without extrahepatic disease has experienced a revolution with the establishment of directly acting antiviral drugs. Interferon free directly acting antiviral drug regimens are currently considered as standard of care in patients with extrahepatic disease. Longitudinal studies are further requested to assess the unmet needs including drugs addressing other potential targets in the viral genome and life cycle.
Conflicts of Interest
None to be declared
References
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins (review). J. Clin. Pathol. 55, 4–13 (2002).
- Mohammed RHA, ElMakhzangy HI, Gamal A et al. Prevalence of rheumatologic manifestations of chronic hepatitis C virus infection among Egyptians. Clin. Rheumatol. 29, 1373–1380 (2010).
- Sansonno D, Carbone A, De Re V et al. Hepatitis C virus infection, cryoglobulinaemia, and beyond. Rheumatology. 46, 572–578 (2007).
- WHO-2016 “Global Health Sector Strategy on Viral Hepatitis, 2016–2021” (2016).
- Mohammed RHA, Hesham I, El-Makhzangy. Book Chapter: Hepatitis C Related Vasculitides. Advances in the Etiology, Pathogenesis and Pathology of Vasculitis. Dr. Luis M Amezcua-Guerra (Ed.), InTech. (2011).
- Sherman AC, Sherman KE. Extrahepatic Manifestations of Hepatitis C Infection: Navigating CHASM. Curr HIV/AIDS Rep. 12(3), 353–361 (2015).
- Ashfaq U, Javed T, Rehman S et al. An overview of HCV molecular biology, replication and immune responses. J. Virol. 8, 161 (2011).
- Goffard A, Callens N, Bartosch B et al. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 79, 8400–8409 (2005).
- Slater-Handshy T, Droll DA, Fan X et al. HCV E2 glycoprotein: mutagenesis of N-linked glycosylation sites and its effects on E2 expression and processing. Virology. 319, 36–48 (2004).
- Polyak SJ, McArdle S, Liu SL et al. Evolution of hepatitis C virus quasispecies in hypervariable region 1 and the putative interferon sensitivity-determining region during interferon therapy and natural infection. J. Virol. 72, 4288–4296 (1998).
- van Doorn LJ, Kleter GE, Stuyver L et al. Sequence analysis of hepatitis C virus genotypes 1 to 5 reveals multiple novel subtypes in the Benelux countries. J. Gen. Virol. 76(7), 1871–1876 (1995).
- Scarselli E, Ansuini H, Cerino R et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. Embo. J. 21, 5017–5025 (2002).
- Pileri P, Uematsu Y, Campagnoli S et al. Binding of hepatitis C virus to CD81. Science. 282, 938–941 (1998).
- Hsu M, Zhang J, Flint M et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. 100, 7271–7276 (2003).
- Racanelli V, Sansonno D, Piccoli C et al. Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus-infected patients. J. Immunol. 167(1), 21–9 (2001).
- Grakoui A, McCourt DW, Wychowski C et al. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J. Virol. 67(5), 2832–2843 (1993).
- Grakoui A, Wychowski C, Lin C et al. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67(3), 1385–1395 (1993).
- Williford SE, McGivern DR. Mechanism of Action of Direct-Acting Antivirals: New Insights into the HCV Life Cycle. Book Chapter in Hepatitis C Virus II.
- Meylan E, Curran J, Hofmann K et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 437(7062), 1167–1172 (2005).
- Li K, Foy E, Ferreon JC et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. U S A. 102(8), 2992–2997 (2005).
- Lin W, Choe WH, Hiasa Y et al. Hepatitis C virus expression suppresses interferon signaling by degrading STAT1. Gastroenterology. 128, 1034–1041 (2005).
- Bode JG, Ludwig S, Ehrhardt C et al. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. Faseb. J. 17, 488–490 (2003).
- Flamm SL. Chronic hepatitis C virus infection. Jama. 289, 2413–2417 (2003).
- Seifert U, Liermann H, Racanelli V et al. Hepatitis C virus mutation affects proteasomal epitope processing. J. Clin. Invest. 114, 250–259 (2004).
- Wedemeyer H, He XS, Nascimbeni M et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169, 3447–3458 (2002).
- Spangenberg HC, Viazov S, Kersting N et al. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 42, 828–837 (2005).
- Grakoui A, McCourt DW, Wychowski C et al. A second hepatitis C virus-encoded proteinase. Proc. Natl. Acad. Sci. USA. 90, 10583–10587 (1993).
- Reed KE, Grakoui A, Rice CM. Hepatitis C virus-encoded NS2-3 protease: cleavage-site mutagenesis and requirements for bimolecular cleavage. J. Virol. 69, 4127–4136 (1995).
- Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N. Engl. J. Med. 327, 1490–5 (1992).
- Giannini E, Borro P, Botta F et al. Serum thrombopoietin levels are linked to liver function in untreated patients with hepatitis C virus-related chronic hepatitis. J. Hepatol. 37, 572–577 (2002).
- Zignego AL, Ferri C, Giannelli F et al. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann. Intern. Med. 137, 571–580 (2002).
- Stein JV, López-Fraga M, Elustondo FA et al. APRIL modulates B and T cell immunity. J. Clin. Invest. 109, 1587–98 (2002).
- Sene D, Limal N, Ghillani-Dalbin P et al. Hepatitis C virus-associated B-cell proliferation—the role of serum B lymphocyte stimulator (BLyS/BAFF). Rheumatology (Oxford). 46, 65–69 (2002).
- Gross JA, Johnston J, Mudri S et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 404(6781), 995–9 (2002).
- Fabris M, Quartuccio L, Sacco S et al. B-Lymphocyte stimulator (BLyS) upregulation in mixed cryoglobulinaemia syndrome and hepatitis-C virus infection. Rheumatology (Oxford). 46, 37–43 (2002).
- De Vita S, De Re V, Sansonno D et al. Lack of HCV infection in malignant cells refutes the hypothesis of a direct transforming action of the virus in the pathogenesis of HCV-associated B-cell NHLs. Tumori. 88, 400–406 (2002).
- Fried MW, Shiffman ML, Reddy KR et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347(13), 975–982.
- Tan SL, Katze MG. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology. 284, 1–12 (2001).
- Gale MJ, Korth MJ, Tang NM et al. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 230, 217–227 (1997).
- Enomoto N, Sakuma I, Asahina Y et al. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. Sensitivity to interferon is conferred by amino acid substitutions in the NS5A region. J. Clin. Invest. 96, 224–230 (1995).
- Gotte M, Feld J. Direct-acting antiviral agents for hepatitis C: structural and mechanistic insights. Nat. Rev. Gastroenterol. Hepatol. 13, 338–351 (2016).
- Rong L, Dahari H, Ribeiro RM et al. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci. Transl. Med. 2(30), 30–32 (2010).
- Lam BP, Jeffers T, Younoszai Z et al. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Therap. Adv. Gastroenterol. 8, 298–312 (2015).
- Feld JJ, Jacobson IM, Hezode C et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 373, 2599–2607 (2015).
- Foster GR, Afdhal N, Roberts SK et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 373, 2608–2617 (2015).
- Curry MP, O’Leary JG, Bzowej N et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N. Engl. J. Med. 373, 2618–2628 (2015).
- Ramos-Casals M, Zignego AL, Ferri C et al. (ISG-EHCV). Evidence-based recommendations on the management of extrahepatic manifestations of chronic hepatitis C virus infection. J. Hepatol. 66, 1282–1299 (2017).
- EASL Recommendations on Treatment of Hepatitis. J. Hepatol. 66, 153–194 (2017).
- Hepatitis C guidance. AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 62, 932–954 (2015).
- Isaacs D, Abdelaziz N, Keller M et al. Measuring the response of extrahepatic symptoms and quality of life to antiviral treatment in patients with hepatitis C. Hepat. Res. Treat. 2013, 910519 (2013).
- Fadda P, La Civita L, Zignego AL. Hepatitis C virus infection and arthritis. A clinico-serological investigation of arthritis in patients with or without cryoglobulinemic syndrome. Reumatismo. 54, 316–323 (2002).
- Zuckerman E, Keren D, Rozenbaum M et al. Hepatitis C virus-related arthritis: characteristics and response to therapy with interferon alpha. Clin. Exp. Rheumatol. 18, 579–584 (2000).
- Ramos-Casals M, Stone JH, Cid MC et al. The cryoglobulinaemias. Lancet. 379, 348–360 (2012).
- Ramos-Casals M, Muñoz S, Medina F et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J. Rheumatol. 36, 1442–1448 (2009).
- Doffoel-Hantz V, Loustaud-Ratti V, Ramos-Casals M et al. Evolution of Sjogren syndrome associated with hepatitis C virus when chronic hepatitis C is treated by interferon or the association of interferon and ribavirin. Rev. Med. Interne. 26, 88–94 (2005).
- Chen MH, Chen MH, Tsai CY et al. Incidence and antiviral response of hepatitis C virus reactivation in lupus patients undergoing immunosuppressive therapy. Lupus. 24, 1029–1036 (2015).
- Perlemuter G, Cacoub P, Sbai A et al. Hepatitis C virus infection in systemic lupus erythematosus: a case-control study. J. Rheumatol. 30, 1473–1478 (2003).
- Onishi S, Nagashima T, Kimura H et al. Systemic lupus erythematosus and Sjogren’s syndrome induced in a case by interferon alpha used for the treatment of hepatitis C. Lupus. 19, 753–755 (2010).
- Younossi ZM, Stepanova M, Henry L et al. An In-Depth Analysis of Patient-Reported Outcomes in Patients With Chronic Hepatitis C Treated With Different Anti-Viral Regimens. Am. J. Gastroenterol. 111, 808–816 (2016).
- Spiegel BMR, Younossi ZM, Hays RD et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 41, 790–800 (2005).
- Ferri C, Ramos-Casals M, Zignego AL et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun. Rev. 15(12), 1145–1160 (2016).
- Ghetie D, Mehraban N, Sibley CH. Cold hard facts of cryoglobulinemia: updates on clinical features and treatment advances. Rheum. Dis. Clin. North. Am. 241, 93–108 (2015).
- Asselah T, Boyer N, Saadoun D et al. Direct acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver. Int. 36, 47–57 (2016).
- Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non- Hodgkin’s lymphomas: what do we know? Ther. Adv. Hematol. 7, 94–107 (2016).
- Cacoub P, Comarmond C, Domont F et al. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther. Adv. Infect. Dis. 3, 3–14 (2016).
- Hausfater P, Cacoub P, Assogba U et al. Plasma exchange and interferon-alpha pharmacokinetics in patients with hepatitis C virus-associated systemic vasculitis. Nephron. 91, 627–630 (2002).
- Sene D, Ghillani-Dalbin P, Amoura Z et al. Rituximab may form a complex with IgMkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis. Rheum. 60, 3848–3855 (2009).
- Hsu M, Zhang J, Flint M et al. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. 100, 7271–7276 (2003).
- Li Q. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl. Acad. Sci. 106, 16410–16415 (2009).
- Baugh JM, Garcia-Rivera JA, Gallay PA. Host targeting agents in the treatment of hepatitis C: a beginning and an end? Antiviral. Res. 100, 555–561 (2013).
- Ashfaq U, Javed T, Rehman S et al. An overview of HCV molecular biology, replication and immune responses. Virology. Journal. 8, 161 (2011).
- Agnello V, Abel G, Elfahal M et al. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 96, 12766–12771 (1999).
- Gardner JP, Durso RJ, Arrigale RR et al. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc. Natl. Acad. Sci. USA. 100, 4498–4503 (2003).